Abstract
Mitochondria are crucial for cellular ATP production. They are highly dynamic organelles, whose morphology and function are controlled through mitochondrial fusion and fission. The specific roles of mitochondria in podocytes, the highly specialized cells of the kidney glomerulus, remain less understood. Given the significant structural, functional, and molecular similarities between mammalian podocytes and Drosophila nephrocytes, we employed fly nephrocytes to explore the roles of mitochondria in cellular function. Our study revealed that alterations in the Pink1–Park (mammalian PINK1–PRKN) pathway can disrupt mitochondrial dynamics in Drosophila nephrocytes. This disruption led to either fragmented or enlarged mitochondria, both of which impaired mitochondrial function. The mitochondrial dysfunction subsequently triggered defective intracellular endocytosis, protein aggregation, and cellular damage. These findings underscore the critical roles of mitochondria in nephrocyte functionality.
Keywords:
mitochondria; Pink1; Parkin; Marf (MFN); endocytosis; reactive oxygen species (ROS); nephrocytes; Drosophila 1. Introduction
Mitochondria are the primary source of cellular ATP production [1], whose morphology and function are controlled by a balance between two opposing actions mitochondrial fusion and fission [2]. Mitochondrial fusion is controlled by Mitofusin 1 (MFN1) and Mitofusin 2 (MFN2) through the PTEN-induced kinase 1 (PINK1)–Parkin RBR E3 ubiquitin-protein ligase (PRKN) pathway; while mitochondrial fission is driven by Dynamin-related protein 1 (DRP1) [3,4,5]. Maintaining mitochondrial health is crucial, and the quality control and selective removal of damaged mitochondria play a tremendously important role in this process. Mitochondrial fission facilitates the isolation and removal of damaged mitochondria through mitophagy. Alongside regulating mitochondrial dynamics, the PINK1-PRKN pathway plays a crucial role in facilitating mitophagy to ensure the removal of damaged mitochondria, thus maintaining cellular health [6]. The dynamic regulation of mitochondrial fusion and fission plays a crucial role in maintaining cellular energy balance and ensuring mitochondrial health and functionality. Imbalances of mitochondrial dynamics have been associated with a range of diseases, including neurodegenerative disorders and metabolic diseases [7,8,9,10,11].
Kidneys are energy-demanding organs due to their constant filtering and reabsorption processes, and therefore highly dependent on mitochondria to produce ATP through cellular respiration, providing the necessary energy to support kidney functions [12,13,14]. Mitochondria are also involved in regulating pH levels within kidney cells and maintaining the balance of sodium, potassium, calcium, and other ions to ensure proper kidney function and fluid balance [15,16]. Furthermore, mitochondria facilitate the stability and dynamics of the podocyte cytoskeleton [17]. Podocytes are specialized cells in the glomeruli of the kidney, that are key to the filtration of blood to form urine and remove waste. Structural integrity provided by the cytoskeleton is crucial to the podocyte filtration function, which is dependent on elaborate foot processes that form interdigitating extensions with neighboring podocytes [18].
Drosophila pericardial nephrocytes, referred to here as nephrocytes, have striking structural and functional similarities to mammalian podocytes [19,20,21,22]. Both cells form highly specialized structures called slit diaphragms, which along with the basement membrane act as size- and charge-dependent filtration barriers [19]. The mitochondrial Coenzyme Q (CoQ) pathway plays a key role in the electron transport chain to efficiently produce ATP [23]. Deficiency for CoQ2, a component of the CoQ pathway, led to a decline in nephrocyte function, mitochondrial dysfunction, and abnormal localization of the slit diaphragm in Drosophila nephrocytes. Either expressing the human COQ2 gene or dietary supplementation with coenzyme Q10, the product of the CoQ pathway, restored these nephrocyte defects [23]. This and other studies [24,25] underscored the value of the Drosophila nephrocyte as a model system for studying mitochondrial functions.
Here, we used Drosophila to explore the roles of mitochondria in nephrocytes in vivo. Genetic modification of mitochondrial dynamic control induced fragmented or enlarged mitochondria, either of which resulted in reduced mitochondrial function. The dysfunctional mitochondria caused defective intracellular endocytosis, and protein aggregation, and culminated in cellular damage and filtration dysfunction.
2. Materials and Methods
2.1. Drosophila Lines
All fly stocks were maintained at 25 °C and fed a standard diet (Meidi Laboratories, Baltimore, MD, USA). Drosophila stocks were sourced from the Bloomington Drosophila Stock Center (Indiana University Bloomington, Bloomington, IN, USA). The following lines were utilized in the experiments: Dot-Gal4 (ID 6903), UAS-mito-GFP (ID 8442), UAS-Pink1-RNAi (ID 31170 and 41671), UAS-park-RNAi (ID 31259 and 37509), UAS-Marf-RNAi (ID 31157 and 55189), UAS-Pink1-OE (ID 51648), UAS-park-OE (ID 51651), UAS-Marf-OE (ID 67157), UAS-Rab5-RNAi (ID 30518 and 34832), UAS-Rab5-YFP (ID 24616), UAS- Rab7-YFP (ID 23270), UAS-Rab11-YFP (ID 9790). w1118 (ID 3605) flies served as control.
2.2. Mitochondrial Membrane Potential Measurement in Drosophila Nephrocytes
The mitochondrial membrane potential was measured using a tetramethylrhodamine, methyl ester (TMRM) assay kit (Abcam, Cambridge, UK; ab228569). Nephrocytes were dissected from 4-day-old adult female flies and maintained at room temperature in artificial hemolymph [24]. Cells were incubated with TMRM (1 µg/mL; Invitrogen, Waltham, MA, USA) for 1 h and subsequently washed three times with phosphate-buffered saline (1XPBS) containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) (PBST). Mitochondrial membrane potential was determined based on TMRM fluorescence levels in nephrocytes, measured using fluorescence confocal microscopy in live cells (ZEISS LSM 900; see details below).
2.3. Transmission Electron Microscopy (TEM) for Drosophila Nephrocytes
Nephrocytes were dissected from 4-day-old adult female flies and maintained in artificial hemolymph. The cells were initially fixed with 8% paraformaldehyde in 1XPBS for 10 min, then transferred to a fixation buffer containing 4% paraformaldehyde and 2.5% glutaraldehyde. Subsequent processing and analysis of the cells were conducted [26] using an FEI Tecnai T12 TEM at the Electron Microscopy Core Imaging Facility at the Center for Innovative Biomedical Resources (CIBR) (University of Maryland School of Medicine, Baltimore, MD, USA).
