The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction
Abstract
1. Introduction
2. Overview of Retinoic Acid
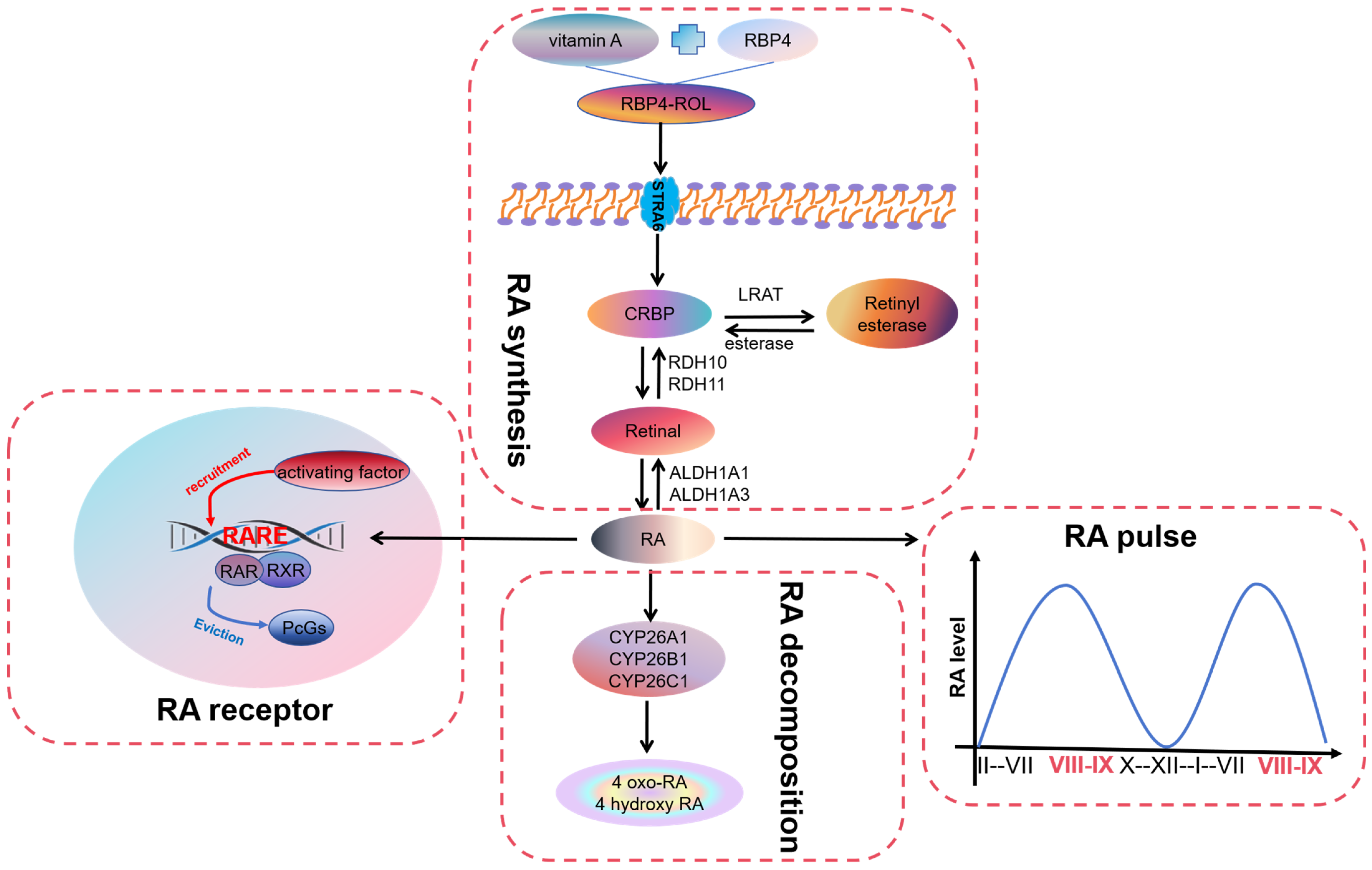
2.1. Synthesis of Retinoic Acid
2.2. Pulse of Retinoic Acid
2.3. Receptors for Retinoic Acid
2.4. Degradation of Retinoic Acid
3. Retinoic Acid in Spermatogenesis
3.1. Retinoic Acid in the Differentiation of Spermatogonia
3.2. Retinoic Acid in the Initiation of Meiosis in Spermatogenic Cells
3.3. Retinoic Acid in the Late Meiotic Process in Spermatogenic Cells
3.4. Retinoic Acid in the Formation and Release of Spermatozoa
4. The Application of Retinoic Acid in Male Reproduction
4.1. The Use of Retinoic Acid in the Treatment of Male Reproductive Disorders
4.2. The Use of Retinoic Acid in Male Contraception
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Yu, K.; Deng, S.L.; Sun, T.C.; Li, Y.Y.; Liu, Y.X. Melatonin Regulates the Synthesis of Steroid Hormones on Male Reproduction: A Review. Molecules 2018, 23, 447. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Leung, M.; Solanki, A.K.; Lobo, G.P. Mapping of the extracellular RBP4 ligand binding domain on the RBPR2 receptor for Vitamin A transport. Front. Cell Dev. Biol. 2023, 11, 1105657. [Google Scholar] [CrossRef]
- Montenegro, D.; Zhao, J.; Kim, H.J.; Shmarakov, I.O.; Blaner, W.S.; Sparrow, J.R. Products of the visual cycle are detected in mice lacking retinol binding protein 4, the only known vitamin A carrier in plasma. J. Biol. Chem. 2022, 298, 102722. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Gely-Pernot, A.; Féret, B.; Dennefeld, C.; Benoit, G.; Davidson, I.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16582–16587. [Google Scholar] [CrossRef] [PubMed]
- Deltour, L.; Haselbeck, R.J.; Ang, H.L.; Duester, G. Localization of class I and class IV alcohol dehydrogenases in mouse testis and epididymis: Potential retinol dehydrogenases for endogenous retinoic acid synthesis. Biol. Reprod. 1997, 56, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, A.; Fan, X.; Deltour, L.; Foglio, M.H.; Martras, S.; Farrés, J.; Parés, X.; Duester, G. Stimulation of retinoic acid production and growth by ubiquitously expressed alcohol dehydrogenase Adh3. Proc. Natl. Acad. Sci. USA 2002, 99, 5337–5342. [Google Scholar] [CrossRef]
- Sandell, L.L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.; Young, K.; Rey, J.P.; Ma, J.X.; Staehling-Hampton, K.; Trainor, P.A. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124. [Google Scholar] [CrossRef]
