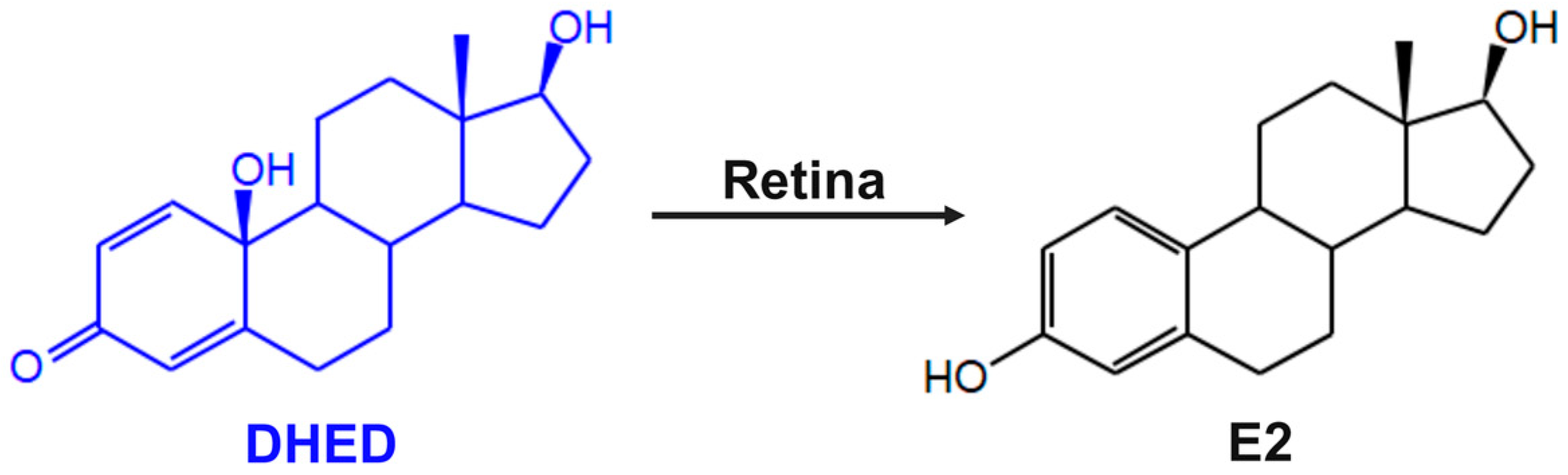
The Prodrug DHED Delivers 17β-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. IOP Measurements and Elevation to Induce Glaucoma
2.4. Eye Drop Treatments
2.5. Assessment of Visual Function
2.6. RGC Quantification
2.7. Optic Nerve Axon Counts
2.8. Measurement of Serum E2
2.9. Targeted Proteomics
2.10. Statistical Analyses
3. Results
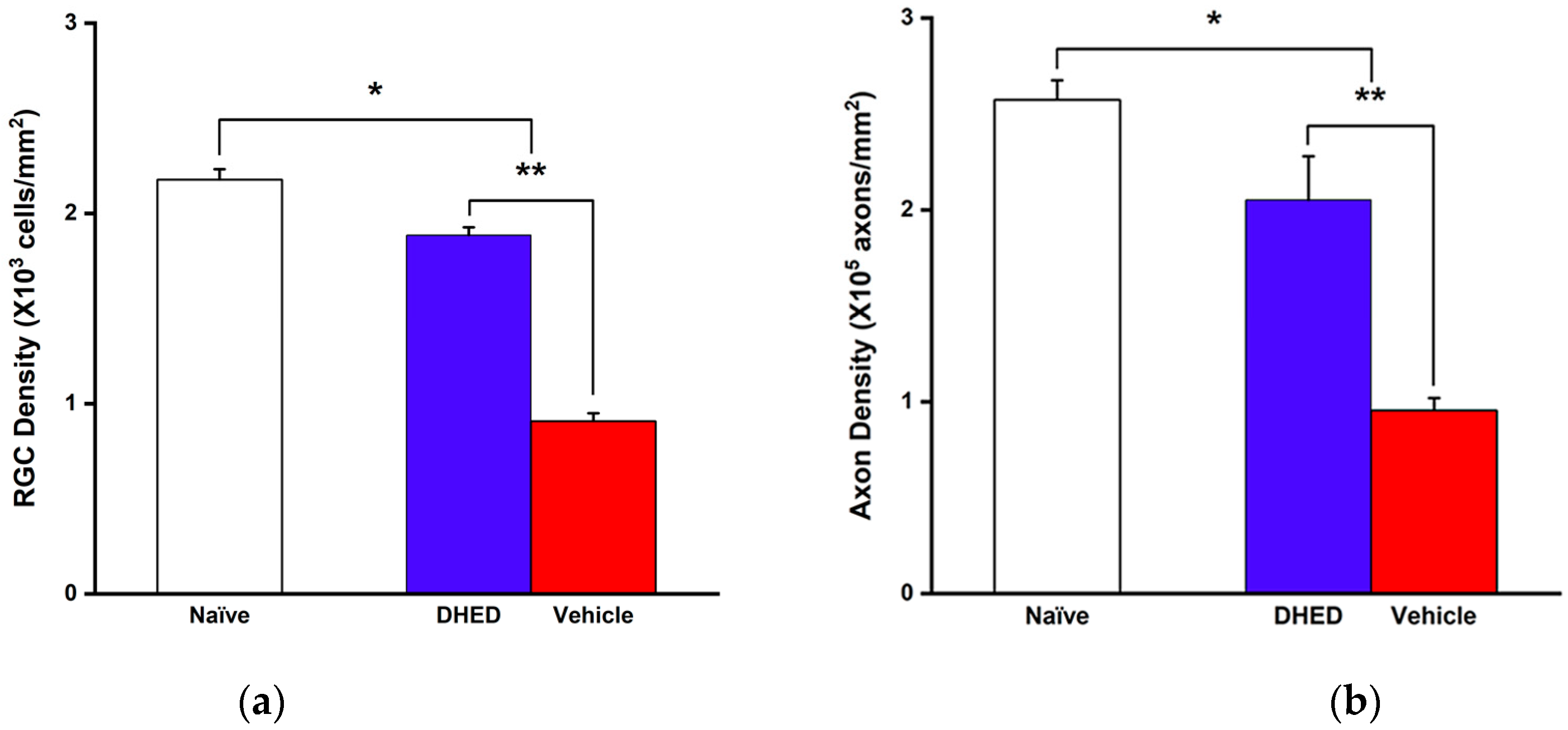
3.1. Impact of Topical DHED Treatments on OHT-Induced Visual Decline Based on OMR, RGC and Axonal Loss
3.2. Impact of Topical DHED Treatments on Visual Function Based on Targeted Proteomics in the Experimental Model of Glaucoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular neurodegenerative diseases: Interconnection between retina and cortical areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Pillar, S.; Moisseiev, E.; Sokolovska, J.; Grzybowski, A. Recent developments in diabetic retinal neurodegeneration: A literature review. J. Diabetes Res. 2020, 2020, 5728674. [Google Scholar] [CrossRef] [PubMed]
- Barresi, C.; Chhablani, J.; Dolz-Marco, R.; Gallego-Pinazo, R.; Berni, A.; Bandello, F.; Borrelli, E. Retinal neurodegeneration in age-related macular degeneration. Eur. J. Ophthalmol. 2024, 34, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Liu, Z.; Zhao, J. Global, regional and national burden of glaucoma: An update analysis from the Global Burden of Disease Study. Int. Ophthalmol. 2024, 44, 234. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of glaucoma: The past, present, and predictions for the future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.G.; Liebmann, J.M.; Levin, L.A. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog. Retin. Eye Res. 2017, 56, 107–147. [Google Scholar] [CrossRef]
- Alqawlaq, S.; Flanagan, J.G.; Sivak, J.M. All roads lead to glaucoma: Induced retinal injury cascades contribute to a common neurodegenerative outcome. Exp. Eye Res. 2019, 183, 88–97. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Matamoros, J.A.; Salobrar-García, E.; Elvira-Hurtado, L.; López-Cuenca, I.; Sánchez-Puebla, L.; Salazar, J.J.; Ramírez, J.M. Glaucoma: From pathogenic mechanisms to retinal glial cell response to damage. Front. Cell. Neurosci. 2024, 18, 1354569. [Google Scholar] [CrossRef]
- Morgan, J.E. Retina ganglion cell degeneration in glaucoma: An opportunity missed? A review. Clin. Exp. Ophthalmol. 2012, 40, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol. Aspects Med. 2023, 92, 101193. [Google Scholar] [CrossRef] [PubMed]
- Kelada, M.; Hill, D.; Yap, T.E.; Manzar, H.; Cordeiro, M.F. Innovations and revolutions in reducing retinal ganglion cell loss in glaucoma. Expert Rev. Ophthalmol. 2021, 16, 33–46. [Google Scholar] [CrossRef]
