Abstract
Huntington’s disease (HD) is a rare but progressive and devastating neurodegenerative disease characterized by involuntary movements, cognitive decline, executive dysfunction, and neuropsychiatric conditions such as anxiety and depression. It follows an autosomal dominant inheritance pattern. Thus, a child who has a parent with the mutated huntingtin (mHTT) gene has a 50% chance of developing the disease. Since the HTT protein is involved in many critical cellular processes, including neurogenesis, brain development, energy metabolism, transcriptional regulation, synaptic activity, vesicle trafficking, cell signaling, and autophagy, its aberrant aggregates lead to the disruption of numerous cellular pathways and neurodegeneration. Essential heavy metals are vital at low concentrations; however, at higher concentrations, they can exacerbate HD by disrupting glial–neuronal communication and/or causing dysbiosis (disturbance in the gut microbiota, GM), both of which can lead to neuroinflammation and further neurodegeneration. Here, we discuss in detail the interactions of iron, manganese, and copper with glial–neuron communication and GM and indicate how this knowledge may pave the way for the development of a new generation of disease-modifying therapies in HD.
1. Introduction
Huntington’s disease (HD) is a relentlessly progressive and debilitating adult-onset neurodegenerative disorder characterized by a well-defined clinical triad: motor dysfunction including choreoathetosis (involuntary twitching, twisting or squirming movements), where severe cases can cause permanent disability, cognitive decline including memory impairment and executive dysfunction, and psychiatric disturbances including anxiety and depression [1,2]. Pneumonia, followed by cardiovascular diseases, are the common causes of death [1,3]. Suicide is also more prevalent in patients with HD compared to the general population [1].
HD is an autosomal dominant disease with characteristic cytosine, adenine, and guanine (CAG) trinucleotide repeats on the short arm of chromosome 4p16.3 within the huntingtin (HTT) gene, leading to the production of a mutant huntingtin protein (mHTT) [2,4]. HTT is involved in many critical cellular processes including neurogenesis and brain development, energy metabolism, transcriptional regulation, synaptic activity, vesicle trafficking, cell signaling, and autophagy [2,4]. It is not surprising, therefore, that aberrant aggregates of this protein lead to the disruption of numerous cellular pathways, triggering a cascade of neurodegeneration [1,2,4].
HD is a rare neurodegenerative disorder (about 4 per 10,000 worldwide) with lower prevalence in Asia and higher prevalence in Europe, North America, and Australia, possibly due to the HTT gene haplotypes [2,5,6]. It typically manifests in mid-life, between the ages of 30 and 50 years, but can occur even before the age of 20, where it is termed juvenile HD. Diagnosis is made based on motor, cognitive, and behavioral tests and is confirmed via genetic testing using DNA analysis. Since no cure is available, treatment is aimed at improving the quality of life and decreasing complications.
Recent advances in molecular biology, focusing not only on the cellular pathways dysregulated by mHTT but also exploring the potential influence of external factors such as heavy metal exposure and gut microbiota (GM), have paved the way for the development of a new generation of disease-modifying therapies (DMTs) for HD [7,8]. In this review, following a brief description of HD pathology, we explore the roles of heavy metals in its etiology and focus on the potential manipulation of the GM as a novel therapeutic strategy.
2. HD Pathophysiology
Three significant categories of risk factors associated with CAG repeats have been identified in HD. The first and foremost is the length of the repeat, as the longer the repeats (>35), the earlier the onset of symptoms. Indeed, abnormal CAG triplet repeats lead to an abnormally elongated polyglutamine (polyQ) tract, which results in neurodegenerative diseases including HD [9]. CAG length is also a significant factor for the progression of the disease, especially in cognitive, motor, and neurological disturbances. The CAG repeats not only provide information on the age of clinical onset but also predict the age of death, as the course of the disease commonly lasts 15 to 20 years [1]. Second is the instability of CAG, and the third are the genetic modifiers that play an essential role in the progression of the disease [1]. The primary pathophysiological features of HD are the degeneration of neurons in the caudate, putamen, and cerebral cortex. The brain, particularly in the striatum, atrophies, showing extensive neuronal loss. It is believed that the choreiform movements and the development of dystonia and akinesia are due to the degeneration and loss of substance-P in the medium spiny neurons of the basal ganglia, and cognitive and behavioral dysfunctions are due to cortical atrophy [1]. Several theories have been suggested as reasons for pathogenesis. These include the accumulation of mHTT aggregates, leading to an impairment of the ubiquitin–proteosome pathway, transcriptional dysregulation, excitotoxicity due to increased release of glutamate and glutamate agonist from the cortical afferents, mitochondrial dysfunction and altered energy metabolism, changes in axonal transport, and synaptic dysfunction [1,10]. These contentions are because HTT is essential not only for the embryonic brain development but also for the adult brain function. Furthermore, mHTT may cause a gain of function or toxicity, or loss of function, either of which can contribute to the HD pathology [11].
mHTT is also a strong activator of glial cells, the brain’s immune cells, leading to chronic neuroinflammation [12]. While the initial activation of the glia is for neuroprotection, the overstimulation of these cells results in a neuroinflammatory response, which can cause neuronal damage and/or cell death, hence contributing to disease progression [12,13]. Recent research suggests the potential contributions of environmental factors like heavy metals such as iron (Fe), manganese (Mn), and copper (Cu) to HD pathology [12,13,14]. Heavy metal exposure further disrupts post-transcriptional mechanisms, exacerbating the problems caused by mHTT and decreasing the clearance rate of misfolded proteins, hence creating a vicious cycle that accelerates the neurodegeneration process [15]. Heavy metals may also indirectly influence neuroinflammation and/or mHTT clearance, causing further damage via their interaction with the GM, as discussed in more detail below.
The characteristic involuntary movements are progressive as they initially begin in the distal extremities and gradually move to proximal and axial muscles with greater amplitude and could extend to facial muscles. Whereas in the early stages, the symptoms manifest as hyperkinetic with involuntary chorea, in later stages, hypokinesia and dystonia predominate. In the later stages of the disease, the patient becomes bedridden due to severe rigidity and contractures in the extremities. Dysarthria and dysphagia and trouble in speaking and swallowing, respectively, develop during the course of the disease, which could lead to aspiration and pneumonia, the main cause of death in HD. Dystonia, characterized by increased muscle tone with slower movements, leads to abnormal posturing such as torticollis (stiff neck) and is usually one of the early signs of motor involvement in HD. Tics and ataxia may also develop. The progression of motor disturbances over time can lead to difficulties in walking and standing and frequent falls [1].
In addition to the motor symptoms, behavioral and cognitive disturbances manifest early on. Thus, initially, patients may present with impulsivity, poor attention, and irritability, leading to outbursts of anger and aggression. Later, emotional blandness with prominent apathy, loss of intuition, and creativity ensues. These are likely due to degeneration in the fronto-striatal pathway. Apathy, which is also progressive, is the most common feature of the disease. Mood disorders including depression are also common, which can lead to suicide in HD. Psychosis and cognitive decline to the point of unawareness appear later. Cognitive decline usually manifests before the onset of motor disturbances. The prominent cognitive changes include difficulty in planning, organizing, and multitasking, which may progress to dementia. Interestingly, it is believed that memory loss in HD is due to an inefficient search of memory (subcortical in nature) rather than a deficient in memory formation. In addition, more common features of cortical dementia such as apraxia and aphasia (speech disorders) are avoided in HD. Nonetheless, there is severe slowness in the psychomotor processes [1].
