Brain RFamide Neuropeptides in Stress-Related Psychopathologies
Abstract
1. Introduction
1.1. Stress and Stress-Related Neuro-Circuitries
1.2. RFamide Peptides as Promising CNS Targets for the Treatment of Stress-Related Disorders
2. The RFamide Peptide Family and Their Receptor Promiscuity
3. Discovery of the RFamide Peptides
3.1. NPFF Peptides
3.2. LPXRFamide/RFRP Peptides
3.3. PrRPs
3.4. QRFPs
3.5. Kisspeptins
4. Distribution of RFamide Peptides and Their Receptors in the CNS
4.1. NPFF and NPAF
4.1.1. Cell Bodies
4.1.2. Fibers
4.2. RFRPs
4.2.1. Cell Bodies
4.2.2. Fibers
4.2.3. Distribution of NPFF Receptors
4.2.4. The Chemical Nature of NPFFR-Bearing Cells
4.3. PrRP
4.3.1. Cell Bodies
4.3.2. Fibers
4.3.3. Distribution of PrRP Receptors
4.3.4. The Chemical Nature of PrRPR-Bearing Cells
4.4. QRFP
4.4.1. Cell Bodies and Fibers
4.4.2. Distribution of QRFP Receptors
4.4.3. The Chemical Nature of QRFPR-Bearing Cells
4.5. Kisspeptins
4.5.1. Cell Bodies
4.5.2. Fibers
4.5.3. Distribution of Kiss1R
4.5.4. The Chemical Nature of the Kiss1R-Bearing Cells
5. Coexpression of RFamides with Other Neurotransmitters
| RFamide Peptide | Area | Coexpression | Origin of Tissue/Cells | Method |
|---|---|---|---|---|
| NPFF | magnocellular PVN, SON | few cells, AVP | colchicine-treated male rats | single IHC, consecutive 10 µm-thick sections [236]. |
| rostral NTS | 80% TH (adrenaline); 80% NPY; 20% cholecystokinin. | male mice | dual IHC; NPY-GFP transgenic mice/IHC [237]. | |
| subpostrema | 95% glutamate; 10% GABA/glycine. | mice | dual ISH, VGLUT2; dual ISH, VGAT [238]. | |
| spinal cord laminae I-II | 85% somatostatin; 38% GRP; 4.6% substance P. | male and female mice | dual IHC; dual ISH; dual ISH [239]. | |
| RFRP | hypothalamus | glutamate; galanin. | mice | single-cell RNA sequencing, VGLUT2 [173] |
| ARC | KP | OVX + estrogen rats | dual IHC [52] | |
| DMN | 12% neurokinin B | male and female mice | dual ISH [240] | |
| PrRP | NTS, ventrolateral medulla | all cells, TH (noradrenaline). | male rats male rats male and female rats | PrRP ISH/TH IHC [179]; dual IHC [241]; dual ISH [187]. |
| NTS & ventrolateral medulla | 76% and 93% nesfatin-1/NUCB2 | male rats | dual IHC [242] | |
| NTS, ventrolateral medulla | glutamate ~80% and ~16%, respectively. | male rats | VGLUT2 ISH/TH IHC [243] | |
| QRFP | PeN, medial preoptic area | 77.9% glutamate; 7.2% GABA/glycine. | mCherry Q-hM3D transgenic mice | mCherry/VGLUT2 or VGAT ISH [34] |
| medial preoptic area | 80% BDNF; 80% PACAP | mCherry Q-hM3D transgenic mice | mCherry/ISH [34] | |
| medial hypothalamus | glutamate orexin | single-cell RNA sequencing, VGLUT2 [173] | ||
| Kisspeptin | ARC, KNDy neurons | all cells, dynorphin; 75% neurokinin B. | OVX + estrogen and ovary-intact ewes | dual IHC [244] |
| 96% dynorphin; 90% neurokinin B. | OVX +/− estrogen mice | dual ISH [245] | ||
| 75% neurokinin B. | post-mortem men | dual IHC [246] | ||
| 90% glutamate; 50% GABA. | KP-ß-galactosidase transgenic mice | ß-galactosidase IHC/VGLUT2 ISH or GAD-67 ISH [247] | ||
| AVPV | 33% dynorphin; 10% neurokinin B. 20% glutamate. 75% GABA. | OVX mice +/− estrogen male and female KP-beta-galactosidase transgenic mice | dual ISH [245] GAD-67 ISH/ß-galactosidase IHC [247] |
5.1. NPFF Peptides
5.2. RFRPs
5.3. PrRPs
5.4. QRFPs
5.5. Kisspeptins
6. Functional Role of RFamide Peptides Based upon Knockout (KO) Mice Models
7. RFamide Peptides in Stress and Stress-Related Diseases
7.1. NPFF Peptides
7.2. RFRPs
7.3. PrRP
7.4. QRFPs
7.5. Kisspeptins
8. Summary and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A1:2 | noradrenergic cell groups |
| ACTH | adrenocorticotropic hormone |
| Adr | adrenaline |
| Amb | ambigous nucleus |
| AMY | amygdala |
| AP | area postrema |
| ARC | arcuate nucleus |
| AV3V | anteroventral periventricular area |
| AVP | arginine vasopressin |
| AVPV | rostral periventricular region of the third ventricle |
| BDNF | brain-derived neurotrophic factor |
| BNST | bed nucleus of the stria terminalis |
| C1, 2 | adrenergic cell groups |
| CNS | central nervous system |
| CORT | glucocorticoids: in humans, cortisol; in rodents, corticosterone |
| CPP | conditioned place preference |
| CRH | corticotropin-releasing hormone |
| CXCR4 | C-X-C chemokine receptor type 4 receptor |
| DMN | dorsomedial hypothalamic nucleus |
| DMX | dorsal motor nucleus of the vagus nerve (nerve X) |
| DREADDs | designer receptors exclusively activated by designer drugs |
| ERK | extracellular signal-regulated kinase ½ |
| FSH | folliculus-stimulating hormone |
| GABA | gamma-amonibutyric acid |
| GAD | glutamic acid decarboxylase |
| GFP | green fluorescent protein |
| GnIH | gonadotropin inhibitory hormone |
| GnRH | gonadotropin-releasing hormone |
| GPCR | G-protein coupled receptor |
| GRP | gastrin-releasing peptide |
| HC | hippocampus |
| HPA | hypothalamic–pituitary–adrenocortical axis |
| HPG | hypothalamic–pituitary–gonadal axis |
| HTH | hypothalamus |
| ICV | intracerebroventricularly |
| IHC | immunohistochemistry |
| IML | intermediolateral cell column of the spinal cord |
| IR | immunoreactivity |
| ISH | in situ hybridization |
| IT | intrathecally |
| KISS1R | kisspeptin receptor |
| KNDy | neurons coexpressing kisspeptin, neurokinin B, and dynorphin |
| KO | knockout |
| KP | kisspeptin |
| LC | locus coeruleus |
| LH | luteinizing hormone |
| LHA | lateral hypothalamic area |
| LPXRFamide | leucine(L)–proline(P)–X-RFamide |
| MAPK | mitogen-activated protein kinase |
| MCH | melanin-concentrating hormone |
| NA | noradrenaline |
| NK | neurokinin |
| NPAF | neuropeptide AF |
| NPFF | neuropeptide FF |
| NPFFR | neuropeptide FF receptor |
| NPSF | neuropeptide SF |
| NPVF | neuropeptide VF, the human equivalent of RFRP-3 |
| NPY | neuropeptide Y |
| NTS | nucleus of the solitary tract |
| NUCB2 | nucleobindin-2 protein |
| OVX | ovariectomized |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PAG | periaqueductal gray matter |
| PeN | periventricular hypothalamic nucleus |
| PFC | prefrontal cortex |
| POA | preoptic area of the hypothalamus |
| POMC | pro-opio–melanocortin |
| PRL | prolactin |
| PrRP | prolactin-releasing peptide |
| PrRPR/PRLHR | PrRP receptor |
| PTSD | post-traumatic stress disorder |
| PVN | paraventricular hypothalamic nucleus |
| QRFP | pyroglutamylated RFamide peptide/QRFP receptor |
| QRFPR | QRFP receptor |
| RFRP | RFamide-related peptide/RFRP receptor |
| RFRPR | RFRP receptor |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| SAM | sympathoadrenomedullary system |
| SON | supraoptic nucleus |
| TH | tyrosine hydroxylase |
| VGAT | vesicular GABA transporter |
| VGLUT | vesicular glutamate transporter |
| VMN | ventromedial hypothalamic nucleus |
References
- McEwen, B.S. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin. Neurosci. 2006, 8, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pace, S.A.; Myers, B. Hindbrain Adrenergic/Noradrenergic Control of Integrated Endocrine and Autonomic Stress Responses. Endocrinology 2023, 165, bqad178. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Tasker, J.G.; Ziegler, D.R.; Cullinan, W.E. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharm. Biochem. Behav. 2002, 71, 457–468. [Google Scholar] [CrossRef]
- van de Poll, Y.; Cras, Y.; Ellender, T.J. The neurophysiological basis of stress and anxiety—Comparing neuronal diversity in the bed nucleus of the stria terminalis (BNST) across species. Front. Cell Neurosci. 2023, 17, 1225758. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Central control of autonomic function and involvement in neurodegenerative disorders. Handb. Clin. Neurol. 2013, 117, 45–57. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef] [PubMed]
- Chami, R.; Monteleone, A.M.; Treasure, J.; Monteleone, P. Stress hormones and eating disorders. Mol. Cell Endocrinol. 2019, 497, 110349. [Google Scholar] [CrossRef]
- Fan, Y.; Pestke, K.; Feeser, M.; Aust, S.; Pruessner, J.C.; Böker, H.; Bajbouj, M.; Grimm, S. Amygdala-Hippocampal Connectivity Changes During Acute Psychosocial Stress: Joint Effect of Early Life Stress and Oxytocin. Neuropsychopharmacology 2015, 40, 2736–2744. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Mantas, I.; Saarinen, M.; Xu, Z.D.; Svenningsson, P. Update on GPCR-based targets for the development of novel antidepressants. Mol. Psychiatry 2022, 27, 534–558. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, D.; Nagai, T.; Yoshimoto, J.; Kaibuchi, K. Neuromodulator regulation and emotions: Insights from the crosstalk of cell signaling. Front. Mol. Neurosci. 2024, 17, 1376762. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. 2022, 7, 48. [Google Scholar] [CrossRef]
- Hökfelt, T.; Broberger, C.; Xu, Z.Q.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides--an overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Ma, X.M.; Levy, A.; Lightman, S.L. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: A study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology 1997, 138, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Matuska, R.; Zelena, D.; Könczöl, K.; Papp, R.S.; Durst, M.; Guba, D.; Török, B.; Varnai, P.; Tóth, Z.E. Colocalized neurotransmitters in the hindbrain cooperate in adaptation to chronic hypernatremia. Brain Struct. Funct. 2020, 225, 969–984. [Google Scholar] [CrossRef]
- Smith, C.M.; Walker, A.W.; Hosken, I.T.; Chua, B.E.; Zhang, C.; Haidar, M.; Gundlach, A.L. Relaxin-3/RXFP3 networks: An emerging target for the treatment of depression and other neuropsychiatric diseases? Front. Pharm. 2014, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, M.; Rathmann, D.; Beck-Sickinger, A.G. RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential. Pharmaceuticals 2011, 4, 1248–1280. [Google Scholar] [CrossRef]
- Desprat, C.; Zajac, J.M. Hypothermic Effects of Neuropeptide FF Analogues in Mice. Pharmacol. Biochem. Behav. 1997, 58, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, Y.-Q.; He, F.; Guo, J.; Chen, Q.; Wang, R. Inhibition of neuropeptide FF (NPFF)-induced hypothermia and anti-morphine analgesia by RF9, a new selective NPFF receptors antagonist. Regul. Pept. 2008, 147, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jászberényi, M.; Bagosi, Z.; Thurzó, B.; Földesi, I.; Szabó, G.; Telegdy, G. Endocrine, behavioral and autonomic effects of neuropeptide AF. Horm. Behav. 2009, 56, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Guo, J.; He, F.; Peng, Y.-l.; Chang, M.; Wang, R. In vivo inhibition of neuropeptide FF agonism by BIBP3226, an NPY Y1 receptor antagonist. Peptides 2006, 27, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Quelven, I.; Roussin, A.; Zajac, J.M. Comparison of pharmacological activities of Neuropeptide FF1 and Neuropeptide FF2 receptor agonists. Eur. J. Pharm. 2005, 508, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Narimatsu, Y.; Fukumura, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. Int. J. Mol. Sci. 2020, 21, 8606. [Google Scholar] [CrossRef] [PubMed]