- Neufeld, A.H. New conceptual approaches for pharmacological neuroprotection in glaucomatous neuronal degeneration. J. Glaucoma 1998, 7, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Vishwaraj, C.R.; Kavitha, S.; Venkatesh, R.; Shukla, A.G.; Chandran, P.; Tripathi, S. Neuroprotection in glaucoma. Indian J. Ophthalmol. 2022, 70, 380–385. [Google Scholar]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Hsueh, Y.J.; Chen, Y.N.; Tsao, Y.T.; Cheng, C.M.; Wu, W.C.; Chen, H.C. The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Pandino, I.; Giammaria, S.; Zingale, G.A.; Roberti, G.; Michelessi, M.; Coletta, M.; Manni, G.; Agnifili, L.; Vercellin, A.V.; Harris, A.; et al. Ubiquitin proteasome system and glaucoma: A survey of genetics and molecular biology studies supporting a link with pathogenic and therapeutic relevance. Mol. Asp. Med. 2023, 94, 101226. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Prokosch, V. The relationship between mitochondria and neurodegeration in the eye: A review. Appl. Sci. 2021, 11, 7385. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Sadri, A. Is target-based drug discovery efficient? discovery and “off-target” mechanisms of all drugs. J. Med. Chem. 2023, 66, 12651–12677. [Google Scholar] [CrossRef]
- Swinney, D.C. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin. Pharmacol. Ther. 2013, 93, 299–301. [Google Scholar] [CrossRef]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug. Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Szabo, M.; Svensson Akusjärvi, S.; Saxena, A.; Liu, J.; Chandrasekar, G.; Kitambi, S.S. Cell and small animal models for phenotypic drug discovery. Drug. Des. Devel. Ther. 2017, 11, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental models of glaucoma: A powerful translational tool for the future development of new therapies for glaucoma in humans-a review of the literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Prusky, G.T.; Douglas, R.M.; Yasumura, D.; Matthes, M.T.; Lowe, R.J.; Duncan, J.L.; Yang, H.; Ahern, K.; Daniello, K.M.; et al. Discordant anatomical, electrophysiological, and visual behavioral profiles of retinal degeneration in rat models of retinal degenerative disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 6232. [Google Scholar] [CrossRef] [PubMed]
- Prusky, G.T.; West, P.W.; Douglas, R.M. Behavioral assessment of visual acuity in mice and rats. Vis. Res. 2000, 40, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-Estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 2013, 19, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Sato, K.; Fujita, K.; Daigaku, R.; Tawarayama, H.; Murayama, N.; Moritoh, S.; Yabana, T.; Shiga, Y.; Omodaka, K.; et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci. Rep. 2017, 7, 6885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sainulabdeen, A.; Akers, K.; Adi, V.; Sims, J.R.; Yarsky, E.; Yan, Y.; Yu, Y.; Ishikawa, H.; Leung, C.K.; et al. Oral scutellarin treatment ameliorates retinal thinning and visual deficits in experimental glaucoma. Front. Med. 2021, 8, 681169. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Prokai, L. A novel prodrug approach for central nervous system-selective estrogen therapy. Molecules 2019, 24, 4197. