Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy
Abstract
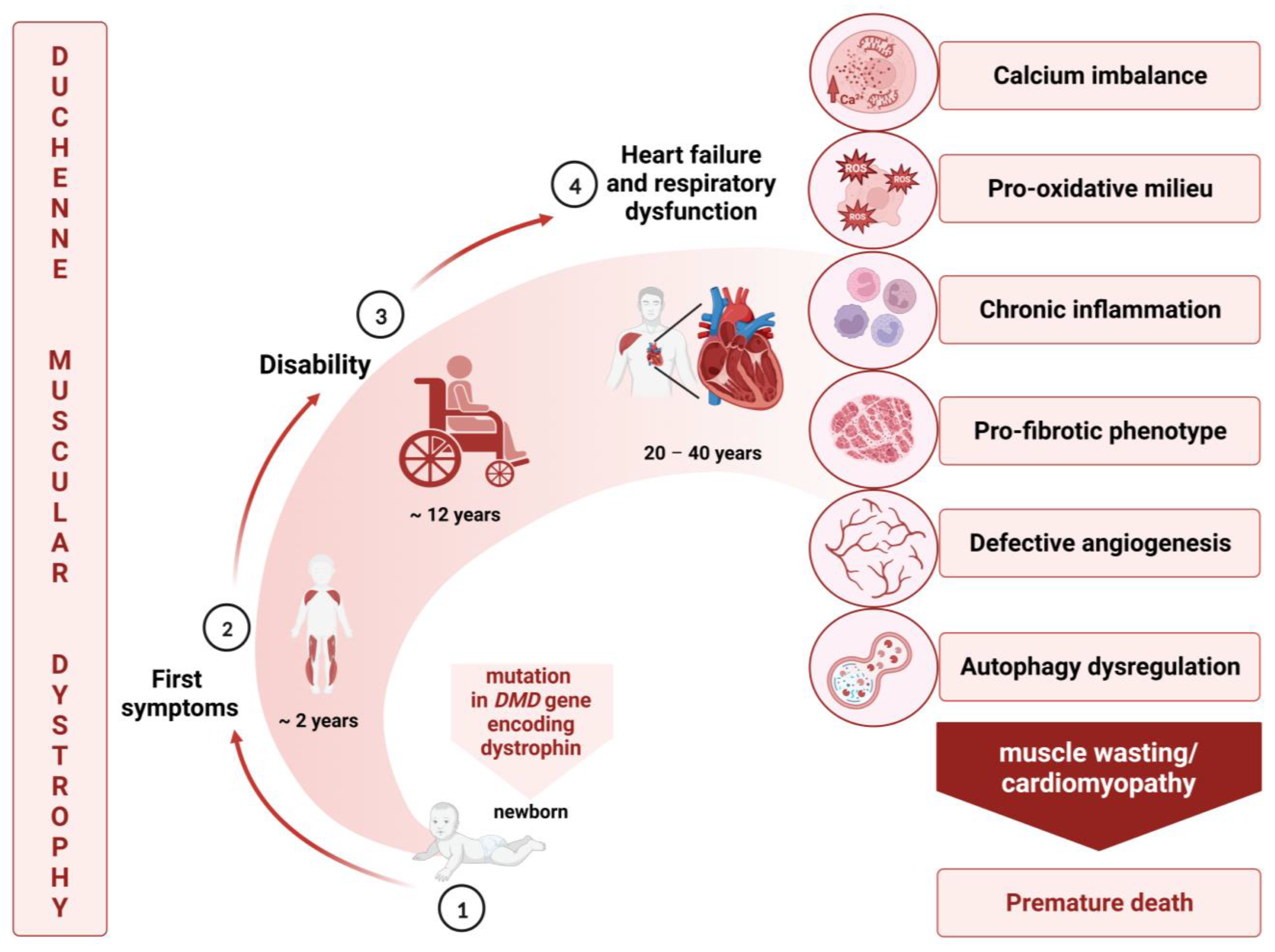
1. Duchenne Muscular Dystrophy: General Overview
2. Cardiovascular Complications in Duchenne Muscular Dystrophy
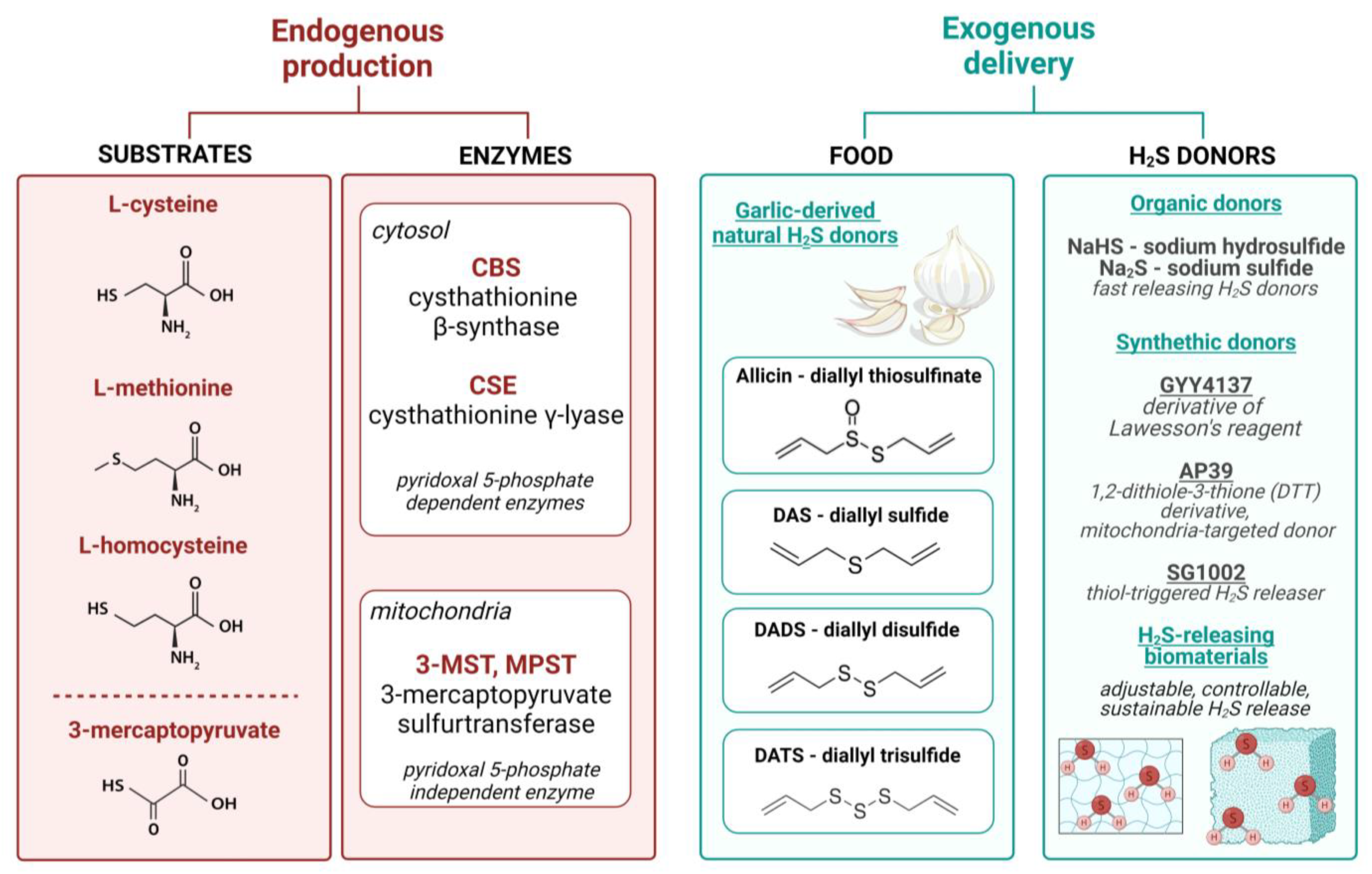
3. Hydrogen Sulfide—Does the Method of Delivery Matter?
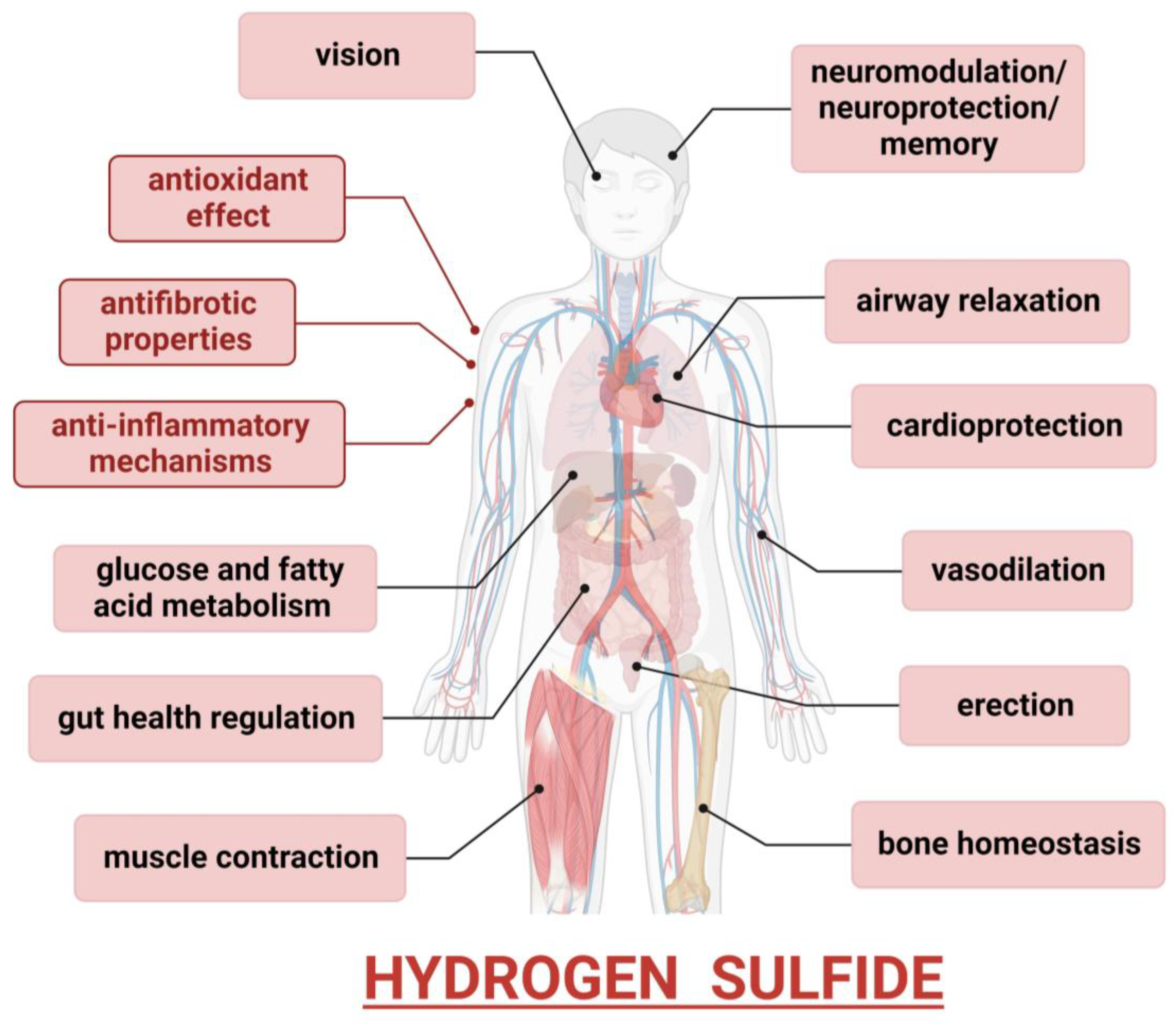
4. Hydrogen Sulfide—A Cytoprotective Gas
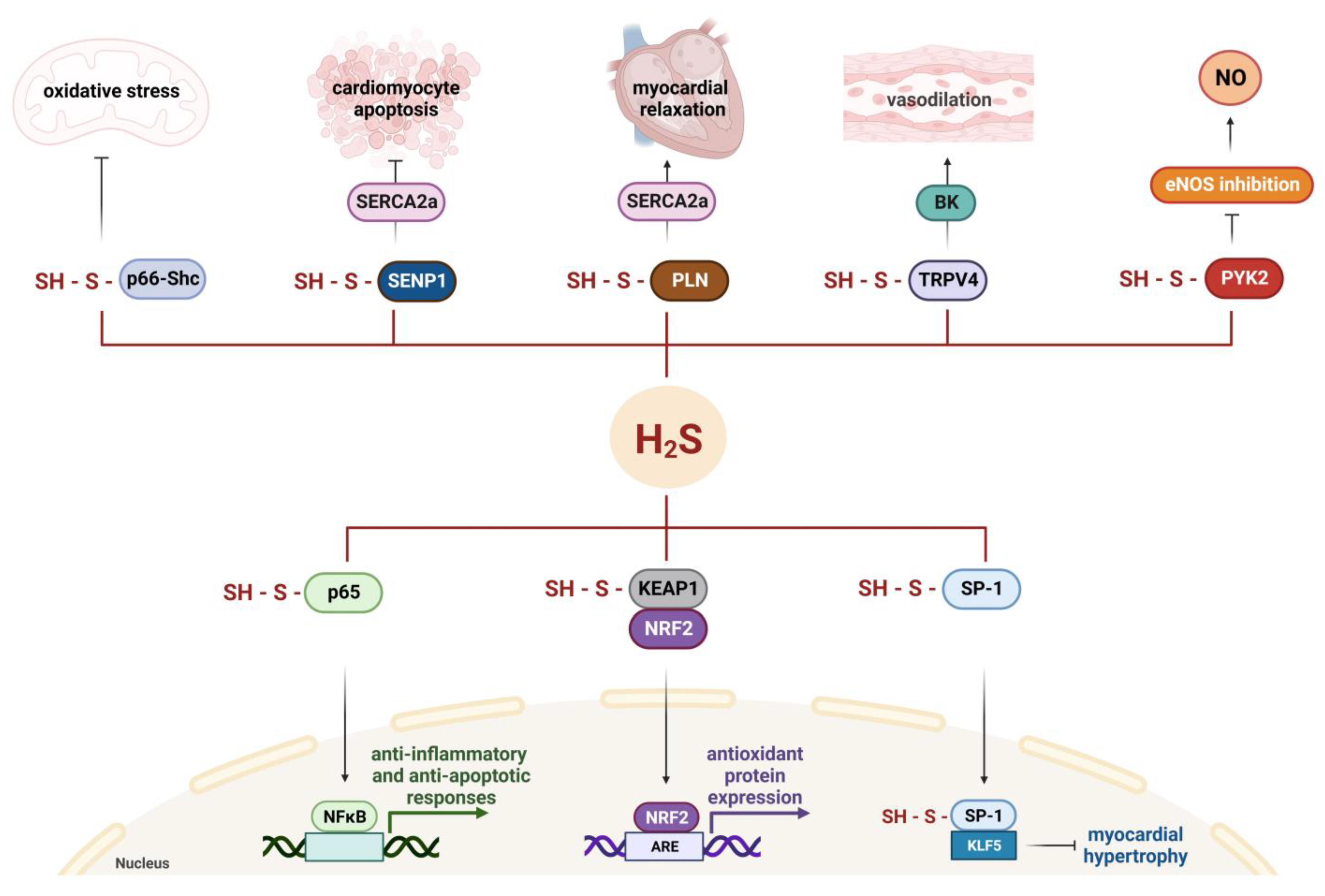
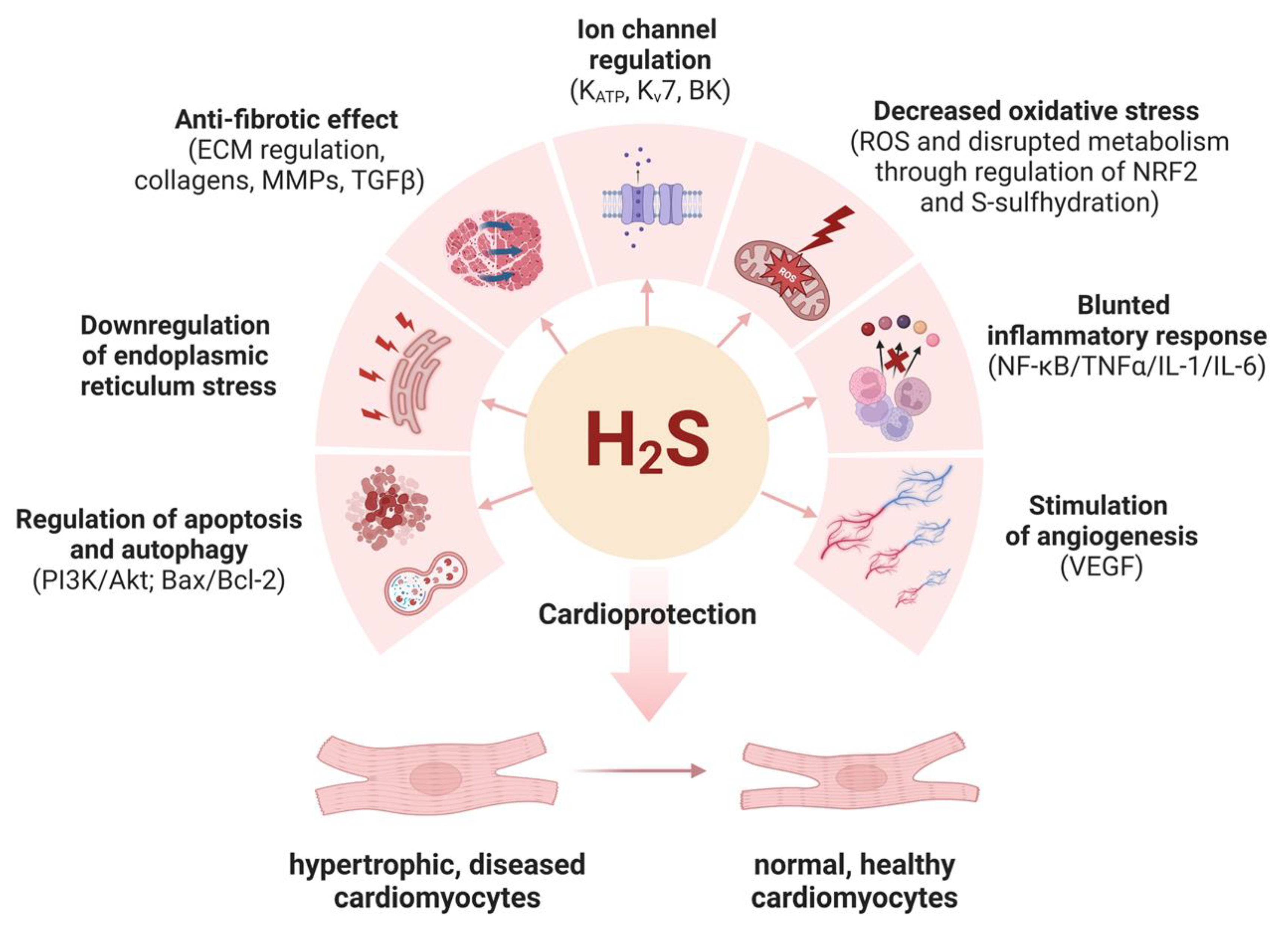
5. Molecular Mechanisms of Cardioprotective Effects of Hydrogen Sulfide
5.1. H2S Regulates the Activity of Ion Channels
5.2. S-Sulfhydration Contributes to H2S-Triggered Cardioprotection
5.3. H2S Downregulates Oxidative Stress
5.4. H2S Has Anti-Inflammatory Functions
5.5. H2S Is Anti-Fibrotic
5.6. H2S Promotes Angiogenesis
5.7. H2S Protects against Apoptosis
6. H2S as a (Cardio)Protective Factor in DMD
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.A.; Flanigan, K.M. Update in Duchenne and Becker Muscular Dystrophy. Curr. Opin. Neurol. 2019, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Łoboda, A.; Dulak, J. Muscle and Cardiac Therapeutic Strategies for Duchenne Muscular Dystrophy: Past, Present, and Future. Pharmacol. Rep. 2020, 72, 1227–1263. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P. The Discovery of Dystrophin, the Protein Product of the Duchenne Muscular Dystrophy Gene. FEBS J. 2020, 287, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of More than 7,000 Duchenne Muscular Dystrophy Mutations. Hum. Mutat. 2015, 36, 395–402. [Google Scholar] [CrossRef]
- Koenig, M.; Hoffman, E.P.; Bertelson, C.J.; Monaco, A.P.; Feener, C.; Kunkel, L.M. Complete Cloning of the Duchenne Muscular Dystrophy (DMD) cDNA and Preliminary Genomic Organization of the DMD Gene in Normal and Affected Individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne Muscular Dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef]
- Emery, A.E.H. The Muscular Dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The Protein Product of the Duchenne Muscular Dystrophy Locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne Muscular Dystrophy: Myonecrosis, Inflammation and Oxidative Stress. Dis. Model. Mech. 2020, 13, dmm043638. [Google Scholar] [CrossRef]
- Matsuo, M. Antisense Oligonucleotide-Mediated Exon-Skipping Therapies: Precision Medicine Spreading from Duchenne Muscular Dystrophy. JMA J. 2021, 4, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant, P.D.J.; Bushby, P.K.; Bann, C.M.; Alman, P.B.A.; Apkon, P.S.D.; Blackwell, A.; Case, L.E.; Cripe, P.L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Respiratory, Cardiac, Bone Health, and Orthopaedic Management. Lancet Neurol. 2018, 17, 347. [Google Scholar] [CrossRef]
- Shih, J.A.; Folch, A.; Wong, B.L. Duchenne Muscular Dystrophy: The Heart of the Matter. Curr. Heart Fail. Rep. 2020, 17, 57–66. [Google Scholar] [CrossRef] [PubMed]
- van Westering, T.L.E.; Betts, C.A.; Wood, M.J.A. Current Understanding of Molecular Pathology and Treatment of Cardiomyopathy in Duchenne Muscular Dystrophy. Molecules 2015, 20, 8823–8855. [Google Scholar] [CrossRef] [PubMed]
- Florczyk-Soluch, U.; Polak, K.; Dulak, J. The Multifaceted View of Heart Problem in Duchenne Muscular Dystrophy. Cell. Mol. Life Sci. 2021, 78, 5447–5468. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-Induced Production of Mitochondrial Reactive Oxygen Species: Potential Mechanisms and Relevance for Cardiovascular Disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef]
- Duboc, D.; Meune, C.; Pierre, B.; Wahbi, K.; Eymard, B.; Toutain, A.; Berard, C.; Vaksmann, G.; Weber, S.; Bécane, H.-M. Perindopril Preventive Treatment on Mortality in Duchenne Muscular Dystrophy: 10 Years’ Follow-Up. Am. Heart J. 2007, 154, 596–602. [Google Scholar] [CrossRef]
- Allen, H.D.; Flanigan, K.M.; Thrush, P.T.; Dvorchik, I.; Yin, H.; Canter, C.; Connolly, A.M.; Parrish, M.; McDonald, C.M.; Braunlin, E.; et al. A Randomized, Double-Blind Trial of Lisinopril and Losartan for the Treatment of Cardiomyopathy in Duchenne Muscular Dystrophy. PLoS Curr. 2013, 5, ecurrents.md.2cc69a1dae4be7dfe2bcb420024ea865. [Google Scholar] [CrossRef]
- Bangalore, S.; Fakheri, R.; Toklu, B.; Ogedegbe, G.; Weintraub, H.; Messerli, F.H. Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers in Patients Without Heart Failure? Insights From 254,301 Patients From Randomized Trials. Mayo Clin. Proc. 2016, 91, 51–60. [Google Scholar] [CrossRef]
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E.; Cripe, L.H.; Duan, D.; Finder, J.D.; Groh, W.J.; Hoffman, E.P.; Judge, D.P.; et al. Contemporary Cardiac Issues in Duchenne Muscular Dystrophy. Working Group of the National Heart, Lung, and Blood Institute in Collaboration with Parent Project Muscular Dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Ploutz, M.; Moore, R.; Ashiki, M.; Wisotzkey, B.; Taylor, B.; Spurney, C.; Taylor, M.; Jefferies, J. Spironolactone Therapy for Cardiomyopathy in Duchenne Muscular Dystrophy. J. Am. Coll. Cardiol. 2017, 69, 870. [Google Scholar] [CrossRef]
- Raman, S.V.; Hor, K.N.; Mazur, W.; Halnon, N.J.; Kissel, J.T.; He, X.; Tran, T.; Smart, S.; McCarthy, B.; Taylor, M.D.; et al. Eplerenone for Early Cardiomyopathy in Duchenne Muscular Dystrophy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2015, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.V.; Hor, K.N.; Mazur, W.; He, X.; Kissel, J.T.; Smart, S.; McCarthy, B.; Roble, S.L.; Cripe, L.H. Eplerenone for Early Cardiomyopathy in Duchenne Muscular Dystrophy: Results of a Two-Year Open-Label Extension Trial. Orphanet J. Rare Dis. 2017, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, H.; Ishigaki, K.; Okumura, K.; Tomimatsu, H.; Nakazawa, M.; Saito, K.; Osawa, M.; Nakanishi, T. Beta-Blocker Therapy for Cardiac Dysfunction in Patients with Muscular Dystrophy. Circ. J. 2006, 70, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Viollet, L.; Thrush, P.T.; Flanigan, K.M.; Mendell, J.R.; Allen, H.D. Effects of Angiotensin-Converting Enzyme Inhibitors and/or Beta Blockers on the Cardiomyopathy in Duchenne Muscular Dystrophy. Am. J. Cardiol. 2012, 110, 98–102. [Google Scholar] [CrossRef]
- Bourke, J.P.; Watson, G.; Muntoni, F.; Spinty, S.; Roper, H.; Guglieri, M.; Speed, C.; McColl, E.; Chikermane, A.; Jayawant, S.; et al. Randomised Placebo-Controlled Trial of Combination ACE Inhibitor and Beta-Blocker Therapy to Prevent Cardiomyopathy in Children with Duchenne Muscular Dystrophy? (DMD Heart Protection Study): A Protocol Study. BMJ Open 2018, 8, e022572. [Google Scholar] [CrossRef]
