Exploring the Biosafety Potential of Haberlea rhodopensis Friv. In Vitro Culture Total Ethanol Extract: A Comprehensive Assessment of Genotoxicity, Mitotoxicity, and Cytotoxicity for Therapeutic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Haberlea Rhodopensis Friv. In Vitro Culture Total Ethanol Extract (HRT)
2.2. Cell Culture, Media and Treatment Protocols
2.3. Phase Contrast Light Microscopy
2.4. WST -1 Cell Viability Assay
2.5. DCFH-DA Assay
2.6. Single Cell Gel Electrophoresis (SCGE)
2.7. Fluorescence Activated Cell Sorting Analysis (FACS)
2.7.1. Cell Cycle Analysis via Propidium Iodide (P.I.) Staining
2.7.2. Analysis of Mitochondrial Membrane Potential (MMP) via Rhodamine 123 (Rh123) Staining and FACS
2.8. Analysis of Mitochondrial Morphology via Biotraker 488 Staining and Fluorescent Microscopy
3. Results
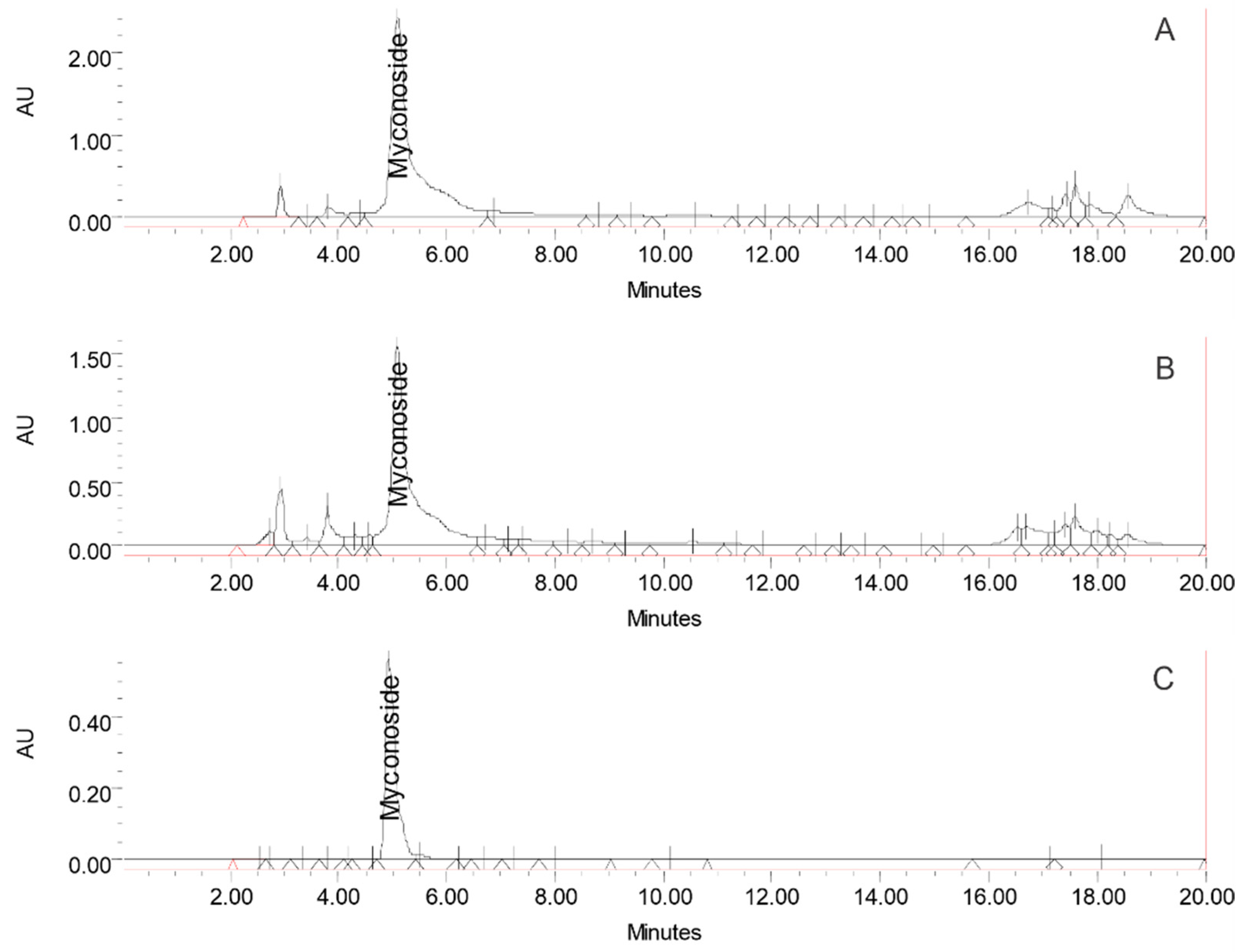
3.1. Biochemical Assessment of the HRT Extracts
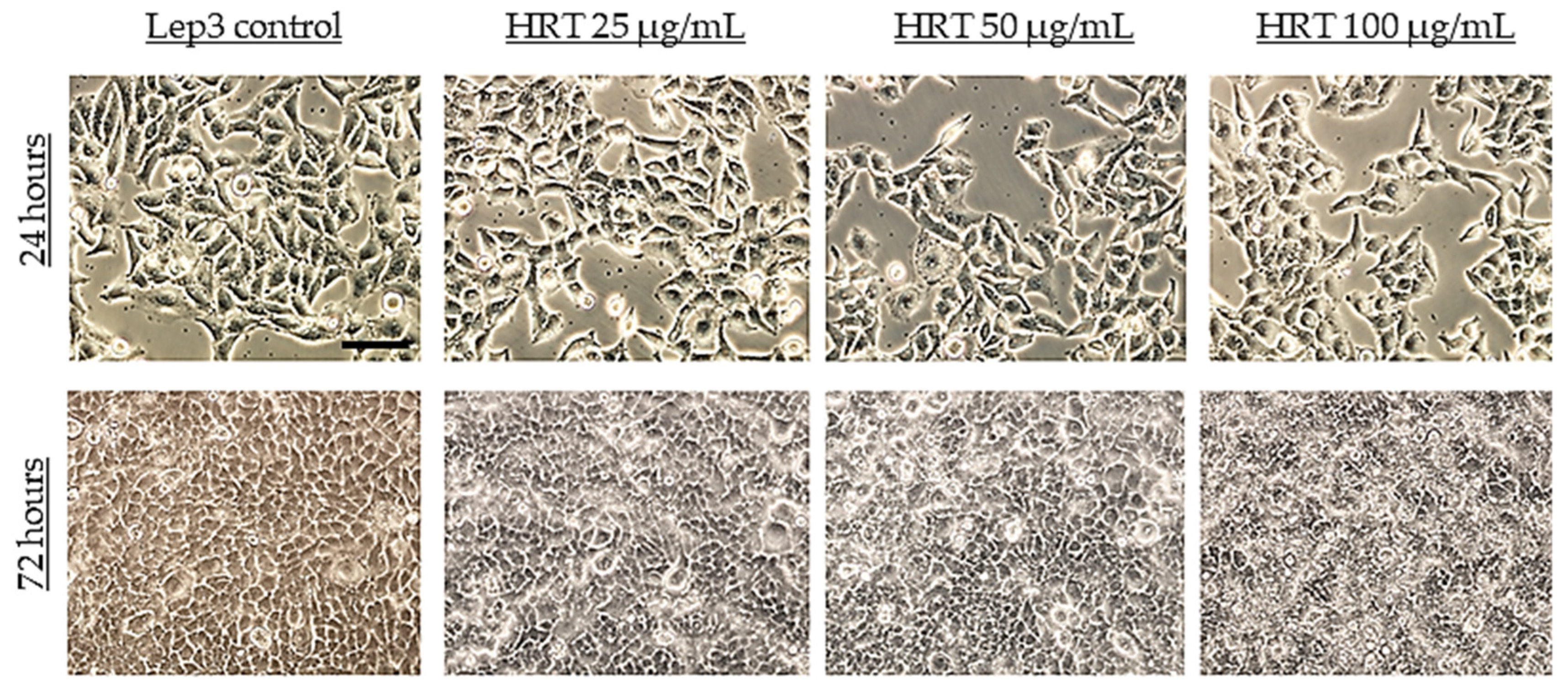
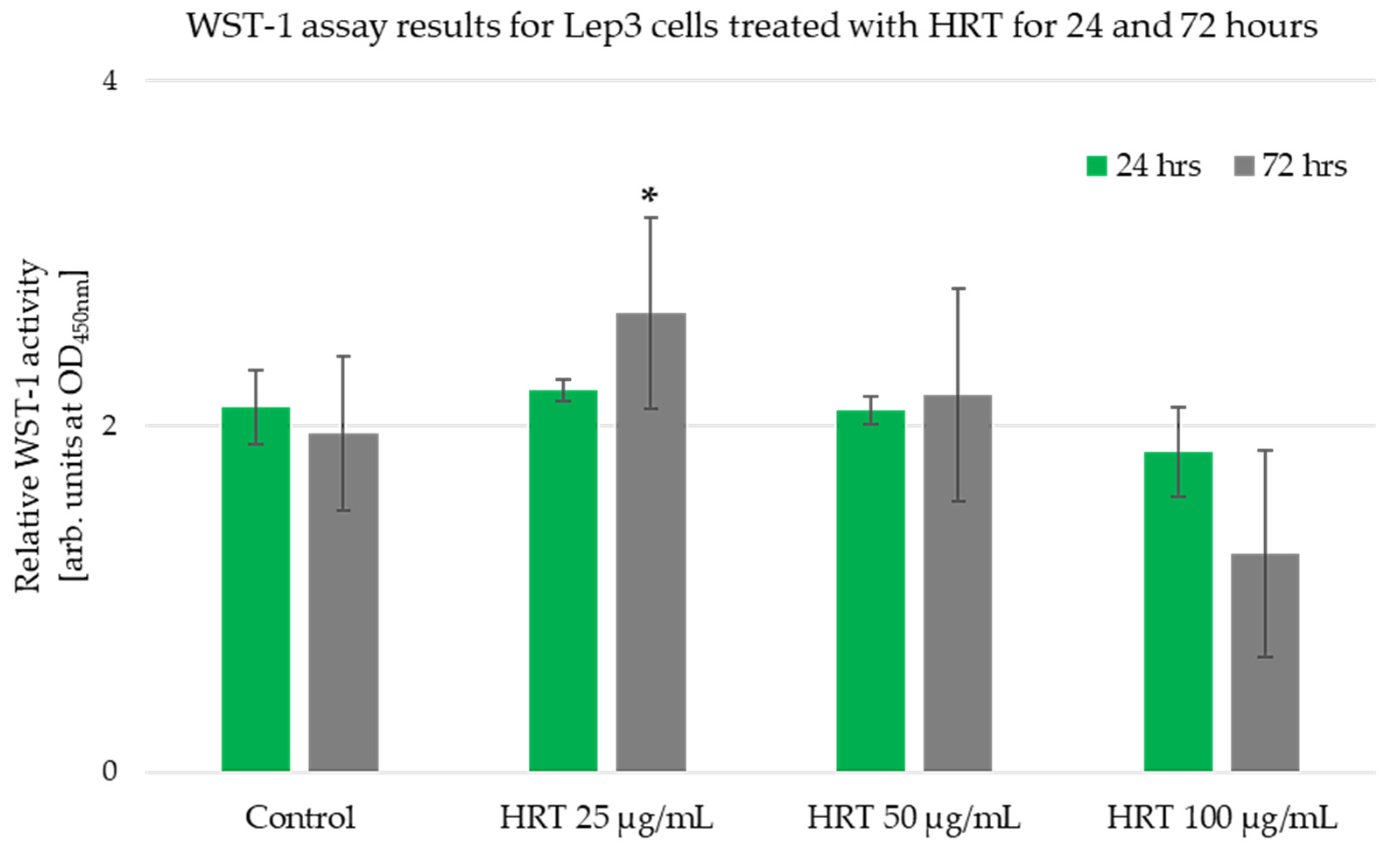
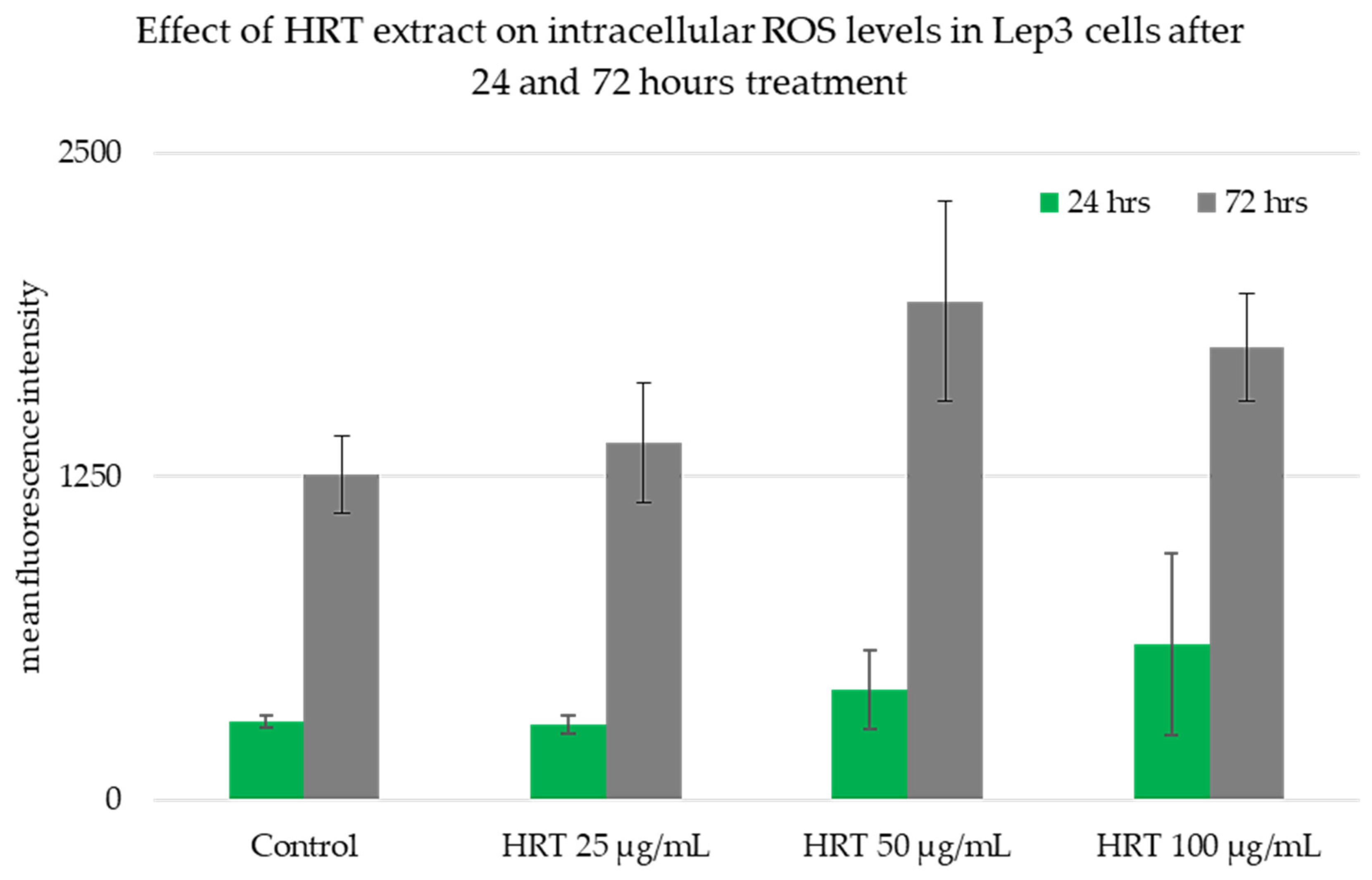
3.2. Cytotoxicity Assessment of the HRT Extracts on the Lep3 Cell Line
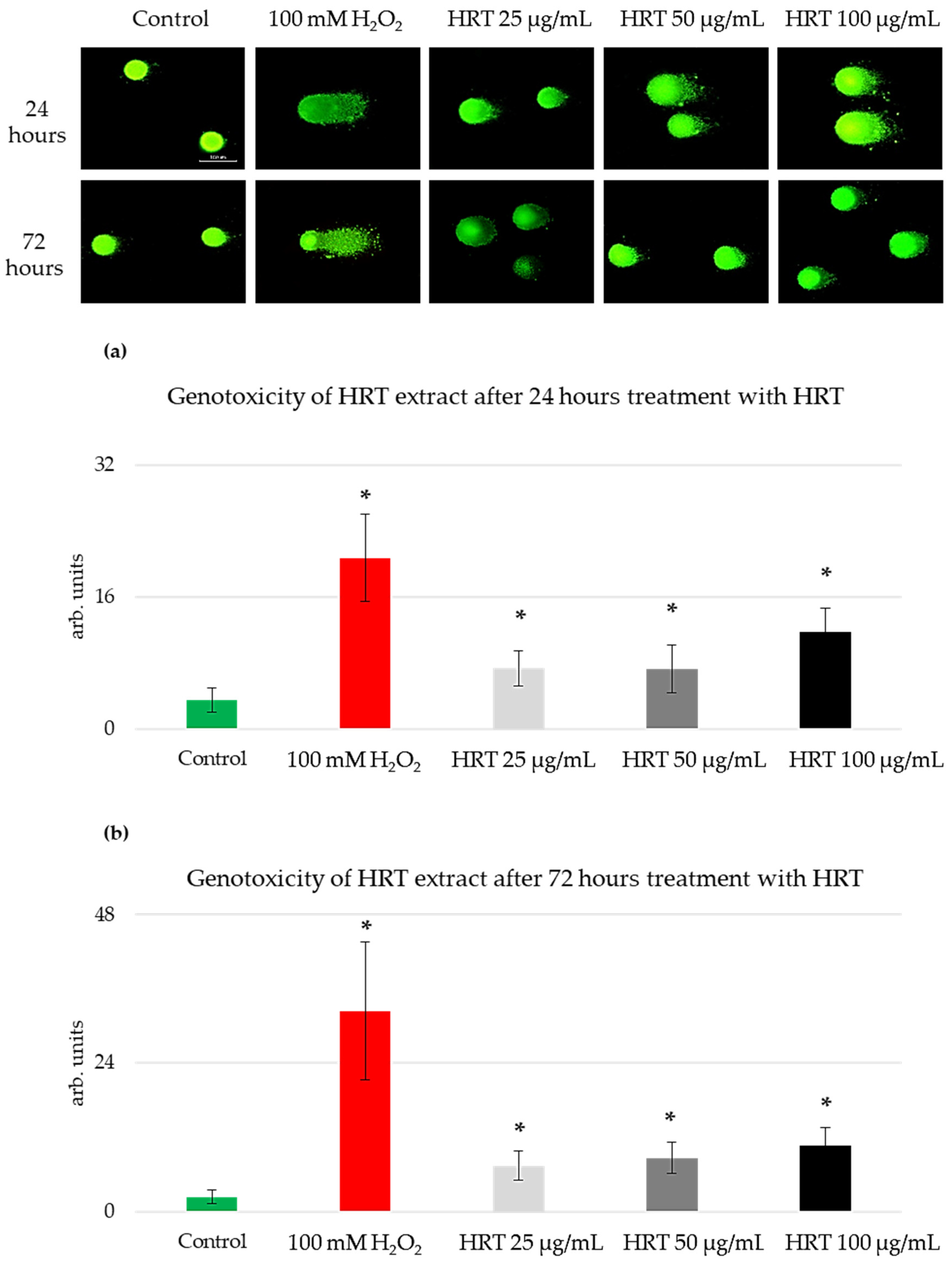
3.3. Genotoxicity Assessment of the HRT Extracts on the Lep3 Cell Line
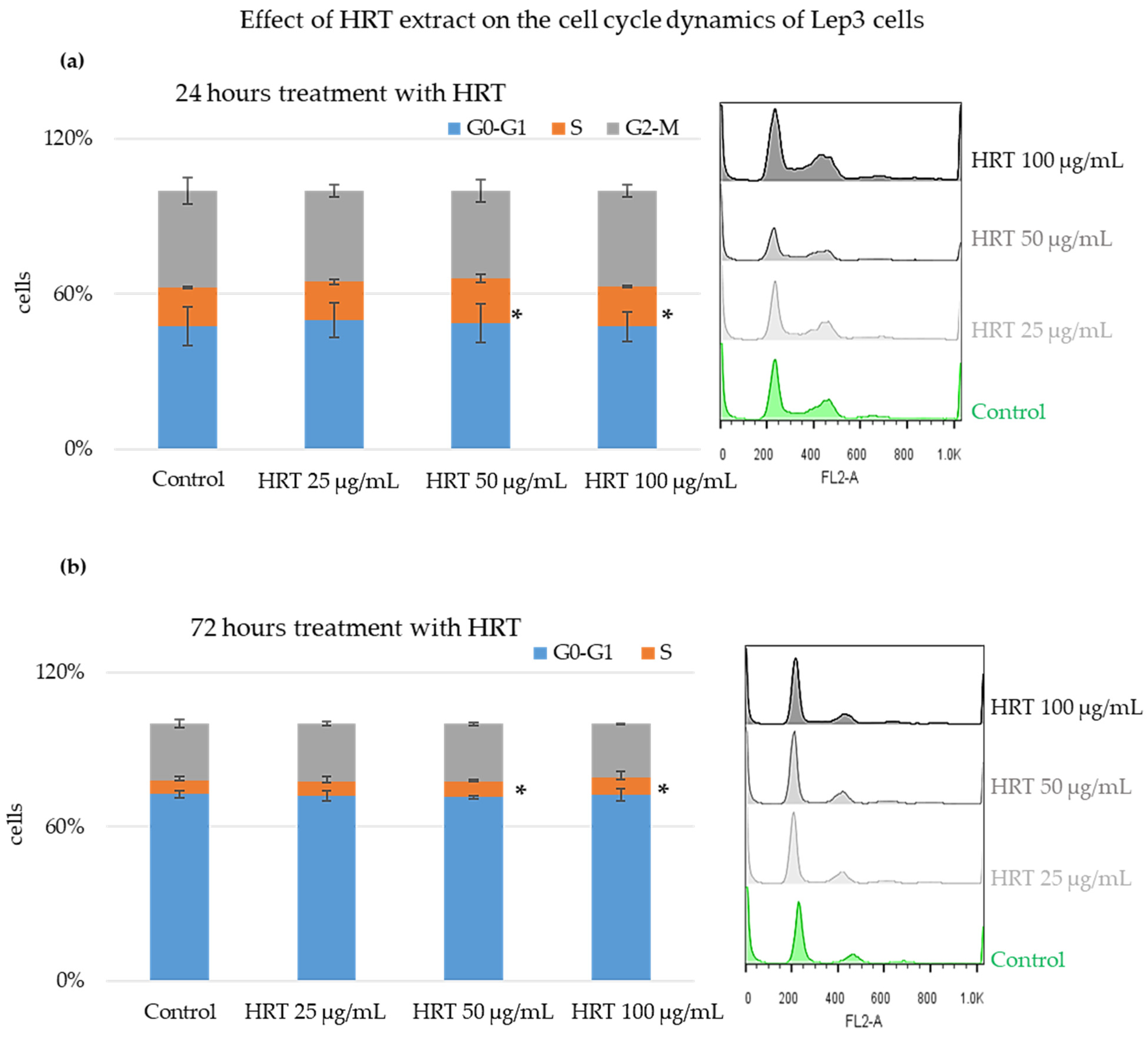
3.4. Cytostatic Effect of the HRT Extract on Lep3 Cells
