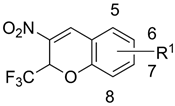
Abstract
The P2Y6 receptor (P2Y6R), a Gq-coupled receptor, is a potential drug discovery target for various inflammatory and degenerative conditions. Antagonists have been shown to attenuate colitis, acute lung injury, etc. In the search for competitive antagonists, we have investigated the SAR of 3-nitro-2-(trifluoromethyl)-2H-chromene derivatives, although high affinity is lacking. We now reveal that long-chain amino-functionalized congeners display greatly enhanced affinity in the antagonism of UDP-induced Ca2+ mobilization in human (h) P2Y6R-transfected 1321N1 astrocytoma cells. A 6-(Boc-amino-n-heptylethynyl) analogue 30 (MRS4940) had an IC50 of 162 nM, which was a 123-fold greater affinity than the corresponding unprotected primary alkylamine, 107-fold greater than the corresponding pivaloyl derivative 30, and 132-fold selective compared to the P2Y14R. However, similar Boc-amino chains attached at the 8-position produced weak µM affinity. Thus, the P2Y6R affinity depended on the chain length, attachment point, and terminal functionality. Off-target activities, at 45 sites, were tested for acylamino derivatives 20, 24, 26, 30, 31, and 37, which showed multiple interactions, particularly at the biogenic amine receptors. The more potent analogues may be suitable for evaluation in inflammation and cancer models, which will be performed in future studies.
1. Introduction
The P2Y6 receptor (P2Y6R) responds to extracellular uridine 5′-diphosphate (UDP) to activate phospholipase C β (PLCβ) and subsequently Ca2+ mobilization through the Gq/11 subfamily of heterotrimeric GTP-binding proteins [1]. UDP is a short-lived, local proinflammatory danger signal, which is cleaved to inactive products by ectonucleotidases [2,3], such as intestinal nucleoside triphosphate diphospho-hydrolase (NTPDase)8 [4]. The P2Y6R occurs in microglia, macrophages, T cells, cardiomyocytes, endothelial cells, and other cell types [5,6,7,8,9], causing the release of inflammatory mediators, such as interleukins IL-6 and IL-8. P2Y6R antagonists are sought for diverse conditions, including diabetes and obesity, pain, cancer, colitis, atherosclerosis, memory loss in aging, psoriasis, and others [8,9,10,11]. P2Y6R knockout (KO) mice, either whole-body or tissue-specific, have been utilized to probe the role of this receptor in various disease models. Both beneficial [4,7,12,13,14] and detrimental effects [15,16,17,18] of genetic knockout (KO) or receptor antagonism have been found.
We have also explored in detail the structure–activity relationship (SAR) of UDP and CDP derivatives as P2Y6R agonists and separated their ability to activate the P2Y6R from activity at the Gi-coupled P2Y14R, which is also UDP-activated [19]. However, the discovery of potent and selective P2Y6R antagonists has lagged behind other purinergic GPCRs, although numerous studies have suggested their potential utility as a drug discovery target [1,4,5,6,8,20,21,22,23,24,25,26,27,28]. Only a few classes of P2Y6R antagonists have been explored. The first selective antagonists were in a series of di-arylisothiocyanates, among which MRS2578 (Figure 1) is the most widely used. This compound does not bind in or near the orthosteric binding site but rather binds covalently to a Cys residue in the intracellular receptor region [6]. This binding induces a mechanism called “redox-induced degradation” by which the receptor is driven to the proteasome for degradation. Another class of P2Y6R antagonists was identified through library screening to discover a 2H-chromene derivative 2a as a reversible P2Y6R antagonist of µM affinity, and its SAR was initially explored by Ito et al. [29]. We have further mapped the SAR, focusing on 6- and 8-position substitutions, e.g., 6,8-difluoro 2b [30,31]. These antagonists contain an alkyl nitro group and are racemic, characteristics that are considered less than optimal for drug development. Nevertheless, we have gradually improved this series’ affinity, including in this work. Machine learning is an alternative approach that was used to identify ABBV-744 3 as a non-surmountable P2Y6R antagonist [32]. Recently, a 2-(1H-pyrazol-3-yl)-1H-benzo[d]imidazole derivative 4 was described as a potent P2Y6R antagonist that attenuated colitis and acute lung injury [33].
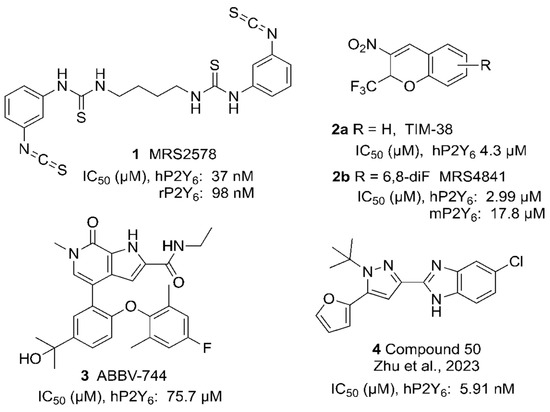
Figure 1.
Selected P2Y6R antagonist structures, with the IC50 values in inhibition of UDP-induced Ca2+ mobilization in hP2Y6R-expressing 1321N1 astrocytoma cells (h, human; r, rat; m, mouse) [33].
This SAR study of the 2H-chromene class of P2Y6R antagonists applies a functionalized congener design approach [34]. Homologous ω-aminoalkyl-functionalized congeners, and their acylated derivatives, of adenosine receptor (AR) ligands were first reported by Jacobson et al. in 1985 as a model for other GPCRs [35]. Strategically chain-extended orthosteric AR agonists and antagonists had enhanced affinity when a terminal amine was present [35]. A later X-ray structure of the A2AAR complex with a xanthine amine congener (XAC) featured its aminoalkyl chain facing outward from the orthosteric binding site and interacting with polar residues [36], as envisioned in an earlier design [35]. A series of aminoalkyl congeners of the M1 muscarinic acetylcholine receptor antagonist telenzepine showed an affinity optimum with a 10-methylene length [37]. We now empirically apply a similar strategy for the P2Y6R, for which there is no experimental structure, as a means of indirectly probing the binding site environment. We considered that the functionalized chains appended might extend into the phospholipid bilayer, as was shown for the A3AR [38].
