Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Recombinant Protein Expression and Click Chemistry
2.3. Drugs and Reagents
2.4. Antibodies
2.5. Binding and Internalization Assays
2.6. Immunofluorescence
2.7. Infectivity Assays
2.8. PLA2 Assay
2.9. Rab9 Knockdown
2.10. Western Blot
2.11. Statistical Analysis
3. Results
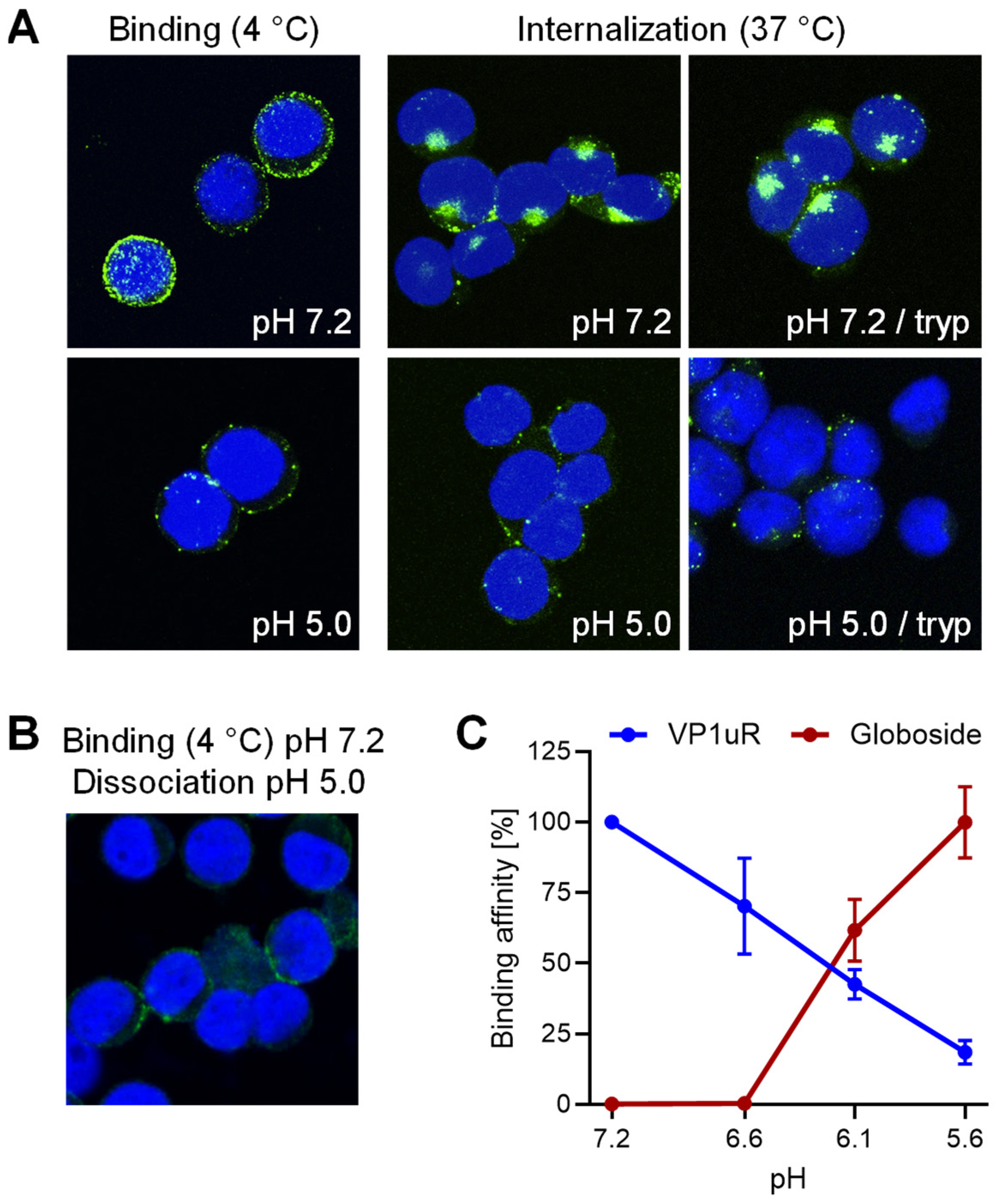
3.1. Interaction of B19V with VP1uR and Globoside Receptors Is Modulated by the pH
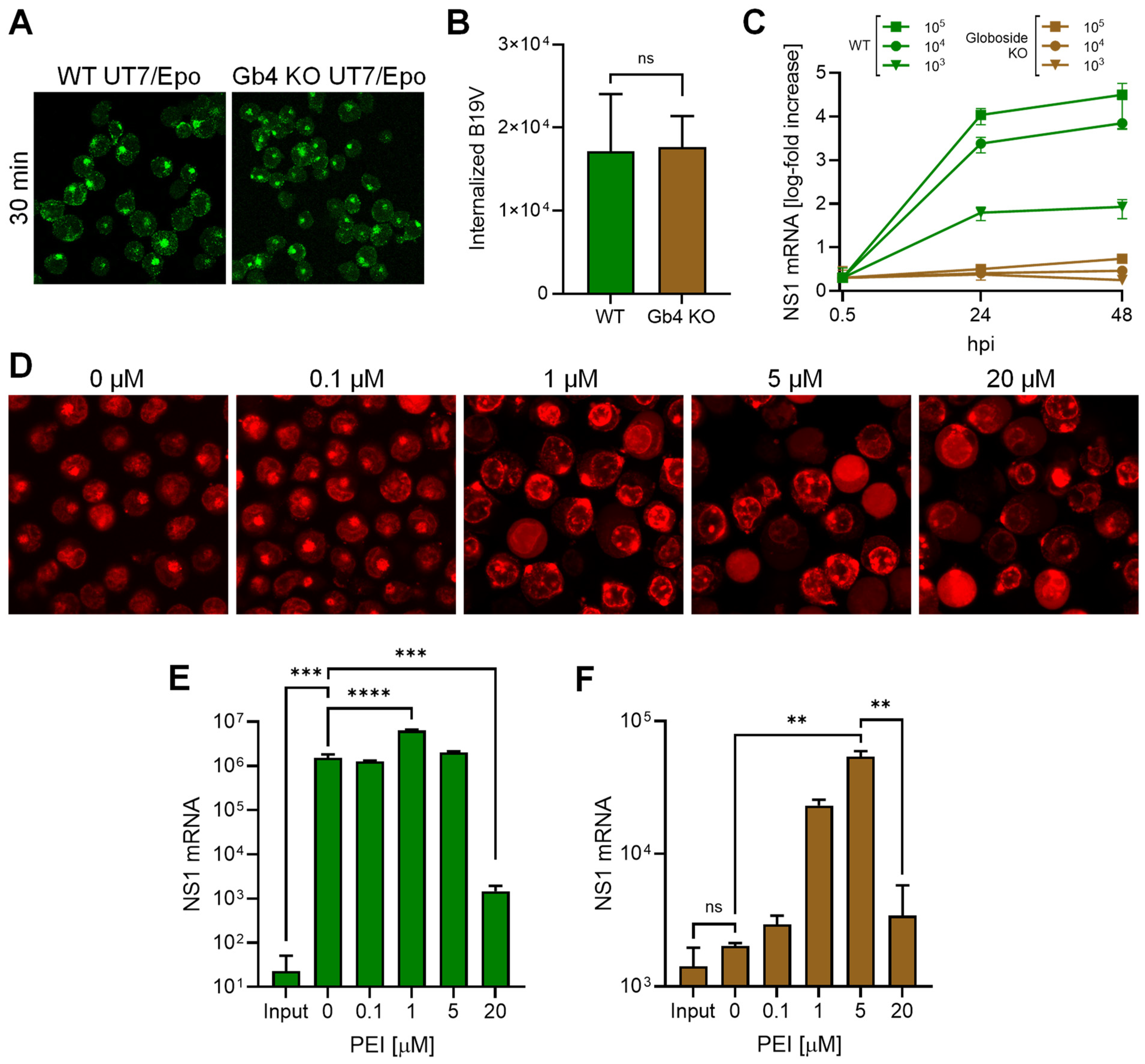
3.2. Promoting Endosomal Leakage with Polyethyleneimine (PEI) Rescues B19V Infection in Globoside Knockout Cells
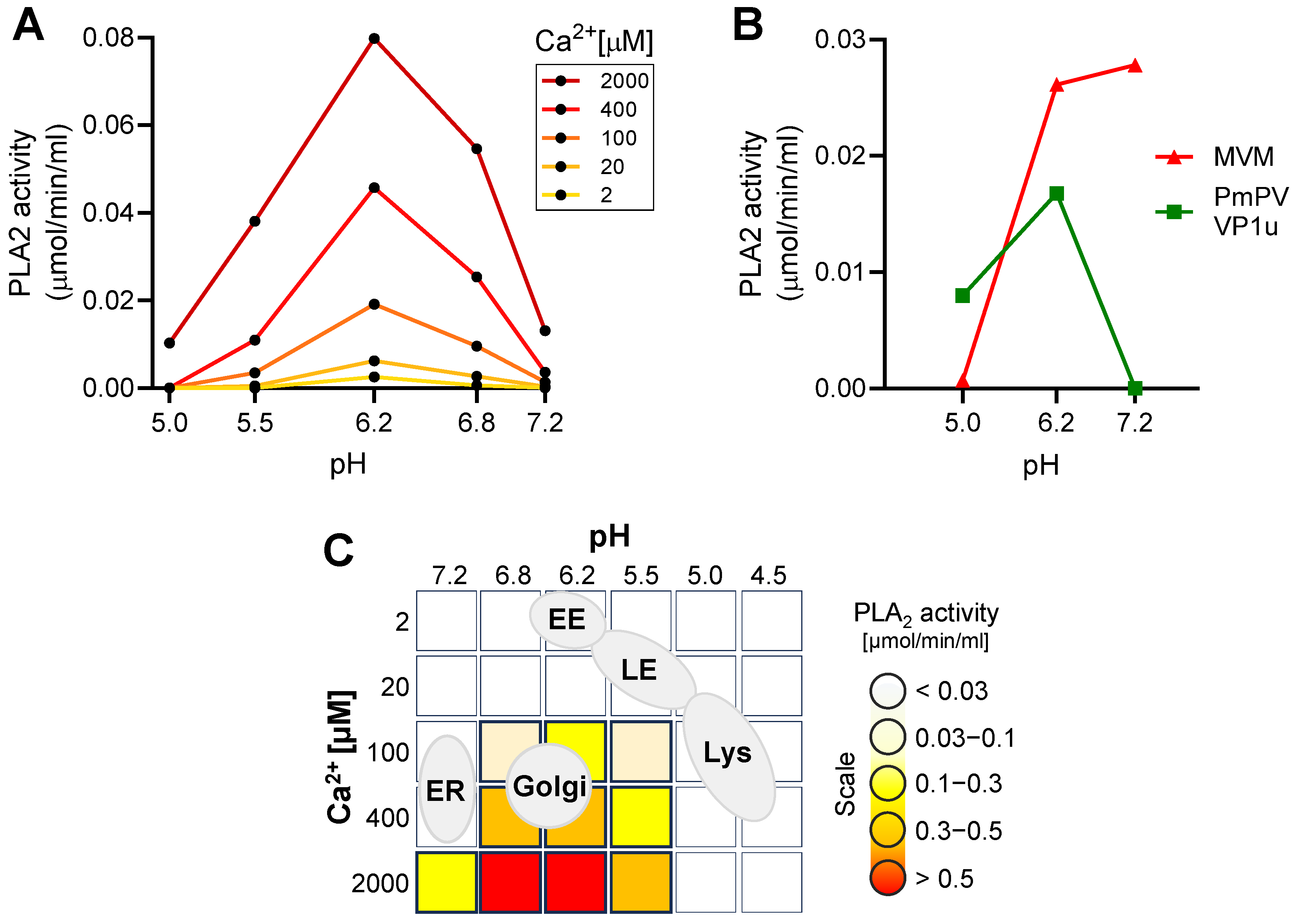
3.3. The Endocytic Compartment Provides Optimal Conditions for Receptor Switching But Not for the Parvoviral PLA2 Activity
3.4. The Escape of Incoming B19V from Endosomes Does Not Compromise the Integrity of the Endosomal Membranes
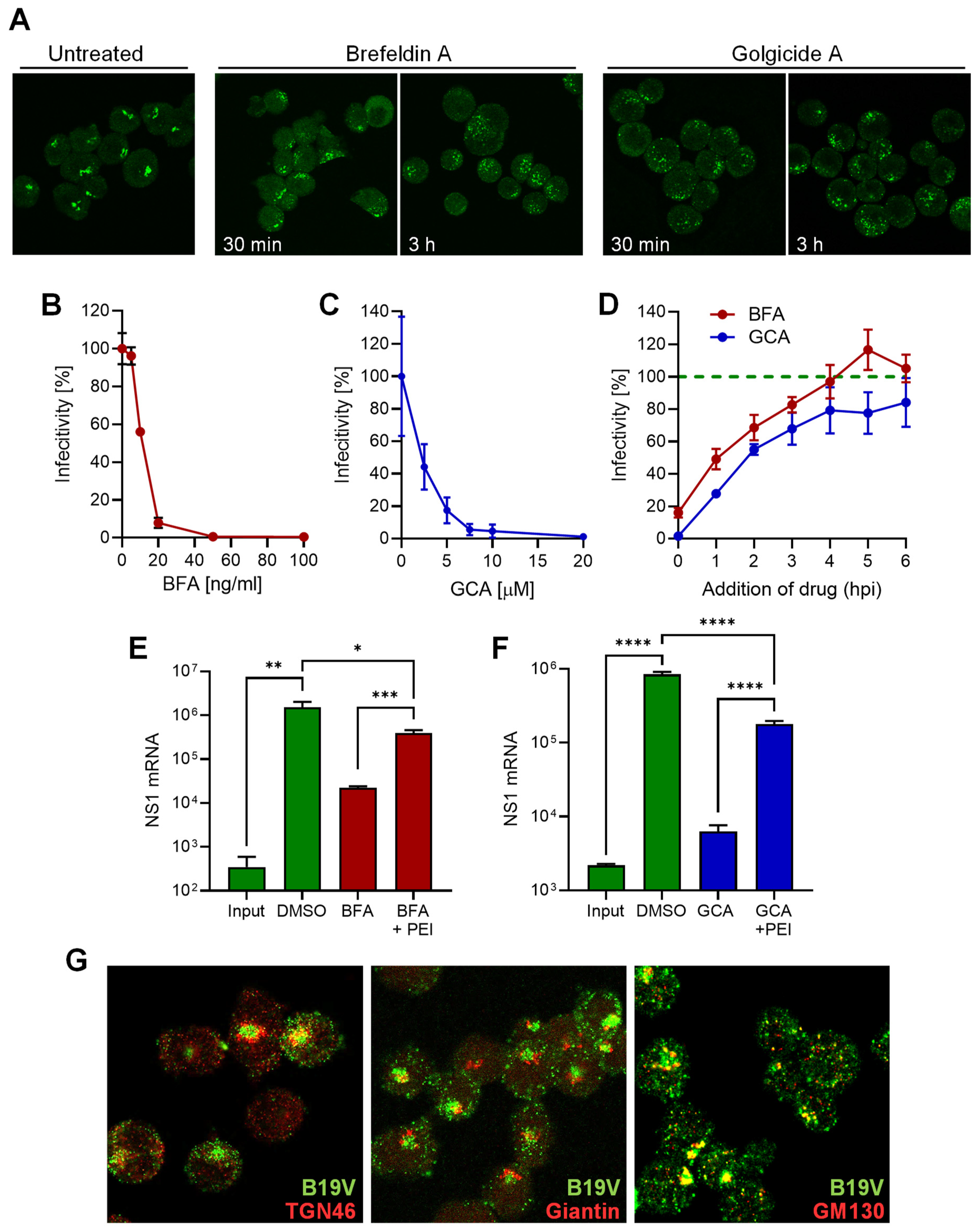
3.5. B19V Entry Involves a Functional Golgi Apparatus
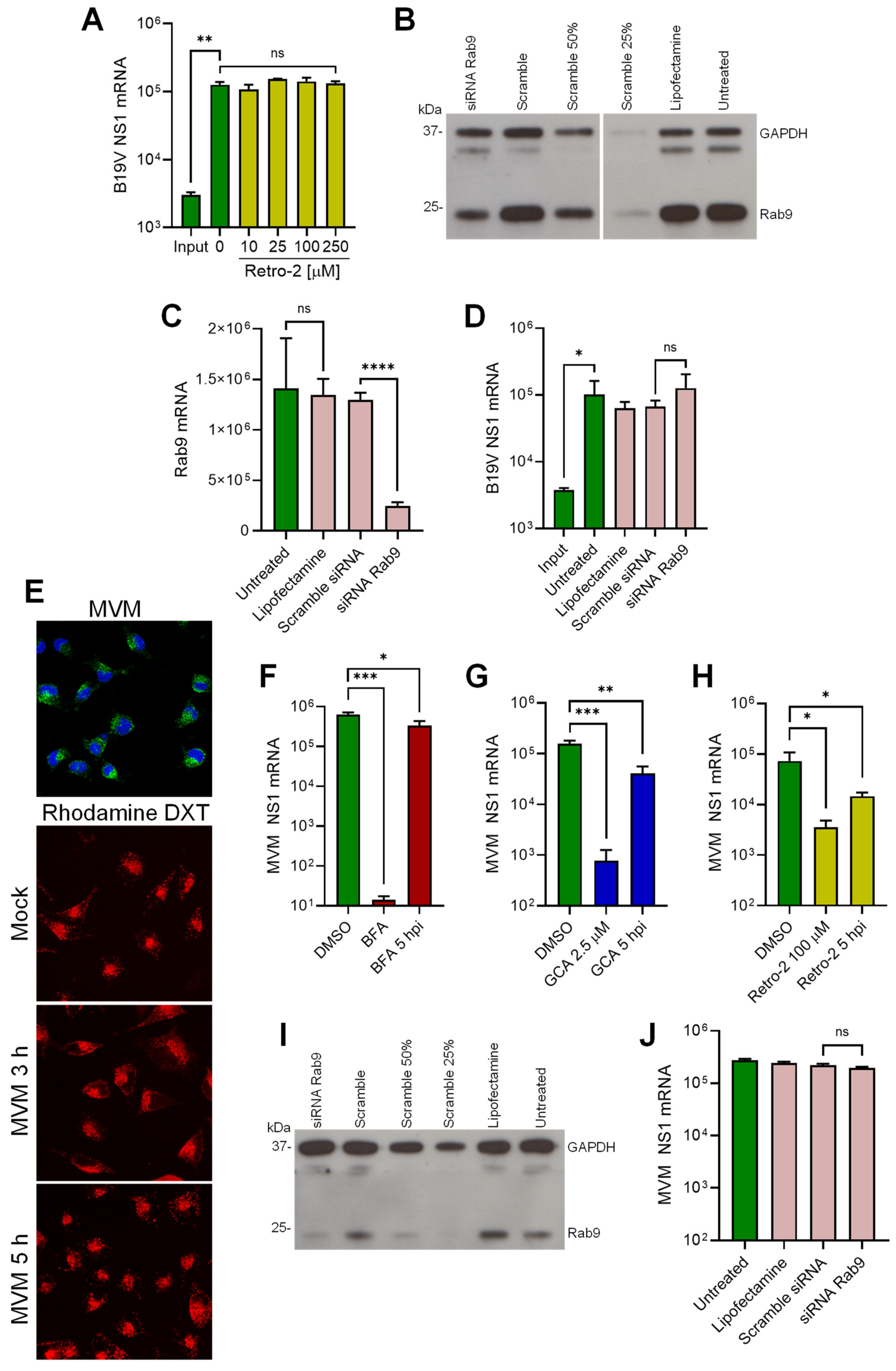
3.6. B19V Does Not Employ Conventional Retrograde Endosome-to-Golgi Transport
3.7. B19V and the Model Parvovirus MVM Share Common Features in Cell Entry
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotmore, S.F.; Tattersall, P. Parvoviruses: Small Does Not Mean Simple. Annu. Rev. Virol. 2014, 1, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Brown, K.E. Human Parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Agbandje, M.; Kajigaya, S.; McKenna, R.; Young, N.S.; Rossmann, M.G. The Structure of Human Parvovirus B19 at 8 Å; Resolution. Virology 1994, 203, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Simpson, A.A.; Rossmann, M.G. The Structure of Human Parvovirus B19. Proc. Natl. Acad. Sci. USA 2004, 101, 11628–11633. [Google Scholar] [CrossRef]