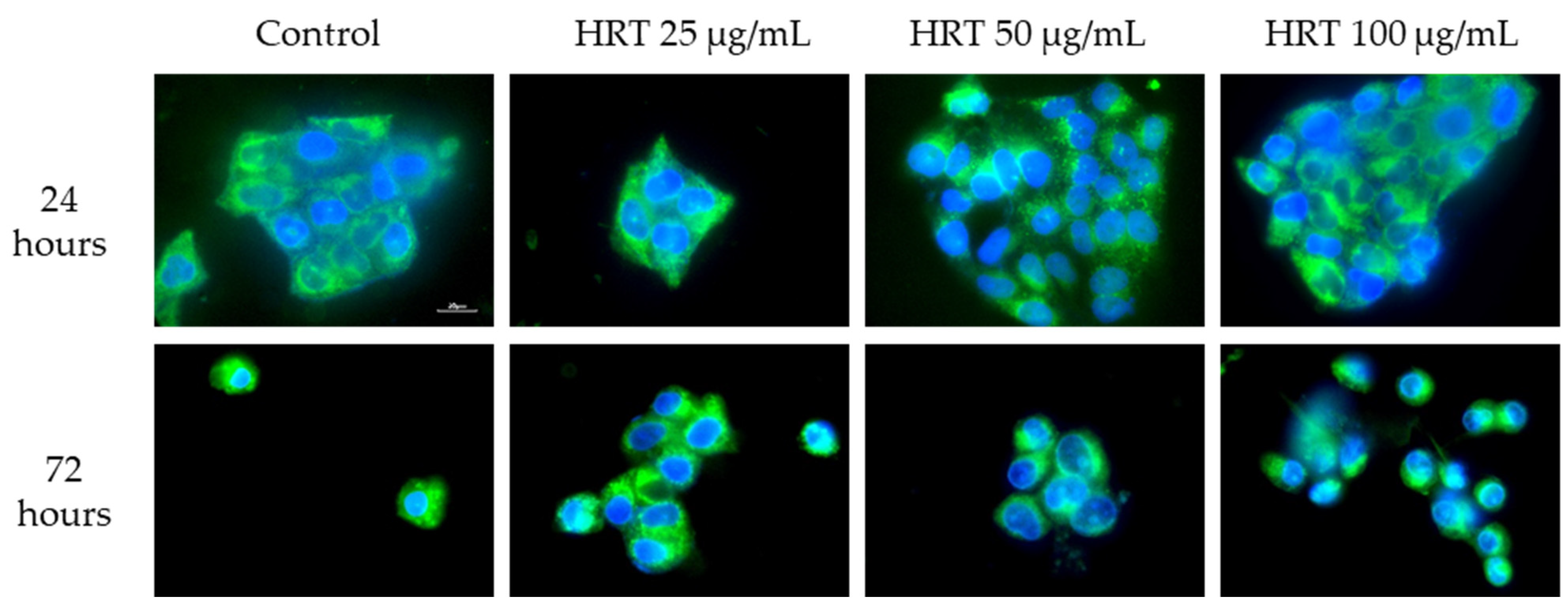
3.5. Mitochondrial Membrane Potential and Morphology Analyses
3.5.1. Mitochondrial Membrane Potential Detection by Staining of Cells with Rh123 and FACS Analyses
3.5.2. Mitochondrial Structure Visualization by Biotracker 488 Staining
4. Discussion
4.1. H. rhodopensis Extracts Demonstrate High Viability and Low Cytotoxicity in Lep3 Cells
4.2. Non-Genotoxic Effects of Low-Dose Haberlea rhodopensis In Vitro Culture Extracts
4.3. Enhanced Mitochondrial Activity and Cell Cycle Distribution in Lep3 Cells Treated with H. rhodopensis In Vitro Extracts for More Extended Periods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedarko, N.S. The biology of aging and frailty. Clin. Geriatr. Med. 2011, 27, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Troen, B.R. The biology of aging. Mt. Sinai J. Med. 2003, 70, 3–22. [Google Scholar] [PubMed]
- Klopack, E.T.; Carroll, J.E.; Cole, S.W.; Seeman, T.E.; Crimmins, E.M. Lifetime exposure to smoking, epigenetic aging, and morbidity and mortality in older adults. Clin. Epigenet. 2022, 14, 72. [Google Scholar] [CrossRef]
