Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Computed Tomography Angiography Scan
2.3. ELISA
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Laboratory and Imaging Markers of Subclinical Atherosclerosis in ART-Treated PLWH
3.2. Plasma Markers in Relationship with HIV-1 Status and Subclinical Atherosclerosis
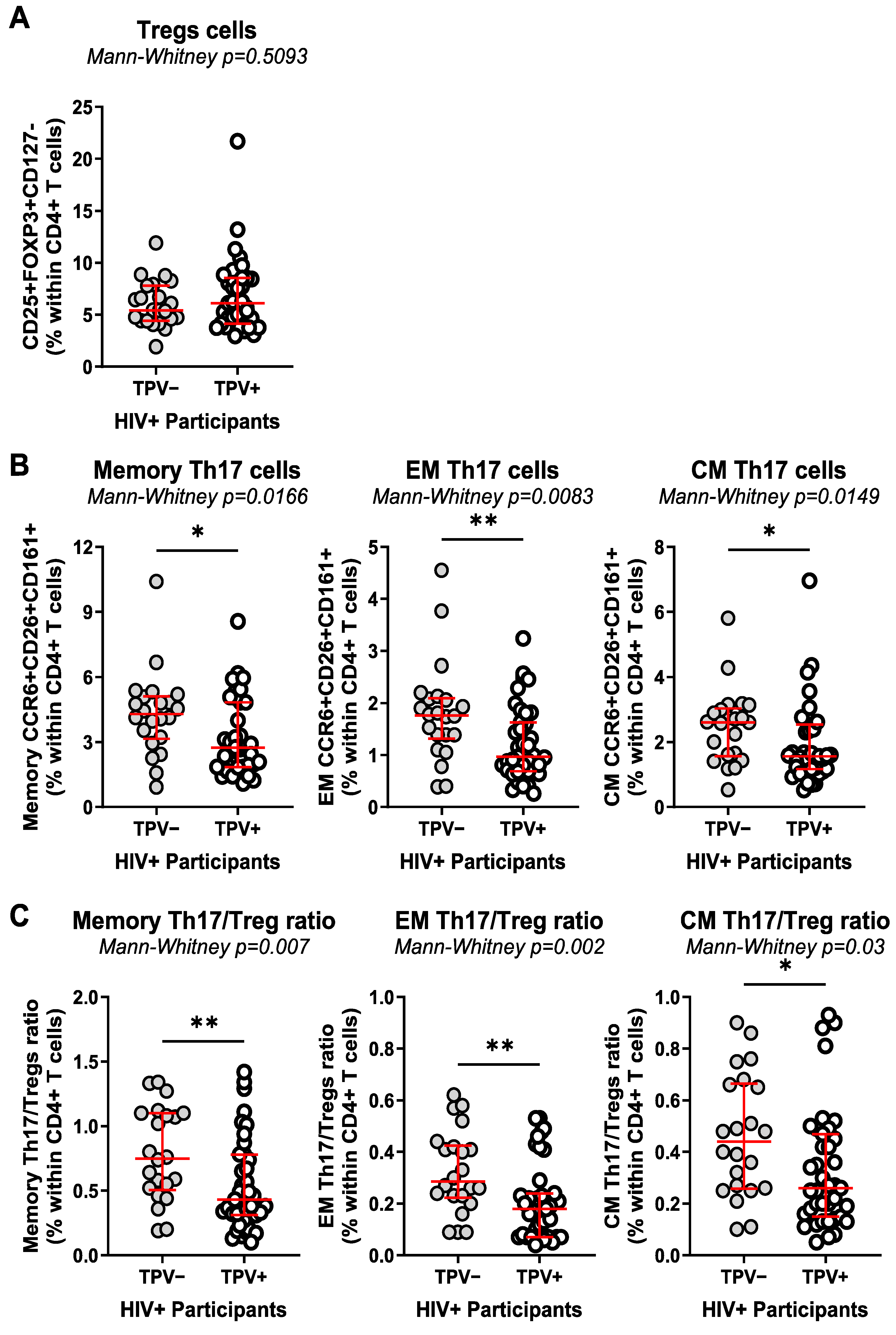
3.3. T-Cell Profile Alterations in Relationship with HIV-1 Status and Subclinical Atherosclerosis
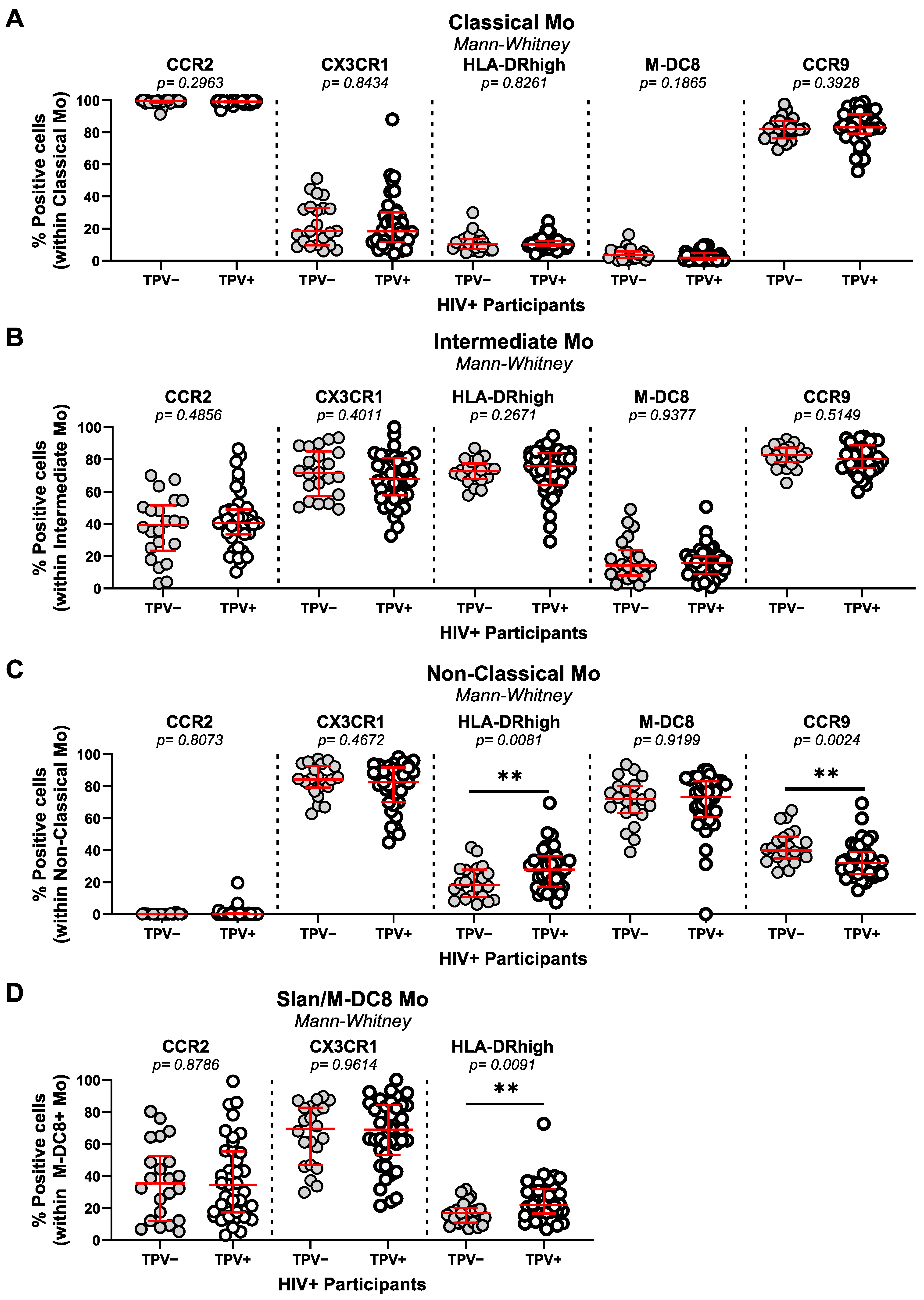
3.4. Monocyte Alterations in Relationship with HIV-1 Status and Subclinical Atherosclerosis
3.5. mDC and pDC Frequencies in Relationship with HIV-1 Status and Subclinical Atherosclerosis
3.6. Multivariate Analysis Identifies an Immunological Signature Associated with Subclinical Atherosclerosis in ART-Treated PLWH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Wagle, A.; Goerlich, E.; Post, W.S.; Woldu, B.; Wu, K.C.; Hays, A.G. HIV and Global Cardiovascular Health. Curr. Cardiol. Rep. 2022, 24, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Kentoffio, K.; Temu, T.M.; Shakil, S.S.; Zanni, M.V.; Longenecker, C.T. Cardiovascular disease risk in women living with HIV. Curr. Opin. HIV AIDS 2022, 17, 270–278. [Google Scholar] [CrossRef]
- Dirajlal-Fargo, S.; Funderburg, N. HIV and cardiovascular disease: The role of inflammation. Curr. Opin. HIV AIDS 2022, 17, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, I.; Sadouni, M.; Mansour, S.; Baril, J.G.; Trottier, B.; Soulez, G.; Chin, S.A.; Leipsic, J.; Tremblay, C.; Durand, M.; et al. Prevalence and Characterization of Subclinical Coronary Atherosclerotic Plaque with CT among Individuals with HIV: Results from the Canadian HIV and Aging Cohort Study. Radiology 2021, 299, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Zdunek, M.A.; Hogh, J.; Kirkegaard-Klitbo, D.M.; Jensen, A.M.R.; Rupert, A.; Troseid, M.; Gerstoft, J.; Nielsen, S.D.; Knudsen, A.D. High incidence of subclinical peripheral artery disease in people with HIV. AIDS 2022, 36, 1355–1362. [Google Scholar] [CrossRef]
- Sarkar, S.; Brown, T.T. CROI 2023: Metabolic and Other Complications of HIV Infection. Top. Antivir. Med. 2023, 31, 538–542. [Google Scholar]
- Henning, R.J.; Greene, J.N. The epidemiology, mechanisms, diagnosis and treatment of cardiovascular disease in adult patients with HIV. Am. J. Cardiovasc. Dis. 2023, 13, 101–121. [Google Scholar]
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; Sighem, A.; De Wolf, F.; Hallett, T.B.; on behalf of the ATHENA Observational Cohort. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect. Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Waters, D.D. HIV infection and coronary heart disease: Mechanisms and management. Nat. Rev. Cardiol. 2019, 16, 745–759. [Google Scholar] [CrossRef]