- Ellacott, K.L.; Lawrence, C.B.; Rothwell, N.J.; Luckman, S.M. PRL-releasing peptide interacts with leptin to reduce food intake and body weight. Endocrinology 2002, 143, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.B.; Liu, Y.L.; Stock, M.J.; Luckman, S.M. Anorectic actions of prolactin-releasing peptide are mediated by corticotropin-releasing hormone receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R101–R107. [Google Scholar] [CrossRef]
- Davis, X.S.; Grill, H.J. The hindbrain is a site of energy balance action for prolactin-releasing peptide: Feeding and thermic effects from GPR10 stimulation of the nucleus tractus solitarius/area postrema. Psychopharmacology 2018, 235, 2287–2301. [Google Scholar] [CrossRef]
- Kampe, J.; Wiedmer, P.; Pfluger, P.T.; Castaneda, T.R.; Burget, L.; Mondala, H.; Kerr, J.; Liaw, C.; Oldfield, B.J.; Tschöp, M.H.; et al. Effect of central administration of QRFP(26) peptide on energy balance and characterization of a second QRFP receptor in rat. Brain Res. 2006, 1119, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Nunn, N.; Worth, A.A.; Bechtold, D.A.; Suter, T.; Gackeheimer, S.; Foltz, L.; Emmerson, P.J.; Statnick, M.A.; Luckman, S.M. The hypothalamic RFamide, QRFP, increases feeding and locomotor activity: The role of Gpr103 and orexin receptors. PLoS ONE 2022, 17, e0275604. [Google Scholar] [CrossRef]
- Moriya, R.; Sano, H.; Umeda, T.; Ito, M.; Takahashi, Y.; Matsuda, M.; Ishihara, A.; Kanatani, A.; Iwaasa, H. RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinology 2006, 147, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.M.; Sunagawa, G.A.; Soya, S.; Abe, M.; Sakurai, K.; Ishikawa, K.; Yanagisawa, M.; Hama, H.; Hasegawa, E.; Miyawaki, A.; et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 2020, 583, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Csabafi, K.; Jászberényi, M.; Bagosi, Z.; Lipták, N.; Telegdy, G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav. Brain Res. 2013, 241, 56–61. [Google Scholar] [CrossRef]
- Murase, T.; Arima, H.; Kondo, K.; Oiso, Y. Neuropeptide FF reduces food intake in rats. Peptides 1996, 17, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Sunter, D.; Hewson, A.K.; Lynam, S.; Dickson, S.L. Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci. Lett. 2001, 313, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Newmyer, B.A.; Cline, M.A. Neuropeptide AF is associated with short-term reduced food intake in rats. Behav. Brain Res. 2011, 219, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Nicklous, D.M.; Simansky, K.J. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1046–R1054. [Google Scholar] [CrossRef]
- Kovács, A.; László, K.; Gálosi, R.; Tóth, K.; Ollmann, T.; Péczely, L.; Lénárd, L. Microinjection of RFRP-1 in the central nucleus of amygdala decreases food intake in the rat. Brain Res. Bull. 2012, 88, 589–595. [Google Scholar] [CrossRef]
- Kovács, A.; László, K.; Gálosi, R.; Ollmann, T.; Péczely, L.; Zagoracz, O.; Bencze, N.; Lénárd, L. Intraamygdaloid microinjection of RFamide-related peptide-3 decreases food intake in rats. Brain Res. Bull. 2014, 107, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Matsuzaki, T.; Iwasa, T.; Yasui, T.; Irahara, M.; Osugi, T.; Tsutsui, K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J. Endocrinol. 2008, 199, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Seal, L.J.; Small, C.J.; Dhillo, W.S.; Stanley, S.A.; Abbott, C.R.; Ghatei, M.A.; Bloom, S.R. PRL-releasing peptide inhibits food intake in male rats via the dorsomedial hypothalamic nucleus and not the paraventricular hypothalamic nucleus. Endocrinology 2001, 142, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Chartrel, N.; Dujardin, C.; Anouar, Y.; Leprince, J.; Decker, A.; Clerens, S.; Do-Régo, J.C.; Vandesande, F.; Llorens-Cortes, C.; Costentin, J.; et al. Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc. Natl. Acad. Sci. USA 2003, 100, 15247–15252. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, S.D.; Barnes, M.J.; Braymer, H.D. Hypothalamic QRFP: Regulation of food intake and fat selection. Horm. Metab. Res. 2013, 45, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, S.; Sakurai, T.; Iwasaki, S.; Teranishi, H.; Yamanaka, A.; Williams, S.C.; Iguchi, H.; Kawasawa, Y.I.; Ikeda, Y.; Sakakibara, I.; et al. A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7438–7443. [Google Scholar] [CrossRef] [PubMed]
- do Rego, J.-C.; Leprince, J.; Chartrel, N.; Vaudry, H.; Costentin, J. Behavioral effects of 26RFamide and related peptides. Peptides 2006, 27, 2715–2721. [Google Scholar] [CrossRef]
- Primeaux, S.D.; Blackmon, C.; Barnes, M.J.; Braymer, H.D.; Bray, G.A. Central administration of the RFamide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides 2008, 29, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Zagorácz, O.; Kovács, A.; László, K.; Ollmann, T.; Péczely, L.; Lénárd, L. Effects of direct QRFP-26 administration into the medial hypothalamic area on food intake in rats. Brain Res. Bull. 2015, 118, 58–64. [Google Scholar] [CrossRef]
- Stengel, A.; Wang, L.; Goebel-Stengel, M.; Taché, Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 2011, 22, 253–257. [Google Scholar] [CrossRef]
- Clarke, I.J.; Qi, Y.; Puspita Sari, I.; Smith, J.T. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front. Neuroendocrinol. 2009, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, S.; Si, L.; Wei, M.; Guo, S.; Chen, Z.; Wang, S.; Qiao, Y. Direct effect of RFRP-3 microinjection into the lateral ventricle on the hypothalamic kisspeptin neurons in ovariectomized estrogen-primed rats. Exp. Med. 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; van den Pol, A.N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci. 2010, 30, 10205–10219. [Google Scholar] [CrossRef] [PubMed]
- Kriegsfeld, L.J.; Jennings, K.J.; Bentley, G.E.; Tsutsui, K. Gonadotrophin-inhibitory hormone and its mammalian orthologue RFamide-related peptide-3: Discovery and functional implications for reproduction and stress. J. Neuroendocr. 2018, 30, e12597. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 2007, 51, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hinuma, S.S.Y.; Fukusumi, S.; Iijima, N.; Matsumoto, Y.; Hosoya, M.; Fujii, R.; Watanabe, T.; Kikuchi, K.; Terao, Y.; Yano, T.; et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat. Cell Biol. 2000, 2, 703–708. [Google Scholar] [CrossRef]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Kinsey-Jones, J.S.; Cheng, Y.; Knox, A.M.; Lin, Y.; Petrou, N.A.; Roseweir, A.; Lightman, S.L.; Milligan, S.R.; Millar, R.P.; et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 2009, 4, e8334. [Google Scholar] [CrossRef] [PubMed]
- Gresham, R.; Li, S.; Adekunbi, D.A.; Hu, M.; Li, X.F.; O’Byrne, K.T. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci. Lett. 2016, 627, 13–17. [Google Scholar] [CrossRef]
- Szawka, R.E.; Ribeiro, A.B.; Leite, C.M.; Helena, C.V.; Franci, C.R.; Anderson, G.M.; Hoffman, G.E.; Anselmo-Franci, J.A. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 2010, 151, 3247–3257. [Google Scholar] [CrossRef]
- Hellier, V.; Brock, O.; Candlish, M.; Desroziers, E.; Aoki, M.; Mayer, C.; Piet, R.; Herbison, A.; Colledge, W.H.; Prévot, V.; et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat. Commun. 2018, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; McLennan, T.; Czieselsky, K.; Herbison, A.E. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. USA 2015, 112, 13109–13114. [Google Scholar] [CrossRef] [PubMed]
- Elhabazi, K.; Humbert, J.P.; Bertin, I.; Schmitt, M.; Bihel, F.; Bourguignon, J.J.; Bucher, B.; Becker, J.A.; Sorg, T.; Meziane, H.; et al. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology 2013, 75, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Panula, P.; Pertovaara, A. A differential modulation of allodynia, hyperalgesia and nociception by neuropeptide FF in the periaqueductal gray of neuropathic rats: Interactions with morphine and naloxone. Neuroscience 1998, 86, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Gouardères, C.; Sutak, M.; Zajac, J.M.; Jhamandas, K. Antinociceptive effects of intrathecally administered F8Famide and FMRFamide in the rat. Eur. J. Pharm. 1993, 237, 73–81. [Google Scholar] [CrossRef]
- Jhamandas, K.; Milne, B.; Sutak, M.; Gouarderes, C.; Zajac, J.M.; Yang, H.Y.T. Facilitation of spinal morphine analgesia in normal and morphine tolerant animals by neuropeptide SF and related peptides. Peptides 2006, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Fratta, W.; Majane, E.A.; Costa, E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acad. Sci. USA 1985, 82, 7757–7761. [Google Scholar] [CrossRef] [PubMed]
- Kavaliers, M.; Innes, D. Sex differences in the effects of neuropeptide FF and IgG from neuropeptide FF on morphine- and stress-induced analgesia. Peptides 1992, 13, 603–607. [Google Scholar] [CrossRef]
- Li, N.; Han, Z.-L.; Fang, Q.; Wang, Z.-L.; Tang, H.-Z.; Ren, H.; Wang, R. Neuropeptide FF and related peptides attenuates warm-, but not cold-water swim stress-induced analgesia in mice. Behav. Brain Res. 2012, 233, 428–433. [Google Scholar] [CrossRef]
- Bonnard, E.; Burlet-Schiltz, O.; Monsarrat, B.; Girard, J.P.; Zajac, J.M. Identification of proNeuropeptide FFA peptides processed in neuronal and non-neuronal cells and in nervous tissue. Eur. J. Biochem. 2003, 270, 4187–4199. [Google Scholar] [CrossRef]
- Altier, N.; Stewart, J. Neuropeptide FF in the VTA blocks the analgesic effects of both intra-VTA morphine and exposure to stress. Brain Res. 1997, 758, 250–254. [Google Scholar] [CrossRef]
- Pertovaara, A.; Ostergård, M.; Ankö, M.L.; Lehti-Koivunen, S.; Brandt, A.; Hong, W.; Korpi, E.R.; Panula, P. RFamide-related peptides signal through the neuropeptide FF receptor and regulate pain-related responses in the rat. Neuroscience 2005, 134, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.L.; Pertovaara, A.; Brandt, A.; Wei, H.; Pietilä, P.; Kalmari, J.; Xu, M.; Kalso, E.; Panula, P. Prolactin-releasing peptide affects pain, allodynia and autonomic reflexes through medullary mechanisms. Neuropharmacology 2004, 46, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Becker, J.A.; Valverde, O.; Ledent, C.; de Kerchove d’Exaerde, A.; Schiffmann, S.N.; Maldonado, R.; Vassart, G.; Parmentier, M. The prolactin-releasing peptide antagonizes the opioid system through its receptor GPR10. Nat. Neurosci. 2005, 8, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Miyazaki, R.; Yamada, T.; Shinozaki, T. Anti-allodynic effects of intrathecally and intracerebroventricularly administered 26RFa, an intrinsic agonist for GRP103, in the rat partial sciatic nerve ligation model. Peptides 2011, 32, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Miyazaki, R.; Yamada, T. Intracerebroventricular administration of 26RFa produces an analgesic effect in the rat formalin test. Peptides 2009, 30, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Wada, T.; Miyazaki, R. Analgesic effects of intrathecally administered 26RFa, an intrinsic agonist for GPR103, on formalin test and carrageenan test in rats. Neuroscience 2008, 157, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nonaka, T.; Nakamura, S.; Araki, M.; Yamamoto, T. Microinjection of 26RFa, an endogenous ligand for the glutamine RF-amide peptide receptor (QRFP receptor), into the rostral ventromedial medulla (RVM), locus coelureus (LC), and periaqueductal grey (PAG) produces an analgesic effect in rats. Peptides 2019, 115, 1–7. [Google Scholar] [CrossRef]