The list of 2H-chromene analogues compared here for human (h) P2Y6R functional inhibition is shown in Table 1, including reference compounds 2 and 4–18. The primary pharmacological assay consisted of the calcium mobilization measurement in hP2Y6R-expressing 1321N1 astrocytoma cells using a fixed UDP (agonist) concentration of 100 nM [30,31]. The newly prepared derivatives (19–41) were synthesized as shown in Figure 2, with the full synthetic procedures and spectral characterization in the Supplementary Materials. The derivatization concentrated on the 6 and 8 positions of original hit compound 2a using 6-I and 8-I intermediates (10 and 11, respectively) subjected to Sonogashira reactions to install various extended alkyne chains, including those bearing a Boc-amino terminal group. The Boc protection was removed using TFA to yield the corresponding alkyl amines.

Table 1.
The inhibition of UDP (100 nM)-induced Ca2+ mobilization in hP2Y6R stably transfected 1321N1 human astrocytoma cells. a The structure of compound 4 appears in Figure 1.
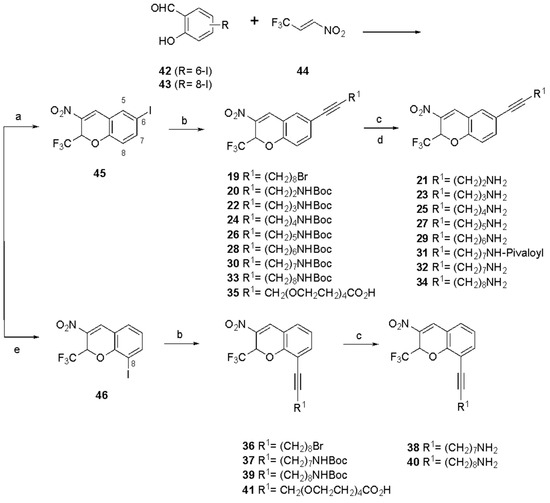
Figure 2.
Synthetic route to P2Y6R antagonists (compounds 19–41). Reagents and conditions: (a) TEA, DCM, rt, 4 h, and 51–89%; (b) alkyne R1-H, PdCl2(PPh3)2, CuI, TEA, DMF, rt, 7 h, and 58–99%; (c) TFA, DCM, rt, and 3 h; (d) pivaloyl chloride, TEA, DMF, rt, and 3 h; and (e) DBU, DMF, μW, 80 °C, 2 h, and 32–99%.
2. Materials and Methods
The representation synthetic methods for compounds 24, 30, and 36 are presented here (Figure 2), and the methods for the other compounds are found in the Supplementary Materials (Figure S1).
2.1. Materials
Unless noted, the reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA). The anhydrous solvents were obtained directly from commercial sources. The Boc-amino-alkynes and other alkynes were obtained from Ambeed (Arlington Heights, IL, USA), except for N-Boc-non-8-yn-1-amine, N-Boc-9-decyn-1-amine, and 10-Br-1-decyne, which were purchased from Acrotein (Hoover, AL, USA). All the reactions were performed using anhydrous solvents and under dry nitrogen. Room temperature or rt refers to 25 ± 2 °C. The 1H-NMR spectra were obtained with a Bruker 400 MHz spectrometer in CDCl3 (7.26 ppm) and MeOD (3.31 ppm). The chemical shifts are expressed as ppm downfield and coupling constants (J) are given in Hz. The TLC analysis utilized glass sheets precoated with silica gel F254 (0.2 mm) from Aldrich. The purity of the final compounds (19–41) was checked using a Hewlett−Packard 1100 HPLC (Agilent Technologies Inc., Palo Alto, CA, USA) equipped with an Agilent Eclipse 5 µm XDB-C18 analytical column (50 × 4.6 mm); the mobile phase: a linear gradient solvent system, 10 mM EAA (triethylammonium acetate):CH3CN from 95:5 to 0:100 in 20 min; and the flow rate was 1.0 mL/min. UV absorption peaks (230, 254, and 280 nm) were detected using a diode array detector. All the derivatives later evaluated pharmacologically showed >95% purity by analytical HPLC. Low-resolution mass spectrometry was performed with a JEOL SX102 spectrometer with 6 kV Xe atoms following desorption from a glycerol matrix or on an Agilent LC/MS 1100 MSD, with an Atlantis C18 column (Waters, Milford, MA, USA). High-resolution mass spectroscopic (HRMS) measurements were carried out on a proteomics-optimized Q-TOF-2 (Micromass-Waters) using external polyalanine calibration and observation of mass accuracies.