- Haddad, C.N.; Ali, S.; Stephanou, D.; Assakura, M.S.; Sahagian, L.; Trogkanis, E. Pharmacological Management of Dilated Cardiomyopathy in Duchenne Muscular Dystrophy: A Systematic Review. Hell. J. Cardiol. 2023, 74, 58–64. [Google Scholar] [CrossRef]
- Lechner, A.; Herzig, J.J.; Kientsch, J.G.; Kohler, M.; Bloch, K.E.; Ulrich, S.; Schwarz, E.I. Cardiomyopathy as Cause of Death in Duchenne Muscular Dystrophy: A Longitudinal Observational Study. ERJ Open Res. 2023, 9, 00176–02023. [Google Scholar] [CrossRef]
- Canonico, F.; Chirivi, M.; Maiullari, F.; Milan, M.; Rizzi, R.; Arcudi, A.; Galli, M.; Pane, M.; Gowran, A.; Pompilio, G.; et al. Focus on the Road to Modelling Cardiomyopathy in Muscular Dystrophy. Cardiovasc. Res. 2022, 118, 1872–1884. [Google Scholar] [CrossRef]
- Quinlan, J.G.; Hahn, H.S.; Wong, B.L.; Lorenz, J.N.; Wenisch, A.S.; Levin, L.S. Evolution of the Mdx Mouse Cardiomyopathy: Physiological and Morphological Findings. Neuromuscul. Disord. 2004, 14, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ascah, A.; Khairallah, M.; Daussin, F.; Bourcier-Lucas, C.; Godin, R.; Allen, B.G.; Petrof, B.J.; Des Rosiers, C.; Burelle, Y. Stress-Induced Opening of the Permeability Transition Pore in the Dystrophin-Deficient Heart Is Attenuated by Acute Treatment with Sildenafil. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H144–H153. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Mikheeva, I.B.; Belosludtsev, K.N. Transport of Ca2+ and Ca2+-Dependent Permeability Transition in Heart Mitochondria in the Early Stages of Duchenne Muscular Dystrophy. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148250. [Google Scholar] [CrossRef] [PubMed]
- Angebault, C.; Panel, M.; Lacôte, M.; Rieusset, J.; Lacampagne, A.; Fauconnier, J. Metformin Reverses the Enhanced Myocardial SR/ER-Mitochondria Interaction and Impaired Complex I-Driven Respiration in Dystrophin-Deficient Mice. Front. Cell Dev. Biol. 2020, 8, 609493. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Talanov, E.Y.; Mikheeva, I.B.; Belosludtseva, N.V.; Serov, D.A.; Tenkov, K.S.; Belosludtseva, E.V.; Belosludtsev, K.N. Effect of the Non-Immunosuppressive MPT Pore Inhibitor Alisporivir on the Functioning of Heart Mitochondria in Dystrophin-Deficient Mdx Mice. Biomedicines 2021, 9, 1232. [Google Scholar] [CrossRef]
- Chang, A.C.Y.; Ong, S.-G.; LaGory, E.L.; Kraft, P.E.; Giaccia, A.J.; Wu, J.C.; Blau, H.M. Telomere Shortening and Metabolic Compromise Underlie Dystrophic Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2016, 113, 13120–13125. [Google Scholar] [CrossRef] [PubMed]
- Mourkioti, F.; Kustan, J.; Kraft, P.; Day, J.W.; Zhao, M.-M.; Kost-Alimova, M.; Protopopov, A.; DePinho, R.A.; Bernstein, D.; Meeker, A.K.; et al. Role of Telomere Dysfunction in Cardiac Failure in Duchenne Muscular Dystrophy. Nat. Cell Biol. 2013, 15, 895–904. [Google Scholar] [CrossRef]
- Ishizaki, M.; Kobayashi, M.; Adachi, K.; Matsumura, T.; Kimura, E. Female Dystrophinopathy: Review of Current Literature. Neuromuscul. Disord. 2018, 28, 572–581. [Google Scholar] [CrossRef]
- Bostick, B.; Yue, Y.; Duan, D. Gender Influences Cardiac Function in the Mdx Model of Duchenne Cardiomyopathy. Muscle Nerve 2010, 42, 600–603. [Google Scholar] [CrossRef]
- Guéniot, L.; Latroche, C.; Thépenier, C.; Chatre, L.; Mazeraud, A.; Fiole, D.; Goossens, P.L.; Chrétien, F.; Jouvion, G. The Female Mdx Mouse: An Unexpected Vascular Story. J. Neurol. Neuromedicine 2016, 1, 41–53. [Google Scholar]
- Schultz, T.I.; Raucci, F.J.; Salloum, F.N. Cardiovascular Disease in Duchenne Muscular Dystrophy. JACC Basic Transl. Sci. 2022, 7, 608–625. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.N. The Golden Retriever Model of Duchenne Muscular Dystrophy. Skelet. Muscle 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, N.; Blutke, A.; Graf, A.; Krause, S.; Burkhardt, K.; Wuensch, A.; Krebs, S.; Kessler, B.; Zakhartchenko, V.; Kurome, M.; et al. Dystrophin-Deficient Pigs Provide New Insights into the Hierarchy of Physiological Derangements of Dystrophic Muscle. Hum. Mol. Genet. 2013, 22, 4368–4382. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Fonteyne, L.; Giesert, F.; Hoppmann, P.; Meier, A.B.; Bozoglu, T.; Baehr, A.; Schneider, C.M.; Sinnecker, D.; Klett, K.; et al. Somatic Gene Editing Ameliorates Skeletal and Cardiac Muscle Failure in Pig and Human Models of Duchenne Muscular Dystrophy. Nat. Med. 2020, 26, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Stepniewski, J.; Pacholczak, T.; Skrzypczyk, A.; Ciesla, M.; Szade, A.; Szade, K.; Bidanel, R.; Langrzyk, A.; Grochowski, R.; Vandermeeren, F.; et al. Heme Oxygenase-1 Affects Generation and Spontaneous Cardiac Differentiation of Induced Pluripotent Stem Cells. IUBMB Life 2018, 70, 129–142. [Google Scholar] [CrossRef]
- Martyniak, A.; Andrysiak, K.; Motais, B.; Coste, S.; Podkalicka, P.; Ferdek, P.; Stępniewski, J.; Dulak, J. Generation of microRNA-378a-Deficient hiPSC as a Novel Tool to Study Its Role in Human Cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 160, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Jeż, M.; Martyniak, A.; Andrysiak, K.; Mucha, O.; Szade, K.; Kania, A.; Chrobok, Ł.; Palus-Chramiec, K.; Sanetra, A.M.; Lewandowski, M.H.; et al. Role of Heme-Oxygenase-1 in Biology of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Cells 2021, 10, 522. [Google Scholar] [CrossRef]
- Andrysiak, K.; Machaj, G.; Priesmann, D.; Woźnicka, O.; Martyniak, A.; Ylla, G.; Krüger, M.; Pyza, E.; Potulska-Chromik, A.; Kostera-Pruszczyk, A.; et al. Dysregulated Iron Homeostasis in Dystrophin-Deficient Cardiomyocytes: Correction by Gene Editing and Pharmacological Treatment. Cardiovasc. Res. 2023, cvad182. [Google Scholar] [CrossRef]
- Rovina, D.; Castiglioni, E.; Niro, F.; Mallia, S.; Pompilio, G.; Gowran, A. “Betwixt Mine Eye and Heart a League Is Took”: The Progress of Induced Pluripotent Stem-Cell-Based Models of Dystrophin-Associated Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 6997. [Google Scholar] [CrossRef]
- Kamdar, F.; Das, S.; Gong, W.; Klaassen Kamdar, A.; Meyers, T.A.; Shah, P.; Ervasti, J.M.; Townsend, D.; Kamp, T.J.; Wu, J.C.; et al. Stem Cell-Derived Cardiomyocytes and Beta-Adrenergic Receptor Blockade in Duchenne Muscular Dystrophy Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 1159–1174. [Google Scholar] [CrossRef]