- Anderson, M.J.; Jones, S.E.; Fisher-Hoch, S.P.; Lewis, E.; Hall, S.M.; Bartlett, C.L.R.; Cohen, B.J.; Mortimer, P.P.; Pereira, M.S. Human parvovirus, the cause of Erythema infectiousum (Fifth disease)? Lancet 1983, 321, 1378. [Google Scholar] [CrossRef]
- Servey, J.T.; Reamy, B.V.; Hodge, J. Clinical Presentations of Parvovirus B19 Infection. Am. Fam. Physician 2007, 75, 373–376. [Google Scholar] [PubMed]
- Giorgio, E.; De Oronzo, M.A.; Iozza, I.; Di Natale, A.; Cianci, S.; Garofalo, G.; Giacobbe, A.M.; Politi, S. Parvovirus B19 during Pregnancy: A Review. J. Prenat. Med. 2010, 4, 63–66. [Google Scholar] [PubMed]
- Cotmore, S.F.; Tattersall, P. Parvoviral Host Range and Cell Entry Mechanisms. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2007; Volume 70, pp. 183–232. ISBN 978-0-12-373728-1. [Google Scholar]
- Ros, C.; Bayat, N.; Wolfisberg, R.; Almendral, J. Protoparvovirus Cell Entry. Viruses 2017, 9, 313. [Google Scholar] [CrossRef]
- Quattrocchi, S.; Ruprecht, N.; Bönsch, C.; Bieli, S.; Zürcher, C.; Boller, K.; Kempf, C.; Ros, C. Characterization of the Early Steps of Human Parvovirus B19 Infection. J. Virol. 2012, 86, 9274–9284. [Google Scholar] [CrossRef]
- Mani, B.; Baltzer, C.; Valle, N.; Almendral, J.M.; Kempf, C.; Ros, C. Low pH-Dependent Endosomal Processing of the Incoming Parvovirus Minute Virus of Mice Virion Leads to Externalization of the VP1 N-Terminal Sequence (N-VP1), N-VP2 Cleavage, and Uncoating of the Full-Length Genome. J. Virol. 2006, 80, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Zádori, Z.; Szelei, J.; Lacoste, M.-C.; Li, Y.; Gariépy, S.; Raymond, P.; Allaire, M.; Nabi, I.R.; Tijssen, P. A Viral Phospholipase A2 Is Required for Parvovirus Infectivity. Dev. Cell 2001, 1, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Farr, G.A.; Zhang, L.; Tattersall, P. Parvoviral Virions Deploy a Capsid-Tethered Lipolytic Enzyme to Breach the Endosomal Membrane during Cell Entry. Proc. Natl. Acad. Sci. USA 2005, 102, 17148–17153. [Google Scholar] [CrossRef]
