Abstract
The NLRP3 inflammasome is a critical component of the innate immune response. NLRP3 activation is a tightly controlled process involving an initial priming to express NLRP3, pro-IL-1 β, and pro-IL-18, followed by an activation signal. The precise mechanism of activation is not fully understood due to the diverse range of activators, yet it effectively orchestrates the activation of caspase-1, which subsequently triggers the release of proinflammatory cytokines IL-1β and IL-18. NLRP3 dysregulation can lead to a variety of inflammatory diseases, highlighting its significant role in immune response and disease pathogenesis. NLRP3 is divided into three domains: the PYD, the NACHT, and the LRR domains. This review focuses on the LRR domain of NLRP3, detailing its structural characteristics, its function in pathogen sensing, its role in the degradation process, and its involvement in inflammasome auto-inhibition and activation. Additionally, we discuss the impact of mutations within the LRR domain found in atypical Cryopyrin-Associated Periodic Syndromes (CAPS), highlighting the clinical relevance of this domain.
1. Introduction
Innate immune cells recognize pathogens or danger-associated molecular patterns (PAMPs and DAMPs) through pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs) and NOD-like receptors (NLRs) [1].
NLR proteins are mostly cytosolic receptors that can sense a wide range of microbial molecules [2,3], leading to either the induction of an inflammatory response or the regulation and/or inhibition of inflammation [4]. Additionally, NLRs have been found to support immune homeostasis in tissues [5]. There are 23 NLRs described in humans and 33 in mice [6]. They all share common features, including (i) an N-terminal effector domain; (ii) a central nucleotide-binding domain termed NACHT (NAIP, CIITA, HETE, and TP1) or NOD, which is involved in ATP-dependent oligomerization; and (iii) a C-terminal portion consisting of a repetition of Leucine-Rich Repeats (LRRs; Figure 1). NLRs can be classified into subfamilies based on their N-terminal effector domain: the NLRA and NLRC families have acidic transactivation and caspase recruitment (CARD) domains, the NLRB family possesses a baculoviral inhibitory repeat (BIR)-like domain, the NLRP family contains a pyrin domain (PYD) and the NLRX family is defined by a mitochondrial targeting sequence (MTS) [7].
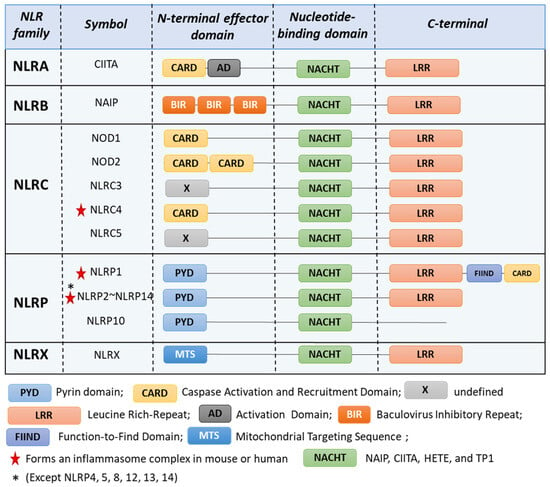
Figure 1.
NOD-like receptors (NLRs) families. NLRs are characterized by a N-terminal, a central nucleotide-binding domain, and a C-terminal domain. NLRC4, NLRP1 [8], NLRP2 [9], NLRP3 [10], NLRP6 [11], NLRP7 [12], NLRP9 [13], NLRP10 [14], and NLRP11 [15] can form inflammasome.
NLRP3, previously “CIAS1” or cryopyrin, is a member of the NLRP protein family. Its activation leads to the formation of the inflammasome complex [16], which are multi-protein complexes that, once assembled in an activated conformation, recruit and activate caspase-1. Activated caspase-1 subsequently cleaves various substrates, resulting in the release of proinflammatory cytokines such as IL-1β and IL-18 and the cleavage of gasdermin-D (GSDMD) into fragments that disrupt the membrane by forming pore, leading to pyroptotic cell death and facilitating cytokine release [17,18].
NLRP3 activation is tightly regulated, and three activation mechanisms have been identified. The canonical activation requires both priming and activation steps (Figure 2). The priming signal can be referred to as a PAMP. It is mediated through activation of PRRs [10] or cytokine receptors [19,20,21]. The priming process serves two main purposes: first, it upregulates the expression of inflammasome components such as NLRP3, pro-IL-1β, and pro-IL-18 [19] via signaling pathway involving MYD88, TRIF, and NF-kB [22,23], and second, it leads to post-translational modifications (PTMs) of NLRP3 [24,25]. Following the activation signal, NLRP3 forms the inflammasome complex with NEK7, ASC, and caspase-1. NLRP3 is unique in its broad range of activators, which include pathogens components [26,27,28,29,30,31,32,33,34], endogenous signals [35,36,37,38,39,40,41,42,43,44,45], and crystals [46,47,48]. However, the exact mechanisms by which these various stimuli activate NLRP3 remain unknown. While it is proposed that these signals converge on potassium (K+) efflux [36], studies have also shown K+ efflux-independent NLRP3 activation [49], suggesting a complex regulatory network involving both K+ efflux-dependent and independent pathways.
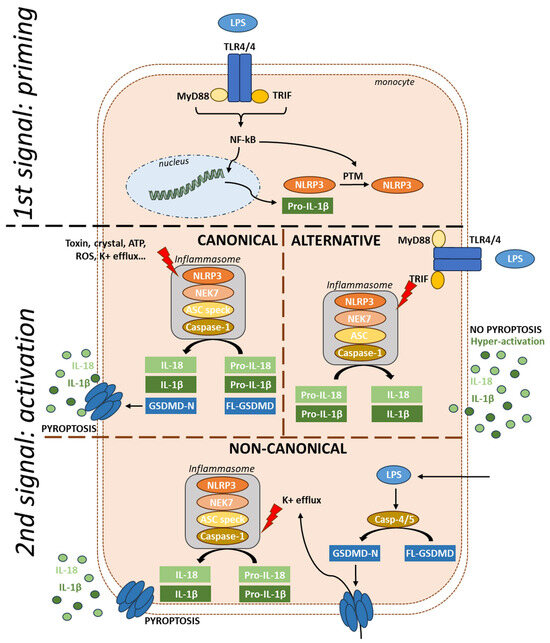
Figure 2.
Activation mechanisms of NLRP3. The priming signal induces the production of NLRP3 and pro-IL-1β via TLR-NF-kB signaling, followed by a series of post-translational modifications. In canonical activation, the second signal, triggered by various activators, leads to the formation of the inflammasome with NEK7, ASC, and caspase-1. Once activated, the inflammasome cleaves pro-cytokines into their active forms and also cleaves gasdermin, leading to pyroptosis. In the alternative pathway, LPS via TLR4 signaling leads to the production of NLRP3 and pro-IL-1β. Dual activation through TRIF then activates the inflammasome, resulting in hyperactivation without pyroptosis and no ASC speck formation. In the non-canonical pathway, intracellular LPS serves as the second signal, activating caspases 4 and 5, which cleave gasdermin, leading to pyroptosis. Pores in the membrane cause K+ efflux, triggering NLRP3 inflammasome formation. FL-GSDMD: Full-length gasdermin; GSDMD-N: Gasdermin-N-terminal; NF-kB: Nuclear Factor kappa-light-chain-enhancer of activated B cells.
An alternative activation pathway has been described in human monocytes treated with LPS (Figure 2). In this context, the TRIF pathway controls the assembly of an alternative NLRP3 inflammasome that does not form the typical ASC speck and which triggers IL-1β release independently of cell death in a gradual manner [50]. NLRP3-dependent IL-1β release by living cells, termed hyperactivation as it results in a high amount of IL-1β over time, has also been reported in mouse cells treated with oxidized lipids [51].
In the non-canonical pathway (Figure 2), cytoplasmic LPS, independently of TLR signaling, is sufficient to form the inflammasome [52]. LPS directly binds and activates caspase-4 and 5, leading to gasdermin cleavage and pyroptosis [53]. Pores in the membrane cause K+ efflux, triggering NLRP3 inflammasome formation [54].
NLRP3 activation is intricately regulated due to its pivotal role in cellular homeostasis, and dysregulation in this process can lead to pathological conditions. The NLRP3 inflammasome is implicated in several frequent inflammatory diseases, including gout [55,56], rheumatoid arthritis [57], Crohn’s disease [58], and, more occasionally, pulmonary fibrosis [46].
Metabolic stressors such as ceramide and fatty acids accumulation, commonly observed in obesity, can also trigger NLRP3 activation, contributing to establishing an inflammatory landscape that promotes insulin resistance and facilitates the onset of type 2 diabetes [44,45]. Additionally, NLRP3 activation can also be triggered by oxidized low-density lipoprotein, leading to atherosclerosis [43]. NLRP3 is also proposed to be activated in several neurodegenerative disorders, including Alzheimer’s disease [40,41,42], Parkinson’s disease [59,60] and epilepsy [61,62]. Furthermore, gain-of-function heterozygous variants in NLRP3 are associated with a rare monogenic auto-inflammatory disease called Cryopyrin-Associated Periodic Syndrome (CAPS) and characterized by systemic inflammation and various clinical features, including mainly urticaria, arthromyalgia, buccal aphthous, and neuroinflammation with eye, hearing, and meningitis involvement [63].
There are two different annotation systems for NLRP3, which may be confusing in the literature as the first exon has two potential start codons [64]. In one system, NLRP3 is annotated as having 1036 amino acids (UniProt), starting the count from the first ATG. However, in the other system, the count begins from the second ATG, which has a better Kozak consensus, resulting in a length of 1 034 amino acids. Furthermore, the first methionine residue is not present in the murine form [65]. This discrepancy of 2 amino acids can result in variations across different publications. For instance, a well-known mutation in NLRP3 is either called the R260W [66] or the R262W mutation [67]. Furthermore, NLRP3 is conserved across species, but there are slight differences between the human and murine forms. Murine NLRP3 consists of 1 033 amino acids and shares 89.6% similarity and 82.7% identity with its human counterpart [68]. This disparity is significant, leading to activation differences. For instance, LPS alone can activate NLRP3 in human cells through an alternative activation pathway but not in murine cells [50,69]. Additionally, while low doses of dynasore, a dynamin inhibitor, can activate NLRP3 in human cells, a 16-fold higher dose is required to activate murine NLRP3 [70]. These findings suggest that human cells are overall more sensitive to inflammasome activation than murine cells.
NLRP3 is divided into three domains: the PYD domain, responsible for interactions with downstream proteins [71] of the inflammasome complex; the NACHT domain containing ATPase activity; and the LRR domain. This review aims to provide a comprehensive overview of current knowledge and recent discoveries regarding the involvement of the LRR domain in modulating NLRP3 inflammasome activation.
