Platelet Function, Platelet Size and Content of Reticulated Platelets: Interactions in Patients Receiving Dual Antiplatelet Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Collection and Patients
2.2. Platelet Count, Mean Platelet Volume (MPV), and Platelet Large Cell Ratio (PLC-R)
2.3. Reticulated Platelets (RP)
2.4. Platelet Forward Scattering (FSC)
2.5. Platelet Function
2.6. Interleukin 6 (IL6)
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Control | CHD | ACS | |
|---|---|---|---|
| n | 66 | 55 | 95 a |
| Age (mean ± SD), years | 62 ± 13 | 65 ± 11 | 62 ± 10 p(CHD) = 0.052 |
| Male/female, n/n | 30/36 | 37/18 * | 78/17 *** p(CHD) = 0.038 |
| Hypertension, n (%) | 47 (71%) | 51 (93%) ** | 84 (88%) ** p(CHD) = 0.398 |
| Diabetes mellitus, n (%) | 10 (15%) | 15 (27%) | 22 (23%) p(CHD) = 0.573 |
| Hypercholesterolemia, n (%) | 22 (33%) | 27 (49%) | 30 (32%) p(CHD) = 0.033 |
References
- Yee, D.L.; Sun, C.W.; Bergeron, A.L.; Dong, J.G.; Bray, P.F. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood 2005, 106, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Yakushkin, V.V.; Zyuryaev, I.T.; Khaspekova, S.G.; Sirotkina, O.V.; Ruda, M.Y.; Mazurov, A.V. Glycoprotein IIb-IIIa content and platelet aggregation in healthy volunteers and patients with acute coronary syndrome. Platelets 2011, 22, 243–251. [Google Scholar] [CrossRef]
- De Gaetano, G.; Santimone, I.; Gianfagna, F.; Iacoviello, L.; Cerletti, C. Variability of platelet indices and function: Acquired and genetic factors. In Handbook of Experimental Pharmacology 2010: Antiplatelet Agents; Gresele, P., Born, G.V.R., Patrono, C., Page, C.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 395–434. [Google Scholar]
- Wisman, P.P.; Roest, M.; Asselbergs, F.W.; de Groot, P.G.; Moll, F.L.; van der Graaf, Y.; de Borst, G.J. Platelet-reactivity tests identify patients at risk of secondary cardiovascular events: A systematic review and meta-analysis. Thromb. Haemost. 2014, 12, 736–747. [Google Scholar] [CrossRef]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Reny, J.L.; Fontana, P.; Hochholzer, W.; Neumann, F.J.; ten Berg, J.; Janssen, P.W.; Geisler, T.; Gawaz, M.; Marcucci, R.; Gori, A.M.; et al. Vascular risk levels affect the predictive value of platelet reactivity for the occurrence of MACE in patients on clopidogrel. Thromb. Haemost. 2016, 115, 823–825. [Google Scholar] [CrossRef]
- Larsen, P.D.; Holley, A.S.; Sasse, A.; Al-Sinan, A.; Fairley, S.; Harding, S.A. Comparison of multiplate and verifyNow platelet function tests in predicting clinical outcome in patients with acute coronary syndromes. Thromb. Res. 2017, 152, 14–19. [Google Scholar] [CrossRef]