2.4. Measurement of Mitochondrial Dimensions
To measure the mitochondrial size in nephrocytes, we utilized Dot-Gal4, UAS-mito-GFP fly lines crossed with the overexpression (OE), or RNAi (IR) lines targeting Pink1, park, or Marf. Images were conducted using ZEISS LSM900 and analyzed with ImageJ software [27] (National Institutes of Health, Bethesda, MD, USA; version 1.52a). Each mitochondrion was manually outlined using the Freehand selection tool and measured. The average size of mitochondria was calculated based on 30 mitochondria from a single nephrocyte. For quantitation, a total of 30 nephrocytes per genotype were analyzed, derived from six 4-day-old adult female flies.
2.5. Assessment of Dextran and Albumin Uptake in Drosophila Nephrocytes
Ex vivo assays measuring dextran and albumin uptake were conducted to evaluate the filtration function of nephrocytes. Nephrocytes were dissected from 4-day-old adult female flies and maintained in artificial hemolymph. Cells were incubated with dextran, Texas Red, 10,000 MW (0.05 mg/mL; Invitrogen, D1828) or albumin, Alexa Fluor 488 (0.05 mg/mL; Invitrogen, A13100) for 20 min, followed by fixation with 4% paraformaldehyde in 1XPBS (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min. The uptake capacity of dextran and albumin was determined by the fluorescence levels in the nephrocytes, which were assessed using ZEISS.
2.6. Fluorescent Immunochemistry
Four-day-old adult female flies were dissected and heat-fixed for 20 s in 100 °C artificial hemolymph to prepare them for immunohistochemistry. Primary antibodies used included a mouse monoclonal anti-Pyd antibody (PYD2) sourced from Developmental Studies Hybridoma Bank (DSHB; University of Iowa, Iowa City, IA, USA) at 1:100 dilution in PBST; and, a mouse monoclonal anti-ubiquitinylated proteins antibody, clone FK2 (Enzo Life Sciences, Farmingdale, NY, USA) at 1:500 dilution in PBST. Secondary antibodies, Alexa Fluor 555 (Thermo Fisher Scientific) and Alexa Fluor 488 (Thermo Fisher Scientific), were each used at 1:1000 dilution in PBST. DAPI (Thermo Fisher Scientific) was utilized to visualize the nuclei at a dilution of 1:1000 in PBST. The nephrocytes were washed three times with PBST, and blocked in PBST containing 2% bovine serum albumin (BSA; Sigma-Aldrich) for 30 min, followed by overnight incubation with primary antibodies at 4 °C. The following day, cells were washed three times with 1XPBST, incubated with secondary antibodies and DAPI at room temperature for 2 h, then washed three times with 1XPBST, and mounted with Vectashield mounting medium (Vector Laboratories, Malvern, PA, USA). Images were captured using ZEISS LSM 900.
2.7. Reactive Oxygen Species Assay in Drosophila Nephrocytes
Levels of reactive oxygen species (ROS) were measured with a dihydroethidium (DHE) assay kit (Invitrogen, D11347). Nephrocytes were dissected from 4-day-old adult female flies and maintained in artificial hemolymph. The cells were incubated with DHE (1 µg/mL; Invitrogen) for 30 min and subsequently washed three times with PBST. The ROS levels were determined based on the DHE fluorescence levels in the nuclei of the nephrocytes, using ZEISS LSM 900.
2.8. Fluorescence Confocal Microscopy of Drosophila Nephrocytes
Imaging for dextran uptake, albumin uptake, UAS-mito-GFP, UAS-Rab5-YFP, UAS-Rab7-YFP, UAS-Rab11-YFP fly strains, fluorescent immunocytochemistry for anti-FK2, DHE, and DAPI was carried out confocal technology; a ZEISS LSM900 microscope with a 20× Plan-Apochromat 0.8 N.A. air objective (ZEN Blue, edition 3.0, acquisition software). Fluorescent immunocytochemistry for anti-Pyd and TMRM and fluorescent imaging for the UAS-mito-GFP fly strains were carried out using a ZEISS LSM900 microscope with a 63× Plan-Apochromat 1.4 N.A. oil objective under Airyscan SR mode (ZEN Blue, Zeiss, Jena, Germany; edition 3.0, acquisition software). For quantitative comparison of intensities, uniform settings were selected to prevent oversaturation and were consistently applied across all images captured for that assay. ImageJ Software [27] (version 1.52a) was used for image processing.
2.9. Statistical Analysis of Drosophila Assays
Statistical analyses were conducted using PAST.exe software (Natural History Museum, Norway; version 2.17C). Initially, data were assessed for normality using the Shapiro–Wilk test (α = 0.05). Data with normal distribution were then analyzed by the Kruskal–Wallis H-test followed by a Dunn’s test for comparisons between multiple groups. For quantitation, 30 nephrocytes per genotype were examined from 6 adult female flies. The results are presented as mean ± SD. Statistical significance (*) was defined as p < 0.05.
3. Results
3.1. Mitochondria Highly Existed in Drosophila Nephrocytes
Mitochondria are essential organelles that are responsible for oxidative phosphorylation, the primary mechanism of cellular ATP production [28,29]. Mitochondrial function is closely tied to dynamic changes in size and shape, which are regulated by processes of fission and fusion [30]. To investigate the role of mitochondria in Drosophila nephrocyte function, we employed the UAS-GAL4 system combined with UAS-mito-GFP, which specifically labels the mitochondria to visualize their morphology. We found that mitochondria are highly abundant in Drosophila nephrocytes (Figure 1A). The mitochondria showed both round and elongated shapes, indicative of mitochondrial dynamics (Figure 1B,B’). Mitochondrial membrane potential is a critical component for ATP production through oxidative phosphorylation [31]. We used tetramethylrhodamine methyl ester (TMRM) to detect mitochondrial membrane potential as a measure of mitochondrial function in the Drosophila nephrocytes. We observed that TMRM was highly expressed and co-located with mitochondria in the nephrocytes, indicative of highly active mitochondria (Figure 1B,B’). Imaging by transmission electron microscopy (TEM) supported the presence of both round and elongated mitochondria in the nephrocytes (Figure 1C). Overall, the data show that mitochondria are abundantly present in nephrocytes with signs of the expected dynamics and activity.