- Bosnes, I.; Nordahl, H.M. Lifestyle predictors of successful aging: A 20-year prospective HUNT study. PLoS ONE 2019, 14, e0219200. [Google Scholar] [CrossRef] [PubMed]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Herath, D.; Cranfield, J.; Henson, S. Who consumes functional foods and nutraceuticals in Canada?: Results of cluster analysis of the 2006 survey of Canadians’ Demand for Food Products Supporting Health and Wellness. Appetite 2008, 51, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic–Implications for consumers and regulatory oversight. PharmaNutrition 2021, 18, 100282. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Vasileva, B.; Staneva, D.; Grozdanova, T.; Petkov, H.; Trusheva, B.; Alipieva, K.; Popova, M.; Miloshev, G.; Bankova, V.; Georgieva, M. Natural Deep Eutectic Extracts of Propolis, Sideritis scardica, and Plantago major Reveal Potential Antiageing Activity during Yeast Chronological Lifespan. Oxid. Med. Cell. Longev. 2022, 2022, 8368717. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Wang, D.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation. Biotechnol. Adv. 2018, 36, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Hille, J.; Woerdenbag, H.J.; Benina, M.; Mehterov, N.; Toneva, V.; Fernie, A.R.; Mueller-Roeber, B. Natural products from resurrection plants: Potential for medical applications. Biotechnol. Adv. 