A Short Sequence Targets Transmembrane Proteins to Primary Cilia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cortical Neuron Differentiation
2.3. Hypothalamic Neuron Differentiation
2.4. Cloning of Fusion Protein Constructs Containing Candidate CTSs
2.5. Transient Transfection in Cell Lines and hPSC-Derived Neurons
2.6. Generation of Stable hPSC Lines Carrying the Ciliary Targeting Construct
2.7. Immunofluorescence and Imaging
2.8. Quantification of Ciliary Labeling Efficiency
2.9. Cilia Length Quantification
2.10. Statistical Analysis
3. Results
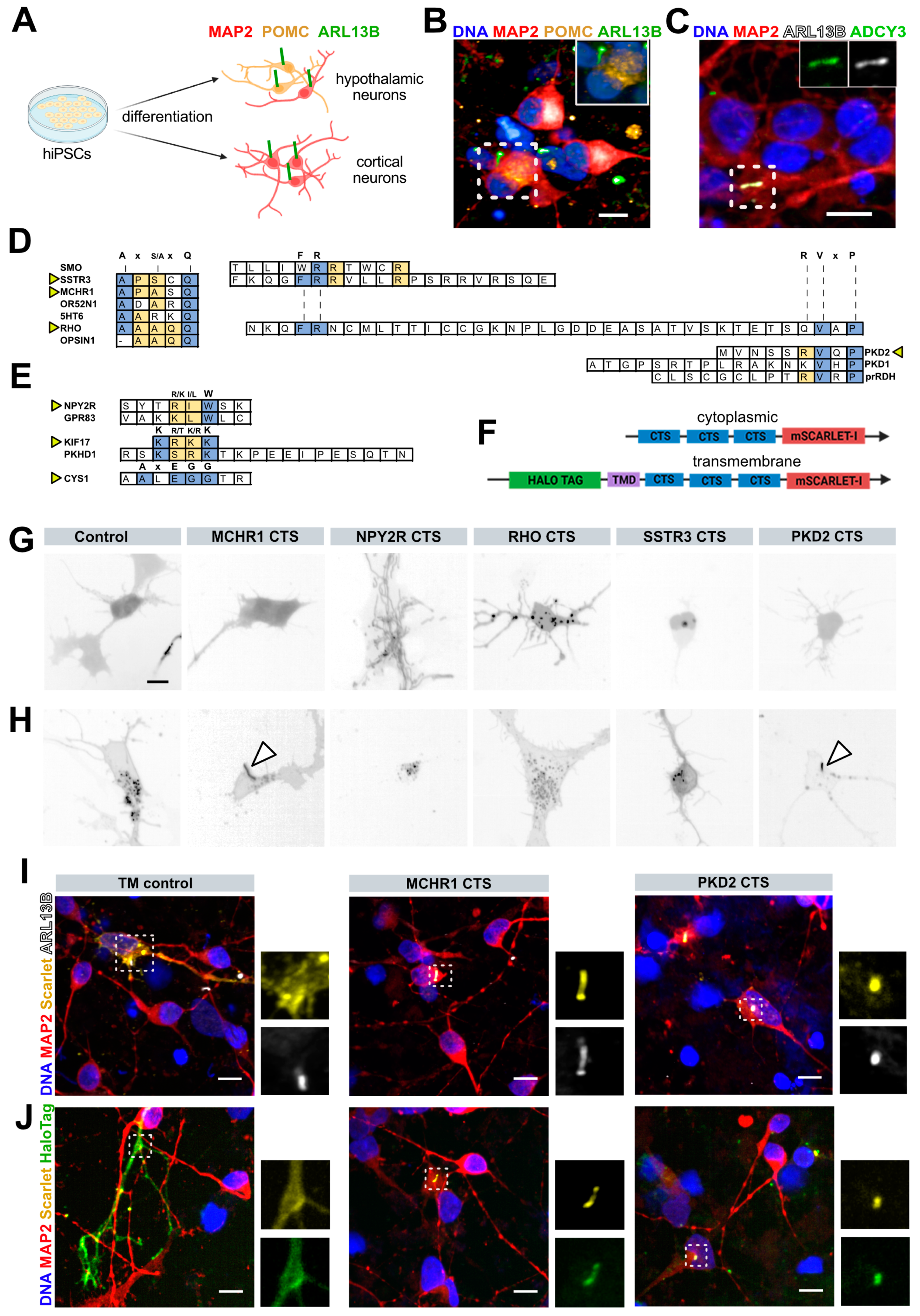
3.1. Identification of CTS Sufficient for Ciliary Targeting in Human Neurons
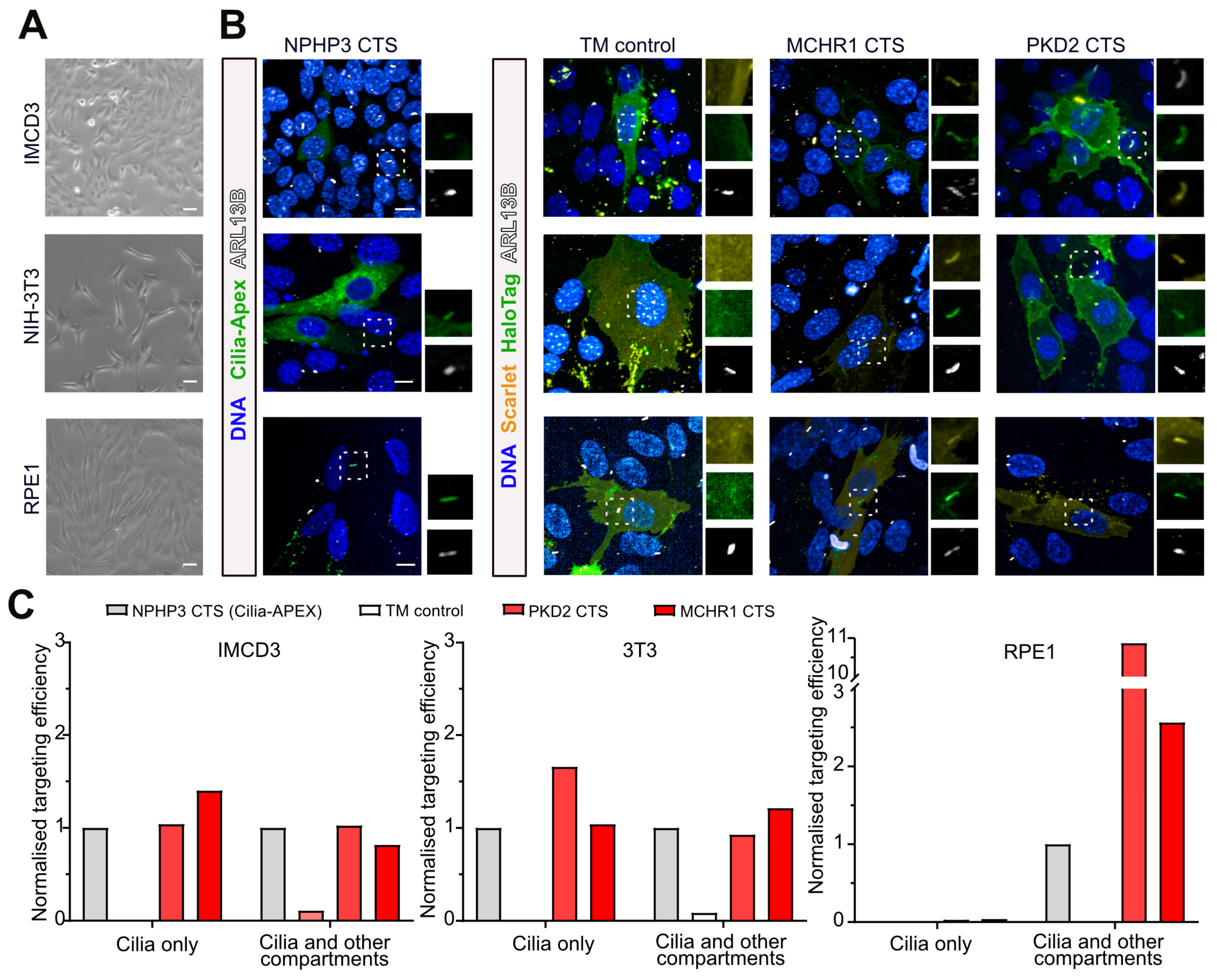
3.2. Ciliary Targeting across Diverse Ciliated Cell Lines
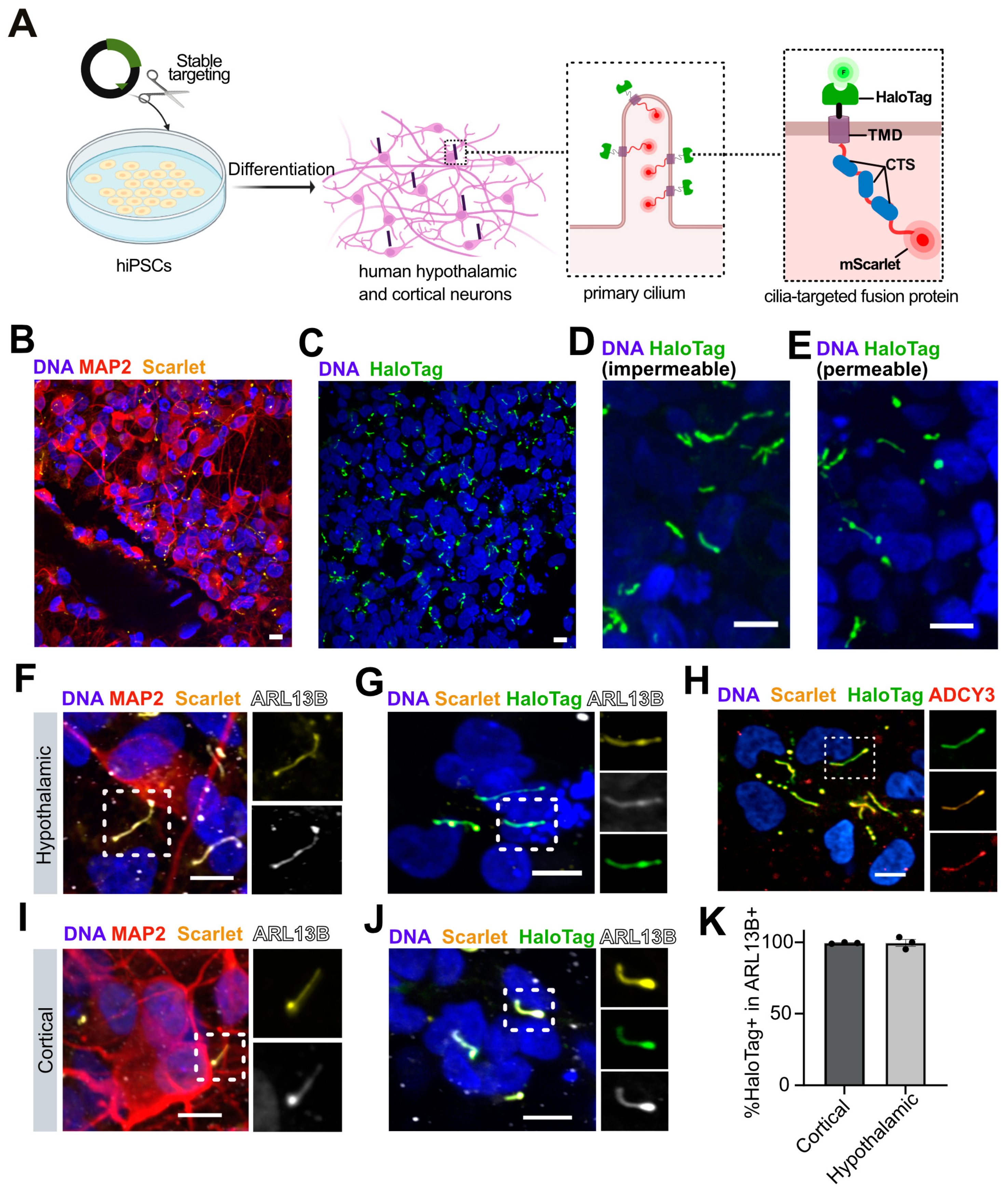
3.3. Generation of Stably Cilia-Tagged Human Pluripotent Stem Cell Lines
3.4. Efficient Ciliary Targeting in Stable Cell Lines
3.5. Image-Based Screening for Regulators of Primary Cilia Length (PCL)
4. Discussion
5. Limitations of Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bernard, A.; Comblain, F.; Yue, X.; Paillart, C.; Zhang, S.; Reiter, J.F.; Vaisse, C. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Investig. 2021, 131, e142064. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.R.; Watts, A.J.; Roper, V.C.; Croyle, M.J.; van Groen, T.; Wyss, J.M.; Nagy, T.R.; Kesterson, R.A.; Yoder, B.K. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007, 17, 1586–1594. [Google Scholar] [CrossRef]
- Brewer, K.M.; Brewer, K.K.; Richardson, N.C.; Berbari, N.F. Neuronal cilia in energy homeostasis. Front. Cell Dev. Biol. 2022, 10, 1082141. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Katoh, Y. Architecture of the IFT ciliary trafficking machinery and interplay between its components. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoek, H.; Klena, N.; Jordan, M.A.; Alvarez Viar, G.; Righetto, R.D.; Schaffer, M.; Erdmann, P.S.; Wan, W.; Geimer, S.; Plitzko, J.M.; et al. In situ architecture of the ciliary base reveals the stepwise assembly of intraflagellar transport trains. Science 2022, 377, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Huang, K. Transport of Ciliary Membrane Proteins. Front. Cell Dev. Biol. 2019, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V.; Seeley, E.S.; Jin, H. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 2010, 26, 59–87. [Google Scholar] [CrossRef]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 2012, 197, 697–709. [Google Scholar] [CrossRef]