- Sonntag, F.; Bleker, S.; Leuchs, B.; Fischer, R.; Kleinschmidt, J.A. Adeno-Associated Virus Type 2 Capsids with Externalized VP1/VP2 Trafficking Domains Are Generated Prior to Passage through the Cytoplasm and Are Maintained until Uncoating Occurs in the Nucleus. J. Virol. 2006, 80, 11040–11054. [Google Scholar] [CrossRef]
- Stahnke, S.; Lux, K.; Uhrig, S.; Kreppel, F.; Hösel, M.; Coutelle, O.; Ogris, M.; Hallek, M.; Büning, H. Intrinsic Phospholipase A2 Activity of Adeno-Associated Virus Is Involved in Endosomal Escape of Incoming Particles. Virology 2011, 409, 77–83. [Google Scholar] [CrossRef]
- Nonnenmacher, M.E.; Cintrat, J.-C.; Gillet, D.; Weber, T. Syntaxin 5-Dependent Retrograde Transport to the Trans -Golgi Network Is Required for Adeno-Associated Virus Transduction. J. Virol. 2015, 89, 1673–1687. [Google Scholar] [CrossRef]
- Bantel-Schaal, U.; Hub, B.; Kartenbeck, J. Endocytosis of Adeno-Associated Virus Type 5 Leads to Accumulation of Virus Particles in the Golgi Compartment. J. Virol. 2002, 76, 2340–2349. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Weber, T. Adeno-Associated Virus 2 Infection Requires Endocytosis through the CLIC/GEEC Pathway. Cell Host Microbe 2011, 10, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An Essential Receptor for Adeno-Associated Virus Infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Pillay, S.; Carette, J.E. Host Determinants of Adeno-Associated Viral Vector Entry. Curr. Opin. Virol. 2017, 24, 124–131. [Google Scholar] [CrossRef]
- Dudek, A.M.; Zabaleta, N.; Zinn, E.; Pillay, S.; Zengel, J.; Porter, C.; Franceschini, J.S.; Estelien, R.; Carette, J.E.; Zhou, G.L.; et al. GPR108 Is a Highly Conserved AAV Entry Factor. Mol. Ther. 2020, 28, 367–381. [Google Scholar] [CrossRef]