- Pinto, D.S.M.; Da Silva, M. Cardiovascular Disease in the Setting of Human Immunodeficiency Virus Infection. Curr. Cardiol. Rev. 2018, 14, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.M.; Ma, Y.; Scherzer, R.; Rahalkar, S.; Martin, J.N.; Mills, C.; Milush, J.; Deeks, S.G.; Hsue, P.Y. Association of Viral Persistence and Atherosclerosis in Adults With Treated HIV Infection. JAMA Netw. Open 2020, 3, e2018099. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.; Woudberg, N.J.; Kamau, F.; Strijdom, H.; Frias, M.A.; Lecour, S. HIV-related cardiovascular disease: Any role for high-density lipoproteins? Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1221–H1226. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Hanna, D.B.; Kizer, J.R. Recent Insights Into Cardiovascular Disease (CVD) Risk Among HIV-Infected Adults. Curr. HIV/AIDS Rep. 2016, 13, 44–52. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e98–e124. [Google Scholar] [CrossRef]
- MacCann, R.; Landay, A.L.; Mallon, P.W.G. HIV and comorbidities—The importance of gut inflammation and the kynurenine pathway. Curr. Opin. HIV AIDS 2023, 18, 102–110. [Google Scholar] [CrossRef]
- Schnittman, S.R.; Hunt, P.W. Clinical consequences of asymptomatic cytomegalovirus in treated human immunodeficency virus infection. Curr. Opin. HIV AIDS 2021, 16, 168–176. [Google Scholar] [CrossRef]
- Teer, E.; Dominick, L.; Mukonowenzou, N.C.; Essop, M.F. HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation. Cells 2022, 11, 2825. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2021, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Huang, L.H.; Randolph, G.J. Cytokine Circuits in Cardiovascular Disease. Immunity 2019, 50, 941–954. [Google Scholar] [CrossRef]
- Komarowska, I.; Coe, D.; Wang, G.; Haas, R.; Mauro, C.; Kishore, M.; Cooper, D.; Nadkarni, S.; Fu, H.; Steinbruchel, D.A.; et al. Hepatocyte Growth Factor Receptor c-Met Instructs T Cell Cardiotropism and Promotes T Cell Migration to the Heart via Autocrine Chemokine Release. Immunity 2015, 42, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Winkels, H.; Wolf, D. Heterogeneity of T Cells in Atherosclerosis Defined by Single-Cell RNA-Sequencing and Cytometry by Time of Flight. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 549–563. [Google Scholar] [CrossRef]
- Gong, F.; Liu, Z.; Liu, J.; Zhou, P.; Liu, Y.; Lu, X. The paradoxical role of IL-17 in atherosclerosis. Cell Immunol. 2015, 297, 33–39. [Google Scholar] [CrossRef]
- Rothan, C.; Yero, A.; Shi, T.; Farnos, O.; Chartrand-Lefebvre, C.; El-Far, M.; Costiniuk, C.T.; Tsoukas, C.; Tremblay, C.; Durand, M.; et al. Antiretroviral therapy-treated HIV-infected adults with coronary artery disease are characterized by a distinctive regulatory T-cell signature. AIDS 2021, 35, 1003–1014. [Google Scholar] [CrossRef]
- Kundu, S.; Freiberg, M.S.; Tracy, R.P.; So-Armah, K.A.; Koethe, J.R.; Duncan, M.S.; Tindle, H.A.; Beckman, J.A.; Feinstein, M.J.; McDonnell, W.J.; et al. Circulating T Cells and Cardiovascular Risk in People With and Without HIV Infection. J. Am. Coll. Cardiol. 2022, 80, 1633–1644. [Google Scholar] [CrossRef]
- Longenecker, C.T.; Funderburg, N.T.; Jiang, Y.; Debanne, S.; Storer, N.; Labbato, D.E.; Lederman, M.M.; McComsey, G.A. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013, 14, 385–390. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Sinclair, E.; Landay, A.L.; Lurain, N.; Sharrett, A.R.; Gange, S.J.; Xue, X.; Hunt, P.; Karim, R.; Kern, D.M.; et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J. Infect. Dis. 2011, 203, 452–463. [Google Scholar] [CrossRef]
