Abstract
Gastrointestinal diseases are becoming a growing public health problem. One of them is inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD). The incidence of IBD is increasing in developing countries and declining in developed countries, affecting people of all ages. Researchers have been exploring new treatment options including insulin signaling pathways in the inflammation of the gastrointestinal tract. It seems that a better understanding of the mechanism of IGF-1, GLP-1 and TL1A on the gut microbiota and inflammation may provide new advances in future therapeutic strategies for patients with IBD, but also other intestinal diseases. This review aims to synthesize insights into the effects of GLP, IGF and anti-TL1A on inflammation and the gut microbiota, which may enable their future use in therapy for people with intestinal diseases.
1. Introduction
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic diseases with periods of exacerbation and remission. In UC, inflammation is localized to the rectum and/or colon, involving the mucosa and submucosa [1]. In CD, inflammation can be located along the entire length of the gastrointestinal tract, from the mouth, through the esophagus, stomach, small intestine, large intestine, and into the rectum. However, most often it is localized in the terminal segment of the small intestine and involves the entire thickness of the gastrointestinal wall [2]. In developing countries, there is an increase in the incidence of IBD, whereas in developed countries, there is a stabilization of the cases. Diseases can occur at any age, but the peak incidence occurs between the second and fourth decades of life. The incidence occurs at similar levels in both men and women [3,4].
Despite the many therapeutic agents available, which include glucocorticosteroids, immunosuppressants and biologics, there are patients who do not achieve remission of the disease. Therefore, recently, researchers have been focusing on new pharmaceutical options, which include a mechanism for rectal insulin signaling [5]. The purpose of our review is to summarize the mechanisms of the effects of insulin, glucagon-like peptide (GLP) and insulin-like growth factor (IGF) on inflammation and the gut microbiota to date. As a consequence, it will be possible to discover a new therapeutic option not only for patients with IBD, but also for other intestinal diseases.
2. Mechanisms of Intestinal Inflammation
Mechanisms of intestinal inflammation affect each other and are interrelated, including interactions between immune cells, the intestinal microbiota, and the intestinal epithelium [6]. Several mechanisms can be distinguished that predispose to inflammation in the intestines. One of them is an inadequate response of the immune system. One component is immune system cells, such as macrophages, which help the body maintain tissue homeostasis, which is why a link has been observed between abnormal changes in monocytes to macrophages and the occurrence of IBD [7]. Furthermore, macrophages with the M1 phenotype may predispose to IBD, while appropriate therapy that promotes the polarization of M2 macrophages may reduce disease activity [8]. Another type of cell is dendritic cells (DCs), which are found in the intestinal lamina propria that contribute to maintaining intestinal homeostasis [9]. In IBD, colonic DCs show an abnormal immature phenotype; in addition, intestinal mucosa DCs in CD secrete greater amounts of interleukin (IL)-12 and IL-6, and show an increased expression of toll-like receptors (TLR) 4 and TLR2 [10]. Lymphocytes also play an important role in the mechanism of intestinal inflammation. Pro-inflammatory T cells that enter the intestinal tract in IBD, for example, are T helper (Th) 1, which secrete interferon gamma (IFN-γ), which in turn shows the ability to stimulate macrophages to produce and secrete pro-inflammatory cytokines. Another example is Th17, which secretes cytokines and also shows an indirect effect by improving neutrophil recruitment [11,12,13]. In addition, transcription factors and signaling pathways also play an important role in the mechanisms of the inflammatory process, e.g., nuclear factor-κB (NF-κB), signal transducer and activator of transcription 3 (STAT3) and mitogen-activated protein kinase (MAPK) [14,15,16].
2.1. Factors Related to the Intestinal Microbiota That Affect the Maintenance of Homeostasis in the Intestinal Tract
One of the factors predisposing to IBD is intestinal dysbiosis. A decrease in microbial diversity and a change in quality, an increase in the number of pathogenic microorganisms and a decrease in the commensal microbiota affect the appearance of a number of changes in the normal functioning of the intestinal barrier and the maintenance of intestinal homeostasis [17]. There are several factors that regulate the inflammatory process that occurs in the intestines. One of them is the regulation of the immune system. Certain bacterial species have been linked to Th cell development and Treg cell induction [18]. Lactobacillus rhamnosus GG (LGG), on the other hand, can stimulate Th17/Treg balance in the gut [19]. Macrophages also respond to stimuli from the gut microbiota. PGE2 + macrophages are crypt niche cells that increase Wnt/β-catenin signaling in intestinal stem cells via prostaglandin E2 (PGE 2) receptors [20]. Another factor against the appearance of inflammation in the intestine is the proper functioning of the intestinal barrier. It is crucial to preventing the passage of pathogenic microorganisms into the body. Factors that determine the proper function of the intestinal barrier include tight junctions. These are transmembrane proteins that connect neighboring intestinal cells; they include, for example, claudins and zonula occludens [21]. In addition, the protective barrier is formed by a layer of mucus, which consists mainly of water, lipids, electrolytes, and proteins. Some intestinal microorganisms can affect the regulation of the mucus barrier (A. muciniphila). They can increase mucus secretion and the thickness of the mucus layer [22]. Another factor is pattern recognition receptors (PRRs), which recognize structures in pathogens called pathogen-associated molecular patterns (PAMPs) [23]. Examples include TLR4, which recognizes lipopolysaccharides (LPS), components of bacterial cell membranes, and TLR5, which recognizes flagellin, a protein that builds bacterial threads [24,25]. Furthermore, a receptor belonging to the NOD-like receptor (NLR) family, i.e., nucleotide-binding oligomerization domain-containing protein 2 (NOD2), participates in maintaining normal intestinal homeostasis through the regulation of the immune system. NOD2 deficiency is associated with reduced antimicrobial activity by Paneth cells, and an imbalance of the intestinal microbiota can occur [26].
2.2. Receptor-Mediated Insulin Signaling
More recently, it has been shown that metabolism is involved in the regulation of immunity [27]. Insulin is a key hormone that is involved in the regulation of glucose, but also fat and protein metabolism. It acts through the insulin receptor (IR) to influence the regulation of nutrient uptake and storage in liver tissues, adipose tissue and muscle. IR has been shown to exist as two isoforms: IR-A and IR-B. The difference between these isoforms is the exclusion (IR-A) or inclusion (IR-B) of exon 11. This exon encodes a 12-amino acid region that is located at the C-terminus of the subunit that binds the α IR ligand [28]. IR-A has a high affinity for insulin and IGF-2, and about a ten-times-lower affinity for GF-1. In contrast, IR-B binds insulin, but has a much lower affinity for insulin-like growth factor (IGF)-1 and IGF-2 [28]. Energy storage by insulin is mediated by the phosphotidyl-inositol-3 kinase (PI3K) and MAPK pathways. This results in the activation and phosphorylation of insulin receptor substrates (IRSs) [29]. Insulin receptor signaling enhances nutrient uptake through T cells [30].