- Suter, C.; Colakovic, M.; Bieri, J.; Gultom, M.; Dijkman, R.; Ros, C. Globoside and the Mucosal pH Mediate Parvovirus B19 Entry through the Epithelial Barrier. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Di Tommaso, C.; Kempf, C.; Ros, C. The Receptor-Binding Domain in the VP1u Region of Parvovirus B19. Viruses 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Leisi, R.; Von Nordheim, M.; Ros, C.; Kempf, C. The VP1u Receptor Restricts Parvovirus B19 Uptake to Permissive Erythroid Cells. Viruses 2016, 8, 265. [Google Scholar] [CrossRef]
- Leisi, R.; Ruprecht, N.; Kempf, C.; Ros, C. Parvovirus B19 Uptake Is a Highly Selective Process Controlled by VP1u, a Novel Determinant of Viral Tropism. J. Virol. 2013, 87, 13161–13167. [Google Scholar] [CrossRef]
- Bieri, J.; Ros, C. Globoside Is Dispensable for Parvovirus B19 Entry but Essential at a Postentry Step for Productive Infection. J. Virol. 2019, 93, e00972-19. [Google Scholar] [CrossRef]
- Bieri, J.; Leisi, R.; Bircher, C.; Ros, C. Human Parvovirus B19 Interacts with Globoside under Acidic Conditions as an Essential Step in Endocytic Trafficking. PLoS Pathog. 2021, 17, e1009434. [Google Scholar] [CrossRef]
- Bircher, C.; Bieri, J.; Assaraf, R.; Leisi, R.; Ros, C. A Conserved Receptor-Binding Domain in the VP1u of Primate Erythroparvoviruses Determines the Marked Tropism for Erythroid Cells. Viruses 2022, 14, 420. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in Vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.-P. The Proton Sponge: A Trick to Enter Cells the Viruses Did Not Exploit. Chimia 1997, 51, 34. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Canaan, S.; Zádori, Z.; Ghomashchi, F.; Bollinger, J.; Sadilek, M.; Moreau, M.E.; Tijssen, P.; Gelb, M.H. Interfacial Enzymology of Parvovirus Phospholipases A2. J. Biol. Chem. 2004, 279, 14502–14508. [Google Scholar] [CrossRef] [PubMed]