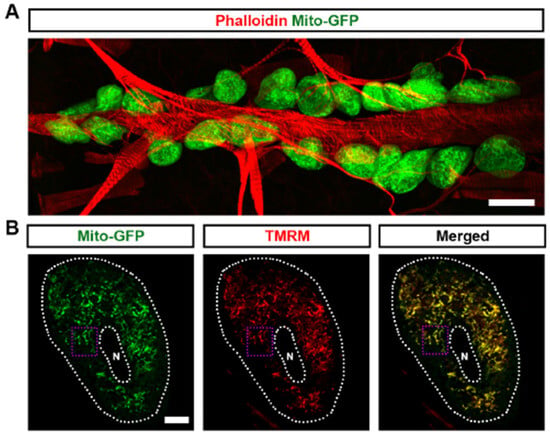
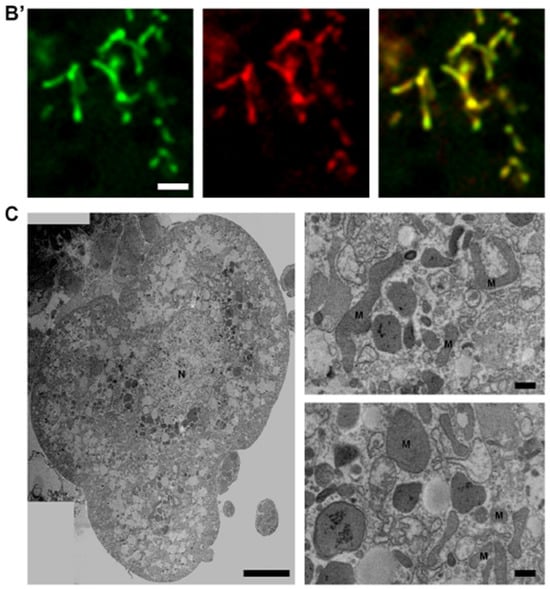
Figure 1.
Mitochondria in Drosophila nephrocytes: (A) UAS-mito-GFP (green) induced by nephrocyte-specific driver Dot-Gal4 to label the mitochondria in Drosophila nephrocytes (Dot > mito-GFP, female, 4-day-old). Phalloidin (red) labels the actin filaments and was used to visualize the fly heart. Scale bar = 20 µm. (B,B’) Dot > mito-GFP (green) was to label the mitochondria in Drosophila nephrocytes (female, 4-day-old). Mitochondrial membrane potential was indicated by tetramethylrhodamine, methyl ester (TMRM; red). White dotted lines, outline the nephrocyte and the nucleus (N); purple dotted box, outlines the area magnified in (B’). Scale bar: (B) = 5 µm; (B’) = 1 µm. (C) Mosaic of transmission electron microscopy (TEM) images showing nephrocyte ultrastructure with nucleus (N) and mitochondria (M). Scale bars: (left) = 5 µm; (right, both) = 2 µm.
Regulation of mitochondrial dynamics in Drosophila nephrocytes by the Pink1–Park pathway. Mitochondrial dynamics are partially regulated by the Pink1–Park pathway, Drosophila ortholog of the human PINK1–PRKN pathway, which also regulates mitophagy to eliminate damaged mitochondria [4]. Consequently, we proceeded to investigate whether the genetic modification of the Pink1–Park pathway in Drosophila nephrocytes influences mitochondrial morphology and function. To achieve this, we combined the nephrocyte-specific driver Dot-Gal4 with either RNAi knockdown (UAS-Pink1-IR, UAS-park-IR, or UAS-Marf-IR) or overexpression (UAS-Pink1-OE, UAS-park-OE, or UAS-Marf-OE) of Pink1, park or Marf in flies. Two independent RNAi lines were examined for each gene, yielding consistent results. Therefore, representative data from one line are displayed in the figures
We first examined the mitochondrial morphology of nephrocytes from the progenies. Pink1 or park overexpression, or Marf silencing changed mitochondrial morphology and significantly reduced mitochondria size; while the opposite was true too, Pink1 or park silencing, or Marf overexpression changed mitochondrial morphology and significantly increased mitochondria size (Figure 2A,B,D,D’). These findings indicate altered mitochondrial fission–fusion dynamics, which were reflected in significantly reduced mitochondrial membrane potential (Figure 2A,C), indicating mitochondrial dysfunction. The data implicate defective mitochondrial dynamics and reduced mitochondrial function when the Pink1–Park pathway is dysregulated, supporting its importance in nephrocyte mitochondrial function.
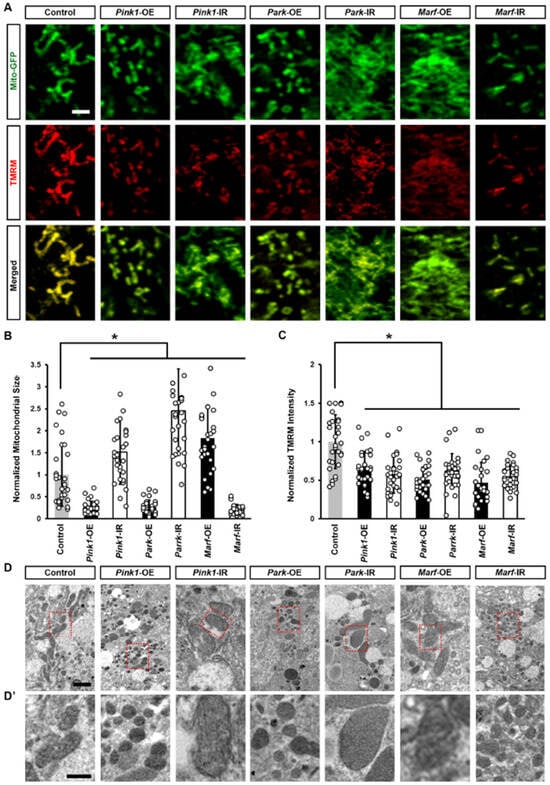
Figure 2.
Defective mitochondrial dynamics resulted in altered mitochondrial morphology and reduced mitochondrial function: (A–D’) Mitochondrial morphology in Drosophila nephrocytes (female, 4-day-old) overexpressing (OE) or inhibiting (RNAi; IR) Pink1, park, or Marf. Control, Dot > mito-GFP. (A) Dot > mito-GFP (green) was to label the mitochondria. Mitochondrial membrane potential was indicated by TMRM (red). Scale bar = 1 µm. (B) Quantitation of mitochondrial size in Pink1/park/Marf-OE/IR nephrocytes. (C) Quantitation of TMRM fluorescence in Pink1/park/Marf-OE/IR nephrocytes. (D,D’) Transmission electron microscopy (TEM) of mitochondria in nephrocytes. Red dotted box outlines the area magnified in (D’). Scale bar: (D) = 5 µm; (D’) = 2 µm. N = 30 mitochondria from 6 flies, per group per assay. Results presented as mean ± SD; statistical significance: *, p < 0.05.