- Eisen, B.; Ben Jehuda, R.; Cuttitta, A.J.; Mekies, L.N.; Shemer, Y.; Baskin, P.; Reiter, I.; Willi, L.; Freimark, D.; Gherghiceanu, M.; et al. Electrophysiological Abnormalities in Induced Pluripotent Stem Cell-Derived Cardiomyocytes Generated from Duchenne Muscular Dystrophy Patients. J. Cell. Mol. Med. 2019, 23, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Loufrani, L.; Matrougui, K.; Gorny, D.; Duriez, M.; Blanc, I.; Lévy, B.I.; Henrion, D. Flow (Shear Stress)-Induced Endothelium-Dependent Dilation Is Altered in Mice Lacking the Gene Encoding for Dystrophin. Circulation 2001, 103, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.; Gatto, I.; Neri, V.; Straino, S.; Smith, R.C.; Silver, M.; Gaetani, E.; Marcantoni, M.; Giarretta, I.; Stigliano, E.; et al. Angiogenic Impairment of the Vascular Endothelium: A Novel Mechanism and Potential Therapeutic Target in Muscular Dystrophy. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Loufrani, L.; Dubroca, C.; You, D.; Li, Z.; Levy, B.; Paulin, D.; Henrion, D. Absence of Dystrophin in Mice Reduces NO-Dependent Vascular Function and Vascular Density: Total Recovery after a Treatment with the Aminoglycoside Gentamicin. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 671–676. [Google Scholar] [CrossRef]
- Hugnot, J.P.; Gilgenkrantz, H.; Chafey, P.; Lambert, M.; Eveno, E.; Kaplan, J.C.; Kahn, A. Expression of the Dystrophin Gene in Cultured Fibroblasts. Biochem. Biophys. Res. Commun. 1993, 192, 69–74. [Google Scholar] [CrossRef]
- Andrysiak, K.; Stępniewski, J.; Dulak, J. Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes, 3D Cardiac Structures, and Heart-on-a-Chip as Tools for Drug Research. Pflug. Arch. 2021, 473, 1061–1085. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A Novel Pathway for the Production of Hydrogen Sulfide from D-Cysteine in Mammalian Cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Sellke, F.W. The Effects of Therapeutic Sulfide on Myocardial Apoptosis in Response to Ischemia-Reperfusion Injury. Eur. J. Cardiothorac. Surg. 2008, 33, 906–913. [Google Scholar] [CrossRef]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen Sulfide (H2S) Releasing Agents: Chemistry and Biological Applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G.; Gojon, G.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a Novel Mitochondria-Targeted Hydrogen Sulfide Donor, Stimulates Cellular Bioenergetics, Exerts Cytoprotective Effects and Protects against the Loss of Mitochondrial DNA Integrity in Oxidatively Stressed Endothelial Cells in Vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a Mitochondria-targeting Hydrogen Sulfide (H2S) Donor, Protects against Myocardial Reperfusion Injury Independently of Salvage Kinase Signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, T.; Liang, K.; Chandrawati, R. Metal-Organic Frameworks for Therapeutic Gas Delivery. Adv. Drug Deliv. Rev. 2021, 171, 199–214. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Matson, J.B. Hydrogels for Gasotransmitter Delivery: Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide. Macromol. Biosci. 2023, e2300138. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Q. Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function. Antioxidants 2023, 12, 652. [Google Scholar] [CrossRef]
- Huang, Z.; Zhuang, X.; Xie, C.; Hu, X.; Dong, X.; Guo, Y.; Li, S.; Liao, X. Exogenous Hydrogen Sulfide Attenuates High Glucose-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome Activation by Suppressing TLR4/NF-κB Pathway in H9c2 Cells. Cell Physiol. Biochem. 2016, 40, 1578–1590. [Google Scholar] [CrossRef]
- Liu, M.-H.; Zhang, Y.; He, J.; Tan, T.-P.; Wu, S.-J.; Guo, D.-M.; He, H.; Peng, J.; Tang, Z.-H.; Jiang, Z.-S. Hydrogen Sulfide Protects H9c2 Cardiac Cells against Doxorubicin-Induced Cytotoxicity through the PI3K/Akt/FoxO3a Pathway. Int. J. Mol. Med. 2016, 37, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, X.; Wang, Y.; Zou, Z.; Yuan, A.; Xiao, Z.; Geng, N.; Qiao, Z.; Li, W.; Lu, X.; et al. Hydrogen Sulfide Alleviates Mitochondrial Damage and Ferroptosis by Regulating OPA3-NFS1 Axis in Doxorubicin-Induced Cardiotoxicity. Cell. Signal. 2023, 107, 110655. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, X.; Zhuang, X.; Hu, X.; Wang, L.; Liao, X. Exogenous Hydrogen Sulfide Protects against High Glucose-induced Inflammation and Cytotoxicity in H9c2 Cardiac Cells. Mol. Med. Rep. 2016, 14, 4911–4917. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.K.; Donnelly, E.; Donnarumma, E.; Hossain, F.; Gardner, J.D.; Islam, K.N. H2S Prodrug, SG-1002, Protects against Myocardial Oxidative Damage and Hypertrophy In Vitro via Induction of Cystathionine β-Synthase and Antioxidant Proteins. Biomedicines 2023, 11, 612. [Google Scholar] [CrossRef]
- Al-Owais, M.M.; Hettiarachchi, N.T.; Dallas, M.L.; Scragg, J.L.; Lippiat, J.D.; Holden, A.V.; Steele, D.S.; Peers, C. Inhibition of the Voltage-Gated Potassium Channel Kv1.5 by Hydrogen Sulfide Attenuates Remodeling through S-Nitrosylation-Mediated Signaling. Commun. Biol. 2023, 6, 651. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wu, Z.; Jiang, J.; Liu, C.; Wu, B.; Li, X.; Li, T.; Mo, H.; He, S.; Li, S.; et al. New Mechanism of Lipotoxicity in Diabetic Cardiomyopathy: Deficiency of Endogenous H2S Production and ER Stress. Mech. Ageing Dev. 2017, 162, 46–52. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, G.; Qiu, S.; Lu, J.; Sheng, J.; Manasi; Tan, G.; Wong, P.; Gan, S.U.; Shim, W. Hydrogen Sulfide Suppresses Outward Rectifier Potassium Currents in Human Pluripotent Stem Cell-Derived Cardiomyocytes. PLoS ONE 2012, 7, e50641. [Google Scholar] [CrossRef]