- Freynhofer, M.K.; Gruber, S.C.; Grove, E.L.; Weiss, T.W.; Wojta, J.; Huber, K. Antiplatelet drugs in patients with enhanced platelet turnover: Biomarkers versus platelet function testing. Thromb. Haemost. 2015, 114, 459–468. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and small platelets—(When) do they differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef]
- Bodrova, V.V.; Shustova, O.N.; Khaspekova, S.G.; Mazurov, A.V. Laboratory markers of platelet production and turnover. Biochemistry 2023, 88 (Suppl. S1), S39–S51. [Google Scholar] [CrossRef]
- Hannawi, B.; Hannawi, Y.; Kleiman, N.S. Reticulated platelets: Changing focus from basics to outcomes. J. Thromb. Haemost. 2018, 118, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Jakubowsky, J.A. The pathophysiology and clinical relevance of platelet heterogeneity. Blood 1988, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leytin, V.; Shapiro, H.; Novikov, I.; Radney, J. Flow cytometric analysis of the platelet surface area and surface density of glycoprotein IIb-IIIa of unactivated human platelets of various sizes. Biochem. Biophys. Res. Commun. 1996, 226, 94–100. [Google Scholar] [CrossRef]
- Guthikonda, S.; Alviar, C.L.; Vaduganathan, M.; Arikan, M.; Tellez, A.; DeLao, T.; Granada, J.F.; Dong, J.-F.; Kleiman, N.S.; Lev, E.I. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2008, 52, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Mangalpally, K.; Siqueiros-Garcia, A.; Vaduganathan, M.; Dong, J.-F.; Kleiman, N.S.; Guthikonda, S. Platelet activation patterns in platelet size sub-populations: Differential responses to aspirin in vitro. J. Thromb. Thrombolysis 2010, 30, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Khaspekova, S.G.; Zyuryaev, I.T.; Yakushkin, V.V.; Sirotkina, O.V.; Zaytseva, N.O.; Ruda, M.Y.; Panteleev, M.A.; Mazurov, A.V. Relationships of glycoproteins IIb-IIIa and Ib content with mean platelet volume and their genetic polymorphisms. Blood Coagul. Fibrinolysis 2014, 25, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.; Rabbolini, D.; Morel-Kopp, M.-C.; Fixter, K.; Donikian, D.; Kondo, M.; Chan, O.; Jarvis, S.; Chen, W.; Brighton, T.; et al. The utility of flow cytometric platelet forward scatter as an alternative to mean platelet volume. Platelets 2022, 33, 1139–1145. [Google Scholar] [CrossRef]
- Guthikonda, S.; Lev, E.I.; Patel, R.; Delao, T.; Bergeron, A.L.; Dong, J.-F.; Kleiman, N.S. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J. Thromb. Haemost. 2007, 5, 490–496. [Google Scholar] [CrossRef]
- Bodrova, V.V.; Shustova, O.N.; Khaspekova, S.G.; Mazurov, A.V. Platelet reticulated forms, size indexes and functional activity. Interactions in healthy volunteers. Platelets 2022, 33, 398–403. [Google Scholar] [CrossRef]
