Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes
Abstract
1. Introduction
2. Podocytes in Innate Immunity
2.1. Toll-like Receptors (TLRs) Signaling
2.2. Nucleotide-Binding Oligomerization Domain (NOD)-like Receptors (NLRs) Signaling
2.3. C-Type Lectin Receptors (CLRs) Signaling
2.4. Cyclic GMP-AMP (cGAS)-Stimulator of Interferon Genes (STING) Signaling
2.5. Retinoic Acid-Inducible Gene (RIG)-I-like Receptors (RLRs) Signaling
3. Immune Response in Podocytes from Different Glomerular Diseases
3.1. Diabetic Kidney Disease (DKD)
3.2. Alport Syndrome (AS)
3.3. Membranous Nephropathy (MN)
3.4. Minimal Change Disease (MCD)
3.5. Fabry Disease
3.6. Focal Segmental Glomerulosclerosis (FSGS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2023.
- St John, P.L.; Abrahamson, D.R. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001, 60, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Byron, A.; Randles, M.J.; Humphries, J.D.; Mironov, A.; Hamidi, H.; Harris, S.; Mathieson, P.W.; Saleem, M.A.; Satchell, S.C.; Zent, R.; et al. Glomerular cell cross-talk influences composition and assembly of extracellular matrix. J. Am. Soc. Nephrol. 2014, 25, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Villegas, G.; Teichman, J.; Mundel, P.; Tufro, A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am. J. Physiol. Renal Physiol. 2006, 291, F422–F428. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.G.; Foster, R.R.; Saleem, M.; Mathieson, P.W.; Gillatt, D.A.; Bates, D.O.; Harper, S.J. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am. J. Physiol. Renal Physiol. 2004, 286, F767–F773. [Google Scholar] [CrossRef] [PubMed]
- van Roeyen, C.R.C.; Eitner, F.; Boor, P.; Moeller, M.J.; Raffetseder, U.; Hanssen, L.; Bücher, E.; Villa, L.; Banas, M.C.; Hudkins, K.L.; et al. Induction of progressive glomerulonephritis by podocyte-specific overexpression of platelet-derived growth factor-D. Kidney Int. 2011, 80, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Sanchez-Russo, L.; Riella, L.V.; Verlato, A.; Manrique, J.; Granata, S.; Fiaccadori, E.; Pesce, F.; Zaza, G.; Cravedi, P. Immune abnormalities in IgA nephropathy. Clin. Kidney J. 2023, 16, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Li, X.K. The Role of Immune Modulation in Pathogenesis of IgA Nephropathy. Front. Med. 2020, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, X.; Peng, X.; Zhao, M.; Mi, L.; Lei, J.; Xu, K. Immune podocytes in the immune microenvironment of lupus nephritis (Review). Mol. Med. Rep. 2023, 28, 204. [Google Scholar] [CrossRef]
- Xipell, M.; Lledó, G.M.; Egan, A.C.; Tamirou, F.; del Castillo, C.S.; Rovira, J.; Gómez-Puerta, J.A.; García-Herrera, A.; Cervera, R.; Kronbichler, A.; et al. From systemic lupus erythematosus to lupus nephritis: The evolving road to targeted therapies. Autoimmun. Rev. 2023, 22, 103404. [Google Scholar] [CrossRef]
- Gupta, S.; Kaplan, M.J. Bite of the wolf: Innate immune responses propagate autoimmunity in lupus. J. Clin. Investig. 2021, 131, e144918. [Google Scholar] [CrossRef] [PubMed]
- Herrada, A.A.; Escobedo, N.; Iruretagoyena, M.; Valenzuela, R.A.; Burgos, P.I.; Cuitino, L.; Llanos, C. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.K.; Mullen, G.E.; Leifer, C.A.; Mazzoni, A.; Davies, D.R.; Segal, D.M. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003, 24, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M. Toll-Like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Cai, S.Y.; Shao, J.Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chadban, S.J.; Zhao, C.Y.; Chen, X.; Kwan, T.; Panchapakesan, U.; Pollock, C.A.; Wu, H. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 2014, 9, e97985. [Google Scholar] [CrossRef] [PubMed]
- Banas, M.C.; Banas, B.; Hudkins, K.L.; Wietecha, T.A.; Iyoda, M.; Bock, E.; Hauser, P.; Pippin, J.W.; Shankland, S.J.; Smith, K.D.; et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J. Am. Soc. Nephrol. 2008, 19, 704–713. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Avalos, A.M.; Mao, S.Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Papadimitraki, E.; Tzardi, M.; Bertsias, G.; Sotsiou, E.; Boumpas, D. Glomerular expression of toll-like receptor-9 in lupus nephritis but not in normal kidneys: Implications for the amplification of the inflammatory response. Lupus 2009, 18, 831–835. [Google Scholar] [CrossRef]
- Machida, H.; Ito, S.; Hirose, T.; Takeshita, F.; Oshiro, H.; Nakamura, T.; Mori, M.; Inayama, Y.; Yan, K.; Kobayashi, N.; et al. Expression of Toll-like receptor 9 in renal podocytes in childhood-onset active and inactive lupus nephritis. Nephrol. Dial. Transplant. 2010, 25, 2430–2537. [Google Scholar] [CrossRef] [PubMed]