- Yang, H.; Mao, Y.; Tan, B.; Luo, S.; Zhu, Y. The Protective Effects of Endogenous Hydrogen Sulfide Modulator, S-Propargyl-Cysteine, on High Glucose-Induced Apoptosis in Cardiomyocytes: A Novel Mechanism Mediated by the Activation of Nrf2. Eur. J. Pharmacol. 2015, 761, 135–143. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wang, C.-C.; Lai, T.-Y.; Tsu, H.-N.; Wang, C.-H.; Liang, H.-Y.; Kuo, W.-W. Antioxidant Effects of Diallyl Trisulfide on High Glucose-Induced Apoptosis Are Mediated by the PI3K/Akt-Dependent Activation of Nrf2 in Cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Q.; Chen, Y.; Ma, Y.; Wang, D. Involvement of MicroRNA-133a in the Protective Effect of Hydrogen Sulfide against Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress and Cardiomyocyte Apoptosis. Pharmacology 2019, 103, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J.; Zhang, Y.; Xi, Y.; Wen, X.; Yuan, D.; Wang, Y.; Wei, C.; Wang, R.; Wu, L.; et al. Exogenous H2S Restores Ischemic Post-Conditioning-Induced Cardioprotection through Inhibiting Endoplasmic Reticulum Stress in the Aged Cardiomyocytes. Cell Biosci. 2017, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, R.; Jin, H.; Liu, D.; Tang, X.; Tang, C.; Du, J. Hydrogen Sulfide Attenuates Hyperhomocysteinemia-Induced Cardiomyocytic Endoplasmic Reticulum Stress in Rats. Antioxid. Redox Signal. 2010, 12, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Yang, C.-T.; Zheng, D.-D.; Mo, L.-Q.; Lan, A.-P.; Yang, Z.-L.; Hu, F.; Chen, P.-X.; Liao, X.-X.; Feng, J.-Q. Hydrogen Sulfide Protects H9c2 Cells against Doxorubicin-Induced Cardiotoxicity through Inhibition of Endoplasmic Reticulum Stress. Mol. Cell. Biochem. 2012, 363, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lin, J.; Xu, W.; Shen, N.; Mo, L.; Zhang, C.; Feng, J. Hydrogen Sulfide Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibition of the P38 MAPK Pathway in H9c2 Cells. Int. J. Mol. Med. 2013, 31, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The Vasorelaxant Effect of H(2)S as a Novel Endogenous Gaseous K(ATP) Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Foster, M.N.; Coetzee, W.A. KATP Channels in the Cardiovascular System. Physiol. Rev. 2016, 96, 177–252. [Google Scholar] [CrossRef] [PubMed]
- McNair, A.; Andreasen, F.; Nielsen, P.E. Antihypertensive Effect of Diazoxide given Intravenously in Small Repeated Doses. Eur. J. Clin. Pharmacol. 1983, 24, 151–156. [Google Scholar] [CrossRef]
- Jahangir, A.; Terzic, A. K Channel Therapeutics at the Bedside. J. Mol. Cell. Cardiol. 2005, 39, 99–112. [Google Scholar] [CrossRef]
- Bienengraeber, M.; Olson, T.M.; Selivanov, V.A.; Kathmann, E.C.; O’Cochlain, F.; Gao, F.; Karger, A.B.; Ballew, J.D.; Hodgson, D.M.; Zingman, L.V.; et al. ABCC9 Mutations Identified in Human Dilated Cardiomyopathy Disrupt Catalytic KATP Channel Gating. Nat. Genet. 2004, 36, 382–387. [Google Scholar] [CrossRef]
- Graciotti, L.; Becker, J.; Granata, A.L.; Procopio, A.D.; Tessarollo, L.; Fulgenzi, G. Dystrophin Is Required for the Normal Function of the Cardio-Protective KATP Channel in Cardiomyocytes. PLoS ONE 2011, 6, e27034. [Google Scholar] [CrossRef]
- Voitychuk, O.I.; Strutynskyi, R.B.; Yagupolskii, L.M.; Tinker, A.; Moibenko, O.O.; Shuba, Y.M. Sarcolemmal Cardiac KATP Channels as a Target for the Cardioprotective Effects of the Fluorine-Containing Pinacidil Analogue, Flocalin: Cardioprotective Effects of Flocalin. Br. J. Pharmacol. 2011, 162, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Veres, G.; Radovits, T.; Gero, D.; Módis, K.; Miesel-Gröschel, C.; Horkay, F.; Karck, M.; Szabó, C. Cardioprotective Effects of Hydrogen Sulfide. Nitric Oxide 2011, 25, 201–210. [Google Scholar] [CrossRef]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen Sulfide-Induced Relaxation of Resistance Mesenteric Artery Beds of Rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2316–H2323. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.R.; Kang, M.; Akbarali, H.I. Hydrogen Sulfide as an Allosteric Modulator of ATP-Sensitive Potassium Channels in Colonic Inflammation. Mol. Pharmacol. 2013, 83, 294–306. [Google Scholar] [CrossRef]
- Jiang, B.; Tang, G.; Cao, K.; Wu, L.; Wang, R. Molecular Mechanism for H2S-Induced Activation of KATP Channels. Antioxid. Redox Signal. 2010, 12, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, J.; Mo, L.; Ke, X.; Zhang, W.; Zheng, D.; Pan, W.; Wu, S.; Feng, J.; Song, M.; et al. ATP-Sensitive K+ Channels Contribute to the Protective Effects of Exogenous Hydrogen Sulfide against High Glucose-Induced Injury in H9c2 Cardiac Cells. Int. J. Mol. Med. 2016, 37, 763–772. [Google Scholar] [CrossRef][Green Version]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Di Cesare Mannelli, L.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The Novel H 2 S-Donor 4-Carboxyphenyl Isothiocyanate Promotes Cardioprotective Effects against Ischemia/Reperfusion Injury through Activation of mitoK ATP Channels and Reduction of Oxidative Stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef]
- Bian, J.-S.; Yong, Q.C.; Pan, T.-T.; Feng, Z.-N.; Ali, M.Y.; Zhou, S.; Moore, P.K. Role of Hydrogen Sulfide in the Cardioprotection Caused by Ischemic Preconditioning in the Rat Heart and Cardiac Myocytes. J. Pharmacol. Exp. Ther. 2006, 316, 670–678. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Starinets, V.S.; Belosludtseva, N.V.; Mikheeva, I.B.; Chelyadnikova, Y.A.; Penkina, D.K.; Vedernikov, A.A.; Belosludtsev, K.N. The Effect of Uridine on the State of Skeletal Muscles and the Functioning of Mitochondria in Duchenne Dystrophy. Int. J. Mol. Sci. 2022, 23, 10660. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Reiter, M.; Gastonguay, C.; McGivern, J.V.; Guan, X.; Ge, Z.-D.; Mack, D.L.; Childers, M.K.; Ebert, A.D.; Strande, J.L. Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 549–562. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by Hydrogen Sulphide Involves Activation of Kv7 Potassium Channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.; Müller, C.T.; Riccardi, D.; Kemp, P.J. Hydrogen Sulfide Inhibits Human BK(Ca) Channels. Adv. Exp. Med. Biol. 2009, 648, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Wong, W.T.; Sayed, N.; Luo, J.; Tsang, S.Y.; Bian, Z.X.; Lu, Y.; Cheang, W.S.; Yao, X.; Chen, Z.Y.; et al. NaHS Relaxes Rat Cerebral Artery in Vitro via Inhibition of L-Type Voltage-Sensitive Ca2+ Channel. Pharmacol. Res. 2012, 65, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Cheang, W.S.; Wong, W.T.; Shen, B.; Lau, C.W.; Tian, X.Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y.; Huang, Y. 4-Aminopyridine-Sensitive K+ Channels Contributes to NaHS-Induced Membrane Hyperpolarization and Relaxation in the Rat Coronary Artery. Vasc. Pharmacol. 2010, 53, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Starinets, V.S.; Belosludtseva, N.V.; Mikheeva, I.B.; Chelyadnikova, Y.A.; Igoshkina, A.D.; Vafina, A.B.; Vedernikov, A.A.; Belosludtsev, K.N. BKCa Activator NS1619 Improves the Structure and Function of Skeletal Muscle Mitochondria in Duchenne Dystrophy. Pharmaceutics 2022, 14, 2336. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Belosludtsev, K.N. Ion Channels of the Sarcolemma and Intracellular Organelles in Duchenne Muscular Dystrophy: A Role in the Dysregulation of Ion Homeostasis and a Possible Target for Therapy. Int. J. Mol. Sci. 2023, 24, 2229. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Kang, M.; Hashimoto, A.; Gade, A.; Akbarali, H.I. Interaction between Hydrogen Sulfide-Induced Sulfhydration and Tyrosine Nitration in the KATP Channel Complex. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G532–G539. [Google Scholar] [CrossRef]