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Nguyen, V.; De La Cruz, D.L.; Guerra, R.; Zaman, K.; Rahlouni, F.; Prokai, L. Retina-targeted delivery of 17β-estradiol by the topically applied DHED prodrug. Pharmaceutics 2020, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Zaman, K.; Nguyen, V.; De La Cruz, D.L.; Prokai, L. Proteomics-based retinal target engagement analysis and retina-targeted delivery of 17β-estradiol by the DHED prodrug for ocular neurotherapy in males. Pharmaceutics 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Moore, C.G.; Deppmeier, L.M.; Gold, B.G.; Meshul, C.K.; Johnson, E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997, 64, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Prokai, L. Estrogen and neuroprotection. In Sex Hormones in Neurodegenerative Processes; Drevensek, G., Ed.; IntechOpen: Riyeka, Croatia, 2017. [Google Scholar]
- Prokai-Tatrai, K.; Zaman, K.; Prokai, L. Neuroprotection by estrogens. In Natural Molecules in Neuroprotection and Neurotoxicity, 1st ed.; de Oliviera, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Kretschmer, F.; Tariq, M.; Chatila, W.; Wu, B.; Badea, T.C. Comparison of optomotor and optokinetic reflexes in mice. J. Neurophysiol. 2017, 118, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.T. In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol. Biol. 2011, 682, 3–13. [Google Scholar] [PubMed]
- Stradleigh, T.W.; Ishida, A.T. Fixation strategies for retinal immunohistochemistry. Prog. Retin. Eye. Res. 2015, 48, 181–202. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef]
- Stoughton, R.B.; Friend, S.H. How molecular profiling could revolutionize drug discovery. Nat. Rev. Drug. Discov. 2005, 4, 345–350. [Google Scholar] [CrossRef]
- Prokai, L.; Zaman, K.; Prokai-Tatrai, K. Mass spectrometry-based retina proteomics. Mass Spectrom. Rev. 2023, 42, 1032–1062. [Google Scholar] [CrossRef]
- Ban, N.; Siegfried, C.J.; Apte, R.S. Monitoring neurodegeneration in glaucoma: Therapeutic implications. Trends Mol. Med. 2018, 24, 7–17. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Bixler, G.V.; Kutzler, L.; Brucklacher, R.M.; Bronson, S.K.; Kimball, S.R.; Freeman, W.M. Multi-modal proteomic analysis of retinal protein expression alterations in a rat model of diabetic retinopathy. PLoS ONE 2011, 6, e16271. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Nguyen, V.; Prokai-Tatrai, K.; Prokai, L. Proteomics-based identification of retinal protein networks impacted by elevated intraocular pressure in the hypertonic saline injection model of experimental glaucoma. Int. J. Mol. Sci. 2023, 24, 12592. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Nguyen, V.; Szarka, S.; Garg, P.; Sabnis, G.; Bimonte-Nelson, H.A.; McLaughlin, K.J.; Talboom, J.S.; Conrad, C.D.; Shughrue, P.J.; et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci. Transl. Med. 2015, 7, 297ra113. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Otmani, A.; Kokkali, E.; Lardner, E.; Morgan, J.E.; Williams, P.A. Retinal ganglion cell degeneration in a rat magnetic bead model of ocular hypertensive glaucoma. Transl. Vis. Sci. Technol. 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Pribyl, T.; Rohácová, J. Effect of estrogen on the increase of anterior pituitary weight and 125-I-thyroxine binding to pituitary proteins: Inhibition by theophylline. Endocrinol. Exp. 1971, 5, 237–244. [Google Scholar] [PubMed]