IR appears to be expressed in the intestinal epithelium, although its role in growth or function is not clearly defined [28]. In addition, the insulin pathway exhibits regulatory effects in mucosal immunology [31]. Zhang et al. showed that high glucose intake promotes Th17 lymphocyte differentiation. This occurs through a mechanism of transforming growth factor-beta (TGF-β) activation via an increase in reactive oxygen species (ROS) in T lymphocytes. In addition, high amounts of glucose promoted the exacerbation of autoimmunity in mice with artificially induced colitis [32]. Yassin et al. in their study presented INSR in the intestinal epithelium as a possible therapeutic target for IBD. The researchers suggest that INSR may accelerate tissue regeneration and increase the resistance of the EIC to damage from inflammation. These actions appear to be facilitated by increased expression of cytoprotective proteins such as Car3 [33]. In contrast, in a study on mice with dextran sulfate sodium (DSS)-induced intestinal inflammation, Li et al. showed that the activation of the insulin pathway increased the expression of enhancer of zeste homolog 2 (EZH2) in mucosal T cells during inflammation. This promoted the differentiation of tissue-resident memory (TRM) T cells, but also upregulated intestinal intraepithelial lymphocytes (IELs) and inflammatory cytokines. Consequently, it promoted the exacerbation of colitis during the inflammatory phase [5]. Due to the discrepancy in some of the findings, further research is needed to clarify and define the role of IR in intestinal diseases
3. Regulation of Inflammation in the Intestines
3.1. The Role of Insulin in Regulating Inflammation
According to Makhijani et al., disturbed insulin-dependent metabolic homeostasis leads to immune dysfunction, and sensitization to the action of this hormone may bring benefits in reducing inflammation [34]. Systemic inflammation can lead to the development of many diseases, including cardiovascular diseases, cancer, non-alcoholic liver disease and diabetes. Due to the common nature of the problem, inflammatory diseases have been considered a more important cause of death worldwide [35]. Hyperglycemia, an early complication of diabetes, can also indue inflammatory programming of macrophages [36,37]. Hyperglycemia in type 2 diabetes (T2DM) may occur as a result of insulin resistance and inappropriate insulin secretion, and glycemic control can be achieved by administering insulin in an appropriate dose [37]. It is believed that insulin may play an important immunomodulatory and anti-inflammatory role in the body [38].
As available data indicate, insulin can exert homeostatic and anti-inflammatory effects in the human body. However, the molecular mechanism by which insulin can reduce inflammation in the body is still not fully understood. There are likely several potential mechanisms by which insulin may reduce inflammation in the body. One of them is its ability to lower blood glucose levels [39]. Hyperglycemia in diabetes may induce toxicity due to the greater flow of glucose through the glycolytic pathway [40]. Additionally, hyperglycemia caused by oxidative stress and cytokine release leads to the expression of cell adhesion molecules on activated endothelial cells in diabetic patients [41]. As a result of oxidative stress, mitochondria become damaged and a number of factors are activated, including the activation of TLRs, which may lead to the dysfunction of pancreatic β-cells [42]. Previous findings have already shown that insulin can inhibit the expression of TLRs at the transcriptional level, probably through its inhibitory effect on the PU.1 transcription factor [43].
It has also been shown that the concentrations of cell adhesion molecules (intercellular adhesion molecule 1—ICAM; vascular cell adhesion molecule 1—VCAM; E-selectin) positively correlate with an increase in the level of glycated hemoglobin (HbA1c) in young people with type 2 diabetes and are associated with blood pressure and microalbuminuria, which are markers of vascular damage [44]. In patients with type 2 diabetes who were introduced to intensive insulin therapy using continuous subcutaneous infusion, a reduction in pro-inflammatory cytokines was observed. Regardless of the initial HbA1c level, a reduction in interleukin-6 receptor (IL-6R), regulated upon activation normal T-cell expressed and secreted (RANTES) and ICAM-1 levels was observed in all groups. These cytokines were significantly involved in the tumor necrosis factor (TNF) signaling pathway [45]. ICAM-1 is a key molecule in the progression of inflammation and leads to endothelial cell damage [46]; therefore, inhibiting ICAM-1 release through insulin supply may likely prevent the development of complications.
It is worth noting that lowering glucose levels is associated with a lower number of advanced glycation end products (AGEs) and a reduction in the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) via RAGE (receptor for AGEs), and thus reducing inflammation [47]. Insulin can suppress LPS/TLR 4signals in leukocytes via the mTORC2-Akt-FoxO signaling axis [48]. It is likely that the reduction in FOXO1 and TLR transcription may serve to suppress insulin-mediated inflammation [34].
Another interesting effect is the effect of insulin on the production of nitric oxide (NO) from the vascular endothelium, which, through increased blood flow, increases glucose uptake in skeletal muscles [49]. In diabetic patients, vascular resistance to NO develops, and insulin supply may reduce this resistance by reducing oxidative stress and peroxygen production [50]. In patients with IBD, the induction of endogenous NO production by enterocytes with supplements may result in improved epithelial integrity and the amelioration of colitis [51].
A study by Huang et al. conducted in an animal model showed that insulin can alleviate encephalopathies in sepsis by inhibiting astrocyte NFκB and microglial MAPK [52]. A study by Chang et al. showed that insulin is a key regulator of NLPRP3 inflammasome activation. It is likely that insulin exerts its anti-inflammatory effects by attenuating ASC speckle formation to reduce inflammasome activation and reduce pro-inflammatory cytokine secretion in an INSR- and IGF1R-dependent manner. Moreover, insulin administration reduced the production of IL-1β by THP-1 cells [38].
3.2. Reducing Inflammation in the Gut by Influencing the Gut Microbiota
Different glycemic states can alter intestinal physiology [53]. A state of elevated blood glucose may cause increased intestinal permeability through glucose transporter 2 (GLUT2)-dependent transcriptional reprogramming of the intestinal epithelium and alteration of tight junction integrity and adhesion [54]. In an in vitro study, Dubois et al. showed that high glucose levels induce morphological and functional changes such as increased permeability, reduced mucus secretion and increased alkaline phosphatase activity. The authors of the study emphasized that the main therapeutic goal in maintaining intestinal health is adequate glycemic control [55].