2. Structure of NLRP3 LRR Domain
There are two recognized versions of the NLRP3 gene nomenclature: one version includes 10 exons [72], while the other comprises a total of 9 exons [73,74]. In the latter version, 6 of these exons are forming the LRR domain (Figure 3). Except for exon 9, which encodes only one LRR, each exon within the LRR domain has a length of 171 base pairs and encodes 2 LRRs. Consequently, the LRR domain comprises a repetitive sequence of 11 LRR patterns, each consisting of either 28 or 29 amino acids, characterized by a high leucine content and a consensus sequence of xLxxLxLxxN/CxLxxxxxxxLxxxLxxxxx [72] (Figure 4A,B). This repetitive pattern forms a uniform and curved horseshoe-like structure with parallel beta sheets on the concave side and α-helical elements on the convex side (Figure 4A,C in blue) [72]. The precise alternating arrangement of LRRs and the exon-exon border always positioned at the same position facilitates the generation of functional isoforms through alternative splicing [72], as the exon-exon junction and the reading frame are conserved without disrupting the 3D structure. Beyond its high leucine content, NLRP3 contains a significant amount of redox-sensitive cysteine (4.3% in human NLRP3 and 4.6% in murine NLRP3), compared to average NLR proteins at around 2.3%. These conserved cysteine residues are located in the LRR domain (Figure 4A,C in red), suggesting that the LRR domain may function as a sensor for ROS [75].
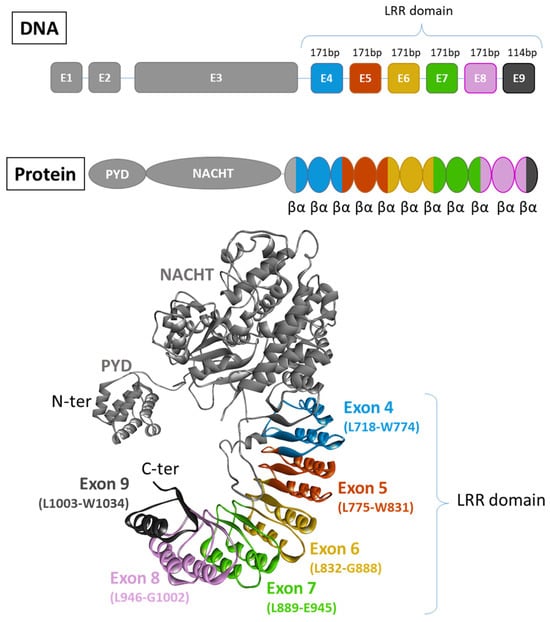
Figure 3.
Exon organisation of NLRP3. There are two recognized versions of the NLRP3 gene nomenclature: one version includes 10 exons [72], while the other comprises a total of 9 exons [73,74]. In this latter version, 6 of them contribute to the formation of the LRR domain. Each exon code for 2 completes LRR motifs composed of 2 α-helix and 2 β-sheets (PDB model: 7PZC [76]).
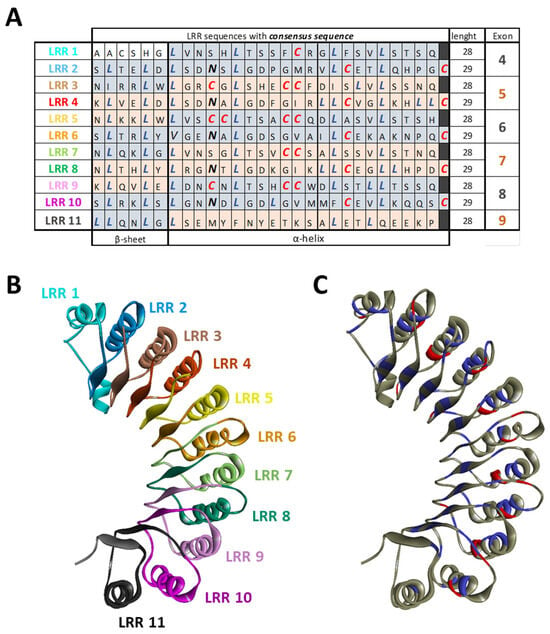
Figure 4.
LRR organization of NLRP3. (A) Each exon code for 2 completes LRR motifs alternating 28 or 29 amino acids. NLRP3 is composed of 11 LRRs that are characterized by a high leucine (blue) and cysteine (red) content and a consensus sequence. (B) The structure of the LRR domain with each LLR repeat is highlighted. (C) Structure of the LRR domain with leucine in blue and cysteine in red. (PDB model: 7PZC).
In 2002, three different NLRP3 isoforms were initially identified through reverse transcription and specific amplification of the NLRP3 gene from human blood. Sequencing revealed that the most prevalent isoform was the long isoform containing all nine exons. Additionally, shorter and intermediate isoforms were identified, with the shorter variant lacking exons 4 to 9, resulting in a loss of the LRR domain, and the intermediate variant lacking exons 4 and 6 [73] (Figure 5).
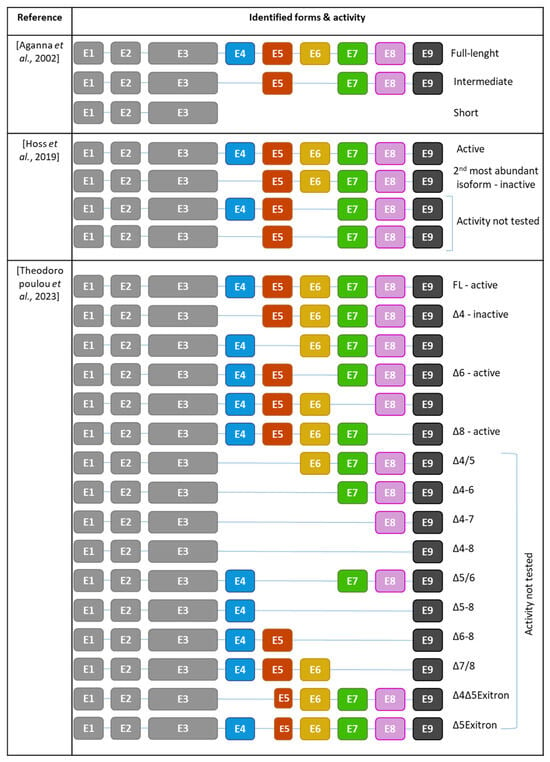
Figure 5.
Repertoire of NLRP3 isoforms identified and their activities. E: exon [72,73,74].
Subsequent RNAseq analysis on human monocyte-derived macrophages from healthy donors confirmed the predominance of the full-length isoform and uncovered additional isoforms. Exon 4 was found to be the most frequently skipped exon, and therefore, NLRP3Δ4 emerged as the second most abundant variant after the full-length. Other variants, such as NLRP3Δ6 isoforms and NLRP6Δ4/6, were identified as well [72].
Further analysis of archive data from experiments using human primary monocytes revealed a total of 14 NLRP3 isoforms. Among them, full-length NLRP3 and NLRP3Δ4 isoforms remained the most prevalent [74]. While full-length NLRP3 is an active form, NLRP3Δ4 is inactive supposedly due to its inability to bind NEK7 [72], resulting in the incapacity to recruit ASC, assemble an inflammasome and the absence of IL-1β production [72,74]. Similarly, NLRP3Δ5, NLRP3Δ7, and NLRP3Δ9 have lost their competence to form an active inflammasome. In contrast, NLRP3Δ6 and NLRP3Δ8 retain their ability to form the inflammasome and produce IL-1β [74]. This observation highlights that not all LRRs are equally important. While exons 6 and 8 are dispensable for inflammasome activation, exons 4, 5, 7, and 9 are essential. This suggests that the specific amino acid composition within these exons plays a crucial role in enabling NLRP3 activation. Given the observed loss of interaction with downstream proteins such as NEK7, LRR is required to form a functional inflammasome that nucleates ASC filaments.
Interestingly, even gain-of-function variants in the NACHT domain responsible for CAPS, such as R260W, do not rescue the activity of non-active isoforms, reinforcing the necessity of these LRR exons to form a functional inflammasome. Importantly, the activity of full-length NLRP3 was not affected by the co-expression of non-functional isoforms, suggesting they do not act as dominant negative nor impair the activation of full-length NLRP3 [74]. Finally, LPS stimulation increased the expression of the full-length NLRP3 isoform, indicating that the repertoire of NLRP3 isoforms can be modulated by exogenous signals [74].
3. Pathogen Sensing
Pieces of evidence indicate that pathogen recognition in various species is mediated by LRR domains. In plants, immune proteins encoded by R-genes feature LRR domains. Sequence analysis revealed that their specificity to pathogens signals depend on these domains [77]. In mammals, TLRs also possess LRR domains with a characteristic 3D structure similar to that of NLRs. They bind ligands as extracellular domains on their concave surface [78].
Similarly to TLRs, NLR responses are highly agonist-specific. Within the NLRC subfamily, NOD1 and NOD2 not forming inflammasome can detect bacterial components to activate NF-kB. Early research demonstrated the crucial role of LRR domains in bacterial sensing for NOD1 and NOD2 [79]. Truncation of the LRR in NOD2 led to mutants unable to respond to bacterial components, and similarly, NOD1 lacking LRR lost its responsiveness to LPS [80]. This suggests that LRR domains in NLRC proteins play an essential role in recognition, highlighting the critical importance of LRR integrity [79].
In the NLRP subfamily, NLRP1 responds to several bacteria, including Shigella flexneri, Listeria monocytogenes, and Bacillus anthracis, to form inflammasome [81,82] but also detect dsRNA through the direct involvement of LRR [83] supporting the detection role of LRR among NLRP proteins.
However, the role of the LRR domain of NLRP3 in DAMP sensing might not be as straightforward. Indeed, NLRP3 inflammasome is activated by a wide array of structurally and chemically diverse triggers. In addition to fungal [26,27,28,29], bacterial [30,31,32,32,33,34] and viral recognition [34], NLRP3 also responds to endogenous signals such as ATP [35], ROS [37], K+ efflux [36], crystals of monosodium urate (MSU) [55], extracellular matrix hyaluronan [39], amyloid-b [40,41,42], oxidized low-density lipoprotein [43], fatty acids [44,45] and oxidized mitochondrial DNA [38]. Furthermore, NLRP3 can sense environmental triggers like asbestos and silica [46,47,48].
Experiments have demonstrated that the LRR domain is dispensable for the response to nigericin, SiO2, alum, and F. novidia U112 in murine [84] and human cells [85]. However, the response to L. monocytogenes is impaired when the LRR domain is absent. Additionally, replacing the LRR domain of NLRP3 with that of NLRP2 results in a 50% reduction in activation by H2O2, suggesting the LRR domain of NLRP3 might possess features to recognize H2O2 [85]. Moreover, NLRP3, lacking half of its LRR domain, lost its ability to respond to MSU, a common trigger used to mimic gout [85].
Rather than direct activation, it appears that the diverse and highly various triggers for NLRP3 activation converge on cellular events such as cytosolic potassium (K+) efflux, production of ROS, or mitochondrial damage. Because these cellular events are interconnected and can mutually activate each other, identifying the causative event of NLRP3 activation is challenging. Several NLRP3 triggers, such as nigericin [86], ATP [87,88], and maitotoxin [32], induce membrane permeation, leading to K+ efflux. The physiological intracellular concentration of K+ has been shown to be too high for active NLRP3 conformation, and inhibition of K+ efflux prevents NLRP3 activation in both murine and human macrophages [89]. Therefore, it was suggested that cytosolic K+ efflux may be the critical common trigger for NLRP3 activation [36]. For K+ efflux-dependent stimuli, the use of ROS scavengers has shown no effect on inflammasome activation, suggesting that ROS are not directly implicated [36]. However, imiquimod, a small molecule known to be a ligand of TLR7, activates NLRP3 in a K+-independent but ROS-dependent manner, suggesting at least two independent pathways for NLRP3 activation [49]. Recent observations reconcile these two models by the demonstration that NLRP3 interaction with vesicles was critical for its activation regardless of K+ involvement. K+-dependent and independent NLRP3 activators increase the levels of phosphatidylinositol 4-phosphate (PI4P) in membranes, and this increase facilitates the binding of NLRP3 to PI4P, which is crucial for its activation [90,91,92,93].