- Naik, J.S.; Osmond, J.M.; Walker, B.R.; Kanagy, N.L. Hydrogen Sulfide-Induced Vasodilation Mediated by Endothelial TRPV4 Channels. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1437–H1444. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen Sulfide, an Enhancer of Vascular Nitric Oxide Signaling: Mechanisms and Implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef] [PubMed]
- Chatzianastasiou, A.; Bibli, S.-I.; Andreadou, I.; Efentakis, P.; Kaludercic, N.; Wood, M.E.; Whiteman, M.; Di Lisa, F.; Daiber, A.; Manolopoulos, V.G.; et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and -Independent Mechanisms. J. Pharmacol. Exp. Ther. 2016, 358, 431–440. [Google Scholar] [CrossRef]
- Ravani, S.; Chatzianastasiou, A.; Papapetropoulos, A. Using Mechanism-Based Combinations of H2S-Donors to Maximize the Cardioprotective Action of H2S. Naunyn Schmiedebergs Arch. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Bibli, S.-I.; Szabo, C.; Chatzianastasiou, A.; Luck, B.; Zukunft, S.; Fleming, I.; Papapetropoulos, A. Hydrogen Sulfide Preserves Endothelial Nitric Oxide Synthase Function by Inhibiting Proline-Rich Kinase 2: Implications for Cardiomyocyte Survival and Cardioprotection. Mol. Pharmacol. 2017, 92, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Mazza, R.; Pasqua, T.; Cerra, M.C.; Angelone, T.; Gattuso, A. Akt/eNOS Signaling and PLN S-Sulfhydration Are Involved in H2S-Dependent Cardiac Effects in Frog and Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R443–R451. [Google Scholar] [CrossRef]
- Peng, S.; Wang, M.; Zhang, S.; Liu, N.; Li, Q.; Kang, J.; Chen, L.; Li, M.; Pang, K.; Huang, J.; et al. Hydrogen Sulfide Regulates SERCA2a SUMOylation by S-Sulfhydration of SENP1 to Ameliorate Cardiac Systole-Diastole Function in Diabetic Cardiomyopathy. Biomed. Pharmacother. 2023, 160, 114200. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.-X.; et al. Hydrogen Sulfide Cytoprotective Signaling Is Endothelial Nitric Oxide Synthase-Nitric Oxide Dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous Hydrogen Sulfide Regulation of Myocardial Injury Induced by Isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.-I.; Kimura, H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Nagy, P.; Winterbourn, C.C. Rapid Reaction of Hydrogen Sulfide with the Neutrophil Oxidant Hypochlorous Acid to Generate Polysulfides. Chem. Res. Toxicol. 2010, 23, 1541–1543. [Google Scholar] [CrossRef]
- Jain, S.K.; Huning, L.; Micinski, D. Hydrogen Sulfide Upregulates Glutamate-Cysteine Ligase Catalytic Subunit, Glutamate-Cysteine Ligase Modifier Subunit, and Glutathione and Inhibits Interleukin-1β Secretion in Monocytes Exposed to High Glucose Levels. Metab. Syndr. Relat. Disord. 2014, 12, 299–302. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Nikolaou, E.; Filippidis, G.; Liakopoulos, V.; Stefanidis, I. Mistimed H2S Upregulation, Nrf2 Activation and Antioxidant Proteins Levels in Renal Tubular Epithelial Cells Subjected to Anoxia and Reoxygenation. Biomed. Rep. 2020, 13, 3. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects Against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the Keap1 Ubiquitin Ligase Substrate Adaptor Through Formation of a Disulfide Bond Between Cys-226 and Cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of Activation of the Transcription Factor Nrf2 by Redox Stressors, Nutrient Cues, and Energy Status and the Pathways through Which It Attenuates Degenerative Disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Wang, M.; Tang, J.; Zhang, S.; Pang, K.; Zhao, Y.; Liu, N.; Huang, J.; Kang, J.; Dong, S.; Li, H.; et al. Exogenous H2S Initiating Nrf2/GPx4/GSH Pathway through Promoting Syvn1-Keap1 Interaction in Diabetic Hearts. Cell Death Discov. 2023, 9, 394. [Google Scholar] [CrossRef]
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The Biologic Effect of Hydrogen Sulfide and Its Function in Various Diseases. Medicine 2018, 97, e13065. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Li, X.-H.; Zhang, T.; Fu, J.; Cui, X.-D. Hydrogen Sulfide Upregulates Heme Oxygenase-1 Expression in Rats with Volume Overload-Induced Heart Failure. Biomed. Rep. 2013, 1, 454–458. [Google Scholar] [CrossRef]
- Hua, W.; Chen, Q.; Gong, F.; Xie, C.; Zhou, S.; Gao, L. Cardioprotection of H2S by Downregulating iNOS and Upregulating HO-1 Expression in Mice with CVB3-Induced Myocarditis. Life Sci. 2013, 93, 949–954. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nicholson, C.K.; Lambert, J.P.; Barr, L.A.; Kuek, N.; Herszenhaut, D.; Tan, L.; Murohara, T.; Hansen, J.M.; Husain, A.; et al. Sodium Sulfide Attenuates Ischemic-Induced Heart Failure by Enhancing Proteasomal Function in an Nrf2-Dependent Manner. Circ. Heart Fail. 2016, 9, e002368. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, W.; Zhang, M.; Jin, M.; Xu, W.; Zhou, X. Gas Signaling Molecule Hydrogen Sulfide Attenuates Doxorubicin-Induced Dilated Cardiomyopathy. Oncotarget 2017, 8, 95425–95431. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-H.; Liu, F.; Chen, Y.; Zhu, Y.-C. Hydrogen Sulfide Decreases the Levels of ROS by Inhibiting Mitochondrial Complex IV and Increasing SOD Activities in Cardiomyocytes under Ischemia/Reperfusion. Biochem. Biophys. Res. Commun. 2012, 421, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.; Steegborn, C. The Lifespan-Regulator p66Shc in Mitochondria: Redox Enzyme or Redox Sensor? Antioxid. Redox Signal. 2010, 13, 1417–1428. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric Oxide, Oxidative Stress, and p66Shc Interplay in Diabetic Endothelial Dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-Z.; Shi, M.-M.; Xie, L.; Wu, Z.-Y.; Li, G.; Hua, F.; Bian, J.-S. Sulfhydration of p66Shc at Cysteine59 Mediates the Antioxidant Effect of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 21, 2531–2542. [Google Scholar] [CrossRef]
- Benedetti, F.; Curreli, S.; Krishnan, S.; Davinelli, S.; Cocchi, F.; Scapagnini, G.; Gallo, R.C.; Zella, D. Anti-Inflammatory Effects of H2S during Acute Bacterial Infection: A Review. J. Transl. Med. 2017, 15, 100. [Google Scholar] [CrossRef]