There are many data indicating the relationship between impaired glycemia and intestinal dysbiosis [56,57,58,59,60]. In a study on an animal model conducted by Wang et al., a positive effect of intensive insulin therapy on the improvement of intestinal morphological parameters was noted, including its length, villus height, microvilli length and crypt depth. Moreover, a beneficial effect in modulating the intestinal microbiota at various taxonomic levels has been demonstrated. Bacterioidetes decreased, while Firmicutes actinobacteria and Deferribacteres increased significantly [61].
An analysis of the fecal microbiota of pregnant women suffering from gestational diabetes (GDM) showed a higher content of Clostridiales, Lactobacillus and Bacteroidetes. Then, after insulin treatment, a higher Firmicutes/Bacteroidetes ratio was found compared to women who controlled GDM with diet, which was also observed in meconium and the first feces of newborns [62].
A summary of the anti-inflammatory effects of insulin is presented in Figure 1.
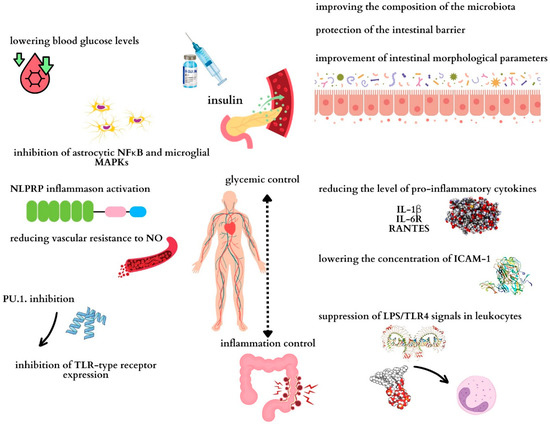
Figure 1.
Potential mechanisms through which insulin may exert anti-inflammatory effects on the intestine. Maintaining proper glycemia determines the control of inflammation in the body and therefore in the intestines. NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; ICAM-1: intercellular adhesion molecule 1; NO: nitric oxide; TLR4: Toll-like receptor 4; LPS: lipopolysaccharide; IL-1β: interleukin 1 beta; IL-6R: interleukin-6 receptor; RANTES: regulated on activation, normal T-cell expressed and secreted.
3.3. Other Factors That Reduce Inflammation in the Gut
3.3.1. A High-Fiber Diet
A diet rich in dietary fiber has a positive effect on the composition of the intestinal microbiota and may reduce inflammation in the intestines. Fiber metabolism also leads to the release of feluric acid, which has antioxidant and anti-inflammatory properties [63].
The fermentation of whey protein with a glycolic acid and pentozophosphorus acid results in microbiological fermentation allowing the extraction of sugar cane sugar (SCFA). Bacteria involved in the development of SCA include Bacteroides spp. Prevotella spp., and Akkermansia muciniphila [64,65]. Among the SCAs, acetic acid, propionic acid and butyric acid stand out, but butyric acid is considered to have the greatest anti-inflammatory role in the body. The bacteria are from the Firmicutes type, including Roseburia, Eubacterium rectale and Faecalibacterium prausnitzii. Butyrate inhibits Th17 cell differentiation and promotes Treg differentiation and secretion of IL-10 and the secretory IgA [65]. It is probably the case that butyrate can improve insulin sensitivity and regulate glucose metabolism. Butyrate’s effect on insulin signaling is related to its metabolism. In a study by Rios-Morales et al., butyrate increased the levels of proteins and metabolites involved in butyrate oxidation itself. The inhibition of butyrate oxidation increased its availability in the body, and its higher concentration led to histone deacetylase inhibition leading to improved insulin sensitivity [66]. HDAC can regulate glucose metabolism, insulin release expression and also insulin signaling [67]. Additionally, SCFAs can affect insulin signaling by affecting β-cells and intestinal cells thereby altering the function of certain proteins [68].
Studies indicate that SCFAs act on G protein-coupled receptors (GPCRs): GPR43/FFAR2 and GPR41/FFAR3. These receptors are found in many tissues in the body, including pancreatic β-cells [69].
FFAAR2 is present in pancreatic islet α and β cells as well as intestinal enteroendocrine cells, and FFAR3 expression additionally occurs on immune cells, intestinal neurons and sympathetic ganglia, among others [66].
The role of SCFAs in reducing inflammation, improving insulin signaling and glucose metabolism still needs to be thoroughly investigated.
3.3.2. Dietary Supplements
Supplements used to reduce inflammation in the intestine include probiotics, butyrate, lactoferrin, palmitoylethanolamide, phosphatidocholine, silymarin and omega 3 acids. It is believed that these substances, in addition to their anti-inflammatory effect, can support maintaining the microbial balance in the intestines and strengthen the barrier function of the mucosa (Table 1) [70].

Table 1.
The influence of selected dietary supplement on the reduction in inflammation.
3.3.3. Physical Activity
A recent meta-analysis of 10 observational studies found that physical activity is inversely associated with the risk of developing IBD [77]. A sedentary lifestyle may cause a Th1/Th2 imbalance, which directly affects the secretion of pro-inflammatory and anti-inflammatory cytokines [78]. Moderate exercise has been associated with a reduction in the levels of cytokines such as IL-1 and TNF-α and an increase in anti-inflammatory factors IL-6, IL-10 and IL-1ra (interleukin-1 receptor antagonist) released by myokines [79].
3.3.4. Adequate Amount of Sleep
Disturbances in circadian rhythms have been associated with worsening of the severity of colitis. It is likely that improving the quality and treatment of sleep disorders may help to alleviate inflammation in the gut [80]. Sleep deprivation may lead to cognitive impairment and activation of the TLR4/NF-κB signaling pathway and deterioration of the intestinal microbiota, thus leading to inflammation [81]. Bermingham et al. showed that social jet lag caused by, among others, shift work and the lack of a fixed sleep rhythm can lead to intestinal dysbiosis and inflammation [82]. Ensuring good quality and duration of sleep seems to be important to reduce intestinal inflammation.
4. Effects of Insulin-like Growth Factor 1 (IGF-1) on Intestinal Inflammation and Gut Microbiota
IGF is a large family with three ligands (IGF-1, IGF-2, insulin) and two receptors (IGF-1 (IGF-IR) and IGF-2 (IGF-IIR)). In the gastrointestinal tract, the IGF system exists as a paracrine, endocrine and autocrine regulator for cell proliferation, survival and apoptosis. In addition, IGF is important in carbohydrate and protein metabolism, but also affects energy balance. Most of the circulating IGF-1 is synthesized in the liver through the regulation of growth hormone (GH) [83]. So far, it has been shown that the most common metabolites of the gut microbiota are short-chain fatty acids, serotonin, polyamines and ATP. However, recent studies have documented that the microbiota also affects insulin-IGF-1 levels [84,85,86]. Low IGF-1 levels appear to be associated with the induction of bacterial translocation growth [87]. In addition, it has been observed that the use of antibiotic therapy in mice reduces serum IGF-1. As a consequence, there is a decrease in bone mass. In contrast, after treatment with SCFAs, the amount of IGF-1 returns to normal, but also bone mass returns to levels like those in mice not treated with antibiotics. The study authors suggest that it is SCFAs that may be involved in the induction of IGF-1 by the gut microbiota [88].
