Soluble CD40 Ligand as a Promising Biomarker in Cancer Diagnosis
Abstract
1. Introduction
2. Biomarker Discovery and Significance
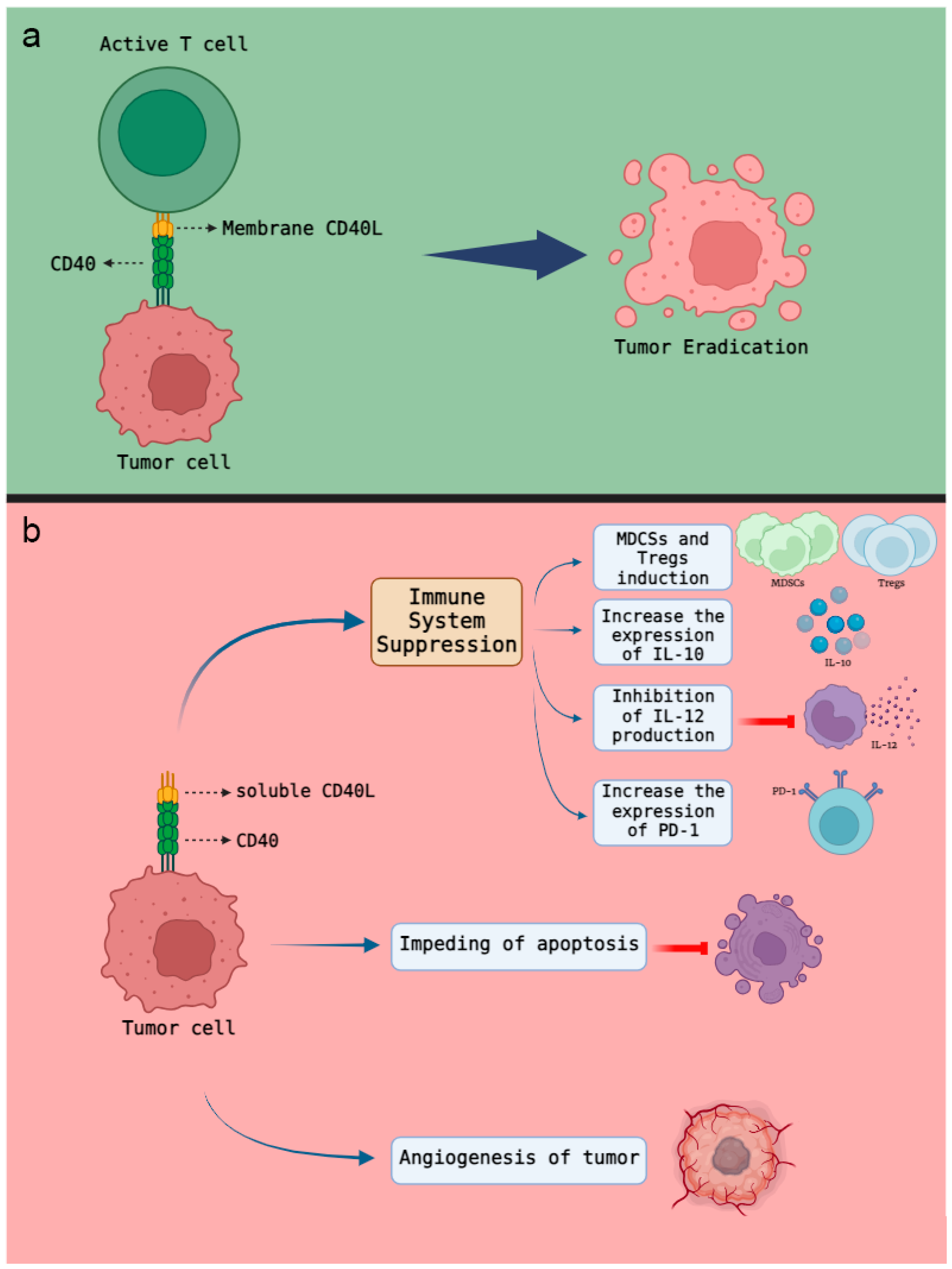
3. CD40 Ligand and Cancer
4. CD40 Roles in Inflammatory Responses
5. CD40 Interaction with Tumor Cells
6. sCD40L as a Biomarker
7. Clinical Applications and Future Directions
8. Cellular and Gene Therapy Based on CD40L
9. Recombinant Proteins Based on CD40L
10. Overview of the Clinical Effectiveness of CD40L-Targeted Therapy
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciardiello, F.; Arnold, D.; Casali, P.G.; Cervantes, A.; Douillard, J.-Y.; Eggermont, A.; Eniu, A.; McGregor, K.; Peters, S.; Piccart, M.; et al. Delivering precision medicine in oncology today and in future—The promise and challenges of personalised cancer medicine: A position paper by the European Society for Medical Oncology (ESMO). Ann. Oncol. 2014, 25, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Biomarkers: Hopes and challenges in the path from discovery to clinical practice. Transl. Res. 2012, 159, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Immune-related biomarkers in myocardial infarction; diagnostic/prognostic value and therapeutic potential. J. Biochem. Mol. Toxicol. 2023, 37, e23489. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Matsushima, M.; Hashimoto, N.; Imaizumi, K.; Hasegawa, Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011, 73, 69. [Google Scholar] [PubMed]
- Zhang, B.; Wu, T.; Chen, M.; Zhou, Y.; Yi, D.; Guo, R. The CD40/CD40L system: A new therapeutic target for disease. Immunol. Lett. 2013, 153, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Vanderkerken, M. Molecular Interactions Controlling Immune Homeostasis in the Marginal Zone. Ph.D. Thesis, Ghent University, Gent, Belgium, 2019. [Google Scholar]
- Jimenez-Luna, C.; Torres, C.; Ortiz, R.; Dieguez, C.; Martinez-Galan, J.; Melguizo, C.; Prados, J.C.; Caba, O. Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget 2018, 9, 16573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohan, A.; Guleria, R. Biomarkers in cancer screening, research and detection: Present and future: A review. Biomarkers 2006, 11, 385–405. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, H.; Li, H. Medical imaging biomarker discovery and integration towards AI-based personalized radiotherapy. Front. Oncol. 2022, 11, 764665. [Google Scholar] [CrossRef]
- Sethi, S.; Ali, S.; Philip, P.A.; Sarkar, F.H. Clinical advances in molecular biomarkers for cancer diagnosis and therapy. Int. J. Mol. Sci. 2013, 14, 14771–14784. [Google Scholar] [CrossRef] [PubMed]
- Abu-Asab, M.S.; Chaouchi, M.; Alesci, S.; Galli, S.; Laassri, M.; Cheema, A.K.; Atouf, F.; VanMeter, J.; Amri, H. Biomarkers in the age of omics: Time for a systems biology approach. Omics J. Integr. Biol. 2011, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P. Cancer biomarkers: Can we turn recent failures into success? J. Natl. Cancer Inst. 2010, 102, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef]
- Vermersch, P.; Berger, T.; Gold, R.; Lukas, C.; Rovira, A.; Meesen, B.; Chard, D.; Comabella, M.; Palace, J.; Trojano, M. The clinical perspective: How to personalise treatment in MS and how may biomarkers including imaging contribute to this? Mult. Scler. J. 2016, 22 (Suppl. 2), 18–33. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.M.; Leehans, A.; Ravishankar, P.; Daily, A. Multiomic Approaches for Cancer Biomarker Discovery in Liquid Biopsies: Advances and Challenges. Biomark. Insights 2023, 18, 11772719231204508. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, A.; Corrales, F.; Čolović, M.; Krstić, D.; Oliver-Martos, B.; Martínez-Cáceres, E.; Jakasa, I.; Gajski, G.; Brun, V.; Kyriacou, K. Analytical techniques for multiplex analysis of protein biomarkers. Expert. Rev. Proteom. 2020, 17, 257–273. [Google Scholar] [CrossRef] [PubMed]