3. Current and Prospective Treatments
Beyond symptom management with dopaminergic and other medications [1,16,17], evolving therapeutics for HD target the molecular aspects with the intention of developing disease-modifying drugs [2,18]. These techniques include direct DNA/gene therapies to manipulate the HTT gene and correct the CAG repeat [19]. Thus, the potential of genome editing such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR/Cas9 system have been suggested [20]. RNA modulation may also be a promising approach, and antisense oligonucleotide (ASO) therapies and RNA interference (RNAi) therapies are currently undergoing clinical trials [16,20]. However, using ASOs to lower HTT by targeting transcripts has not been successful in human clinical trials [21]. Although gene therapy might be a promising future intervention, treatments addressing the functional aspects of HTT could be incorporated into current HD therapies. Attempts at enhancing neurogenesis are also being considered [2]. This is because HTT promotes BDNF expression and enhances BDNF vesicular trafficking along microtubules, and mHTT dysregulates these functions by suppressing BDNF transcription, resulting in lower central BDNF levels [22,23,24]. Indeed, it has been suggested that BDNF may serve as a gauge in detecting the severity of HD [25]. Thus, BDNF provides an attractive target for pharmacotherapeutial developments in HD [24]. In addition, targeting mHTT, therapies using potent small molecules, ubiquitin proteasome, or the autophagy-lysosomal systems are also under consideration [26,27].
Other disease-modifying therapies target aberrant downstream pathways such as excitotoxicity, mitochondrial dysfunction, and neuroinflammation [1].
Excitotoxicity, due to an imbalance between excitatory and inhibitory neurotransmitters, has been a subject of intense studies for more than two decades [28,29]. Excessive stimulation by glutamate, the excitatory neurotransmitter, can result in cell death via calcium-mediated mitochondrial dysfunction. The increased cytoplasmic calcium directly targets the mitochondria and alters its membrane potential. This compromises the electron transport chain, a vital pathway for energy production within mitochondria. Consequently, the cell experiences reduced ATP synthesis, hindering its ability to maintain essential cellular functions. Furthermore, the mitochondrial dysfunction compromises the antioxidative processes and leads to the overproduction of reactive oxygen species (ROS) [16,20]. Calcium dysregulation also activates apoptotic cell death pathways involving caspase-9 and caspase-3, accelerating programmed cell death [30].
Therefore, targeting the Glutamate/GABA imbalance may be a viable option in addressing some HD symptoms. This contention is further supported by the presence of aberrant NMDA receptor distribution in HD pathogenesis. Specifically, a reduction in palmitoylation, a post-translational modification, was observed in striatal NR2B-containing NMDA receptors of YAC128 mice, a model of HD. This decrease in palmitoylation correlated with an increase in extrasynaptic NMDA receptors, signifying a potential mislocalization of these receptors away from their typical synaptic sites, hence contributing to the vulnerability of striatal neurons in HD. These findings highlight the potential of targeting NMDA receptor palmitoylation as a therapeutic strategy for HD [29]. Medications like memantine and amantadine, both NMDA receptor antagonists, have shown effectiveness in at least the motor symptoms in HD [16,31,32].
As mentioned above, glial cells in general, and micro- and astroglia in particular, are major contributory cells to neuroinflammation, which is one of the aberrant pathways involved in the pathophysiology of HD [33,34]. Hence, below, we briefly discuss the potential role of glial cells in HD pathology.
4. Glial Cells—HD
Glial cells, outnumbering the neurons by 10 to 1, were once considered only to be structural support for the neurons. However, they are involved in numerous critical brain functions, including myelination, the formation of the blood–brain barrier (BBB), the development and remodeling of synapses, energetic support for neurons, the control of metabolism, the regulation of neurotransmitters and neuroendocrine function, the control of the fluid/electrolyte homeostasis, detoxification, and immune response [35]. Their dysregulation has been associated with neuropsychiatric and neurodegenerative diseases including HD [2,13,36,37]. Recently, we proposed that glial nAChRs may be a suitable target for intervention in Parkinson’s disease (PD) [38]. It would be of interest to determine if this hypothesis can extend to HD.
Four major glial cells (microglia, astrocytes, oligodendrocytes and synantocytes or NG2 cells) have been identified to date. We briefly discuss each with their relevance to neurodegenerative diseases in general, and HD in particular. Moreover, heavy metal interactions with these cells directly or indirectly via GM are also touched upon.
4.1. Microglia—HD
Microglia, constituting 10–15% of all central nervous system (CNS) cells, are considered the resident immune cells, as they constantly survey the environment and react quickly to any kind of insult. They play a vital role in maintaining homeostasis in the brain; however, their overactivation leads to neuroinflammation, which, as alluded to above, may be responsible for the manifestation of neuropsychiatric and/or neurodegenerative diseases [35]. Microglia also regulate the number of neuronal precursor cells, neurogenesis, and the formation and elimination of neuronal synapse and mediate infiltration of T cells into the brain [39].
Depending on the status of their activity, microglia are referred to as resting, activated, or phagocytic. Whereas at the resting or inactive state, they are highly ramified, when activated, they contract, assume an enlarged cell body, and proliferate. This happens in response to injury or insult, allowing them to carry their phagocytic activity, whereby debris is eliminated, and repair and recovery can ensue. This essential function can become detrimental if microglia are overactivated, causing neuroinflammation, followed by neurological anomalies [40,41,42,43].
Microglia express various receptors such as the calcium-sensing receptor (CASR), low-density lipoprotein receptor-related protein 1 (LRP1), triggering receptor expressed on myeloid cells-2 (TREM2), nicotinic cholinergic receptors (nAChRs), and toll-like receptors 2 and 4 (TLR2 and TLR4) [43]. TLRs are the subject of intense investigation as potential targets for neuropsychiatric/neurodegenerative diseases as they facilitate the removal of debris or pathogens by initiating the innate immune response [39,44,45,46].
Importantly, heavy metals (discussed in detail below) can activate microglia and trigger neuroinflammation and neuronal death [47].
4.2. Astroglia (Astrocytes)—HD
Astroglia, or astrocytes, have a wide distribution in the brain and may constitute up to 60% of the total cells in certain areas of the brain. They provide nutrients for the neurons, remove waste, monitor and regulate pH homeostasis, and are key components of the BBB. Moreover, they have extensive synaptic connections with the neurons and help to maintain neuronal integrity [39,48,49,50]. They are also key mediators of excitotoxic glutamate reuptake [51,52]. More recently, it was reported that astrocytes are the necessary source of TNF-α for the mediation of homeostatic synaptic plasticity [53]. Astrocytes contain their own neurotrophic factor, referred to as glial cell line-derived neurotrophic factor (GDNF), a protein that, like brain-derived neurotrophic factor (BDNF), provides trophic support for the growth and differentiation of synapses and promotes cell survival [49,53]. Astrocytes also express high levels of glial fibrillary astrocytic protein (GFAP), which is important for astrocyte–neuron communication, and helps to maintain the mechanical strength, shape, and movement of the cell and is commonly used as a marker for their identification [53,54].
Interestingly, astrocytes can become reactive by polarized microglia to help with defense mechanisms and the removal of pathogens [39]. However, also in this case, the overstimulation of these cells will result in the production of pro-inflammatory cytokines and contribute synergistically to neuronal dysregulation and/or death [55,56,57]. In this regard, heavy metals can cause astrocyte dysfunction, triggering neuronal, as well as oligodendrocyte, malfunction [58,59,60]. Moreover, as the BBB controls the transport of nutrients and metabolites into the brain and limits the access of harmful substances, its disruption is associated with the pathophysiology of major neurological disorders. For example, lead-induced damage of the BBB has been implicated in autism spectrum disorder (ASD), whereas Cu, Mn, and Fe disruption of the BBB have been linked to HD [14,61,62]. Finally, the GM, which, via short-chain fatty acids (SCFAs), maintains the integrity of the BBB, may be highly impacted by gut dysbiosis (discussed below).