- Witzgall, R. Golgi bypass of ciliary proteins. Semin. Cell Dev. Biol. 2018, 83, 51–58. [Google Scholar] [CrossRef]
- Gonçalves, J.; Pelletier, L. The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol. Cells 2017, 40, 243–253. [Google Scholar] [CrossRef]
- Hu, Q.; Milenkovic, L.; Jin, H.; Scott, M.P.; Nachury, M.V.; Spiliotis, E.T.; Nelson, W.J. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 2010, 329, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.L.; Leroux, M.R. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 2017, 47, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.L.; Dishinger, J.F.; Blasius, T.L.; Liu, C.-J.; Margolis, B.; Verhey, K.J. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 2012, 14, 431–437. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.K.; Malarkey, E.B.; Berbari, N.F.; Croyle, M.J.; Haycraft, C.J.; Bell, P.D.; Hohenstein, P.; A Kesterson, R.; Yoder, B.K. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Berbari, N.F.; Johnson, A.D.; Lewis, J.S.; Askwith, C.C.; Mykytyn, K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 2008, 19, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Otis, J.M.; Suciu, S.K.; Catalano, C.; Xing, L.; Constable, S.; Wachten, D.; Gupton, S.; Lee, J.; Lee, A.; et al. Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Dev. Cell 2019, 51, 759–774.e5. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Falcone, J.L.; Curci, S.; Hofer, A.M. Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc. Natl. Acad. Sci. USA 2019, 116, 12066–12071. [Google Scholar] [CrossRef]
- Moore, B.S.; Stepanchick, A.N.; Tewson, P.H.; Hartle, C.M.; Zhang, J.; Quinn, A.M.; Hughes, T.E.; Mirshahi, T. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 13069–13074. [Google Scholar] [CrossRef]
- Nakata, K.; Shiba, D.; Kobayashi, D.; Yokoyama, T. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled-coil domains. Cytoskeleton 2012, 69, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Mick, D.U.; Rodrigues, R.B.; Leib, R.D.; Adams, C.M.; Chien, A.S.; Gygi, S.P.; Nachury, M.V. Proteomics of Primary Cilia by Proximity Labeling. Dev. Cell 2015, 35, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.V.; Jackson, P.K. Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep. 2013, 5, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, C.B.; Yang, A.; Lara, E.; McDonough, J.A.; Blauwendraat, C.; Peng, L.; Oguro, H.; Kanaujiya, J.; Zou, J.; Sebesta, D.; et al. A reference human induced pluripotent stem cell line for large-scale collaborative studies. Cell Stem Cell 2022, 29, 1685–1702.e22. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, F.R.; Salomonis, N.; Sheehan, A.; Huang, M.; Park, J.S.; Spindler, M.J.; Lizarraga, P.; A Weiss, W.; So, P.-L.; Conklin, B.R. A robust method to derive functional neural crest cells from human pluripotent stem cells. Am. J. Stem Cells. 2013, 2, 119–131. [Google Scholar] [PubMed]
- Chen, H.-J.C.; Mazzaferro, S.; Tian, T.; Mali, I.; Merkle, F.T. Differentiation, Transcriptomic Profiling, and Calcium Imaging of Human Hypothalamic Neurons. Curr. Protoc. 2023, 3, e786. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Wen, X.; Chih, B.; Nelson, C.D.; Lane, W.S.; Scales, S.J.; Jackson, P.K. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010, 24, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Fernandopulle, M.S.; Prestil, R.; Grunseich, C.; Wang, C.; Gan, L.; Ward, M.E. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Curr. Protoc. Cell Biol. 2018, 79, e51. [Google Scholar] [CrossRef]
- Tian, R.; Gachechiladze, M.A.; Ludwig, C.H.; Laurie, M.T.; Hong, J.Y.; Nathaniel, D.; Prabhu, A.V.; Fernandopulle, M.S.; Patel, R.; Abshari, M.; et al. CRISPR Interference-Based Platform for Multimodal Genetic Screens in Human iPSC-Derived Neurons. Neuron 2019, 104, 239–255.e12. [Google Scholar] [CrossRef]
- Flores, E.L.; Qi, A.; Reilly, L.; Santiana, M.; Ward, M.; Cookson, M. piNDI Transcription Factor-NGN2 Differentiation of Human iPSC into Cortical Neurons Version 1 v1; ZappyLab, Inc.: New York, NY, USA, 2022. [Google Scholar]
- Ma, R.; Zhao, J.; Du, H.-C.; Tian, S.; Li, L.-W. Removing endotoxin from plasmid samples by Triton X-114 isothermal extraction. Anal. Biochem. 2012, 424, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Maroof, A.; Wataya, T.; Sasai, Y.; Studer, L.; Eggan, K.; Schier, A.F. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 2015, 142, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, P.; Jura, M.; Merkle, F.T. Generation and Characterization of Functional Human Hypothalamic Neurons. Curr. Protoc. Neurosci. 2017, 81, 3.33.1–3.33.24. [Google Scholar] [CrossRef]