- Jackson, A.C.; Tenniswood, M.; Bird, C.E.; Clark, A.F. Effects of androgen and estradiol administration on the weight of the ventral prostate, seminal vesicles, and testes of immature rats. Investig. Urol. 1977, 14, 351–355. [Google Scholar]
- Johnson, N.P.; Gregorich, S.M.; Passaglia, C.L. Spatiotemporal contrast sensitivity of Brown-Norway rats under scotopic and photopic illumination. Neurosci. 2020, 449, 63–73. [Google Scholar] [CrossRef]
- Mahato, B.; Kaya, K.D.; Fan, Y.; Sumien, N.; Shetty, R.A.; Zhang, W.; Davis, D.; Mock, T.; Batabyal, S.; Ni, A.; et al. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature 2020, 581, 83–88. [Google Scholar] [CrossRef]
- Youale, J.; Bigot, K.; Kodati, B.; Jaworski, T.; Fan, Y.; Nsiah, N.Y.; Pappenhagen, N.; Inman, D.M.; Behar-Cohen, F.; Bordet, T.; et al. Neuroprotective effects of transferrin in experimental glaucoma models. Int. J. Mol. Sci. 2022, 23, 12753. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 567–671. [Google Scholar] [CrossRef]
- Sadun, A.A.; Smith, L.E.; Kenyon, K.R. Paraphenylenediamine: A new method for tracing human visual pathways. J. Neuropathol. Exp. Neurol. 1983, 42, 200–206. [Google Scholar] [CrossRef]
- Goyal, V.; Read, A.T.; Ritch, M.D.; Hannon, B.G.; Rodriguez, G.S.; Brown, D.M.; Feola, A.J.; Hedberg-Buenz, A.; Cull, G.A.; Reynaud, J.; et al. AxoNet 2.0: A deep learning-based tool for morphometric analysis of retinal ganglion cell axons. Transl. Vis. Sci. Technol. 2023, 12, 9. [Google Scholar] [CrossRef]
- Szarka, S.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Separation of dansylated 17β-estradiol, 17α -estradiol and estrone on a single HPLC column for simultaneous quantitation by LC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 3399–3406. [Google Scholar] [CrossRef]
- Prokai, L.; Nguyen, V.; Urbanski, H.F. Effect of estradiol replacement on hippocampal concentrations of estrogens in aged rhesus macaques maintained on an obesogenic diet. Biochem. Biophys. Rep. 2023, 35, 101548. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Zaman, K.; Nguyen, V.; Prokai-Tatrai, K. 17β-Estradiol delivered in eye drops: Evidence of impact on protein networks and associated biological processes in the rat retina through quantitative proteomics. Pharmaceutics 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open-source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Koszegi, Z.; Cheong, R.Y.; Abrahám, I.M. Neuroprotective effects of non-classical estrogen-like signaling activators: From mechanism to potential implications. CNS Neurol. Disord. Drug Targets 2013, 12, 1219–1225. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal degeneration and Alzheimer’s disease: An evolving link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef]