3.2. Pink1–Park-Mediated Defective Mitochondrial Dynamics Lead to Reduced Nephrocyte Function and a Disrupted Slit Diaphragm Filtration Structure
Next, we examined changes in the function of Drosophila nephrocytes associated with the altered mitochondrial morphology. We measured nephrocyte filtration function with an ex vivo assay of cell ability to uptake the smaller 10 kDa dextran or the larger molecular weight albumin fluorescent particles. We noted a significant decrease in both 10 kDa dextran and albumin intensity when silencing or overexpressing the Pink1–Park pathway genes in the nephrocytes (Figure 3), indicating nephrocyte functional decline.
Figure 3.
Defective mitochondrial dynamics reduced nephrocyte function: (A–D) Function in Drosophila nephrocytes (female, 4-day-old) overexpressing (OE) or inhibiting (RNAi; IR) Pink1, park, or Marf. Control, Dot > w1118. (A) 10 kDa dextran (red) uptake by nephrocytes. Scale bar = 25 µm. (B) Quantitation of 10 kDa dextran fluorescence in Pink1/park/Marf-OE/IR nephrocytes, relative to control. (C) Albumin (green) uptake by nephrocytes. Scale bar = 25 µm. (D) Quantitation of albumin fluorescence in Pink1/park/Marf-OE/IR nephrocytes, relative to control. N = 30 nephrocytes from 6 flies, per group per assay. Results presented as mean ± SD; statistical significance: *, p < 0.05.
To understand how altered mitochondrial dynamics led to defective nephrocyte uptake function, we examined the slit diaphragm and lacunar channel structures, which are crucial for the filtration function of nephrocytes [32,33]. Immunochemistry for the slit diaphragm protein Polychaetoid (Pyd) in control nephrocytes revealed its typical localization as a well-defined and continuous circumferential ring (Figure 4A, medial optical section) and a uniform and smoothly distributed fingerprint-like pattern on the surface (Figure 4B,B’). Genetically modified Pink1–Park pathway by either silencing or overexpressing Pink1, park, or Marf in the nephrocytes disrupted nephrocyte Pyd localization; such that a significant portion of the Pyd protein is internalized rather than remaining on the surface (Figure 4A). We also observed a disrupted slit diaphragm localization pattern at the nephrocyte surface, no longer resembling a clear fingerprint-like pattern (Figure 4B,B’). To visualize the lacunar channel ultrastructure in Drosophila nephrocytes, we used a transmission electron microscope (TEM). Control nephrocytes showed regularly spaced and shaped lacunar channels along the circumference of the cell (Figure 4C), whereas channel shapes were severely disrupted and the numbers of slit diaphragms were significantly declined in nephrocytes after Pink1, park, or Marf were silenced or overexpressed (Figure 4C,D). Together these data indicate that genetic modification of Pink1–Park-mediated defective mitochondrial dynamics leads to disrupted filtration structures and associated dysfunction in nephrocytes.
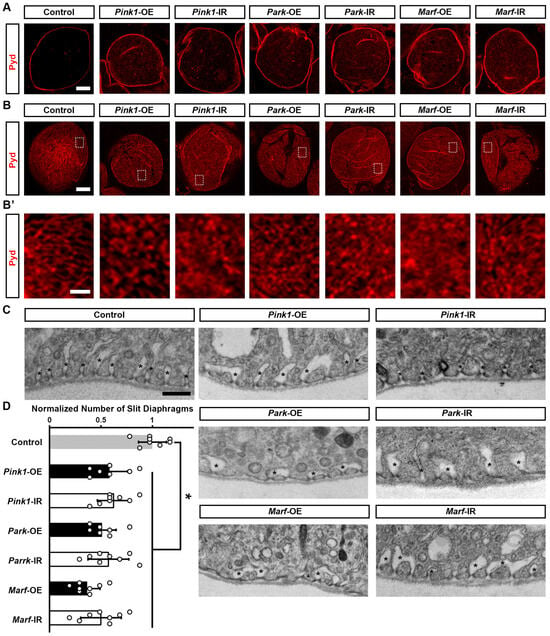
Figure 4.
Defective mitochondrial dynamics in nephrocytes lead to a disrupted slit diaphragm structure: (A–C) Slit diaphragm in Drosophila nephrocytes (female, 4-day-old) overexpressing (OE) or inhibiting (RNAi; IR) Pink1, park, or Marf. Control, Dot > w1118. (A–B’) Distribution of slit diaphragm protein Polychaetoid (Pyd; red) by immunofluorescence in Drosophila nephrocytes. (A) Imaged at medial optical sections. Scale bar = 5 µm. (B,B’) Imaged at nephrocyte surface. While the dotted box outlines the area magnified in (B’). Scale bar: (B) = 5 µm; (B’) = 1 µm. (C) Transmission electron microscopy (TEM) displaying nephrocyte ultrastructure featuring slit diaphragms and lacunar channels around the circumference. Asterisk (*) indicates lacunar channel. Scale bar = 200 nm. (D) Quantitation of numbers of slit diaphragms in Pink1/park/Marf-OE/IR nephrocytes, relative to control. N = 8 flies, per group. Results presented as mean ± SD; statistical significance: *, p < 0.05.
3.3. Pink1–Park-Mediated Defective Mitochondrial Dynamics Lead to Impaired Endocytic Membrane Trafficking
We previously found that endocytic membrane trafficking is essential for maintaining a slit diaphragm structure and nephrocyte function [34]. Therefore, we examined whether the genetically modified Pink1–Park pathway affected the Rab GTPases we had identified as most important for the nephrocyte slit diaphragm [34]. We found a significant reduction in all three—Rab5 (early endosome), Rab7 (late endosome), and Rab11 (recycling endosome)—after silencing or overexpressing Pink1, park, or Marf (Figure 5). These results highlight the critical role of mitochondrial dynamics in endocytic membrane trafficking. Disrupted trafficking following Pink1–Park pathway genetic modification, likely underlies the associated altered slit diaphragm filtration structure and impaired nephrocyte function.
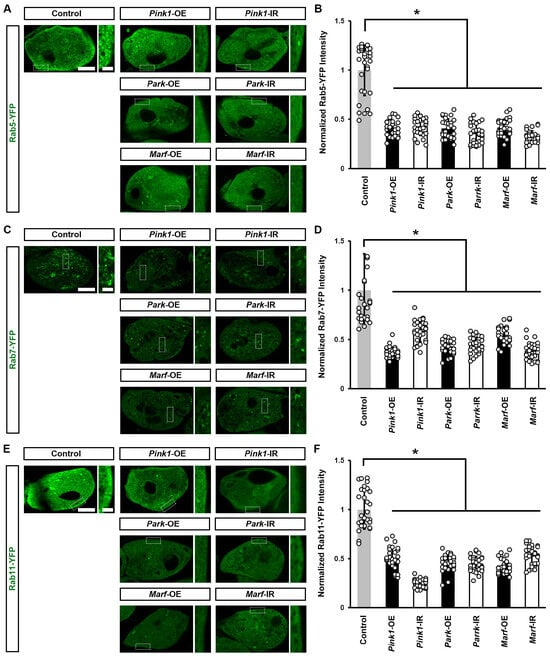
Figure 5.