- McKean, W.B.; Moser, J.C.; Rimm, D.; Hu-Lieskovan, S. Biomarkers in precision cancer immunotherapy: Promise and challenges. Am. Soc. Clin. Oncol. Educ. Book. 2020, 40, e275–e291. [Google Scholar] [CrossRef]
- Bonassi, S.; Neri, M.; Puntoni, R. Validation of biomarkers as early predictors of disease. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Ostermann, A.I.; Newman, J.W.; Schebb, N.H. MS-based targeted metabolomics of eicosanoids and other oxylipins: Analytical and inter-individual variabilities. Free Radic. Biol. Med. 2019, 144, 72–89. [Google Scholar] [CrossRef]
- Fujimoto, H.; Fukuzato, S.; Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Reduced Relapse-Free Survival in Colorectal Cancer Patients with Elevated Soluble CD40 Ligand Levels Improved by Vitamin D Supplementation. Nutrients 2023, 15, 4361. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef]
- Korniluk, A.; Kemona, H.; Dymicka-Piekarska, V. Multifunctional CD40L: Pro- and anti-neoplastic activity. Tumour Biol. 2014, 35, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Sjövik, C.; Soop, A.; Hållström, L.; Frostell, C.; Pisetsky, D.S.; Wallén, H. CD40L expression in plasma of volunteers following LPS administration: A comparison between assay of CD40L on platelet microvesicles and soluble CD40L. Platelets 2015, 26, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Monteiro de Oliveira Novaes, J.A.; Hirz, T.; Guijarro, I.; Nilsson, M.; Pisegna, M.A.; Poteete, A.; Barsoumian, H.B.; Fradette, J.J.; Chen, L.N.; Gibbons, D.L.; et al. Targeting of CD40 and PD-L1 Pathways Inhibits Progression of Oral Premalignant Lesions in a Carcinogen-induced Model of Oral Squamous Cell Carcinoma. Cancer Prev. Res. 2021, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Soong, R.S.; Song, L.; Trieu, J.; Lee, S.Y.; He, L.; Tsai, Y.C.; Wu, T.C.; Hung, C.F. Direct T cell activation via CD40 ligand generates high avidity CD8+ T cells capable of breaking immunological tolerance for the control of tumors. PLoS ONE 2014, 9, e93162. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Zhang, Y.; Zeng, W.; Wang, S.; Ji, P.; Pan, M.; Zhu, C.; Wang, Y. Distinct roles of ICOS and CD40L in human T-B cell adhesion and antibody production. Cell Immunol. 2021, 368, 104420. [Google Scholar] [CrossRef]
- Dustin, M.L. Help to go: T cells transfer CD40L to antigen-presenting B cells. Eur. J. Immunol. 2017, 47, 31–34. [Google Scholar] [CrossRef]
- Laman, J.D.; Claassen, E.; Noelle, R.J. Functions of CD40 and Its Ligand, gp39 (CD40L). Crit. Rev. Immunol. 2017, 37, 371–420. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.J.; Sohn, H.J.; Kim, T.G. CD40 ligand stimulation affects the number and memory phenotypes of human peripheral CD8(+) T cells. BMC Immunol. 2023, 24, 15. [Google Scholar] [CrossRef]
- Kuhn, N.F.; Lopez, A.V.; Li, X.; Cai, W.; Daniyan, A.F.; Brentjens, R.J. CD103(+) cDC1 and endogenous CD8(+) T cells are necessary for improved CD40L-overexpressing CAR T cell antitumor function. Nat. Commun. 2020, 11, 6171. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Wan, P.; Tan, X.; Xiang, Y.; Tong, H.; Yu, M. PI3K/AKT and CD40L Signaling Regulate Platelet Activation and Endothelial Cell Damage in Sepsis. Inflammation 2018, 41, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, N.; Seijkens, T.; Lievens, D.; Kuijpers, M.J.; Winkels, H.; Projahn, D.; Hartwig, H.; Beckers, L.; Megens, R.T.; Boon, L.; et al. Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arter. Thromb. Vasc. Biol. 2016, 36, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, L.A.; Bosch, L.; Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. The CD40-CD40L Dyad as Immunotherapeutic Target in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2021, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef]