4.3. Oligodendrocytes—HD
Oligodendrocytes (OLs), constituting 75% of all glial cells, are well recognized as the primary source of myelination in the CNS [63]. They control extracellular potassium concentration, modulate axonal growth, provide metabolic and trophic supply to myelin, secrete GDNF and BDNF, and, like microglia and astrocytes, express TLRs, which are also necessary for myelin formation [64,65,66]. Myelinated axons, which comprise the white matter, connect various gray matter areas (consisting of neuronal bodies, axon terminals, and dendrites) of the brain to each other and carry nerve impulses between neurons. Abnormality in white matter has been considered as an early indicator in HD [67]. Importantly, heavy metals can cause dysfunction in these cells as well [58,59,68].
4.4. Synantocytes (NG2 Cells)—HD
The fourth subset of major glial cells in CNS, synantocytes, are OL-precursor cells that are almost uniformly distributed in both white and gray matter areas, associate closely with neuronal cell bodies and dendrites, and maintain the ability to keep proliferating in the adult brain [63,69,70]. These cells can also give rise to astrocytes and neurons [63,69,70], and their potential involvement in neurodegenerative diseases is suspected [71,72]. For example, neuroinflammation and increased BBB permeability in experimental autoimmune encephalomyelitis (EAE) have been attributed to NG2 cells in [73], where it was postulated that NG2 cells, via the stimulation of reactive T cells, control IL-12 expression [73]. NG2 cells have been implicated in neuroinflammation [74] and neurovascular unit formation during development [75]. Following acute ischemic stroke, NG2 cells play a key role in angiogenesis and the generation of OLs [75]. Because of their influence on neuronal plasticity and communication with neurons, OLs may provide a novel target for therapeutic interventions in a variety of neurological diseases [75,76,77]. Whether heavy metals interact with NG2 cells is yet to be determined.
5. Gut Microbiota
GM is a complex and dynamic population of trillions of bacteria, fungi, archaea, and eukarya found in the gastrointestinal tract (GI). Microbiome refers to the genetic composition of these cells, which is now estimated to be slightly higher than the human genome [78,79,80]. GM exhibits remarkable diversity that changes over a person’s lifespan following a symbiotic relationship with the host. It plays a vital role in brain development, digestion, nutrient absorption, the fermentation of undigested carbohydrates, the production of essential vitamins and metabolites like SCFAs, the regulation of the immune system, the maintenance of BBB integrity, and overall health [81,82]. Dysbiosis, referring to an imbalance in the composition and function of the GM, has been implicated in a wide range of pathological processes, including digestive, metabolic, autoimmune, and neurological disorders [62,83,84].
The immune system plays a major role in the perpetuation and maintenance of the symbiotic relationship between the host and the beneficial commensal bacterial strains. Due to its substantial influence on physiological processes, as well as its wide implication in various pathological states, the GM is considered to be a new ‘metabolic organ’, having a major influence not only on the digestive system but also on other organs, notably the CNS [8,83,85].
6. Gut–Brain Axis
A bidirectional communication pathway, termed the gut–brain axis (GBA), that links the GM to the CNS is well recognized [86]. This axis facilitates communication through the vagus nerve, the immune system, and microbial metabolites. Dysbiosis has been increasingly implicated in the pathogenesis of various neurological disorders through several mechanisms, the most prominent being the neuroinflammation. In dysbiosis, there is the release of pro-inflammatory mediators such as cytokines (e.g., interleukin-1β, tumor necrosis factor-α) and chemokines from the immune cells, which can then migrate to the CNS via the bloodstream or lymphatic system and exacerbate neuroinflammation, affecting brain development and behavior [84,87,88,89].
Some of the metabolites produced by the GM, such as the SCFA butyrate, contribute to epithelial defense and have antioxidant and anti-inflammatory properties [90,91]. Some other metabolites such as lipopolysaccharides (LPSs) are pro-inflammatory and used to mimic inflammatory diseases [92,93]. An imbalance in the GM may also weaken the intestinal barrier, allowing bacterial products and toxins to translocate into the bloodstream. This phenomenon, known as the leaky gut, highlights the significance of maintaining the integrity of the GM [93,94]. Of direct relevance to the topic of our discussion are the recent reports implicating dysbiosis in HD, which is elaborated below [84,95].
7. Heavy Metals
Heavy metals are essential for a variety of biological functions [38,96,97,98]. For example, iron (Fe) is a critical component of many vital enzymes or coenzymes such as catalases and cytochromes, which mediate cellular processes and drug metabolism. Indeed, catalases, by neutralizing hydrogen peroxide, are critical in providing protection against oxidative stress [38]. Fe is also an essential component of hemoglobin, where its deficiency leads to Fe-deficiency anemia [99]. Similarly, Mn acts as an activator or cofactor for a variety of metalloenzymes that are essential for normal cell growth and development [100,101,102,103,104]. Moreover, the enzymes or coenzymes utilizing Mn play key roles in functions such as gluconeogenesis, the suppression of oxidative stress (Mn superoxide dismutase, SOD) and conversion of glutamate into glutamine (glutamine synthetase) [105,106], all of which have critical biological functions. Copper (Cu) is another metal essential for the synthesis of red blood cells, collagen, bone, and connective tissue and maintenance of nerve cells and the immune system. It is required for adequate growth, cardiovascular integrity, lung elasticity, neovascularization, neuroendocrine function, and Fe metabolism [98,106]. However, at higher concentrations, it can contribute to HD pathology. Below, we discuss the relevance of each essential heavy metal to HD vis-à-vis their interaction with GM and inflammatory processes.
7.1. Iron (Fe)—HD
Ferroptosis is a newly discovered form of programmed cell death distinct from apoptosis and necrosis. It is considered a key contributor to the pathogenesis of neurodegenerative diseases [107,108]. This section focuses on evidence linking it to neurodegeneration, particularly to HD [109].
Ferroptosis, an Fe-dependent form of regulated cell death, is characterized by the excessive peroxidation of polyunsaturated fatty acids (PUFAs) found within cell membranes. Enzymes like acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are key in the catalyzation of these reactions [110]. Mitochondrial dysfunction is a hallmark of ferroptosis and a necessary condition for the perpetuation of these reactions. In fact, increased Fe uptake promotes the generation of destructive hydroxyl radicals through the Fenton reaction, thus perpetuating the chain reaction of lipid peroxidation, ultimately compromising membrane integrity [111], which leads to cell membrane disruption and cell death. Unlike apoptosis and necrosis, ferroptosis presents with its own cellular pathways. While mitochondria are central to the execution of ferroptosis, other organelles contribute through stress-related pathways. The endoplasmic reticulum, Golgi apparatus, and lysosomes can be involved in amplifying the cell death program [112,113].
Ferroptosis is an oxidative process that needs to be controlled via counter regulatory mechanisms. In this context, glutathione, a major antioxidant system in conjunction with its key enzyme glutathione peroxidase 4 (GPX4), plays a major role in protecting cells from uncontrolled ferroptosis by suppressing lipid peroxidation [114]. Fe metabolism, cysteine availability, and lipid homeostasis are tightly intertwined and serve as key regulatory points for ferroptosis induction or inhibition. Unregulated ferroptosis is a major determinant of neuroinflammation and neurodegenerative diseases [109].