- Chen, H.; Kendall, D.A. Artificial transmembrane segments. Requirements for stop transfer and polypeptide orientation. J. Biol. Chem. 1995, 270, 14115–14122. [Google Scholar] [CrossRef]
- Nam, H.-S.; Benezra, R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 2009, 5, 515–526. [Google Scholar] [CrossRef]
- Oceguera-Yanez, F.; Kim, S.-I.; Matsumoto, T.; Tan, G.W.; Xiang, L.; Hatani, T.; Kondo, T.; Ikeya, M.; Yoshida, Y.; Inoue, H.; et al. Engineering the AAVS1 locus for consistent and scalable transgene expression in human iPSCs and their differentiated derivatives. Methods 2016, 101, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Zotova, A.; Lopatukhina, E.; Filatov, A.; Khaitov, M.; Mazurov, D. Gene Editing in Human Lymphoid Cells: Role for Donor DNA, Type of Genomic Nuclease and Cell Selection Method. Viruses 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Skarnes, W.C.; Pellegrino, E.; McDonough, J.A. Improving homology-directed repair efficiency in human stem cells. Methods 2019, 164–165, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.P.; Kiskinis, E.; Eggan, K.; Merkle, F.T. Comprehensive Protocols for CRISPR/Cas9-based Gene Editing in Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2016, 38, 5B.6.1–5B.6.60. [Google Scholar] [CrossRef]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef]
- Wang, J.; Deretic, D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog. Retin. Eye Res. 2014, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.H.; Brown-Glaberman, U.; Wang, J.; Morita, Y.; Alper, S.L.; Bedrick, E.J.; Gattone, V.H.; Deretic, D.; Wandinger-Ness, A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell 2011, 22, 3289–3305. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Okuhara, D.; Yu, Z.; Tian, X.; Cai, Y.; Shibazaki, S.; Somlo, S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 2006, 119, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Marsh-Armstrong, N.; Rattner, A.; Nathans, J. An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase. J. Neurosci. 2004, 24, 2623–2632. [Google Scholar] [CrossRef]
- Chadha, A.; Paniagua, A.E.; Williams, D.S. Comparison of Ciliary Targeting of Two Rhodopsin-Like GPCRs: Role of C-Terminal Localization Sequences in Relation to Cilium Type. J. Neurosci. 2021, 41, 7514–7531. [Google Scholar] [CrossRef] [PubMed]