- Luri-Rey, C.; Gomis, G.; Glez-Vaz, J.; Manzanal, A.; Martinez Riaño, A.; Rodriguez Ruiz, M.E.; Teijeira, A.; Melero, I. Cytotoxicity as a form of immunogenic cell death leading to efficient tumor antigen cross-priming. Immunological Reviews 2024, 321, 143–151. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol. Rev. 2020, 296, 24–35. [Google Scholar] [CrossRef]
- Ritter, A.T.; Shtengel, G.; Xu, C.S.; Weigel, A.; Hoffman, D.P.; Freeman, M.; Iyer, N.; Alivodej, N.; Ackerman, D.; Voskoboinik, I.; et al. ESCRT-mediated membrane repair protects tumor-derived cells against T cell attack. Science 2022, 376, 377–382. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, S.; Li, B.; Sun, L.; Li, H.; Lin, P.; Wang, S.; Teng, W.; Zhou, X.; et al. Decitabine Enhances Vγ9Vδ2 T Cell-Mediated Cytotoxic Effects on Osteosarcoma Cells via the NKG2DL-NKG2D Axis. Front. Immunol. 2018, 9, 1239. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, V.; Zurawski, S.; Montes, M.; Kroll, M.; Bouteau, A.; Wang, Z.; Ellis, J.; Igyártó, B.Z.; Lévy, Y.; Zurawski, G. Anti-CD40 Antibody Fused to CD40 Ligand Is a Superagonist Platform for Adjuvant Intrinsic DC-Targeting Vaccines. Front. Immunol. 2021, 12, 786144. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Pastor Bandeira, I.; de Almeida Franzoi, A.E.; Murillo Wollmann, G.; de Medeiros Junior, W.L.G.; Nogueira Brandão, W.; Schatzmann Peron, J.P.; Becker, J.; Nascimento, O.J.M.; Magno Gonçalves, M.V. Interleukin-31 and soluble CD40L: New candidate serum biomarkers that predict therapeutic response in multiple sclerosis. Neurol. Sci. 2022, 43, 6271–6278. [Google Scholar] [CrossRef]
- Barbé-Tuana, F.M.; Klein, D.; Ichii, H.; Berman, D.M.; Coffey, L.; Kenyon, N.S.; Ricordi, C.; Pastori, R.L. CD40–CD40 Ligand Interaction Activates Proinflammatory Pathways in Pancreatic Islets. Diabetes 2006, 55, 2437–2445. [Google Scholar] [CrossRef]
- Yan, C.; Saleh, N.; Yang, J.; Nebhan, C.A.; Vilgelm, A.E.; Reddy, E.P.; Roland, J.T.; Johnson, D.B.; Chen, S.-C.; Shattuck-Brandt, R.L.; et al. Novel induction of CD40 expression by tumor cells with RAS/RAF/PI3K pathway inhibition augments response to checkpoint blockade. Mol. Cancer 2021, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Iulianna, T.; Kuldeep, N.; Eric, F. The Achilles’ heel of cancer: Targeting tumors via lysosome-induced immunogenic cell death. Cell Death Dis. 2022, 13, 509. [Google Scholar] [CrossRef]
- Troitskaya, O.S.; Novak, D.D.; Richter, V.A.; Koval, O.A. Immunogenic Cell Death in Cancer Therapy. Acta Naturae 2022, 14, 40–53. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Becherini, C.; Lancia, A.; Detti, B.; Lucidi, S.; Scartoni, D.; Ingrosso, G.; Carnevale, M.G.; Roghi, M.; Bertini, N.; Orsatti, C.; et al. Modulation of tumor-associated macrophage activity with radiation therapy: A systematic review. Strahlenther. Onkol. 2023, 199, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; Patel, M.; Kema, I.; Vyboh, K.; Kanagaratham, C.; Radzioch, D.; Thébault, P.; Lapointe, R.; Gilmore, N.; Ancuta, P.; et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T cell expansion in HIV infection. Clin. Exp. Immunol. 2014, 178, 102–111. [Google Scholar] [CrossRef]
- Chand Dakal, T.; Dhabhai, B.; Agarwal, D.; Gupta, R.; Nagda, G.; Meena, A.R.; Dhakar, R.; Menon, A.; Mathur, R.; Mona; et al. Mechanistic basis of co-stimulatory CD40-CD40L ligation mediated regulation of immune responses in cancer and autoimmune disorders. Immunobiology 2020, 225, 151899. [Google Scholar] [CrossRef] [PubMed]