2.2. Chemical Synthetic Procedures
tert-Butyl (6-(3-nitro-2-(trifluoromethyl)-2H-chromen-6-yl)hex-5-yn-1-yl)carbamate (24). The compound was synthesized following the same procedure for compound 36, using N-Boc-hex-5-yn-1-amine as the alkyne. The residue was purified by silica gel column chromatography (hexane:ethyl acetate = 90:10) to afford compound 24 (57% yield) as a yellow solid; 1H NMR (400 MHz, methanol-d4) δ 8.32 (d, J = 2.1 Hz, 1H), 7.54 (d, J = 1.9 Hz, 1H), 7.52–7.43 (m, 1H), 7.02 (d, J = 8.4 Hz, 1H), 6.35 (qd, J = 6.5, 1.6 Hz, 1H), ESI-HRMS calcd. m/z for C16H16N2O3F3 341.1113, found 341.1108.
tert-Butyl (9-(3-nitro-2-(trifluoromethyl)-2H-chromen-6-yl)non-8-yn-1-yl)carbamate (30). The compound was synthesized following the same procedure for compound 36, using N-Boc-non-8-yn-1-amine as the alkyne. The residue was purified by silica gel column chromatography (hexane:ethyl acetate = 90:10) to afford the compound 30 (83% yield) as a yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H), 7.44 (dd, J = 8.5, 2.0 Hz, 1H), 7.38 (d, J = 2.0 Hz, 1H), 6.98 (d, J = 8.4 Hz, 1H), 6.07 (q, J = 6.2 Hz, 1H), 4.52 (s, 1H), 3.10 (h, J = 6.3, 5.2 Hz, 4H), 2.37 (t, J = 7.0 Hz, 2H), 2.23 (t, J = 6.9 Hz, 2H), 1.58 (p, J = 7.1 Hz, 2H), 1.43 (s, 9H), 1.38–1.23 (m, 4H). MS (ESI, m/z) 505.2 [M+H]+; ESI-HRMS calcd. m/z for C24H29N2O5F3 505.1926, found 505.1932 [M + H]+.
8-(10-Bromodec-1-yn-1-yl)-3-nitro-2-(trifluoromethyl)-2H-chromene (36). To a mixture of 8-iodo-3-nitro-2-(trifluoromethyl)-2H-chromene (46) (50 mg, 0.134 mmol), PdCl2(PPh3)2 (4.7 mg, 0.0067 mmol), and copper iodide (2.5 mg, 0.0134 mmol) in DMF (3 mL), triethylamine (37 μL, 0.27 mmol) and 10-bromodec-1-yne (44 mg, 0.202 mmol) were added at room temperature under a N2 atmosphere, and then this reaction mixture was stirred at room temperature for 7 h under a N2 atmosphere. This reaction mixture was diluted with water (5 mL) and extracted with ethyl acetate (10 mL × 3). The combined organic extracts were dried over anhydrous Na2SO4, filtered through a pad of Celite, and evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane:ethyl acetate = 90:10) to afford the compound 36 (32 mg, 52%) as a yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.50 (dd, J = 8 Hz, 1H), 7.28 (dd, J = 8 Hz, 1H), 7.03 (t, J = 8 Hz, 1H), 6.14–6.21 (m, 1H), 3.41 (t, J = 8 Hz, 2H), 2.46 (t, J = 8 Hz, 2H), 1.81–1.90 (m, 2H), 1.45–1.51 (m, 8H).
6-Iodo-3-nitro-2-(trifluoromethyl)-2H-chromene (45). To a mixture of 5-iodosalicylaldehyde (42, 0.50 g, 2.01 mmol) and (1E)-3,3,3-trifluoro-1-nitroprop-1-ene (44, 0.31 g, 2.21 mmol) in dichloromethane (10 mL), triethylamine (42 μL, 0.30 mmol) was added. The mixture was stirred at room temperature for 5 h. This reaction mixture was diluted with water (20 mL) and extracted with dichloromethane (10 mL × 3). The combined organic extracts were dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane:ethyl acetate = 20:1) to afford the compound 45 (0.57 g, 77%) as a yellow solid; HPLC purity 99%; 1H NMR (400 MHz, CDCl3) δ 8.00 (s, 1H), 7.70 (dd, J = 2.12 and 8.56 Hz, 1H), 7.65 (d, J = 2.12 Hz, 1H), 6.84 (d, J = 8.56 Hz, 1H), 6.06 (q, J = 6.16 Hz, 1H); 19F NMR (376 MHz, CDCl3) δ −77.69 (d, J = 6.16 Hz, CF3).
8-Iodo-3-nitro-2-(trifluoromethyl)-2H-chromene (46). To a mixture of 2-hydroxy-3-iodobezaldeyde (43, 20 mg, 0.142 mmol) and (1E)-3,3,3-trifluoro-1-nitroprop-1-ene (44, 90 mg, 0.642 mmol) in DMF (2.0 mL), 1,8-diazabicyclo[5.4.0]undec-7-ene (2.0 μL, 0.014 mmol) was added. The mixture was stirred at 80 °C for 2 h in a microwave reactor. This reaction mixture was diluted with water (5 mL) and extracted with ethyl acetate (10 mL × 3). The combined organic extracts were dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane:ethyl acetate = 95:5) to afford the compound 46. Yield = 41%; HPLC purity 99%; 1H NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 7.87 (dd, J = 1.48 and 7.92 Hz, 1H), 7.35 (dd, J = 1.48 and 7.56 Hz, 1H), 6.88 (t, J = 7.72 Hz, 1H), 6.18 (q, J = 6.16 Hz, 1H); 19F NMR (376 MHz, CDCl3) δ −77.78 (d, J = 6.12 Hz, 3F).
2.3. Assay of hP2Y6R-Induced Ca2+ Transients
To screen for P2Y6R antagonist activity, PLC-mediated calcium mobilization assays were carried out using the recombinant human P2Y6R stably expressed in 1321N1 astrocytoma cells (hP2Y6R-1321N1) according to the previously published method [30]. Briefly, the hP2Y6R-1321N1 cells were seeded on 96-well black plates and incubated overnight. The antagonists were pretreated along with the FLIPR dye (Calcium 6 assay kit, Molecular Devices, San Jose, CA, USA) containing 5 mM probenecid for 45 min, prior to agonist addition followed by an immediate intracellular Ca2+ measurement. UDP was used as a P2Y6R agonist (final concentration 100 nM, EC50 13 nM in PLC activation). Here, probenecid was used as an organic anion transporter inhibitor to prevent the efflux of the dye into the extracellular environment. During incubation, the membrane permeable dye enters the cytoplasm and cleaves the acetoxymethyl protection to enable Ca2+ binding. When the P2Y6R was activated by UDP, the increased calcium concentration was directly measured by intracellular changes in the fluorescence intensity with a Fluorescent Imaging Plate Reader (FLIPRTETRA, Molecular Devices, San Jose, CA, USA).