- Bourque, C.; Zhang, Y.; Fu, M.; Racine, M.; Greasley, A.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. H2S Protects Lipopolysaccharide-Induced Inflammation by Blocking NFκB Transactivation in Endothelial Cells. Toxicol. Appl. Pharmacol. 2018, 338, 20–29. [Google Scholar] [CrossRef]
- Du, J.; Huang, Y.; Yan, H.; Zhang, Q.; Zhao, M.; Zhu, M.; Liu, J.; Chen, S.X.; Bu, D.; Tang, C.; et al. Hydrogen Sulfide Suppresses Oxidized Low-Density Lipoprotein (Ox-LDL)-Stimulated Monocyte Chemoattractant Protein 1 Generation from Macrophages via the Nuclear Factor κB (NF-κB) Pathway. J. Biol. Chem. 2014, 289, 9741–9753. [Google Scholar] [CrossRef]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Stahl, G.L.; Sellke, F.W. Hydrogen Sulfide Therapy Attenuates the Inflammatory Response in a Porcine Model of Myocardial Ischemia/Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2009, 138, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Zhao, G.; Sun, A.; Zong, N.C.; Li, Z.; Zhu, H.; Zou, Y.; Yang, X.; Ge, J. Hydrogen Sulfide Attenuates the Recruitment of CD11b+Gr-1+ Myeloid Cells and Regulates Bax/Bcl-2 Signaling in Myocardial Ischemia Injury. Sci. Rep. 2014, 4, 4774. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, H.; Wu, B.; Zhang, L.; Wu, S.-W.; Wang, J.-N.; Zhang, Y.-E. Hydrogen Sulfide Reduces Recruitment of CD11b+Gr-1+ Cells in Mice With Myocardial Infarction. Cell Transplant. 2017, 26, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, K.; Kida, K.; Marutani, E.; Crimi, E.; Bougaki, M.; Khatri, A.; Kimura, H.; Ichinose, F. Inhaled Hydrogen Sulfide Prevents Endotoxin-Induced Systemic Inflammation and Improves Survival by Altering Sulfide Metabolism in Mice. Antioxid. Redox Signal. 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Toldo, S.; Das, A.; Mezzaroma, E.; Chau, V.Q.; Marchetti, C.; Durrant, D.; Samidurai, A.; Van Tassell, B.W.; Yin, C.; Ockaili, R.A.; et al. Induction of microRNA-21 with Exogenous Hydrogen Sulfide Attenuates Myocardial Ischemic and Inflammatory Injury in Mice. Circ. Cardiovasc. Genet. 2014, 7, 311–320. [Google Scholar] [CrossRef]
- Snijder, P.M.; Frenay, A.R.; de Boer, R.A.; Pasch, A.; Hillebrands, J.L.; Leuvenink, H.G.D.; van Goor, H. Exogenous Administration of Thiosulfate, a Donor of Hydrogen Sulfide, Attenuates Angiotensin II-Induced Hypertensive Heart Disease in Rats. Br. J. Pharmacol. 2015, 172, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Lilyanna, S.; Peh, M.T.; Liew, O.W.; Wang, P.; Moore, P.K.; Richards, A.M.; Martinez, E.C. GYY4137 Attenuates Remodeling, Preserves Cardiac Function and Modulates the Natriuretic Peptide Response to Ischemia. J. Mol. Cell. Cardiol. 2015, 87, 27–37. [Google Scholar] [CrossRef]
- Pan, L.-L.; Wang, X.-L.; Wang, X.-L.; Zhu, Y.-Z. Sodium Hydrosulfide Prevents Myocardial Dysfunction through Modulation of Extracellular Matrix Accumulation and Vascular Density. Int. J. Mol. Sci. 2014, 15, 23212–23226. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Zheng, J.; Huang, X.; Jin, H. Hydrogen Sulfide Attenuates Cardiac Hypertrophy and Fibrosis Induced by Abdominal Aortic Coarctation in Rats. Mol. Med. Rep. 2012, 5, 923–928. [Google Scholar] [CrossRef]
- Nie, L.; Liu, M.; Chen, J.; Wu, Q.; Li, Y.; Yi, J.; Zheng, X.; Zhang, J.; Chu, C.; Yang, J. Hydrogen Sulfide Ameliorates Doxorubicin-Induced Myocardial Fibrosis in Rats via the PI3K/AKT/mTOR Pathway. Mol. Med. Rep. 2021, 23, 299. [Google Scholar] [CrossRef]
- Tran, B.H.; Yu, Y.; Chang, L.; Tan, B.; Jia, W.; Xiong, Y.; Dai, T.; Zhong, R.; Zhang, W.; Le, V.M.; et al. A Novel Liposomal S-Propargyl-Cysteine: A Sustained Release of Hydrogen Sulfide Reducing Myocardial Fibrosis via TGF-Β1/Smad Pathway. Int. J. Nanomed. 2019, 14, 10061–10077. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Tyagi, N.; Sen, U.; Givvimani, S.; Tyagi, S.C. H2S Ameliorates Oxidative and Proteolytic Stresses and Protects the Heart against Adverse Remodeling in Chronic Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H451–H456. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-L.; Liu, X.-H.; Shen, Y.-Q.; Wang, N.-Z.; Xu, J.; Wu, D.; Xiong, Q.-H.; Deng, H.-Y.; Huang, G.-Y.; Zhu, Y.-Z. Inhibition of NADPH Oxidase 4-Related Signaling by Sodium Hydrosulfide Attenuates Myocardial Fibrotic Response. Int. J. Cardiol. 2013, 168, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhu, J.; Xiao, Y.; Huang, Z.; Zhang, Y.; Tang, X.; Xie, L.; Chen, Y.; Shao, Y.; Ferro, A.; et al. Hydrogen Sulfide Donor GYY4137 Protects against Myocardial Fibrosis. Oxid. Med. Cell. Longev. 2015, 2015, 691070. [Google Scholar] [CrossRef]
- Ma, N.; Liu, H.-M.; Xia, T.; Liu, J.-D.; Wang, X.-Z. Chronic Aerobic Exercise Training Alleviates Myocardial Fibrosis in Aged Rats through Restoring Bioavailability of Hydrogen Sulfide. Can. J. Physiol. Pharmacol. 2018, 96, 902–908. [Google Scholar] [CrossRef]
- Mucha, O.; Myszka, M.; Podkalicka, P.; Świderska, B.; Malinowska, A.; Dulak, J.; Łoboda, A. Proteome Profiling of the Dystrophic Mdx Mice Diaphragm. Biomolecules 2023, 13, 1648. [Google Scholar] [CrossRef]
- Holland, A.; Dowling, P.; Meleady, P.; Henry, M.; Zweyer, M.; Mundegar, R.R.; Swandulla, D.; Ohlendieck, K. Label-Free Mass Spectrometric Analysis of the Mdx-4cv Diaphragm Identifies the Matricellular Protein Periostin as a Potential Factor Involved in Dystrophinopathy-Related Fibrosis. Proteomics 2015, 15, 2318–2331. [Google Scholar] [CrossRef]
- Murphy, S.; Dowling, P.; Zweyer, M.; Mundegar, R.R.; Henry, M.; Meleady, P.; Swandulla, D.; Ohlendieck, K. Proteomic Analysis of Dystrophin Deficiency and Associated Changes in the Aged Mdx-4cv Heart Model of Dystrophinopathy-Related Cardiomyopathy. J. Proteom. 2016, 145, 24–36. [Google Scholar] [CrossRef]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin Is Essential for Cardiac Healing after Acute Myocardial Infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef]
- Oka, T.; Xu, J.; Kaiser, R.A.; Melendez, J.; Hambleton, M.; Sargent, M.A.; Lorts, A.; Brunskill, E.W.; Dorn, G.W.; Conway, S.J.; et al. Genetic Manipulation of Periostin Expression Reveals a Role in Cardiac Hypertrophy and Ventricular Remodeling. Circ. Res. 2007, 101, 313–321. [Google Scholar] [CrossRef]
- Kundu, S.; Pushpakumar, S.; Khundmiri, S.J.; Sen, U. Hydrogen Sulfide Mitigates Hyperglycemic Remodeling via Liver Kinase B1-Adenosine Monophosphate-Activated Protein Kinase Signaling. Biochim. Biophys. Acta 2014, 1843, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