- Djureinovic, D.; Wang, M.; Kluger, H.M. Agonistic CD40 Antibodies in Cancer Treatment. Cancers 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jochems, C.; Talaie, T.; Anderson, A.; Jales, A.; Tsang, K.Y.; Madan, R.A.; Gulley, J.L.; Schlom, J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012, 120, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Frankish, J.; Mukherjee, D.; Romano, E.; Billian-Frey, K.; Schröder, M.; Heinonen, K.; Merz, C.; Redondo Müller, M.; Gieffers, C.; Hill, O.; et al. The CD40 agonist HERA-CD40L results in enhanced activation of antigen presenting cells, promoting an anti-tumor effect alone and in combination with radiotherapy. Front. Immunol. 2023, 14, 1160116. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.J., Jr.; Fisher, D.A.; Wallace, P.K.; Channon, J.Y.; Noelle, R.J.; Gui, J.; Ernstoff, M.S. A Randomized Trial of Ex vivo CD40L Activation of a Dendritic Cell Vaccine in Colorectal Cancer Patients: Tumor-Specific Immune Responses Are Associated with Improved Survival. Clin. Cancer Res. 2010, 16, 5548–5556. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Richmond, A. Hiding in the dark: Pan-cancer characterization of expression and clinical relevance of CD40 to immune checkpoint blockade therapy. Mol. Cancer 2021, 20, 146. [Google Scholar] [CrossRef]
- Salomon, R.; Dahan, R. Next Generation CD40 Agonistic Antibodies for Cancer Immunotherapy. Front. Immunol. 2022, 13, 940674. [Google Scholar] [CrossRef]
- Grioni, M.; Brevi, A.; Cattaneo, E.; Rovida, A.; Bordini, J.; Bertilaccio, M.T.S.; Ponzoni, M.; Casorati, G.; Dellabona, P.; Ghia, P.; et al. CD4+ T cells sustain aggressive chronic lymphocytic leukemia in Eμ-TCL1 mice through a CD40L-independent mechanism. Blood Adv. 2021, 5, 2817–2828. [Google Scholar] [CrossRef]
- Biagi, E.; Popat, U.; Raphael, R.; Eric, Y.; Giampietro, D.; Lawrence, R.; Andreef, M.; Brenner, M. Immunotherapy of Chronic Lymphocytic Leukemia using CD40L and IL2 Expressing Autologous Tumor Cells. Blood 2004, 104, 768. [Google Scholar] [CrossRef]
- Ara, A.; Ahmed, K.A.; Xiang, J. Multiple effects of CD40-CD40L axis in immunity against infection and cancer. Immunotargets Ther. 2018, 7, 55–61. [Google Scholar] [CrossRef]
- Grazia, G.A.; Bastos, D.R.; Villa, L.L. CD40/CD40L expression and its prognostic value in cervical cancer. Braz. J. Med. Biol. Res. 2023, 56, e13047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Y.; Han, X.; Yan, J.; Wu, Y.; Song, P.; Wang, Y.; Li, X.; Zhang, H. Functional 2D Iron-Based Nanosheets for Synergistic Immunotherapy, Phototherapy, and Chemotherapy of Tumor. Adv. Healthc. Mater. 2022, 11, 2200776. [Google Scholar] [CrossRef]
- Angelou, A.; Antoniou, E.; Garmpis, N.; Damaskos, C.; Theocharis, S.; Margonis, G.A. The Role of Soluble CD40L Ligand in Human Carcinogenesis. Anticancer. Res. 2018, 38, 3199–3201. [Google Scholar] [CrossRef] [PubMed]
- Caggiari, L.; Guidoboni, M.; Vaccher, E.; Barzan, L.; Franchin, G.; Gloghini, A.; Martorelli, D.; Zancai, P.; Bortolin, M.T.; Mazzucato, M.; et al. High serum levels of soluble CD40-L in patients with undifferentiated nasopharyngeal carcinoma: Pathogenic and clinical relevance. Infect. Agent. Cancer 2007, 2, 5. [Google Scholar] [CrossRef]
- Lima, P.M.A.; Torres, L.C.; Martins, M.R.; da Matta, M.C.; Lima, J.T.O.; de Mello, M.J.G.; da Silva, L.M.; Cintra, E.B., Jr.; Lira, C.C.R.; da Fonte, E.J.A.; et al. Soluble levels of sCD40L and s4-1BB are associated with a poor prognosis in elderly patients with colorectal cancer. J. Surg. Oncol. 2020, 121, 901–905. [Google Scholar] [CrossRef]
- Mazzei, G.J.; Edgerton, M.D.; Losberger, C.; Lecoanet-Henchoz, S.; Graber, P.; Durandy, A.; Gauchat, J.F.; Bernard, A.; Allet, B.; Bonnefoy, J.Y. Recombinant soluble trimeric CD40 ligand is biologically active. J. Biol. Chem. 1995, 270, 7025–7028. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Yang, J.; Martens, J.W.; Mills, E.A.; Saad, A.; Chilukuri, P.; Dowling, C.A.; Mao-Draayer, Y. Elevated sCD40L in Secondary Progressive Multiple Sclerosis in Comparison to Non-progressive Benign and Relapsing Remitting Multiple Sclerosis. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211050712. [Google Scholar] [CrossRef]
- Younes, A.; Snell, V.; Consoli, U.; Clodi, K.; Zhao, S.; Palmer, J.L.; Thomas, E.K.; Armitage, R.J.; Andreeff, M. Elevated levels of biologically active soluble CD40 ligand in the serum of patients with chronic lymphocytic leukaemia. Br. J. Haematol. 1998, 100, 135–141. [Google Scholar] [CrossRef]