Chena et al. showed that IGF-1 contributes to mucosal regeneration through the β-arrestin2-mediated extracellular signal-associated kinase signaling pathway [89]. Xu et al. showed that the intravenous injection of human tonsil-derived MSCs (T-MSCs) had an effect on alleviating colitis. Moreover, the analysis showed that administration of T-MSCs increased endogenous IGF-1 in treated mice. In contrast, the administration of IGF-1 receptor inhibitors reduced the therapeutic functions of T-MSCs. Additional stimulation of IGF-1 had the effect of reducing apoptosis and promoting cell proliferation. The authors suggest that acting on endogenous IGF-1 secretion can be used as a therapeutic strategy by maintaining the integrity and promoting the regeneration of the colonic epithelium [90]. In addition, another study showed that MSCs supplemented with IGF-1, among others, induced cell cycle regulation of intestinal stem cells and induced a balance of anti- and pro-inflammatory cytokines. The result was a mitigation of intestinal damage [91]. Similarly, another study using MSCs and IGF-1 observed that IGF-1 maximized the therapeutic effect of MSCs by attenuating inflammation, but also promoting colon regeneration [92]. An analysis of parenteral nutrition in rats showed that both GH and IGF-1 administration reduced total body weight loss and nitrogen excretion. In addition, they induced an increase in protein synthesis. In addition, the results showed that GH action increased IGF-1-mRNA levels in the liver and IGF-1 levels in plasma. In contrast, IGF-1 action increased IGF-1 in plasma, but did not change IGF-1-mRNA levels in the liver [93]. In another study, an attempt was made to treat experimental colitis through the use of GH. The process was found to increase IGF-1 levels and body weight in mice, but without affecting colitis [94]. Bohin et al. in a study in mice, showed that IGF-1 signaling through mTORC1 activation potentially induced regeneration of intestinal crypts [95]. Another study showed that the action of IGF-1 combined with fibroblast growth factor 2 (FGF-2) can increase cell diversity in small intestinal organoids in mice [96]. One study confirmed that IGF-1 levels were reduced in patients. In contrast, prednisolone treatment affected its increase [97]. Sipos et al. showed that CD patients exhibit increased expression of the insulin-like growth factor 1 receptor (IGF1R) signaling pathway in submucosal fibroblast cells and subserosal adipocytes [98]. It appears that IGF-1 release may be the main therapeutic mechanism in the treatment of experimental colitis with ghrelin [99]. However, studies on the mechanisms of IGF-1 in IBD patients are still lacking.
5. Effects of Glucagon-like Peptide-1 (GLP-1) on Intestinal Inflammation and Gut Microbiota
Glucagon-like peptide-1 (GLP1) is an incretin hormone that controls glycemia, slows intestinal motility, and shows receptor expression in various types of mucosal cells. It is produced in the L cells of the small intestine and large intestine, while it is released after nutrients are delivered to the intestinal lumen [100].
The effects of GLP1 on the gut are multidirectional and can affect, among other things, the integrity of the intestinal barrier. In their study, Anbazhagan et al. showed that GLP-1-SSM administered as an injection can reduce intestinal mucosal inflammation and the incidence of diarrhea in patients with IBD [101]. It can also reduce intestinal permeability and intensify the mucosal healing process [102]. In another study, Funayama et al. observed that a centrally administered GLP-1 analog showed the ability to improve colon permeability [103]. GLP1 can also improve intestinal barrier function by modulating the intestinal microbiota and stimulating crypt cell division [104]. GLP-1 receptor agonists up-regulate chemokine ligand 20 (CCL20), mucin 5b (MUC5) and IL-33, with which they show the ability to influence the maintenance of homeostasis in the gut [105]. Ebbesen et al. also indicate that GLP1 may exhibit regenerative capacity of damaged epithelial barriers [106]. The GLP-1R agonist (liraglutide) had a beneficial effect on TJ proteins (zonula occludens-1 (ZO-1), occludins) [107]. Arvanitakis et al. came to a similar conclusion in their review that by administering glucagon-like peptide 1 receptor agonists (GLP-1 RAs) to patients with IBD, the benefits of therapy can be maximized, among other things, by maintaining and adequately functioning TJ [108]. In another study, similarly, liraglutide showed the ability to reduce endothelial nitric oxide synthase (eNOS) expression and to recover normal TJ protein function [109]. The protection of the intestinal mucosa in the context of IBD is very important, as patients experience damage to the intestinal barrier, which predisposes pathogenic microorganisms to enter the body, thus causing inflammation [6]. Furthermore, GLP1 and its agonists can reduce anti-inflammatory cytokine production by, among other things, reducing NF-κB phosphorylation [110]. Amato et al. also showed that the expression of IL-6, IL-8, IL-1β and TNF-α decreased after teduglutide treatment [111]. In addition to reducing pro-inflammatory cytokine secretion, liraglutide therapy also attenuated neutrophil infiltration into the intestine [112]. GLP-1 also shows the ability to reduce the expression of CD69 and CD11b and the production of IL-4 and IL-8 by LPS-induced eosinophils [113]. GLP 1 also affects changes in the gut microbiota. In their paper, Everard et al. present a link between bacterial metabolites and the triggering of GLP1 secretion. However, they emphasize that certain bacteria, e.g., Bifidobacterium spp, can modulate this relationship both indirectly and directly [114]. In Angelini et al., similar conclusions were reached by the gut microbiota (SCFAs) modulating the activity of enteroendocrine cells, increasing, for example, GLP1 secretion [115]. On the other hand, SCFAs, such as butyric acid, show anti-inflammatory properties [116].