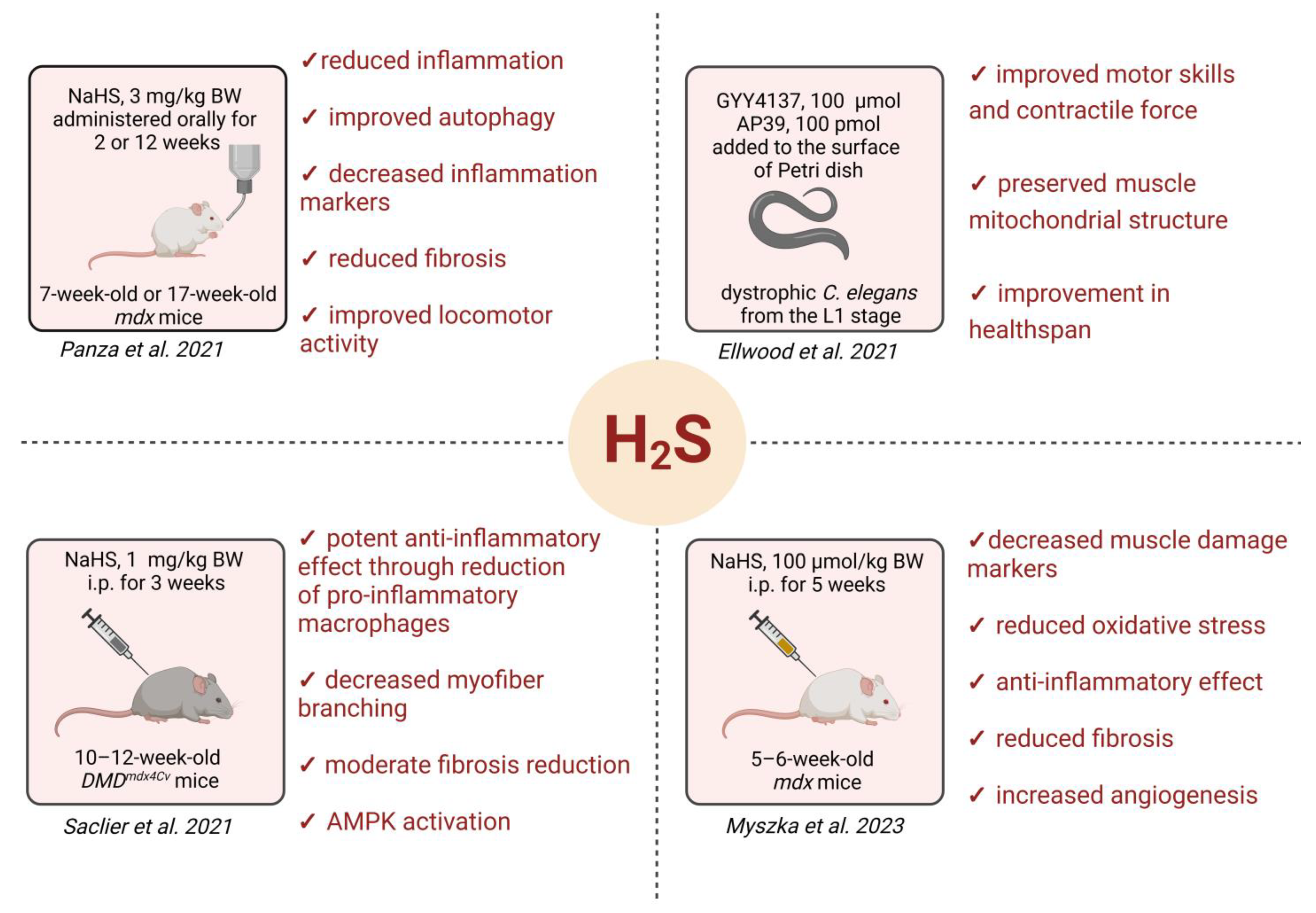
- Myszka, M.; Mucha, O.; Podkalicka, P.; Waśniowska, U.; Dulak, J.; Łoboda, A. Sodium Hydrosulfide Moderately Alleviates the Hallmark Symptoms of Duchenne Muscular Dystrophy in Mdx Mice. Eur. J. Pharmacol. 2023, 955, 175928. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and Molecular Implications of Osteopontin in Heart Failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Givvimani, S.; Bhatnagar, S.; Qipshidze, N.; Tyagi, S.C.; Kumar, A. Osteopontin-Stimulated Expression of Matrix Metalloproteinase-9 Causes Cardiomyopathy in the Mdx Model of Duchenne Muscular Dystrophy. J. Immunol. 2011, 187, 2723–2731. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen Sulfide Is an Endogenous Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Cai, W.-J.; Li, N.; Ding, Y.-J.; Chen, Y.; Zhu, Y.-C. The Hydrogen Sulfide Donor NaHS Promotes Angiogenesis in a Rat Model of Hind Limb Ischemia. Antioxid. Redox Signal. 2010, 12, 1065–1077. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Zhuo, Y.; Gong, Q.; Rose, P.; Zhu, Y. Hypoxia-Inducible Factor-1α Is Involved in the pro-Angiogenic Effect of Hydrogen Sulfide under Hypoxic Stress. Biol. Pharm. Bull. 2010, 33, 1550–1554. [Google Scholar] [CrossRef]
- Bir, S.C.; Kolluru, G.K.; McCarthy, P.; Shen, X.; Pardue, S.; Pattillo, C.B.; Kevil, C.G. Hydrogen Sulfide Stimulates Ischemic Vascular Remodeling through Nitric Oxide Synthase and Nitrite Reduction Activity Regulating Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor-Dependent Angiogenesis. J. Am. Heart Assoc. 2012, 1, e004093. [Google Scholar] [CrossRef]
- Givvimani, S.; Munjal, C.; Gargoum, R.; Sen, U.; Tyagi, N.; Vacek, J.C.; Tyagi, S.C. Hydrogen Sulfide Mitigates Transition from Compensatory Hypertrophy to Heart Failure. J. Appl. Physiol. 2011, 110, 1093. [Google Scholar] [CrossRef]
- Qipshidze, N.; Metreveli, N.; Mishra, P.K.; Lominadze, D.; Tyagi, S.C. Hydrogen Sulfide Mitigates Cardiac Remodeling During Myocardial Infarction via Improvement of Angiogenesis. Int. J. Biol. Sci. 2012, 8, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.-B.; Liu, S.-Y.; Zhang, C.-C.; Fu, W.; Cai, W.-J.; Wang, Y.; Shen, Q.; Wang, M.-J.; Chen, Y.; Zhang, L.-J.; et al. VEGFR2 Functions As an H2S-Targeting Receptor Protein Kinase with Its Novel Cys1045–Cys1024 Disulfide Bond Serving As a Specific Molecular Switch for Hydrogen Sulfide Actions in Vascular Endothelial Cells. Antioxid. Redox Signal. 2013, 19, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.; Kondo, K.; Bhushan, S.; Bir, S.C.; Kevil, C.G.; Murohara, T.; Lefer, D.J.; Calvert, J.W. Hydrogen Sulfide Attenuates Cardiac Dysfunction after Heart Failure via Induction of Angiogenesis. Circ. Heart Fail. 2013, 6, 1077–1086. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G.; Gojon, G.; et al. H2S Protects against Pressure Overload-Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef]
- Cai, W.-J.; Wang, M.-J.; Moore, P.K.; Jin, H.-M.; Yao, T.; Zhu, Y.-C. The Novel Proangiogenic Effect of Hydrogen Sulfide Is Dependent on Akt Phosphorylation. Cardiovasc. Res. 2007, 76, 29–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Xue, W.-L.; Jin, S.; Li, M.-Y.; Zhang, C.-C.; Yu, B.; Zhu, L.; Liang, K.; Chen, Y.; et al. YB-1 Recruits Drosha to Promote Splicing of Pri-miR-192 to Mediate the Proangiogenic Effects of H2S. Antioxid. Redox Signal. 2022, 36, 760–783. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-L.; Chen, R.-Q.; Zhang, Q.-Q.; Li, X.-H.; Cao, L.; Li, M.-Y.; Li, Y.; Lin, G.; Chen, Y.; Wang, M.-J.; et al. Hydrogen Sulfide Rescues High Glucose-Induced Migration Dysfunction in HUVECs by Upregulating miR-126-3p. Am. J. Physiol. Cell Physiol. 2020, 318, C857–C869. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, P.K.; Xiong, X.; Dwivedi, S.K.D.; Mustafi, S.B.; Leigh, N.R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine β-Synthase Regulates Endothelial Function via Protein S-Sulfhydration. FASEB J. 2016, 30, 441–456. [Google Scholar] [CrossRef]
- Meng, G.; Xiao, Y.; Ma, Y.; Tang, X.; Xie, L.; Liu, J.; Gu, Y.; Yu, Y.; Park, C.-M.; Xian, M.; et al. Hydrogen Sulfide Regulates Krüppel-Like Factor 5 Transcription Activity via Specificity Protein 1 S-Sulfhydration at Cys664 to Prevent Myocardial Hypertrophy. J. Am. Heart Assoc. 2016, 5, e004160. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Tsang, A.; Yellon, D.M. The Reperfusion Injury Salvage Kinase Pathway: A Common Target for Both Ischemic Preconditioning and Postconditioning. Trends Cardiovasc. Med. 2005, 15, 69–75. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Pan, T.-T.; Neo, K.L.; Lee, S.W.; Khin, E.S.W.; Moore, P.K.; Bian, J.-S. Cardioprotection Induced by Hydrogen Sulfide Preconditioning Involves Activation of ERK and PI3K/Akt Pathways. Pflug. Arch. 2008, 455, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Yao, L.-L.; Huang, X.-W.; Wang, Y.-G.; Cao, Y.-X.; Zhang, C.-C.; Zhu, Y.-C. Hydrogen Sulfide Protects Cardiomyocytes from Hypoxia/Reoxygenation-Induced Apoptosis by Preventing GSK-3β-Dependent Opening of mPTP. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1310–H1319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xiao, J.; Kang, B.; Zhu, X.; Xin, N.; Wang, Z. PI3K/SGK1/GSK3β Signaling Pathway Is Involved in Inhibition of Autophagy in Neonatal Rat Cardiomyocytes Exposed to Hypoxia/Reoxygenation by Hydrogen Sulfide. Exp. Cell Res. 2016, 345, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological Postconditioning against Myocardial Infarction with a Slow-Releasing Hydrogen Sulfide Donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef]
- Panza, E.; Vellecco, V.; Iannotti, F.A.; Paris, D.; Manzo, O.L.; Smimmo, M.; Mitilini, N.; Boscaino, A.; de Dominicis, G.; Bucci, M.; et al. Duchenne’s Muscular Dystrophy Involves a Defective Transsulfuration Pathway Activity. Redox Biol. 2021, 45, 102040. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, R.A.; Hewitt, J.E.; Torregrossa, R.; Philp, A.M.; Hardee, J.P.; Hughes, S.; van de Klashorst, D.; Gharahdaghi, N.; Anupom, T.; Slade, L.; et al. Mitochondrial Hydrogen Sulfide Supplementation Improves Health in the C. Elegans Duchenne Muscular Dystrophy Model. Proc. Natl. Acad. Sci. USA 2021, 118, e2018342118. [Google Scholar] [CrossRef]