Defective mitochondrial dynamics in nephrocytes impaired endocytic membrane trafficking: (A–F) Endocytic membrane trafficking in Drosophila nephrocytes (female, 4-day-old) overexpressing (OE) or inhibiting (RNAi; IR) Pink1, park or Marf. Control, (A) Dot > Rab5-YFP, (B) Dot > Rab7-YFP (C) Dot > Rab11-YFP. (A) Early endosome visualized by Rab5-YFP (green) in nephrocytes. Scale bar = 5 µm. Boxed areas are shown magnified to the right (scale bar: 1 μm). (B) Quantitation of Rab5-YFP fluorescence in Pink1/park/Marf-OE/IR nephrocytes. (C) Late endosomes visualized by Rab7-YFP (green) in nephrocytes. Scale bar = 5 µm. Boxed areas are shown magnified to the right (scale bar: 1 μm). (D) Quantitation of Rab7-YFP fluorescence in Pink1/park/Marf-OE/IR nephrocytes. (E) Recycling endosomes visualized by Rab11-YFP (green) in nephrocytes. Scale bar = 5 µm. Boxed areas are shown magnified to the right (scale bar: 1 μm). (F) Quantitation of Rab11-YFP fluorescence in Pink1/park/Marf-OE/IR nephrocytes, relative to control. N = 30 nephrocytes from 6 flies, per group per assay. Results presented as mean ± SD; statistical significance: *, p < 0.05.
3.4. Pink1–Park-Mediated Defective Mitochondrial Dynamics Lead to Induced Cellular Protein Aggregation and Increased ROS
To understand why defective mitochondrial dynamics caused Drosophila nephrocyte damage, we further investigated cellular changes induced by impaired endocytic membrane trafficking. Reactive oxygen species (ROS) production and oxidative stress have been implicated in mitochondrial dysfunction [35]. We examined the effect of silencing or overexpressing Pink1, park, or Marf on ROS levels in Drosophila nephrocytes. Dihydroethidium (DHE) was used as a redox indicator [23,36]. Reduced DHE fluoresces blue, but it shifts to red fluorescence upon oxidation, and intercalates into DNA. We observed a significant increase in DHE red fluorescence intensity in the nephrocyte nucleus after silencing or overexpressing Pink1, park, or Marf (Figure 6A,B). Furthermore, these nephrocytes contained aggregates of ubiquitinylated proteins, another indicator of defective endocytic membrane trafficking and autophagy (Figure 6C,D).
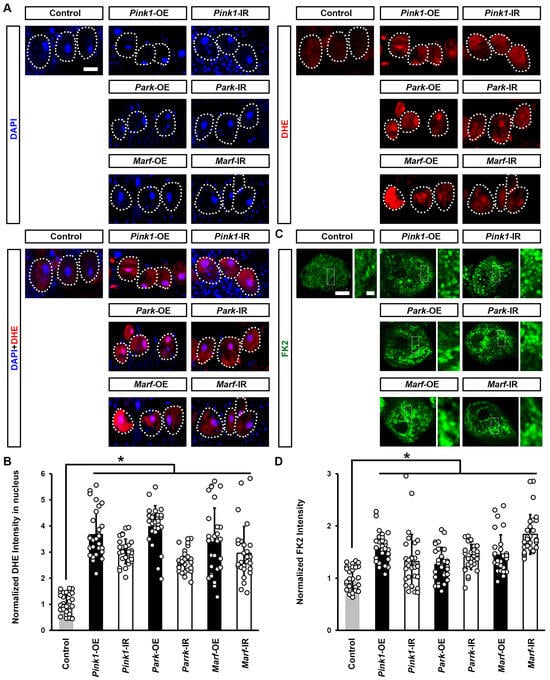
Figure 6.
Defective mitochondrial dynamics in nephrocytes induced protein aggregation and increased ROS: (A–C) Reactive oxygen species (ROS) and protein aggregation in Drosophila nephrocytes (female, 4-day-old) overexpressing (OE) or inhibiting (RNAi; IR) Pink1, park, or Marf. Control, Dot > w1118. (A) Dihydroethidium (DHE; red) labels reactive oxygen species (ROS); and, DAPI (blue) was used to visualize the nuclei in nephrocytes. Scale bar = 25 µm. (B) Quantitation of DHE fluorescence in Pink1/park/Marf-OE/IR nephrocytes. (C) Anti-ubiquitinylated proteins antibody, clone FK2 (green) was used to label protein aggregates in nephrocytes. Scale bar = 5 µm. Boxed areas are shown magnified to the right (scale bar: 1 μm). (D) Quantitation of FK2 fluorescence in Pink1/park/Marf-OE/IR nephrocytes, relative to control. N = 30 nephrocytes from 6 flies, per group per assay. Results are presented as mean ± SD. Statistical significance (*) is defined as p < 0.05.
3.5. Defective Endocytosis Leads to Reduced Nephrocyte Function, Induced Cellular Protein Aggregation, and Increased ROS
To understand whether induced cellular protein aggregation and increased ROS were caused by defective endocytosis induced by mitochondrial dysfunction, we knocked down the endocytic-associated gene Rab5 in flies. We noted a significant decline in both 10 kDa dextran and albumin intensity when silencing Rab5 in the nephrocytes (Figure 7A–C), indicating nephrocyte functional decline. We further observed a disrupted slit diaphragm localization pattern at the nephrocyte surface (Figure 7D) and a significant increase in DHE red fluorescence intensity in the nephrocyte nucleus and protein aggregation after silencing Rab5 (Figure 7E–H). Previous studies suggest that defective endocytosis can impair autophagy and lead to protein aggregation [37,38]. Protein aggregation in cells has been linked to increased ROS levels [39,40]. Together, these findings suggest that defects in endocytosis caused by mitochondrial dysfunction can lead to protein accumulation, which in turn may increase ROS levels.