6. Tumor Necrosis Factor-like Ligand 1A (TL1A)
Tumor necrosis factor (TNF)-like cytokine 1A (TL1A) belongs to the TNF family. It is a type 2 transmembrane protein first described in 2002. TL1A expression is increased in macrophages and dendritic cells [117]. It is secreted by cells that present the antigen after stimulation [118]. It can regulate inflammation and intestinal homeostasis through the TL1A/DR3/DcR3 pathway [119]. Anti-TL1A treatment targets TL1A (Tumor Necrosis Factor-like ligand 1A), which regulates mucosal defense in the intestines, and therefore inflammation, and is involved in the pathogenesis of IBD [120]. Therefore, the human immunoglobulin G1 monoclonal antibody (anti-TL1A antibody) is a potential therapeutic agent for moderate to severe UC [121]. The use of such therapies has been shown to potentially inhibit endogenous TL1A activity [122]. Furthermore, TL1A and its functional death receptor 3 (DR3) may play a role in intestinal fibrosis during ongoing inflammation [123]. DR3 signaling directly affects fibroblasts, influencing activation and migration, and ultimately intestinal fibrosis [124]. Therefore, the modified stable ligand TL1A shows the ability to stimulate T cells by specific binding to DR3 [125]. Jacob et al. also indicate that intestinal fibrosis mediated by TL1A may depend on the intestinal microflora [126]. Bamias’ work shows that TL1A and DR3 enhance Th1, Th2 and Th17 responses [127]. Therefore, the anti-TL1A antibody can alleviate intestinal fibrosis [128]. A study by Yang et al. in animal models showed that TL1A disrupts intestinal epithelial barrier function and affects TJ expression regulation [129]. In another article, the authors found that the activation of DR3 signaling results in loss of ILC3, which is associated with increased inflammation [130]. Anti-TL1A treatment is a promising therapeutic strategy.
7. Conclusions
Interactions between immune system cells, intestinal microbiota and the intestinal epithelium are important mechanisms of IBD pathogenesis. Recognition of the complex mechanisms of IBD pathogenesis and potential therapeutic interventions, including the effects of insulin, IGF-1, GLP-1 and TL1A on inflammation and intestinal microbiota, may contribute to the development of more effective therapeutic strategies for patients with inflammatory bowel diseases. There is research suggesting that insulin may exert anti-inflammatory effects by reducing blood glucose levels and regulating signaling pathways related to the immune response. Furthermore, insulin can influence the composition and diversity of microorganisms in the intestines. In turn, the use of GLP-1 receptor agonists may improve the function of the intestinal barrier, and IGF-1 may promote the regeneration of the intestinal epithelium and reduce inflammation. Treatment with anti-TL1A antibodies also seems to be a promising strategy in patients with moderate to severe ulcerative colitis. Further in vitro and clinical studies may be helpful to better understand the role of these factors in maintaining intestinal homeostasis and reducing inflammation in IBD patients.
Author Contributions
Conceptualization, R.F.; writing—original draft preparation, S.J.-C., A.S.-D., K.F. and R.F. writing—review and editing, S.J.-C., A.S.-D., K.F. and R.F.; supervision, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Li, T.; Han, B.; Wang, L.; Sun, L.; Cai, Y.; Yu, M.; Xiao, W.; Yang, H. Activation of mucosal insulin receptor exacerbates intestinal inflammation by promoting tissue resident memory T cells differentiation through EZH2. J. Transl. Med. 2024, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Yan, W.; Xu, L. Macrophage polarization in inflammatory bowel disease. Cell Commun. Signal 2023, 21, 367. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, J.; Ayansola, H.; Masatoshi, H.; Zhang, B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front. Immunol. 2021, 11, 623691. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bris, R.; Saez, A.; Herrero-Fernandez, B.; Rius, C.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor. Rev. 2023, 69, 28–42. [Google Scholar] [CrossRef]
- Wang, B.; Shen, J. NF-κB Inducing Kinase Regulates Intestinal Immunity and Homeostasis. Front. Immunol. 2022, 13, 895636. [Google Scholar] [CrossRef] [PubMed]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, T.; Wu, J.; Fan, D.; Liu, B.; Zhu, X.; Guo, H.; Du, Y.; Liu, F.; Tian, Y.; et al. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022, 32, 555–569. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243, Erratum in Gut 2023, 72, e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, S.Q.; Lin, Z.M.; He, S.J.; Zuo, J.P. NOD-like receptors in autoimmune diseases. Acta Pharmacol. Sin. 2021, 42, 1742–1756. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Chen, S.; He, R.; Chao, G.; Zhang, S. TLR5 Signaling in the Regulation of Intestinal Mucosal Immunity. J. Inflamm. Res. 2023, 16, 2491–2501. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Jin, T.; Yi, C.; Ocansey, D.K.W.; Mao, F. The role of NOD2 in intestinal immune response and microbiota modulation: A therapeutic target in inflammatory bowel disease. Int. Immunopharmacol. 2022, 113, 109466. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Delgoffe, G.M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021, 21, 785–797. [Google Scholar] [CrossRef]
- Andres, S.F.; Simmons, J.G.; Mah, A.T.; Santoro, M.A.; Van Landeghem, L.; Lund, P.K. Insulin receptor isoform switching in intestinal stem cells, progenitors, differentiated lineages and tumors: Evidence that IR-B limits proliferation. J. Cell Sci. 2013, 126, 5645–5656. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681. [Google Scholar] [CrossRef]
- Yassin, M.; Sadowska, Z.; Tritsaris, K.; Kissow, H.; Hansen, C.H.F.; Forman, J.L.; Rogler, G.; Troelsen, J.T.; Pedersen, A.E.; Olsen, J. Rectal Insulin Instillation Inhibits Inflammation and Tumor Development in Chemically Induced Colitis. J. Crohn’s Colitis 2018, 12, 1459–1474. [Google Scholar] [CrossRef]
- Makhijani, P.; Basso, P.J.; Chan, Y.T.; Chen, N.; Baechle, J.; Khan, S.; Furman, D.; Tsai, S.; Winer, D.A. Regulation of the immune system by the insulin receptor in health and disease. Front. Endocrinol. 2023, 14, 1128622. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Matuschik, L.; Riabov, V.; Schmuttermaier, C.; Sevastyanova, T.; Weiss, C.; Klüter, H.; Kzhyshkowska, J. Hyperglycemia Induces Inflammatory Response of Human Macrophages to CD163-Mediated Scavenging of Hemoglobin-Haptoglobin Complexes. Int. J. Mol. Sci. 2022, 23, 1385. [Google Scholar] [CrossRef]
- Mouri, M.I.; Badireddy, M. Hyperglycemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430900/ (accessed on 24 April 2023).