- Dishinger, J.F.; Kee, H.L.; Jenkins, P.M.; Fan, S.; Hurd, T.W.; Hammond, J.W.; Truong, Y.N.; Margolis, B.; Martens, J.R.; Verhey, K.J. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 2010, 12, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Follit, J.A.; Li, L.; Vucica, Y.; Pazour, G.J. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 2010, 188, 21–28. [Google Scholar] [CrossRef]
- Tao, B.; Bu, S.; Yang, Z.; Siroky, B.; Kappes, J.C.; Kispert, A.; Guay-Woodford, L.M. Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J. Am. Soc. Nephrol. 2009, 20, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Barbeito, P.; Tachibana, Y.; Martin-Morales, R.; Moreno, P.; Mykytyn, K.; Kobayashi, T.; Garcia-Gonzalo, F.R. HTR6 and SSTR3 ciliary targeting relies on both IC3 loops and C-terminal tails. Life Sci. Alliance 2021, 4, e202000746. [Google Scholar] [CrossRef]
- Schou, K.B.; Pedersen, L.B.; Christensen, S.T. Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 2015, 16, 1099–1113. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Snapp, E. Design and use of fluorescent fusion proteins in cell biology. Curr. Protoc. Cell Biol. 2005, 21, 21.4.1–21.4.13. [Google Scholar] [CrossRef] [PubMed]
- Bindels, D.S.; Haarbosch, L.; van Weeren, L.; Postma, M.; Wiese, K.E.; Mastop, M.; Aumonier, S.; Gotthard, G.; Royant, A.; Hink, M.A.; et al. mScarlet: A bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 2017, 14, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M.; et al. HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef]
- Sharpe, H.J.; Stevens, T.J.; Munro, S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 2010, 142, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Munro, S. A comparison of the transmembrane domains of Golgi and plasma membrane proteins. Biochem. Soc. Trans. 1995, 23, 527–530. [Google Scholar] [CrossRef]
- Hung, V.; Udeshi, N.D.; Lam, S.S.; Loh, K.H.; Cox, K.J.; Pedram, K.; Carr, S.A.; Ting, A.Y. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 2016, 11, 456–475. [Google Scholar] [CrossRef]
- May, E.A.; Kalocsay, M.; D’Auriac, I.G.; Schuster, P.S.; Gygi, S.P.; Nachury, M.V.; Mick, D.U. Time-resolved proteomics profiling of the ciliary Hedgehog response. J. Cell Biol. 2021, 220, e202007207. [Google Scholar] [CrossRef] [PubMed]