4. Degradation
NLRP3 is degraded by both autophagy- and proteasome-dependant mechanisms [94,95] (Figure 6). Accumulative studies have highlighted the importance of PTMs within the LRR domain in the degradation of NLRP3. LPS priming has been shown to influence the half-life of NLRP3, extending it from 4 h to over 6 h [25]. This suggests that LPS alters the PTM status of NLRP3 to switch to a “ready-to-be-activated” conformation and increase its cellular level by reducing its degradation.
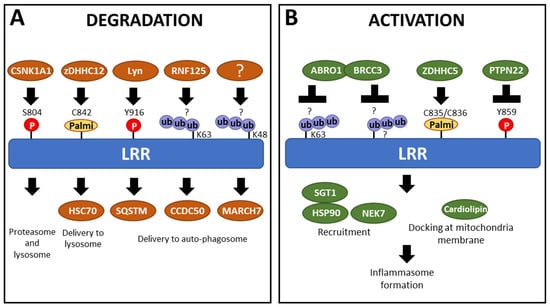
Figure 6.
Partners alter the PTM status of the LRR domain, participating in either (A) degradation or (B) activation of NLRP3. Ub = ubiquitination; Palmi = palmitoylation; P = phosphorylation; ? = amino acids carrying ubiquitination are unknown.
Studies have demonstrated that, in a resting state, NLRP3 bears degradative and non-degradative polyubiquitination with K63 and K48 ubiquitin chains [24,96] that keep NLRP3 both inactive and at low levels [94]. Therefore, upon activation, NLRP3 undergoes deubiquitination [24,97]. The LRR domain, rich in lysine residues, is a favorable site for ubiquitination, suggesting it might lead to NLRP3 degradation. However, intracellular levels of human NLRP3 variants lacking exons in the LRR domain [72,73,74] and murine NLRP3 mutants lacking LRR parts [84] are consistently lower compared to WT NLRP3. Moreover, in an overexpression system, NLRP3 lacking exons in the LRR domain are more prone to proteasomal degradation than the full-length NLRP3. This suggests that the C-terminal LRR domain plays a protective role, helping to prevent NLRP3 degradation [84].
Phosphorylation also participates in maintaining NLRP3 levels. For example, Y859 (Y861 in the other annotation) is phosphorylated under steady-state conditions. This phosphorylation is removed during the activation step by the phosphatase PTPN22 [98]. In mice, cells lacking PTPN22 show an increased proportion of NLRP3 in autophagosomes, suggesting that phosphorylation at Y859 induces NLRP3 degradation. When the tyrosine residue is mutated to a non-phosphorylable phenylalanine, the accumulation of NLRP3 in the autophagosome is prevented, further supporting the involvement of Y859 phosphorylation in the regulation of NLRP3 degradation. Specifically, SQSTM1, an autophagosome cargo protein, binds to phosphorylated NLRP3, facilitating its degradation through autophagy [99]. However, a fraction of NLRP3 is phosphorylated under steady-state conditions but is not recruited to phagophores, suggesting that multiple signals are involved in NLRP3 degradation, and Y859 phosphorylation alone is not sufficient. Moreover, the interaction between NLRP3 and SQSTM1 also depends on the pyrin domain [99], indicating that degradation is influenced by the accumulation of multiple signals.
Inflammasome activators also trigger PTMs controlling NLRP3 degradation, a mechanism that participates in its negative control and/or the resolution of the inflammation. The kinase Lyn phosphorylates NLRP3 following ATP activation at position Y916 (Y918 in the other annotation). This phosphorylation increases NLRP3 ubiquitination, leading to its degradation by proteasome, suggesting Lyn phosphorylation starts a process of ubiquitination, which leads to NLRP3 degradation [100]. Indeed, E3 ubiquitin ligase and deubiquitinase target sequentially NLRP3 following activation, determining its fate. For example, RNF125 adds K63 ubiquitin chains on the LRR domain, which controls the recruitment of Cbl-b, which in turn adds K48 ubiquitination chains on the NACHT domain targeting NLRP3 to degradation and represses inflammasome activation [101]. Similarly, the E3 ubiquitin ligase MARCH7 is implicated in the autophagy-mediated degradation of NLRP3, associated with K48-polyubiquitination within the LRR domain [102]. On the opposite, the recruitment of the BRCC36 deubiquitinase (as well as its mouse homolog BRCC3) by the ubiquitinated-LRR domain mediates its K63-deubiquitination following activation stimuli and promotes inflammasome assembly [96,103,104]. Noteworthy, K63 polyubiquitination of the LRR could also directly facilitate NLRP3 delivery to autophagosomes [105]. Indeed, NLRP3 interaction with CCDC50, an autophagy receptor highly expressed in immune cells [106], is significantly increased following LPS treatment. Although the specific binding motif has not been identified, CCDC50 triggers the degradation of K63-ubiquitinated NLRP3, with the LRR domain proving essential and sufficient for CCDC50 binding [105]. In addition to phosphorylation and ubiquitination, palmitoylation also plays a crucial role in regulating NLRP3 stability and degradation. Specifically, palmitoylation of the LRR domain at the cysteine C842 (C844 in the other annotation) by ZDHHC12 upon activation enhances the ability of the Heat Shock Cognate protein 70 (HSC70) to recognize NLRP3, which in turn promotes its degradation through the lysosomal pathway [107].
5. Activation/Auto-Inhibition
In the context of NLRP3 regulation, addressing auto-inhibition alongside activation is crucial for understanding the functions of the LRR domain.
The auto-inhibitory role of LRR domains is highly documented in various proteins. In plants, LRR domains are proposed to self-inhibit NLRs until pathogen sensing triggers its cleavage [108]. Similarly, the crystal structure of murine NLRC4 suggests that the LRR domain maintains NLRC4 in a monomeric inactive form [109]. Experimental evidence supports this observation, as NLRC4ΔLRR is constitutively active and oligomerizes spontaneously, regardless of flagellin presence [110,111]. Similarly, NLRP1ΔLRR binds ATP equally in the presence or absence of stimuli, enabling oligomerization in the absence of agonists [112]. Consistent with these findings, deletion of LRR domains in both NOD1 and NOD2 results in constitutive activation [79].
NLRP10 is the only member of the NLR family lacking a LRR domain (Figure 1) [113]. It was long thought to inhibit the NLRP3 inflammasome [114], but NLRP10 has recently been shown to form an inflammasome in response to mitochondrial damage in several murine and human models [14,115]. Both the PYD and NACHT domains are necessary for NLRP10 inflammasome formation, and the absence of the LRR domain does not prevent ASC recruitment [115].
Cryo-EM analysis of both human and murine NLRP3 reveals that inactive NLRP3 (bound to ADP/MCC950) forms various spheric oligomeric structures, including barrel-hexamer [116], decamer [76], dodecamer [117], and even tetradecamer and hexadecamer configurations [118] (Figure 7A). These NLRP3 “cages” sequestered the PYD domain within the complex core inaccessible to ASC and are maintained by LRR domains with interaction surfaces occurring in both “back-to-back” and “face-to-face” orientations (Figure 7A, left and middle). Specifically, the negatively charged C-terminal of one LRR domain interacts with the positively charged concave surface of another LRR [117], leading to the intertwining of LRR domains [76]. Multiple cage structures observed in cryo-EM suggest that inactive NLRP3 undergoes oligomerization, with LRR domains playing a crucial role in maintaining the overall structure. Further supporting the importance of LRR domains, an acidic loop (residues 686–725) acting in the LRR-LRR concave interface in the closed conformation becomes disordered in the active form [119]. Consistent with this hypothesis, NLRP3 mutants lacking the LRR domain show reduced self-oligomerization [120], and specific mutations in the “back-to-back” or “face-to-face” interfaces results in impaired cage formation with a higher quantity of free NLRP3 [118]. Notably, NLRP3 oligomers containing fewer NLRP3 units, such as hexameric cages, present a “head-to-face” surface interaction between a NACHT and LRR domain rather than the typical “face-to-face” surface between 2 LRR domains (Figure 7A, right). Several PTMs of NLRP3 upon priming may participate in the disruption of these auto-inhibited complexes, including phosphorylation of the LRR domain at S804 by CSNK1A1, and Y859, as only unphosphorylated forms are found in cage complexes [99,104,118].
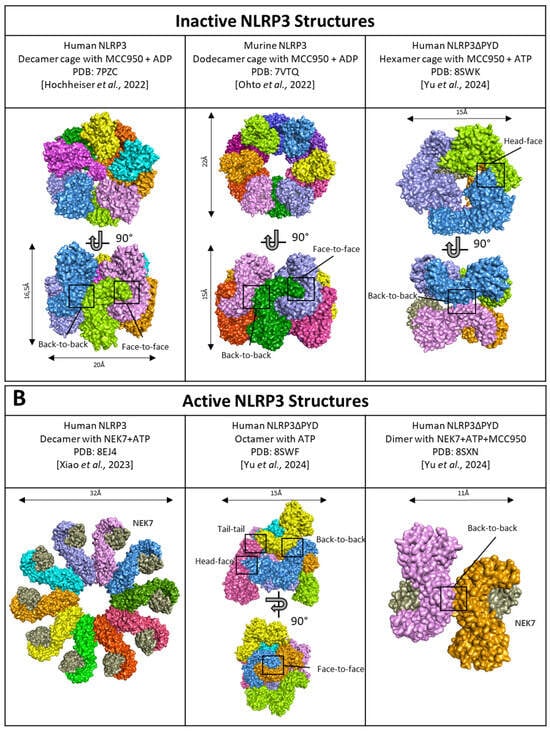
Figure 7.
Structure of (A) inactive NLRP3 cages and (B) active NLRP3. NLRP3 monomers are represented in several colors. NEK7, a partner of activation is in grey. Black boxes highlight the interaction surfaces. PDB ID: 7PZC [76]; 7VTQ [117]; 8EJ4 [119]; 8SWF/8SXN/8SWK [116].
Activated NLRP3 adopts a radial flower-like oligomeric structure, where LRR domains are not engaged in disk assembly. Instead, LRR domains bind NEK7 and lose their interactions with each other [119]. Notably, they do not interact with central caspase-1, which suggests LRR domains are not directly engaged in the catalytic part of the inflammasome, but rather, the binding to NEK7 potentially promotes the transition of NLRP3 from a closed to an open conformation controlled by ATP binding or stabilizes the latter (Figure 7B, left). Therefore, the role of LRR domains appears to precede inflammasome activation. Indeed, NLRP3 lacking the LRR domain does not exhibit constitutive activation under basal conditions [85]. The recent description of open NLRP3 oligomers in the presence of ATP revealed an octameric structure with conserved “back-to-back” and “face-to-face” interface interactions of the LRR domains (Figure 7B, middle). Additionally, new interface interactions termed “tail-to-tail” and “head-to-face” were identified by cryo-EM. This structure revealed a novel organization of NLRP3 oligomers proposed to be an intermediate form between inactive and active forms (Figure 7B, middle). Notably, whether in the open or closed conformation, NEK7 clashes with adjacent NLRP3, indicating that NEK7 presence leads to the dissociation of the oligomeric cage. This results in dimers of NLRP3/NEK7 with “back-to-back” interactions maintained through the LRR domains (Figure 7B, right) [116]. Consistent with earlier findings [119], the acidic loop located on the concave face of the LRR, which facilitates “face-to-face” interactions, is disordered in the open conformation [116]. However, it’s important to note that this recent model lacks the PYD domain, which could potentially influence the overall structure of the activated inflammasome.