- Batsford, S.; Duermueller, U.; Seemayer, C.; Mueller, C.; Hopfer, H.; Mihatsch, M. Protein level expression of Toll-like receptors 2, 4 and 9 in renal disease. Nephrol. Dial. Transplant. 2011, 26, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Samih, M.A.; Dzhindzhikhashvili, M.; Liu, H.; Balsam, L.; Rubinstein, S. Toll-like receptor 9 and vascular endothelial growth factor levels in human kidneys from lupus nephritis patients. J. Nephrol. 2012, 25, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Xia, H.; Liang, Y.; Ye, Y.; Lu, Y.; Xu, X.; Duan, A.; He, J.; Chen, Z.; Wu, Y.; et al. Toll-like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci. Rep. 2016, 6, 22579. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.A.; Ichii, O.; Hosny Ali Elewa, Y.; Nakamura, T.; Otani, Y.; Hosotani, M.; Kon, Y. Overexpression of toll-like receptor 9 correlates with podocyte injury in a murine model of autoimmune membranoproliferative glomerulonephritis. Autoimmunity 2018, 51, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Muruve, D.A. The inflammasomes in kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Meunier, E.; Broz, P. Evolutionary Convergence and Divergence in NLR Function and Structure. Trends Immunol. 2017, 38, 744–757. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Tsolis, R.M. NOD1 and NOD2: Beyond Peptidoglycan Sensing. Trends Immunol. 2017, 38, 758–767. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, T.J.; Ren, G.L.; Cai, L.; Meng, X.M. Novel insights into NOD-like receptors in renal diseases. Acta Pharmacol. Sin. 2022, 43, 2789–2806. [Google Scholar] [CrossRef]
- Schneider, M.; Zimmermann, A.G.; Roberts, R.A.; Zhang, L.; Swanson, K.V.; Wen, H.; Davis, B.K.; Allen, I.C.; Holl, E.K.; Ye, Z.; et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-kappaB. Nat. Immunol. 2012, 13, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Benko, S.; Magalhaes, J.G.; Philpott, D.J.; Girardin, S.E. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 2010, 185, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Stokman, G.; Kors, L.; Bakker, P.J.; Rampanelli, E.; Claessen, N.; Teske, G.J.D.; Butter, L.; van Andel, H.; van den Bergh Weerman, M.A.; Larsen, P.W.B.; et al. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. J. Exp. Med. 2017, 214, 2405–2420. [Google Scholar] [CrossRef]
- Anand, P.K.; Malireddi, R.K.; Lukens, J.R.; Vogel, P.; Bertin, J.; Lamkanfi, M.; Kanneganti, T.D. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 2012, 488, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Gao, B.; Song, A.Q.; Zhu, Y.J.; Zhou, J.; Li, W.Z.; Yin, Y.Y.; Wu, W.N. Spinal cord NLRP1 inflammasome contributes to dry skin induced chronic itch in mice. J. Neuroinflamm. 2020, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, L.; Paudel, S.; Jin, L.; Jeyaseelan, S. The NLRP6 inflammasome in health and disease. Mucosal Immunol. 2020, 13, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.; He, Z.; Xu, Y.; Li, X.; Ding, J.; Lu, M.; Hu, G. Kynurenine regulates NLRP2 inflammasome in astrocytes and its implications in depression. Brain Behav. Immun. 2020, 88, 471–481. [Google Scholar] [CrossRef]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Bujko, K.; Cymer, M.; Thapa, A.; Adamiak, M.; Ratajczak, J.; Abdel-Latif, A.K.; Kucia, M. The Nlrp3 inflammasome as a “rising star” in studies of normal and malignant hematopoiesis. Leukemia 2020, 34, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Lech, M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013, 84, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; He, F.F.; Tang, H.; Lei, C.T.; Chen, S.; Meng, X.F.; Su, H.; Zhang, C. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015, 2015, 504761. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, X.X.; Rui, H.L.; Li, L.J.; Zhao, J.; Yang, M.; Sun, L.J.; Dong, H.R.; Cheng, H.; Chen, Y.P. Artificially Cultivated Ophiocordyceps sinensis Alleviates Diabetic Nephropathy and Its Podocyte Injury via Inhibiting P2X7R Expression and NLRP3 Inflammasome Activation. J. Diabetes Res. 2018, 2018, 1390418. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Fan, B.; Han, H.; Zhen, J.; Shang, J.; Wang, X.; Li, X.; Shi, W.; Tang, W.; Bao, C.; et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int. 2013, 84, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Drouin, M.; Saenz, J.; Chiffoleau, E. C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity. Front. Immunol. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, I.M.; Brown, G.D. C-type lectins in immunity: Recent developments. Curr. Opin. Immunol. 2015, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Hoving, J.C.; Wilson, G.J.; Brown, G.D. Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol. 2014, 16, 185–194. [Google Scholar] [CrossRef]
- Sato, K.; Yang, X.L.; Yudate, T.; Chung, J.S.; Wu, J.; Luby-Phelps, K.; Kimberly, R.P.; Underhill, D.; Cruz, P.D., Jr.; Ariizumi, K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 2006, 281, 38854–38866. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, L.; Rosen, D.B.; Bover, L.; Watanabe, G.; Bao, M.; Lanier, L.L.; Liu, Y.J. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007, 5, e248. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Baker, E.; Sutherland, G.R.; Phillips, J.H.; Lanier, L.L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl. Acad. Sci. USA 1999, 96, 9792–9796. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van Het Hof, B.; van Kooyk, Y.; Geijtenbeek, T.B. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 2007, 26, 605–616. [Google Scholar] [CrossRef]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.S.; Willment, J.A.; Lin, H.H.; Williams, D.L.; Gordon, S.; Brown, G.D. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J. Biol. Chem. 2004, 279, 14792–14802. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Thibault, N.; Veilleux, P.; Gareau-Pagé, G.; Beaulieu, A.D. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: A new mechanism of action for SHP-2. Mol. Immunol. 2006, 43, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhou, T.; Wang, X.; Shang, M.; Zhang, Y.; Luo, M.; Xu, C.; Yuan, W. DC-SIGN expression on podocytes and its role in inflammatory immune response of lupus nephritis. Clin. Exp. Immunol. 2016, 183, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanaka, M.; Watanabe, N.; Ito, M.; Pastan, I.; Koizumi, M.; Matsusaka, T. C-type lectin-like receptor (CLEC)-2, the ligand of podoplanin, induces morphological changes in podocytes. Sci. Rep. 2022, 12, 22356. [Google Scholar] [CrossRef]