2.4. Assay of hP2Y14R Binding
The affinity of three P2Y6R antagonists was tested at the hP2Y14R using whole-cell binding of a specific fluorescent antagonist MRS4174 [31] in CHO cells stably overexpressing the hP2Y14R (CHO-hP2Y14R). Briefly, 1 × 104 cells were seeded on a 96-well plate for 24 h prior to the assay and incubated at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.5 mg/mL selective antibiotic G418 sulfate. On the day of the assay, three unlabeled P2Y6R antagonists (24, 30, and 37) were diluted separately in the serum-free media with a 10-fold serial dilution (1 nM–100 μM). The cells were first incubated with unlabeled ligands for 30 min at 37 °C and 5% CO2 followed by another 30 min of incubation with 20 nM fluorescent antagonist (MRS 4174). After the incubation, the cells were washed three times with Dulbecco’s phosphate-buffered saline (1X DPBS) without Ca2+/Mg2+ to remove the residual unlabeled and labeled fluorescent ligands. The cells were detached using Cellstripper and resuspended in 1X DPBS for a flow cytometry analysis. The competitive binding assays were performed on a CytoFLEX flow cytometer (Beckman Coulter, CA, USA).
2.5. Cell Culture and Cell Viability
The 1321N1 astrocytoma (hP2Y6R-overexpressing) and HeLa (non-transfected) cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Logan, UT, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS, HyClone, UT, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin in 5% CO2 at 37 °C. The cell viability was determined using an MTT kit (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. Briefly, 1 × 104 cells were seeded in triplicate in 96-well plates. The hP2Y6R-astrocytoma and HeLa cells were treated with a P2Y6R agonist and antagonists at a range of concentrations and incubated for 12 h. The cell viability was measured at 490 nm using a SpectraMax-M5 Spectrometer (Molecular Devices, San Jose, CA, USA).
2.6. Statistical Analysis
The pharmacological parameters were determined using the Prism 10.2.2 software (GraphPad, San Diego, CA, USA). The values are expressed as the mean ± standard error mean. The data were analyzed by analysis of variance (ANOVA) followed by post hoc analysis to check the statistical difference among the groups with p < 0.05 being considered significant.
3. Results
Functional Antagonism
As reported in our previous studies [30,40], the mono-halo substitution of the core 3-nitro-2-(trifluoromethyl)-2H-chromene heterocycle in 5–11 indicates a preference for 8- and 6-position substitution, compared to the 5 and 7 positions (Table 1). A comparison of the triethylsilyl-alkynyl groups placed at the 5, 6, and 8 positions (12–14) is consistent with the substitution preference at the 8 and 6 positions, with sub-µM affinity in derivatives 13 and 14. Furthermore, the presence of a p-substituted phenyl ring with various terminal groups in derivatives 15–18 indicated the relative freedom of substitution at the 6 position, suggesting that it is located in a region on the receptor with no strict steric constraints. Previously, both hydrophilic (e.g., 16 and 17) and hydrophobic terminal groups are tolerated, although none were found to greatly enhance the affinity.
Table 1 shows the structures and P2Y6R inhibitory affinity of newly synthesized (19–41) compounds, as well as their lipophilicity and aqueous solubility predictions. Sigmoidal inhibition curves for selected derivatives appear in Figure 3. There is no indication of P2Y6R agonist activity for any tested antagonists. The high affinity reported for compound 4, in the reduction of IP3 production in response to 10 µM UDP in hP2Y6R-HEK cells [33], was not confirmed in our assay (Ca2+ mobilization in response to 100 nM UDP in hP2Y6R-1321N1 cells). Compound 4 appeared to be only a weak hP2Y6R antagonist (18.9 ± 8.5% inhibition at 100 µM).
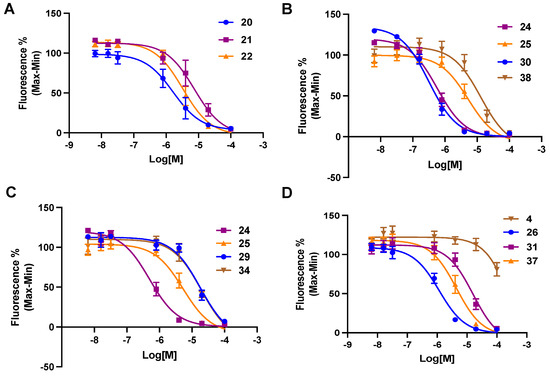
Figure 3.
Representative curves (A–D), single experiments run in triplicate, for inhibition by selected 3-nitro-2-(trifluoromethyl)-2H-chromene derivatives, 20–22, 24–26, 29–31, 34, 37, and 38, and 1H-benzo[d]imidazole derivative 4 of calcium mobilization by agonist UDP (100 nM) in hP2Y6R-expressing astrocytoma cells. Mean IC50 ± SEM (n = 3) values are shown in Table 1.
Given the unknown receptor binding site location for this series, we expanded the SAR by probing the distal changes while maintaining the CF3 and NO2 groups as constant. Instead of a rigid phenyl ring, we introduced flexible alkyl or oxyalkyl (PEG) groups on the anchoring ethynyl moiety. Varying both the length and the terminal functionality of these groups had characteristic effects on the hP2Y6R affinity. The 6 position (19–35) was more extensively modified than the 8 position (36–41). A progression from a chain length of 3 to 8 methylenes extending the ethynyl moiety was examined, and the affinity values are also shown graphically, comparing the terminal primary amines and terminal Boc-amines (Figure S2, Supplementary Materials). The pattern of Boc-amino at the chain terminus (6 position) being more potent than unblocked amino was consistent for different chain lengths (except for 8 methylenes). The progression of affinity with increasing chain length was not smooth (7 > 4 > 3 > 6 > 8 methylenes), which probably indicates specific interactions with groups on the receptor rather than tethering away from the receptor. Thus, there was no clear definition of the environment around the appended chains or evidence that they reach polar head groups of the phospholipid bilayer [38], as was our original hypothesis.