- Jaworowski, A.; Hearps, A.C.; Angelovich, T.A.; Hoy, J.F. How Monocytes Contribute to Increased Risk of Atherosclerosis in Virologically-Suppressed HIV-Positive Individuals Receiving Combination Antiretroviral Therapy. Front. Immunol. 2019, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Zhang, W.; Xu, Y. A myriad of roles of dendritic cells in atherosclerosis. Clin. Exp. Immunol. 2021, 206, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Wallis, Z.K.; Williams, K.C. Monocytes in HIV and SIV Infection and Aging: Implications for Inflamm-Aging and Accelerated Aging. Viruses 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Woollard, K.J.; Geissmann, F. Monocytes in atherosclerosis: Subsets and functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hofer, T.P.; Zawada, A.M.; Rotter, B.; Krezdorn, N.; Noessner, E.; Devaux, Y.; Heine, G.; Ziegler-Heitbrock, L. Epigenetics in non-classical monocytes support their pro-inflammatory gene expression. Immunobiology 2020, 225, 151958. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Ishai, A.; Li, D.; Takx, R.A.; Hur, S.; Kaiser, Y.; Pampaloni, M.; Rupert, A.; Hsu, D.; Sereti, I.; et al. Association of Arterial and Lymph Node Inflammation With Distinct Inflammatory Pathways in Human Immunodeficiency Virus Infection. JAMA Cardiol. 2017, 2, 163–171. [Google Scholar] [CrossRef]
- Subramanian, S.; Tawakol, A.; Burdo, T.H.; Abbara, S.; Wei, J.; Vijayakumar, J.; Corsini, E.; Abdelbaky, A.; Zanni, M.V.; Hoffmann, U.; et al. Arterial inflammation in patients with HIV. JAMA 2012, 308, 379–386. [Google Scholar] [CrossRef]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Hanna, D.B.; Lin, J.; Post, W.S.; Hodis, H.N.; Xue, X.; Anastos, K.; Cohen, M.H.; Gange, S.J.; Haberlen, S.A.; Heath, S.L.; et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J. Infect. Dis. 2017, 215, 1352–1361. [Google Scholar] [CrossRef]
- Westhorpe, C.L.; Maisa, A.; Spelman, T.; Hoy, J.F.; Dewar, E.M.; Karapanagiotidis, S.; Hearps, A.C.; Cheng, W.J.; Trevillyan, J.; Lewin, S.R.; et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol. Cell Biol. 2014, 92, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.A.L.; Hanna, D.B.; Ehinger, E.; Xue, X.; Baas, L.; Gawaz, M.P.; Geisler, T.; Anastos, K.; Cohen, M.H.; Gange, S.J.; et al. Loss of CXCR4 on non-classical monocytes in participants of the Women’s Interagency HIV Study (WIHS) with subclinical atherosclerosis. Cardiovasc. Res. 2019, 115, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Angelovich, T.A.; Trevillyan, J.M.; Hoy, J.F.; Wong, M.E.; Agius, P.A.; Hearps, A.C.; Jaworowski, A. Monocytes from men living with HIV exhibit heightened atherogenic potential despite long-term viral suppression with antiretroviral therapy. AIDS 2020, 34, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.C.; Mau, M.; Hodis, H.N.; Kewcharoen, J.; Li, Y.; Siriwardhana, C.; Souza, S.A.; Mitchell, B.I.; Bowler, S.; SahBandar, I.; et al. Short Communication: Carotid Artery Plaque Burden in HIV Is Associated with Soluble Mediators and Monocytes. AIDS Res. Hum. Retroviruses 2020, 36, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, V.; McKay, H.S.; Brusca, R.M.; Palella, F.J.; Kingsley, L.A.; Witt, M.D.; Hodis, H.N.; Tracy, R.P.; Post, W.S.; Haberlen, S.A. Inflammatory biomarkers and subclinical carotid atherosclerosis in HIV-infected and HIV-uninfected men in the Multicenter AIDS Cohort Study. PLoS ONE 2019, 14, e0214735. [Google Scholar] [CrossRef] [PubMed]