- Schlom, J.; Jochems, C.; Gulley, J.L.; Huang, J. The role of soluble CD40L in immunosuppression. Oncoimmunology 2013, 2, e22546. [Google Scholar] [CrossRef]
- Mielczarek-Palacz, A.; Sikora, J.; Kondera-Anasz, Z.; Hauza, G. Imbalance in serum soluble CD30/CD30L and CD40/CD40L systems are associated with ovarian tumors. Hum. Immunol. 2013, 74, 70–74. [Google Scholar] [CrossRef]
- Tsirakis, G.; Pappa, C.A.; Psarakis, F.E.; Fragioudaki, M.; Tsioutis, C.; Stavroulaki, E.; Boula, A.; Alexandrakis, M.G. Serum concentrations and clinical significance of soluble CD40 ligand in patients with multiple myeloma. Med. Oncol. 2012, 29, 2396–2401. [Google Scholar] [CrossRef]
- Roselli, M.; Mineo, T.C.; Basili, S.; Martini, F.; Mariotti, S.; Aloe, S.; Del Monte, G.; Ambrogi, V.; Spila, A.; Palmirotta, R.; et al. Soluble CD40 ligand plasma levels in lung cancer. Clin. Cancer Res. 2004, 10, 610–614. [Google Scholar] [CrossRef]
- Holzer, G.; Pfandlsteiner, T.; Blahovec, H.; Trieb, K.; Kotz, R. Serum concentrations of sCD30 and sCD40L in patients with malignant bone tumours. Wien. Med. Wochenschr. 2003, 153, 40–42. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.C.; Pang, X.Q.; Hua, C.; Li, L.; Zhang, X.G. Expression of CD40 and CD40L in gastric cancer tissue and its clinical significance. Int. J. Mol. Sci. 2009, 10, 3900–3917. [Google Scholar] [CrossRef]
- Eltaher, S.M.; El-Gil, R.; Fouad, N.; Mitwali, R.; El-Kholy, H. Evaluation of serum levels and significance of soluble CD40 ligand in screening patients with hepatitis C virus-related hepatocellular carcinoma. East. Mediterr. Health J. 2016, 22, 603–610. [Google Scholar] [CrossRef]
- Chung, H.W.; Lim, J.B. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. J. Transl. Med. 2014, 12, 102. [Google Scholar] [CrossRef]
- Herold, Z.; Herold, M.; Herczeg, G.; Fodor, A.; Szasz, A.M.; Dank, M.; Somogyi, A. High plasma CD40 ligand level is associated with more advanced stages and worse prognosis in colorectal cancer. World J. Clin. Cases 2022, 10, 4084–4096. [Google Scholar] [CrossRef]
- Silva, R.; Torres, L.C.; da Fonte, E.J.A.; Mello, M.J.G.; Lima, J.T.O.; da Matta, M.C. Analysis of physical activity and plasma levels of soluble CD40 and CD40L in older people with gastrointestinal tract cancer. Exp. Gerontol. 2022, 160, 111677. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Van Nuffel, A.M.T.; Benteyn, D.; Corthals, J.; Aerts, C.; Heirman, C.; Van Riet, I.; Bonehill, A.; Thielemans, K.; Neyns, B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 2013, 24, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Van Nuffel, A.M.; Corthals, J.; Heirman, C.; Tuyaerts, S.; Benteyn, D.; De Coninck, A.; Van Riet, I.; Verfaillie, G.; Vandeloo, J.; et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J. Immunother. 2011, 34, 448–456. [Google Scholar] [CrossRef]
- Gray, J.E.; Chiappori, A.; Williams, C.C.; Tanvetyanon, T.; Haura, E.B.; Creelan, B.C.; Kim, J.; Boyle, T.A.; Pinder-Schenck, M.; Khalil, F.; et al. A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunol. Immunother. 2018, 67, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Creelan, B.C.; Antonia, S.; Noyes, D.; Hunter, T.B.; Simon, G.R.; Bepler, G.; Williams, C.C.; Tanvetyanon, T.; Haura, E.B.; Schell, M.J.; et al. Phase II trial of a GM-CSF-producing and CD40L-expressing bystander cell line combined with an allogeneic tumor cell-based vaccine for refractory lung adenocarcinoma. J. Immunother. 2013, 36, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wierda, W.G.; Cantwell, M.J.; Woods, S.J.; Rassenti, L.Z.; Prussak, C.E.; Kipps, T.J. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000, 96, 2917–2924. [Google Scholar] [CrossRef]