Regarding HD, it has been shown that the aggregation and accumulation of mHTT increases the susceptibility of basal ganglia neurons to ferroptotic cell death [115]. Fe overload not only disrupts mitochondrial functions, leading to impaired energy production and increased oxidative stress, but also generates highly reactive free radicals that damage lipids, proteins, and DNA and disrupt the redox balance making neurons more susceptible to ferroptotic cell death, thus exacerbating HD [14,116,117,118]. Indeed, elevated levels of lipid peroxidation products have been detected in both cellular HD models and HD patients [119].
A direct interaction between Fe and mHTT is also evident whereby Fe enhances mHTT aggregation and its neurotoxic effect. This creates a vicious cycle that accelerates neurodegeneration [27,120]. In the same way, mHTT might interfere with System Xc-, an antiporter that exchanges glutamate (excitatory neurotransmitter) for cystine (precursor for glutathione synthesis), leading to decreased glutathione (GSH) levels, a crucial antioxidant that protects cells from ferroptosis [27,120].
It is worth noting that alterations in the GM composition and/or function could impact the absorption and metabolism of dietary Fe in the GI tract. Moreover, changes in Fe metabolism may, in turn, affect Fe levels in the brain and contribute to the neurodegenerative process in HD [121,122,123].
Based on these findings, several therapeutic perspectives have been explored. Among them are the ferroptosis inhibitors and Fe chelators, which have shown promising neuroprotective effects in HD models [16,124,125,126].
7.2. Manganese (Mn)—HD
Another essential heavy metal implicated in HD pathophysiology is Mn. As alluded to earlier, Mn is a crucial cofactor for many enzymes and is necessary for amino acid, cholesterol, glucose, and carbohydrate metabolism; reactive oxygen species scavenging; bone formation; reproduction; and the immune response [127,128]. Mn deficiency can lead to weakness, seizures, infertility, and bone malformation. Mn overload, on the other hand, concentrates in the brain, especially in the basal ganglia, resulting in Parkinsonism [129]. Early life exposure to high levels of Mn is thought to impact neurodevelopment, especially cognitive behavior in children [130]. Importantly, high Mn exposure and alteration in the GM has been linked to oxidative stress and neuroinflammation, which are implicated in HD [131,132].
The potential link between Mn, insulin/IGF signaling, and HD, whereby Mn deficiency was shown to share cellular consequences such as increased oxidative stress and mitochondrial dysfunction with HD, was reviewed recently [133]. It was concluded that Mn can mimic some actions of insulin/IGF signaling in HD models, thereby providing protection in instances where HD symptoms might be precipitated by Mn deficiency [133].
7.3. Copper (Cu)—HD
Cu toxicity has also been linked to HD [134]. Cu, as mentioned earlier, is an essential metal that plays a critical role in various neurochemical processes, where its dysregulation is detrimental [135]. Studies highlight its potential contribution to neurodegeneration in HD via the enhancement of mHTT toxicity [15]. Cu may also disrupt proteostasis, the process of protein folding and degradation, further contributing to cellular dysfunction [136]. It is noteworthy that Wilson’s disease also involves the disruption of Cu metabolism and its deposition in the basal ganglia. People suffering from this genetic disorder present with extrapyramidal signs and symptoms ranging from movement disorders (tremor, dystonia, parkinsonism) to cognitive and speech impairment and psychiatric symptoms, similar to what is observed in HD [137]. Thus, like Fe, Cu may promote mHTT aggregation and toxicity. Cu also modulates the interaction between huntingtin inclusions and the autophagy adaptor protein, which is responsible for the clearance of the toxic aggregate [15,138,139,140]. A study using the drosophila model of HD showed that D-penicillamine, a Cu chelator, significantly reduced the formation of amyloid-like huntingtin aggregates, suggesting a potential therapeutic avenue for mitigating the toxicity associated with huntingtin aggregation [15].
In summary, epidemiological and clinical studies have shown a strong correlation between aberrant metal exposure and several neurological diseases, including HD [141]. For example, toxic effects of long-term exposure to copper, zinc, and their mixture, in a C. elegans-based HD model, was recently reported [134]. Similarly, cadmium, Fe, Mn, and Cu have been implicated [141,142,143]. Thus, it would be of significant clinical relevance to investigate whether the prevalence of HD correlates with exposure to high levels of heavy metals in select populations.
8. Heavy Metals and GM—HD
Building upon the intriguing link between heavy metal dysregulation and HD, recent research is exploring the potential influence of the GM in HD pathophysiology. The GM is in fact increasingly recognized for its role in brain health and disease [93,95]. The GM can both influence and be influenced by heavy metal exposure. Certain gut bacteria can facilitate the absorption and accumulation of heavy metals like Fe, lead (Pb), and Cu in the body [62]. Conversely, heavy metal exposure can disrupt the composition and function of the GM, triggering inflammatory responses that can indirectly impact the basal ganglia and exacerbate HD pathology [144]. The basal ganglia, a control center for movement, cognition, and emotional regulation, is critically affected in HD and is particularly susceptible to GM-derived neuroinflammation [144]. Thus, by promoting a healthy GM composition through dietary interventions or prebiotics/probiotics, the absorption of heavy metals like Fe and Cu may be curtailed, thereby mitigating their potential contribution to HD pathology.
The intricate relationship between the GM and HD pathophysiology is a burgeoning area of research with significant therapeutic potential. Recent studies suggest a multifaceted interplay between gut bacteria, the immune system, and the CNS that may contribute to HD progression [84,145,146,147]. One key mechanism in this scenario involves SCFAs produced by beneficial bacteria like Bifidobacterium and Faecalibacterium prausnitzii that exert neuroprotective effects [62,148,149]. Thus, in a mouse model of HD, SCFA supplementation can improve motor function, reduce mHTT aggregation, and mitigate neuroinflammation. Conversely, dysbiosis, leading to LPS production, has been linked to an increase in mHTT aggregation and neuronal death [150].
Another critical link in GBA is a bidirectional communication pathway involving the vagus nerve, immune signaling, and the production of neurotransmitters. Dysbiosis can trigger chronic low-grade inflammation in the gut, leading to the activation of immune cells and the release of pro-inflammatory cytokines. These inflammatory signals can then travel up to the brain via the vagus nerve, promoting neuroinflammation and further compromising neuronal health in the basal ganglia [151]. Interestingly, mHTT was shown to be widely expressed in the intestines, which would allow it to interact with the GM, hence affecting the progression of HD [146]. GM involvement in HD pathology has also been verified in several animal models [145,152].
It was mentioned earlier that a leaky BBB, characterized by increased permeability, allows the passage of harmful bacterial products and inflammatory molecules into the brain. It is noteworthy that dysbiosis can also disrupt the tight junctions of the BBB, potentially accelerating neurodegeneration in HD [153].
9. Conclusions
Neurodegenerative diseases exact a tremendous toll on those affected and their caregivers. Although, in most cases, the etiology is unknown, in the case of HD, a mutation in huntingtin gene is the main culprit. In this regard, efforts are underway to quantify the mutant protein in the cerebrospinal fluid with the aim of developing effective therapies [154]. In addition, exposure to high levels of essential heavy metals such as Fe, Mn, and Cu may exacerbate HD symptoms by disrupting neuronal communications, particularly glia–neuron interaction. High levels of heavy metals, via their interaction with the GM and induction of dysbiosis, can also promote neuroinflammation and, hence, indirectly contribute to HD pathology (Figure 1). Understanding the intricate coordination of the GBA and specific effects of each heavy metal on this axis may provide further therapeutic intervention in this devastating disease [8,155,156].