A terminal Br group in 19 and 36 was associated with moderate µM affinity. At the 6 position, the Boc-amino derivatives tended to have greater antagonist potency than the corresponding amino derivatives. The presence of the Boc-amino-protecting group in the 6-position-derivatized 30 enhanced the affinity by 17-fold compared to the primary amino derivative 29. However, removing the Boc-amino-protecting group in the 8-position-derivatized 40 did not affect the affinity, which was weak in both cases (IC50 20–30 µM). Curiously, the extension of Boc derivative 30 by one single carbon in 33 reduced it by 160-fold. At both the 6 and 8 positions, the introduction of a hydrophilic PEG chain terminating in a carboxylic acid greatly reduced the affinity, which is consistent with a mainly hydrophobic environment.
Prediction of the physicochemical and pharmacokinetic properties was performed using the Stardrop Software (Version 7.4.0, Table 1 and Table S1, Supplementary Materials) [41]. Among the reference compounds, nonpolar compounds 2 and 5–14 were predicted to cross the BBB. Among the newly synthesized analogues, only the two ω-bromo-alkyl analogues 19 and 36, both lacking H-bond donors, were predicted to cross the blood–brain barrier (BBB), and all except the carboxylate analogues 35 and 41 were predicted to have intestinal bioavailability. Most other compounds had only one H-bond donor, which contributed to human intestinal permeability and high flexibility. However, the potent compound 30 had low predicted aqueous solubility (0.86 mg/mL), while the ω-aminoalkyl analogues had a predicted aqueous solubility of ~100 mg/mL and pKa of 8.7–9. Although a nitro group is unusual in pharmaceuticals, there are examples of approved drugs containing this functionality [41].
The original lead compound 2a was shown to be P2Y6R-selective compared to all the other P2YRs [29]. The antagonistic affinity at one other P2YR, i.e., hP2Y14R, was determined here for the selected compounds (Figure 4) using a previously reported fluorescent binding assay in whole cells [31]. The P2Y14R affinity was generally weak. Broader off-target binding interactions at 45 different membrane-bound proteins were assayed by the NIMH Psychoactive Drug Screening Program (PDSP) [42] for acylamino derivatives 20, 24, 26, 30, 31, and 37 (Table 2 and Table S2). Curiously, while the highest affinity P2Y6R antagonist 30 displayed seven off-target interactions, including relatively potent interactions at the α2A- and α2B-adrenergic receptors, the corresponding pivaloyl derivative 31 (only one O atom different) had only two weak interactions (Ki, µM): α2A, 3.5; σ2, 3.3. In contrast, 6,8-difluoro analogue 2b had no off-target interactions.
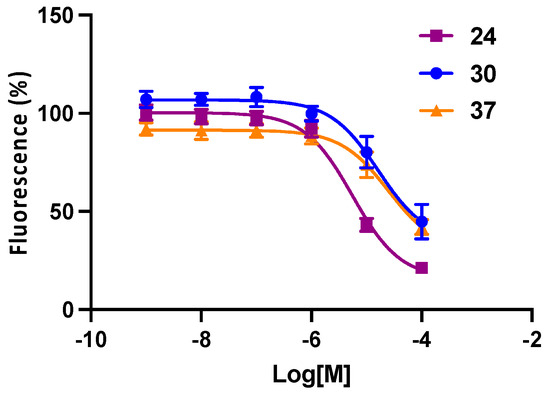
Figure 4.
Affinity of selected P2Y6R antagonists in a fluorescent assay of hP2Y14R binding; screening for CHO-hP2Y14 (flow cytometer, competitive binding) by the method described [31]. Mean IC50 ± SEM (n = 3) values (µM) were as follows: 24, 5.6 ± 0.7; 30, 21.4 ± 5.8; and 37, 31.2 ± 12.2.

Table 2.
Off-target interactions of selected antagonists, determined in binding assays at 45 target sites by the PDSP (mean, n = 2) [42].
Although previous members of this chemical series were shown to be inactive in hP2Y14R binding, including 2a, 7, 8, 15, and 16 [29,43], three Boc-containing antagonists, 24, 30, and 37, displayed < µM IC50 values (Figure 4). The most potent hP2Y6R antagonists in this series, 24 and 30, were 10- and 132-fold selective, respectively, compared to the hP2Y14R. The inhibition at most other P2YRs by 2a, 13, 14, and 17 was shown to be minor at high µM concentrations [29,43].
Potent P2Y6R agonist 5-iodouridine-5′-O-diphosphate (MRS2693, EC50 15 nM in PLC activation) and three antagonists were tested for effects on cell viability using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay [44] in two cell lines, hP2Y6R-1321N1 human astrocytoma cells and HeLa cells (Figure 5, Table S3). The minor effects, following 12 h exposure, were slightly greater in the HeLa cells than in the astrocytoma cells. The agonist (≤10 µM) had no significant effect in the astrocytoma cells, but there was a modest increased (4–6%) viability at ≥1 µM in the HeLa cells. The antagonist MRS4841 2b [43] had only minor inhibition of viability at 10 µM (22% in the HeLa cells and 12% in the hP2Y6R-1321N1 cells). However, MRS4940 30 at 1 µM inhibited viability by 29% in the HeLa cells and 23% in the hP2Y6R-1321N1 cells. The minor cell viability inhibition by 2b and 30 at concentrations higher than their receptor affinity appeared to be dose-dependent, but there was no indication of P2Y6R-dependent effects on proliferation. Thus, we expect that this series of antagonists will be useful as pharmacological tool compounds.