- Su, Z.; Li, Y.; Lv, H.; Cui, X.; Liu, M.; Wang, Z.; Zhang, Y.; Zhen, J.; Tang, W.; Wang, X.; et al. CLEC14A protects against podocyte injury in mice with adriamycin nephropathy. FASEB J. 2021, 35, e21711. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Rohl, I.; Hopfner, K.P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, Y.; Wang, H.; Yan, Y.; Ding, C.; Sun, J. Chicken STING Mediates Activation of the IFN Gene Independently of the RIG-I Gene. J. Immunol. 2015, 195, 3922–3936. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Yang, Y.; Li, S.; Wang, Y.Y.; Li, Y.; Diao, F.; Lei, C.; He, X.; Zhang, L.; Tien, P.; et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 2008, 29, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Zhang, C.; Chen, Z.J.; Bai, X.C.; Zhang, X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 2019, 567, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Cho, M.G.; Kim, E.Y.; Kwon, D.; Kang, S.J.; Lee, J.H. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp. Mol. Med. 2020, 52, 643–657. [Google Scholar] [CrossRef]
- Yatim, N.; Jusforgues-Saklani, H.; Orozco, S.; Schulz, O.; Barreira da Silva, R.; Reis e Sousa, C.; Green, D.R.; Oberst, A.; Albert, M.L. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8⁺ T cells. Science 2015, 350, 328–334. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zimmerman, S.E.; Weyemi, U. Genomic instability and metabolism in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef]
- Burdette, D.L.; Vance, R.E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 2013, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Takahashi, M.; Sharma, S. Cytosolic sensing of immuno-stimulatory DNA, the enemy within. Curr. Opin. Immunol. 2018, 50, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Chiang, S.H.; Decker, S.J.; Chang, L.; Uhm, M.; Larsen, M.J.; Rubin, J.R.; Mowers, J.; White, N.M.; Hochberg, I.; et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat. Med. 2013, 19, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wong, K.I.; Sun, X.; Reilly, S.M.; Uhm, M.; Liao, Z.; Skorobogatko, Y.; Saltiel, A.R. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell 2018, 172, 731–743.e12. [Google Scholar] [CrossRef]
- Ding, L.; Dong, G.; Zhang, D.; Ni, Y.; Hou, Y. The regional function of cGAS/STING signal in multiple organs: One of culprit behind systemic lupus erythematosus? Med. Hypotheses 2015, 85, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Park, J.; Takamatsu, H.; Konaka, H.; Aoki, W.; Aburaya, S.; Ueda, M.; Nishide, M.; Koyama, S.; Hayama, Y.; et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1507–1515. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J.; et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019, 30, 784–799.e5. [Google Scholar] [CrossRef]
- Maekawa, H.; Inagi, R.; Nangaku, M.; Inoue, T.; Inoue, R.; Nishi, H. SUN-155 Mitochondrial DNA leakage causes inflammation via the cGAS-STING axis in cisplatin-induced acute kidney injury. Kidney Int. Rep. 2019, 4, S222. [Google Scholar] [CrossRef]
- Maekawa, H.; Inoue, T.; Ouchi, H.; Jao, T.M.; Inoue, R.; Nishi, H.; Fujii, R.; Ishidate, F.; Tanaka, T.; Tanaka, Y.; et al. Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep. 2019, 29, 1261–1273.e6. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cervantes, C.; Liu, J.; He, S.; Zhou, H.; Zhang, B.; Cai, H.; Yin, D.; Hu, D.; Li, Z.; et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 12196–12201. [Google Scholar] [CrossRef] [PubMed]
- Zang, N.; Cui, C.; Guo, X.; Song, J.; Hu, H.; Yang, M.; Xu, M.; Wang, L.; Hou, X.; He, Q.; et al. cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience 2022, 25, 105145. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Geng, K.; Law, B.Y.-K.; Wang, P.; Pu, Y.L.; Chen, Q.; Xu, H.W.; Tan, X.Z.; Jiang, Z.Z.; Xu, Y. Lipotoxicity-induced mtDNA release promotes diabetic cardiomyopathy by activating the cGAS-STING pathway in obesity-related diabetes. Cell Biol. Toxicol. 2022, 39, 277–299. [Google Scholar] [CrossRef]
- Davis, S.E.; Khatua, A.K.; Popik, W. Nucleosomal dsDNA Stimulates APOL1 Expression in Human Cultured Podocytes by Activating the cGAS/IFI16-STING Signaling Pathway. Sci. Rep. 2019, 9, 15485. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G. Nucleic acid immunity. Adv. Immunol. 2017, 133, 121–169. [Google Scholar] [PubMed]
- Goubau, D.; Deddouche, S.; e Sousa, C.R. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef]