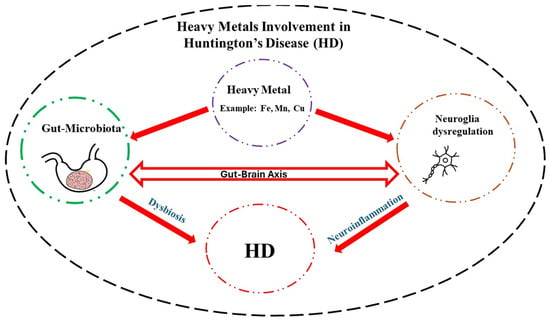
Figure 1.
Schematic diagram depicting how heavy metals, via their interactions with the neuroglia and gut microbiota, may contribute to Huntington’s disease (HD) pathology. A detailed understanding of this interaction can pave the way for novel therapeutic interventions in this rare but devastating neurological disease.
Author Contributions
Conceptualization, Y.T., M.A. and B.G.; Writing—original draft preparation, S.B. and N.E.K.; Review and editing, Y.T., M.A. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
Y.T. was supported in part by NIH/NIAAA R03 AA022479 and NIH/NIGMS (2 SO6 GM08016-39), while M.A. was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS) R01ES10563 and R01ES07331.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ajitkumar, A.; De Jesus, O. Huntington Disease; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- D’Egidio, F.; Castelli, V.; Lombardozzi, G.; Ammannito, F.; Cimini, A.; d’Angelo, M. Therapeutic Advances in Neural Regeneration for Huntington’s Disease. Neural Regen. Res. 2024, 19, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, A.-W.; Roos, R.A.C. Aspiration Pneumonia and Death in Huntington’s Disease. PLoS Curr. 2012, 4, RRN1293. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S. Huntington’s Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, M.D.; Wexler, N.S.; Wexler, A.R.; Tabrizi, S.J.; Douglas, I.; Evans, S.J.W.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.C.; Hurley, E.P.; Parsons, M.P. Huntingtin and the Synapse. Front. Cell. Neurosci. 2021, 15, 689332. [Google Scholar] [CrossRef]
- Kim, A.; Lalonde, K.; Truesdell, A.; Gomes Welter, P.; Brocardo, P.S.; Rosenstock, T.R.; Gil-Mohapel, J. New Avenues for the Treatment of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 8363. [Google Scholar] [CrossRef]
- Khoshnan, A. Gut Microbiota as a Modifier of Huntington’s Disease Pathogenesis. J. Huntingt. Dis. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Estevam, B.; Matos, C.A.; Nóbrega, C. PolyQ Database—An Integrated Database on Polyglutamine Diseases. Database 2023, 2023, baad060. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Wright, G.E.; Hayden, M.R. Huntington Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- van der Plas, E.; Schultz, J.L.; Nopoulos, P.C. The Neurodevelopmental Hypothesis of Huntington’s Disease. J. Huntingt. Dis. 2020, 9, 217–229. [Google Scholar] [CrossRef]
- Crotti, A.; Glass, C.K. The Choreography of Neuroinflammation in Huntington’s Disease. Trends Immunol. 2015, 36, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Donley, D.W.; Realing, M.; Gigley, J.P.; Fox, J.H. Iron Activates Microglia and Directly Stimulates Indoleamine-2,3-Dioxygenase Activity in the N171-82Q Mouse Model of Huntington’s Disease. PLoS ONE 2021, 16, e0250606. [Google Scholar] [CrossRef] [PubMed]
- Lobato, A.G.; Ortiz-Vega, N.; Zhu, Y.; Neupane, D.; Meier, K.K.; Zhai, R.G. Copper Enhances Aggregational Toxicity of Mutant Huntingtin in a Drosophila Model of Huntington’s Disease. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2024, 1870, 166928. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.; Kennedy, C.J.; Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Current and Possible Future Therapeutic Options for Huntington’s Disease. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221092517. [Google Scholar] [CrossRef] [PubMed]
- Paleacu, D. Tetrabenazine in the Treatment of Huntington’s Disease. Neuropsychiatr. Dis. Treat. 2007, 3, 545–551. [Google Scholar] [PubMed]
- Sheridan, C. Questions Swirl around Failures of Disease-Modifying Huntington’s Drugs. Nat. Biotechnol. 2021, 39, 650–652. [Google Scholar] [CrossRef]
- Alkanli, S.S.; Alkanli, N.; Ay, A.; Albeniz, I. CRISPR/Cas9 Mediated Therapeutic Approach in Huntington’s Disease. Mol. Neurobiol. 2023, 60, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Muñoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential Disease-Modifying Therapies for Huntington’s Disease: Lessons Learned and Future Opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs 2022, 36, 105–119. [Google Scholar] [CrossRef]
- Yu, C.; Li, C.H.; Chen, S.; Yoo, H.; Qin, X.; Park, H. Decreased BDNF Release in Cortical Neurons of a Knock-in Mouse Model of Huntington’s Disease. Sci. Rep. 2018, 8, 16976. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, S.; Zhang, R.; Kang, K.; Zhang, X.; Xu, Y.; Zhao, C.; Zhao, M. Functional Analysis of Brain Derived Neurotrophic Factor (BDNF) in Huntington’s Disease. Aging 2021, 13, 6103–6114. [Google Scholar] [CrossRef] [PubMed]
- Speidell, A.; Bin Abid, N.; Yano, H. Brain-Derived Neurotrophic Factor Dysregulation as an Essential Pathological Feature in Huntington’s Disease: Mechanisms and Potential Therapeutics. Biomedicines 2023, 11, 2275. [Google Scholar] [CrossRef]
- Plinta, K.; Plewka, A.; Pawlicki, K.; Zmarzły, N.; Wójcik-Pędziwiatr, M.; Rudziński, M.; Krzak-Kubica, A.; Doręgowska-Stachera, M.; Rudzińska-Bar, M. The Utility of BDNF Detection in Assessing Severity of Huntington’s Disease. J. Clin. Med. 2021, 10, 5181. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Mishra, S.K.; Mishra, R.; Mishra, A.; Kumar, A. Discovery of a Potent Small Molecule Inhibiting Huntington’s Disease (HD) Pathogenesis via Targeting CAG Repeats RNA and Poly Q Protein. Sci. Rep. 2019, 9, 16872. [Google Scholar] [CrossRef]
- Jarosińska, O.D.; Rüdiger, S.G.D. Molecular Strategies to Target Protein Aggregation in Huntington’s Disease. Front. Mol. Biosci. 2021, 8, 769184. [Google Scholar] [CrossRef]
- Raymond, L.A. Excitotoxicity in Huntington Disease. Clin. Neurosci. Res. 2003, 3, 121–128. [Google Scholar] [CrossRef]
- Kang, R.; Wang, L.; Sanders, S.S.; Zuo, K.; Hayden, M.R.; Raymond, L.A. Altered Regulation of Striatal Neuronal N-Methyl-D-Aspartate Receptor Trafficking by Palmitoylation in Huntington Disease Mouse Model. Front. Synaptic Neurosci. 2019, 11, 3. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Liu, Y.; Li, Y.-Y.; Chen, C.; Liu, L.-M.; Wu, Y.-M.; Li, S.; Yang, C. Glutamate and GABA Imbalance Promotes Neuronal Apoptosis in Hippocampus after Stress. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 499–512. [Google Scholar] [CrossRef]
- Saigoh, K.; Hirano, M.; Mitsui, Y.; Oda, I.; Ikegawa, A.; Samukawa, M.; Yoshikawa, K.; Yamagishi, Y.; Kusunoki, S.; Nagai, Y. Memantine Administration Prevented Chorea Movement in Huntington’s Disease: A Case Report. J. Med. Case Rep. 2023, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Verhagen Metman, L.; Morris, M.J.; Farmer, C.; Gillespie, M.; Mosby, K.; Wuu, J.; Chase, T.N. Huntington’s Disease: A Randomized, Controlled Trial Using the NMDA-Antagonist Amantadine. Neurology 2002, 59, 694–699. [Google Scholar] [CrossRef]