- Csabafi, K.; Bagosi, Z.; Dobó, É.; Szakács, J.; Telegdy, G.; Szabó, G. Kisspeptin modulates pain sensitivity of CFLP mice. Peptides 2018, 105, 21–27. [Google Scholar] [CrossRef]
- Spampinato, S.; Trabucco, A.; Biasiotta, A.; Biagioni, F.; Cruccu, G.; Copani, A.; Colledge, W.H.; Sortino, M.A.; Nicoletti, F.; Chiechio, S. Hyperalgesic activity of kisspeptin in mice. Mol. Pain. 2011, 7, 90. [Google Scholar] [CrossRef]
- Kotlinska, J.; Pachuta, A.; Dylag, T.; Silberring, J. Neuropeptide FF (NPFF) reduces the expression of morphine- but not of ethanol-induced conditioned place preference in rats. Peptides 2007, 28, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Pachuta, A.; Silberring, J. Neuropeptide FF (NPFF) reduces the expression of cocaine-induced conditioned place preference and cocaine-induced sensitization in animals. Peptides 2008, 29, 933–939. [Google Scholar] [CrossRef]
- Lénárd, L.; Kovács, A.; Ollmann, T.; Péczely, L.; Zagoracz, O.; Gálosi, R.; László, K. Positive reinforcing effects of RFamide-related peptide-1 in the rat central nucleus of amygdala. Behav. Brain Res. 2014, 275, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Bagosi, Z.; Jászberényi, M. The action of neuropeptide AF on passive avoidance learning. Involvement of neurotransmitters. Neurobiol. Learn. Mem. 2016, 127, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; László, K.; Zagoracz, O.; Ollmann, T.; Péczely, L.; Gálosi, R.; Lénárd, L. Effects of RFamide-related peptide-1 (RFRP-1) microinjections into the central nucleus of amygdala on passive avoidance learning in rats. Neuropeptides 2017, 62, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zagorácz, O.; Ollmann, T.; Péczely, L.; László, K.; Kovács, A.; Berta, B.; Kállai, V.; Kertes, E.; Lénárd, L. QRFP administration into the medial hypothalamic nuclei improves memory in rats. Brain Res. 2020, 1727, 146563. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Khonacha, S.; Janahmadi, M.; Motamedi, F. Kisspeptin-13 Improves Spatial Memory Consolidation and Retrieval against Amyloid-β Pathology. Iran. J. Pharm. Res. 2019, 18, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Han, R.W.; Chang, M.; Wang, R. Kisspeptin-13 enhances memory and mitigates memory impairment induced by Aβ1-42 in mice novel object and object location recognition tasks. Neurobiol. Learn. Mem. 2015, 123, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Adamik, Á. The action of kisspeptin-13 on passive avoidance learning in mice. Involvement of transmitters. Behav. Brain Res. 2013, 243, 300–305. [Google Scholar] [CrossRef]
- Kotlinska, J.; Pachuta, A.; Dylag, T.; Silberring, J. The role of neuropeptide FF (NPFF) in the expression of sensitization to hyperlocomotor effect of morphine and ethanol. Neuropeptides 2007, 41, 51–58. [Google Scholar] [CrossRef]
- Marco, N.; Stinus, L.; Allard, M.; Le Moal, M.; Simonnet, G. Neuropeptide FLFQRFamide receptors within the ventral mesenchephalon and dopaminergic terminal areas: Localization and functional antiopioid involvement. Neuroscience 1995, 64, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Cador, M.; Marco, N.; Stinus, L.; Simonnet, G. Interaction between neuropeptide FF and opioids in the ventral tegmental area in the behavioral response to novelty. Neuroscience 2002, 110, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kaewwongse, M.; Takayanagi, Y.; Onaka, T. Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. J. Neuroendocr. 2011, 23, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Vergoni, A.V.; Watanobe, H.; Guidetti, G.; Savino, G.; Bertolini, A.; Schiöth, H.B. Effect of repeated administration of prolactin releasing peptide on feeding behavior in rats. Brain Res. 2002, 955, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ibos, K.E.; Bodnár, É.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-Like Behavior and Hypolocomotion by Activating the HPA Axis and Increasing GABA Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef]
- Fukusumi, S.; Fujii, R.; Hinuma, S. Recent advances in mammalian RFamide peptides: The discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides 2006, 27, 1073–1086. [Google Scholar] [CrossRef]
- Osugi, T.; Ukena, K.; Sower, S.A.; Kawauchi, H.; Tsutsui, K. Evolutionary origin and divergence of PQRFamide peptides and LPXRFamide peptides in the RFamide peptide family. Insights from novel lamprey RFamide peptides. Febs J. 2006, 273, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Ukena, K.; Tsutsui, K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: Structure, localization, and function. Mass. Spectrom. Rev. 2005, 24, 469–486. [Google Scholar] [CrossRef]
- Gouardères, C.; Tafani, J.A.M.; Mazarguil, H.; Zajac, J.M. Autoradiographic Characterization of Rat Spinal Neuropeptide FF Receptors by Using [125I][D.Tyr1, (NMe)Phe3]NPFF. Brain Res. Bull. 1997, 42, 231–238. [Google Scholar] [CrossRef]
- Kotani, M.; Mollereau, C.; Detheux, M.; Le Poul, E.; Brézillon, S.; Vakili, J.; Mazarguil, H.; Vassart, G.; Zajac, J.M.; Parmentier, M. Functional characterization of a human receptor for neuropeptide FF and related peptides. Br. J. Pharm. 2001, 133, 138–144. [Google Scholar] [CrossRef]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, X.M.; Martin, W.J.; McDonald, T.P.; Clements, M.K.; Jiang, Q.; Zeng, Z.; Jacobson, M.; Williams, D.L., Jr.; Yu, H.; et al. Identification and Characterization of Novel Mammalian Neuropeptide FF-like Peptides That Attenuate Morphine-induced Antinociception. J. Biol. Chem. 2001, 276, 36961–36969. [Google Scholar] [CrossRef]
- Mollereau, C.; Mazarguil, H.; Marcus, D.; Quelven, I.; Kotani, M.; Lannoy, V.; Dumont, Y.; Quirion, R.; Detheux, M.; Parmentier, M.; et al. Pharmacological characterization of human NPFF1 and NPFF2 receptors expressed in CHO cells by using NPY Y1 receptor antagonists. Eur. J. Pharmacol. 2002, 451, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Habata, Y.; Hosoya, M.; Kawamata, Y.; Kitada, C.; Hinuma, S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Res. 2003, 1593, 151–157. [Google Scholar] [CrossRef]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharm. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Fukusumi, S.; Yoshida, H.; Fujii, R.; Maruyama, M.; Komatsu, H.; Habata, Y.; Shintani, Y.; Hinuma, S.; Fujino, M. A New Peptidic Ligand and Its Receptor Regulating Adrenal Function in Rats*. J. Biol. Chem. 2003, 278, 46387–46395. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, L.; Gustafson, E.L.; Yadav, D.; Laverty, M.; Murgolo, N.; Vassileva, G.; Zeng, M.; Laz, T.M.; Behan, J.; et al. Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. J. Biol. Chem. 2003, 278, 27652–27657. [Google Scholar] [CrossRef]
- Lee, D.K.; Nguyen, T.; Lynch, K.R.; Cheng, R.; Vanti, W.B.; Arkhitko, O.; Lewis, T.; Evans, J.F.; George, S.R.; O’Dowd, B.F. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene 2001, 275, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.I.; Chamberlain, L.; Elshourbagy, N.A.; Michalovich, D.; Moore, D.J.; Calamari, A.; Szekeres, P.G.; Sarau, H.M.; Chambers, J.K.; Murdock, P.; et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001, 276, 28969–28975. [Google Scholar] [CrossRef]
- Stafford, L.J.; Xia, C.; Ma, W.; Cai, Y.; Liu, M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002, 62, 5399–5404. [Google Scholar]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Gouardères, C.; Mazarguil, H.; Mollereau, C.; Chartrel, N.; Leprince, J.; Vaudry, H.; Zajac, J.M. Functional differences between NPFF1 and NPFF2 receptor coupling: High intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPgammaS binding. Neuropharmacology 2007, 52, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Moulédous, L.; Froment, C.; Dauvillier, S.; Burlet-Schiltz, O.; Zajac, J.M.; Mollereau, C. GRK2 protein-mediated transphosphorylation contributes to loss of function of μ-opioid receptors induced by neuropeptide FF (NPFF2) receptors. J. Biol. Chem. 2012, 287, 12736–12749. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Brownjohn, P.W.; Dyer, B.S.; Beltramo, M.; Walker, C.S.; Hay, D.L.; Painter, G.F.; Tyndall, J.D.A.; Anderson, G.M. Anxiogenic and Stressor Effects of the Hypothalamic Neuropeptide RFRP-3 Are Overcome by the NPFFR Antagonist GJ14. Endocrinology 2015, 156, 4152–4162. [Google Scholar] [CrossRef]
- Liu, X.; Herbison, A.E. RF9 excitation of GnRH neurons is dependent upon Kiss1r in the adult male and female mouse. Endocrinology 2014, 155, 4915–4924. [Google Scholar] [CrossRef]
- Maletínská, L.; Tichá, A.; Nagelová, V.; Špolcová, A.; Blechová, M.; Elbert, T.; Železná, B. Neuropeptide FF analog RF9 is not an antagonist of NPFF receptor and decreases food intake in mice after its central and peripheral administration. Brain Res. 2013, 1498, 33–40. [Google Scholar] [CrossRef]
- Oishi, S.; Misu, R.; Tomita, K.; Setsuda, S.; Masuda, R.; Ohno, H.; Naniwa, Y.; Ieda, N.; Inoue, N.; Ohkura, S.; et al. Activation of Neuropeptide FF Receptors by Kisspeptin Receptor Ligands. ACS Med. Chem. Lett. 2011, 2, 53–57. [Google Scholar] [CrossRef]
- Liu, X.; Herbison, A. Kisspeptin Regulation of Arcuate Neuron Excitability in Kisspeptin Receptor Knockout Mice. Endocrinology 2015, 156, 1815–1827. [Google Scholar] [CrossRef]
- Engström, M.; Brandt, A.; Wurster, S.; Savola, J.M.; Panula, P. Prolactin releasing peptide has high affinity and efficacy at neuropeptide FF2 receptors. J. Pharm. Exp. 2003, 305, 825–832. [Google Scholar] [CrossRef]
- Ma, L.; MacTavish, D.; Simonin, F.; Bourguignon, J.J.; Watanabe, T.; Jhamandas, J.H. Prolactin-releasing peptide effects in the rat brain are mediated through the Neuropeptide FF receptor. Eur. J. Neurosci. 2009, 30, 1585–1593. [Google Scholar] [CrossRef]
- Buffel, I.; Meurs, A.; Portelli, J.; Raedt, R.; De Herdt, V.; Sioncke, L.; Wadman, W.; Bihel, F.; Schmitt, M.; Vonck, K.; et al. Neuropeptide FF and prolactin-releasing peptide decrease cortical excitability through activation of NPFF receptors. Epilepsia 2015, 56, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.J.; Yi-Kung Huang, E.; Cronk, D.; Bagust, J.; Sharma, R.; Walker, R.J.; Wilson, S.; Burke, J.F. A human gene encoding morphine modulating peptides related to NPFF and FMRFamide. FEBS Lett. 1997, 409, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Vilim, F.S.; Aarnisalo, A.A.; Nieminen, M.L.; Lintunen, M.; Karlstedt, K.; Kontinen, V.K.; Kalso, E.; States, B.; Panula, P.; Ziff, E. Gene for pain modulatory neuropeptide NPFF: Induction in spinal cord by noxious stimuli. Mol. Pharm. 1999, 55, 804–811. [Google Scholar]