- Chang, Y.W.; Hung, L.C.; Chen, Y.C.; Wang, W.H.; Lin, C.Y.; Tzeng, H.H.; Suen, J.L.; Chen, Y.H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Changes in Cells Associated with Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 2397. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; George, T.P.; Mujammami, M.; Isnani, A.; Alfadda, A.A. The association of cell adhesion molecules and selectins (VCAM-1, ICAM-1, E-selectin, L-selectin, and P-selectin) with microvascular complications in patients with type 2 diabetes: A follow-up study. Front. Endocrinol. 2023, 14, 1072288. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 5743. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Mohanty, P.; Deopurkar, R.; Sia, C.L.; Korzeniewski, K.; Abuaysheh, S.; Chaudhuri, A.; Dandona, P. Acute modulation of toll-like receptors by insulin. Diabetes Care 2008, 31, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Tryggestad, J.B.; Shah, R.D.; Braffett, B.H.; Bacha, F.; Gidding, S.S.; Gubitosi-Klug, R.A.; Shah, A.S.; Urbina, E.M.; Levitt Katz, L.E.; TODAY Study Group. Circulating adhesion molecules and associations with HbA1c, hypertension, nephropathy, and retinopathy in the Treatment Options for type 2 Diabetes in Adolescent and Youth study. Pediatr. Diabetes 2020, 21, 923–931. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Dai, P.; Liu, L.; Yang, Y.; Liu, X.; Li, Y.; Liao, Z. The effect of short-term intensive insulin therapy on inflammatory cytokines in patients with newly diagnosed type 2 diabetes. J. Diabetes 2022, 14, 192–204. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Nguyen, A.; Wang, C.; He, L.; Li, H.; Hallowell, P.; McNamara, C.; Schmidt, A.M. AGE/RAGE/DIAPH1 axis is associated with immunometabolic markers and risk of insulin resistance in subcutaneous but not omental adipose tissue in human obesity. Int. J. Obes. 2021, 45, 2083–2094. [Google Scholar] [CrossRef]
- Zhang, Z.; Amorosa, L.F.; Coyle, S.M.; Macor, M.A.; Birnbaum, M.J.; Lee, L.Y.; Haimovich, B. Insulin-Dependent Regulation of mTORC2-Akt-FoxO Suppresses TLR4 Signaling in Human Leukocytes: Relevance to Type 2 Diabetes. Diabetes 2016, 65, 2224–2234. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Vascular nitric oxide resistance in type 2 diabetes. Cell Death Dis. 2023, 14, 410. [Google Scholar] [CrossRef]
- Stettner, N.; Rosen, C.; Bernshtein, B.; Gur-Cohen, S.; Frug, J.; Silberman, A.; Sarver, A.; Carmel-Neiderman, N.N.; Eilam, R.; Biton, I.; et al. Induction of Nitric-Oxide Metabolism in Enterocytes Alleviates Colitis and Inflammation-Associated Colon Cancer. Cell Rep. 2018, 23, 1962–1976. [Google Scholar] [CrossRef]
- Huang, C.T.; Lue, J.H.; Cheng, T.H.; Tsai, Y.J. Glycemic control with insulin attenuates sepsis-associated encephalopathy by inhibiting glial activation via the suppression of the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways in septic rats. Brain Res. 2020, 1738, 146822. [Google Scholar] [CrossRef] [PubMed]
- Darra, A.; Singh, V.; Jena, A.; Popli, P.; Nada, R.; Gupta, P.; Bhadada, S.K.; Singh, A.K.; Sharma, V.; Bhattacharya, A.; et al. Hyperglycemia is associated with duodenal dysbiosis and altered duodenal microenvironment. Sci. Rep. 2023, 13, 11038. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Dubois, N.; Muñoz-Garcia, J.; Heymann, D.; Renodon-Cornière, A. High glucose exposure drives intestinal barrier dysfunction by altering its morphological, structural and functional properties. Biochem. Pharmacol. 2023, 216, 115765. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Al Bataineh, M.T.; Künstner, A.; Dash, N.R.; Alsafar, H.S.; Ragab, M.; Schmelter, F.; Sina, C.; Busch, H.; Ibrahim, S.M. Uncovering the relationship between gut microbial dysbiosis, metabolomics, and dietary intake in type 2 diabetes mellitus and in healthy volunteers: A multi-omics analysis. Sci. Rep. 2023, 13, 17943. [Google Scholar] [CrossRef]
- He, J.L.; Zhao, Y.W.; Yang, J.L.; Ju, J.M.; Ye, B.Q.; Huang, J.Y.; Huang, Z.H.; Zhao, W.Y.; Zeng, W.F.; Xia, M.; et al. Enhanced interactions among gut mycobiomes with the deterioration of glycemic control. Med 2024, 18, 909–925.e7. [Google Scholar] [CrossRef]
- Abdellatif, A.M.; Sarvetnick, N.E. Current understanding of the role of gut dysbiosis in type 1 diabetes. J. Diabetes 2019, 11, 632–644. [Google Scholar] [CrossRef]
- Luo, M.; Sun, M.; Wang, T.; Zhang, S.; Song, X.; Liu, X.; Wei, J.; Chen, Q.; Zhong, T.; Qin, J. Gut microbiota and type 1 diabetes: A two-sample bidirectional Mendelian randomization study. Front. Cell Infect. Microbiol. 2023, 13, 1163898. [Google Scholar] [CrossRef]
- Wang, H.; Tang, W.; Zhang, P.; Zhang, Z.; He, J.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to effects of intensive insulin therapy on intestinal morphological alteration in high-fat-diet-treated mice. Acta Diabetol. 2020, 57, 455–467. [Google Scholar] [CrossRef]
- Huang, L.; Sililas, P.; Thonusin, C.; Tongsong, T.; Luewan, S.; Chattipakorn, N.; Chattipakorn, S.C. Association Between Gut Microbiota and Insulin Therapy in Women With Gestational Diabetes Mellitus. Can. J. Diabetes 2022, 46, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Saha, S.; Umar, S. Health Benefits of Dietary Fiber for the Management of Inflammatory Bowel Disease. Biomedicines 2022, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, D.; Huang, J.; Zuo, T. The gut microbiome: Linking dietary fiber to inflammatory diseases. Med. Microecol. 2022, 14, 100070. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Rios-Morales, M.; Vieira-Lara, M.A.; Homan, E.; Homan, E.; Langelaar-Makkinje, M.; Gerding, A.; Li, Z.; Huijkman, N.; Rensen, P.C.N.; Wolters, J.C.; et al. Butyrate oxidation attenuates the butyrate-induced improvement of insulin sensitivity in myotubes. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166476. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Chakraborty, P.; Gangopadhyay, M.; Sahu, R.; Medala, V.; John, A.; Reddy, P.H.; De Feo, V.; et al. The Emerging Role of HDACs: Pathology and Therapeutic Targets in Diabetes Mellitus. Cells. 2021, 10, 1340. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signaling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Liu, J.L.; Segovia, I.; Yuan, X.L.; Gao, Z.H. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic β-Cell Growth and Insulin Secretion. Int. J. Mol. Sci. 2020, 21, 910. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Donato, K.; Bertelli, M. Dietary supplements for intestinal inflammation. J. Prev. Med. Hyg. 2022, 63, E214–E220. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Benvenuti, L.; Di Salvo, C.; Flori, L.; Piccarducci, R.; Daniele, S.; Martelli, A.; Calderone, V.; et al. Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 748021. [Google Scholar] [CrossRef] [PubMed]