- Green, S.W.; Malkovska, I.; O’Sullivan, M.G.; Brown, K.E. Rhesus and Pig-Tailed Macaque Parvoviruses: Identification of Two New Members of the Erythrovirus Genus in Monkeys. Virology 2000, 269, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; D’Abramo, A.M.; Ticknor, C.M.; Tattersall, P. Controlled Conformational Transitions in the MVM Virion Expose the VP1 N-Terminus and Viral Genome without Particle Disassembly. Virology 1999, 254, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, J.V.; Tepikin, A.V.; Petersen, O.H.; Gerasimenko, O.V. Calcium Uptake via Endocytosis with Rapid Release from Acidifying Endosomes. Curr. Biol. 1998, 8, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, X.; Xu, H. Pairing Phosphoinositides with Calcium Ions in Endolysosomal Dynamics: Phosphoinositides Control the Direction and Specificity of Membrane Trafficking by Regulating the Activity of Calcium Channels in the Endolysosomes. BioEssays 2011, 33, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ Signalling in the Golgi Apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kellokumpu, S. Golgi pH, Ion and Redox Homeostasis: How Much Do They Really Matter? Front. Cell Dev. Biol. 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; McKie, V.C.; Anderson, L.J.; Astell, C.R.; Tattersall, P. Identification of the Major Structural and Nonstructural Proteins Encoded by Human Parvovirus B19 and Mapping of Their Genes by Procaryotic Expression of Isolated Genomic Fragments. J. Virol. 1986, 60, 548–557. [Google Scholar] [CrossRef]
- Mastico, R.A.; Talbot, S.J.; Stockley, P.G. Multiple Presentation of Foreign Peptides on the Surface of an RNA-Free Spherical Bacteriophage Capsid. J. Gen. Virol. 1993, 74, 541–548. [Google Scholar] [CrossRef]