- Hockemeyer, D.; Soldner, F.; Beard, C.; Gao, Q.; Mitalipova, M.; DeKelver, R.C.; Katibah, G.; Amora, R.; A Boydston, E.; Zeitler, B.; et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 2009, 27, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Guadiana, S.M.; Semple-Rowland, S.; Daroszewski, D.; Madorsky, I.; Breunig, J.J.; Mykytyn, K.; Sarkisian, M.R. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J. Neurosci. 2013, 33, 2626–2638. [Google Scholar] [CrossRef]
- Macarelli, V.; Leventea, E.; Merkle, F.T. Regulation of the length of neuronal primary cilia and its potential effects on signalling. Trends Cell Biol. 2023, 33, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Kasahara, K.; Miyazaki, I.; Asanuma, M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem. Biophys. Res. Commun. 2009, 388, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hierck, B.P.; Van der Heiden, K.; Alkemade, F.E.; Van de Pas, S.; Van Thienen, J.V.; Groenendijk, B.C.; Bax, W.H.; Van der Laarse, A.; DeRuiter, M.C.; Horrevoets, A.J.; et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 2008, 237, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.L.; Carlson, K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J. Cell Biol. 1969, 40, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Berbari, N.F.; Lewis, J.S.; Bishop, G.A.; Askwith, C.C.; Mykytyn, K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 2008, 105, 4242–4246. [Google Scholar] [CrossRef] [PubMed]
- Madugula, V.; Lu, L. A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes. J. Cell Sci. 2016, 129, 3922–3934. [Google Scholar] [CrossRef]
- Geneva, I.I.; Tan, H.Y.; Calvert, P.D. Untangling ciliary access and enrichment of two rhodopsin-like receptors using quantitative fluorescence microscopy reveals cell-specific sorting pathways. Mol. Biol. Cell 2017, 28, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Leng, Q.; Mixson, A.J. Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J. Gene Med. 2005, 7, 354–365. [Google Scholar] [CrossRef]

| Primary Antibodies | ||
|---|---|---|
| Antibody | Dilution | Catalogue |
| anti-MAP2 | 1:2000 | Abcam (Cambridge, UK) ab5392 |
| anti-ARL13b | 1:700 | Proteintech (Manchester, UK) 17711-1-AP |
| anti-ADCY3 | 1:500 | SantaCruz (Wembley, UK) sc-518057 |
| anti-RFP | 1:500 | Synaptic System (Goettingen, Germany) 409 011 |
| Anti-POMC (ACTH/αMSH) | 1:5000 | Prof. Anne White (University of Manchester, UK) |
| Secondary Antibodies | ||
| Antibody | Dilution | Catalogue |