- Dockray, G.J.; Reeve, J.R., Jr.; Shively, J.; Gayton, R.J.; Barnard, C.S. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature 1983, 305, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Morgan, K.; Pawson, A.J.; Osugi, T.; Chowdhury, V.S.; Minakata, H.; Tsutsui, K.; Millar, R.P.; Bentley, G.E. Identification of Human GnIH Homologs, RFRP-1 and RFRP-3, and the Cognate Receptor, GPR147 in the Human Hypothalamic Pituitary Axis. PLoS ONE 2009, 4, e8400. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Ubuka, T. How to Contribute to the Progress of Neuroendocrinology: Discovery of GnIH and Progress of GnIH Research. Front. Endocrinol. 2018, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Fukusumi, S.; Habata, Y.; Yoshida, H.; Iijima, N.; Kawamata, Y.; Hosoya, M.; Fujii, R.; Hinuma, S.; Kitada, C.; Shintani, Y.; et al. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Res. 2001, 1540, 221–232. [Google Scholar] [CrossRef]
- Hinuma, S.; Habata, Y.; Fujii, R.; Kawamata, Y.; Hosoya, M.; Fukusumi, S.; Kitada, C.; Masuo, Y.; Asano, T.; Matsumoto, H.; et al. A prolactin-releasing peptide in the brain. Nature 1998, 393, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Jarry, H.; Heuer, H.; Schomburg, L.; Bauer, K. Prolactin-releasing peptides do not stimulate prolactin release in vivo. Neuroendocrinology 2000, 71, 262–267. [Google Scholar] [CrossRef]
- Maruyama, M.; Matsumoto, H.; Fujiwara, K.; Noguchi, J.; Kitada, C.; Hinuma, S.; Onda, H.; Nishimura, O.; Fujino, M.; Higuchi, T.; et al. Central administration of prolactin-releasing peptide stimulates oxytocin release in rats. Neurosci. Lett. 1999, 276, 193–196. [Google Scholar] [CrossRef]
- Morales, T.; Sawchenko, P.E. Brainstem prolactin-releasing peptide neurons are sensitive to stress and lactation. Neuroscience 2003, 121, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Rubinek, T.; Hadani, M.; Barkai, G.; Melmed, S.; Shimon, I. Prolactin (PRL)-Releasing Peptide Stimulates PRL Secretion from Human Fetal Pituitary Cultures and Growth Hormone Release from Cultured Pituitary Adenomas1. J. Clin. Endocrinol. Metab. 2001, 86, 2826–2830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Onaka, T. Facilitative role of prolactin-releasing peptide neurons in oxytocin cell activation after conditioned-fear stimuli. Neuroscience 2003, 118, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ozawa, A.; Ishii, S.; Shibusawa, N.; Hashida, T.; Ishizuka, T.; Hosoya, T.; Monden, T.; Satoh, T.; Mori, M. Isolation and Characterization of the Rat Prolactin-Releasing Peptide Gene: Multiple TATA Boxes in the Promoter Region. Biochem. Biophys. Res. Commun. 2001, 281, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, F.; Lectez, B.; Tollemer, H.; Leprince, J.; Dujardin, C.; Rachidi, W.; Chatenet, D.; Baroncini, M.; Beauvillain, J.C.; Vallarino, M.; et al. Anatomical distribution and biochemical characterization of the novel RFamide peptide 26RFa in the human hypothalamus and spinal cord. J. Neurochem. 2006, 99, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. JNCI J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Tng, E.L. Kisspeptin signalling and its roles in humans. Singap. Med. J. 2015, 56, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides 2009, 30, 4–9. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Funes, S.; Hedrick, J.A.; Vassileva, G.; Markowitz, L.; Abbondanzo, S.; Golovko, A.; Yang, S.; Monsma, F.J.; Gustafson, E.L. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 2003, 312, 1357–1363. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garrido, M.A.; Ruiz-Pino, F.; Manfredi-Lozano, M.; Leon, S.; Garcia-Galiano, D.; Castaño, J.P.; Luque, R.M.; Romero-Ruiz, A.; Castellano, J.M.; Diéguez, C.; et al. Obesity-induced hypogonadism in the male: Premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology 2014, 155, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.J.; Vigo, E.; Casanueva, F.F.; Aguilar, E.; et al. Changes in Hypothalamic KiSS-1 System and Restoration of Pubertal Activation of the Reproductive Axis by Kisspeptin in Undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, L.; Majane, E.A.; Yang, H.Y.; Panula, P. Immunohistochemical distribution and partial characterization of FLFQPQRFamidelike peptides in the central nervous system of rats. J. Comp. Neurol. 1989, 286, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, L.; Panula, P. Origin and distribution of neuropeptide-FF-like immunoreactivity in the spinal cord of rats. J. Comp. Neurol. 1991, 307, 107–119. [Google Scholar] [CrossRef]
- Langlieb, J.; Sachdev, N.S.; Balderrama, K.S.; Nadaf, N.M.; Raj, M.; Murray, E.; Webber, J.T.; Vanderburg, C.; Gazestani, V.; Tward, D.; et al. The molecular cytoarchitecture of the adult mouse brain. Nature 2023, 624, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.D.; Buijs, R.M.; Mactavish, D.; Jhamandas, J.H. Neuropeptide FF distribution in the human and rat forebrain: A comparative immunohistochemical study. J. Comp. Neurol. 2006, 496, 572–593. [Google Scholar] [CrossRef]
- Bao, A.M.; Meynen, G.; Swaab, D.F. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res. Rev. 2008, 57, 531–553. [Google Scholar] [CrossRef]
- Hammack, S.E.; Braas, K.M.; May, V. Chemoarchitecture of the bed nucleus of the stria terminalis: Neurophenotypic diversity and function. Handb. Clin. Neurol. 2021, 179, 385–402. [Google Scholar] [CrossRef]
- Imamura, K.; Takumi, T. Mood phenotypes in rodent models with circadian disturbances. Neurobiol. Sleep. Circadian Rhythm. 2022, 13, 100083. [Google Scholar] [CrossRef] [PubMed]
- Sundblom, D.M.; Kalso, E.; Tigerstedt, I.; Wahlbeck, K.; Panula, P.; Fyhrquist, F. Neuropeptide FF-like immunoreactivity in human cerebrospinal fluid of chronic pain patients and healthy controls. Peptides 1997, 18, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Sundblom, D.M.; Hyrkkö, A.; Fyhrquist, F. Pulsatile secretion of neuropeptide FF into human blood. Peptides 1998, 19, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Sundblom, D.M.; Panula, P.; Fyhrquist, F. Neuropeptide FF-like immunoreactivity in human plasma. Peptides 1995, 16, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Aarnisalo, A.A.; Karhunen, T.; Vanhatalo, S.; Panula, P. Peptide GEGLSS-like immunoreactivity in the rat central nervous system. Brain Res. Bull. 1997, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kirouac, G.J. The Paraventricular Nucleus of the Thalamus as an Integrating and Relay Node in the Brain Anxiety Network. Front. Behav. Neurosci. 2021, 15, 627633. [Google Scholar] [CrossRef] [PubMed]
- Bagley, E.E.; Ingram, S.L. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173, 108131. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Iijima, N.; Kakihara, K.; Hinuma, S.; Tanaka, M.; Ibata, Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003, 982, 156–167. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Mei, D.F.; Bentley, G.E.; Ubuka, T.; Mason, A.O.; Inoue, K.; Ukena, K.; Tsutsui, K.; Silver, R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc. Natl. Acad. Sci. USA 2006, 103, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T. A mammalian gonadotropin-inhibitory hormone homolog RFamide-related peptide 3 regulates pain and anxiety in mice. Cell Tissue Res. 2023, 391, 159–172. [Google Scholar] [CrossRef]
- Singh, P.; Anjum, S.; Srivastava, R.K.; Tsutsui, K.; Krishna, A. Central and peripheral neuropeptide RFRP-3: A bridge linking reproduction, nutrition, and stress response. Front. Neuroendocrinol. 2022, 65, 100979. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, J.D.; Simonneaux, V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 2009, 30, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Oldfield, B.J.; Clarke, I.J. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J. Neuroendocr. 2009, 21, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharm. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Carotenuto, M.; Monda, V.; Valenzano, A.; Villano, I.; Precenzano, F.; Tafuri, D.; Salerno, M.; Filippi, N.; Nuccio, F.; et al. Orexin System: The Key for a Healthy Life. Front. Physiol. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Diniz, G.B.; Bittencourt, J.C. The Melanin-Concentrating Hormone as an Integrative Peptide Driving Motivated Behaviors. Front. Syst. Neurosci. 2017, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Gouardères, C.; Puget, A.; Zajac, J.M. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: A comparative autoradiographic study. Synapse 2004, 51, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Marusich, J.; Li, J.X.; Zhang, Y. Neuropeptide FF and Its Receptors: Therapeutic Applications and Ligand Development. J. Med. Chem. 2020, 63, 12387–12402. [Google Scholar] [CrossRef] [PubMed]
- Ankö, M.L.; Ostergård, M.; Lintunen, M.; Panula, P. Alternative splicing of human and mouse NPFF2 receptor genes: Implications to receptor expression. FEBS Lett. 2006, 580, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Kanaya, M.; Ozawa, H. Expression analysis of neuropeptide FF receptors on neuroendocrine-related neurons in the rat brain using highly sensitive in situ hybridization. Histochem. Cell Biol. 2021, 155, 465–475. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Whittle, N.; Fadok, J.; MacPherson, K.P.; Nguyen, R.; Botta, P.; Wolff, S.B.E.; Müller, C.; Herry, C.; Tovote, P.; Holmes, A.; et al. Central amygdala micro-circuits mediate fear extinction. Nat. Commun. 2021, 12, 4156. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.A.; Zeisel, A.; Bakker, J.; Girach, F.; Hellysaz, A.; Tomer, R.; Alpár, A.; Mulder, J.; Clotman, F.; Keimpema, E.; et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 2017, 20, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Macosko, E.Z.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.; Resch, J.M.; McCarroll, S.A.; et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Torz, L.; Niss, K.; Lundh, S.; Rekling, J.C.; Quintana, C.D.; Frazier, S.E.D.; Mercer, A.J.; Cornea, A.; Bertelsen, C.V.; Gerstenberg, M.K.; et al. NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model. Int. J. Mol. Sci. 2022, 23, 3260. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Tao, P.-L.; Huang, E.Y.-K. Distribution of neuropeptide FF (NPFF) receptors in correlation with morphine-induced reward in the rat brain. Peptides 2010, 31, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Kataoka, Y.; Kakihara, K.; Bamba, H.; Tamada, Y.; Hayashi, S.; Matsuda, T.; Tanaka, M.; Honjyo, H.; Hosoya, M.; et al. Cytochemical study of prolactin-releasing peptide (PrRP) in the rat brain. Neuroreport 1999, 10, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Morales, T.; Hinuma, S.; Sawchenko, P.E. Prolactin-releasing peptide is expressed in afferents to the endocrine hypothalamus, but not in neurosecretory neurones. J. Neuroendocr. 2000, 12, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Roland, B.L.; Sutton, S.W.; Wilson, S.J.; Luo, L.; Pyati, J.; Huvar, R.; Erlander, M.G.; Lovenberg, T.W. Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology 1999, 140, 5736–5745. [Google Scholar] [CrossRef]