- Andrejeva, J.; Childs, K.; Young, D.; Carlos, T.; Stock, N.; Goodbourn, S.; Randall, R. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 2004, 101, 17264–17269. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Hur, S. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu. Rev. Immunol. 2019, 37, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Roth, A.; Hornung, V.; Hagmann, C.A.; Wimmenauer, V.; Barchet, W.; Coch, C.; Janke, M.; Mihailovic, A.; Wardle, G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 2009, 31, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.J.; Sparrer, K.M.; van Gent, M.; Lässig, C.; Huang, T.; Osterrieder, N.; Hopfner, K.-P.; Gack, M.U. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat. Immunol. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, X.; Dunker, W.; Song, Y.; Karijolich, J. RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection. Nat. Commun. 2018, 9, 4841. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Dhir, S.; Borowski, L.S.; Jimenez, L.; Teitell, M.; Rötig, A.; Crow, Y.J.; Rice, G.I.; Duffy, D.; Tamby, C.; et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018, 560, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Tigano, M.; Vargas, D.C.; Tremblay-Belzile, S.; Fu, Y.; Sfeir, A. Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature 2021, 591, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jin, P.; Shen, C.; Lin, W.; Yu, L.; Hu, X.; Meng, T.; Zhang, L.; Peng, L.; Xiao, X.; et al. Single-cell RNA sequencing reveals the transcriptomic landscape of kidneys in patients with ischemic acute kidney injury. Chin. Med. J. 2023, 136, 1177–1187. [Google Scholar] [CrossRef]
- Urabe, A.; Doi, S.; Nakashima, A.; Ike, T.; Morii, K.; Sasaki, K.; Doi, T.; Arihiro, K.; Masaki, T. Klotho deficiency intensifies hypoxia-induced expression of IFN-α/β through upregulation of RIG-I in kidneys. PLoS ONE 2021, 16, e0258856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ni, J.; Li, J.; Huo, C.; Miao, N.; Yin, F.; Cheng, Q.; Xu, D.; Xie, H.; Chen, P.; et al. RIG-I aggravates interstitial fibrosis via c-Myc-mediated fibroblast activation in UUO mice. J. Mol. Med. 2020, 98, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Aizawa-Yashiro, T.; Tsuruga, K.; Tanaka, H.; Matsumiya, T.; Yoshida, H.; Tatsuta, T.; Xing, F.; Hayakari, R.; Satoh, K. Melanoma differentiation-associated gene 5 regulates the expression of a chemokine CXCL10 in human mesangial cells: Implications for chronic inflammatory renal diseases. Tohoku J. Exp. Med. 2012, 228, 17–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamashita, M.; Millward, C.A.; Inoshita, H.; Saikia, P.; Chattopadhyay, S.; Sen, G.C.; Emancipator, S.N. Antiviral innate immunity disturbs podocyte cell function. J. Innate Immun. 2013, 5, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Shimada, M.; Narita-Kinjo, I.; Nagawa, D.; Kitayama, K.; Hamadate, M.; Miura, N.; Nozaka, M.; Kawamura, Y.; Fujita, T.; et al. PolyIC Induces Retinoic Acid-inducible Gene-I and Melanoma Differentiation-associated Gene 5 and Modulates Inflammation in Podocytes. In Vivo 2021, 35, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yao, X.; Hou, M.; Duan, M.; Xing, L.; Huang, J.; Wang, Y.; Zhu, B.; Chen, Q.; Wang, H. ApoL1 induces kidney inflammation through RIG-I/NF-kappaB activation. Biochem. Biophys. Res. Commun. 2020, 527, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Hägele, H.; Allam, R.; Pawar, R.D.; Anders, H.-J. Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrol. Dial. Transplant. 2009, 24, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Tanaka, H.; Matsumiya, T.; Yoshida, H.; Tanji, K.; Tsuruga, K.; Oki, E.; Aizawa-Yashiro, T.; Ito, E.; Satoh, K. Retinoic acid-inducible gene-I is induced by double-stranded RNA and regulates the expression of CC chemokine ligand (CCL) 5 in human mesangial cells. Nephrol. Dial. Transplant. 2010, 25, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Navarro, J.F. Inflammation and diabetic nephropathy. Curr. Diabetes Rep. 2006, 6, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R. Linking metabolism and immunology: Diabetic nephropathy is an inflammatory disease. J. Am. Soc. Nephrol. 2005, 16, 1537–1538. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Fontanella, A.M.; Merscher, S.; Fornoni, A. Lipid deposition and metaflammation in diabetic kidney disease. Curr. Opin. Pharmacol. 2020, 55, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.P.; Mohan, M.; McClelland, A.; Tikellis, C.; Ziemann, M.; Kaspi, A.; Gray, S.P.; Pickering, R.; Tan, S.M.; Ali-Shah, S.T.; et al. Lipoxins Regulate the Early Growth Response-1 Network and Reverse Diabetic Kidney Disease. J. Am. Soc. Nephrol. JASN 2018, 29, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Park, A.S.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.M.; Todd, J.N.; Sandholm, N.; Cole, J.B.; Chen, W.-M.; Andrews, D.; Pezzolesi, M.G.; McKeigue, P.M.; Hiraki, L.T.; Qiu, C.; et al. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J. Am. Soc. Nephrol. 2019, 30, 2000–2016. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, G.; Zhong, H.; Chen, M.; Chen, T.; Gao, L.; Wu, H.; Guo, J. RIG-I inhibits pancreatic β cell proliferation through competitive binding of activated Src. Sci. Rep. 2016, 6, 28914. [Google Scholar] [CrossRef]