- Ellwood, R.A.; Slade, L.; Lewis, J.; Torregrossa, R.; Sudevan, S.; Piasecki, M.; Whiteman, M.; Etheridge, T.; Szewczyk, N.J. Sulfur Amino Acid Supplementation Displays Therapeutic Potential in a C. Elegans Model of Duchenne Muscular Dystrophy. Commun. Biol. 2022, 5, 1255. [Google Scholar] [CrossRef]
- Saclier, M.; Ben Larbi, S.; My Ly, H.; Moulin, E.; Mounier, R.; Chazaud, B.; Juban, G. Interplay between Myofibers and Pro-Inflammatory Macrophages Controls Muscle Damage in Mdx Mice. J. Cell Sci. 2021, 134, jcs258429. [Google Scholar] [CrossRef]
| Type of Drugs | Abbreviation | Exemplary Drugs | Mechanism of Action | Recommendations for Drug Use | References |
|---|---|---|---|---|---|
| angiotensin-converting enzyme inhibitors | ACEis | perindopril, enalapril, captopril | inhibition of Ang II formation and metabolism | ACEis should be used even in asymptomatic DMD boys with normal LV systolic function by the age of 10 years | [16,17,18,21] |
| angiotensin receptor blockers | ARBs | losartan | competitive inhibition of Ang II binding to the angiotensin 1 receptor | ARBs should be used even in asymptomatic DMD boys with normal LV systolic function by the age of 10 years | [19,20,21] |
| mineralocorticoid receptor antagonists (aldosterone antagonists) | MRAs | eplerenone, spironolactone | blocking the endogenous MR, aldosterone, at its receptors | early MRA treatment can increase the chance of improving the cardiac condition | [22,23,24] |
| beta-adrenergic receptor | β-AR | bisoprolol, metoprolol, carvedilol | nonselective or selective inhibition of β-adrenergic receptor | second-line therapy in patients with tachycardia and/or no effect of ACEis | [25,26,27] |
| Cardiac Disease | Cellular Model | Type and Concentration of H2S Donor | Time of Stimulation with H2S Donor | Additional Information about Cell Stimulation | Major Molecular Mechanism of H2S-Mediated Cardioprotection | References |
|---|---|---|---|---|---|---|
| DCM | Rat H9C2 cells; HG-induced cardiotoxicity model (33 mM glucose; 48 h) | NaHS (50 µM), SPRC (5–25 µM) | 4 h | Cells were pre-treated with H2S donors for 4 h before culturing in HG medium | Activation of AKT/NRF2 | [78] |
| DCM | Rat H9C2 cells; HG-induced cardiotoxicity model (33 mM glucose; 36 h) | DATS (1–10 µM) | 12–48 h | Cells were treated with HG and DATS (1, 5, or 10 μM) for 36 h or DATS (10 μM) for 12–48 h | Activation of PI3K/AKT/NRF2 | [79] |
| DCM | Rat H9C2 cells; HG-induced cardiotoxicity model (33 mM glucose; 24 h) | NaHS (400 µM) | 30 min | Cells were pre-treated with 400 μM of NaHS for 30 min before culturing in HG medium | Suppression of TLR4/NF-κB pathway: alleviation of HG-induced activation of NLRP3 inflammasome, TLR4, and NF-κB | [70] |
| DCM | Rat H9C2 cells; HG-induced cardiotoxicity model (33 mM glucose; 24 h) | NaHS (400 µM) | 30 min | Cells were pre-treated with 400 µM of NaHS for 30 min before culturing in HG medium | Inhibition of the p38MAPK/NF-κB, COX-2 and iNOS signaling pathways | [73] |
| DCM | Human AC16 cells; cardiac lipotoxicity model (500 µM PA; 24 h) | NaHS (100 µM) | 24 h | NaHS treatment was repeated every 6 h during the entire treatment period of 24 h | Inhibition of ER stress: downregulation of stress marker proteins including GRP78, CHOP, and caspase-12 | [76] |
| I/R injury | Rat H9C2 cells; hypoxia/reoxygenation model (H/R model: 0.1% O2 + 5% CO2 in 1% FBS serum-starvation medium for 4 h. After hypoxia, the cells were re-oxygenated in 95% O2 + 5% CO2) | NaHS (200 µM) | Not specified directly | Initially, H2S in different concentrations from 50 to 200 μM were tested, and then 200 μM was used for subsequent experiments | Inhibition of ER stress: downregulation of stress marker proteins including GRP78, CHOP, and eIF2α. The involvement of miR-133a in the H2S effect was demonstrated. | [80] |
| I/R injury | Old rat H9C2 cells (aging: 30 μM of H2O2; 2 h and subsequent culture for 3 days); hypoxia/reoxygenation model (H/R model: aged cardiac cells were exposed to a hypoxic culture medium for 3 h and reoxygenated for 6 h) | NaHS (100 µM) | 6 h | NaHS was added for a 6 h reoxygenation phase | Inhibition of ER stress: decreased the expression of GRP78, CHOP, cleaved caspase-12, ATF4, ATF6, and XBP-1, and the phosphorylation of PERK, eIF2α, and IRE1α | [81] |
| HHcy-induced MI | Rat H9C2 cells; hyperhomocysteine-induced ER stress model (Hcy 0.1–2.5 mM; 6 h) | NaHS (100–1000 µM) | 30 min | Cells were pre-treated for 30 min with NaHS and then supplemented with Hcy for 6 h | Inhibition of ER stress: decreased CHOP expression induced by Hcy | [82] |
| DOX-induced cardiotoxicity | Rat H9C2 cells (5 µM DOX; 24 h) | NaHS (400 µM) | 30 min | Cells were treated with NaHS for 30 min before exposure to DOX | Inhibition of ER stress: blocking of DOX-induced overexpression of GRP78 and CHOP | [83] |
| DOX-induced cardiotoxicity | Rat H9C2 cells (5 µM DOX; 60 min) | NaHS (400 µM) | 30 min | H9c2 cells were pre-treated with NaHS for 30 min before DOX treatment | Decreased expression level of phospho-p38 MAPK | [84] |
| DOX-induced cardiotoxicity | Rat H9C2 cells (5 µM DOX; 24 h) | NaHS (100 µM) | 30 min | Cells were pre-treated with NaHS for 30 min, followed by exposure to DOX for 24 h | Activation of PI3K/AKT/FoxO3a pathways | [71] |
| DOX-induced cardiotoxicity | Rat H9C2 cells (1 μM DOX; 24 h) | NaHS (30 μM) | 30 min | Cells were pre-treated NaHS for 30 min, then the supernatant was substituted with medium containing DOX | Increased S-sulfhydration and downregulation of OPA3 ubiquitination | [72] |
| HF/cardiac hypertrophy | Murine HL-1 cells (starvation in 1% FBS-containing media; oxidative stress induction: 500 μM H2O2, 1 h) | SG-1002 (10 μM) | 1 h | Serum-starved cells were treated for 1 h with SG-1002, H2O2, or in combination | Inhibition of oxidative stress: induction of antioxidant proteins, catalase and SOD1 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łoboda, A.; Dulak, J. Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy. Cells 2024, 13, 158. https://doi.org/10.3390/cells13020158
Łoboda A, Dulak J. Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy. Cells. 2024; 13(2):158. https://doi.org/10.3390/cells13020158
Chicago/Turabian StyleŁoboda, Agnieszka, and Józef Dulak. 2024. "Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy" Cells 13, no. 2: 158. https://doi.org/10.3390/cells13020158
APA StyleŁoboda, A., & Dulak, J. (2024). Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy. Cells, 13(2), 158. https://doi.org/10.3390/cells13020158