- Doring, Y.; Zernecke, A. Plasmacytoid dendritic cells in atherosclerosis. Front. Physiol. 2012, 3, 230. [Google Scholar] [CrossRef] [PubMed]
- Doring, Y.; Manthey, H.D.; Drechsler, M.; Lievens, D.; Megens, R.T.; Soehnlein, O.; Busch, M.; Manca, M.; Koenen, R.R.; Pelisek, J.; et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Niessner, A.; Sato, K.; Chaikof, E.L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 2006, 114, 2482–2489. [Google Scholar] [CrossRef]
- Sage, A.P.; Murphy, D.; Maffia, P.; Masters, L.M.; Sabir, S.R.; Baker, L.L.; Cambrook, H.; Finigan, A.J.; Ait-Oufella, H.; Grassia, G.; et al. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 2014, 130, 1363–1373. [Google Scholar] [CrossRef]
- Yun, T.J.; Lee, J.S.; Machmach, K.; Shim, D.; Choi, J.; Wi, Y.J.; Jang, H.S.; Jung, I.H.; Kim, K.; Yoon, W.K.; et al. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab. 2016, 23, 852–866. [Google Scholar] [CrossRef]
- Chehimi, J.; Campbell, D.E.; Azzoni, L.; Bacheller, D.; Papasavvas, E.; Jerandi, G.; Mounzer, K.; Kostman, J.; Trinchieri, G.; Montaner, L.J. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 2002, 168, 4796–4801. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Manches, O.; Wilen, C.; Gopal, R.; Huq, R.; Wu, V.; Sunseri, N.; Bhardwaj, N. CD4 Receptor is a Key Determinant of Divergent HIV-1 Sensing by Plasmacytoid Dendritic Cells. PLoS Pathog. 2016, 12, e1005553. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.P.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Giguere, K.; Chartrand-Lefebvre, C.; Baril, J.G.; Conway, B.; El-Far, M.; Falutz, J.; Harris, M.; Jenabian, M.A.; Leipsic, J.; Loutfy, M.; et al. Baseline characteristics of a prospective cohort study of aging and cardiovascular diseases among people living with HIV. HIV Med. 2023, 24, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Boldeanu, I.; Nepveu, S.; Durand, M.; Chin, A.S.; Kauffmann, C.; Mansour, S.; Soulez, G.; Tremblay, C.; Chartrand-Lefebvre, C. In vivo coronary artery plaque assessment with computed tomography angiography: Is there an impact of iterative reconstruction on plaque volume and attenuation metrics? Acta Radiol. 2017, 58, 660–669. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. The use of baseline covariates in crossover studies. Biostatistics 2010, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- He, S.; Kahles, F.; Rattik, S.; Nairz, M.; McAlpine, C.S.; Anzai, A.; Selgrade, D.; Fenn, A.M.; Chan, C.T.; Mindur, J.E.; et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 2019, 566, 115–119. [Google Scholar] [CrossRef]
- Surma, S.; Banach, M. Fibrinogen and Atherosclerotic Cardiovascular Diseases-Review of the Literature and Clinical Studies. Int. J. Mol. Sci. 2021, 23, 193. [Google Scholar] [CrossRef]
- Fontaine, J.; Poudrier, J.; Roger, M. Short communication: Persistence of high blood levels of the chemokines CCL2, CCL19, and CCL20 during the course of HIV infection. AIDS Res. Hum. Retroviruses 2011, 27, 655–657. [Google Scholar] [CrossRef]