- Wierda, W.G.; Castro, J.E.; Aguillon, R.; Sampath, D.; Jalayer, A.; McMannis, J.; Prussak, C.E.; Keating, M.; Kipps, T.J. A phase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154. Leukemia 2010, 24, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, P.U.; Loskog, A.S.; Lindqvist, C.A.; Mangsbo, S.M.; Fransson, M.; Wanders, A.; Gardmark, T.; Totterman, T.H. AdCD40L immunogene therapy for bladder carcinoma--the first phase I/IIa trial. Clin. Cancer Res. 2010, 16, 3279–3287. [Google Scholar] [CrossRef]
- Schiza, A.; Wenthe, J.; Mangsbo, S.; Eriksson, E.; Nilsson, A.; Totterman, T.H.; Loskog, A.; Ullenhag, G. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J. Transl. Med. 2017, 15, 79. [Google Scholar] [CrossRef]
- Loskog, A.; Maleka, A.; Mangsbo, S.; Svensson, E.; Lundberg, C.; Nilsson, A.; Krause, J.; Agnarsdottir, M.; Sundin, A.; Ahlstrom, H.; et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br. J. Cancer 2016, 114, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.F.; Purdon, T.J.; van Leeuwen, D.G.; Lopez, A.V.; Curran, K.J.; Daniyan, A.F.; Brentjens, R.J. CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response. Cancer Cell 2019, 35, 473–488. [Google Scholar] [CrossRef]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Moreno, R.; Alemany, R.; Loskog, A. IL-6 Signaling Blockade during CD40-Mediated Immune Activation Favors Antitumor Factors by Reducing TGF-beta, Collagen Type I, and PD-L1/PD-1. J. Immunol. 2019, 202, 787–798. [Google Scholar] [CrossRef]
- Funakoshi, S.; Longo, D.L.; Beckwith, M.; Conley, D.K.; Tsarfaty, G.; Tsarfaty, I.; Armitage, R.J.; Fanslow, W.C.; Spriggs, M.K.; Murphy, W.J. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood 1994, 83, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Longo, D.L.; Taub, D.D.; Ferris, D.K.; Young, L.S.; Eliopoulos, A.G.; Agathanggelou, A.; Cullen, N.; Macartney, J.; Fanslow, W.C.; et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 1999, 93, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H.; Dutcher, J.P.; Anderson, J.E.; Eckhardt, S.G.; Stephans, K.F.; Razvillas, B.; Garl, S.; Butine, M.D.; Perry, V.P.; Armitage, R.J.; et al. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001, 19, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, M.; Richards, D.M.; Heinonen, K.; Kluge, M.; Marschall, V.; Merz, C.; Redondo Muller, M.; Schnyder, T.; Sefrin, J.P.; Sykora, J.; et al. A Single-Chain-Based Hexavalent CD27 Agonist Enhances T Cell Activation and Induces Anti-Tumor Immunity. Front. Oncol. 2018, 8, 387. [Google Scholar] [CrossRef]
- Merz, C.; Sykora, J.; Marschall, V.; Richards, D.M.; Heinonen, K.; Redondo Muller, M.; Thiemann, M.; Schnyder, T.; Fricke, H.; Hill, O.; et al. The Hexavalent CD40 Agonist HERA-CD40L Induces T-Cell-mediated Antitumor Immune Response Through Activation of Antigen-presenting Cells. J. Immunother. 2018, 41, 385–398. [Google Scholar] [CrossRef]
- Gieffers, C.; Kluge, M.; Merz, C.; Sykora, J.; Thiemann, M.; Schaal, R.; Fischer, C.; Branschadel, M.; Abhari, B.A.; Hohenberger, P.; et al. APG350 induces superior clustering of TRAIL receptors and shows therapeutic antitumor efficacy independent of cross-linking via Fcgamma receptors. Mol. Cancer Ther. 2013, 12, 2735–2747. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.M.; Marschall, V.; Billian-Frey, K.; Heinonen, K.; Merz, C.; Redondo Muller, M.; Sefrin, J.P.; Schroder, M.; Sykora, J.; Fricke, H.; et al. HERA-GITRL activates T cells and promotes anti-tumor efficacy independent of FcgammaR-binding functionality. J. Immunother. Cancer 2019, 7, 191. [Google Scholar] [CrossRef]
- Lin, J.H.; Huffman, A.P.; Wattenberg, M.M.; Walter, D.M.; Carpenter, E.L.; Feldser, D.M.; Beatty, G.L.; Furth, E.E.; Vonderheide, R.H. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 2020, 217, e20190673. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Schmittnaegel, M.; Rigamonti, N.; Pais-Ferreira, D.; Mueller, P.; Buchi, M.; Ooi, C.H.; Kreuzaler, M.; Hirschmann, P.; Guichard, A.; et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.H.; Diamond, M.S.; Hay, C.A.; Byrne, K.T.; Vonderheide, R.H. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc. Natl. Acad. Sci. USA 2020, 117, 8022–8031. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Role of CD40L | Immune Cells Involved | Treatment Sensitization | References |