- Aida, K.; Nishida, Y.; Tanaka, S.; Maruyama, T.; Shimada, A.; Awata, T.; Suzuki, M.; Shimura, H.; Takizawa, S.; Ichijo, M.; et al. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes 2011, 60, 884–889. [Google Scholar] [CrossRef] [PubMed]
- He, Q.-Q.; Huang, Y.; Nie, L.; Ren, S.; Xu, G.; Deng, F.; Cheng, Z.; Zuo, Q.; Zhang, L.; Cai, H.; et al. MAVS integrates glucose metabolism and RIG-I-like receptor signaling. Nat. Commun. 2023, 14, 5343. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Tian, X.; Men, L.; Li, S.; Chen, Y.; Xue, M.; Hu, Y.; Zhou, P.; Long, G.; Shi, Y.; et al. Spleen tyrosine kinase promotes NLR family pyrin domain containing 3 inflammasome-mediated IL-1beta secretion via c-Jun N-terminal kinase activation and cell apoptosis during diabetic nephropathy. Mol. Med. Rep. 2018, 18, 1995–2008. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism 2021, 118, 154748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, X.; Jiang, M.; Wang, Z.F.; Zhao, T.; Cao, S.M.; Li, Q.M. GLP-1RAs inhibit the activation of the NLRP3 inflammasome signaling pathway to regulate mouse renal podocyte pyroptosis. Acta Diabetol. 2024, 61, 225–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, P.; Tang, M.; Song, Y.; Liu, C.; Zhao, B. Dapagliflozin alleviates renal podocyte pyroptosis via regulation of the HO-1/NLRP3 axis. Mol. Med. Rep. 2023, 28, 200. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, J.A.; Jha, J.C.; Sharma, A.; Dai, A.; Choi, J.S.; de Haan, J.B.; Cooper, M.E.; Jandeleit-Dahm, K. Adverse renal effects of NLRP3 inflammasome inhibition by MCC950 in an interventional model of diabetic kidney disease. Clin. Sci. 2022, 136, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Knauf, F.; Asplin, J.R.; Granja, I.; Schmidt, I.M.; Moeckel, G.W.; David, R.J.; Flavell, R.A.; Aronson, P.S. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013, 84, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Fontanella, A.; Tolerico, M.; Mallela, S.K.; Molina, J.; Kim, J.J.; Burke, G.; Merscher, S.; Fornoni, A. STING activation causes proteinuria in mice and contributes to glomerular disease. Kidney Int. Rep. 2022, 7, S155–S156. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Fontanella, A.; Tolerico, M.; Mallela, S.; Molina, J.; Zuo, Y.; Boulina, M.; Kim, J.-J.; Varona Santos, J.; Ge, M.; et al. Activation of Stimulator of Interferon Genes (STING) Causes Proteinuria and Contributes to Glomerular Diseases. J. Am. Soc. Nephrol. 2022, 33, 2153–2173. [Google Scholar] [CrossRef] [PubMed]
- Khedr, S.; Dissanayake, L.V.; Palygin, O.; Staruschenko, A. Potential Role of cGAS-STING Pathway in the Induction of Diabetic Kidney Disease. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Qi, H.; Casalena, G.; Shi, S.; Yu, L.; Ebefors, K.; Sun, Y.; Zhang, W.; D’Agati, V.; Schlondorff, D.; Haraldsson, B.; et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes 2017, 66, 763–778. [Google Scholar] [CrossRef]
- Casalena, G.A.; Yu, L.; Gil, R.; Rodriguez, S.; Sosa, S.; Janssen, W.; Azeloglu, E.U.; Leventhal, J.S.; Daehn, I.S. The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes. Cell Commun. Signal 2020, 18, 105. [Google Scholar] [CrossRef]
- Yang, N.; Wang, M.; Lin, K.; Wang, M.; Xu, D.; Han, X.; Zhao, X.; Wang, Y.; Wu, G.; Luo, W.; et al. Dectin-1 deficiency alleviates diabetic cardiomyopathy by attenuating macrophage-mediated inflammatory response. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166710. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J. Renal, auricular, and ocular outcomes of Alport syndrome and their current management. Pediatr. Nephrol. 2018, 33, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Warady, B.A.; Agarwal, R.; Bangalore, S.; Chapman, A.; Levin, A.; Stenvinkel, P.; Toto, R.D.; Chertow, G.M. Alport Syndrome Classification and Management. Kidney Med. 2020, 2, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Delimont, D.; Dufek, B.M.; Meehan, D.T.; Zallocchi, M.; Gratton, M.A.; Phillips, G.; Cosgrove, D. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS ONE 2014, 9, e99083. [Google Scholar] [CrossRef] [PubMed]
- Dufek, B.; Meehan, D.T.; Delimont, D.; Cheung, L.; Gratton, M.A.; Phillips, G.; Song, W.; Liu, S.; Cosgrove, D. Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int. 2016, 90, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Rodgers, K.; Meehan, D.; Miller, C.; Bovard, K.; Gilroy, A.; Gardner, H.; Kotelianski, V.; Gotwals, P.; Amatucci, A.; et al. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am. J. Pathol. 2000, 157, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.; Mulay, S.R.; Miosge, N.; Gross, O.; Anders, H.J. Tumour necrosis factor-α drives Alport glomerulosclerosis in mice by promoting podocyte apoptosis. J. Pathol. 2012, 226, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Chimenz, R.; Chirico, V.; Basile, P.; Carcione, A.; Conti, G.; Monardo, P.; Lacquaniti, A. HMGB-1 and TGFβ-1 highlight immuno-inflammatory and fibrotic processes before proteinuria onset in pediatric patients with Alport syndrome. J. Nephrol. 2021, 34, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, C.; Schachter, A.; Klickstein, L.; Liu, X.; Jennings, L.; Finkel, N. Urinary Monocyte Chemoattractant Protein-1 in Patients With Alport Syndrome. Kidney Int. Rep. 2022, 7, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.H.; Suzuki, K.; Downes, M.; Welch, G.L.; De Jesus, P.; Miraglia, L.J.; Orth, A.P.; Chanda, S.K.; Evans, R.M.; Verma, I.M. Tumor suppressor protein (p)53, is a regulator of NF-kappaB repression by the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 17117–17122. [Google Scholar] [CrossRef]