NEK7, a key partner in inflammasome activation, is essential to NLRP3 inflammasome assembly in mouse macrophages [121,122]. Deletion experiments with ΔNEK7 cells have demonstrated altered caspase-1 cleavage [123] and impaired IL-1β production [124]. Co-immunoprecipitation assays and cryo-EM analysis have revealed that NEK7 binding depends on both the NACHT and LRR domains, although the precise involvement of the NACHT domain is unclear [123]. Notably, an intact LRR domain is essential for this interaction [120,121]. Supporting the concept of “cage opening,” the NEK7-NLRP3 interaction is moderately enhanced after LPS priming and significantly boosted following ATP activation [121], suggesting increased interaction between NEK7 and the LRR domain upon NLRP3 activation. The authors proposed that NEK7 may be hindered from interacting with the LRR domain under steady-state conditions due to neighboring LRR occupancy and auto-inhibition [119].
Further proving the significance of the LRR domain in the activation of the inflammasome, some LRR mutations have been proposed to either enhance or reduce the affinity for NEK7, rendering them pathogenic, such as R918Q and G755R, or hypomorphic, such as D946G, respectively [122,125]. In addition, NEK7 recruitment is also controlled by PTMs in the LRR domain. Following its transient phosphorylation upon priming signal, NLRP3 is dephosphorylated at S804 upon activation by nigericin. Phospho-mimetic substitution S804D prevents the recruitment of NEK7 and the inflammasome formation [104] (Figure 6A). The role of NLRP3 dephosphorylation at Y859 by PTPN22 on NEK7 recruitment remains to be investigated but could constitute an additional mechanism for the positive role of PTPN22 on inflammasome activity (Figure 6B). Indeed, the location of Y859 and S804 within the LRR domain, at the interface with NEK7, suggests a potential mechanism where steric hindrance or charge repulsion could inhibit binding between LRR and NEK7 [104,123]. Consequently, PTMs enhancing the affinity of the LRR domain for NEK7 could destabilize the inactive NLRP3 oligomers through interactions with the acidic loop, thereby promoting activation. Additionally, palmitoylation within the LRR domain was also shown to have an impact on NEK7 binding. Specifically, palmitoylations at positions C835 and C836 (C837/C838 in the other annotation) by ZDHHC5 promote NLRP3 activation following both priming and boost signals (Figure 6A,B). Modeling analysis suggests that palmitoylations at these sites may enhance the interaction between NEK7 and the LRR domain, facilitating the activation process [126]. The variant R918Q has been modeled to exhibit increased affinity for NEK7 [125]. This variant displays higher levels of palmitoylations on C835/C836, attributed to decreased recruitment of the Acylprotein thioesterase 1 and alpha/beta hydrolase domain-containing protein 17A depalmitoylase (ABHD17A). This reduced recruitment enhances palmitoylation and consequently strengthens binding to NEK7 [126].
Intriguingly, inactive NLRP3 isoforms lacking exon 4 lose the ability to bind NEK7 [72], indicating the critical importance of amino acids other than Y859 (in exon 6), S804 (in exon 5) or R918 (in exon 7) for NEK7 binding, or the necessity of an intact LRR domain for NEK7 interaction [120]. However, some studies suggest that the LRR domain may not be necessary for interaction with NEK7 and subsequent inflammasome activation [84,85] and that NLRP3 can also form the inflammasome independently of NEK7 [23,127]. Notably, long priming that activates IKKβ has been reported to enable NEK7-independent inflammasome activation as the predominant pathway in human myeloid cells [23]. Further studies would be required to investigate the putative differential role of the LRR domain upon the IKKβ-dependent vs NEK7-dependent inflammasome activation pathway.
In addition to NEK7 recruitment, the LRR domain also facilitates the recruitment of other partners upon activation, such as the AKT kinase. During the priming phase, AKT phosphorylates serine 5 in the PYD domain, preventing NLRP3 from interacting with the adaptor protein ASC [128]. Furthermore, the SGT1-HSP90 (co-chaperon-like ubiquitin-ligase-associated protein and heat-shock protein 90) complex is also recruited by the LRR domain to aid inflammasome activation [129], and disruption of this interaction inhibits NLRP3 activation [130]. Furthermore, HSP90β and SGT1 are essential for the self-activation of NLRP3 variants that carry activating mutations, further proving the involvement of the LRR domain in recruiting activating partners [131] (Figure 6B). Interestingly, SGT1 interacts minimally with non-active NLRP3 isoforms but interacts with full-length NLRP3 and isoforms lacking exon 6 or 8, which are the only active isoforms [74]. It suggests that the LRR domain influences interactions with proteins upstream of inflammasome assembly. Additionally, NLRP3 is reported to be recruited at the mitochondria and the endoplasmic reticulum after the inflammasome activation with MSU or nigericin [132]. Cardiolipin, a phospholipid from the outer mitochondrial membrane, interacts with the LRR domain, providing a docking site to localize activated NLRP3 at mitochondria. This interaction is crucial for inflammasome activation, indicating the involvement of the LRR domain in the activation process [133].
The evidence suggests that the LRR domain plays a crucial role in the activation process of the inflammasome. Cells expressing NLRP3 deletion mutant lacking the LRR domain were unable to secrete IL-1β in response to combined priming and activation signals. This indicates that the LRR domain is not only required for maintaining the endogenous stability of inactive oligomers but is also essential for NLRP3 inflammasome activation [120].
6. Typical and Atypical Cryopyrin-Associated Periodic Syndrome
Gain-of-function variants in NLRP3 are associated with CAPS, a rare monogenic autoinflammatory disease also named NLRP3-AID for NLRP3-associated autoinflammatory disease, which was first described in 2001 [64]. Most cases follow an autosomal dominant inheritance pattern, but somatic mutations are not rare and lead to sporadic acquired cases either in children, such as in the most severe form entitled NOMID/CINCA, or in adults [134]. Most pathogenic variants ultimately lead to overactivation of the NLRP3 inflammasome, resulting in the overproduction of IL-1β and, thus, cell pyroptosis. Normally, the NLRP3 inflammasome requires a priming signal and an activation signal to be active. The mechanism leading to the overactivation of the inflammasome depends on the location of the mutation, which can cause either a bypass of the priming signal, the activation signal, or both [135]. In the classical CAPS description, patients typically display episodes of fever and/or chills, pseudo-urticarial lesions triggered by environmental cold exposure, arthromyalgia +/− arthritis associated with neuroinflammatory features such as aseptic meningitis, sensorineural hearing loss, conjunctivitis, and uveitis [63]. While pseudo-urticarial lesions are a central symptom of classical CAPS and play a crucial role in clinical suspicion by clinicians, some patients may lack cutaneous involvement but can present with deafness, defining a subset classified as atypical CAPS [136,137].
Although the majority of variants are found in the NACHT domain, variants in the LRR domain are present in patients with atypical CAPS phenotypes associated with low or absence of cutaneous involvement and a higher risk of deafness [136,137]. This observation raises the question of a correlation between the location of the mutation and the atypical clinical phenotype with less frequent cutaneous features such as urticaria. We performed a literature review focusing on CAPS patients carrying NLRP3 variants in the LRR domain. We found 45 patients reported; the NLRP3 variants described were G755R (n = 5), G755A (n = 1), G809S (n = 1), K829T (n = 1), Y859C (n = 15), Y859H (n = 5), R918Q (n = 14), R918X (n = 2), L1016F (n = 1) (Table 1; Figure 8). The median age at symptom onset was 5 years, ranging from 0 to 32 years, and the median age at diagnosis was 34 years, ranging from 0.3 to 70 years. The median delay in diagnosis was 20 years, ranging from 0.3 to 49 years. Skin involvement with pseudo-urticarial lesions was found in 41.9% of patients (13/31), and hearing loss in 89.7% of cases (35/39). Atypical forms of CAPS were particularly prevalent in patients with the K829T, Y859C, Y859H, and R918Q variants of NLRP3, with 94.1% (32/34) of hearing loss and only 30.7% (8/26) of skin manifestations. In contrast, a significant cohort of CAPS patients with NLRP3 mutated in the NACHT domain reported 42% of patients with hearing loss and 97% of patients with skin involvement [138]. Therefore, patients harboring NLRP3 variants in the LRR domain tend to exhibit a higher incidence of hearing loss and lower prevalence of skin involvement compared to those with variants in the NACHT domain [137]. This observation supports a genotype-phenotype correlation depending on the domain carrying the variant: mutations in the NACHT domain would lead to typical CAPS presentation, while mutations in the LRR domain would be more likely to cause the atypical form of CAPS. These observations also suggest distinct pathophysiological mechanisms for NACHT-mutated and LRR-mutated NLRP3 variants. Consistently, functional analysis of NLRP3 natural variants by Cosson et al. [135] showed that gain-of-function mutations in the LRR domain correspond to a new group of mutants activated by the activation signal independently of any priming. In opposition, most disease-causing variants from the NACHT domain are either constitutively activated or require only the priming signal to be active [135]. These observations suggest that atypical CAPS phenotype is preferentially caused by variations localized in the LRR domain of NLRP3 that drive priming-independent but activation-dependent assembly of the inflammasome [135].

Table 1.
A literature review focusing on pathogenic LRR variants was performed using the PubMed database. A total of 1113 articles were retrieved. After removing duplicates and excluding articles describing patients with mutations in the NACHT domain, those without genetic analysis, those describing previously reported patients, and those with other pathogenic mutations explaining the symptoms, a total of 18 articles describing 45 patients were included in the final analysis.
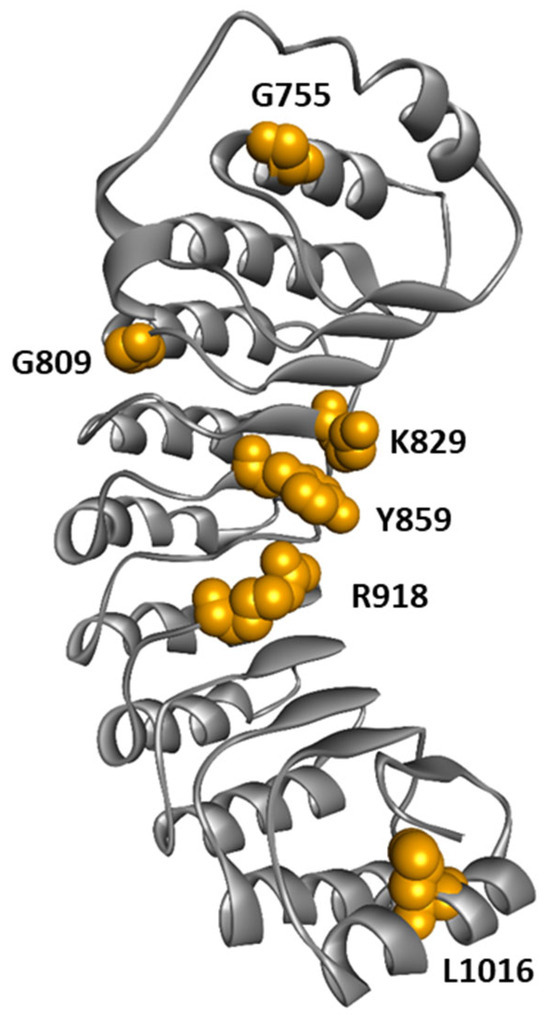
Figure 8.
