n-3 Polyunsaturated Fatty Acids Are Associated with Stable Nitric Oxide Metabolites in Highly Trained Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Approval
2.3. Study Procedure
2.4. Plasma Fatty Acid Profile Analysis
2.5. Determination of NOx
2.6. Exercise Test on a Cycle Ergometer “Until Exhaustion”
2.7. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philip, N.; Yun, X.; Pi, H. Fatty acid metabolism promotes TRPV4 activity in lung microvascular endothelial cells in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Elherik, K.; Bolton-Smith, C.; Barr, R.; Hill, A.; Murrie, I. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc. Res. 2003, 59, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Nettleton, J.A.; Herrington, D.M.; Johnson, W.C.; Tsai, M.Y.; Siscovick, D. Relation of omega-3 fatty acid and dietary fish intake with brachial artery flow-mediated vasodilatation in the multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2010, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gąsior, Z.; Poprzęcki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport. Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Yonemitsu, Y. Vascular endothelial growth factor–Basic science and its clinical implications. Pathophysiology 2004, 1, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D. Omega-3 Polyunsaturated Fatty Acids in Physical Performance Optimization. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J. Regulation of nitric oxide synthesis by dietary factors. Annu. Rev. Nutr. 2002, 22, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V. Omega-3 fatty acids and the cardiometabolic syndrome. J. CardioMetabolic Syndr. 2008, 3, 244–253. [Google Scholar] [CrossRef]
- Jensen, L.; Bangsbo, J.; Hellsten, Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J. Physiol. 2004, 557, 571–582. [Google Scholar] [CrossRef]
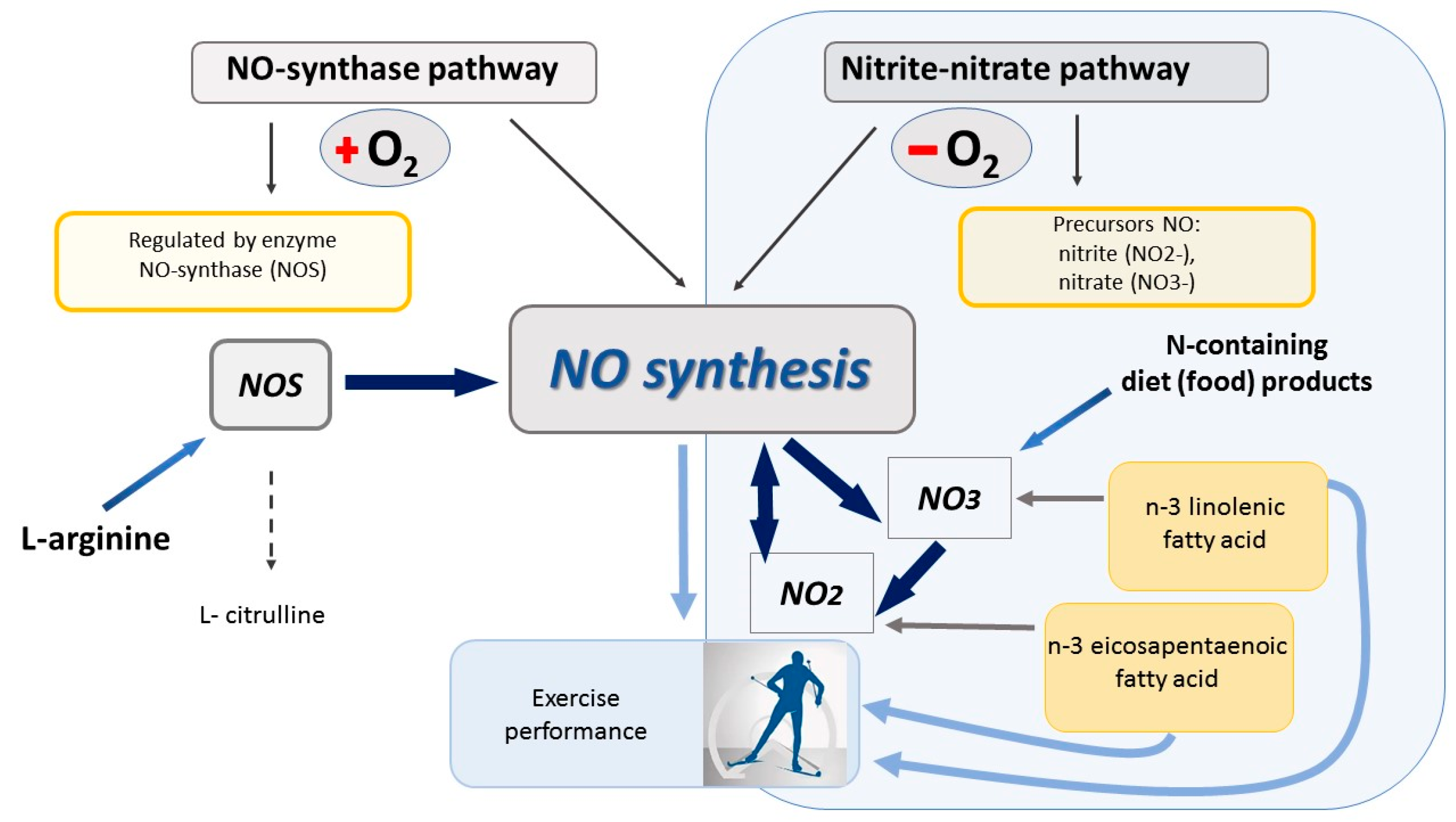