- Fukuda, R.; Suico, M.A.; Kai, Y.; Omachi, K.; Motomura, K.; Koga, T.; Komohara, Y.; Koyama, K.; Yokota, T.; Taura, M.; et al. Podocyte p53 Limits the Severity of Experimental Alport Syndrome. J. Am. Soc. Nephrol. 2016, 27, 144–157. [Google Scholar] [CrossRef]
- Kaseda, S.; Sannomiya, Y.; Horizono, J.; Kuwazuru, J.; Suico, M.A.; Ogi, S.; Sasaki, R.; Sunamoto, H.; Fukiya, H.; Nishiyama, H.; et al. Novel Keap1-Nrf2 Protein-Protein Interaction Inhibitor UBE-1099 Ameliorates Progressive Phenotype in Alport Syndrome Mouse Model. Kidney360 2022, 3, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gu, X.; Zheng, Q.; Liu, Y.; Suhas, T.; Du, W.; Xie, L.; Fang, Z.; Zhao, Y.; Yang, M.; et al. Tauroursodeoxycholic acid ameliorates renal injury induced by COL4A3 mutation. Kidney Int. 2024, in press. [CrossRef] [PubMed]
- Sethi, S.; Beck, L.H., Jr.; Glassock, R.J.; Haas, M.; De Vriese, A.S.; Caza, T.N.; Hoxha, E.; Lambeau, G.; Tomas, N.M.; Madden, B.; et al. Mayo Clinic consensus report on membranous nephropathy: Proposal for a novel classification. Kidney Int. 2023, 104, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G. Primary Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H., Jr.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Kumar, V.; Kumar, A.; Yadav, A.K.; Nada, R.; Kumar, H.; Kumar, V.; Rathi, M.; Kohli, H.S.; Gupta, K.L.; et al. PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol. Dial. Transplant. 2015, 31, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Debiec, H.; Madden, B.; Charlesworth, M.C.; Morelle, J.; Gross, L.; Ravindran, A.; Buob, D.; Jadoul, M.; Fervenza, F.C.; et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020, 97, 163–174. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.; Debiec, H.; Morelle, J.; Charlesworth, M.C.; Gross, L.; Negron, V.; Buob, D.; Chaudhry, S.; Jadoul, M.; et al. Protocadherin 7-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1249–1261. [Google Scholar] [CrossRef]
- Caza, T.N.; Hassen, S.I.; Kuperman, M.; Sharma, S.G.; Dvanajscak, Z.; Arthur, J.; Edmondson, R.; Storey, A.; Herzog, C.; Kenan, D.J.; et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021, 100, 171–181. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, C.H.; Huang, Y.C.; Chan, C.J.; Chen, D.C.; Tsai, F.J. Genetic susceptibility to idiopathic membranous nephropathy in high-prevalence Area, Taiwan. Biomedicine 2014, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, Y.; Huang, X.; Li, P.; Liang, X.; Wang, R.; Bao, K. Identification of hub genes and their correlation with immune infiltrating cells in membranous nephropathy: An integrated bioinformatics analysis. Eur. J. Med. Res. 2023, 28, 525. [Google Scholar] [CrossRef]
- Cameron, J.S. Nephrotic syndrome in the elderly. Semin. Nephrol. 1996, 16, 319–329. [Google Scholar] [PubMed]
- Barisoni, L.; Schnaper, H.W.; Kopp, J.B. A Proposed Taxonomy for the Podocytopathies: A Reassessment of the Primary Nephrotic Diseases. Clin. J. Am. Soc. Nephrol. 2007, 2, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, R.; Bonanni, A.; Caridi, G.; Canepa, A.; Ghiggeri, G.M. Molecular and Cellular Mechanisms for Proteinuria in Minimal Change Disease. Front. Med. 2018, 5, 170. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.W.; Wei, C.L.; Tan, L.K.; Tan, P.H.; Chiang, G.S.; Lee, C.G.; Jordan, S.C.; Yap, H.K. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J. Am. Soc. Nephrol. 2007, 18, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.H.; West, L.; Zheng, W. Effect of interleukin-8 on glomerular sulfated compounds and albuminuria. Pediatr. Nephrol. 1997, 11, 274–279. [Google Scholar] [CrossRef]
- Kim, A.H.; Chung, J.J.; Akilesh, S.; Koziell, A.; Jain, S.; Hodgin, J.B.; Miller, M.J.; Stappenbeck, T.S.; Miner, J.H.; Shaw, A.S. B cell-derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight 2017, 2, e81836. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, J.; Beldi-Ferchiou, A.; Audureau, E.; Azzaoui, I.; Molinier-Frenkel, V.; Frontera, V.; Karras, A.; Moktefi, A.; Pillebout, E.; Zaidan, M.; et al. Circulating plasmablasts and high level of BAFF are hallmarks of minimal change nephrotic syndrome in adults. Nephrol. Dial. Transplant. 2020, 36, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.B.; Keller, K.H.; Lerner, G.; Rosales, I.; Collins, A.B.; Sekulic, M.; Waikar, S.S.; Chandraker, A.; Riella, L.V.; Alexander, M.P.; et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J. Am. Soc. Nephrol. 2022, 33, 238–252. [Google Scholar] [CrossRef]