- Hille, L.; Lenz, M.; Vlachos, A.; Grüning, B.; Hein, L.; Neumann, F.-J.; Nührenberg, T.G.; Trenk, D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020, 18, 2034–2046. [Google Scholar] [CrossRef]
- Lador, A.; Leshem, L.D.; Spectre, G.; Abelow, A.; Kornowski, R.; Lev, E.I. Characterization of surface antigens of reticulated immature platelets. J. Thromb. Thrombolysis 2017, 44, 291–297. [Google Scholar] [CrossRef]
- McBane, R.D.; Gonzalez, C.; Hodge, D.O.; Wysokinski, W.E. Propensity for young reticulated platelet recruitment into arterial thrombi. J. Thromb. Thrombolysis 2014, 37, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.C.; Hoefer, T.; Knowles, R.B.; Tucker, A.T.; Hayman, M.A.; Ferreira, P.M.; Chan, M.V.; Warner, T.D. Newly formed reticulated platelets undermine pharmacokinetically short-lived antiplatelet therapies. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Cesari, F.; Marcucci, R.; Caporale, R.; Paniccia, R.; Romano, E.; Gensini, G.-F.; Abbate, R.; Gori, A.-M. Relationship between high platelet turnover and platelet function in high-risk patients with coronary artery disease on dual antiplatelet therapy. J. Thromb. Haemost. 2008, 99, 930–935. [Google Scholar] [CrossRef]
- Sansanayudh, N.; Numthavaj, P.; Muntham, D.; Yamwong, S.; McEvoy, M.; Attia, J.; Sritara, P.; Thakkinstian, A. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb. Haemost. 2015, 114, 1299–1309. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, R.; Zhang, Y.; Shi, D. The prognostic value of reticulated platelets in patients with coronary artery disease: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2020, 7, 57–61. [Google Scholar] [CrossRef]
- Kaushansky, K. Thrombopoiesis. Semin. Hematol. 2015, 52, 4–11. [Google Scholar] [CrossRef]
- Baatout, S. Interleukin-6 and megakaryocytopoiesis: An update. Ann. Hematol. 1996, 73, 157–162. [Google Scholar] [CrossRef]
- Senaran, H.; Ileri, M.; Altinbaş, A.; Koşar, A.; Yetkin, E.; Oztürk, M.; Karaaslan, Y.; Kirazli, S. Thrombopoietin and mean platelet volume in coronary artery disease. Clin. Cardiol. 2001, 24, 405–408. [Google Scholar] [CrossRef]
| MPV, fl | PLC-R % | FSC, a.u. | RP % | |
|---|---|---|---|---|
| Control | 8.7 ± 1.1 | 27.2 ± 7.6 | 20,320 ± 4556 | 12.8 ± 5.5 |
| r (MPV) | - | 0.904 | 0.779 | 0.506 |
| r (PLC-R) | 0.841 | 0.537 | ||
| r (FSC) | 0.694 | |||
| CHD | 8.7 ± 0.8 | 27.8 ± 6.1 | 20,144 ± 4997 | 11.6 ± 4.1 |
| r (MPV) | - | 0.891 | 0.538 | 0.348 |
| r (PLC-R) | 0.642 | 0.480 | ||
| r (FSC) | 0.467 | |||
| ACS | 8.4 ± 0.8 * | 25.9 ± 6.6 | 19,753 ± 4060 | 12.2 ± 5.2 |
| r (MPV) | - | 0.922 | 0.628 | 0.278 |
| r (PLC-R) | 0.779 | 0.486 | ||
| r (FSC) | 0.650 |
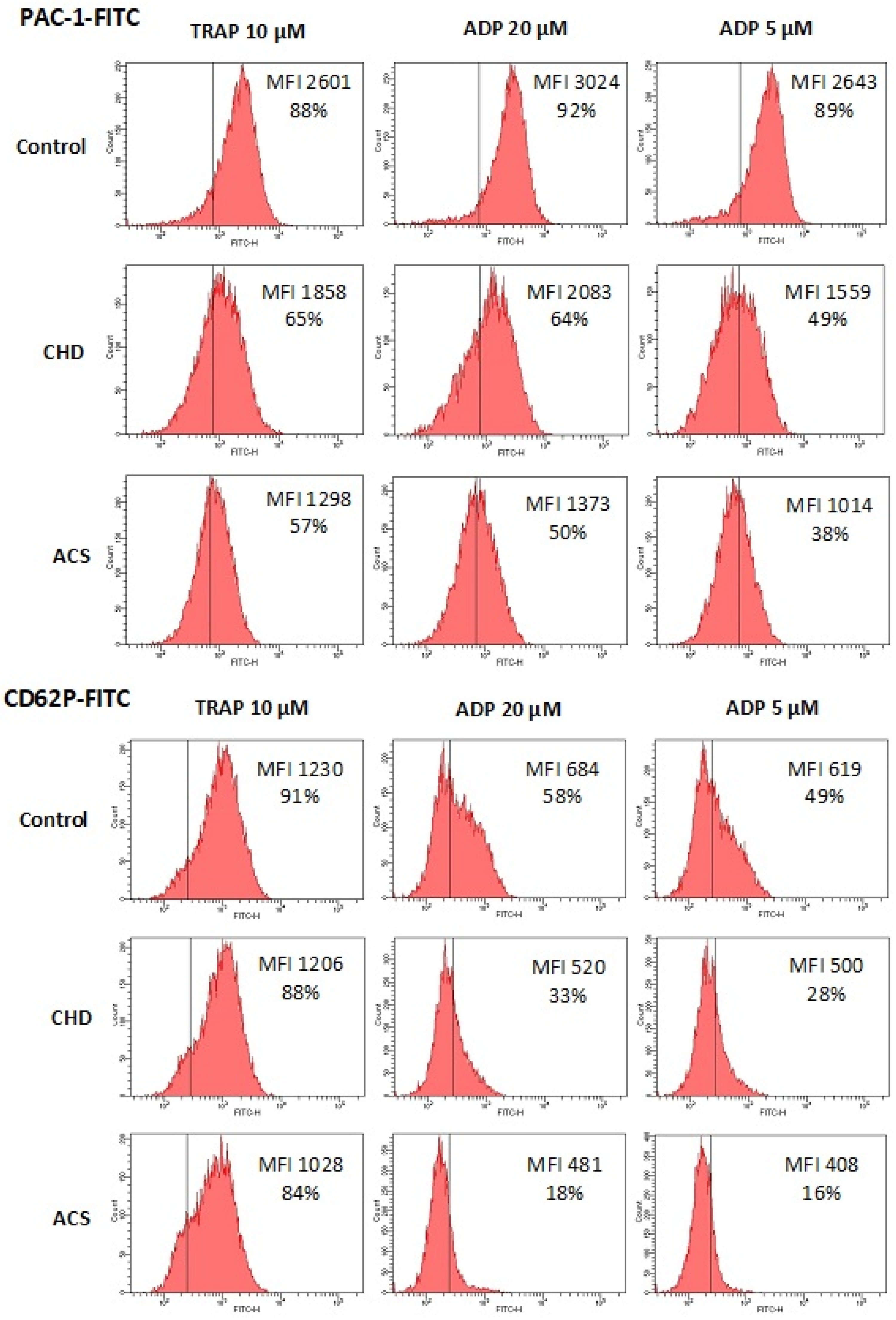