- Minami, S.; Nakata, T.; Tokita, R.; Onodera, H.; Imaki, J. Cellular localization of prolactin-releasing peptide messenger RNA in the rat brain. Neurosci. Lett. 1999, 266, 73–75. [Google Scholar] [CrossRef]
- Dodd, G.T.; Luckman, S.M. Physiological Roles of GPR10 and PrRP Signaling. Front. Endocrinol. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Fukusumi, S.; Hosoya, M.; Kawamata, Y.; Habata, Y.; Hinuma, S.; Sekiguchi, M.; Kitada, C.; Kurokawa, T.; Nishimura, O.; et al. Tissue distribution of prolactin-releasing peptide (PrRP) and its receptor. Regul. Pept. 1999, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Danila, D.C.; Katai, M.; Swearingen, B.; Klibanski, A. Expression of prolactin-releasing peptide and its receptor messenger ribonucleic acid in normal human pituitary and pituitary adenomas. J. Clin. Endocrinol. Metab. 1999, 84, 4652–4655. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.T.; Kokay, I.C.; Lang, T.; Grattan, D.R.; Curlewis, J.D. Quantification of prolactin-releasing peptide (PrRP) mRNA expression in specific brain regions of the rat during the oestrous cycle and in lactation. Brain Res. 2003, 973, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Iijima, N.; Yano, T.; Kakihara, K.; Hayashi, S.; Hinuma, S.; Honjo, H.; Hayashi, S.; Tanaka, M.; Ibata, Y. Gonadal regulation of PrRP mRNA expression in the nucleus tractus solitarius and ventral and lateral reticular nuclei of the rat. Mol. Brain Res. 2001, 87, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, H.; An, X.F.; Ma, S.L.; Chen, B.Y. Expression of brain prolactin releasing peptide (PrRP) changes in the estrous cycle of female rats. Neurosci. Lett. 2007, 419, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.E.; Zelena, D.; Mergl, Z.; Kirilly, E.; Várnai, P.; Mezey, E.; Makara, G.B.; Palkovits, M. Chronic repeated restraint stress increases prolactin-releasing peptide/tyrosine-hydroxylase ratio with gender-related differences in the rat brain. J. Neurochem. 2008, 104, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Abe, T.; Matsumoto, K.; Tomita, M. Does prolactin releasing peptide receptor regulate prolactin-secretion in human pituitary adenomas? Neurosci. Lett. 2000, 291, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Kudo, K.; Kanba, S.; Arita, J. Distribution of prolactin-releasing peptide-immunoreactive neurons in the rat hypothalamus. Neurosci. Lett. 1999, 267, 113–116. [Google Scholar] [CrossRef]
- Vas, S.; Papp, R.S.; Könczöl, K.; Bogáthy, E.; Papp, N.; Ádori, C.; Durst, M.; Sípos, K.; Ocskay, K.; Farkas, I.; et al. Prolactin-Releasing Peptide Contributes to Stress-Related Mood Disorders and Inhibits Sleep/Mood Regulatory Melanin-Concentrating Hormone Neurons in Rats. J. Neurosci. 2023, 43, 846–862. [Google Scholar] [CrossRef]
- Lagerström, M.C.; Fredriksson, R.; Bjarnadóttir, T.K.; Fridmanis, D.; Holmquist, T.; Andersson, J.; Yan, Y.L.; Raudsepp, T.; Zoorob, R.; Kukkonen, J.P.; et al. Origin of the prolactin-releasing hormone (PRLH) receptors: Evidence of coevolution between PRLH and a redundant neuropeptide Y receptor during vertebrate evolution. Genomics 2005, 85, 688–703. [Google Scholar] [CrossRef]
- Kimura, A.; Ohmichi, M.; Tasaka, K.; Kanda, Y.; Ikegami, H.; Hayakawa, J.; Hisamoto, K.; Morishige, K.; Hinuma, S.; Kurachi, H.; et al. Prolactin-releasing peptide activation of the prolactin promoter is differentially mediated by extracellular signal-regulated protein kinase and c-Jun N-terminal protein kinase. J. Biol. Chem. 2000, 275, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Pražienková, V.; Popelová, A.; Kuneš, J.; Maletínská, L. Prolactin-Releasing Peptide: Physiological and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 5297. [Google Scholar] [CrossRef]
- Sun, B.; Fujiwara, K.; Adachi, S.; Inoue, K. Physiological roles of prolactin-releasing peptide. Regul. Pept. 2005, 126, 27–33. [Google Scholar] [CrossRef]
- Lin, S.H.; Leslie, F.M.; Civelli, O. Neurochemical properties of the prolactin releasing peptide (PrRP) receptor expressing neurons: Evidence for a role of PrRP as a regulator of stress and nociception. Brain Res. 2002, 952, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Totsune, K.; Murakami, O.; Sone, M.; Noshiro, T.; Hayashi, Y.; Sasano, H.; Shibahara, S. Expression of prolactin-releasing peptide and its receptor in the human adrenal glands and tumor tissues of adrenocortical tumors, pheochromocytomas and neuroblastomas. Peptides 2002, 23, 1135–1140. [Google Scholar] [CrossRef]
- Lin, S.H.; Arai, A.C.; España, R.A.; Berridge, C.W.; Leslie, F.M.; Huguenard, J.R.; Vergnes, M.; Civelli, O. Prolactin-releasing peptide (PrRP) promotes awakening and suppresses absence seizures. Neuroscience 2002, 114, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xu, X.; Chen, R.; Jia, W.B.; Xu, P.F.; Liu, X.Q.; Zhang, Y.; Liu, X.F.; Zhang, Y. The thalamic reticular nucleus-lateral habenula circuit regulates depressive-like behaviors in chronic stress and chronic pain. Cell Rep. 2023, 42, 113170. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Takayanagi, Y.; Yoshida, M.; Nishimori, K.; Kusama, M.; Onaka, T. Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. J. Neuroendocr. 2013, 25, 455–465. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Onaka, T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int. J. Mol. Sci. 2021, 23, 150. [Google Scholar] [CrossRef]
- Bruzzone, F.; Lectez, B.; Alexandre, D.; Jégou, S.; Mounien, L.; Tollemer, H.; Chatenet, D.; Leprince, J.; Vallarino, M.; Vaudry, H.; et al. Distribution of 26RFa binding sites and GPR103 mRNA in the central nervous system of the rat. J. Comp. Neurol. 2007, 503, 573–591. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Karteris, E.; Chen, J.; Rucinski, M.; Ziolkowska, A.; Ahmed, N.; Kagerer, S.; Jöhren, O.; Lehnert, H.; Malendowicz, L.K.; et al. QRFP induces aldosterone production via PKC and T-type calcium channel-mediated pathways in human adrenocortical cells: Evidence for a novel role of GPR103. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1049–E1058. [Google Scholar] [CrossRef]
- Ishigame, N.; Kageyama, K.; Takayasu, S.; Furumai, K.; Nakada, Y.; Daimon, M. Regulation of the expression of corticotropin-releasing factor gene by pyroglutamylated RFamide peptide in rat hypothalamic 4B cells. Endocr. J. 2016, 63, 919–927. [Google Scholar] [CrossRef]
- Davies, J.; Chen, J.; Pink, R.; Carter, D.; Saunders, N.; Sotiriadis, G.; Bai, B.; Pan, Y.; Howlett, D.; Payne, A.; et al. Orexin receptors exert a neuroprotective effect in Alzheimer’s disease (AD) via heterodimerization with GPR103. Sci. Rep. 2015, 5, 12584. [Google Scholar] [CrossRef]
- Leprince, J.; Bagnol, D.; Bureau, R.; Fukusumi, S.; Granata, R.; Hinuma, S.; Larhammar, D.; Primeaux, S.; Sopkova-de Oliveiras Santos, J.; Tsutsui, K.; et al. The Arg-Phe-amide peptide 26RFa/glutamine RF-amide peptide and its receptor: IUPHAR Review 24. Br. J. Pharm. 2017, 174, 3573–3607. [Google Scholar] [CrossRef]
- Perez, D.M. From plants to man: The GPCR “tree of life”. Mol. Pharm. 2005, 67, 1383–1384. [Google Scholar] [CrossRef]
- Lectez, B.; Jeandel, L.; El-Yamani, F.Z.; Arthaud, S.; Alexandre, D.; Mardargent, A.; Jégou, S.; Mounien, L.; Bizet, P.; Magoul, R.; et al. The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus. Endocrinology 2009, 150, 2342–2350. [Google Scholar] [CrossRef]
- Rometo, A.M.; Krajewski, S.J.; Lou Voytko, M.; Rance, N.E. Hypertrophy and Increased Kisspeptin Gene Expression in the Hypothalamic Infundibular Nucleus of Postmenopausal Women and Ovariectomized Monkeys. J. Clin. Endocrinol. Metab. 2007, 92, 2744–2750. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J. Neuroendocr. 2009, 21, 305–311. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006, 147, 5817–5825. [Google Scholar] [CrossRef]
- Kim, J.; Semaan, S.J.; Clifton, D.K.; Steiner, R.A.; Dhamija, S.; Kauffman, A.S. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011, 152, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Cunningham, M.J.; Rissman, E.F.; Clifton, D.K.; Steiner, R.A. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005, 146, 3686–3692. [Google Scholar] [CrossRef]
- Pineda, R.; Plaisier, F.; Millar, R.P.; Ludwig, M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology 2017, 104, 223–238. [Google Scholar] [CrossRef]
- Yeo, S.H.; Kyle, V.; Morris, P.G.; Jackman, S.; Sinnett-Smith, L.C.; Schacker, M.; Chen, C.; Colledge, W.H. Visualisation of Kiss1 Neurone Distribution Using a Kiss1-CRE Transgenic Mouse. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Yamada, S.; Takatsu, Y.; Matsui, H.; Kinoshita, M.; Takase, K.; Sugiura, H.; Ohtaki, T.; Matsumoto, H.; Uenoyama, Y.; et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 2007, 53, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Nagae, M.; Tsuchida, H.; Inoue, N.; Tsukamura, H. Role of KNDy Neurons Expressing Kisspeptin, Neurokinin B, and Dynorphin A as a GnRH Pulse Generator Controlling Mammalian Reproduction. Front. Endocrinol. 2021, 12, 724632. [Google Scholar] [CrossRef]
- Hrabovszky, E.; Ciofi, P.; Vida, B.; Horvath, M.C.; Keller, E.; Caraty, A.; Bloom, S.R.; Ghatei, M.A.; Dhillo, W.S.; Liposits, Z.; et al. The kisspeptin system of the human hypothalamus: Sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci. 2010, 31, 1984–1998. [Google Scholar] [CrossRef]
- Lee, D.K.; Nguyen, T.; O’Neill, G.P.; Cheng, R.; Liu, Y.; Howard, A.D.; Coulombe, N.; Tan, C.P.; Tang-Nguyen, A.T.; George, S.R.; et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999, 446, 103–107. [Google Scholar] [CrossRef]
- Franssen, D.; Tena-Sempere, M. The kisspeptin receptor: A key G-protein-coupled receptor in the control of the reproductive axis. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 107–123. [Google Scholar] [CrossRef]
- Ahow, M.; Min, L.; Pampillo, M.; Nash, C.; Wen, J.; Soltis, K.; Carroll, R.S.; Glidewell-Kenney, C.A.; Mellon, P.L.; Bhattacharya, M.; et al. KISS1R signals independently of Gαq/11 and triggers LH secretion via the β-arrestin pathway in the male mouse. Endocrinology 2014, 155, 4433–4446. [Google Scholar] [CrossRef]
- Szereszewski, J.M.; Pampillo, M.; Ahow, M.R.; Offermanns, S.; Bhattacharya, M.; Babwah, A.V. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Gα(q/11) and β-arrestin-dependent manner. PLoS ONE 2010, 5, e12964. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Chen, L.H.; Chiu, W.J.; Tsai, C.L. Kisspeptin Regulates Cell Invasion and Migration in Endometrial Cancer. J. Endocr. Soc. 2024, 8, bvae001. [Google Scholar] [CrossRef] [PubMed]