- Bailey, S.J.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. Eur. J. Sport Sci. 2012, 12, 309–320. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Takada, Y.; Watanabe, K. Effect of Omega-3 Polyunsaturated Fatty Acids Intake on Eosinophil Airway Inflammation in University Athletes. J. Clin. Med. Res. 2022, 14, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Lyudinina, A.Y.; Bushmanova, E.A.; Varlamova, N.G.; Bojko, E.R. Dietary and plasma blood α-linolenic acid as modulator of fat oxidation and predictor of aerobic performance. J. Int. Soc. Sports Nutr. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Parshukova, O.I.; Varlamova, N.G.; Potolitsyna, N.N.; Lyudinina, A.Y.; Bojko, E.R. Features of metabolic support of physical performance in highly trained cross-country skiers of different qualifications during physical activity at maximum load. Cells 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.L.; Taintor, R.R.; Boockvar, K.S.; Hibbs, J.B.J. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol. 1996, 268, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Zhang, Q.; Wang, M.; Liu, F.Z.; Zhao, S.M.; Ma, J.; Luo, N.; Li, N.; Li, Y.S.; Xu, G.W. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch. Biochem. Biophys. 2007, 466, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Buikema, H.; Gilst, W.H.; Henning, R.H. Caveolae and endothelial dysfunction: Filling the caves in cardiovascular disease. Eur. J. Pharmacol. 2008, 585, 256–260. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Dialti, V.; Mos, L.; Sechi, L.A. The vascular response to vasodilators is related to the membrane content of polyunsaturated fatty acids in hypertensive patients. J. Hypertens. 2015, 33, 993–1000. [Google Scholar] [CrossRef]
- Chapkin, R.S.; McMurray, D.N.; Davidson, L.A.; Patil, B.S.; Fan, Y.; Lupton, J.R. Bioactive dietary long-chain fatty acids: Emerging mechanisms of action. Br. J. Nutr. 2008, 100, 1152–1157. [Google Scholar] [CrossRef]
- Ma, D.W.; Seo, J.; Davidson, L.A. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004, 18, 1040–1042. [Google Scholar] [CrossRef]