- Sapp, E.; Kegel, K.B.; Aronin, N.; Hashikawa, T.; Uchiyama, Y.; Tohyama, K.; Bhide, P.G.; Vonsattel, J.P.; Difiglia, M. Early and Progressive Accumulation of Reactive Microglia in the Huntington Disease Brain. J. Neuropathol. Exp. Neurol. 2001, 60, 161–172. [Google Scholar] [CrossRef]
- Vonsattel, J.P.G.; Keller, C.; Pilar Amaya, M.D. Neuropathology of Huntington’s Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 89, pp. 599–618. ISBN 978-0-444-51898-9. [Google Scholar]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; Da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N. Editorial: Glial Cells as an Emerging Therapeutic Target in the Pathobiology of Central Nervous System Disorders: Friend or Foe? Front. Cell. Neurosci. 2023, 17, 1191743. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyteand Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- Carvalho, F.V.; Landis, H.E.; Getachew, B.; Diogenes Amaral Silva, V.; Ribeiro, P.R.; Aschner, M.; Tizabi, Y. Iron Toxicity, Ferroptosis and Microbiota in Parkinson’s Disease: Implications for Novel Targets. In Advances in Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2024; Volume 11, pp. 105–132. ISBN 978-0-443-21560-5. [Google Scholar]
- Pathak, D.; Sriram, K. Neuron-Astrocyte Omnidirectional Signaling in Neurological Health and Disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Munitic, I.; Vidatic, L.; Papić, E.; Rački, V.; Nimac, J.; Jurak, I.; Novotni, G.; Rogelj, B.; Vuletic, V.; et al. Overlapping Neuroimmune Mechanisms and Therapeutic Targets in Neurodegenerative Disorders. Biomedicines 2023, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Saitgareeva, A.R.; Bulygin, K.V.; Gareev, I.F.; Beylerli, O.A.; Akhmadeeva, L.R. The Role of Microglia in the Development of Neurodegeneration. Neurol. Sci. 2020, 41, 3609–3615. [Google Scholar] [CrossRef]
- Soares, É.N.; Costa, A.C.D.S.; Ferrolho, G.D.J.; Ureshino, R.P.; Getachew, B.; Costa, S.L.; Da Silva, V.D.A.; Tizabi, Y. Nicotinic Acetylcholine Receptors in Glial Cells as Molecular Target for Parkinson’s Disease. Cells 2024, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhu, W.; Liao, X.; Liu, W.; Hou, Y.; Wan, J. Expression of Toll-like Receptors in the Cerebellum during Pathogenesis of Prion Disease. Front. Behav. Neurosci. 2024, 18, 1341901. [Google Scholar] [CrossRef]
- Fatoba, O.; Itokazu, T.; Yamashita, T. Microglia as Therapeutic Target in Central Nervous System Disorders. J. Pharmacol. Sci. 2020, 144, 102–118. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The Role of Toll-like Receptors and Neuroinflammation in Parkinson’s Disease. J. Neuroinflamm. 2022, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, M.I.; Acosta-Saavedra, L.C.; Hernández-Kelly, L.C.; Loaeza-Loaeza, J.; Ortega, A. Microglial Activation in Metal Neurotoxicity: Impact in Neurodegenerative Diseases. BioMed Res. Int. 2023, 2023, 1–27. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef]
- Kotliarova, A.; Sidorova, Y.A. Glial Cell Line-Derived Neurotrophic Factor Family Ligands, Players at the Interface of Neuroinflammation and Neuroprotection: Focus Onto the Glia. Front. Cell. Neurosci. 2021, 15, 679034. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Stoklund Dittlau, K.; Freude, K. Astrocytes: The Stars in Neurodegeneration? Biomolecules 2024, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Heir, R.; Abbasi, Z.; Komal, P.; Altimimi, H.F.; Franquin, M.; Moschou, D.; Chambon, J.; Stellwagen, D. Astrocytes Are the Source of TNF Mediating Homeostatic Synaptic Plasticity. J. Neurosci. 2024, 44, e2278222024. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional Roles of Reactive Astrocytes in Neuroinflammation and Neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Pamphlett, R.; Bishop, D.P. The Toxic Metal Hypothesis for Neurological Disorders. Front. Neurol. 2023, 14, 1173779. [Google Scholar] [CrossRef]
- Cheli, V.T.; Correale, J.; Paez, P.M.; Pasquini, J.M. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro 2020, 12, 175909142096268. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in Heavy Metal Neurotoxicity and Neurodegeneration. Brain Res. 2021, 1752, 147234. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Bodnya, C.; Ilieva, I.; Neely, M.D.; Aschner, M.; Bowman, A.B. Huntington’s Disease Associated Resistance to Mn Neurotoxicity Is Neurodevelopmental Stage and Neuronal Lineage Dependent. Neurotoxicology 2019, 75, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Bennani, S.; El Kouhen, N.; Getachew, B.; Aschner, M. Interaction of Heavy Metal Lead with Gut Microbiota: Implications for Autism Spectrum Disorder. Biomolecules 2023, 13, 1549. [Google Scholar] [CrossRef]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Bsibsi, M.; Nomden, A.; Van Noort, J.M.; Baron, W. Toll-like Receptors 2 and 3 Agonists Differentially Affect Oligodendrocyte Survival, Differentiation, and Myelin Membrane Formation. J. Neurosci. Res. 2012, 90, 388–398. [Google Scholar] [CrossRef]
- Kumar, V. Toll-Like Receptors in Adaptive Immunity. In Toll-like Receptors in Health and Disease; Kumar, V., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2021; Volume 276, pp. 95–131. ISBN 978-3-031-06511-8. [Google Scholar]
- Sanchez-Petidier, M.; Guerri, C.; Moreno-Manzano, V. Toll-like Receptors 2 and 4 Differentially Regulate the Self-Renewal and Differentiation of Spinal Cord Neural Precursor Cells. Stem Cell Res. Ther. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tong, H.; Yang, T.; Liu, L.; Li, X.-J.; Li, S. Insights into White Matter Defect in Huntington’s Disease. Cells 2022, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef]
- Hill, R.A.; Patel, K.D.; Goncalves, C.M.; Grutzendler, J.; Nishiyama, A. Modulation of Oligodendrocyte Generation during a Critical Temporal Window after NG2 Cell Division. Nat. Neurosci. 2014, 17, 1518–1527. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Dimou, L.; Gallo, V. NG 2-glia and Their Functions in the Central Nervous System. Glia 2015, 63, 1429–1451. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, W.; Zhou, M. Spatial Organization of NG2 Glial Cells and Astrocytes in Rat Hippocampal CA1 Region. Hippocampus 2014, 24, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Errede, M.; Girolamo, F.; Morando, S.; Ivaldi, F.; Panini, N.; Bendotti, C.; Perris, R.; Furlan, R.; Virgintino, D.; et al. NG2, a Common Denominator for Neuroinflammation, Blood–Brain Barrier Alteration, and Oligodendrocyte Precursor Response in EAE, Plays a Role in Dendritic Cell Activation. Acta Neuropathol. 2016, 132, 23–42. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Yang, Q.; Gu, H.; Yin, Y.; Li, Y.; Hou, J.; Chen, R.; Sun, Q.; Sun, Y.; et al. NG2 Glia Regulate Brain Innate Immunity via TGF-Β2/TGFBR2 Axis. BMC Med. 2019, 17, 204. [Google Scholar] [CrossRef]