- Galaway, F.A.; Stockley, P.G. MS2 Viruslike Particles: A Robust, Semisynthetic Targeted Drug Delivery Platform. Mol. Pharm. 2013, 10, 59–68. [Google Scholar] [CrossRef]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A Causes Disassembly of the Golgi Complex and Accumulation of Secretory Proteins in the Endoplasmic Reticulum. J. Biol. Chem. 1988, 263, 18545–18552. [Google Scholar] [CrossRef]
- Sáenz, J.B.; Sun, W.J.; Chang, J.W.; Li, J.; Bursulaya, B.; Gray, N.S.; Haslam, D.B. Golgicide A Reveals Essential Roles for GBF1 in Golgi Assembly and Function. Nat. Chem. Biol. 2009, 5, 157–165. [Google Scholar] [CrossRef]
- Linstedt, A.D.; Hauri, H.P. Giantin, a Novel Conserved Golgi Membrane Protein Containing a Cytoplasmic Domain of at Least 350 kDa. MBoC 1993, 4, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Rojas, R. Retrograde Transport from Endosomes to the Trans-Golgi Network. Nat. Rev. Mol. Cell Biol. 2006, 7, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Stechmann, B.; Bai, S.-K.; Gobbo, E.; Lopez, R.; Merer, G.; Pinchard, S.; Panigai, L.; Tenza, D.; Raposo, G.; Beaumelle, B.; et al. Inhibition of Retrograde Transport Protects Mice from Lethal Ricin Challenge. Cell 2010, 141, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, D.; Soldati, T.; Riederer, M.A.; Goda, Y.; Zerial, M.; Pfeffer, S.R. Rab9 Functions in Transport between Late Endosomes and the Trans Golgi Network. EMBO J. 1993, 12, 677–682. [Google Scholar] [CrossRef]
- Bruce, L.J. Molecular Mechanism of P1 Antigen Expression. Blood 2018, 131, 1505–1506. [Google Scholar] [CrossRef]
- Brown, K.E.; Anderson, S.M.; Young, N.S. Erythrocyte P Antigen: Cellular Receptor for B19 Parvovirus. Science 1993, 262, 114–117. [Google Scholar] [CrossRef]
- Ning, K.; Zou, W.; Xu, P.; Cheng, F.; Zhang, E.Y.; Zhang-Chen, A.; Kleiboeker, S.; Qiu, J. Identification of AXL as a Co-Receptor for Human Parvovirus B19 Infection of Human Erythroid Progenitors. Sci. Adv. 2023, 9, eade0869. [Google Scholar] [CrossRef]