|---|---|---|---|---|
| Breast cancer |
| Dendritic cells, Macrophages | … | [53,54] |
| ||||
| ||||
| Lung cancer |
| T cells, NK cells, Macrophages | Chemotherapy, Radiotherapy | [54,55] |
| ||||
| ||||
| Prostate cancer |
| Immune cells, Macrophages | … | [56] |
| ||||
| ||||
| Colorectal cancer |
| Dendritic cells, T cells | … | [54,57] |
| ||||
| ||||
| Melanoma |
| T cells, NK cells | … | [54,58] |
| ||||
| Bladder cancer |
| T cells, NK cells, Macrophages | Chemotherapy, Radiotherapy | [54,59] |
| ||||
| ||||
| Non-Hodgkin lymphoma |
| Dendritic cells, T cells | … | [54,60] |
| ||||
| ||||
| Leukemia |
| Immune cells, Macrophages | … | [61,62] |
| ||||
| ||||
| Pancreatic cancer |
| Dendritic cells, Macrophages | … | [63] |
| ||||
| ||||
| Liver cancer |
| T cells, NK cells, Macrophages | Chemotherapy, Radiotherapy | [63] |
| ||||
| ||||
| Stomach cancer |
| Dendritic cells, T cells | … | [63,64] |
| ||||
| ||||
| Ovarian cancer |
| T cells, NK cells | … | [59,63] |
| ||||
| Cervical cancer |
| Immune cells, Macrophages | … | [58] |
| ||||
|
| Cancer Type | sCD40L Level | Measured in | Note | References |
|---|---|---|---|---|
| Prostate cancer | Serum levels N/A (Elevated) | Serum | No significant difference was observed in pre- and post-treatment outcomes of the PSA targeting vaccine. | [55] |
| Breast cancer | Serum levels N/A (Elevated) | Serum | [55] | |
| Gastric cancer | Patients: 3.57 ± 1.63 ng/mL Standards: 1.94 ± 0.86 ng/mL p value < 0.01 (Elevated) a | Serum | sCD40L inhibits cancer cell growth and apoptosis. | [65,77] |
| Malignant bone tumors | Patients: 1.9 ± 1.7 ng/mL Standards: 0.037 ± 0.04 ng/mL p value < 0.05 (Elevated) a | Serum | [76] | |
| Hepatitis C virus-related hepatocellular carcinoma | Patients: 9462 ± 2385 pg/mL Standards: 3280 ± 938 pg/mL p value < 0.001 (Elevated) a | Serum |
| [78] |
| ||||
| Multiple myeloma | Patients: 710.8 pg/mL Standards: N/A p value < 0.001 (Elevated) b | Serum |
| [74] |
| ||||
| Nasopharyngeal carcinoma | Patients: 15.2 ± 6.4 ng/mL Standards: 6.3 ± 3.6 ng/mL p value < 0.001 (Elevated) a | Serum | sCD40L levels may be useful in identifying UNPC patients with occult distant metastases. | [66] |
| Ovarian cancer | Patients: 0.056 ± 0.043 ng/mL Standards: 0.21 ± 0.008 ng/mL p value < 0.001 (Elevated) a | Serum Ovarian cyst fluid |
| [73] |
| ||||
| Pancreatic ductal adenocarcinoma | Patients: 30,044.2 ± 9747.9 ng/mL Standards: 9170.5 ± 5449.8 ng/mL p value < 0.001 (Elevated) a | Serum | Serum sCD40L is correlated with immunosuppression and angiogenesis in PDAC carcinogenesis/progression and is a promising diagnostic and prognostic biomarker for PDAC superior to CA19-9 and CEA. | [79] |
| Lung cancer | Patients: 0.46 (0.18–0.96) ng/mL Standards: 0.13 (0.05–0.44) ng/mL p value < 0.001 (Elevated) c | Plasma | sCD40L levels were significantly higher in squamous cancer compared with adenocarcinoma. | [75] |
| Chronic lymphocytic leukemia | Patients: 0.80 ng/mL Standard: 0.29 ng/mL p value < 0.001(Elevated) b | Serum | sCD40L inhibits apoptosis by interfering with the downstream signaling of Fas and its ligands. | [71] |
| Colorectal cancer | Plasma levels N/A (Elevated) | Plasma | The level of sCD40L in the plasma is linked to disease progression and the development of distant metastases. | [67,80] |
| Older people with gastrointestinal tract cancer | Plasma level N/A (Reduced) | Plasma | … | [81] |
| Drug/Intervention | Brief Summary | Phase | Condition | Study Registration Date | Study Record Updates (Last Verified) | Enrollment (Actual) | NCT |
|---|---|---|---|---|---|---|---|