- Hu, X.; Geng, P.; Zhao, X.; Wang, Q.; Liu, C.; Guo, C.; Dong, W.; Jin, X. The NG2-Glia Is a Potential Target to Maintain the Integrity of Neurovascular Unit after Acute Ischemic Stroke. Neurobiol. Dis. 2023, 180, 106076. [Google Scholar] [CrossRef]
- Timmermann, A.; Tascio, D.; Jabs, R.; Boehlen, A.; Domingos, C.; Skubal, M.; Huang, W.; Kirchhoff, F.; Henneberger, C.; Bilkei-Gorzo, A.; et al. Dysfunction of NG2 Glial Cells Affects Neuronal Plasticity and Behavior. Glia 2023, 71, 1481–1501. [Google Scholar] [CrossRef]
- Vélez-Fort, M.; Maldonado, P.P.; Butt, A.M.; Audinat, E.; Angulo, M.C. Postnatal Switch from Synaptic to Extrasynaptic Transmission between Interneurons and NG2 Cells. J. Neurosci. 2010, 30, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, W.R.; Chen, D.; Garud, N.R. Comparative Population Genetics in the Human Gut Microbiome. Genome Biol. Evol. 2022, 14, evab116. [Google Scholar] [CrossRef]
- Chatterjee, G.; Negi, S.; Basu, S.; Faintuch, J.; O’Donovan, A.; Shukla, P. Microbiome Systems Biology Advancements for Natural Well-Being. Sci. Total Environ. 2022, 838, 155915. [Google Scholar] [CrossRef] [PubMed]
- VanEvery, H.; Franzosa, E.A.; Nguyen, L.H.; Huttenhower, C. Microbiome Epidemiology and Association Studies in Human Health. Nat. Rev. Genet. 2023, 24, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut Reactions: How the Blood–Brain Barrier Connects the Microbiome and the Brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Sharma, G.; Biswas, S.S.; Mishra, J.; Navik, U.; Kandimalla, R.; Reddy, P.H.; Bhatti, G.K.; Bhatti, J.S. Gut Microbiota Dysbiosis and Huntington’s Disease: Exploring the Gut-Brain Axis and Novel Microbiota-Based Interventions. Life Sci. 2023, 328, 121882. [Google Scholar] [CrossRef]
- Tong, H.; Yang, T.; Xu, S.; Li, X.; Liu, L.; Zhou, G.; Yang, S.; Yin, S.; Li, X.-J.; Li, S. Huntington’s Disease: Complex Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 3845. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.-J.; Zhu, Z.-Q. To Re-Examine the Intersection of Microglial Activation and Neuroinflammation in Neurodegenerative Diseases from the Perspective of Pyroptosis. Front. Aging Neurosci. 2023, 15, 1284214. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont Release from Dysbiotic Gut Microbiota Biofilms in Intestinal Inflammatory Diseases: A Role for Iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Follmer, C. Gut Microbiome Imbalance and Neuroinflammation: Impact of COVID-19 on Parkinson’s Disease. Mov. Disord. 2020, 35, 1495–1496. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Canani, R.B. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky Gut: Mechanisms, Measurement and Clinical Implications in Humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut Dysbiosis in Huntington’s Disease: Associations among Gut Microbiota, Cognitive Performance and Clinical Outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiącek, D. Dietary Intake of Toxic Heavy Metals with Major Groups of Food Products—Results of Analytical Determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef]
- Rieder, G.S.; Duarte, T.; Delgado, C.P.; Rodighiero, A.; Nogara, P.A.; Orian, L.; Aschner, M.; Dalla Corte, C.L.; Da Rocha, J.B.T. Interplay between Diphenyl Diselenide and Copper: Impact on D. Melanogaster Survival, Behavior, and Biochemical Parameters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 281, 109899. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Yousefi Babadi, V.; Sadeghi, L.; Shirani, K.; Malekirad, A.A.; Rezaei, M. The Toxic Effect of Manganese on the Acetylcholinesterase Activity in Rat Brains. J. Toxicol. 2014, 2014, 946372. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Andrade, V.; Mateus, M.L.; Batoréu, M.C.; Aschner, M.; Dos Santos, A.M. Toxic Mechanisms Underlying Motor Activity Changes Induced by a Mixture of Lead, Arsenic and Manganese. EC Pharmacol. Toxicol. 2017, 3, 31–42. [Google Scholar] [PubMed]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-Induced Neurotoxicity: A Review of Its Behavioral Consequences and Neuroprotective Strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Burton, N.C.; Schneider, J.S.; Syversen, T.; Guilarte, T.R. Effects of Chronic Manganese Exposure on Glutamatergic and GABAergic Neurotransmitter Markers in the Nonhuman Primate Brain. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 111, 131–139. [Google Scholar] [CrossRef]
- Aschner, M.; Gannon, M. Manganese (Mn) Transport across the Rat Blood-Brain Barrier: Saturable and Transferrin-Dependent Transport Mechanisms. Brain Res. Bull. 1994, 33, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zheng, K.; Li, S.; Ren, C.; Shen, Y.; Tian, L.; Zhu, H.; Zhou, Z.; Jiang, Y. Insight into the Potential Role of Ferroptosis in Neurodegenerative Diseases. Front. Cell. Neurosci. 2022, 16, 1005182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, W.K.; Bae, K.-H.; Lee, S.C.; Lee, E.-W. Lipid Metabolism and Ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef]
- Tian, H.-Y.; Huang, B.-Y.; Nie, H.-F.; Chen, X.-Y.; Zhou, Y.; Yang, T.; Cheng, S.-W.; Mei, Z.-G.; Ge, J.-W. The Interplay between Mitochondrial Dysfunction and Ferroptosis during Ischemia-Associated Central Nervous System Diseases. Brain Sci. 2023, 13, 1367. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tang, D.; Wang, Y.; Li, X.; Bao, H.; Tang, C.; Dong, X.; Li, X.; Yang, Q.; Yan, Y.; et al. The Mechanism of Ferroptosis and Its Related Diseases. Mol. Biomed. 2023, 4, 33. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.d.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Johnson, E.B.; Parker, C.S.; Scahill, R.I.; Gregory, S.; Papoutsi, M.; Zeun, P.; Osborne-Crowley, K.; Lowe, J.; Nair, A.; Estevez-Fraga, C.; et al. Altered Iron and Myelin in Premanifest Huntington’s Disease More than 20 Years before Clinical Onset: Evidence from the Cross-Sectional HD Young Adult Study. EBioMedicine 2021, 65, 103266. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron Imbalance in Neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef]
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, H.; Shi, X.-J.; Cheng, Y. Blood Oxidative Stress Marker Aberrations in Patients with Huntington’s Disease: A Meta-Analysis Study. Oxid. Med. Cell. Longev. 2020, 2020, 9187195. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Impaired Redox Signaling in Huntington’s Disease: Therapeutic Implications. Front. Mol. Neurosci. 2019, 12, 68. [Google Scholar] [CrossRef]
- Chen, J.; Marks, E.; Lai, B.; Zhang, Z.; Duce, J.A.; Lam, L.Q.; Volitakis, I.; Bush, A.I.; Hersch, S.; Fox, J.H. Iron Accumulates in Huntington’s Disease Neurons: Protection by Deferoxamine. PLoS ONE 2013, 8, e77023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, G.; Liang, Z.; Qi, T.; Deng, K.; Yu, J.; Peng, Y.; Zheng, J.; Song, Y.; Chang, X. Microbiota-Assisted Iron Uptake Promotes Immune Tolerance in the Intestine. Nat. Commun. 2023, 14, 2790. [Google Scholar] [CrossRef] [PubMed]