- Reiser, J.; von Gersdorff, G.; Loos, M.; Oh, J.; Asanuma, K.; Giardino, L.; Rastaldi, M.P.; Calvaresi, N.; Watanabe, H.; Schwarz, K.; et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Investig. 2004, 113, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Cara-Fuentes, G.; Wasserfall, C.H.; Wang, H.; Johnson, R.J.; Garin, E.H. Minimal change disease: A dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr. Nephrol. 2014, 29, 2333–2340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cara-Fuentes, G.; Venkatareddy, M.; Verma, R.; Segarra, A.; Cleuren, A.C.; Martínez-Ramos, A.; Johnson, R.J.; Garg, P. Glomerular endothelial cells and podocytes can express CD80 in patients with minimal change disease during relapse. Pediatr. Nephrol. 2020, 35, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, J.; Min, W.; Ai, J.; Lin, X.; Miao, J.; Zhou, S.; Liang, Y.; Chen, S.; Ren, Q.; et al. B7-1 mediates podocyte injury and glomerulosclerosis through communication with Hsp90ab1-LRP5-β-catenin pathway. Cell Death Differ. 2022, 29, 2399–2416. [Google Scholar] [CrossRef] [PubMed]
- Hengel, F.E.; Dehde, S.; Lassé, M.; Zahner, G.; Seifert, L.; Schnarre, A.; Kretz, O.; Demir, F.; Pinnschmidt, H.O.; Grahammer, F.; et al. Autoantibodies Targeting Nephrin in Podocytopathies. N. Engl. J. Med. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Desnick, R.J.; Brady, R.O. Fabry disease in childhood. J. Pediatr. 2004, 144, S20–S26. [Google Scholar] [CrossRef]
- Ries, M.; Ramaswami, U.; Parini, R.; Lindblad, B.; Whybra, C.; Willers, I.; Gal, A.; Beck, M. The early clinical phenotype of Fabry disease: A study on 35 European children and adolescents. Eur. J. Pediatr. 2003, 162, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Vernon, J.; Kopach, O.; Minett, M.S.; Mills, K.; Clayton, P.T.; Meert, T.; Wood, J.N. The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci. Lett. 2015, 594, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Thurberg, B.L.; Rennke, H.; Colvin, R.B.; Dikman, S.; Gordon, R.E.; Collins, A.B.; Desnick, R.J.; O’Callaghan, M. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002, 62, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Ortiz, A.; Sánchez-Niño, M.D. Lyso-Gb3 Increases αvβ3 Integrin Gene Expression in Cultured Human Podocytes in Fabry Nephropathy. J. Clin. Med. 2020, 9, 3659. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Blomberg, L.; Brodesser, S.; Liebau, M.C.; Schermer, B.; Benzing, T.; Kurschat, C.E. Enzyme Replacement Therapy Clears Gb3 Deposits from a Podocyte Cell Culture Model of Fabry Disease but Fails to Restore Altered Cellular Signaling. Cell Physiol. Biochem. 2019, 52, 1139–1150. [Google Scholar] [CrossRef]
- Wu, H.; Behera, T.R.; Gong, J.; Shen, Q. Coexistence of Fabry disease with IgM nephropathy: A case report. Medicine 2019, 98, e17566. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Kang, E.; Kim, Y.M.; Go, H.; Kim, K.Y.; Jung, J.Y.; Kang, M.; Kim, G.H.; Kim, J.M.; Choi, I.H.; et al. Fabry disease: Characterisation of the plasma proteome pre- and post-enzyme replacement therapy. J. Med. Genet. 2017, 54, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Laffer, B.; Lenders, M.; Ehlers-Jeske, E.; Heidenreich, K.; Brand, E.; Köhl, J. Complement activation and cellular inflammation in Fabry disease patients despite enzyme replacement therapy. Front. Immunol. 2024, 15, 1307558. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Taguchi, A.; Nishikawa, Y.; Guili, C.; Mikame, M.; Nameta, M.; Yamaguchi, Y.; Ueno, M.; Imai, N.; Ito, Y.; et al. Medullary thick ascending limb impairment in the Gla(tm)Tg(CAG-A4GALT) Fabry model mice. FASEB J. 2018, 32, 4544–4559. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, P.; Feriozzi, S.; Braun, F. The role of tubular cells in the pathogenesis of Fabry nephropathy. Front. Cardiovasc. Med. 2024, 11, 1386042. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, R.; Cardona-Muñoz, E.G.; Ortiz-Lazareno, P.; Andrade-Sierra, J.; Gómez-Hermosillo, L.F.; Casillas-Moreno, J.; Campos-Bayardo, T.I.; Román-Rojas, D.; García-Sánchez, A.; Miranda-Díaz, A.G. The Role of the Oxidative State and Innate Immunity Mediated by TLR7 and TLR9 in Lupus Nephritis. Int. J. Mol. Sci. 2023, 24, 15234. [Google Scholar] [CrossRef] [PubMed]
- Matafora, V.; Cuccurullo, M.; Beneduci, A.; Petrazzuolo, O.; Simeone, A.; Anastasio, P.; Mignani, R.; Feriozzi, S.; Pisani, A.; Comotti, C.; et al. Early markers of Fabry disease revealed by proteomics. Mol. Biosyst. 2015, 11, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, L.; Vázquez-Mosquera, M.E.; Colón-Mejeras, C.; Bravo, S.B.; Barbosa-Gouveia, S.; Álvarez, J.V.; Sánchez-Martínez, R.; López-Mendoza, M.; López-Rodríguez, M.; Villacorta-Argüelles, E.; et al. Characterization of the plasma proteomic profile of Fabry disease: Potential sex- and clinical phenotype-specific biomarkers. Transl. Res. 2024, 269, 47–63. [Google Scholar] [CrossRef]
- Doykov, I.D.; Heywood, W.E.; Nikolaenko, V.; Śpiewak, J.; Hällqvist, J.; Clayton, P.T.; Mills, P.; Warnock, D.G.; Nowak, A.; Mills, K. Rapid, proteomic urine assay for monitoring progressive organ disease in Fabry disease. J. Med. Genet. 2020, 57, 38–47. [Google Scholar] [CrossRef]
- Braun, F.; Abed, A.; Sellung, D.; Rogg, M.; Woidy, M.; Eikrem, O.; Wanner, N.; Gambardella, J.; Laufer, S.D.; Haas, F.; et al. Accumulation of α-synuclein mediates podocyte injury in Fabry nephropathy. J. Clin. Investig. 2023, 133, e157782. [Google Scholar] [CrossRef]