- Park, J.; Yamaura, T.; Kawamoto, J.; Kurihara, T.; Sato, S.B. Reciprocal Modulation of Surface Expression of Annexin A2 in a Human Umbilical Vein Endothelial Cell-Derived Cell Line by Eicosapentaenoic Acid and Docosahexaenoic Acid. PLoS ONE 2014, 9, e85045. [Google Scholar] [CrossRef][Green Version]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef]
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R.; Harrison, R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763. [Google Scholar] [CrossRef]
- Cubrilo, D.; Djordjevic, D.; Zivkovic, V.; Djuric, D.; Blagojevic, B.; Spasic, M.; Jakovljevic, V. Oxidative stress and nitrite dynamics under maximal load in elite athletes: Relation to sport type. Mol. Cell. Biochem. 2011, 355, 273–279. [Google Scholar] [CrossRef]
- Parshukova, O.I.; Nutrikhin, A.V.; Boyko, E.R. The significance of the determination of nitric oxide in highly trained cross-country skiers. Physiother. Sports Med. 2017, 6, 31–36. (In Russian) [Google Scholar]
- Lira, V.A.; Brown, D.L.; Lira, A.K.; Kavazis, A.N.; Soltow, Q.A.; Zeanah, E.H.; David, S. Criswell Nitric oxide and AMPK cooperatively regulate PGC-1α in skeletal muscle cells. J. Physiol. 2010, 588, 3551–3566. [Google Scholar] [CrossRef]
- Ashmore, T.; Roberts, L.D.; Morash, A.J.; Kotwica, A.O.; Finnerty, J.; West, J.A.; Murfitt, S.A.; Fernandez, B.O.; Branco, C. Nitrate enhances skeletal muscle fatty acid oxidation via a nitric oxide-cGMP-PPARmediated mechanism. BMC Biol. 2015, 13, 110. [Google Scholar] [CrossRef]
- Sureda, A.; Tauler, P.; Aguily, A.; Fuentespina, E.; Cyrdova, A. Blood cell NO synthesis in response to exercise. Nitric Oxide 2006, 15, 5–12. [Google Scholar] [CrossRef]
- Dreissigacker, U.; Wendt, M.; Wittke, T.; Tsikas, D.; Maassen, N. Positive correlation between plasma nitrite and performance during high-intensive exercise but not oxidative stress in healthy men. Nitric Oxide 2010, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.A. Comparative Analysis of the Fatty Acid Profile in the Diet and Blood of Athletes and Students. Hum. Physiol. 2022, 48, 563–568. [Google Scholar]