- DaFonseca, S.; Niessl, J.; Pouvreau, S.; Wacleche, V.S.; Gosselin, A.; Cleret-Buhot, A.; Bernard, N.; Tremblay, C.; Jenabian, M.A.; Routy, J.P.; et al. Impaired Th17 polarization of phenotypically naive CD4+ T-cells during chronic HIV-1 infection and potential restoration with early ART. Retrovirology 2015, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Bengsch, B.; Seigel, B.; Flecken, T.; Wolanski, J.; Blum, H.E.; Thimme, R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J. Immunol. 2012, 188, 5438–5447. [Google Scholar] [CrossRef] [PubMed]
- Mudd, J.C.; Lederman, M.M. CD8 T cell persistence in treated HIV infection. Curr. Opin. HIV AIDS 2014, 9, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, N.; Han, Y.; Zhu, T.; Xie, J.; Qiu, Z.; Song, X.; Li, Y.; Routy, J.P.; Wang, J.; et al. A higher CD4/CD8 ratio correlates with an ultralow cell-associated HIV-1 DNA level in chronically infected patients on antiretroviral therapy: A case control study. BMC Infect. Dis. 2017, 17, 771. [Google Scholar] [CrossRef] [PubMed]
- Wacleche, V.S.; Tremblay, C.L.; Routy, J.P.; Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Tsou, C.L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef]
- Abd Alla, J.; Langer, A.; Elzahwy, S.S.; Arman-Kalcek, G.; Streichert, T.; Quitterer, U. Angiotensin-converting enzyme inhibition down-regulates the pro-atherogenic chemokine receptor 9 (CCR9)-chemokine ligand 25 (CCL25) axis. J. Biol. Chem. 2010, 285, 23496–23505. [Google Scholar] [CrossRef]
- Franca, C.N.; Izar, M.C.O.; Hortencio, M.N.S.; Do Amaral, J.B.; Ferreira, C.E.S.; Tuleta, I.D.; Fonseca, F.A.H. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef]
- Linton, L.; Karlsson, M.; Grundstrom, J.; Hjalmarsson, E.; Lindberg, A.; Lindh, E.; Glise, H.; Befrits, R.; Janczewska, I.; Karlen, P.; et al. HLA-DR(hi) and CCR9 Define a Pro-Inflammatory Monocyte Subset in IBD. Clin. Transl. Gastroenterol. 2012, 3, e29. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef]
- Wan, W.; Lim, J.K.; Lionakis, M.S.; Rivollier, A.; McDermott, D.H.; Kelsall, B.L.; Farber, J.M.; Murphy, P.M. Genetic deletion of chemokine receptor Ccr6 decreases atherogenesis in ApoE-deficient mice. Circ. Res. 2011, 109, 374–381. [Google Scholar] [CrossRef]
- Levast, B.; Barblu, L.; Coutu, M.; Prevost, J.; Brassard, N.; Peres, A.; Stegen, C.; Madrenas, J.; Kaufmann, D.E.; Finzi, A. HIV-1 gp120 envelope glycoprotein determinants for cytokine burst in human monocytes. PLoS ONE 2017, 12, e0174550. [Google Scholar] [CrossRef] [PubMed]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef]
- Chege, D.; Sheth, P.M.; Kain, T.; Kim, C.J.; Kovacs, C.; Loutfy, M.; Halpenny, R.; Kandel, G.; Chun, T.W.; Ostrowski, M.; et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 2011, 25, 741–749. [Google Scholar] [CrossRef]
- Caruso, M.P.; Falivene, J.; Holgado, M.P.; Zurita, D.H.; Laufer, N.; Castro, C.; Nico, A.; Maeto, C.; Salido, J.; Perez, H.; et al. Impact of HIV-ART on the restoration of Th17 and Treg cells in blood and female genital mucosa. Sci. Rep. 2019, 9, 1978. [Google Scholar] [CrossRef]