- Zhou, X.; Li, F.; Ge, J.; Sarkisian, S.R., Jr.; Tomita, H.; Zaharia, A.; Chodosh, J.; Cao, W. Retinal ganglion cell protection by 17-β-estradiol in a mouse model of inherited glaucoma. Dev. Neurobiol. 2007, 67, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ma, X.; Zhao, Q.; Li, Y.; Xing, Y.; Deng, Q.; Shen, Y. The neuroprotective effects of novel estrogen receptor GPER1 in mouse retinal ganglion cell degeneration. Exp. Eye Res. 2019, 189, 107826. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, Y.; Munemasa, Y.; Hayashi, Y.; Kuribayashi, J.; Koseki, N.; Kojima, K.; Kumai, T.; Ueno, S. Axonal protection by 17β-estradiol through thioredoxin-1 in tumor necrosis factor-induced optic neuropathy. Endocriology 2011, 152, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cavaliere, F.; Watanabe, C.; Nucci, C.; Bagetta, G.; Corasaniti, M.T.; Sakurada, S.; Morrone, L.A. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog. Brain Res. 2008, 173, 583–590. [Google Scholar] [PubMed]
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Vajaranant, T.S.; Pasquale, L.R. Estrogen deficiency accelerates aging of the optic nerve. Menopause 2012, 19, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Gossman, C.A.; Christie, J.; Webster, M.K.; Linn, D.M.; Linn, C.L. Neuroprotective strategies in glaucoma. Curr. Pharm. Des. 2016, 22, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Y.; Yao, K. Protection of retinal ganglion cells in glaucoma: Current status and future. Exp. Eye Res. 2021, 205, 108506. [Google Scholar] [CrossRef]
- Pascale, A.; Drago, F.; Govoni, S. Protecting the retinal neurons from glaucoma: Lowering ocular pressure is not enough. Pharmacol. Res. 2012, 66, 19–32. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Pharmaceutical approaches to normal tension glaucoma. Pharmaceuticals 2023, 16, 1172. [Google Scholar] [CrossRef]
- Lešták, J.; Pitrová, Š.; Marešová, K. Highlights of hypertensive and normotensive glaucoma. Cesk. Slov. Ofralmol. 2020, 76, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Yin, Z.; Madore, C.; Pitts, K.M.; Letcher, S.M.; Tang, J.; Jiang, S.; Gauthier, C.D.; Silveira, S.R.; Schroeder, C.M.; et al. Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity 2022, 55, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.V.; Soto, I.; Kim, K.-Y.; Bushong, E.A.; Oglesby, E.; Valiente-Soriano, F.J.; Yang, Z.; Davis, C.-H.O.; Bedont, J.L.; Son, J.L.; et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. USA 2011, 108, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Garone, G.; Travaglini, L.; Vasco, G.; Galosi, S.; Rios, L.; Castiglioni, C.; Barassi, C.; Battaglia, D.; Gambardella, M.L.; et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Park. Relat. Disord. 2019, 61, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, J. Structural basis of properties, mechanisms, and channelopathy of cyclic nucleotide-gated channels. Channels 2023, 17, 2273165. [Google Scholar] [CrossRef] [PubMed]