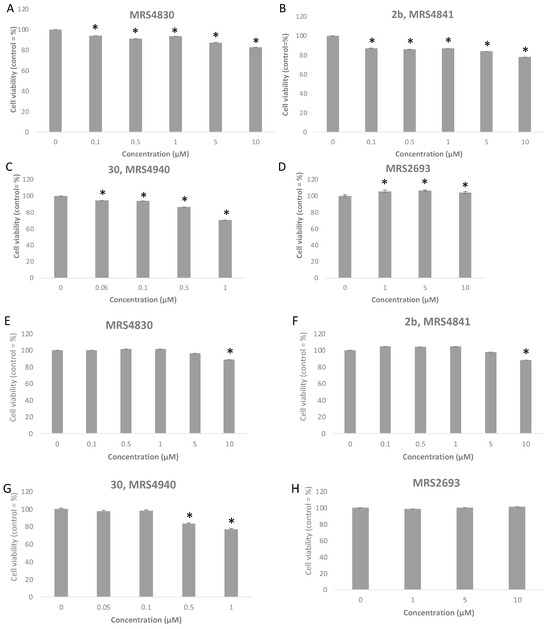
Figure 5.
Cell viability of two cell lines (HeLa, (A–D); P2Y6R-astrocytoma, (E–H)) treated with antagonists MRS4830 (6-fluoro-3-nitro-2-(trifluoromethyl)-2H-chromene), 2b, and 30 and with agonist MRS2693 at a range of concentrations up to 10 µM, following exposure for 12 h. Viability was determined using the MTT assay [44], showing mean ± SEM as a percentage of control. * p < 0.05. Percent data are in Supplementary Materials.
4. Discussion
In conclusion, we followed our previous results that the 2H-chromenes could be derivatized most effectively, with respect to receptor affinity, at the 6 and 8 positions. Further SAR exploration of 2H-chromene derivatives as P2Y6R antagonists has led to the identification of potent analogues containing an extended alkyl chain terminating in various functional groups, either charged or protected with a Boc-amino group. Curiously, the Boc-protected analogues were consistently more potent than the free amines, suggesting a hydrophobic environment around the extended chains, unlike earlier studies of GPCR-functionalized congeners. Consistently, carboxylates on extended polar PEG chains greatly reduced the affinity. The affinity was not dependent on the methylene chain length. The highest affinity was observed for the 6-(Boc-aminoheptyl) analogue MRS4940 30 (IC50 162 nM) and 4-(Boc-aminobutyl) analogue MRS4944 24 (IC50 568 nM). Compound 30 was 132-fold selective compared to the P2Y14R. When the identical chain found in 30 was placed at the 8 position in 37, the affinity was 31-fold lower. However, 30 also slightly inhibited the cell viability at 2.5 µM (15-fold higher than the IC50) and displayed off-target interactions with two adrenergic receptors. The µM (or in some cases with greater affinity) off-target inhibition of binding at various receptors by multiple analogues, mainly α-adrenergic and dopamine receptors, is also a limitation of the use of these compounds. Multiple compounds at 10 µM displayed a small reduction in the cell viability in two cancer cell lines.
5. Conclusions
We have improved the affinity of previous 3-nitro-2-(trifluoromethyl)-2H-chromene derivatives as P2Y6R antagonists. The addition of a favored 6-ethynyl-linked chain indicated a hydrophobic receptor environment surrounding the pharmacophore. Although the P2Y6R affinity depended on the chain length, attachment point, and terminal functionality of the added chain, there was not a smooth progression of affinity with increased length. Nevertheless, some patterns were evident, such as the high affinity of Boc-protected amines. Thus, despite the limitations of this 2H-chromene series, and given the difficulty of discovery of new chemotypes as antagonists, some of these analogues may now be evaluated further to probe the applicability of P2Y6R antagonists for potential treatment of neurodegeneration, cancer, and inflammatory and other conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cells13161366/s1, Additional synthesis of P2Y6 antagonists, pharmacological procedures, and results prediction using StarDrop software, version 7.6.1. Figure S1: Synthetic methods for non-chromene derivative 4; Figure S2: Dependence of affinity on chain length and terminal group; Table S1: StarDrop (calculation of ADMET properties); Table S2: PDSP off-target screening; Table S3: Cell viability data.