- El-Far, M.; Tremblay, C.L. Gut microbial diversity in HIV infection post combined antiretroviral therapy: A key target for prevention of cardiovascular disease. Curr. Opin. HIV AIDS 2018, 13, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gullaksen, S.; Funck, K.L.; Laugesen, E.; Hansen, T.K.; Dey, D.; Poulsen, P.L. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc. Dis. Res. 2019, 16, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Andersen, G.O.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Sim, J.H.; Mukerji, S.S.; Russo, S.C.; Lo, J. Gastrointestinal Dysfunction and HIV Comorbidities. Curr. HIV/AIDS Rep. 2021, 18, 57–62. [Google Scholar] [CrossRef]
- Sereti, I.; Verburgh, M.L.; Gifford, J.; Lo, A.; Boyd, A.; Verheij, E.; Verhoeven, A.; Wit, F.; Schim van der Loeff, M.F.; Giera, M.; et al. Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. 2023, 42, 113336. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, L.; Yan, Z.; Jiang, W.; Wu, B.; Li, D.; Xiong, X. Identification of heterogeneous subsets of aortic interleukin-17A-expressing CD4(+) T cells in atherosclerotic mice. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221117933. [Google Scholar] [CrossRef]
- Shakil, S.S.; Temu, T.M.; Kityo, C.; Nazzinda, R.; Erem, G.; Kentoffio, K.; Bittencourt, M.; Ntusi, N.A.B.; Zanni, M.V.; Longenecker, C.T. Sex modulates the association between inflammation and coronary atherosclerosis among older Ugandan adults with and without HIV. AIDS 2022, 37, 579–586. [Google Scholar] [CrossRef]
- Turcotte, I.; El-Far, M.; Sadouni, M.; Chartrand-Lefebvre, C.; Filali-Mouhim, A.; Fromentin, R.; Chamberland, A.; Jenabian, M.A.; Baril, J.G.; Trottier, B.; et al. Association between the development of sub-clinical cardiovascular disease and HIV reservoir markers in people with HIV on suppressive ART. Clin. Infect. Dis. 2022, 76, 1318–1321. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009, 11, 620–624. [Google Scholar] [CrossRef]
- Sallusto, F.; Zielinski, C.E.; Lanzavecchia, A. Human Th17 subsets. Eur. J. Immunol. 2012, 42, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Spor, A.; Felin, J.; Fak, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Yap, Z.H.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Yero, A.; Bouassa, R.M.; Ancuta, P.; Estaquier, J.; Jenabian, M.A. Immuno-metabolic control of the balance between Th17-polarized and regulatory T-cells during HIV infection. Cytokine Growth Factor. Rev. 2023, 69, 1–13. [Google Scholar] [CrossRef]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32ra36. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Petitjean, G.; Dunyach-Remy, C.; Didier, C.; Girard, P.M.; Manea, M.E.; Campa, P.; Meyer, L.; Rouzioux, C.; Lavigne, J.P.; et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog. 2013, 9, e1003453. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Amanatidou, V.; Papadakis, E.G.; Spandidos, D.A. Genetic diversity of CX3CR1 gene and coronary artery disease: New insights through a meta-analysis. Atherosclerosis 2009, 207, 8–15. [Google Scholar] [CrossRef]
- Chow, D.C.; Kagihara, J.M.; Zhang, G.; Souza, S.A.; Hodis, H.N.; Li, Y.; Mitchell, B.I.; Nakamoto, B.K.; Kallianpur, K.J.; Keating, S.M.; et al. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin. Trials 2016, 17, 114–122. [Google Scholar] [CrossRef]
- Ancuta, P.; Wang, J.; Gabuzda, D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J. Leukoc. Biol. 2006, 80, 1156–1164. [Google Scholar] [CrossRef]