Structure of NLRP3-LRR highlighting amino acids mutated in CAPS patients.
It remains unclear, however, how disease variants located in the LRR domain lead to abnormal inflammasome activation. At the molecular level, Q918, T755, and A755 mutations were described to enhance the interaction of the LRR domain with NEK7, potentially promoting NLRP3 activation [125]. In contrast, C859 and H859 were not predicted to impact NEK7-LRR interaction [136]. In cultured monocytes from patients with a Y859C genotype, the kinetics of IL-1β production demonstrate an increased production at an early time point and sustained production over time compared to cells with WT NLRP3 [136]. These observations might be explained by the role of Y859 in controlling NLRP3 degradation by phagolysosome, as shown in mice [98]. Alternatively, these mutations might disrupt the interaction between an acidic loop and the concave side of the LRR observed in the auto-inhibitory spheric cage complex [76], destabilizing this inactive complex.
In conclusion, our literature review on CAPS patients shows that patients displaying pathogenic NLRP3 variants in the LRR domain present with more hearing loss and less frequent cutaneous urticaria lesions compared to patients with classical CAPS. Whether atypical forms of CAPS are more specifically related to NLRP3 variants whose activation is independent of priming signal remains to be confirmed.
7. Conclusions/Discussion
NLRP3 is composed of three domains: the N-terminal PYD domain, the central NACHT domain, and the C-terminal LRR domain. Given the implication of NLRP3 in various diseases, including auto-inflammatory syndromes [55,56,57,64] and neuro-inflammatory diseases [40,41,42,59,60,61,62], deciphering the mechanism controlling NLRP3 inflammasome activation and degradation remains critical.
Compared to other proteins with LRR domains, the specific ligand for NLRP3 remains unidentified. Indeed, NLRP3 is responsive to a diverse array of stimuli, including microbial compounds, endogenous signals, and environmental triggers. Although evidence suggests that the LRR domain may be required to detect certain activator signals, no specific ligand-binding pocket has been discovered. It appears more likely that the known activators converge on cellular events, which lead to its activation. Specifically, NLRP3 activation is often associated with mechanisms involving K+ efflux, suggesting that this process could serve as the converging point and primary activator of NLRP3.
Pieces of evidence indicate that the LRR domain plays a critical role in regulating NLRP3. On one hand, interactions between LRR domains appear to maintain the integrity of the auto-inhibitory structure in steady-state oligomeric structures. On the other hand, PTMs accumulated within the LRR domains are documented to regulate both the activation and degradation of NLRP3.
Mutations in NLRP3 associated with CAPS provide valuable insights into the functional roles of its LRR domain. While most mutations are found in the NACHT domain, those in the LRR domain lead to atypical symptoms, indicating a genotype-phenotype correlation. Unlike NACHT mutations, which typically activate NLRP3 by bypassing the activation signal, LRR mutations demonstrate a distinct activation profile that appears to be priming-independent and activation signal-dependent [135]. This unique activation pattern underscores the pivotal role of the LRR domain in the activation process of NLRP3. An intriguing aspect of these LRR domain mutations is the presentation of hearing loss as a primary symptom in affected patients. NLRP3 is expressed in immune cells in the cochlea [155], and K+ is known to play a critical role in auditory system functions [156]. Thus, the prevalent hearing loss observed in patients with LRR mutations might be explained by K+ efflux, which may act as the activation signal in the cochlea.
LRR-mutated patients are relatively rare, but their atypical clinical presentation suggests the actual incidence of these mutations may be underestimated. The delay in diagnosis might contribute to the evolution toward AA amyloidosis [157]. Early diagnosis is thus essential to initiate specific treatment as soon as possible to prevent outcomes such as deafness. In this context, IL-1β inhibitors have proven to be effective in reducing symptoms of the disease and in halting hearing loss progression [158,159,160].
The existence of several isoforms with unknown roles may impede cell- or tissue-specific expression and activity. It is widely accepted that among immune cells, mononuclear phagocytic cells express high levels of NLRP3 and are particularly responsive to activators that trigger inflammasome formation. Most studies are conducted in mouse models, human cell lines that have been genetically modified to overexpress NLRP3, or primary human monocytes. However, the exploration of NLRP3 regulation in other cell types remains limited, but future research could reveal cell-specific functions of the LRR domain. For example, NLRP3 is implicated in the formation of neutrophil extracellular traps [161,162], the differentiation of T cells, and the development of B cells [163], indicating a broader range of influence beyond inflammasome response. Further research into NLRP3 activity in different cell types might reveal cell-specific roles of the LRR domain.
Author Contributions
Writing—original draft preparation, M.C.; Table, Y.Y.J.Z.; Writing—review and editing L.L.C., S.G.-L., M.P.R. and B.F.P.; supervision, M.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABHD17A | α/β-hydrolase Domain Containing 17A |
| AID | associated autoinflammatory disease |
| AS | Alternative splicing |
| ASC | Apoptosis-associated Speck-like protein containing a CARD |
| ATP | Adenosine TriPhosphate |
| BIR | Baculoviral Inhibitory Repeat |
| CAPS | Cryopyrin-Associated Periodic Syndrome |
| CARD | Caspase Recruitment Domain |
| Cbl-b | Casitas B-lineage Lymphoma-b |
| CCDC50 | Coiled-Coil Domain Containing 50 |
| CIAS1 | Cold-Induced Autoinflammatory Syndrome 1 |
| CINCA | Chronic Infantile Neurological Cutaneous and Articular syndrome |
| CLR | C-type Lectin Receptor |
| Cryo-EM | Cryo-electronic microscopy |
| CSNK1A1 | Casein Kinase 1, Alpha 1 |
| DAMP | Danger-Associated Molecular Pattern |
| dsRNA | double-strand RNA |
| GSDMD | Gasdermin-D |
| H2O2 | Hydrogen peroxide |
| HSC70 | Heat Shock Cognate 70 |
| HSP90 | Heat Shock Protein 90 |
| IL-1β | Interleukin 1β |
| IL-18 | Interleukin 18 |
| K+ | Potassium ion |
| LPS | Lipopolysaccharide |
| LRR | Leucine-Rich Repeat |
| MARCH7 | Membrane-Associated RING-CH type finger protein 7 |
| MSU | crystals of monosodium urate |
| MTS | Mitochondrial Targeting Sequence |
| MyD88 | Myeloid differentiation primary response 88 |
| NACHT | Nucleotide-binding and oligomerization domain |
| NALP | NACHT, LRR, and PYD domains-containing protein |
| NEK7 | NIMA-related kinase 7 |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLR | NOD-Like Receptor |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| NOMID | Neonatal Onset Multisystem Inflammatory Disease |
| PAMP | Pathogen-Associated Molecular Pattern |
| PI4P | phosphatidylinositol 4-phosphate |
| PRR | Pathogen-Recognition Receptor |
| PTM | Post-Translational Modification |
| PTPN22 | Protein Tyrosine Phosphatase, Non-Receptor Type 22 |
| PYD | Pyrin domain |
| RNAseq | Ribonucleic Acid sequencing |
| RNF125 | Ring Finger Protein 125 |
| ROS | Reactive Oxygen Species |
| RLR | RIG-I-Like Receptor |
| SiO2 | Silica |
| SGT1 | Suppressor of G2 allele of SKP1 |
| SQSTM1 | Sequestosome 1 |
| TLR | Toll-Like Receptor |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| ZDHHC12 | Zinc Finger DHHC-Type Containing 12 |
References
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Ratner, D.; Orning, M.P.A.; Lien, E. Bacterial secretion systems and regulation of inflammasome activation. J. Leukoc. Biol. 2017, 101, 165–181. [Google Scholar] [CrossRef]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef]
- Bi, P.Y.; Killackey, S.A.; Schweizer, L.; Girardin, S.E. NLRX1: Versatile functions of a mitochondrial NLR protein that controls mitophagy. Biomed. J. 2024, 47, 100635. [Google Scholar] [CrossRef] [PubMed]
- Parlato, M.; Yeretssian, G. NOD-Like Receptors in Intestinal Homeostasis and Epithelial Tissue Repair. Int. J. Mol. Sci. 2014, 15, 9594–9627. [Google Scholar] [CrossRef]
- Conforti-Andreoni, C.; Ricciardi-Castagnoli, P.; Mortellaro, A. The inflammasomes in health and disease: From genetics to molecular mechanisms of autoinflammation and beyond. Cell. Mol. Immunol. 2011, 8, 135–145. [Google Scholar] [CrossRef]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR Gene Family: A Standard Nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef]
- Barry, K.; Murphy, C.; Mansell, A. NLRP1- A CINDERELLA STORY: A perspective of recent advances in NLRP1 and the questions they raise. Commun. Biol. 2023, 6, 1274. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Luc, N.; Dorfleutner, A.; Stehlik, C. Inflammasomes and Their Activation. Crit Rev Immunol 2010, 30, 463–487. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Kern, L.; Elinav, E. The NLRP6 inflammasome. Immunology 2021, 162, 281–289. [Google Scholar] [CrossRef]
- Carriere, J.; Dorfleutner, A.; Stehlik, C. NLRP7: From inflammasome regulation to human disease. Immunology 2021, 163, 363–376. [Google Scholar] [CrossRef]
- Zhu, S.; Ding, S.; Wang, P.; Wei, Z.; Pan, W.; Palm, N.W.; Yang, Y.; Yu, H.; Li, H.-B.; Wang, G.; et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 2017, 546, 667–670. [Google Scholar] [CrossRef]