- Tong, M.H.; Yang, Q.E.; Davis, J.C.; Griswold, M.D. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc. Natl. Acad. Sci. USA 2013, 110, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fernandez, L.A.; del Mazo, J. The cytosolic aldehyde dehydrogenase gene (Aldh1) is developmentally expressed in Leydig cells. FEBS Lett. 1997, 407, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Sperkova, Z.; Napoli, J.L. Cellular expression of retinal dehydrogenase types 1 and 2: Effects of vitamin A status on testis mRNA. J. Cell. Physiol. 2001, 186, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Beedle, M.T.; Stevison, F.; Zhong, G.; Topping, T.; Hogarth, C.; Isoherranen, N.; Griswold, M.D. Sources of all-trans retinal oxidation independent of the aldehyde dehydrogenase 1A isozymes exist in the postnatal testisdagger. Biol. Reprod. 2019, 100, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Topping, T.; Griswold, M.D. Global Deletion of ALDH1A1 and ALDH1A2 Genes Does Not Affect Viability but Blocks Spermatogenesis. Front. Endocrinol. 2022, 13, 871225. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.W.; Li, D.X.; Ling, Z.J.; Tan, L.L.; Zhang, Y.F.; Zhang, J.; Li, H.; Chang, W.; Zhu, H.L.; Zhang, J.; et al. Loss of Atg5 in Sertoli cells enhances the susceptibility of cadmium-impaired testicular spermatogenesis in mice. Food. Chem. Toxicol. 2023, 179, 113967. [Google Scholar] [CrossRef]
- Xing, H.; Chen, S.; Wang, X.; Li, J.; Ren, F. 3-Monochloropropane-1,2-diol causes spermatogenesis failure in male rats via Sertoli cell dysfunction but not testosterone reduction. Toxicol. Lett. 2022, 360, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed]
- Nourashrafeddin, S.; Rashidi, B.H. Gonadotropin Regulation of Retinoic Acid Activity in the Testis. Acta Med. Iran. 2018, 56, 34–42. [Google Scholar]
- Leblond, C.P.; Clermont, Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 1952, 55, 548–573. [Google Scholar] [CrossRef]
- Afsartala, Z.; Rezvanfar, M.A.; Hodjat, M.; Tanha, S.; Assadollahi, V.; Bijangi, K.; Abdollahi, M.; Ghasemzadeh-Hasankolaei, M. Amniotic membrane mesenchymal stem cells can differentiate into germ cells in vitro. In Vitro Cell Dev. Biol. Anim. 2016, 52, 1060–1071. [Google Scholar] [PubMed]
- Costa, G.M.J.; Leal, M.C.; Franca, L.R. Morphofunctional evaluation of the testis, duration of spermatogenesis and spermatogenic efficiency in the Japanese fancy mouse (Mus musculus molossinus). Zygote 2017, 25, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Murray, E.; Sinha, A.; Laumas, A.; Li, J.; Lesman, D.; Nie, X.; Hotaling, J.; Guo, J.; Cairns, B.R.; et al. Dissecting mammalian spermatogenesis using spatial transcriptomics. Cell Rep. 2021, 37, 109915. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thumfart, K.M.; Mansuy, I.M. What are Sertoli cells? Historical, methodological, and functional aspects. Andrology 2023, 11, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015, 92, 37. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.L.; Kent, T.; Hogarth, C.A.; Schlatt, S.; Prasad, B.; Haenisch, M.; Walsh, T.; Muller, C.H.; Griswold, M.D.; Amory, J.K.; et al. Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations. J. Lipid. Res. 2015, 56, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Gewiss, R.; Topping, T.; Griswold, M.D. Cycles, waves, and pulses: Retinoic acid and the organization of spermatogenesis. Andrology 2020, 8, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Freinkman, E.; de Rooij, D.G.; Page, D.C. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E10132–E10141. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Evans, R.M. The RXR heterodimers and orphan receptors. Cells 1995, 83, 841–850. [Google Scholar] [CrossRef]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Griswold, M.D. The regulation of retinoic acid receptor mRNA levels during spermatogenesis. Mol. Endocrinol. 1990, 4, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.J.; Braun, K.W.; McLean, D.J.; Wright, R.W.; Griswold, M.D.; Kim, K.H. Potential functions of retinoic acid receptor A in Sertoli cells and germ cells during spermatogenesis. Ann. N. Y. Acad. Sci. 2007, 1120, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Condrea, D.; Souali-Crespo, S.; Féret, B.; Klopfenstein, M.; Faisan, S.; Mark, M.; Ghyselinck, N.B.; Vernet, N. Retinoic Acid Receptor Alpha Is Essential in Postnatal Sertoli Cells but Not in Germ Cells. Cells 2022, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Pasceri, P.; Conlon, R.A.; Rossant, J.; Giguère, V. Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech. Dev. 1995, 53, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gely-Pernot, A.; Raverdeau, M.; Célébi, C.; Dennefeld, C.; Feret, B.; Klopfenstein, M.; Yoshida, S.; Ghyselinck, N.B.; Mark, M. Spermatogonia differentiation requires retinoic acid receptor gamma. Endocrinology 2012, 153, 438–449. [Google Scholar] [CrossRef]
- Vernet, N.; Dennefeld, C.; Klopfenstein, M.; Ruiz, A.; Bok, D.; Ghyselinck, N.B.; Mark, M. Retinoid X receptor beta (RXRB) expression in Sertoli cells controls cholesterol homeostasis and spermiation. Reproduction 2008, 136, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Brocard, J.; Kastner, P.; Chambon, P. Two novel RXR alpha isoforms from mouse testis. Biochem. Biophys. Res. Commun. 1996, 229, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Evans, E.; Onken, J.; Kent, T.; Mitchell, D.; Petkovich, M.; Griswold, M.D. CYP26 Enzymes Are Necessary Within the Postnatal Seminiferous Epithelium for Normal Murine Spermatogenesis. Biol. Reprod. 2015, 93, 19. [Google Scholar] [CrossRef] [PubMed]