- Umar, S.; Palasiewicz, K.; Van Raemdonck, K.; Volin, M.V.; Romay, B.; Ahmad, I.; Tetali, C.; Sweiss, N.; Amin, M.A.; Zomorrodi, R.K.; et al. CCL25 and CCR9 is a unique pathway that potentiates pannus formation by remodeling RA macrophages into mature osteoclasts. Eur. J. Immunol. 2021, 51, 903–914. [Google Scholar] [CrossRef]
- Xu, Z.; Mei, F.; Liu, H.; Sun, C.; Zheng, Z. C-C Motif Chemokine Receptor 9 Exacerbates Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction. J. Am. Heart Assoc. 2016, 5, e003342. [Google Scholar] [CrossRef] [PubMed]
- Parsa, N.; Zaheri, P.M.; Hewitt, R.G.; Karimi Akhormeh, A.; Taravatmanesh, S.; Wallin, L. The rapid CD4 + T-lymphocyte decline and human immunodeficiency virus progression in females compared to males. Sci. Rep. 2020, 10, 16816. [Google Scholar] [CrossRef] [PubMed]
| HIV+ (n = 61) | HIV− (n = 21) | p-Value | |
|---|---|---|---|
| Demographics and Clinical Information | |||
| Age (years) * | 55.38 ± 6.99 | 56.94 ± 7.99 | 0.39 |
| Male & | 61 (100%) | 17 (81%) | 0.003 |
| BMI (kg/m2) # | 24.43 (22.10–28.29) | 25.72 (24.34–28.33) | 0.2023 |
| Framingham Risk Score (FRS) # | 10 (6–14.25) | 8 (8–12.5) | 0.67 |
| Current Statin Treatment & | 14 (23%) | 5 (23.8%) | 1 |
| Smoking & | 0.502 | ||
| Never | 18 (29.5%) | 8 (38.1%) | |
| Current Smoker | 23 (37.7%) | 5 (23.8%) | |
| Former Smoker | 20 (32.8%) | 8 (38.1%) | |
| Coronary Artery Disease Parameters | |||
| Total Plaque Volume (TPV; mm3) # | 108.30 (0–698) | 49.70 (0–279.1) | 0.32 |
| Low Attenuated Plaque Volume (LAPV; mm3) # | 34.40 (0–194.5) | 10.53 (0–118.9) | 0.39 |
| Laboratory Parameters | |||
| White Blood Cells (×109/L) # | 5.6 (4.7–7.1) | 6.15 (5.65–7) | 0.2580 |
| Lymphocytes (×109/L) * | 1.90 ± 0.66 | 1.87 ± 0.47 | 0.85 |
| LDL (mmol/L) # | 2.58 (2.13–3.40) | 3.10 (2.30–3.82) | 0.21 |
| HDL (mmol/L) # | 1.23 (1.07–1.3) | 1.44 (1.30–1.6) | 0.002 |
| Triglycerides (mmol/L) # | 1.73 (1.19–2.99) | 1.21 (0.89–1.68) | 0.008 |
| TPV+ (n = 39) | TPV− (n = 22) | p-Value | |
|---|---|---|---|
| Demographics and Clinical Parameters | |||
| Age (years) * | 56.24 ± 6.35 | 53.85 ± 7.93 | 0.1625 |
| Male & | 39 (100%) | 22 (100%) | NA |
| BMI (kg/m2) # | 24.05 (21.05–28.38) | 25.29 (22.91–28.27) | 0.23 |
| Framingham Risk Score # | 11.00 (7–18) | 8.00 (5.5–13) | 0.085 |
| Current Statin Treatment & | 12 (30.8%) | 2 (9.1%) | 0.064 |
| Smoking & | 0.005 | ||
| Never | 7 (17.9%) | 11 (50%) | |
| Current Smoker | 20 (51.3%) | 3 (13.6%) | |
| Former Smoker | 12 (30.8%) | 8 (36.4%) | |
| Coronary Artery Disease Parameters | |||
| Total Plaque Volume (mm3) # | 608.70 (142.6–881.8) | 0 | NA |
| Low Attenuated Plaque Volume (mm3) # | 164.80 (36–296) | 0 | NA |
| HIV Disease Parameters | |||
| Undetectable Viral Load & | 37 (94.8%) | 19 (86.36%) | 0.34 |
| Duration of HIV (years) # | 19.16 (14.29–24.4) | 14.71 (4.58–24.14) | 0.06 |
| Duration of ART (years) * | 16.14 ± 5.68 | 10.69 ± 7.82 | 0.003 |
| Antecedent/Current Protease Inhibitor Use & | 39 (100%) | 14 (63.64%) | <0.0001 |
| Nadir CD4 (×109/L) # | 195 (100–330) | 170 (78–255) | 0.397 |
| Laboratory Parameters | |||
| White Blood Cells (×109/L) # | 5.90 (4.7–7.8) | 5.40 (4.3–6.85) | 0.27 |
| Lymphocytes (×109/L) * | 1.91 ± 0.60 | 1.89 ± 0.78 | 0.924 |
| D-Dimer (ug/L) # | 300.00 (197.5–410) | 290.00 (175–380) | 0.52 |