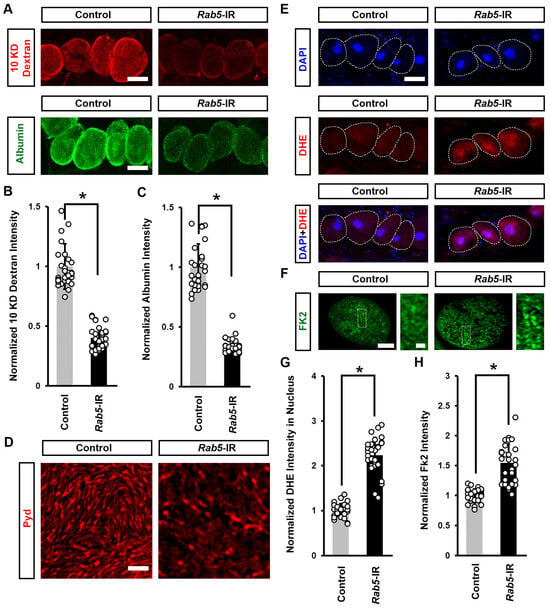
Figure 7.
Defective endocytosis reduced nephrocyte function, induced protein aggregation, and increased ROS: (A–H) Function, protein aggregation, and ROS in Drosophila nephrocytes (female, 4-day-old) inhibiting (RNAi; IR) Rab5. Control, Dot > w1118. (A) 10 kDa dextran (red) and albumin (green) uptake by nephrocytes. Scale bar = 25 µm. (B) Quantitation of 10 kDa dextran fluorescence in Rab4-IR nephrocytes. (C) Quantitation of albumin fluorescence in Rab5-IR nephrocyte. (D) Localization of slit diaphragm protein Polychaetoid (Pyd; red) by immunofluorescence in Drosophila nephrocytes. Imaged at nephrocyte surface. Scale bar = 1 µm. (E) Dihydroethidium (DHE; red) labels reactive oxygen species (ROS); and, DAPI (blue) was used to visualize the nuclei in nephrocytes. Scale bar = 25 µm. (F) Quantitation of DHE fluorescence in Rab5-IR nephrocytes. (G) Anti-ubiquitinylated proteins antibody, clone FK2 (green) was used to label protein aggregates in nephrocytes. Scale bar = 5 µm. Boxed areas are shown magnified to the right (scale bar: 1 μm). (H) Quantitation of FK2 fluorescence in Rab5-IR nephrocytes, relative to control. N = 30 nephrocytes from 6 flies, per group per assay. Results are presented as mean ± SD. Statistical significance (*) is defined as p < 0.05.
Altogether, these findings demonstrate that a defective Pink1–Park pathway—through silencing or overexpressing Pink1, park, or Marf—leads to altered mitochondrial dynamics, which disrupts endocytosis mechanisms, which induces protein aggregation and further increases cellular ROS levels, which likely plays a role in the abnormal uptake function observed in nephrocytes (Figure 8). However, mitochondrial dysfunction can also enhance glycolysis [41,42], leading to increased production of pyruvate, which can be converted to lactate. This process regenerates NAD+ from NADH and contributes to increased ROS levels.
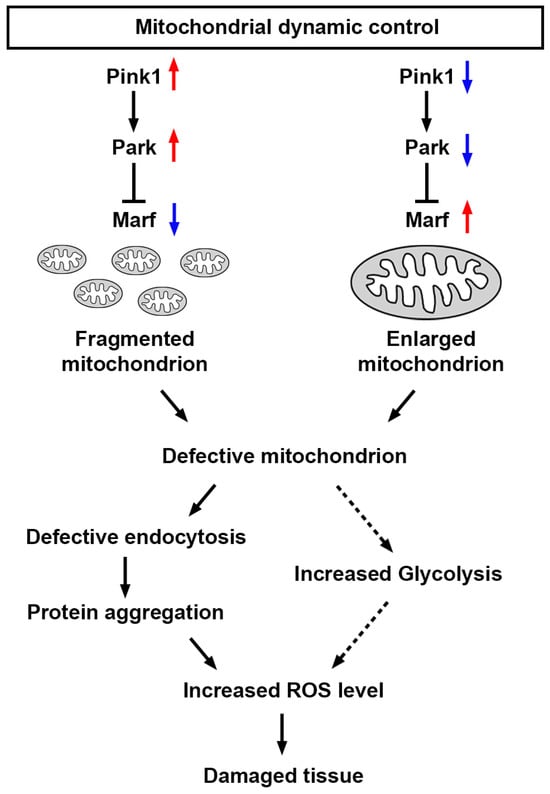
Figure 8.
Model of nephrocyte damage mediated by mitochondrial dynamics Schematic representation of the regulation of mitochondrial dynamics. Mitochondrial dynamics can be disrupted by the genetic modification of the Pink1–Park pathway (Pink1/park/Marf overexpression or silencing; red upward arrow indicates increase; blue downward arrow indicates decrease). The resulting changes in mitochondrial morphology lead to mitochondrial dysfunction, which impairs the endocytic trafficking pathway, resulting in protein aggregation, and increased reactive oxygen species (ROS), culminating in further tissue damage. Marf, Mitochondrial assembly regulatory factor; Park, Parkin; Pink1, PTEN-induced putative kinase 1.
4. Discussion
The Drosophila nephrocyte, a podocyte-like cell, features a filtration slit diaphragm and exhibits remarkable structural and functional similarities with the mammalian podocyte [19,43,44]. Both nephrocytes and podocytes form highly specialized filtration barriers [39]. Filtered molecules that pass through these filters in nephrocytes enter the lacuna channel, analogous to the urinary space in the Bowman’s capsule of human kidneys. While in human kidneys, filtered molecules undergo reabsorption or excretion, in nephrocytes, these molecules are absorbed and processed by the cells. Proteins are degraded for amino acid recycling, and waste is detoxified and stored in vacuoles. Although there are differences in processing post-filtration, nephrocytes perform a true filtration function that closely resembles that of podocytes [45]. Since being identified more than a decade ago, the Drosophila nephrocyte has emerged as an invaluable model for exploring development processes and kidney diseases [32,44,46].
Here, we explored the roles of mitochondria in Drosophila nephrocytes, focusing on how molecular regulators of mitochondrial dynamics through fission and fusion pathways maintain mitochondrial function. These pathways are highly conserved from Drosophila to humans [4,47,48,49]. We found that genetic manipulation of the Pink1–Park pathway disrupted mitochondrial dynamics, leading to abnormalities in the slit diaphragm and functional defects in the nephrocytes. Additionally, mitochondrial dysfunction in nephrocytes triggered defects in endocytosis, protein aggregation, increased levels of ROS, and, ultimately, cell damage (Figure 7). Our findings illustrate that dysfunction in mitochondrial dynamics in Drosophila nephrocytes leads to various functional defects, underscoring the critical role of mitochondria in nephrocyte development and kidney diseases. This research provides a valuable model for studying the mechanisms underlying mitochondrial-related kidney diseases and offers insights into potential therapeutic strategies.