| CD40 ligand expressing MSLN-CAR T cells | CD40 Ligand Expressing MSLN-CAR T Cell Therapy in MSLN Positive Advanced/Metastatic Solid Tumors | Phase 1 Phase 2 | Advanced or Metastatic Solid Tumors | 20 January 2023 | May 2024 | 30 | NCT05693844 |
| Autologous B-CLL vaccine expressing CD40L and IL2 | Treatment of B-Chronic Lymphocytic Leukemia (B-CLL) with Autologous CD40 Ligand and IL-2-Expressing Tumor Cells | Phase 1 | Chronic Lymphocytic Leukemia (CLL) | December 2006 | 1 January 2014 | 15 | NCT00458679 |
| Autologous IL2 and CD40 Ligand-Expressing Tumor Cells + Lenalidomide | Treatment of B-CLL with Autologous IL2 and CD40 Ligand-Expressing Tumor Cells + Lenalidomide | Phase 1 | Chronic Lymphocytic Leukemia (CLL) | February 2013 | February 2016 | 15 | NCT01604031 |
| Combination of Flt3L And CD40L | Treatment Of Patients with Metastatic Melanoma and Renal Cancer with a Combination of Flt3L and CD40L | Phase 1 | Kidney Cancer Melanoma | March 2001 | April 2002 | unknown | NCT00020540 |
| Recombinant CD40-ligand | Vaccination of HLA-A1 and/or -A2+ Stage III or IV Melanoma Patients with Tumor Peptide—Loaded Autologous Dendritic Cells That Are Generated in the Absence or Presence of CD40 Ligand | Phase 1 Phase 2 | Melanoma | October 2002 | May 2015 | 62 | NCT00053391 |
| MEM-288 Intratumoral Injection | Intratumoral injection of MEM-288, conditionally replicative oncolytic adenovirus vector encoding transgenes for human interferon beta (IFNβ) and a recombinant chimeric form of CD40-ligand (MEM40) plus Nivolumab | Phase 1 | Solid Tumors | 23 December 1996 | 4 September 2014 | 61 | NCT00001564 |
| MEDI5083 | MEDI5083 is a novel fusion protein containing a hexameric recombinant human CD40L structure covalently linked to human IgG4p Fc | Phase 1 | Advanced Solid Tumors | 21 March 2017 | July 2020 | 204 | NCT03089645 |
| GM.CD40L | GM-CSF-Producing and CD40L-Expressing Bystander Cell Line (GM.CD40L) Vaccine in Combination with CCL21 for Patients with Stage IV Adenocarcinoma of the Lung | Phase 1 Phase 2 | Lung Cancer Adenocarcinoma | 26 March 2012 | August 2019 | 73 | NCT01433172 |
| LOAd703 | Oncolytic adenovirus serotype 5/35 encoding TMZ-CD40L and 4-1BBL | Phase 1 Phase 2 | Pancreatic Adenocarcinoma Ovarian Cancer Biliary Carcinoma Colorectal Cancer | 1 March 2018 | January 2024 | 50 | NCT03225989 |
| Avrend | Trimeric soluble human CD40L | Phase 1 | Advanced solid tumors NHL | unknown | unknown | unknown | unknown |
| Ad-CD40L | Adenovirus carrying human CD40L gene | Phase 1 Phase 2 | Malignant Melanoma | September 2011 | February 2016 | 30 | NCT01455259 |
| Ad-CD40L-CLL cells | Autologous CLL B Cells Transduced to Express Chimeric CD154 (ISF35) | Phase 1 | chronic Lymphocytic Leukemia | June 2006 | October 2008 | 9 | NCT00779883 |
| TriMixDC-MEL | A Phase II Trial Using a Universal GM-CSF-Producing and CD40L-Expressing Bystander Cell Line (GM.CD40L) in the Formulation of Autologous Tumor Cell-Based Vaccines for Patients with Malignant Melanoma | Phase 2 | Melanoma | October 2004 | September 2012 | 43 | NCT00101166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazoki, A.; Dadfar, S.; Shadab, A.; Haghmorad, D.; Oksenych, V. Soluble CD40 Ligand as a Promising Biomarker in Cancer Diagnosis. Cells 2024, 13, 1267. https://doi.org/10.3390/cells13151267
Pazoki A, Dadfar S, Shadab A, Haghmorad D, Oksenych V. Soluble CD40 Ligand as a Promising Biomarker in Cancer Diagnosis. Cells. 2024; 13(15):1267. https://doi.org/10.3390/cells13151267
Chicago/Turabian StylePazoki, Alireza, Sepehr Dadfar, Alireza Shadab, Dariush Haghmorad, and Valentyn Oksenych. 2024. "Soluble CD40 Ligand as a Promising Biomarker in Cancer Diagnosis" Cells 13, no. 15: 1267. https://doi.org/10.3390/cells13151267
APA StylePazoki, A., Dadfar, S., Shadab, A., Haghmorad, D., & Oksenych, V. (2024). Soluble CD40 Ligand as a Promising Biomarker in Cancer Diagnosis. Cells, 13(15), 1267. https://doi.org/10.3390/cells13151267


_Wang.png)