Author Contributions
Conceptualization, discussion, and interpretation of results: P.O., A.P., J.M.M., and K.A.J.; performed experiments: P.O., A.P., Y.-H.J., S.A.L., and J.H.P.; writing and editing: P.O., A.P., J.M.M., and K.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIDDK Intramural Research Program grant number ZIADK031116.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Acknowledgments
Supported by the NIDDK Intramural Research grant ZIADK31116. We thank Bryan L. Roth, National Institute of Mental Health’s Psychoactive Drug Screening Program (Univ. North Carolina at Chapel Hill, Contract # HHSN-271-2008-00025-C) for the screening data.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashida, M.; Iqbal, J. Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5′-nucleotidase, and alkaline phosphatase inhibitors. Med. Res. Rev. 2014, 34, 703–743. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.B.; Lovászi, M.; Braganhol, E.; Pacher, P.; Haskó, G. Ectonucleotidases in inflammation, immunity, and cancer. J. Immunol. 2021, 206, 1983–1990. [Google Scholar] [CrossRef]
- Salem, M.; Lecka, J.; Pelletier, J.; Gomes Marconato, D.; Dumas, A.; Vallières, L.; Brochu, G.; Robaye, B.; Jobin, C.; Sévigny, J. NTPDase8 protects mice from intestinal inflammation by limiting P2Y6 receptor activation: Identification of a new pathway of inflammation for the potential treatment of IBD. Gut 2022, 71, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wihlborg, A.K.; Balogh, J.; Wang, L.; Borna, C.; Dou, Y.; Joshi, B.V.; Lazarowski, E.; Jacobson, K.A.; Arner, A.; Erlinge, D. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of utp in man during myocardial infarction. Circ. Res. 2006, 98, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Nishimura, A.; Shimoda, K.; Tanaka, T.; Kato, Y.; Shibata, T.; Tanaka, H.; Kurose, H.; Azuma, Y.T.; Ihara, H.; et al. Redox-dependent internalization of the purinergic P2Y6 receptor limits colitis progression. Sci. Signal. 2022, 15, eabj0644. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Shigemoto-Mogami, Y.; Nasu-Tada, K.; Shinozaki, Y.; Ohsawa, K.; Tsuda, M.; Joshi, B.V.; Jacobson, K.A.; Kohsaka, S.; Inoue, K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 2007, 446, 1091–1095. [Google Scholar] [CrossRef]
- Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y purinergic receptors, endothelial dysfunction, and cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 6855. [Google Scholar] [CrossRef] [PubMed]
- Kauffenstein, G.; Tamareille, S.; Prunier, F.; Roy, C.; Ayer, A.; Toutain, B.; Billaud, M.; Isakson, B.E.; Grimaud, L.; Loufrani, L.; et al. Central role of P2Y6 UDP receptor in arteriolar myogenic tone. Arterioscleros. Thromb. Vasc. Biol. 2016, 36, 1598–1606. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Yang, G. P2Y6 receptor mediated cell proliferation and migration in human hepatocellular carcinoma cells. Purinergic Signal. 2011, 7, 413–420. [Google Scholar]
- Yin, L.; Zhang, E.; Mao, T.; Zhu, Y.; Ni, S.; Li, Y.; Liu, C.; Fang, Y.; Ni, K.; Lu, Y.; et al. Macrophage P2Y6R activation aggravates psoriatic inflammation through IL-27-mediated Th1 responses. Acta Pharmaceut. Sinica B, 2024; in press. [Google Scholar] [CrossRef]
- Mamedova, L.; Joshi, B.V.; Gao, Z.G.; von Kügelgen, I.; Jacobson, K.A. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem. Pharmacol. 2004, 67, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Milde, S.; Brown, G.C. Knockout of the p2y6 receptor prevents peri-infarct neuronal loss after transient, focal ischemia in mouse brain. Int. J. Mol. Sci. 2022, 23, 2304. [Google Scholar] [CrossRef] [PubMed]
- Dundee, J.M.; Puigdellívol, M.; Butler, R.; Cockram, T.O.J.; Brown, G.C. p2y6 receptor-dependent microglial phagocytosis of synapses mediates synaptic and memory loss in aging. Aging Cell 2023, 22, e13761. [Google Scholar] [CrossRef] [PubMed]
- Clouet, S.; Di Pietrantonio, L.; Daskalopoulos, E.P.; Esfahani, H.; Horckmans, M.; Vanorlé, M.; Lemaire, A.; Balligand, J.L.; Beauloye, C.; Boeynaems, J.M.; et al. Loss of mouse P2Y6 nucleotide receptor is associated with physiological macrocardia and amplified pathological cardiac hypertrophy. J. Biol. Chem. 2016, 291, 15841–15852. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Nishimura, A.; Sunggip, C.; Ito, T.; Nishiyama, K.; Kato, Y.; Tanaka, T.; Tozaki-Saitoh, H.; Tsuda, M.; Nishida, M. Modulation of P2Y6R expression exacerbates pressure overload-induced cardiac remodeling in mice. Sci. Rep. 2020, 10, 13926. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Shinozaki, Y.; Kashiwagi, K.; Shigetomi, E.; Robaye, B.; Koizumi, S. Müller cell-mediated neurite outgrowth of the retinal ganglion cells via P2Y6 receptor signals. J. Neurochem. 2016, 136, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.F.; Singh, V.; Dixit, M.; Ginsburg-Shmuel, T.; Fonseca, B.; Pintor, J.; Youdim, M.B.H.; Fischer, B. A promising drug candidate for the treatment of glaucoma based on a P2Y6-receptor agonist. Purinergic Signal. 2018, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Oliva, P.; Scortichini, M.; Dobelmann, C.; Jain, S.; Gopinatth, V.; Toti, K.S.; Phung, N.B.; Junker, A.; Jacobson, K.A. Structure-activity relationships of pyrimidine nucleotides containing a 5′-α, β-methylene diphosphonate at the P2Y6 receptor. Bioorg. Med. Chem. Lett. 2021, 45, 128137. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Paeger, L.; Bremser, S.; Evers, N.; Hinze, Y.; Idzko, M.; Kloppenburg, P.; Brüning, J.C. Hypothalamic UDP increases in obesity and promotes feeding via P2Y6-dependent activation of AgRP neurons. Cell 2015, 162, 1404–1417. [Google Scholar] [CrossRef]