| Fibrinogen (G/L) # | 3.23 (2.73–3.78) | 2.67 (2.27–3) | 0.005 |
| LDL (mmol/L) # | 2.40 (1.99–3.48) | 2.90 (2.23–3.40) | 0.35 |
| HDL (mmol/L) # | 1.21 (1.07–1.37) | 1.25 (0.98–1.37) | 0.96 |
| Triglycerides (mmol/L) # | 2.07 (1.26–3.1) | 1.38 (1.09–2.03) | 0.109 |
| Serology | |||
| Co-Infection CMV # | 36 (92.3%) | 17 (77.3%) | 0.39 |
| Models | OR | Memory Th17/Treg Ratio | EM Th17/Treg Ratio | CM Th17/Treg Ratio | CCR9+ HLA-DRlow Non-Classical Monocytes | CCR9low HLA-DRhigh Non-Classical Monocytes | M-DC8+ HLA-DRhigh Monocytes |
|---|---|---|---|---|---|---|---|
| Crude Model | OR (CI) | 0.49 (0.27–0.86) | 0.44 (0.24–0.79) | 0.57 (0.33–0.99) | 0.30 (0.15–0.63) | 3.56 (1.45–8.74) | 2.71 (1.24–5.91) |
| Adj. p | 0.03 | 0.03 | 0.05 | 0.01 | 0.02 | 0.10 | |
| Model 1 | OR (CI) | 0.47 (0.22–1.02) | 0.43 (0.18–1.03) | 0.55 (0.27–1.11) | 0.24 (0.09–0.62) | 6 (1.77–20.28) | 5.39 (1.56–18.6) |
| Adj. p | 0.1 | 0.1 | 0.1 | 0.015 | 0.015 | 0.04 | |
| %Δ OR | 3% | 2% | 4% | 22% | 70% | 98% | |
| Model 2 | OR (CI) | 0.45 (0.21–0.94) | 0.41 (0.18–0.94) | 0.52 (0.26–1.03) | 0.2 (0.07–0.56) | 6.44 (1.89–21.95) | 6.06 (1.68–21.93) |
| Adj. p | 0.07 | 0.07 | 0.07 | 0.01 | 0.01 | 0.03 | |
| %Δ OR | 9% | 6% | 9% | 35% | 80% | 123% | |
| Model 3 | OR (CI) | 0.41 (0.19–0.91) | 0.38 (0.16–0.94) | 0.48 (0.24–0.97) | 0.17 (0.06–0.52) | 8.09 (2.06–31.67) | 7.13 (1.8–28.22) |
| Adj. p | 0.06 | 0.06 | 0.06 | 0.01 | 0.01 | 0.03 | |
| %Δ OR | 17% | 13% | 16% | 44% | 126% | 162% | |
| Model 4 | OR (CI) | 0.45 (0.23–0.89) | 0.47 (0.24–0.92) | 0.49 (0.26–0.94) | 0.26 (0.11–0.6) | 5 (1.72–14.56) | 3.75 (1.4–10.04) |
| Adj. p | 0.05 | 0.05 | 0.05 | 0.01 | 0.01 | 0.05 | |
| %Δ OR | 7% | 6% | 14% | 15% | 40% | 40% | |
| Model 5 | OR (CI) | 0.45 (0.24–0.86) | 0.45 (0.23–0.87) | 0.5 (0.27–0.94) | 0.26 (0.11–0.62) | 3.07 (1.14–8.25) | 2.75 (1.12–6.74) |
| Adj. p | 0.04 | 0.04 | 0.04 | 0.02 | 0.1 | 0.15 | |
| %Δ OR | 8% | 3% | 13% | 13% | 14% | 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiche Salinas, T.R.; Zhang, Y.; Gosselin, A.; Rosario, N.F.; El-Far, M.; Filali-Mouhim, A.; Routy, J.-P.; Chartrand-Lefebvre, C.; Landay, A.L.; Durand, M.; et al. Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection. Cells 2024, 13, 157. https://doi.org/10.3390/cells13020157
Wiche Salinas TR, Zhang Y, Gosselin A, Rosario NF, El-Far M, Filali-Mouhim A, Routy J-P, Chartrand-Lefebvre C, Landay AL, Durand M, et al. Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection. Cells. 2024; 13(2):157. https://doi.org/10.3390/cells13020157
Chicago/Turabian StyleWiche Salinas, Tomas Raul, Yuwei Zhang, Annie Gosselin, Natalia Fonseca Rosario, Mohamed El-Far, Ali Filali-Mouhim, Jean-Pierre Routy, Carl Chartrand-Lefebvre, Alan L. Landay, Madeleine Durand, and et al. 2024. "Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection" Cells 13, no. 2: 157. https://doi.org/10.3390/cells13020157
APA StyleWiche Salinas, T. R., Zhang, Y., Gosselin, A., Rosario, N. F., El-Far, M., Filali-Mouhim, A., Routy, J.-P., Chartrand-Lefebvre, C., Landay, A. L., Durand, M., Tremblay, C. L., & Ancuta, P., on behalf of the Canadian HIV and Aging Cohort Study (CHACS). (2024). Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection. Cells, 13(2), 157. https://doi.org/10.3390/cells13020157