- Katz, H.R.; Austen, K.F. Plasma Membrane and Intracellular Expression of Globotetraosylceramide (Globoside) in Mouse Bone Marrow-Derived Mast Cells. J. Immunol. 1986, 136, 3819–3824. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and Functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Meissner, W.; Kostin, S.; Niemann, A.; Elsasser, H.-P. Intracellular Route and Transcriptional Competence of Polyethylenimine–DNA Complexes. J. Control. Release 2002, 82, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.L.; Chapman, M.S. Adeno-Associated Virus (AAV) Cell Entry: Structural Insights. Trends Microbiol. 2022, 30, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Gerber, M.; Kempf, C. Conformational Changes in the VP1-Unique Region of Native Human Parvovirus B19 Lead to Exposure of Internal Sequences That Play a Role in Virus Neutralization and Infectivity. J. Virol. 2006, 80, 12017–12024. [Google Scholar] [CrossRef] [PubMed]
- Bönsch, C.; Kempf, C.; Ros, C. Interaction of Parvovirus B19 with Human Erythrocytes Alters Virus Structure and Cell Membrane Integrity. J. Virol. 2008, 82, 11784–11791. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Zhao, Y.; Nguyen, T.H.; Campbell, R.E.; Johnson, J.D. Fluorescent Biosensors Illuminate Calcium Levels within Defined Beta-Cell Endosome Subpopulations. Cell Calcium 2015, 57, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.L.; Parrish, C.R. Cellular Uptake and Infection by Canine Parvovirus Involves Rapid Dynamin-Regulated Clathrin-Mediated Endocytosis, Followed by Slower Intracellular Trafficking. J. Virol. 2000, 74, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Antila, M.; Jaatinen, A.; Vihinen-Ranta, M.; Vuento, M. Release of Canine Parvovirus from Endocytic Vesicles. Virology 2003, 316, 267–280. [Google Scholar] [CrossRef]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.E.; Fraisier, V.; Florent, J.