- Correnti, M.; Gammella, E.; Cairo, G.; Recalcati, S. Iron Absorption: Molecular and Pathophysiological Aspects. Metabolites 2024, 14, 228. [Google Scholar] [CrossRef]
- Patanè, G.T.; Putaggio, S.; Tellone, E.; Barreca, D.; Ficarra, S.; Maffei, C.; Calderaro, A.; Laganà, G. Ferroptosis: Emerging Role in Diseases and Potential Implication of Bioactive Compounds. Int. J. Mol. Sci. 2023, 24, 17279. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting Ferroptosis Opens New Avenues for the Development of Novel Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Ji, M.; Wu, C.; Zhang, Y.; Ji, S. Targeting Ferroptosis in Neuroimmune and Neurodegenerative Disorders for the Development of Novel Therapeutics. Biomed. Pharmacother. 2024, 176, 116777. [Google Scholar] [CrossRef] [PubMed]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Getachew, B.; Aschner, M. Butyrate Protects and Synergizes with Nicotine against Iron- and Manganese-Induced Toxicities in Cell Culture. Neurotox. Res. 2024, 42, 3. [Google Scholar] [CrossRef]
- Evans, G.R.; Masullo, L.N. Manganese Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Porru, S.; Esplugues, A.; Llop, S.; Delgado-Saborit, J.M. The Effects of Heavy Metal Exposure on Brain and Gut Microbiota: A Systematic Review of Animal Studies. Environ. Pollut. 2024, 348, 123732. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Martins, A.C.; Oliveira-Paula, G.H.; Skalny, A.V.; Zaitseva, I.P.; Bowman, A.B.; Kirichuk, A.A.; Santamaria, A.; Tizabi, Y.; Tinkov, A.A. Manganese in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder: The State of the Art. Curr. Res. Toxicol. 2024, 6, 100170. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.R.; Bowman, A.B. Manganese and the Insulin-IGF Signaling Network in Huntington’s Disease and Other Neurodegenerative Disorders. In Neurotoxicity of Metals; Aschner, M., Costa, L.G., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, Switzerland, 2017; Volume 18, pp. 113–142. ISBN 978-3-319-60188-5. [Google Scholar]
- Cordeiro, L.M.; Soares, M.V.; Da Silva, A.F.; Dos Santos, L.V.; De Souza, L.I.; Da Silveira, T.L.; Baptista, F.B.O.; De Oliveira, G.V.; Pappis, C.; Dressler, V.L.; et al. Toxicity of Copper and Zinc Alone and in Combination in Caenorhabditis Elegans Model of Huntington’s Disease and Protective Effects of Rutin. Neurotoxicology 2023, 97, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Royer, A.; Sharman, T. Copper Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Opazo, C.M.; Lotan, A.; Xiao, Z.; Zhang, B.; Greenough, M.A.; Lim, C.M.; Trytell, H.; Ramírez, A.; Ukuwela, A.A.; Mawal, C.H.; et al. Nutrient Copper Signaling Promotes Protein Turnover by Allosteric Activation of Ubiquitin E2D Conjugases. bioRxiv 2021. [Google Scholar]
- Immergluck, J.; Anilkumar, A.C. Wilson Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Xiao, G.; Fan, Q.; Wang, X.; Zhou, B. Huntington Disease Arises from a Combinatory Toxicity of Polyglutamine and Copper Binding. Proc. Natl. Acad. Sci. USA 2013, 110, 14995–15000. [Google Scholar] [CrossRef]
- Pfalzer, A.C.; Yan, Y.; Kang, H.; Totten, M.; Silverman, J.; Bowman, A.B.; Erikson, K.; Claassen, D.O. Alterations in Metal Homeostasis Occur Prior to Canonical Markers in Huntington Disease. Sci. Rep. 2022, 12, 10373. [Google Scholar] [CrossRef]
- Rosas, H.D.; Chen, Y.I.; Doros, G.; Salat, D.H.; Chen, N.; Kwong, K.K.; Bush, A.; Fox, J.; Hersch, S.M. Alterations in Brain Transition Metals in Huntington Disease: An Evolving and Intricate Story. Arch. Neurol. 2012, 69, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miah, M.R.; Aschner, M. Metals and Neurodegeneration. F1000Research 2016, 5, 366. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, G.F.; Jiménez, J.A.; Thomas, M.G.; Kingsley, B.A.; McIIvin, M.; Saito, M.A.; Korley, E.M. Heterozygous Huntingtin Promotes Cadmium Neurotoxicity and Neurodegeneration in Striatal Cells via Altered Metal Transport and Protein Kinase C Delta Dependent Oxidative Stress and Apoptosis Signaling Mechanisms. Neurotoxicology 2019, 70, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-Associated Metabolic Pathways Affected by Heavy Metals and Metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.-S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Love, C.J.; Masson, B.A.; Gubert, C.; Hannan, A.J. The Microbiota-Gut-Brain Axis in Huntington’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 167, pp. 141–184. ISBN 978-0-323-99176-6. [Google Scholar]
- Wronka, D.; Karlik, A.; Misiorek, J.O.; Przybyl, L. What the Gut Tells the Brain—Is There a Link between Microbiota and Huntington’s Disease? Int. J. Mol. Sci. 2023, 24, 4477. [Google Scholar] [CrossRef] [PubMed]
- Ekwudo, M.N.; Gubert, C.; Hannan, A.J. The Microbiota–Gut–Brain Axis in Huntington’s Disease: Pathogenic Mechanisms and Therapeutic Targets. FEBS J. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Getachew, B.; Csoka, A.B.; Bhatti, A.; Copeland, R.L.; Tizabi, Y. Butyrate Protects Against Salsolinol-Induced Toxicity in SH-SY5Y Cells: Implication for Parkinson’s Disease. Neurotox. Res. 2020, 38, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Csoka, A.B.; Garden, A.R.; Copeland, R.L.; Tizabi, Y. Sodium Butyrate Protects Against Ethanol-Induced Toxicity in SH-SY5Y Cell Line. Neurotox. Res. 2021, 39, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Love, C.J.; Kodikara, S.; Mei Liew, J.J.; Renoir, T.; Lê Cao, K.-A.; Hannan, A.J. Gene-Environment-Gut Interactions in Huntington’s Disease Mice Are Associated with Environmental Modulation of the Gut Microbiome. iScience 2022, 25, 103687. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood–Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Vauleon, S.; Schutz, K.; Massonnet, B.; Gruben, N.; Manchester, M.; Buehler, A.; Schick, E.; Boak, L.; Hawellek, D.J. Quantifying Mutant Huntingtin Protein in Human Cerebrospinal Fluid to Support the Development of Huntingtin-Lowering Therapies. Sci. Rep. 2023, 13, 5332. [Google Scholar] [CrossRef]
- Juarez, D.; Handal-Silva, A.; Morán-Perales, J.L.; Torres-Cifuentes, D.M.; Flores, G.; Treviño, S.; Moreno-Rodriguez, A.; Guevara, J.; Diaz, A. New Insights into Sodium Phenylbutyrate as a Pharmacotherapeutic Option for Neurological Disorders. Synapse 2024, 78, e22301. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Li, X.; Yu, J.-T.; Wang, Y.-J. Therapeutics for Neurodegenerative Diseases by Targeting the Gut Microbiome: From Bench to Bedside. Transl. Neurodegener. 2024, 13, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).