2014, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Djilianov, D.; Ivanov, S.; Georgieva, T.; Moyankova, D.; Berkov, S.; Petrova, G.; Mladenov, P.; Christov, N.; Hristozova, N.; Peshev, D.; et al. A Holistic Approach to Resurrection Plants. Haberlea rhodopensis—A Case Study. Biotechnol. Biotechnol. Equip. 2009, 23, 1414–1416. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N. The ancient Thracian endemic plant Haberlea rhodopensis Friv. and related species: A review. J. Ethnopharmacol. 2020, 249, 112359. [Google Scholar] [CrossRef] [PubMed]
- Hayrabedyan, S.; Todorova, K.; Zasheva, D.; Moyankova, D.; Georgieva, D.; Todorova, J.; Djilianov, D. Haberlea rhodopensis has Potential as a New Drug Source Based on its Broad Biological Modalities. Biotechnol. Biotechnol. Equip. 2013, 27, 3553–3560. [Google Scholar] [CrossRef]
- Gupta, S.; Petrov, V.; Garg, V.; Mueller-Roeber, B.; Fernie, A.R.; Nikoloski, Z.; Gechev, T. The genome of Haberlea rhodopensis provides insights into the mechanisms for tolerance to multiple extreme environments. Cell. Mol. Life Sci. 2024, 81, 117. [Google Scholar] [CrossRef] [PubMed]
- Amirova, K.M.; Dimitrova, P.A. Biotechnologically-Produced Myconoside and Calceolarioside E Induce Nrf2 Expression in Neutrophils. Int. J. Mol. Sci. 2021, 22, 1759. [Google Scholar] [CrossRef] [PubMed]