- Jain, S.; Pydi, S.P.; Toti, K.S.; Robaye, B.; Idzko, M.; Gavrilova, O.; Wess, J.; Jacobson, K.A. Lack of adipocyte purinergic P2Y6 receptor greatly improves whole body glucose homeostasis. Proc. Nat. Acad. Sci. USA 2020, 117, 30763–30774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, W.; Li, Y.; Zhang, Q.; Ji, H.; Li, H.; Hu, Q. The role of P2Y6R in cardiovascular diseases and recent development of P2Y6R antagonists. Drug Disc. Today 2020, 25, 568–573. [Google Scholar] [CrossRef]
- Milde, S.; van Tartwijk, F.W.; Vilalta, A.; Hornik, T.C.; Dundee, J.M.; Puigdellívol, M.; Brown, G.C. Inflammatory neuronal loss in the substantia nigra induced by systemic lipopolysaccharide is prevented by knockout of the P2Y6 receptor in mice. J. Neuroinflamm. 2021, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Zhang, Y.; Liu, Y.; Li, Q.; Tang, H.; Liu, Q. P2Y6 receptor-mediated spinal microglial activation in neuropathic pain. Pain Res. Manag. 2019, 2019, 2612534. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.; Sharda, A.; Warburton, R.; Weinberg, E.O.; Dong, J.; Fang, M.; Sahagian, G.G.; Chen, T.; Xue, C.; Castellot, J.J.; et al. A purinergic P2Y6 receptor agonist prodrug modulates airway inflammation, remodeling, and hyperreactivity in a mouse model of asthma. J. Asthma Allergy 2018, 11, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Placet, M.; Arguin, G.; Molle, C.M.; Babeu, J.P.; Jones, C.; Carrier, J.C.; Robaye, B.; Geha, S.; Boudreau, F.; Gendron, F.P. The G protein-coupled P2Y₆ receptor promotes colorectal cancer tumorigenesis by inhibiting apoptosis. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864 Pt A, 1539–1551. [Google Scholar] [CrossRef]
- Wan, H.; Xie, R.; Xu, J.; He, J.; Tang, B.; Liu, Q.; Wang, S.; Guo, Y.; Yang, X.; Dong, T.X.; et al. Anti-proliferative effects of nucleotides on gastric cancer via a novel P2Y6/SOCE/Ca2+/β-catenin pathway. Sci. Rep. 2017, 7, 2459. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Yan, M.; Search, D.; Zhang, R.; Carson, N.L.; Ryan, C.S.; Smith-Monroy, C.; Zheng, J.; Chen, J.; Kong, Y.; et al. P2Y6 receptor potentiates pro-inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS ONE 2014, 9, e111385. [Google Scholar] [CrossRef]
- Ito, M.; Egashira, S.I.; Yoshida, K.; Mineno, T.; Kumagai, K.; Kojima, H.; Okabe, T.; Nagano, T.; Ui, M.; Matsuoka, I. Identification of novel selective P2Y6 receptor antagonists by high-throughput screening assay. Life Sci. 2017, 180, 137–142. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jain, S.; Gopinatth, V.; Phung, N.B.; Gao, Z.G.; Jacobson, K.A. Structure activity relationship of 3-nitro-2-(trifluoromethyl)-2H-chromene derivatives as P2Y6 receptor antagonists. Bioorg. Med. Chem. Lett. 2021, 41, 128008. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Salmaso, V.; Wen, Z.; Bennett, J.M.; Phung, N.B.; Lieberman, D.I.; Gopinatth, V.; Randle, J.C.R.; Chen, Z.; Salvemini, D. Structure-activity relationships of heterocyclic P2Y14 receptor antagonists: Removal of the zwitterionic character with piperidine bioisosteres. J. Med. Chem. 2021, 64, 5099–5122. [Google Scholar] [CrossRef]
- Puhl, A.C.; Lewicki, S.A.; Gao, Z.G.; Pramanik, A.; Makarov, V.; Ekins, S.; Jacobson, K.A. Use of machine learning to search for ligands of P2Y6 and other P2Y receptors. Purinergic Signal. 2024, in press. [CrossRef]
- Zhu, Y.; Zhou, M.; Cheng, X.; Wang, H.; Li, Y.; Guo, Y.; Wang, Y.; Tian, S.; Mao, T.; Zhang, Z.; et al. Discovery of selective P2Y6R antagonists with high affinity and in vivo efficacy for inflammatory disease therapy. J. Med. Chem. 2023, 66, 6315–6332. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A. Functionalized congener approach to the design of ligands for G protein–coupled receptors (GPCRs). Bioconjug. Chem. 2009, 20, 1816–1835. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Ukena, D.; Padgett, W.; Kirk, K.L.; Daly, J.W. Molecular probes for extracellular adenosine receptors. Biochem. Pharmacol. 1987, 36, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef]
- Karton, Y.; Baumgold, J.; Handen, J.S.; Jacobson, K.A. Molecular probes for muscarinic receptors: Derivatives of the M1-antagonist telenzepine. Bioconjug. Chem. 1992, 3, 234–240. [Google Scholar] [CrossRef]
- Pradhan, B.; Pavan, M.; Fisher, C.L.; Salmaso, V.; Wan, T.C.; Keyes, R.F.; Rollison, N.; Suresh, R.R.; Kumar, T.S.; Gao, Z.G.; et al. Lipid trolling to optimize A3 adenosine receptor positive allosteric modulators (PAMs). J. Med. Chem. 2024, 67, 12221–12247. [Google Scholar] [CrossRef] [PubMed]
- Robaye, B.; Boeynaems, J.M.; Communi, D. Slow desensitization of the human P2Y6 receptor. Eur. J. Pharmacol. 1997, 329, 231–236. [Google Scholar] [CrossRef]
- Jung, Y.H.; Shah, Q.; Lewicki, S.A.; Pramanik, A.; Gopinatth, V.; Pelletier, J.; Sévigny, J.; Iqbal, J.; Jacobson, K.A. Synthesis and pharmacological characterization of multiply substituted 2H-chromene derivatives as P2Y6 receptor antagonists. Bioorg. Med. Chem. Lett. 2022, 75, 128981. [Google Scholar] [CrossRef]
- Segall, M.D. Multi-parameter optimization: Identifying high quality compounds with a balance of properties. Curr. Pharmaceut. Des. 2012, 18, 1292–1310. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Besnard, J.; Ruda, G.F.; Setola, V.; Abecassis, K.; Rodriguiz, R.M.; Huang, X.P.; Norval, S.; Sassano, M.F.; Shin, A.I.; Webster, L.A.; et al. Automated design of ligands to polypharmacological profiles. Nature 2012, 492, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).