| Alexa donkey anti-rabbit 488 | 1:500 | Thermo Fisher Scientific (Altrincham, UK) A21206 |
| Alexa donkey anti-mouse 555 | 1:500 | Thermo Fisher Scientific (Altrincham, UK) A31570 |
| Alexa donkey anti-chicken 647 | 1:500 | Stratech (Ely, UK) 703-606-155 |
| Gene Name | Protein Reference Sequence | Candidate CTS from Literature | References | Selected CTS Position | Selected CTS Sequence |
|---|---|---|---|---|---|
| CYS1 (Cystin 1) | NP_001032237.1 | MGSGSSRSSR; RRRRS; AALEGGTR | [49] | 1–44, disordered region. The second residue (G) is a myristoylation site | MGSGSSRSSRTLRRRRSPESLPAGPGAAALEGGTRRRVPVAAAE |
| KIF17 (Kinesin family member 17) | NP_001116291.1 | KRKK | [47] | 1011–1022, disordered region | FTKAKRKKSKSN |
| MCHR1 (Melanin concentrating hormone receptor 1) | NP_005288.4 | APASQ | [17] | 235–248, third cytoplasmic loop | TSSVAPASQRSIR |
| NPY2R (Neuropeptide Y receptor Y2) | NP_000901.1 | [R,K][I,L]W | [23] | 237–248, third cytoplasmic loop | SYTRIWSKLKN |
| PKD2 (Polycystin 2/PC2) | NP_000288.1 | SxRVxP included in aa 5–72 | [43,44] | 1–76, disordered cytoplasmic domain | MVNSSRVQPQQPGDAKRPPAPRAPDPGRLMAGCAAVGASLAAPGGLCEQRGLEIEMQRIRQAAARDPPAGAAASPS |
| RHO (Rhodopsin) | NP_000530.1 | AAAQQ; FR; SQVAPA | [17,42] | 228–242, third cytoplasmic loop (partial), and 310–348, last cytoplasmic loop | FTVKEAAAQQQESATNKQFRNCMLTTICCGKNPLGDDEASATVSKTETSQVAPA |
| SSTR3 (Somatostatin receptor 3) | NP_001042.1 | APSCQ | [17,50] | 232–257, third cytoplasmic loop | KVRSAGRRVWAPSCQRRRRSERRVTR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macarelli, V.; Harding, E.C.; Gershlick, D.C.; Merkle, F.T. A Short Sequence Targets Transmembrane Proteins to Primary Cilia. Cells 2024, 13, 1156. https://doi.org/10.3390/cells13131156
Macarelli V, Harding EC, Gershlick DC, Merkle FT. A Short Sequence Targets Transmembrane Proteins to Primary Cilia. Cells. 2024; 13(13):1156. https://doi.org/10.3390/cells13131156
Chicago/Turabian StyleMacarelli, Viviana, Edward C. Harding, David C. Gershlick, and Florian T. Merkle. 2024. "A Short Sequence Targets Transmembrane Proteins to Primary Cilia" Cells 13, no. 13: 1156. https://doi.org/10.3390/cells13131156
APA StyleMacarelli, V., Harding, E. C., Gershlick, D. C., & Merkle, F. T. (2024). A Short Sequence Targets Transmembrane Proteins to Primary Cilia. Cells, 13(13), 1156. https://doi.org/10.3390/cells13131156