- Traykova, B.; Stanilova, M. Soilless Propagation of Haberlea rhodopensis Friv. Using Different Hydroponic Systems and Substrata. Ecol. Balk. 2020, 12, 111–121. [Google Scholar]
- Georgiev, V.G.; Pavlov, A.I. Standardized Plant Extract from Biomass of In Vitro Cultures, Method for Preparation and Use Thereof. U.S. Patent Application 17/912,237 WO2021184086A1, 22 June 2023. [Google Scholar]
- Krasteva, N.; Staneva, D.; Vasileva, B.; Miloshev, G.; Georgieva, M. Bioactivity of PEGylated Graphene Oxide Nanoparticles Combined with Near-Infrared Laser Irradiation Studied in Colorectal Carcinoma Cells. Nanomaterials 2021, 11, 3061. [Google Scholar] [CrossRef]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Walsh, M.L.; Bockus, B.J.; Chen, L.B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 1981, 88, 526–535. [Google Scholar] [CrossRef]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a probe of mitochondrial membrane potential: Evaluation of proton flux through F0 during ATP synthesis. Biochim. Et Biophys. Acta 2003, 1606, 137–146. [Google Scholar] [CrossRef]
- Gokerkucuk, E.B.; Tramier, M.; Bertolin, G. Imaging Mitochondrial Functions: From Fluorescent Dyes to Genetically-Encoded Sensors. Genes 2020, 11, 125. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre d’Hellencourt, C.; Gonthier, M.P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. 