- McGrogan, A.; Franssen, C.F.M.; de Vries, C.S. The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol. Dial. Transplant. 2010, 26, 414–430. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Kopp, J.B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017, 12, 502–517. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Kaskel, F.J.; Falk, R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 2011, 365, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- de Cos, M.; Meliambro, K.; Campbell, K.N. Novel Treatment Paradigms: Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2023, 8, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.W., 3rd; Mitrofanova, A.; Fontanella, A.; Ciancio, G.; Roth, D.; Ruiz, P.; Abitbol, C.; Chandar, J.; Merscher, S.; Fornoni, A. The podocyte: Glomerular sentinel at the crossroads of innate and adaptive immunity. Front. Immunol. 2023, 14, 1201619. [Google Scholar] [CrossRef] [PubMed]
- Savin, V.J.; Sharma, R.; Sharma, M.; McCarthy, E.T.; Swan, S.K.; Ellis, E.; Lovell, H.; Warady, B.; Gunwar, S.; Chonko, A.M.; et al. Circulating Factor Associated with Increased Glomerular Permeability to Albumin in Recurrent Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 1996, 334, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Savin, V.J.; McCarthy, E.T.; Sharma, R.; Charba, D.; Sharma, M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl. Res. 2008, 151, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.; Vinogradov, A.; Cao, V.; Gindis, A.; Berns, A.; Alentov, I.; Sergeeva, N. Serum levels of plasminogen activator urokinase receptor and cardiotrophin-like cytokine factor 1 in patients with nephrotic syndrome. Clin. Nephrol. 2022, 97, 103–110. [Google Scholar] [CrossRef]
- Sharma, M.; Zhou, J.; Gauchat, J.-F.; Sharma, R.; McCarthy, E.T.; Srivastava, T.; Savin, V.J. Janus kinase 2/signal transducer and activator of transcription 3 inhibitors attenuate the effect of cardiotrophin-like cytokine factor 1 and human focal segmental glomerulosclerosis serum on glomerular filtration barrier. Transl. Res. 2015, 166, 384–398. [Google Scholar] [CrossRef]
- Müller-Deile, J.; Sarau, G.; Kotb, A.M.; Jaremenko, C.; Rolle-Kampczyk, U.E.; Daniel, C.; Kalkhof, S.; Christiansen, S.H.; Schiffer, M. Novel diagnostic and therapeutic techniques reveal changed metabolic profiles in recurrent focal segmental glomerulosclerosis. Sci. Rep. 2021, 11, 4577. [Google Scholar] [CrossRef]
- Wei, C.; Trachtman, H.; Li, J.; Dong, C.; Friedman, A.L.; Gassman, J.J.; McMahan, J.L.; Radeva, M.; Heil, K.M.; Trautmann, A.; et al. Circulating suPAR in two cohorts of primary FSGS. J. Am. Soc. Nephrol. 2012, 23, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Yacov, N.; Feldman, B.; Volkov, A.; Ishai, E.; Breitbart, E.; Mendel, I. Treatment with lecinoxoids attenuates focal and segmental glomerulosclerosis development in nephrectomized rats. Basic Clin. Pharmacol. Toxicol. 2019, 124, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Abid, Q.; Best Rocha, A.; Larsen, C.P.; Schulert, G.; Marsh, R.; Yasin, S.; Patty-Resk, C.; Valentini, R.P.; Adams, M.; Baracco, R. APOL1-Associated Collapsing Focal Segmental Glomerulosclerosis in a Patient With Stimulator of Interferon Genes (STING)-Associated Vasculopathy with Onset in Infancy (SAVI). Am. J. Kidney Dis. 2020, 75, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Raman, A.; Coffey, N.J.; Sheng, X.; Wahba, J.; Seasock, M.J.; Ma, Z.; Beckerman, P.; Laczkó, D.; Palmer, M.B.; et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J. Clin. Investig. 2021, 131, e136329. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.; Shao, N.; Gao, P.; Tang, H.; Su, H.; Zhang, C.; Meng, X.-F. The Expression and Significance of NLRP3 Inflammasome in Patients with Primary Glomerular Diseases. Kidney Blood Press. Res. 2015, 40, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Y.; Wang, L.; Li, G. Mechanisms of TMEM30A/NLRP3 Inflammasome Pathway-Mediated Podocyte Pyroptosis in FSGS: SA-PO1003. J. Am. Soc. Nephrol. 2023, 34, 1010. [Google Scholar] [CrossRef]
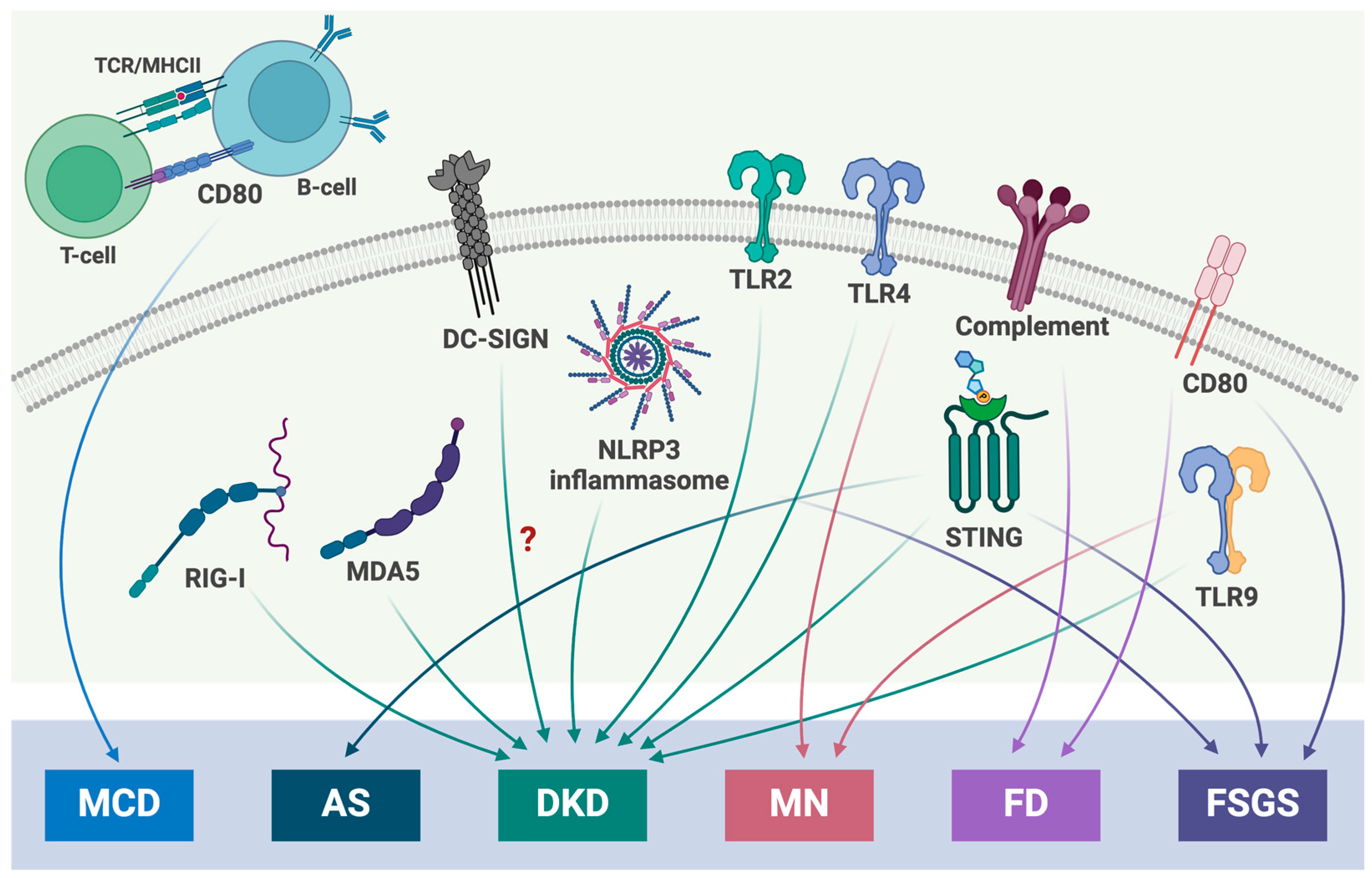
| PRRs | PAMPs/DAMPs | Downstream Effector | Glomerular Disease |
|---|---|---|---|
| DC-SIGN | Mannose | TLRs; NF-κB; INF1β | DKD? |
| MDA5 | mtRNA | INF1β | DKD |
| NLRP3 | mtDNA; oxmtDNA; ROS; high glucose | Caspase1; IL1β; IL-18; P2XR | DKD; FSGS |
| RIG-I | mtRNA | INF1β | DKD |
| STING | dsDNA; mtDNA | IL6; INF1β; TNFα; NF-κB | AS; DKD |
| TLR2 | Porins; HA protein; tGPI mucin; PGN | MyD88; TIRAP | DKD; FSGS |
| TLR4 | dsDNA; LPS | MyD88; TIRAP; TRIF; TRAM (TIR) | DKD; MN; FSGS |
| TLR9 | dsDNA; HMGB1 | MyD88; NF-κB | AS; DKD; MN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, W.; Njeim, R.; Carrazco, A.; Burke, G.W.; Mitrofanova, A. Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes. Cells 2024, 13, 1157. https://doi.org/10.3390/cells13131157
Issa W, Njeim R, Carrazco A, Burke GW, Mitrofanova A. Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes. Cells. 2024; 13(13):1157. https://doi.org/10.3390/cells13131157
Chicago/Turabian StyleIssa, Wadih, Rachel Njeim, Arianna Carrazco, George W. Burke, and Alla Mitrofanova. 2024. "Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes" Cells 13, no. 13: 1157. https://doi.org/10.3390/cells13131157
APA StyleIssa, W., Njeim, R., Carrazco, A., Burke, G. W., & Mitrofanova, A. (2024). Role of the Innate Immune Response in Glomerular Disease Pathogenesis: Focus on Podocytes. Cells, 13(13), 1157. https://doi.org/10.3390/cells13131157