- Zheng, D.; Mohapatra, G.; Kern, L.; He, Y.; Shmueli, M.D.; Valdés-Mas, R.; Kolodziejczyk, A.A.; Próchnicki, T.; Vasconcelos, M.B.; Schorr, L.; et al. Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation. Nat. Immunol. 2023, 24, 585–594. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Gil-Marqués, M.L.; Kharbanda, V.; Zajac, A.S.; Miller, K.A.; Wood, T.E.; Hachey, A.C.; Egger, K.T.; Goldberg, M.B. NLRP11 is a pattern recognition receptor for bacterial lipopolysaccharide in the cytosol of human macrophages. Sci. Immunol. 2023, 8, eabo4767. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef]
- Xing, Y.; Yao, X.; Li, H.; Xue, G.; Guo, Q.; Yang, G.; An, L.; Zhang, Y.; Meng, G. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling–Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J. Immunol. 2017, 199, 1561–1566. [Google Scholar] [CrossRef]
- Guarda, G.; Zenger, M.; Yazdi, A.S.; Schroder, K.; Ferrero, I.; Menu, P.; Tardivel, A.; Mattmann, C.; Tschopp, J. Differential Expression of NLRP3 among Hematopoietic Cells. J. Immunol. 2011, 186, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Schmacke, N.A.; O’duill, F.; Gaidt, M.M.; Szymanska, I.; Kamper, J.M.; Schmid-Burgk, J.L.; Mädler, S.C.; Mackens-Kiani, T.; Kozaki, T.; Chauhan, D.; et al. IKKβ primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network. Immunity 2022, 55, 2271–2284.e7. [Google Scholar] [CrossRef] [PubMed]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef]
- Han, S.; Lear, T.B.; Jerome, J.A.; Rajbhandari, S.; Snavely, C.A.; Gulick, D.L.; Gibson, K.F.; Zou, C.; Chen, B.B.; Mallampalli, R.K. Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase. J. Biol. Chem. 2015, 290, 18124–18133. [Google Scholar] [CrossRef]
- Joly, S.; Ma, N.; Sadler, J.J.; Soll, D.R.; Cassel, S.L.; Sutterwala, F.S. Cutting Edge: Candida albicans Hyphae Formation Triggers Activation of the Nlrp3 Inflammasome. J. Immunol. 2009, 183, 3578–3581. [Google Scholar] [CrossRef]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An Essential Role for the NLRP3 Inflammasome in Host Defense against the Human Fungal Pathogen Candida albicans. Cell Host Microbe. 2009, 5, 487–497. [Google Scholar] [CrossRef]
- Kumar, H.; Kumagai, Y.; Tsuchida, T.; Koenig, P.A.; Satoh, T.; Guo, Z.; Jang, M.H.; Saitoh, T.; Akira, S.; Kawai, T. Involvement of the NLRP3 Inflammasome in Innate and Humoral Adaptive Immune Responses to Fungal β-Glucan. J. Immunol. 2009, 183, 8061–8067. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschläger, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef]
- Martinon, F.; Agostini, L.; Meylan, E.; Tschopp, J. Identification of Bacterial Muramyl Dipeptide as Activator of the NALP3/Cryopyrin Inflammasome. Curr. Biol. 2004, 14, 1929–1934. [Google Scholar] [CrossRef]
- Craven, R.R.; Gao, X.; Allen, I.C.; Gris, D.; Wardenburg, J.B.; McElvania-TeKippe, E.; Ting, J.P.; Duncan, J.A. Staphylococcus aureus α-Hemolysin Activates the NLRP3-Inflammasome in Human and Mouse Monocytic Cells. PLoS ONE 2009, 4, e7446. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gurcel, L.; Abrami, L.; Girardin, S.; Tschopp, J.; van der Goot, F.G. Caspase-1 Activation of Lipid Metabolic Pathways in Response to Bacterial Pore-Forming Toxins Promotes Cell Survival. Cell 2006, 126, 1135–1145. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Body-Malapel, M.; Amer, A.; Park, J.-H.; Whitfield, J.; Franchi, L.; Taraporewala, Z.F.; Miller, D.; Patton, J.T.; Inohara, N.; et al. Critical Role for Cryopyrin/Nalp3 in Activation of Caspase-1 in Response to Viral Infection and Double-stranded RNA. J. Biol. Chem. 2006, 281, 36560–36568. [Google Scholar] [CrossRef]
- Zha, Q.-B.; Wei, H.-X.; Li, C.-G.; Liang, Y.-D.; Xu, L.-H.; Bai, W.-J.; Pan, H.; He, X.-H.; Ouyang, D.-Y. ATP-Induced Inflammasome Activation and Pyroptosis Is Regulated by AMP-Activated Protein Kinase in Macrophages. Front. Immunol. 2016, 7, 597. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Yamasaki, K.; Muto, J.; Taylor, K.R.; Cogen, A.L.; Audish, D.; Bertin, J.; Grant, E.P.; Coyle, A.J.; Misaghi, A.; Hoffman, H.M.; et al. NLRP3/Cryopyrin Is Necessary for Interleukin-1β (IL-1β) Release in Response to Hyaluronan, an Endogenous Trigger of Inflammation in Response to Injury. J. Biol. Chem. 2009, 284, 12762–12771. [Google Scholar] [CrossRef]
- Saresella, M.; La Rosa, F.; Piancone, F.; Zoppis, M.; Marventano, I.; Calabrese, E.; Rainone, V.; Nemni, R.; Mancuso, R.; Clerici, M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 23. [Google Scholar] [CrossRef]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Cassel, S.L.; Eisenbarth, S.C.; Iyer, S.S.; Sadler, J.J.; Colegio, O.R.; Tephly, L.A.; Carter, A.B.; Rothman, P.B.; Flavell, R.A.; Sutterwala, F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9035–9040. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauernfeind, F.; Halle, A.; O Samstad, E.; Kono, H.; Rock, K.L.; A Fitzgerald, K.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Groß, C.J.; Mishra, R.; Schneider, K.S.; Médard, G.; Wettmarshausen, J.; Dittlein, D.C.; Shi, H.; Gorka, O.; Koenig, P.-A.; Fromm, S.; et al. K + Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 2016, 45, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Springstead, J.R.; Kagan, J.C. By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity 2017, 47, 697–709.e3. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Boucher, D.; Bierschenk, D.; Tebartz, C.; Whitney, P.G.; D’Silva, D.B.; Tanzer, M.C.; Monteleone, M.; Robertson, A.A.; Cooper, M.; et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015, 45, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Burgk, J.L.; Gaidt, M.M.; Schmidt, T.; Ebert, T.S.; Bartok, E.; Hornung, V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015, 45, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.F.; Kingsbury, S.R.; Conaghan, P.G.; Conaghan, P.G. The role of the NLRP3 inflammasome in gout. J. Inflamm. Res. 2011, 4, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kastbom, A.; Verma, D.; Eriksson, P.; Skogh, T.; Wingren, G.; Soderkvist, P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (The Swedish TIRA project). Rheumatology 2007, 47, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.-C.; Lemire, M.; Fortin, G.; Louis, E.; Silverberg, M.S.; Collette, C.; Baba, N.; Libioulle, C.; Belaiche, J.; Bitton, A.; et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 2009, 41, 71–76. [Google Scholar] [CrossRef]
- Fan, Z.; Pan, Y.-T.; Zhang, Z.-Y.; Yang, H.; Yu, S.-Y.; Zheng, Y.; Ma, J.-H.; Wang, X.-M. Systemic activation of NLRP3 inflammasome and plasma α-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J. Neuroinflammation 2020, 17, 11. [Google Scholar] [CrossRef]
- Nguyen, L.T.N.; Nguyen, H.D.; Kim, Y.J.; Nguyen, T.T.; Lai, T.T.; Lee, Y.K.; Ma, H.-I.; Kim, Y.E. Role of NLRP3 Inflammasome in Parkinson’s Disease and Therapeutic Considerations. J. Park. Dis. 2022, 12, 2117–2133. [Google Scholar] [CrossRef]
- Mohseni-Moghaddam, P.; Roghani, M.; Khaleghzadeh-Ahangar, H.; Sadr, S.S.; Sala, C. A literature overview on epilepsy and inflammasome activation. Brain Res. Bull. 2021, 172, 229–235. [Google Scholar] [CrossRef]
- Pohlentz, M.S.; Müller, P.; Cases-Cunillera, S.; Opitz, T.; Surges, R.; Hamed, M.; Vatter, H.; Schoch, S.; Becker, A.J.; Pitsch, J. Characterisation of NLRP3 pathway-related neuroinflammation in temporal lobe epilepsy. PLoS ONE 2022, 17, e0271995. [Google Scholar] [CrossRef]
- Welzel, T.; Kuemmerle-Deschner, J.B. Diagnosis and Management of the Cryopyrin-Associated Periodic Syndromes (CAPS): What Do We Know Today? J. Clin. Med. 2021, 10, 128. [Google Scholar] [CrossRef]
- Hoffman, H.M.; Mueller, J.L.; Broide, D.H.; Wanderer, A.A.; Kolodner, R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat. Genet. 2001, 29, 301–305. [Google Scholar] [CrossRef]
- Milhavet, F.; Cuisset, L.; Hoffman, H.M.; Slim, R.; El-Shanti, H.; Aksentijevich, I.; Lesage, S.; Waterham, H.; Wise, C.; de Menthiere, C.S.; et al. The infevers autoinflammatory mutation online registry: Update with new genes and functions. Hum. Mutat. 2008, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Alejandre, N.; Ruiz-Palacios, A.; García-Aparicio, A.M.; Blanco-Kelly, F.; Bermúdez, S.; Fernández-Sanz, G.; Romero, F.I.; Aróstegui, J.I.; Ayuso, C.; Jiménez-Alfaro, I.; et al. Description of a new family with cryopyrin-associated periodic syndrome: Risk of visual loss in patients bearing the R260W mutation. Rheumatology 2014, 53, 1095–1099. [Google Scholar] [CrossRef]
- Ramreddy, N.; Hopkins, A.; Lozada, C. A Rare Hereditary Disease: Muckle-Wells Syndrome. Rheumatol. Rep. 2016, 8. Available online: http://www.pagepress.org/journals/index.php/rr/article/view/6535 (accessed on 4 April 2024). [CrossRef]
- Anderson, J.P.; Mueller, J.L.; Rosengren, S.; Boyle, D.L.; Schaner, P.; Cannon, S.B.; Goodyear, C.S.; Hoffman, H.M. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 2004, 338, 25–34. [Google Scholar] [CrossRef]
- Zito, G.; Buscetta, M.; Cimino, M.; Dino, P.; Bucchieri, F.; Cipollina, C. Cellular Models and Assays to Study NLRP3 Inflammasome Biology. Int. J. Mol. Sci. 2020, 21, 4294. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, L.; Meng, G. The NLRP3 inflammasome activation in human or mouse cells, sensitivity causes puzzle. Protein Cell 2013, 4, 565–568. [Google Scholar] [CrossRef]
- Chu, L.H.; Gangopadhyay, A.; Dorfleutner, A.; Stehlik, C. An updated view on the structure and function of PYRIN domains. Apoptosis 2015, 20, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Hoss, F.; Mueller, J.L.; Ringeling, F.R.; Rodriguez-Alcazar, J.F.; Brinkschulte, R.; Seifert, G.; Stahl, R.; Broderick, L.; Putnam, C.D.; Kolodner, R.D.; et al. Alternative splicing regulates stochastic NLRP3 activity. Nat. Commun. 2019, 10, 3238. [Google Scholar] [CrossRef] [PubMed]