2015, 12, 10. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- Møller, P. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin. Pharmacol. Toxicol. 2005, 96 (Suppl. 1), 1–42. [Google Scholar] [PubMed]
- Ladeira, C.; Smajdova, L. The use of genotoxicity biomarkers in molecular epidemiology: Applications in environmental, occupational and dietary studies. AIMS Genet. 2017, 4, 166–191. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, M.; Amiri, M.; Ebrahimzadeh-bideskan, A.; Hajavi, J. DNA damage assessment in the lymphocytes of construction painters by comet assay. Toxicol. Ind. Health 2016, 32, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Moyankova, D.; Djilianov, D.; Uzunova, K.; Miloshev, G. Methanol extracts from the resurrection plant Haberlea rhodopensis ameliorate cellular vitality in chronologically ageing Saccharomyces cerevisiae cells. Biogerontology 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Rasesemola, R.M.; Mmusi-Phetoe, R.M.; Havenga, Y. Social determinants of health in non-communicable diseases prevention policies in South Africa. Curationis 2023, 46, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, J.; Lan, X.; Zeng, W.; Zhou, J.; Huang, T.E.; Xiao, W.L.; Wang, Q.Q.; Sun, S.; Su, W.; et al. Handelin extends lifespan and healthspan of Caenorhabditis elegans by reducing ROS generation and improving motor function. Biogerontology 2022, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Peeva, M.I.; Georgieva, M.G.; Balacheva, A.A.; Pavlov, A.; Tzvetkov, N.T. In Vitro Investigation of the Cytotoxic and Antiproliferative Effects of Haberlea rhodopensis Total Extract: A Comparative Study. Cosmetics 2024, 11, 46. [Google Scholar] [CrossRef]
- Ozgun, G.S.; Ozgun, E. The cytotoxic concentration of rosmarinic acid increases MG132-induced cytotoxicity, proteasome inhibition, autophagy, cellular stresses, and apoptosis in HepG2 cells. Hum. Exp. Toxicol. 2020, 39, 514–523. [Google Scholar] [CrossRef]
- Diao, M.; Liang, Y.; Zhao, J.; Zhao, C.; Zhang, J.; Zhang, T. Enhanced cytotoxicity and antioxidant capacity of kaempferol complexed with α-lactalbumin. Food Chem. Toxicol. 2021, 153, 112265. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, B.; Krasteva, N.; Hristova-Panusheva, K.; Ivanov, P.; Miloshev, G.; Pavlov, A.; Georgiev, V.; Georgieva, M. Exploring the Biosafety Potential of Haberlea rhodopensis Friv. In Vitro Culture Total Ethanol Extract: A Comprehensive Assessment of Genotoxicity, Mitotoxicity, and Cytotoxicity for Therapeutic Applications. Cells 2024, 13, 1118. https://doi.org/10.3390/cells13131118