- Kianifar, H.; Jafari, S.A.; Khalesi, M.; Kiani, M.; Sadeghi, T.; Jaafari, M.R.; Amani, F. The Impact of Silymarin on the Symptom Severity in Pediatric Patients with Inflammatory Bowel Disease: A Randomized Clinical Trial. Int. J. Pediatr. 2023, 11, 18274–18286. [Google Scholar] [CrossRef]
- Marton, L.T.; Goulart, R.A.; Carvalho, A.C.A.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Trindade, L.M.; Souza, A.; Torres, L.; Andrade, M.E.R.; Cardoso, V.N.; Generoso, S.V. Non-pharmacologic strategies for the management of intestinal inflammation. Biomed. Pharmacother. 2022, 145, 112414. [Google Scholar] [CrossRef]
- Tiong, H.T.; Fan, D.; Frampton, C.; Ananthakrishnan, A.N.; Gearry, R.B. Physical Activity Is Associated With A Decreased Risk Of Developing Inflammatory Bowel Disease: A Systematic Review And Meta-Analysis. J. Crohn’s Colitis 2024, 18, 1476–1485. [Google Scholar] [CrossRef]
- Protano, C.; Gallè, F.; Volpini, V.; De Giorgi, A.; Mazzeo, E.; Ubaldi, F.; Romano Spica, V.; Vitali, M.; Valeriani, F. Physical activity in the prevention and management of inflammatory bowel disease: A systematic review. J. Public Health 2024. [Google Scholar] [CrossRef]
- Davis, S.P.; Crane, P.B.; Bolin, L.P.; Johnson, L.A. An integrative review of physical activity in adults with inflammatory bowel disease. Intest. Res. 2022, 20, 43–52. [Google Scholar] [CrossRef]
- Qazi, T.; Farraye, F.A. Sleep and Inflammatory Bowel Disease: An Important Bi-Directional Relationship. Inflamm. Bowel Dis. 2019, 25, 843–852. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Stensrud, S.; Asnicar, F.; Valdes, A.M.; Franks, P.W.; Wolf, J.; Hadjigeorgiou, G.; Davies, R.; Spector, T.D.; Segata, N.; et al. Exploring the relationship between social jetlag with gut microbial composition, diet and cardiometabolic health, in the ZOE PREDICT 1 cohort. Eur. J. Nutr. 2023, 62, 3135–3147. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, J.F. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol. Metab. Clin. N. Am. 2012, 41, 409–423. [Google Scholar] [CrossRef]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- García Navas, P.; Ruíz Del Prado, M.Y.; Villoslada Blanco, P.; Recio Fernández, E.; Ruíz Del Campo, M.; Pérez Matute, P. Composition of the microbiota in patients with growth hormone deficiency before and after treatment with growth hormone. An. Pediatr. 2024, 100, 404–411. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, F.; Tao, J.; Tan, S.; Zeng, L.; Peng, X.; Wu, B. Insulin-Like Growth Factor-1 Contributes to Mucosal Repair by β-Arrestin2-Mediated Extracellular Signal-Related Kinase Signaling in Experimental Colitis. Am. J. Pathol. 2015, 185, 2441–2453. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Chen, J.; Chen, S.; Li, Z.; Liu, H.; Bai, Y.; Zhi, F. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics 2020, 10, 12204–12222. [Google Scholar] [CrossRef]
- Chen, H.; Min, X.H.; Wang, Q.Y.; Leung, F.W.; Shi, L.; Zhou, Y.; Yu, T.; Wang, C.M.; An, G.; Sha, W.H.; et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci. Rep. 2015, 5, 8718. [Google Scholar] [CrossRef]
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Saito, H.; Fukushima, R.; Hashiguchi, Y.; Lin, M.T.; Inoue, T.; Fukatsu, K.; Muto, T.; Takenaka, A.; Takahashi, S.; et al. Effects of growth hormone and insulin-like growth factor 1 (IGF-1) treatments on the nitrogen metabolism and hepatic IGF-1-messenger RNA expression in postoperative parenterally fed rats. J. Parenter. Enteral Nutr. 1996, 20, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Soendergaard, C.; Kvist, P.H.; Thygesen, P.; Reslow, M.; Nielsen, O.H.; Kopchick, J.J.; Holm, T.L. Characterization of Growth Hormone Resistance in Experimental and Ulcerative Colitis. Int. J. Mol. Sci. 2017, 18, 2046. [Google Scholar] [CrossRef] [PubMed]
- Bohin, N.; McGowan, K.P.; Keeley, T.M.; Carlson, E.A.; Yan, K.S.; Samuelson, L.C. Insulin-like Growth Factor-1 and mTORC1 Signaling Promote the Intestinal Regenerative Response After Irradiation Injury. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 797–810. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Grønbek, H.; Thøgersen, T.; Frystyk, J.; Vilstrup, H.; Flyvbjerg, A.; Dahlerup, J.F. Low free and total insulinlike growth factor I (IGF-I) and IGF binding protein-3 levels in chronic inflammatory bowel disease: Partial normalization during prednisolone treatment. Am. J. Gastroenterol. 2002, 97, 673–678. [Google Scholar] [CrossRef]
- Sipos, F.; Galamb, O.; Herszényi, L.; Molnár, B.; Solymosi, N.; Zágoni, T.; Berczi, L.; Tulassay, Z. Elevated insulin-like growth factor 1 receptor, hepatocyte growth factor receptor and telomerase protein expression in mild ulcerative colitis. Scand. J. Gastroenterol. 2008, 43, 289–298. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kuśnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambroży, T.; Gil, K.; Olszanecki, R.; et al. Essential Role of Growth Hormone and IGF-1 in Therapeutic Effect of Ghrelin in the Course of Acetic Acid-Induced Colitis. Int. J. Mol. Sci. 2017, 18, 1118. [Google Scholar] [CrossRef]