- Lyudinina, A.Y.; Markov, A.L.; Bojko, E.R. Essential Fatty Acid Associated with Heart Rate Variability in Highly Trained Male Cross-Country Skiers: A Pilot Study. Physiologia 2024, 4, 54–63. [Google Scholar] [CrossRef]
- Nishimura, M.; Nanbu, A.; Komori, T.; Ohtsuka, K.; Takahashi, H.; Yoshimura, M. Eicosapentaenoic acid stimulates nitric oxide production and decreases cardiac noradrenalin in diabetic rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 618–624. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Dong, Y.; Wang, S.; Song, P.; Viollet, B.; Zou, M.H. Activation of the AMP-activated protein kinase by eicosapentaenoic acid (EPA, 20:5 n-3) improves endothelial function in vivo. PLoS ONE 2012, 7, e35508. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Jacob, R.F.; Corbalan, J.J.; Malinski, T. Combination Eicosapentaenoic Acid and Statin Treatment Reversed Endothelial Dysfunction in HUVECs Exposed to Oxidized LDL. J. Clin. Lipidol. 2014, 8, 342–343. [Google Scholar] [CrossRef]
- Ishida, T.; Naoe, S.; Nakakuki, M.; Kawano, H.; Imada, K. Eicosapentaenoic Acid Prevents Saturated Fatty Acid-Induced Vascular Endothelial Dysfunction: Involvement of Long-Chain Acyl-CoA Synthetase. J. Atheroscler. Thromb. 2015, 22, 1172–1185. [Google Scholar] [CrossRef]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]

| Characteristics of Athletes | M ± SD |
|---|---|
| Age, years | 21.7 ± 3.9 |
| Height, cm | 176.1 ± 4.8 |
| Body mass, kg | 72.8 ± 4.1 |
| Body-weight index, kg/m2 | 22.8 ± 1.1 |
| Fat mass, % | 9.6 ± 2.7 |
| Maximum oxygen consumption, L/min | 4.1 ± 0.6 |
| Heart rate | 60.4 ± 11.3 |
| Systolic blood pressure, mm Hg | 117.0 ± 11.2 |
| Diastolic blood pressure, mm Hg | 71.8 ± 8.4 |
| Metabolites | M ± SD | Reference Range |
|---|---|---|
| α-Linolenic acid (C18:3 n-3, mol% (mg/mL) | 0.3 ± 0.2 0.005 ± 0.002 | >0.6 mol% |
| Eicosapentaenoic acid (C20:5 n-3, mol% (mg/mL) | 0.7 ± 0.4 0.012 ± 0.008 | >1.4 mol% |
| Docosahexaenoic acid (C22:6 n-3, mol% (mg/mL) | 1.4 ± 0.7 0.023 ± 0.011 | >2.4 mol% |