- Navenot, J.M.; Wang, Z.; Chopin, M.; Fujii, N.; Peiper, S.C. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: A potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res. 2005, 65, 10450–10456. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Honda, S.; Iijima, N.; Ozawa, H. Mapping of Kisspeptin Receptor mRNA in the Whole Rat Brain and its Co-Localisation with Oxytocin in the Paraventricular Nucleus. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; de Tassigny, X.; Doran, J.; Colledge, W.H. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S.; Fraley, G.S.; Smith, J.T.; Acohido, B.V.; Popa, S.M.; Cunningham, M.J.; Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004, 80, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Moore, A.M.; Onslow, K.; Hileman, S.M.; Hardy, S.L.; Bowdridge, E.C.; Walters, B.A.; Agus, S.; Griesgraber, M.J.; Aerts, E.G.; et al. Lesions of KNDy and Kiss1R Neurons in the Arcuate Nucleus Produce Different Effects on LH Pulse Patterns in Female Sheep. Endocrinology 2023, 164, bqad148. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Iijima, N.; Ozawa, H. Characterisation of Kiss1r (Gpr54)-Expressing Neurones in the Arcuate Nucleus of the Female Rat Hypothalamus. J. Neuroendocr. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Faron-Górecka, A.; Latocha, K.; Pabian, P.; Kolasa, M.; Sobczyk-Krupiarz, I.; Dziedzicka-Wasylewska, M. The Involvement of Prolactin in Stress-Related Disorders. Int. J. Environ. Res. Public Health 2023, 20, 3257. [Google Scholar] [CrossRef]
- Eiden, L.E.; Hernández, V.S.; Jiang, S.Z.; Zhang, L. Neuropeptides and small-molecule amine transmitters: Cooperative signaling in the nervous system. Cell Mol. Life Sci. 2022, 79, 492. [Google Scholar] [CrossRef]
- Levine, A.S.; Jewett, D.C.; Kotz, C.M.; Olszewski, P.K. Behavioral plasticity: Role of neuropeptides in shaping feeding responses. Appetite 2022, 174, 106031. [Google Scholar] [CrossRef] [PubMed]
- Osório, C.; Probert, T.; Jones, E.; Young, A.H.; Robbins, I. Adapting to Stress: Understanding the Neurobiology of Resilience. Behav. Med. 2017, 43, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Wittmann, G.; Turi, G.F.; Liposits, Z.; Fekete, C. Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology 2005, 146, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Raadsheer, F.C.; Hoogendijk, W.J.; Stam, F.C.; Tilders, F.J.; Swaab, D.F. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 1994, 60, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Quillet, R.; Ayachi, S.; Bihel, F.; Elhabazi, K.; Ilien, B.; Simonin, F. RF-amide neuropeptides and their receptors in Mammals: Pharmacological properties, drug development and main physiological functions. Pharm. 2016, 160, 84–132. [Google Scholar] [CrossRef]
- Boersma, C.J.; Sonnemans, M.A.; Van Leeuwen, F.W. Immunocytochemical localization of neuropeptide FF (FMRF amide-like peptide) in the hypothalamo-neurohypophyseal system of Wistar and Brattleboro rats by light and electron microscopy. J. Comp. Neurol. 1993, 336, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S.; Pizano, K.; Matson, K.; Shan, Y.; Reynolds, D.; Wray, S. An Inhibitory Circuit From Brainstem to GnRH Neurons in Male Mice: A New Role for the RFRP Receptor. Endocrinology 2021, 162, bqab030. [Google Scholar] [CrossRef]
- Zhang, L.; Koller, J.; Gopalasingam, G.; Qi, Y.; Herzog, H. Central NPFF signalling is critical in the regulation of glucose homeostasis. Mol. Metab. 2022, 62, 101525. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Bell, A.; Polgár, E.; Watanabe, M.; Todd, A.J. Expression of Neuropeptide FF Defines a Population of Excitatory Interneurons in the Superficial Dorsal Horn of the Mouse Spinal Cord that Respond to Noxious and Pruritic Stimuli. Neuroscience 2019, 416, 281–293. [Google Scholar] [CrossRef]
- Poling, M.C.; Quennell, J.H.; Anderson, G.M.; Kauffman, A.S. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J. Neuroendocr. 2013, 25, 876–886. [Google Scholar] [CrossRef]
- Maruyama, M.; Matsumoto, H.; Fujiwara, K.; Noguchi, J.; Kitada, C.; Fujino, M.; Inoue, K. Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology 2001, 142, 2032–2038. [Google Scholar] [CrossRef]
- Könczöl, K.; Bodnár, I.; Zelena, D.; Pintér, O.; Papp, R.S.; Palkovits, M.; Nagy, G.M.; Tóth, Z.E. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem. Int. 2010, 57, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Stornetta, R.L.; Sevigny, C.P.; Guyenet, P.G. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J. Comp. Neurol. 2002, 444, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Lehman, M.N.; Smith, J.T.; Coolen, L.M.; de Oliveira, C.V.; Jafarzadehshirazi, M.R.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- Hrabovszky, E.; Sipos, M.T.; Molnár, C.S.; Ciofi, P.; Borsay, B.; Gergely, P.; Herczeg, L.; Bloom, S.R.; Ghatei, M.A.; Dhillo, W.S.; et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 2012, 153, 4978–4989. [Google Scholar] [CrossRef] [PubMed]
- Cravo, R.M.; Margatho, L.O.; Osborne-Lawrence, S.; Donato, J., Jr.; Atkin, S.; Bookout, A.L.; Rovinsky, S.; Frazão, R.; Lee, C.E.; Gautron, L.; et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011, 173, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.D.; Buijs, R.M.; Jhamandas, J.H.; Swaab, D.F. The hypothalamic neuropeptide FF network is impaired in hypertensive patients. Brain Behav. 2014, 4, 453–467. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; MacTavish, D.; Harris, K.H. Neuropeptide FF (NPFF) control of magnocellular neurosecretory cells of the rat hypothalamic paraventricular nucleus (PVN). Peptides 2006, 27, 973–979. [Google Scholar] [CrossRef]
- Engelmann, M.; Landgraf, R.; Wotjak, C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Front. Neuroendocr. 2004, 25, 132–149. [Google Scholar] [CrossRef]
- Härfstrand, A.; Fuxe, K.; Terenius, L.; Kalia, M. Neuropeptide Y-immunoreactive perikarya and nerve terminals in the rat medulla oblongata: Relationship to cytoarchitecture and catecholaminergic cell groups. J. Comp. Neurol. 1987, 260, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.T., Jr.; Bohn, M.C.; Sawchenko, P.E. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp. Neurol. 1990, 292, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Nahvi, R.J.; Sabban, E.L. Sex Differences in the Neuropeptide Y System and Implications for Stress Related Disorders. Biomolecules 2020, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Palkovits, M.; Gallatz, K. Fine structure of the area subpostrema in rat. Open gate for the medullary autonomic centers. Ideggyogy. Sz. 2007, 60, 83–88. [Google Scholar]
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lönnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Kókai, É.; Polgár, E.; Quillet, R.; Titterton, H.F.; Weir, G.A.; Watanabe, M.; Todd, A.J. Antibodies Against the Gastrin-releasing Peptide Precursor Pro-Gastrin-releasing Peptide Reveal Its Expression in the Mouse Spinal Dorsal Horn. Neuroscience 2023, 510, 60–71. [Google Scholar] [CrossRef]
- Robinson, S.L.; Thiele, T.E. A role for the neuropeptide somatostatin in the neurobiology of behaviors associated with substances abuse and affective disorders. Neuropharmacology 2020, 167, 107983. [Google Scholar] [CrossRef]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; Liu, X.T.; Liu, B.; Liu, X.Y.; Gao, F.; Zeng, X.; Liu, J.; Yang, Q.; Wilhelm, S.; Yin, J.; et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun. 2020, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Kent, P.; Luft, T.; Schwartsmann, G.; Merali, Z. Gastrin-releasing peptide receptor signaling in the integration of stress and memory. Neurobiol. Learn. Mem. 2014, 112, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.Z.; Harbid, A.A.; Inglis, M.A.; Quennell, J.H.; Anderson, G.M. Evidence that hypothalamic RFamide related peptide-3 neurones are not leptin-responsive in mice and rats. J. Neuroendocr. 2014, 26, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, D.; Mac Gillavry, D.W. Mini-review: A possible role for galanin in post-traumatic stress disorder. Neurosci. Lett. 2021, 756, 135980. [Google Scholar] [CrossRef] [PubMed]
- Demsie, D.G.; Altaye, B.M.; Weldekidan, E.; Gebremedhin, H.; Alema, N.M.; Tefera, M.M.; Bantie, A.T. Galanin Receptors as Drug Target for Novel Antidepressants: Review. Biologics 2020, 14, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Clarke, I.J. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol. Metab. 2010, 21, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Schank, J.R. Neurokinin receptors in drug and alcohol addiction. Brain Res. 2020, 1734, 146729. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Cooke, E.K.; Leib, D.E.; Lin, Y.C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons that Control Body Temperature. Cell 2016, 167, 47–59. [Google Scholar] [CrossRef]
- Boucher, M.N.; May, V.; Braas, K.M.; Hammack, S.E. PACAP orchestration of stress-related responses in neural circuits. Peptides 2021, 142, 170554. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef] [PubMed]
- Sargin, D. The role of the orexin system in stress response. Neuropharmacology 2019, 154, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Tsukamura, H. KNDy neurones and GnRH/LH pulse generation: Current understanding and future aspects. J. Neuroendocr. 2023, 35, e13285. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Novak, A.G.; Lehman, M.N. KNDy Neurons of the Hypothalamus and Their Role in GnRH Pulse Generation: An Update. Endocrinology 2023, 165, bqad194. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V). Brain Res. Rev. 2008, 57, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Koller, J.; Gopalasingam, G.; Herzog, H. NPFF signalling is critical for thermosensory and dietary regulation of thermogenesis. Neuropeptides 2022, 96, 102292. [Google Scholar] [CrossRef]
- Zhang, L.; Koller, J.; Ip, C.K.; Gopalasingam, G.; Bajaj, N.; Lee, N.J.; Enriquez, R.F.; Herzog, H. Lack of neuropeptide FF signalling in mice leads to reduced repetitive behavior, altered drinking behavior, and fuel type selection. Faseb J. 2021, 35, e21980. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.; Velasco, I.; Vázquez, M.J.; Barroso, A.; Beiroa, D.; Heras, V.; Ruiz-Pino, F.; Manfredi-Lozano, M.; Romero-Ruiz, A.; Sanchez-Garrido, M.A.; et al. Sex-Biased Physiological Roles of NPFF1R, the Canonical Receptor of RFRP-3, in Food Intake and Metabolic Homeostasis Revealed by its Congenital Ablation in mice. Metabolism 2018, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ip, C.K.; Lee, I.J.; Qi, Y.; Reed, F.; Karl, T.; Low, J.K.; Enriquez, R.F.; Lee, N.J.; Baldock, P.A.; et al. Diet-induced adaptive thermogenesis requires neuropeptide FF receptor-2 signalling. Nat. Commun. 2018, 9, 4722. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wu, K.H.; Jhang, J.J.; Jhang, J.L.; Yu, Z.; Tsai, S.C.; Chen, J.C.; Hsu, P.H.; Li, H.Y. Hypothalamic NPFFR2 attenuates central insulin signaling and its knockout diminishes metabolic dysfunction in mouse models of diabetes mellitus. Clin. Nutr. 2024, 43, 603–619. [Google Scholar] [CrossRef]