Numerous genetic mutations have been linked to monogenic forms of nephrotic syndrome (NS), including several genes involved in the mitochondrial CoQ pathway [20,23,50]. However, significant gaps in understanding the roles of these genes in kidney cell biology and disease mechanisms remain. For instance, a deficiency in CoQ2, a component of the CoQ pathway, results in mitochondrial dysfunction and mislocalization of slit diaphragms [45,46]. Our current study suggests this might be due to endocytosis defects. Additionally, mitochondrial dysfunction has been implicated in diabetic nephropathy [51,52]. In previous studies using Drosophila, chronic exposure to high dietary sucrose mimicked several features of diabetic nephropathy [24,25,53]. Chronic sucrose intake also led to morphological abnormalities in the mitochondria of Drosophila nephrocytes, linked to defects in mitochondrial fission [24]. Genetic or pharmacological intervention at the Pink1–Park pathway attenuated the mitochondrial dysfunction and slowed the decline in nephrocyte function in the high-sucrose-fed flies [24]. Here, we showed the trajectory from disrupted mitochondrial dynamics to damaged tissue, through reduced endocytic trafficking, associated increased protein aggregates, and increased ROS (Figure 7). These, and previous, findings highlight the critical role of mitochondrial health in kidney function and provide a valuable framework for exploring therapeutic interventions in mitochondrial-related kidney diseases.
Author Contributions
J.-y.Z. and Z.H. designed the study; J.-y.Z. and J.D. carried out the experiments; J.-y.Z., J.v.d.L. and Z.H. analyzed and interpreted the data; J.-y.Z. and J.v.d.L. prepared the figures; J.-y.Z., J.v.d.L. and Z.H. drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Maryland Baltimore, Institute for Clinical & Translational Research (ICTR) Pilot Grant (J.Z.), the National Kidney Foundation mini-Grant (J.Z.), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK098410 and R01-DK120908 (Z.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
We are grateful to the Bloomington Drosophila Stock Center at Indiana University for providing the Drosophila stocks and to the Electron Microscopy Core Imaging Facility at the Center for Innovative Biomedical Resources (CIBR) (University of Maryland School of Medicine, MD, USA) for their assistance in acquiring TEM images.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kühlbrandt, W. Structure and Function of Mitochondrial Membrane Protein Complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Al Ojaimi, M.; Salah, A.; El-Hattab, A.W. Mitochondrial Fission and Fusion: Molecular Mechanisms, Biological Functions, and Related Disorders. Membranes 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 Coordinately Regulate Mitochondrial Fusion and Are Essential for Embryonic Development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Dodson, M.W.; Huang, H.; Guo, M. The Parkinson’s Disease Genes Pink1 and Parkin Promote Mitochondrial Fission and/or Inhibit Fusion in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 14503–14508. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sibilla, C.; Liu, R.; Yun, J.; Hay, B.A.; Blackstone, C.; Chan, D.C.; Harvey, R.J.; Guo, M. Clueless/CLUH Regulates Mitochondrial Fission by Promoting Recruitment of Drp1 to Mitochondria. Nat. Commun. 2022, 13, 1582. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin Mitochondrial Quality Control: A Source of Regional Vulnerability in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- McInnes, J. Insights on Altered Mitochondrial Function and Dynamics in the Pathogenesis of Neurodegeneration. Transl. Neurodegener. 2013, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ying, J.; Wang, X.; Zhao, T.; Yoon, S.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Hua, F. Mitochondrial Dynamics: A Key Role in Neurodegeneration and a Potential Target for Neurodegenerative Disease. Front. Neurosci. 2021, 15, 654785. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Mukherjee, A.; Nagotu, S. Mitochondrial Dynamics and Its Impact on Human Health and Diseases: Inside the DRP1 Blackbox. J. Mol. Med. 2022, 100, 1–21. [Google Scholar] [CrossRef]
- Ong, S.-B.; Hall, A.R.; Hausenloy, D.J. Mitochondrial Dynamics in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2013, 19, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial Dynamics in Health and Disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Pacheu-Grau, D.; Rucktäschel, R.; Deckers, M. Mitochondrial Dysfunction and Its Role in Tissue-Specific Cellular Stress. Cell Stress 2018, 2, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Nomura, M.; Jofuku, A.; Kato, H.; Suzuki, S.O.; Masuda, K.; Otera, H.; Nakanishi, Y.; Nonaka, I.; Goto, Y.-I.; et al. Mitochondrial Fission Factor Drp1 Is Essential for Embryonic Development and Synapse Formation in Mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Hoshijima, M.; Seo, K.; Bedja, D.; Sysa-Shah, P.; Andrabi, S.A.; Chen, W.; Höke, A.; Dawson, V.L.; Dawson, T.M.; et al. Parkin-Independent Mitophagy Requires Drp1 and Maintains the Integrity of Mammalian Heart and Brain. EMBO J. 2014, 33, 2798–2813. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, S.; Thongboonkerd, V. Mitochondrial Dysfunction and Kidney Stone Disease. Front. Physiol. 2020, 11, 566506. [Google Scholar] [CrossRef] [PubMed]
- Gujarati, N.A.; Vasquez, J.M.; Bogenhagen, D.F.; Mallipattu, S.K. The Complicated Role of Mitochondria in the Podocyte. Am. J. Physiol. Renal Physiol. 2020, 319, F955–F965. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Altintas, M.M. Podocytes. F1000Research 2016, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Weavers, H.; Prieto-Sánchez, S.; Grawe, F.; Garcia-López, A.; Artero, R.; Wilsch-Bräuninger, M.; Ruiz-Gómez, M.; Skaer, H.; Denholm, B. The Insect Nephrocyte Is a Podocyte-like Cell with a Filtration Slit Diaphragm. Nature 2009, 457, 322–326. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, J.-Y.; Richman, A.; Zhao, Z.; Zhang, F.; Ray, P.E.; Han, Z. A Drosophila Model System to Assess the Function of Human Monogenic Podocyte Mutations That Cause Nephrotic Syndrome. Hum. Mol. Genet. 2017, 26, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Huber, T.B. Flying Podocytes. Kidney Int. 2009, 75, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Cagan, R. The Drosophila Nephrocyte: Back on Stage. J. Am. Soc. Nephrol. 2013, 24, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Fu, Y.; Richman, A.; Zhao, Z.; Ray, P.E.; Han, Z. A Personalized Model of COQ2 Nephropathy Rescued by the Wild-Type COQ2 Allele or Dietary Coenzyme Q10 Supplementation. J. Am. Soc. Nephrol. 2017, 28, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; van de Leemput, J.; Han, Z. Promoting Mitochondrial Dynamics by Inhibiting the PINK1/PRKN Pathway to Relieve Diabetic Nephropathy. Dis. Model. Mech. 2024; ahead of print. [Google Scholar] [CrossRef]