| NOx, µmol/L | 22.0 ± 5.6 | 17–35 |
| NO2, µmol/L | 10.2 ± 5.2 | 0–5 |
| NO3, µmol/L | 11.8 ± 6.3 | 12–25 |
| Parameters | Spearman Correlation (Rs) | p Level |
|---|---|---|
| C18:3 n3 and NOx | 0.255 | 0.115 |
| C18:3 n3 and NO2 | −0.304 | 0.059 |
| C18:3 n3 and NO3 | 0.461 | 0.003 |
| C20:5 and NOx | 0.116 | 0.481 |
| C20:5 and NO2 | 0.449 | 0.004 |
| C20:5 and NO3 | −0.315 | 0.050 |
| C22:6 and NOx | 0.025 | 0.877 |
| C22:6 and NO2 | −0.192 | 0.239 |
| C22:6 and NO3 | 0.195 | 0.233 |
| α-Linolenic Acid (C18:3 n-3, mol%) | Eicosapentaenoic Acid (C20:5 n-3, mol%) | Docosahexaenoic Acid (C22:6 n-3, mol%) | |
|---|---|---|---|
| Nitrites—NO2 | |||
| I Group | 0.37 ± 0.39 | 0.56 ± 0.37 | 1.47 ± 0.89 |
| II Group | 0.24 ± 0.09 | 0.79 ± 0.38 | 1.38 ± 0.60 |
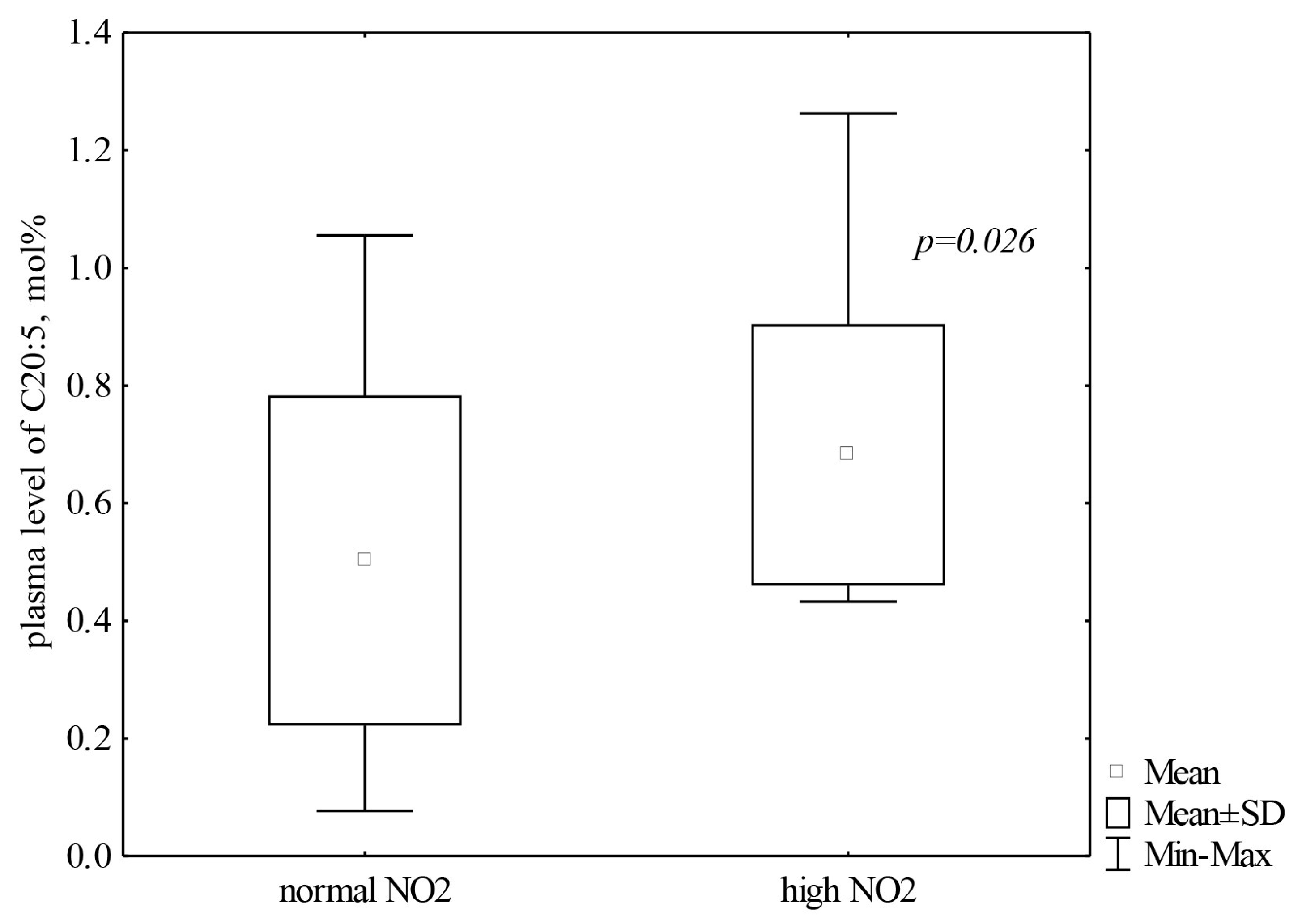
| p-level | 0.242 | 0.0178 | 0.819 |
| Nitrates—NO3 | |||
| III Group | 0.4 ± 0.35 | 0.83 ± 0.53 | 1.51 ± 0.82 |
| VI Group | 0.21 ± 0.06 | 0.77 ± 0.25 | 0.91 ± 0.25 |
| p-level | 0.0169 | 0.168 | 0.354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyudinina, A.Y.; Parshukova, O.I.; Bojko, E.R. n-3 Polyunsaturated Fatty Acids Are Associated with Stable Nitric Oxide Metabolites in Highly Trained Athletes. Cells 2024, 13, 1110. https://doi.org/10.3390/cells13131110
Lyudinina AY, Parshukova OI, Bojko ER. n-3 Polyunsaturated Fatty Acids Are Associated with Stable Nitric Oxide Metabolites in Highly Trained Athletes. Cells. 2024; 13(13):1110. https://doi.org/10.3390/cells13131110
Chicago/Turabian StyleLyudinina, Aleksandra Y., Olga I. Parshukova, and Evgeny R. Bojko. 2024. "n-3 Polyunsaturated Fatty Acids Are Associated with Stable Nitric Oxide Metabolites in Highly Trained Athletes" Cells 13, no. 13: 1110. https://doi.org/10.3390/cells13131110
APA StyleLyudinina, A. Y., Parshukova, O. I., & Bojko, E. R. (2024). n-3 Polyunsaturated Fatty Acids Are Associated with Stable Nitric Oxide Metabolites in Highly Trained Athletes. Cells, 13(13), 1110. https://doi.org/10.3390/cells13131110