- Aganna, E.; Martinon, F.; Hawkins, P.N.; Ross, J.B.; Swan, D.C.; Booth, D.R.; Booth, D.R.; Lachmann, H.J.; Gaudet, R.; Woo, P.; et al. Association of mutations in theNALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002, 46, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, K.; Spel, L.; Zaffalon, L.; Delacrétaz, M.; Hofer, M.; Martinon, F. NLRP3 leucine-rich repeats control induced and spontaneous inflammasome activation in cryopyrin-associated periodic syndrome. J. Allergy Clin. Immunol. 2023, 151, 222–232.e9. [Google Scholar] [CrossRef] [PubMed]
- Neuwirt, E.; Gorka, O.; Saller, B.S.; Groß, C.J.; Madl, T.; Groß, O. NLRP3 as a sensor of metabolism gone awry. Curr. Opin. Biotechnol. 2021, 68, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hochheiser, I.V.; Pilsl, M.; Hagelueken, G.; Moecking, J.; Marleaux, M.; Brinkschulte, R.; Latz, E.; Engel, C. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 2022, 604, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Song, D.H.; Lee, J. Sensing of microbial molecular patterns by Toll-like receptors. Immunol. Rev. 2012, 250, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Jéhanno, M.; Mengin-Lecreulx, D.; Sansonetti, P.J.; Alzari, P.M.; Philpott, D.J. Identification of the Critical Residues Involved in Peptidoglycan Detection by Nod1. J. Biol. Chem. 2005, 280, 38648–38656. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Chamaillard, M.; Ogura, Y.; Zhu, L.; Qiu, S.; Masumoto, J.; Ghosh, P.; Moran, A.; Predergast, M.M.; Tromp, G.; et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004, 23, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef]
- Levinsohn, J.L.; Newman, Z.L.; Hellmich, K.A.; Fattah, R.; Getz, M.A.; Liu, S.; Sastalla, I.; Leppla, S.H.; Moayeri, M. Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome. PLOS Pathog. 2012, 8, e1002638. [Google Scholar] [CrossRef] [PubMed]
- Bauernfried, S.; Scherr, M.J.; Pichlmair, A.; Duderstadt, K.E.; Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 2021, 371, eabd0811. [Google Scholar] [CrossRef]
- Hafner-Bratkovič, I.; Sušjan, P.; Lainšček, D.; Tapia-Abellán, A.; Cerović, K.; Kadunc, L.; Angosto-Bazarra, D.; Pelegrin, P.; Jerala, R. NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat. Commun. 2018, 9, 5182. [Google Scholar] [CrossRef]
- Rahman, T.; Nagar, A.; Duffy, E.B.; Okuda, K.; Silverman, N.; Harton, J.A. NLRP3 Sensing of Diverse Inflammatory Stimuli Requires Distinct Structural Features. Front. Immunol. 2020, 11, 1828. [Google Scholar] [CrossRef]
- Perregaux, D.; Gabel, C. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994, 269, 15195–15203. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X7Receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Colomar, A.; Marty, V.; Médina, C.; Combe, C.; Parnet, P.; Amédée, T. Maturation and Release of Interleukin-1β by Lipopolysaccharide-primed Mouse Schwann Cells Require the Stimulation of P2X7 Receptors. J. Biol. Chem. 2003, 278, 30732–30740. [Google Scholar] [CrossRef] [PubMed]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Zhang, Z.; Venditti, R.; Ran, L.; Liu, Z.; Vivot, K.; Schürmann, A.; Bonifacino, J.S.; De Matteis, M.A.; Ricci, R. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nat. Immunol. 2023, 24, 30–41. [Google Scholar] [CrossRef]
- Lee, B.; Hoyle, C.; Wellens, R.; Green, J.P.; Martin-Sanchez, F.; Williams, D.M.; Matchett, B.J.; Seoane, P.I.; Bennett, H.; Adamson, A.; et al. Disruptions in endocytic traffic contribute to the activation of the NLRP3 inflammasome. Sci. Signal. 2023, 16, eabm7134. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 2018, 564, 71–76. [Google Scholar] [CrossRef]
- Hollingsworth, L.R.; Veeraraghavan, P.; Paulo, J.A.; Harper, J.W.; Rauch, I. Spatiotemporal Proteomic Profiling of Cellular Responses to NLRP3 Agonists. bioRxiv 2024. Available online: http://biorxiv.org/lookup/doi/10.1101/2024.04.19.590338 (accessed on 25 June 2024).
- Song, H.; Liu, B.; Huai, W.; Yu, Z.; Wang, W.; Zhao, J.; Han, L.; Jiang, G.; Zhang, L.; Gao, C.; et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016, 7, 13727. [Google Scholar] [CrossRef] [PubMed]
- Mehto, S.; Jena, K.K.; Nath, P.; Chauhan, S.; Kolapalli, S.P.; Das, S.K.; Sahoo, P.K.; Jain, A.; Taylor, G.A.; Chauhan, S. The Crohn’s Disease Risk Factor IRGM Limits NLRP3 Inflammasome Activation by Impeding Its Assembly and by Mediating Its Selective Autophagy. Mol. Cell 2019, 73, 429–445.e7. [Google Scholar] [CrossRef]
- Py, B.F.; Kim, M.-S.; Vakifahmetoglu-Norberg, H.; Yuan, J. Deubiquitination of NLRP3 by BRCC3 Critically Regulates Inflammasome Activity. Mol. Cell 2013, 49, 331–338. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Yin, R.; Liu, T.; Yang, S.; Zhou, L.; Pan, X.; Ma, A. K63 ubiquitin chains target NLRP3 inflammasome for autophagic degradation in ox-LDL-stimulated THP-1 macrophages. Aging 2020, 12, 1747–1759. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Gutte, P.M.; Grütter, M.G.; Beer, H.-D.; et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Investig. 2016, 126, 1783–1800. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Lang, S.; Gottier, C.; Dai, X.; Rawlings, D.J.; Chan, A.C.; Rogler, G.; Scharl, M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017, 13, 1590–1601. [Google Scholar] [CrossRef]
- Tang, J.; Xiao, Y.; Lin, G.; Guo, H.; Deng, H.-X.; Tu, S.; Langdon, W.Y.; Yang, H.; Tao, L.; Li, Y.; et al. Tyrosine phosphorylation of NLRP3 by the Src family kinase Lyn suppresses the activity of the NLRP3 inflammasome. Sci. Signal. 2021, 14, eabe3410. [Google Scholar] [CrossRef]
- Tang, J.; Tu, S.; Lin, G.; Guo, H.; Yan, C.; Liu, Q.; Huang, L.; Tang, N.; Xiao, Y.; Pope, R.M.; et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J. Exp. Med. 2020, 217, e20182091. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, X.; Xiao, Y.; Zhang, W.; Wang, Y.; Ma, W.; Wang, X.; Song, P.; Lai, L.; Chen, H.; et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 2019, 38, e100376. [Google Scholar] [CrossRef]
- Niu, T.; De Rosny, C.; Chautard, S.; Rey, A.; Patoli, D.; Groslambert, M.; Cosson, C.; Lagrange, B.; Zhang, Z.; Visvikis, O.; et al. NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly. Nat. Commun. 2021, 12, 5862. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Wang, Y.; Tian, T.; Jia, P.; Ye, Y.; He, M.; Yang, Z.; Li, C.; Guo, D.; et al. CCDC50 suppresses NLRP3 inflammasome activity by mediating autophagic degradation of NLRP3. EMBO Rep. 2022, 23, e54453. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Tian, T.; Jia, P.; Lin, Y.; Li, Z.; Wang, Y.; Ye, Y.; Li, C.; Guo, D. The regulation of NLRP3 inflammasome activation by CCDC50-mediated autophagy. Autophagy 2023, 19, 365–366. [Google Scholar] [CrossRef]
- Wang, L.; Cai, J.; Zhao, X.; Ma, L.; Zeng, P.; Zhou, L.; Liu, Y.; Yang, S.; Cai, Z.; Zhang, S.; et al. Palmitoylation prevents sustained inflammation by limiting NLRP3 inflammasome activation through chaperone-mediated autophagy. Mol. Cell 2023, 83, 281–297.e10. [Google Scholar] [CrossRef]
- Wang, G.F.; Ji, J.; EI-Kasmi, F.; Dangl, J.L.; Johal, G.; Balint-Kurti, P.J. Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity. Mackey D, editor. PLoS Pathog. 2015, 11, e1004674. [Google Scholar]
- Hu, Z.; Yan, C.; Liu, P.; Huang, Z.; Ma, R.; Zhang, C.; Wang, R.; Zhang, Y.; Martinon, F.; Miao, D.; et al. Crystal Structure of NLRC4 Reveals Its Autoinhibition Mechanism. Science 2013, 341, 172–175. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Poyet, J.-L.; Srinivasula, S.M.; Tnani, M.; Razmara, M.; Fernandes-Alnemri, T.; Alnemri, E.S. Identification of Ipaf, a Human Caspase-1-activating Protein Related to Apaf-1. J. Biol. Chem. 2001, 276, 28309–28313. [Google Scholar] [CrossRef] [PubMed]
- Faustin, B.; Lartigue, L.; Bruey, J.-M.; Luciano, F.; Sergienko, E.; Bailly-Maitre, B.; Volkmann, N.; Hanein, D.; Rouiller, I.; Reed, J.C. Reconstituted NALP1 Inflammasome Reveals Two-Step Mechanism of Caspase-1 Activation. Mol. Cell 2007, 25, 713–724. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, M.; Imamura, R.; Kinoshita, T.; Kondo, C.; Konaka, K.; Suda, T. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int. Immunol. 2004, 16, 777–786. [Google Scholar] [CrossRef]
- Murphy, N.; Grehan, B.; Lynch, M.A. Glial Uptake of Amyloid Beta Induces NLRP3 Inflammasome Formation via Cathepsin-Dependent Degradation of NLRP10. NeuroMolecular Med. 2014, 16, 205–215. [Google Scholar] [CrossRef]
- Próchnicki, T.; Vasconcelos, M.B.; Robinson, K.S.; Mangan, M.S.J.; De Graaf, D.; Shkarina, K.; Lovotti, M.; Standke, L.; Kaiser, R.; Stahl, R.; et al. Mitochondrial damage activates the NLRP10 inflammasome. Nat. Immunol. 2023, 24, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Matico, R.E.; Miller, R.; Chauhan, D.; Van Schoubroeck, B.; Grauwen, K.; Suarez, J.; Pietrak, B.; Haloi, N.; Yin, Y.; et al. Structural basis for the oligomerization-facilitated NLRP3 activation. Nat. Commun. 2024, 15, 1164. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Kamitsukasa, Y.; Ishida, H.; Zhang, Z.; Murakami, K.; Hirama, C.; Maekawa, S.; Shimizu, T. Structural basis for the oligomerization-mediated regulation of NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2121353119. [Google Scholar] [CrossRef]
- Andreeva, L.; David, L.; Rawson, S.; Shen, C.; Pasricha, T.; Pelegrin, P.; Wu, H. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 2021, 184, 6299–6312.e22. [Google Scholar] [CrossRef]
- Xiao, L.; Magupalli, V.G.; Wu, H. Cryo-EM structures of the active NLRP3 inflammasome disc. Nature 2023, 613, 595–600. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, J.; Cai, J.; Kelley, N.; He, Y. The leucine-rich repeat (LRR) domain of NLRP3 is required for NLRP3 inflammasome activation in macrophages. J. Biol. Chem. 2022, 298, 102717. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016, 17, 250–258. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Schmid-Burgk, J.L.; Chauhan, D.; Schmidt, T.; Ebert, T.S.; Reinhardt, J.; Endl, E.; Hornung, V. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 103–109. [Google Scholar] [CrossRef]