| Agonist | Index | Control | CHD p (Control) < 0.001 a | ACS p (Control) < 0.001 b |
|---|---|---|---|---|
| Activated GP IIb-IIIa (PAC-1 binding) | ||||
| No agonists | MFI, a.u | 365 ± 84 | 373 ± 90 | 346 ± 84 |
| TRAP 10 µM | MFI, a.u. | 2830 ± 1124 | 1802 ± 718 | 1405 ± 462 *** |
| PAC-1+, % | 88 ± 7 | 68 ± 15 | 59 ± 12 *** | |
| TRAP 10 µM Epi 20 µM | MFI, a.u | n.d. | 2784 ± 971 | 2531 ± 704 |
| PAC-1+, % | n.d. | 87 ± 8 | 85 ± 7 | |
| ADP 20 µM | MFI, a.u | 3186 ± 1184 | 1934 ± 780 | 1425 ± 507 *** |
| PAC-1+, % | 90 ± 5 | 61 ± 19 | 48 ± 12 *** | |
| ADP 20 µM Epi 20 µM | MFI, a.u | n.d. | 2883 ± 1008 | 2572 ± 805 * |
| PAC-1+, % | n.d. | 86 ± 9 | 83 ± 8 | |
| ADP, 5 µM | MFI, a.u | 2940 ± 1103 | 1705 ± 694 | 1237 ± 445 *** |
| PAC-1+, % | 87 ± 6 | 55 ± 20 | 37 ± 11 *** | |
| ADP 5 µM Epi 20 µM | MFI, a.u | n.d. | 2686 ± 989 | 2280 ± 725 ** |
| PAC-1+, % | n.d. | 84 ± 10 | 78 ± 10 *** | |
| ADP 2.5 µM | MFI, a.u | 2690 ± 1044 | n.d. | n.d. |
| PAC-1+, % | 83 ± 8 | n.d. | n.d. | |
| P-selectin (CD62P binding) | ||||
| No agonists | MFI, a.u. | 338 ± 143 | 320 ± 128 | 348 ± 138 |
| CD62P+, % | 8 ± 4 | 10 ± 6 | 9 ± 5 | |
| TRAP 10 µM | MFI, a.u. | 1443 ± 455 | 1277 ± 334 | 1166 ± 317 * |
| CD62P+, % | 90 ± 8 | 89 ± 6 | 87 ± 8 | |
| TRAP 10 µM Epi 20 µM | MFI, a.u | n.d. | 1484 ± 373 | 1491 ± 381 |
| CD62P+, % | n.d | 93 ± 4 | 92 ± 5 | |
| ADP 20 µM | MFI, a.u | 723 ± 202 | 537 ± 177 | 427 ± 97 *** |
| CD62P+, % | 57 ± 15 | 35 ± 17 | 21 ± 10 *** | |
| ADP 20 µM Epi, 20 µM | MFI, a.u | n.d. | 784 ± 265 | 648 ± 171 *** |
| CD62P, % | n.d. | 61 ± 17 | 52 ± 16 *** | |
| ADP 5 µM | MFI, a.u | 643 ± 179 | 496 ± 153 | 393 ± 84 *** |
| CD62P+, % | 50 ± 16 | 31 ± 16 | 18 ± 10 *** | |
| ADP 5 µM Epi 20 µM | MFI, a.u | n.d. | 720 ± 238 | 594 ± 149 *** |
| CD62P+, % | n.d. | 57 ± 18 | 47 ± 16 *** | |
| ADP 2.5 µM | MFI, a.u | 594 ± 156 | n.d. | n.d. |
| CD62P+, % | 46 ± 15 | n.d. | n.d. | |
| Agonist | MPV | P-LCR | FSC | RP % |
|---|---|---|---|---|
| PAC-1, MFI | ||||
| TRAP 10 µM | 0.356 ** | 0.385 ** | 0.412 *** | 0.450 *** |
| ADP 20 µM | 0.460 *** | 0.473 *** | 0.528 *** | 0.536 *** |
| ADP 5 µM | 0.450 *** | 0.471 *** | 0.492 *** | 0.520 *** |
| ADP 2.5 µM | 0.471 *** | 0.473 *** | 0.485 *** | 0.519 *** |
| CD62P, MFI | ||||
| TRAP, 10 µM | 0.320 ** | 0.458 *** | 0.546 *** | 0.557 *** |
| ADP 20 µM | 0.300 * | 0.321 ** | 0.448 *** | 0.546 *** |
| ADP 5 µM | 0.330 ** | 0.330 ** | 0.431 *** | 0.551 *** |
| ADP 2.5 µM | 0.321 ** | 0.385 ** | 0.413 *** | 0.547 *** |
| CHD (ASA + Clopidogrel) | ||||
| Agonist | MPV | P-LCR | FSC | RP % |
| PAC-1, MFI | ||||
| TRAP 10 µM | 0.290 * | 0.484 *** | 0.410 ** | 0.141 |
| TRAP 10 µM + Epi 20 µM | 0.233 | 0.475 *** | 0.370 ** | 0.235 |
| ADP 20 µM | 0.217 | 0.447 *** | 0.360 ** | 0.163 |