- Kim, K.; Cha, S.J.; Choi, H.-J.; Kang, J.S.; Lee, E.Y. Dysfunction of Mitochondrial Dynamics in Drosophila Model of Diabetic Nephropathy. Life 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, P.; van de Leemput, J.; Zhao, Z.; Han, Z. Slit Diaphragm Maintenance Requires Dynamic Clathrin-Mediated Endocytosis Facilitated by AP-2, Lap, Aux and Hsc70-4 in Nephrocytes. Cell Biosci. 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Frederick, R.L.; Shaw, J.M. Moving Mitochondria: Establishing Distribution of an Essential Organelle. Traffic 2007, 8, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- de Boer, L.; de Vries, M.C.; Smeitink, J.A.M.; Koopman, W.J.H. Disorders of Mitochondrial Homeostasis, Dynamics, Protein Import, and Quality Control. In Physician’s Guide to the Diagnosis, Treatment, and Follow-up of Inherited Metabolic Diseases; Springer International Publishing: Cham, Switzerland, 2022; pp. 889–913. ISBN 9783030677268. [Google Scholar]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- van de Leemput, J.; Wen, P.; Han, Z. Using Drosophila Nephrocytes to Understand the Formation and Maintenance of the Podocyte Slit Diaphragm. Front. Cell Dev. Biol. 2022, 10, 837828. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhang, F.; Fu, Y.; Zhu, J.-Y.; Han, Z. Exocyst Genes Are Essential for Recycling Membrane Proteins and Maintaining Slit Diaphragm in Drosophila Nephrocytes. J. Am. Soc. Nephrol. 2020, 31, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, J.-Y.; Zhang, F.; Richman, A.; Zhao, Z.; Han, Z. Comprehensive Functional Analysis of Rab GTPases in Drosophila Nephrocytes. Cell Tissue Res. 2017, 368, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [PubMed]
- McCormick, R.; Pearson, T.; Vasilaki, A. Manipulation of Environmental Oxygen Modifies Reactive Oxygen and Nitrogen Species Generation during Myogenesis. Redox Biol. 2016, 8, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, Å.B.; Johansen, T. Autophagy and Endocytosis—Interconnections and Interdependencies. J. Cell Sci. 2020, 133, jcs228114. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Abada, A.; Elazar, Z. Endocytosis and Autophagy: Exploitation or Cooperation? Cold Spring Harb. Perspect. Biol. 2014, 6, a018358. [Google Scholar] [CrossRef] [PubMed]
- Lévy, E.; El Banna, N.; Baïlle, D.; Heneman-Masurel, A.; Truchet, S.; Rezaei, H.; Huang, M.-E.; Béringue, V.; Martin, D.; Vernis, L. Causative Links between Protein Aggregation and Oxidative Stress: A Review. Int. J. Mol. Sci. 2019, 20, 3896. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.-L.; Zhang, C.-W.; Li, L. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2018, 2018, 9163040. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Schurr, A.; Portincasa, P. Mitochondrial Transport in Glycolysis and Gluconeogenesis: Achievements and Perspectives. Int. J. Mol. Sci. 2021, 22, 12620. [Google Scholar] [CrossRef] [PubMed]
- Haythorne, E.; Lloyd, M.; Walsby-Tickle, J.; Tarasov, A.I.; Sandbrink, J.; Portillo, I.; Exposito, R.T.; Sachse, G.; Cyranka, M.; Rohm, M.; et al. Altered Glycolysis Triggers Impaired Mitochondrial Metabolism and MTORC1 Activation in Diabetic β-Cells. Nat. Commun. 2022, 13, 6754. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Huber, T.B. Insights into Human Kidney Function from the Study of Drosophila. Pediatr. Nephrol. 2023, 38, 3875–3887. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila Melanogaster: A Simple Genetic Model of Kidney Structure, Function and Disease. Nat. Rev. Nephrol. 2022, 18, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, X. The Drosophila Nephrocyte Has a Glomerular Filtration System. Nat. Rev. Nephrol. 2014, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Gautam, N.K. Drosophila Renal Organ as a Model for Identification of Targets and Screening of Potential Therapeutic Agents for Diabetic Nephropathy. Curr. Drug Targets 2018, 19, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.L.; Masoudi, R.; Haddadi, M. The Distinctive Role of Tau and Amyloid Beta in Mitochondrial Dysfunction through Alteration in Mfn2 and Drp1 MRNA Levels: A Comparative Study in Drosophila Melanogaster. Gene 2020, 754, 144854. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Mark Cooper, J.; Chau, K.-Y.; Rojo, M.; Schapira, A.H.V.; Taanman, J.-W. Mitofusin 1 and Mitofusin 2 Are Ubiquitinated in a PINK1/Parkin-Dependent Manner upon Induction of Mitophagy. Hum. Mol. Genet. 2013, 22, 1697. [Google Scholar] [CrossRef]
- Guo, M. Drosophila as a Model to Study Mitochondrial Dysfunction in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009944. [Google Scholar] [PubMed]
- Hermle, T.; Braun, D.A.; Helmstädter, M.; Huber, T.B.; Hildebrandt, F. Modeling Monogenic Human Nephrotic Syndrome in the Drosophila Garland Cell Nephrocyte. J. Am. Soc. Nephrol. 2017, 28, 1521–1533. [Google Scholar] [PubMed]
- Zhang, P.N.; Zhou, M.Q.; Guo, J.; Zheng, H.J.; Tang, J.; Zhang, C.; Liu, Y.N.; Liu, W.J.; Wang, Y.X. Mitochondrial Dysfunction and Diabetic Nephropathy: Nontraditional Therapeutic Opportunities. J. Diabetes Res. 2021, 2021, 1010268. [Google Scholar]
- Liu, S.; Yuan, Y.; Xue, Y.; Xing, C.; Zhang, B. Podocyte Injury in Diabetic Kidney Disease: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2022, 10, 832887. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Sweetwyne, M.T.; Park, A.S.D.; Susztak, K.; Cagan, R.L. Diet-Induced Podocyte Dysfunction in Drosophila and Mammals. Cell Rep. 2015, 12, 636–647. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).