- Lin, Y.T.; Huang, Y.L.; Tsai, S.C.; Chen, J.C. Ablation of NPFFR2 in Mice Reduces Response to Single Prolonged Stress Model. Cells 2020, 9, 2479. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Matsumoto, H.; Nakata, M.; Mera, T.; Fukusumi, S.; Hinuma, S.; Ueta, Y.; Yada, T.; Leng, G.; Onaka, T. Endogenous prolactin-releasing peptide regulates food intake in rodents. J. Clin. Investig. 2008, 118, 4014–4024. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Lennerås, M.; Göransson, M.; Elmgren, A.; Bohlooly, Y.M. GPR10 deficiency in mice results in altered energy expenditure and obesity. Biochem. Biophys. Res. Commun. 2007, 363, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Geddes, B.J.; Zhang, C.; Foley, K.P.; Stricker-Krongrad, A. The prolactin-releasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J. Mol. Neurosci. 2004, 22, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pražienková, V.; Funda, J.; Pirník, Z.; Karnošová, A.; Hrubá, L.; Kořínková, L.; Neprašová, B.; Janovská, P.; Benzce, M.; Kadlecová, M.; et al. GPR10 gene deletion in mice increases basal neuronal activity, disturbs insulin sensitivity and alters lipid homeostasis. Gene 2021, 774, 145427. [Google Scholar] [CrossRef]
- Talbot, F.; Feetham, C.H.; Mokrosiński, J.; Lawler, K.; Keogh, J.M.; Henning, E.; Mendes de Oliveira, E.; Ayinampudi, V.; Saeed, S.; Bonnefond, A.; et al. A rare human variant that disrupts GPR10 signalling causes weight gain in mice. Nat. Commun. 2023, 14, 1450. [Google Scholar] [CrossRef]
- Watanabe, A.; Okuno, S.; Okano, M.; Jordan, S.; Aihara, K.; Watanabe, T.K.; Yamasaki, Y.; Kitagawa, H.; Sugawara, K.; Kato, S. Altered emotional behaviors in the diabetes mellitus OLETF type 1 congenic rat. Brain Res. 2007, 1178, 114–124. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamasaki, M.; Takao, K.; Soya, S.; Iwasaki, M.; Sasaki, K.; Magoori, K.; Sakakibara, I.; Miyakawa, T.; Mieda, M.; et al. QRFP-Deficient Mice Are Hypophagic, Lean, Hypoactive and Exhibit Increased Anxiety-Like Behavior. PLoS ONE 2016, 11, e0164716. [Google Scholar] [CrossRef]
- El-Mehdi, M.; Takhlidjt, S.; Khiar, F.; Prévost, G.; do Rego, J.L.; do Rego, J.C.; Benani, A.; Nedelec, E.; Godefroy, D.; Arabo, A.; et al. Glucose homeostasis is impaired in mice deficient in the neuropeptide 26RFa (QRFP). BMJ Open Diabetes Res. Care 2020, 8, e000942. [Google Scholar] [CrossRef]
- Tolson, K.P.; Garcia, C.; Yen, S.; Simonds, S.; Stefanidis, A.; Lawrence, A.; Smith, J.T.; Kauffman, A.S. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J. Clin. Investig. 2014, 124, 3075–3079. [Google Scholar] [CrossRef]
- Halvorson, C.L.; De Bond, J.P.; Maloney, S.K.; Smith, J.T. Thermoneutral conditions correct the obese phenotype in female, but not male, Kiss1r knockout mice. J. Biol. 2020, 90, 102592. [Google Scholar] [CrossRef] [PubMed]
- Delmas, S.; Porteous, R.; Bergin, D.H.; Herbison, A.E. Altered aspects of anxiety-related behavior in kisspeptin receptor-deleted male mice. Sci. Rep. 2018, 8, 2794. [Google Scholar] [CrossRef] [PubMed]
- Jászberényi, M.; Bagosi, Z.; Csabafi, K.; Palotai, M.; Telegdy, G. The actions of neuropeptide SF on the hypothalamic-pituitary-adrenal axis and behavior in rats. Regul. Pept. 2014, 188, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, W.E.; Ziegler, D.R.; Herman, J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008, 213, 63–72. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.; Dinan, T.G.; Scott, L.V. Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014, 124, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, J.J.; Zhu, Y.; Kosten, T.; Li, D.P. Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons through NKCC1 Upregulation. Neuroendocrinology 2017, 104, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Simonin, F.; Bourguignon, J.J.; Harris, K.H. Neuropeptide FF and neuropeptide VF inhibit GABAergic neurotransmission in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1872–R1880. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Goncharuk, V. Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front. Endocrinol. 2013, 4, 8. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; MacTavish, D. Central administration of neuropeptide FF causes activation of oxytocin paraventricular hypothalamic neurones that project to the brainstem. J. Neuroendocr. 2003, 15, 24–32. [Google Scholar] [CrossRef]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Pachuta, A.; Bochenski, M.; Silberring, J. Dansyl-PQRamide, a putative antagonist of NPFF receptors, reduces anxiety-like behavior of ethanol withdrawal in a plus-maze test in rats. Peptides 2009, 30, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Liu, T.-Y.; Yang, C.-Y.; Yu, Y.-L.; Chen, T.-C.; Day, Y.-J.; Chang, C.-C.; Huang, G.-J.; Chen, J.-C. Chronic activation of NPFFR2 stimulates the stress-related depressive behaviors through HPA axis modulation. Psychoneuroendocrinology 2016, 71, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.H.; Gibula-Bruzda, E.; Koltunowska, D.; Raoof, H.; Suder, P.; Silberring, J. Modulation of neuropeptide FF (NPFF) receptors influences the expression of amphetamine-induced conditioned place preference and amphetamine withdrawal anxiety-like behavior in rats. Peptides 2012, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Yu, Y.L.; Hong, W.C.; Yeh, T.S.; Chen, T.C.; Chen, J.C. NPFFR2 Activates the HPA Axis and Induces Anxiogenic Effects in Rodents. Int. J. Mol. Sci. 2017, 18, 1810. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA 2009, 106, 11324–11329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Song, C.I.; Hughes, J.K.; Kreisman, M.J.; Parra, R.A.; Haisenleder, D.J.; Kauffman, A.S.; Breen, K.M. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology 2017, 158, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pereira, I.; Llorca-Torralba, M.; Bravo, L.; Camarena-Delgado, C.; Soriano-Mas, C.; Berrocoso, E. The Role of the Locus Coeruleus in Pain and Associated Stress-Related Disorders. Biol. Psychiatry 2022, 91, 786–797. [Google Scholar] [CrossRef]
- Kumar, J.R.; Rajkumar, R.; Jayakody, T.; Marwari, S.; Hong, J.M.; Ma, S.; Gundlach, A.L.; Lai, M.K.P.; Dawe, G.S. Relaxin’ the brain: A case for targeting the nucleus incertus network and relaxin-3/RXFP3 system in neuropsychiatric disorders. Br. J. Pharm. 2017, 174, 1061–1076. [Google Scholar] [CrossRef]
- Szőnyi, A.; Sos, K.E.; Nyilas, R.; Schlingloff, D.; Domonkos, A.; Takács, V.T.; Pósfai, B.; Hegedüs, P.; Priestley, J.B.; Gundlach, A.L.; et al. Brainstem nucleus incertus controls contextual memory formation. Science 2019, 364, eaaw0445. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Parkington, H.C. Gonadotropin inhibitory hormone (GnIH) as a regulator of gonadotropes. Mol. Cell Endocrinol. 2014, 385, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.C.; Muroy, S.E.; Zhao, S.; Bentley, G.E.; Kriegsfeld, L.J.; Kaufer, D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Elife 2015, 4, e04316. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Dalpatadu, S.L.; Wong, D.W.; Parhar, I.S. Neonatal dexamethasone exposure down-regulates GnRH expression through the GnIH pathway in female mice. Neuroscience 2012, 218, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sawchenko, P.E.; Swanson, L.W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982, 257, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Pacak, K.; Palkovits, M.; Kopin, I.J.; Goldstein, D.S. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: In vivo microdialysis studies. Front. Neuroendocr. 1995, 16, 89–150. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.M.; Marks, D.; Kobayashi, K.; Okano, H.; Low, M.J.; Andresen, M.C. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J. Neurosci. 2007, 27, 13292–13302. [Google Scholar] [CrossRef] [PubMed]
- Mera, T.; Fujihara, H.; Kawasaki, M.; Hashimoto, H.; Saito, T.; Shibata, M.; Saito, J.; Oka, T.; Tsuji, S.; Onaka, T.; et al. Prolactin-releasing peptide is a potent mediator of stress responses in the brain through the hypothalamic paraventricular nucleus. Neuroscience 2006, 141, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Maruyama, M.; Noguchi, J.; Horikoshi, Y.; Fujiwara, K.; Kitada, C.; Hinuma, S.; Onda, H.; Nishimura, O.; Inoue, K.; et al. Stimulation of corticotropin-releasing hormone-mediated adrenocorticotropin secretion by central administration of prolactin-releasing peptide in rats. Neurosci. Lett. 2000, 285, 234–238. [Google Scholar] [CrossRef]
- Seal, L.J.; Small, C.J.; Dhillo, W.S.; Kennedy, A.R.; Ghatei, M.A.; Bloom, S.R. Prolactin-releasing peptide releases corticotropin-releasing hormone and increases plasma adrenocorticotropin via the paraventricular nucleus of the hypothalamus. Neuroendocrinology 2002, 76, 70–78. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Onaka, T. The medial amygdala-medullary PrRP-synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology 2014, 155, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Mochiduki, A.; Takeda, T.; Kaga, S.; Inoue, K. Stress response of prolactin-releasing peptide knockout mice as to glucocorticoid secretion. J. Neuroendocr. 2010, 22, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kobayashi, D.; Das, G.; Onaka, T.; Inoue, K.; Itoi, K. Participation of the prolactin-releasing peptide-containing neurones in caudal medulla in conveying haemorrhagic stress-induced signals to the paraventricular nucleus of the hypothalamus. J. Neuroendocr. 2010, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.K.; Resch, Z.T.; Murphy, T.C. A novel action of the newly described prolactin-releasing peptides: Cardiovascular regulation. Brain Res. 2000, 858, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, J.; Saigusa, T.; Sugiyama, N.; Kanba, S.; Nishida, Y.; Sato, Y.; Hinuma, S.; Arita, J. Effects of prolactin-releasing peptide microinjection into the ventrolateral medulla on arterial pressure and sympathetic activity in rats. Brain Res. 2002, 958, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Kalsbeek, A.; Buijs, R.M. Early changes of immunoreactivity to orexin in hypothalamus and to RFamide peptides in brainstem during the development of hypertension. Neurosci. Lett. 2021, 762, 136144. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.K.; Rinaman, L. The role of nucleus of the solitary tract glucagon-like peptide-1 and prolactin-releasing peptide neurons in stress: Anatomy, physiology and cellular interactions. Br. J. Pharm. 2022, 179, 642–658. [Google Scholar] [CrossRef]
- Ohiwa, N.; Chang, H.; Saito, T.; Onaka, T.; Fujikawa, T.; Soya, H. Possible inhibitory role of prolactin-releasing peptide for ACTH release associated with running stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R497–R504. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Mochiduki, A.; Nemoto, H.; Sun, B.; Fujiwara, K.; Matsumoto, H.; Inoue, K. Estrogen suppresses the stress response of prolactin-releasing peptide-producing cells. Neurosci. Lett. 2005, 380, 311–315. [Google Scholar] [CrossRef]