- Grau-Bové, C.; González-Quilen, C.; Cantini, G.; Nardini, P.; Espina, B.; Bani, D.; Terra, X.; Blay, M.; Rodríguez-Gallego, E.; Luconi, M.; et al. GLP1 Exerts Paracrine Activity in the Intestinal Lumen of Human Colon. Int. J. Mol. Sci. 2022, 23, 3523. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine 2017, 13, 659–665. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Zhang, H.; Li, L.; Fan, T.; Jin, Z. The alleviating effect and mechanism of GLP-1 on ulcerative colitis. Aging 2023, 15, 8044–8060. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Sumi, C.; Saito, T.; Toki, Y.; Hatayama, M.; Yamamoto, M.; Shindo, M.; et al. Centrally administered GLP-1 analogue improves intestinal barrier function through the brain orexin and the vagal pathway in rats. Brain Res. 2023, 1809, 148371. [Google Scholar] [CrossRef] [PubMed]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk Microorganisms 2022, 10, 2061. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Søkilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunner’s Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef]
- Ebbesen, M.; Kissow, H.; Hartmann, B.; Kielsen, K.; Sørensen, K.; Stinson, S.E.; Frithioff-Bøjsøe, C.; Esmann Fonvig, C.; Holm, J.C.; Hansen, T.; et al. Glucagon-Like Peptide-1 Is Associated With Systemic Inflammation in Pediatric Patients Treated With Hematopoietic Stem Cell Transplantation. Front. Immunol. 2021, 12, 793588. [Google Scholar] [CrossRef]
- Su, Y.; Liu, N.; Zhang, Z.; Li, H.; Ma, J.; Yuan, Y.; Shi, M.; Liu, J.; Zhao, Z.; Zhang, Z.; et al. Cholecystokinin and glucagon-like peptide-1 analogues regulate intestinal tight junction, inflammation, dopaminergic neurons and α-synuclein accumulation in the colon of two Parkinson’s disease mouse models. Eur. J. Pharmacol. 2022, 926, 175029. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Koufakis, T.; Popovic, D.; Maltese, G.; Mustafa, O.; Doumas, M.; Giouleme, O.; Kotsa, K.; Germanidis, G. GLP-1 Receptor Agonists in Obese Patients with Inflammatory Bowel Disease: From Molecular Mechanisms to Clinical Considerations and Practical Recommendations for Safe and Effective Use. Curr. Obes. Rep. 2023, 12, 61–74. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Ou, D.; Yang, Q. The GLP-1 receptor agonist liraglutide protects against oxidized LDL-induced endothelial inflammation and dysfunction via KLF2. Life 2019, 71, 1347–1354. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef]
- Amato, A.; Mulè, F. Neural Regeneration Research. Mumbai 2019, 11, 1901–1902. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, Z. Liraglutide attenuates intestinal ischemia/reperfusion injury via NF-κB and PI3K/Akt pathways in mice. Life Sci. 2022, 309, 121045. [Google Scholar] [CrossRef] [PubMed]
- Insuela, D.B.R.; Carvalho, V.F. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur. J. Pharmacol. 2017, 812, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Angelini, G.; Russo, S.; Mingrone, G. Incretin hormones, obesity and gut microbiota. Peptides 2024, 178, 171216. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Xu, W.D.; Li, R.; Huang, A.F. Role of TL1A in Inflammatory Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2022, 13, 891328. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, M.; Hokari, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81. [Google Scholar] [CrossRef]
- Siakavellas, S.I.; Sfikakis, P.P.; Bamias, G. The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin. Arthritis Rheum. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Furfaro, F.; Alfarone, L.; Gilardi, D.; Correale, C.; Allocca, M.; Fiorino, G.; Argollo, M.; Zilli, A.; Zacharopoulou, E.; Loy, L.; et al. TL1A: A New Potential Target in the Treatment of Inflammatory Bowel Disease. Curr. Drug Targets 2021, 22, 760–769. [Google Scholar] [CrossRef]
- Danese, S.; Klopocka, M.; Scherl, E.J.; Romatowski, J.; Allegretti, J.R.; Peeva, E.; Vincent, M.S.; Schoenbeck, U.; Ye, Z.; Hassan-Zahraee, M.; et al. Anti-TL1A Antibody PF-06480605 Safety and Efficacy for Ulcerative Colitis: A Phase 2a Single-Arm Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2324–2332.e6. [Google Scholar] [CrossRef]
- Clarke, A.W.; Poulton, L.; Shim, D.; Mabon, D.; Butt, D.; Pollard, M.; Pande, V.; Husten, J.; Lyons, J.; Tian, C.; et al. An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease. mAbs 2018, 10, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Kolios, G.; Bamias, G. TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines With Diverse Functions in Gut Mucosal Immunity. Front. Immunol. 2019, 10, 583. [Google Scholar] [CrossRef]
- Jacob, N.; Kumagai, K.; Abraham, J.P.; Shimodaira, Y.; Ye, Y.; Luu, J.; Blackwood, A.Y.; Castanon, S.L.; Stamps, D.T.; Thomas, L.S.; et al. Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci. Rep. 2020, 10, 18189. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, A.; Chan, S.R.; Harvilla, P.; Mahady, S.; Armstrong, A.A.; Luistro, L.; Tamot, N.; Yamada, D.; Derebe, M.; Pomerantz, S.; et al. A stable, engineered TL1A ligand co-stimulates T cells via specific binding to DR3. Sci. Rep. 2022, 12, 20538. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.; Jacobs, J.P.; Kumagai, K.; Ha, C.W.Y.; Kanazawa, Y.; Lagishetty, V.; Altmayer, K.; Hamill, A.M.; Von Arx, A.; Sartor, R.B.; et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018, 11, 1466–1476. [Google Scholar] [CrossRef]
- Bamias, G. At the Junction of Immunity and Barrier Function: The Immunomodulatory Protein TL1A May Also Regulate Intestinal Permeability. Dig. Dis. Sci. 2019, 64, 1728–1730. [Google Scholar] [CrossRef]
- Li, H.; Song, J.; Niu, G.; Zhang, H.; Guo, J.; Shih, D.Q.; Targan, S.R.; Zhang, X. TL1A blocking ameliorates intestinal fibrosis in the T cell transfer model of chronic colitis in mice. Pathol. Res. Pract. 2018, 214, 217–227. [Google Scholar] [CrossRef]
- Yang, M.; Jia, W.; Wang, D.; Han, F.; Niu, W.; Zhang, H.; Shih, D.Q.; Zhang, X. Effects and Mechanism of Constitutive TL1A Expression on Intestinal Mucosal Barrier in DSS-Induced Colitis. Dig. Dis. Sci. 2019, 64, 1844–1856. [Google Scholar] [CrossRef]
- Li, J.; Shi, W.; Sun, H.; Ji, Y.; Chen, Y.; Guo, X.; Sheng, H.; Shu, J.; Zhou, L.; Cai, T.; et al. Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation. Nat. Commun. 2019, 10, 3371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).