Vasileva B, Krasteva N, Hristova-Panusheva K, Ivanov P, Miloshev G, Pavlov A, Georgiev V, Georgieva M. Exploring the Biosafety Potential of Haberlea rhodopensis Friv. In Vitro Culture Total Ethanol Extract: A Comprehensive Assessment of Genotoxicity, Mitotoxicity, and Cytotoxicity for Therapeutic Applications. Cells. 2024; 13(13):1118. https://doi.org/10.3390/cells13131118
Chicago/Turabian StyleVasileva, Bela, Natalia Krasteva, Kamelia Hristova-Panusheva, Penyo Ivanov, George Miloshev, Atanas Pavlov, Vasil Georgiev, and Milena Georgieva. 2024. "Exploring the Biosafety Potential of Haberlea rhodopensis Friv. In Vitro Culture Total Ethanol Extract: A Comprehensive Assessment of Genotoxicity, Mitotoxicity, and Cytotoxicity for Therapeutic Applications" Cells 13, no. 13: 1118. https://doi.org/10.3390/cells13131118
APA StyleVasileva, B., Krasteva, N., Hristova-Panusheva, K., Ivanov, P., Miloshev, G., Pavlov, A., Georgiev, V., & Georgieva, M. (2024). Exploring the Biosafety Potential of Haberlea rhodopensis Friv. In Vitro Culture Total Ethanol Extract: A Comprehensive Assessment of Genotoxicity, Mitotoxicity, and Cytotoxicity for Therapeutic Applications. Cells, 13(13), 1118. https://doi.org/10.3390/cells13131118