- Thanos, S.; Böhm, M.R.; Meyer zu Hörste, M.; Prokosch-Willing, V.; Hennig, M.; Bauer, D.; Heiligenhaus, A. Role of crystallins in ocular neuroprotection and axonal regeneration. Prog. Ret. Eye Res. 2014, 42, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, N.; Matsuyama, S.; Voss, O.; Doseff, A.I.; Song, K.; Danielpour, D.; Nagaraj, R.H. The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010, 1, e31X. [Google Scholar] [CrossRef] [PubMed]
- Anders, F.; Teister, J.; Liu, A.; Funke, S.; Grus, F.H.; Thanos, S.; von Pein, H.D.; Pfeiffer, N.; Prokosch, V. Intravitreal injection of β-crystallin B2 improves retinal ganglion cell survival in an experimental animal model of glaucoma. PLoS ONE 2017, 12, e0175451. [Google Scholar] [CrossRef]
- Böhm, M.R.; Prokosch, V.; Brückner, M.; Pfrommer, S.; Melkonyan, H.; Thanos, S. βB2-Crystallin promotes axonal regeneration in the injured optic nerve in adult rats. Cell Transplant. 2015, 24, 1829–1844. [Google Scholar] [CrossRef]
- Fischer, R.A.; Risner, M.L.; Roux, A.L.; Wareham, L.K.; Sappington, R.M. Impairment of membrane repolarization accompanies axon transport deficits in glaucoma. Front. Neurosci. 2019, 13, 1139. [Google Scholar] [CrossRef]
- Joyal, J.-S.; Gantner, M.L.; Smith, L.E.H. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T. Mitochondrial dysfunction in the aging retina. Biology 2019, 11, 31. [Google Scholar] [CrossRef]
- Chu, L.; Xiao, L.; Xu, B.; Xu, J. Dissociation of HKII in retinal epithelial cells induces oxidative stress injury in the retina. Int. J. Mol. Med. 2019, 44, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Weh, E.; Lutrzykowska, Z.; Smith, A.; Hager, H.; Pawar, M.; Wubben, T.J.; Besirli, C.G. Hexokinase 2 is dispensable for photoreceptor development but is required for survival during aging and outer retinal stress. Cell Death Dis. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]


| Group | E2 (pg/mL) | SV (mg) | AP (mg) |
|---|---|---|---|
| Naïve | 2.3 ± 0.7 | 507.8 ± 50.1 | 5.2 ± 0.4 |
| Vehicle | 2.4 ± 0.6 | 501.0 ± 34.3 | 5.2 ± 0.6 |
| DHED | 2.2 ± 0.4 | 493.0 ± 15.8 | 5.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapic, A.; Zaman, K.; Nguyen, V.; Neagu, G.C.; Sumien, N.; Prokai, L.; Prokai-Tatrai, K. The Prodrug DHED Delivers 17β-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma. Cells 2024, 13, 1126. https://doi.org/10.3390/cells13131126
Kapic A, Zaman K, Nguyen V, Neagu GC, Sumien N, Prokai L, Prokai-Tatrai K. The Prodrug DHED Delivers 17β-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma. Cells. 2024; 13(13):1126. https://doi.org/10.3390/cells13131126
Chicago/Turabian StyleKapic, Ammar, Khadiza Zaman, Vien Nguyen, George C. Neagu, Nathalie Sumien, Laszlo Prokai, and Katalin Prokai-Tatrai. 2024. "The Prodrug DHED Delivers 17β-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma" Cells 13, no. 13: 1126. https://doi.org/10.3390/cells13131126
APA StyleKapic, A., Zaman, K., Nguyen, V., Neagu, G. C., Sumien, N., Prokai, L., & Prokai-Tatrai, K. (2024). The Prodrug DHED Delivers 17β-Estradiol into the Retina for Protection of Retinal Ganglion Cells and Preservation of Visual Function in an Animal Model of Glaucoma. Cells, 13(13), 1126. https://doi.org/10.3390/cells13131126