- Caseley, E.A.; Lara-Reyna, S.; Poulter, J.A.; Topping, J.; Carter, C.; Nadat, F.; Spickett, G.P.; Savic, S.; McDermott, M.F. An Atypical Autoinflammatory Disease Due to an LRR Domain NLRP3 Mutation Enhancing Binding to NEK7. J. Clin. Immunol. 2022, 42, 158–170. [Google Scholar] [CrossRef]
- Zheng, S.; Que, X.; Wang, S.; Zhou, Q.; Xing, X.; Chen, L.; Hou, C.; Ma, J.; An, P.; Peng, Y.; et al. ZDHHC5-mediated NLRP3 palmitoylation promotes NLRP3-NEK7 interaction and inflammasome activation. Mol. Cell 2023, 83, 4570–4585.e7. [Google Scholar] [CrossRef]
- Machtens, D.A.; Bresch, I.P.; Eberhage, J.; Reubold, T.F.; Eschenburg, S. The Inflammasome Activity of NLRP3 Is Independent of NEK7 in HEK293 Cells Co-Expressing ASC. Int. J. Mol. Sci. 2022, 23, 10269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, C.-S.; Harrison, K.; Hwang, I.-Y.; Nabar, N.R.; Wang, M.; Kehrl, J.H. AKT Regulates NLRP3 Inflammasome Activation by Phosphorylating NLRP3 Serine 5. J. Immunol. 2020, 205, 2255–2264. [Google Scholar] [CrossRef]
- Mayor, A.; Martinon, F.; De Smedt, T.; Pétrilli, V.; Tschopp, J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 2007, 8, 497–503. [Google Scholar] [CrossRef]
- Xu, G.; Fu, S.; Zhan, X.; Wang, Z.; Zhang, P.; Shi, W.; Qin, N.; Chen, Y.; Wang, C.; Niu, M.; et al. Echinatin effectively protects against NLRP3 inflammasome–driven diseases by targeting HSP90. JCI Investig. 2021, 6, e134601. [Google Scholar] [CrossRef]
- Spel, L.; Hou, C.; Theodoropoulou, K.; Zaffalon, L.; Wang, Z.; Bertoni, A.; Hofer, M.; Gattorno, M.; Martinon, F. HSP90β controls NLRP3 autoactivation. Sci. Adv. 2024, 10, eadj6289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Labrousse, M.; Kevorkian-Verguet, C.; Boursier, G.; Rowczenio, D.; Maurier, F.; Lazaro, E.; Aggarwal, M.; Lemelle, I.; Mura, T.; Belot, A.; et al. Mosaicism in autoinflammatory diseases: Cryopyrin-associated periodic syndromes (CAPS) and beyond. A systematic review. Crit. Rev. Clin. Lab. Sci. 2018, 55, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Cosson, C.; Riou, R.; Patoli, D.; Niu, T.; Rey, A.; Groslambert, M.; De Rosny, C.; Chatre, E.; Allatif, O.; Henry, T.; et al. Functional diversity of NLRP3 gain-of-function mutants associated with CAPS autoinflammation. J. Exp. Med. 2024, 221, e20231200. [Google Scholar] [CrossRef] [PubMed]
- Fayand, A.; Cescato, M.; Le Corre, L.; Terré, A.; Wacheux, M.; Zhu, Y.Y.; Melet, A.; Moreau, T.R.; Bodaghi, B.; Bonnet, F.; et al. Pathogenic variants in the NLRP3 LRR domain at position 861 are responsible for a boost-dependent atypical CAPS phenotype. J. Allergy Clin. Immunol. 2023, 152, 1303–1311.e1. [Google Scholar] [CrossRef] [PubMed]
- Putnam, C.D.; Broderick, L.; Hoffman, H.M. The discovery of NLRP3 and its function in cryopyrin-associated periodic syndromes and innate immunity. Immunol. Rev. 2024, 322, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Gérard, L.; Kuemmerle-Deschner, J.; Lachmann, H.J.; Koné-Paut, I.; Cantarini, L.; Woo, P.; Naselli, A.; Bader-Meunier, B.; Insalaco, A.; et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: A series of 136 patients from the Eurofever Registry. Ann. Rheum. Dis. 2015, 74, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.A.; Silva, C.A.; Segundo, G.R.; Aksentijevich, I.; Fujihira, E.; Watanabe, M.; Carneiro-Sampaio, M.; Duarte, A.J.S.; Oliveira, J.B. Phenotype–Genotype Analysis of Cryopyrin-Associated Periodic Syndromes (CAPS): Description of a Rare Non-Exon 3 and a Novel CIAS1 Missense Mutation. J. Clin. Immunol. 2008, 28, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, T.; Sugiura, H.; Arai, T.; Oh-Ishi, T.; Inamo, Y. Anakinra therapy for CINCA syndrome with a novel mutation in exon 4 of the CIAS1 gene. Acta. Paediatr. 2006, 95, 246–249. [Google Scholar] [CrossRef]
- Tanaka, N.; Izawa, K.; Saito, M.K.; Sakuma, M.; Oshima, K.; Ohara, O.; Nishikomori, R.; Morimoto, T.; Kambe, N.; Goldbach-Mansky, R.; et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: Results of an international multicenter collaborative study. Arthritis Rheum. 2011, 63, 3625–3632. [Google Scholar] [CrossRef]
- Saito, M.; Nishikomori, R.; Kambe, N.; Fujisawa, A.; Tanizaki, H.; Takeichi, K.; Imagawa, T.; Iehara, T.; Takada, H.; Matsubayashi, T.; et al. Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood 2008, 111, 2132–2141. [Google Scholar] [CrossRef]
- Watanabe, M.; Nishikomori, R.; Fujimaki, Y.; Heike, T.; Ohara, A.; Saji, T. Live-attenuated vaccines in a cryopyrin-associated periodic syndrome patient receiving canakinumab treatment during infancy. Clin. Case Rep. 2017, 5, 1750–1755. [Google Scholar] [CrossRef]
- Haverkamp, M.H.; van de Vosse, E.; Goldbach-Mansky, R.; Holland, S.M. Impaired cytokine responses in patients with cryopyrin-associated periodic syndrome (CAPS). Clin. Exp. Immunol. 2014, 177, 720–731. [Google Scholar] [CrossRef]
- Ogawa, B.; Aoki, M.; Ohnishi, H.; Ohashi, T.; Hayashi, H.; Kuze, B.; Ito, Y. The Long-Term Efficacy of Cochlear Implantation for Hearing Loss in Muckel-Wells Syndrome. J. Int. Adv. Otol. 2019, 15, 454–458. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, Z.; Li, D.; Zhang, X.; Li, B.; Zhao, X.; Yan, W.; Wu, B.; Wu, N.; Wang, X.; et al. New retinal findings in NLRP3-associated autoinflammatory disease. Orphanet J. Rare Dis. 2023, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.; Van Kempen, M.J.A.; Kuis, W.; Van Amstel, H.K.P. Variant chronic infantile neurologic, cutaneous, articular syndrome due to a mutation within the leucine-rich repeat domain ofCIAS1. Arthritis Rheum. 2004, 50, 2719–2720. [Google Scholar] [CrossRef]
- Parker, T.; Keddie, S.; Kidd, D.; Lane, T.; Maviki, M.; Hawkins, P.N.; Lachmann, H.J.; Ginsberg, L. Neurology of the cryopyrin-associated periodic fever syndrome. Eur. J. Neurol. 2016, 23, 1145–1151. [Google Scholar] [CrossRef]
- Jéru, I.; Marlin, S.; Le Borgne, G.; Cochet, E.; Normand, S.; Duquesnoy, P.; Moal, F.D.; Cuisset, L.; Hentgen, V.; Alnemri, T.F.; et al. Functional consequences of a germline mutation in the leucine-rich repeat domain of NLRP3 identified in an atypical autoinflammatory disorder. Arthritis Rheum. 2010, 62, 1176–1185. [Google Scholar] [CrossRef]
- Matsuo, T.; Yashiro, M.; Yamasaki, O.; Tanaka, T.; Manki, A. Bilateral Optic Disc Swelling as a Plausible Common Ocular Sign of Autoinflammatory Diseases: Report of Three Patients with Blau Syndrome or Cryopyrin-Associated Periodic Syndrome. Life 2021, 11, 1433. [Google Scholar] [CrossRef]
- Schwarzbach, C.J.; Schmitt, W.H.; Szabo, K.; Bäzner, H.; Hennerici, M.G.; Blahak, C. Chorea in a patient with cryopyrin-associated periodic syndrome. Neurology 2016, 86, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Chae, J.J.; Ross, A.M.; Pinto-Patarroyo, G.; Patel, S.K.; Muskett, J.A.; Ratay, J.S.; Chattaraj, P.; et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E7766–E7775. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Muskett, J.A.; Kim, H.J.; Brewer, C.C.; Griffith, A.J. Gradual Symmetric Progression of DFNA34 Hearing Loss Caused by an NLRP3 Mutation and Cochlear Autoinflammation. Otol. Neurotol. 2018, 39, e181–e185. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, Y.H.; Lee, S.; Han, J.H.; Lee, S.-Y.; Seong, J.; Lee, D.-H.; Park, H.-R.; Carandang, M.; Oh, D.; et al. Otological aspects of NLRP3-related autoinflammatory disorder focusing on the responsiveness to anakinra. Rheumatology 2021, 60, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kociszewska, D.; Vlajkovic, S. Age-Related Hearing Loss: The Link between Inflammaging, Immunosenescence, and Gut Dysbiosis. Int. J. Mol. Sci. 2022, 23, 7348. [Google Scholar] [CrossRef] [PubMed]
- Spitzmaul, G.; Rías, E.; Dionisio, L. Potential Mechanisms of Hearing Loss Due to Impaired Potassium Circulation in the Organ of Corti. In Updates on Hearing Loss and Its Rehabilitation; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Rodrigues, F.; Cuisset, L.; Cador-Rousseau, B.; Giurgea, I.; Neven, B.; Buob, D.; Quartier, P.; Hachulla, E.; Lequerré, T.; Cam, G.; et al. AA amyloidosis complicating cryopyrin-associated periodic syndrome: A study of 86 cases including 23 French patients and systematic review. Rheumatology 2022, 61, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, M.; Hallén, B.; Aldén-Raboisson, M.; Olivecrona, H. PW02-039—Long-term anakinra treatment in CAPS: A metaanalys. Pediatr. Rheumatol. 2013, 11, A180. [Google Scholar] [CrossRef]
- Wikén, M.; Hallén, B.; Kullenberg, T.; Koskinen, L.O. Development and effect of antibodies to anakinra during treatment of severe CAPS: Sub-analysis of a long-term safety and efficacy study. Clin. Rheumatol. 2018, 37, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.D.; Yalamanoglu, A.; Boyman, O. Systematic Review of Safety and Efficacy of IL-1-Targeted Biologics in Treating Immune-Mediated Disorders. Front. Immunol. 2022, 13, 888392. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Lee, J.; Yu, J.-W.; Hyun, Y.-M. NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain. Immune Netw. 2023, 23, e27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Patil, G.; Dayal, S. NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases? Cells 2023, 12, 2709. [Google Scholar] [CrossRef]
- Honda, T.S.B.; Ku, J.; Anders, H.-J. Cell type-specific roles of NLRP3, inflammasome-dependent and -independent, in host defense, sterile necroinflammation, tissue repair, and fibrosis. Front. Immunol. 2023, 14, 1214289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).