- Maniscalco, J.W.; Zheng, H.; Gordon, P.J.; Rinaman, L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. J. Neurosci. 2015, 35, 10701–10714. [Google Scholar] [CrossRef]
- Ghazi Ghanim, K.; Saab Kadhim, M.; Hameed Abed Ali, B.; Jawad, R.A. The Relation between Increasing Anxiety and Prolactin-Releasing Peptide in Rats. Arch. Razi Inst. 2023, 78, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G. Anxiolytic effect of the GPR103 receptor agonist peptide P550 (homolog of neuropeptide 26RFa) in mice. Involvement of neurotransmitters. Peptides 2016, 82, 20–25. [Google Scholar] [CrossRef]
- Seifinejad, A.; Li, S.; Mikhail, C.; Vassalli, A.; Pradervand, S.; Arribat, Y.; Pezeshgi Modarres, H.; Allen, B.; John, R.M.; Amati, F.; et al. Molecular codes and in vitro generation of hypocretin and melanin concentrating hormone neurons. Proc. Natl. Acad. Sci. USA 2019, 116, 17061–17070. [Google Scholar] [CrossRef]
- Rao, Y.S.; Mott, N.N.; Pak, T.R. Effects of kisspeptin on parameters of the HPA axis. Endocrine 2011, 39, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, M.; An, X.F.; He, M.; Yu, J.Y. Central administration of kisspeptin-10 inhibits natriuresis and diuresis induced by blood volume expansion in anesthetized male rats. Acta Pharm. Sin. 2010, 31, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Adekunbi, D.A.; Li, X.F.; Lass, G.; Shetty, K.; Adegoke, O.A.; Yeo, S.H.; Colledge, W.H.; Lightman, S.L.; O’Byrne, K.T. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J. Neuroendocr. 2018, 30, e12572. [Google Scholar] [CrossRef]
- Tanaka, M.; Csabafi, K.; Telegdy, G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul. Pept. 2013, 180, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Wall, M.B.; Demetriou, L.; Shah, A.J.; Clarke, S.A.; Narayanaswamy, S.; Nesbitt, A.; Izzi-Engbeaya, C.; Prague, J.K.; Abbara, A.; et al. Kisspeptin modulates sexual and emotional brain processing in humans. J. Clin. Investig. 2017, 127, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Demetriou, L.; Wall, M.B.; Shah, A.J.; Clarke, S.A.; Narayanaswamy, S.; Nesbitt, A.; Izzi-Engbeaya, C.; Prague, J.K.; Abbara, A.; et al. Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight 2018, 3, e121958. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Murakami, M.; Shimizu, F.; Kuwahara, A.; Yasui, T.; Irahara, M. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J. Endocrinol. Investig. 2008, 31, 656–659. [Google Scholar] [CrossRef]
- Kinsey-Jones, J.S.; Li, X.F.; Knox, A.M.; Wilkinson, E.S.; Zhu, X.L.; Chaudhary, A.A.; Milligan, S.R.; Lightman, S.L.; O’Byrne, K.T. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J. Neuroendocr. 2009, 21, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kobayashi, Y.; Omotehara, T.; Tatsumi, A.; Hashimoto, R.; Umemura, Y.; Nagahara, D.; Mantani, Y.; Yokoyama, T.; Kitagawa, H.; et al. Unpredictable chronic stress-induced reproductive suppression associated with the decrease of kisspeptin immunoreactivity in male mice. J. Vet. Med. Sci. 2014, 76, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.; Li, X.F.; Ivanova, D.; Wang, J.; O’Byrne, K.T. Hypothalamic PVN CRH Neurons Signal Through PVN GABA Neurons to Suppress GnRH Pulse Generator Frequency in Female Mice. Endocrinology 2023, 164, bqad075. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.; Stephens, S.B.; Chaing, S.; Munaganuru, N.; Kauffman, A.S.; Breen, K.M. Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice. Endocrinology 2016, 157, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Becari, C.; Lesnikova, A.; Biojone, C.; Salgado, M.C.O.; Salgado, H.C.; Resstel, L.B.M.; Guimarães, F.S.; Castrén, E.; Casarotto, P.C.; et al. Elastase-2 Knockout Mice Display Anxiogenic- and Antidepressant-Like Phenotype: Putative Role for BDNF Metabolism in Prefrontal Cortex. Mol. Neurobiol. 2018, 55, 7062–7071. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.; Rao, B.S.; Nair, D.; Trinh, M.; Mawjee, N.; Tonegawa, S.; Chattarji, S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA 2006, 103, 13208–13213. [Google Scholar] [CrossRef]
- Meczekalski, B.; Niwczyk, O.; Bala, G.; Szeliga, A. Stress, kisspeptin, and functional hypothalamic amenorrhea. Curr. Opin. Pharm. 2022, 67, 102288. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A.; Benedetti, F. Perspectives in affective disorders: Clocks and sleep. Eur. J. Neurosci. 2020, 51, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Torterolo, P.; Scorza, C.; Lagos, P.; Urbanavicius, J.; Benedetto, L.; Pascovich, C.; López-Hill, X.; Chase, M.H.; Monti, J.M. Melanin-Concentrating Hormone (MCH): Role in REM Sleep and Depression. Front. Neurosci. 2015, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, D.A.; Luckman, S.M. The role of RFamide peptides in feeding. J. Endocrinol. 2007, 192, 3–15. [Google Scholar] [CrossRef]
- Lutter, M. Emerging Treatments in Eating Disorders. Neurotherapeutics 2017, 14, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Luisi, S.; Petraglia, F. Is Stress a Cause or a Consequence of Endometriosis? Reprod. Sci. 2020, 27, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Labrouche, S.; Laulin, J.P.; Le Moal, M.; Tramu, G.; Simonnet, G. Neuropeptide FF in the rat adrenal gland: Presence, distribution and pharmacological effects. J. Neuroendocr. 1998, 10, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pirník, Z.; Kořínková, L.; Osacká, J.; Železná, B.; Kuneš, J.; Maletínská, L. Cholecystokinin system is involved in the anorexigenic effect of peripherally applied palmitoylated prolactin-releasing peptide in fasted mice. Physiol. Res. 2021, 70, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Holubová, M.; Hrubá, L.; Popelová, A.; Bencze, M.; Pražienková, V.; Gengler, S.; Kratochvílová, H.; Haluzík, M.; Železná, B.; Kuneš, J.; et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of β-amyloid pathology. Neuropharmacology 2019, 144, 377–387. [Google Scholar] [CrossRef]
- Alexopoulou, F.; Bech, E.M.; Pedersen, S.L.; Thorbek, D.D.; Leurs, U.; Rudkjær, L.C.B.; Fosgerau, K.; Hansen, H.H.; Vrang, N.; Jelsing, J.; et al. Lipidated PrRP31 metabolites are long acting dual GPR10 and NPFF2 receptor agonists with potent body weight lowering effect. Sci. Rep. 2022, 12, 1696. [Google Scholar] [CrossRef]
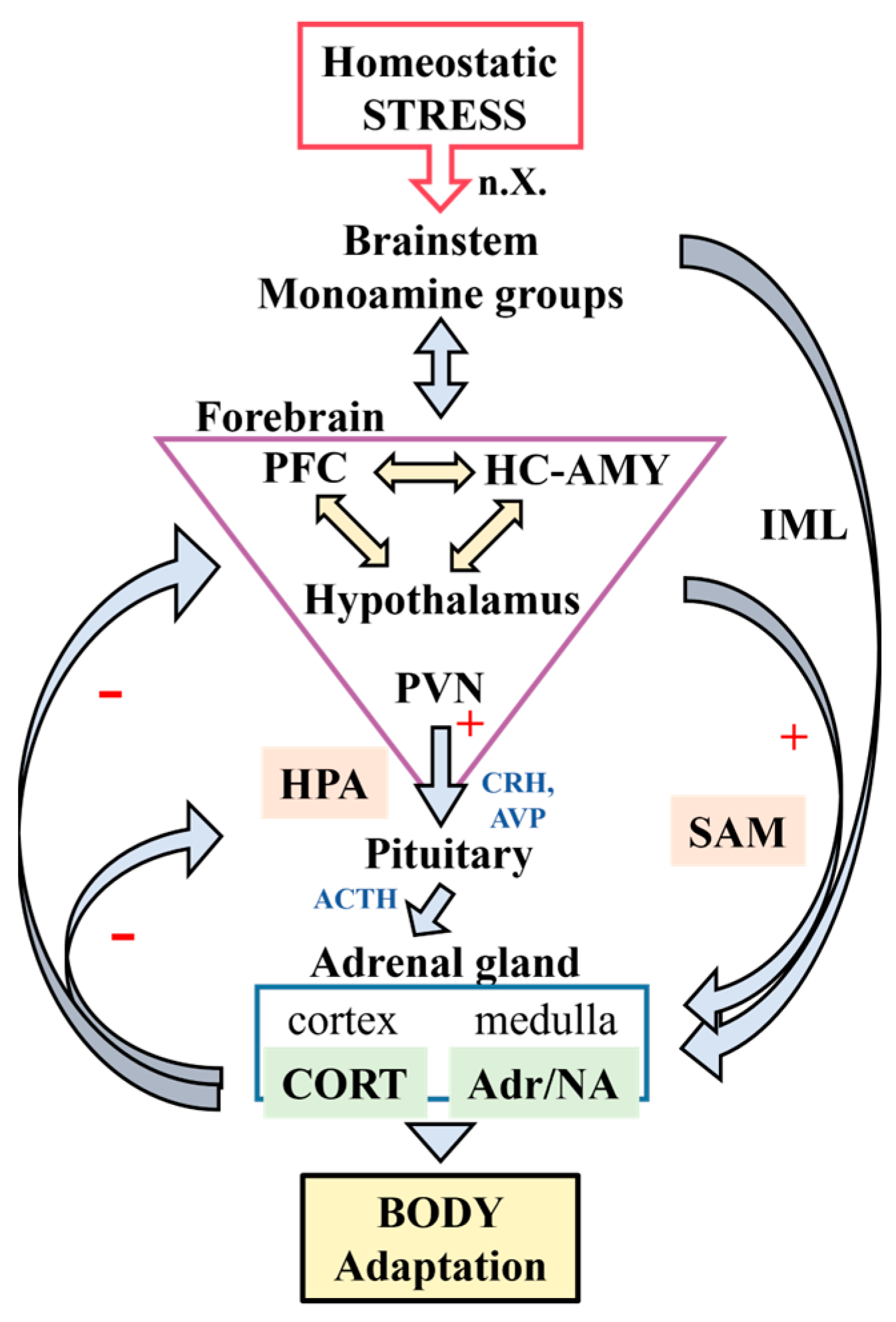
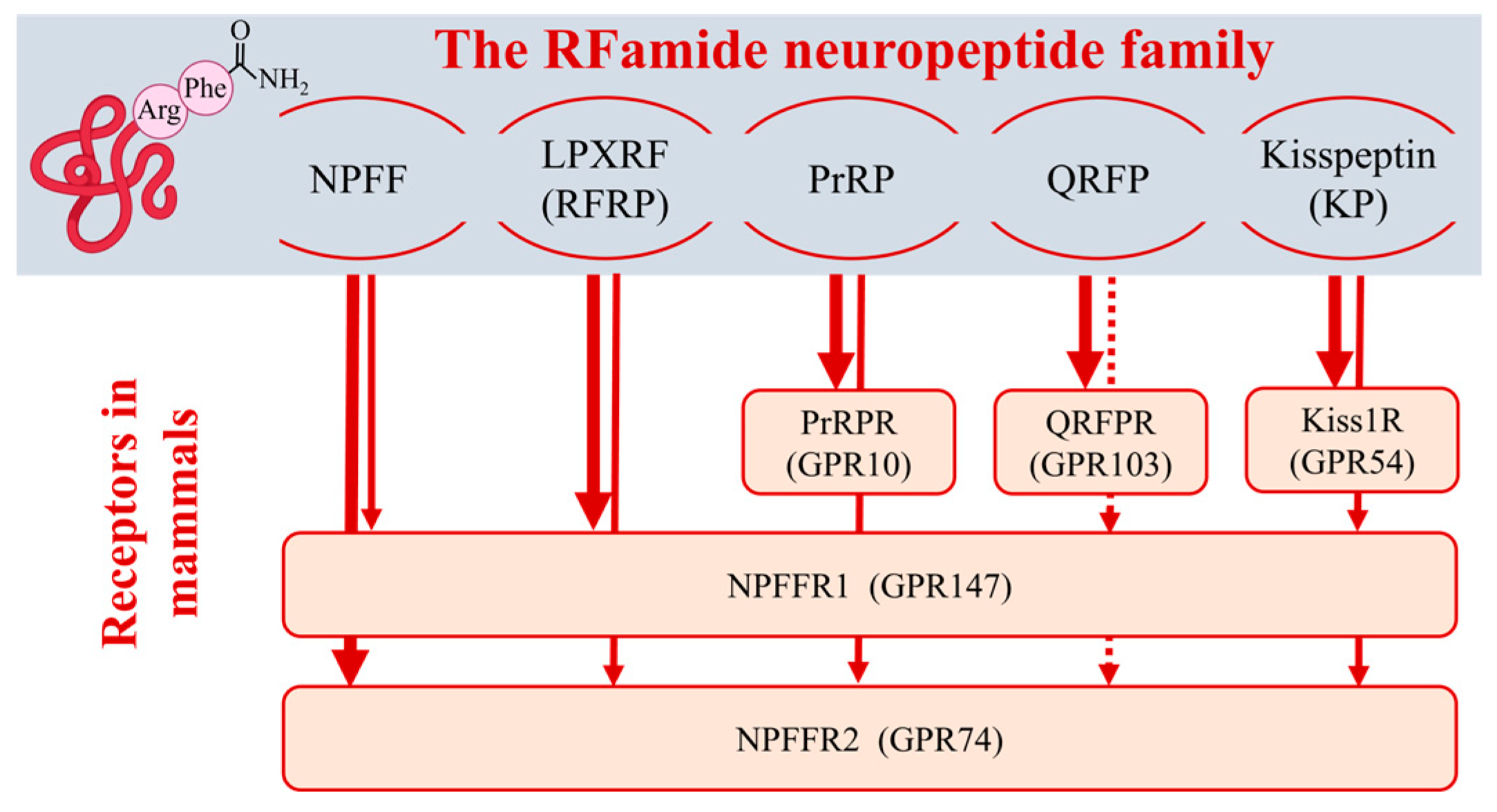
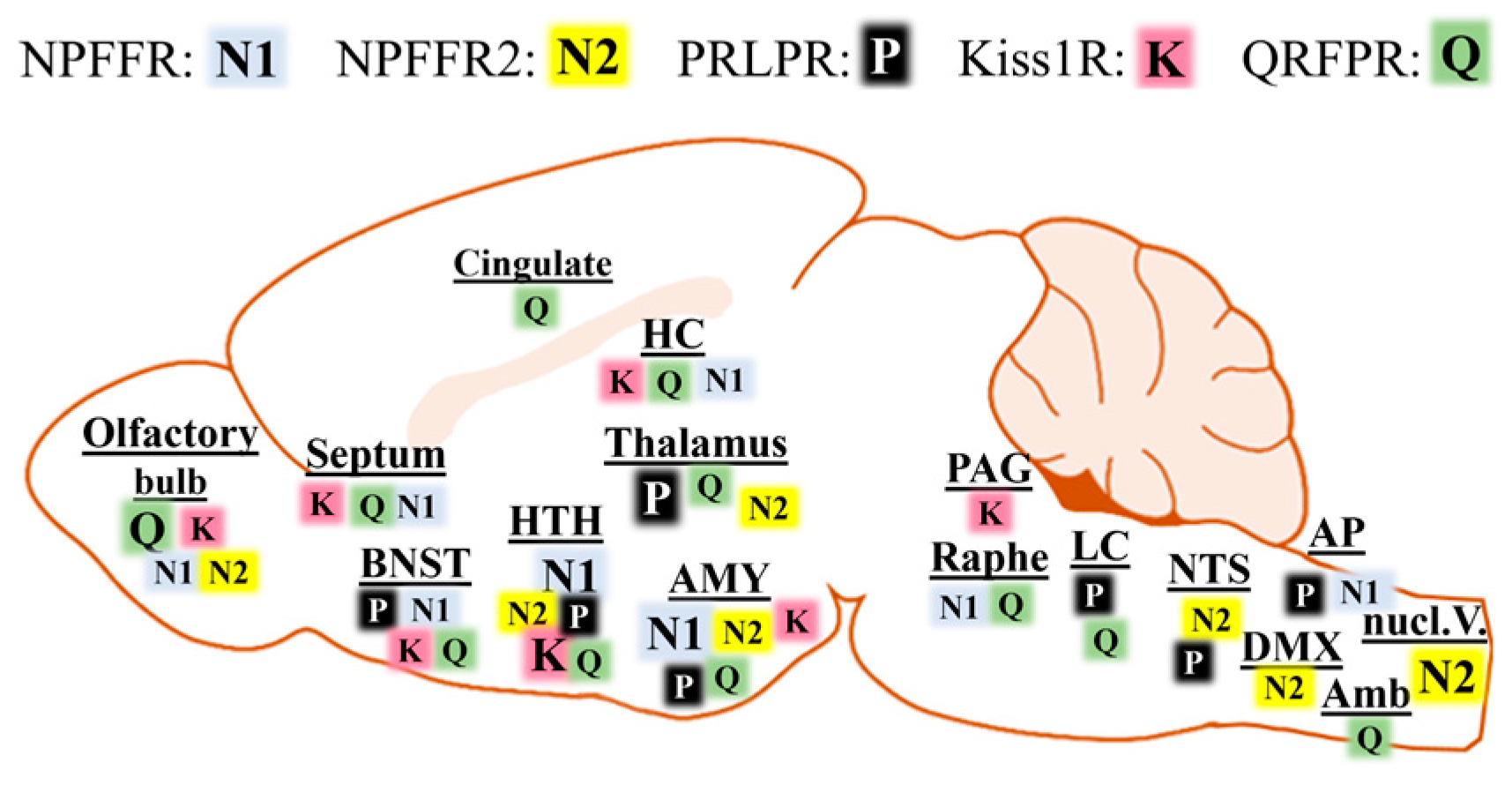
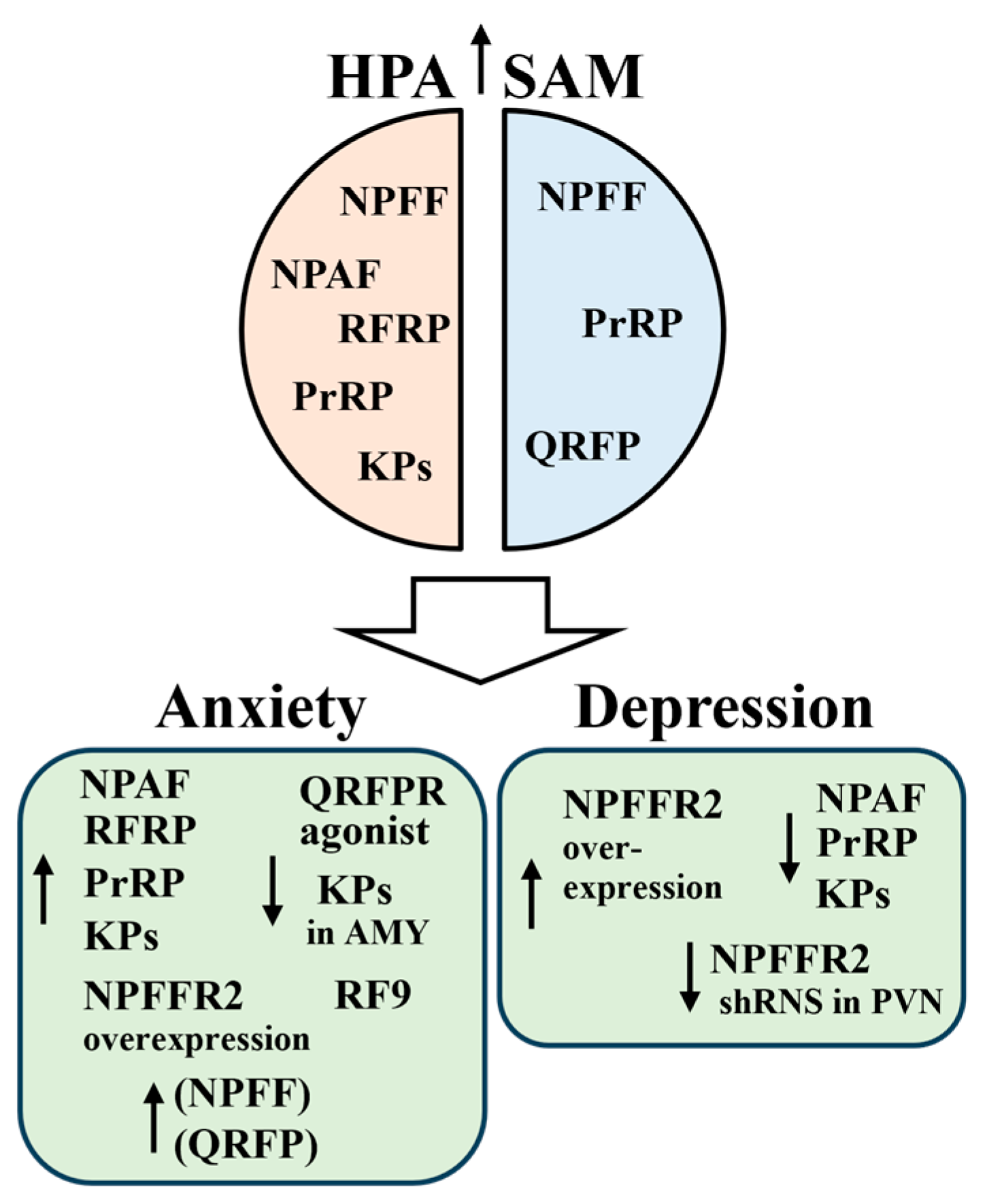
| Effect | NPFF/NPAF | RFP1/RFP3 | PrRP | QRFP | Kisspeptins |
|---|---|---|---|---|---|
| Energy expediture | Hypothermia: NPFF, ICV [22,23]; NPAF, ICV [24]. | Hypothermia: RFP3, ICV [25,26,27] | Hyperthermia: ICV [28], brief hypothermia then long-lasting hyperthermia [29]; Fourth ventricle, NTS, hyperthermia [30]. | No effect: ICV [31,32]; Hypothermia, reduced thermogenesis: ICV, chronic treatment [33]; Hypothermia and hibernation-like state: chemogenetical activation [34]. | Hyperthermia: KP-13, ICV [35]. |
| Food intake | Anorexigenic: NPFF, ICV [36,37]; NPAF, ICV [38]. Orexigenic: NPFF, lateral PBN, high dose [39]. | Anorexigenic: RFP1/3, central AMY [40,41]; Orexigenic: RFP3, ICV in the light phase [42], chronic infusion [27]. | Anorexigenic: ICV [28,29]; DMN [43]; NTS [30]. | Orexigenic: ICV [32,44,45,46,47], high fat intake [48]; medial hypothalamic area [49]. | Anorexigenic: KP-10, ICV [50]. |
| Reproduction | Inhibits GnRH cells: RFP-3, ICV [51]; Inhibits KP cells: RFP-3, ICV [52,53]; Inhibits LH secretion: RFP-1/3, ICV [52,54,55]; Stimulates LH secretion in males: RFRP-3, ICV [54]; Stimulates PRL release: RFRP-1, ICV [56]. | Stimulates LH secretion: KP-54/10, ICV [57]; KP-10, ARC, POA [58]; medial AMY [59]. Stimulates FSH secretion: KP-54, ICV [57]; Stimulates PRL secretion: KP-10, ICV [60]; Stimulates sexual behavior: KP-10, ICV [61]; photostimulation, AVPV [62]; KP-10, medial AMY [59]. | |||
| Pain perception | No effect: NPFF, ICV, on basal nociceptive threshold [63]. Antinociceptive: NPFF, PAG, antiallodynia [64]; NPFF, NPAF, NPSF, IT, analgesic effect, enhanced morphine analgesia [65,66]. Nociceptive NPFF, ICV, VTA, PAG, hyperalgesia, reduced morphine and stress analgesia [23,64,67,68,69,70,71]; NPSF, ICV, reversed morphine analgesia [70]. | Antinociceptive: RFP1, IT, antiallodynia, antinociception [72]; RFP3, ICV, enhanced morphine analgesia [25]. Nociceptive: RFP3, ICV, reduced warm-water-swim stress-induced analgesia [69]; reduced basal nociceptive threshold [63]. | Antinociceptive: PAG, antiallodynia; NTS, antinociception [73]. Nociceptive: ICV, reduced basal nociceptive threshold and morphine analgesia; [63,74] caudal ventrolateral medulla, hyperalgesia [73]. No effect: IT [73]. | Antinociceptive: ICV, IT, antiallodynia [75] and analgesia [76,77]; LC, PAG, analgesia [78]. Nociceptive: ICV, reduced basal nociceptive threshold [63] | Nociceptive: KP-10/13, ICV, reduced basal nociceptive threshold and morphine analgesia [63,79]; KP, IT, hyperalgesia [80] |
| Reward | Negative effect: NPFF, ICV, anti-opioid effect in CPP test [81,82]. | Positive reinforcement: RFP1, central AMY [83]. | No effect: 4th ventricle, food reward [30]. | No effect ICV, food reward [32] | |
| Learning/ Memory | Improved learning: NPAF, ICV, reversed memory impairment [84]. | Improves learning: RFP1, central AMY [85]. | Improves memory Medial hypothalamic injection [86] | Improves memory and learning: reversed memory impairment KP-13, ICV [87,88,89]; KP-13, hippocampus [88]; | |
| Locomotion | Hypoactivity: NPFF, ICV, anti-opioid effect [82,90]; VTA, anti-opioid, anti-novelty effects [91,92]. Hyperactivity: NPAF, ICV [24]. | Hypoactivity: RFP3, ICV, decreased total locomotion [93]. No effect: RFP3, chronic infusion ICV [27]. | No effect: ICV, repeated injection, measured on day 3 [94]. | Hyperactivity ICV [32,46,47] No effect ICV [31] Hypoactivity Chemogenetical activation, long-lasting effect [34] | Hypoactivity: KP-8, ICV [95]. Hyperactivity: KP-13, ICV [35]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.; Szabó, E.; László, K.; Kertes, E.; Zagorácz, O.; Mintál, K.; Tóth, A.; Gálosi, R.; Berta, B.; Lénárd, L.; et al. Brain RFamide Neuropeptides in Stress-Related Psychopathologies. Cells 2024, 13, 1097. https://doi.org/10.3390/cells13131097
Kovács A, Szabó E, László K, Kertes E, Zagorácz O, Mintál K, Tóth A, Gálosi R, Berta B, Lénárd L, et al. Brain RFamide Neuropeptides in Stress-Related Psychopathologies. Cells. 2024; 13(13):1097. https://doi.org/10.3390/cells13131097
Chicago/Turabian StyleKovács, Anita, Evelin Szabó, Kristóf László, Erika Kertes, Olga Zagorácz, Kitti Mintál, Attila Tóth, Rita Gálosi, Bea Berta, László Lénárd, and et al. 2024. "Brain RFamide Neuropeptides in Stress-Related Psychopathologies" Cells 13, no. 13: 1097. https://doi.org/10.3390/cells13131097
APA StyleKovács, A., Szabó, E., László, K., Kertes, E., Zagorácz, O., Mintál, K., Tóth, A., Gálosi, R., Berta, B., Lénárd, L., Hormay, E., László, B., Zelena, D., & Tóth, Z. E. (2024). Brain RFamide Neuropeptides in Stress-Related Psychopathologies. Cells, 13(13), 1097. https://doi.org/10.3390/cells13131097