-C.; Perrais, D.; et al. Shiga Toxin Induces Tubular Membrane Invaginations for Its Uptake into Cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Groza, R.; Ewers, H. Membrane Deformation by the Cholera Toxin Beta Subunit Requires More than One Binding Site. Proc. Natl. Acad. Sci. USA 2020, 117, 17467–17469. [Google Scholar] [CrossRef] [PubMed]
- Ewers, H.; Römer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 Structure Determines SV40-Induced Membrane Invagination and Infection. Nat. Cell Biol. 2010, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rydell, G.E.; Svensson, L.; Larson, G.; Johannes, L.; Römer, W. Human GII.4 Norovirus VLP Induces Membrane Invaginations on Giant Unilamellar Vesicles Containing Secretor Gene Dependent A1,2-Fucosylated Glycosphingolipids. Biochim. Biophys. Acta BBA—Biomembr. 2013, 1828, 1840–1845. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieri, J.; Suter, C.; Caliaro, O.; Bartetzko, S.; Bircher, C.; Ros, C. Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape. Cells 2024, 13, 1254. https://doi.org/10.3390/cells13151254
Bieri J, Suter C, Caliaro O, Bartetzko S, Bircher C, Ros C. Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape. Cells. 2024; 13(15):1254. https://doi.org/10.3390/cells13151254
Chicago/Turabian StyleBieri, Jan, Corinne Suter, Oliver Caliaro, Seraina Bartetzko, Cornelia Bircher, and Carlos Ros. 2024. "Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape" Cells 13, no. 15: 1254. https://doi.org/10.3390/cells13151254
APA StyleBieri, J., Suter, C., Caliaro, O., Bartetzko, S., Bircher, C., & Ros, C. (2024). Globoside Is an Essential Intracellular Factor Required for Parvovirus B19 Endosomal Escape. Cells, 13(15), 1254. https://doi.org/10.3390/cells13151254