| ADP 20 µM + Epi 20 µM | 0.190 | 0.445 *** | 0.319 * | 0.186 |
| ADP 5 µM | 0.208 | 0.445 *** | 0.376 ** | 0.176 |
| ADP 5 µM + Epi 20 µM | 0.184 | 0.437 *** | 0.316 * | 0.151 |
| CD62P, MFI | ||||
| TRAP 10 µM | 0.264 * | 0.489 *** | 0.477 *** | 0.408 ** |
| TRAP 10 µM + Epi 20 µM | 0.235 | 0.495 *** | 0.474 *** | 0.457 *** |
| ADP 20 µM | 0.209 | 0.201 | 0.221 | 0.097 |
| ADP 20 µM + Epi 20 µM | 0.196 | 0.233 | 0.218 | 0.151 |
| ADP 5 µM | 0.216 | 0.198 | 0.248 | 0.077 |
| ADP 5 µM + Epi 20 µM | 0.203 | 0.224 | 0.255 | 0.144 |
| ACS (ASA + Ticagrelor) | ||||
| Agonist | MPV | P-LCR | FSC | RP % |
| PAC-1, MFI | ||||
| TRAP 10 µM | 0.073 | 0.261 * | 0.507 *** | 0.459 *** |
| TRAP 10 µM + Epi 20 µM | 0.004 | 0.172 | 0.376 *** | 0.319 ** |
| ADP 20 µM | 0.119 | 0.283 ** | 0.504 *** | 0.451 *** |
| ADP 20 µM + Epi 20 µM | 0.052 | 0.235 * | 0.440 *** | 0.411 *** |
| ADP 5 µM | 0.094 | 0.282 ** | 0.527 *** | 0.477 *** |
| ADP 5 µM + Epi 20 µM | 0.052 | 0.234 * | 0.447 *** | 0.388 *** |
| CD62P, MFI | ||||
| TRAP 10 µM | 0.156 | 0.369 *** | 0.487 *** | 0.600 *** |
| TRAP 10 µM + Epi 20 µM | 0.161 | 0.418 *** | 0.541 *** | 0.605 *** |
| ADP 20 µM | 0.244 * | 0.116 | 0.052 | 0.061 |
| ADP 20 µM + Epi 20 µM | 0.175 | 0.171 | 0.190 | 0.157 |
| ADP 5 µM | 0.270 * | 0.142 | 0.074 | 0.058 |
| ADP 5 µM + Epi 20 µM | 0.209 * | 0.183 | 0.146 | 0.124. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodrova, V.V.; Shustova, O.N.; Golubeva, N.V.; Alieva, A.K.; Vlodzyanovsky, V.V.; Pevzner, D.V.; Mazurov, A.V. Platelet Function, Platelet Size and Content of Reticulated Platelets: Interactions in Patients Receiving Dual Antiplatelet Therapy. Cells 2024, 13, 1712. https://doi.org/10.3390/cells13201712
Bodrova VV, Shustova ON, Golubeva NV, Alieva AK, Vlodzyanovsky VV, Pevzner DV, Mazurov AV. Platelet Function, Platelet Size and Content of Reticulated Platelets: Interactions in Patients Receiving Dual Antiplatelet Therapy. Cells. 2024; 13(20):1712. https://doi.org/10.3390/cells13201712
Chicago/Turabian StyleBodrova, Valeria V., Olga N. Shustova, Nina V. Golubeva, Amina K. Alieva, Vladislav V. Vlodzyanovsky, Dmitry V. Pevzner, and Alexey V. Mazurov. 2024. "Platelet Function, Platelet Size and Content of Reticulated Platelets: Interactions in Patients Receiving Dual Antiplatelet Therapy" Cells 13, no. 20: 1712. https://doi.org/10.3390/cells13201712
APA StyleBodrova, V. V., Shustova, O. N., Golubeva, N. V., Alieva, A. K., Vlodzyanovsky, V. V., Pevzner, D. V., & Mazurov, A. V. (2024). Platelet Function, Platelet Size and Content of Reticulated Platelets: Interactions in Patients Receiving Dual Antiplatelet Therapy. Cells, 13(20), 1712. https://doi.org/10.3390/cells13201712