- Bellutti, L.; Abby, E.; Tourpin, S.; Messiaen, S.; Moison, D.; Trautmann, E.; Guerquin, M.J.; Rouiller-Fabre, V.; Habert, R.; Livera, G. Divergent Roles of CYP26B1 and Endogenous Retinoic Acid in Mouse Fetal Gonads. Biomolecules 2019, 9, 536. [Google Scholar] [CrossRef]
- Parekh, P.A.; Garcia, T.X.; Waheeb, R.; Jain, V.; Gandhi, P.; Meistrich, M.L.; Shetty, G.; Hofmann, M.C. Undifferentiated spermatogonia regulate Cyp26b1 expression through NOTCH signaling and drive germ cell differentiation. FASEB J. 2019, 33, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; MacLean, G.; Cameron, D.; Clagett-Dame, M.; Petkovich, M. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS ONE 2009, 4, e7501. [Google Scholar] [CrossRef] [PubMed]
- MacLean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Small, C.; Griswold, M.D. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol. Reprod. 2010, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wolgemuth, D.J. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome Res. 2004, 105, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Z.; Garcia, E.; Glubrecht, D.D.; Poon, H.Y.; Mackey, J.R.; Godbout, R. CRABP1 is associated with a poor prognosis in breast cancer: Adding to the complexity of breast cancer cell response to retinoic acid. Mol. Cancer 2015, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.; Hogarth, C. Synchronizing spermatogenesis in the mouse. Biol. Reprod. 2022, 107, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Griswold, M.D. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 1987, 121, 432–434. [Google Scholar] [CrossRef]
- van Pelt, A.M.; de Rooij, D.G. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol. Reprod. 1990, 43, 363–367. [Google Scholar] [CrossRef]
- Busada, J.T.; Chappell, V.A.; Niedenberger, B.A.; Kaye, E.P.; Keiper, B.D.; Hogarth, C.A.; Geyer, C.B. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev. Biol. 2015, 397, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, L.; Hogarth, C.; Wei, G.; Griswold, M.D.; Tong, M.H. Retinoid signaling controls spermatogonial differentiation by regulating expression of replication-dependent core histone genes. Development 2016, 143, 1502–1511. [Google Scholar] [CrossRef]
- Serra, N.D.; Velte, E.K.; Niedenberger, B.A.; Kirsanov, O.; Geyer, C.B. Cell-autonomous requirement for mammalian target of rapamycin (Mtor) in spermatogonial proliferation and differentiation in the mousedagger. Biol. Reprod. 2017, 96, 816–828. [Google Scholar] [CrossRef]
- Agrimson, K.S.; Oatley, M.J.; Mitchell, D.; Oatley, J.M.; Griswold, M.D.; Hogarth, C.A. Retinoic acid deficiency leads to an increase in spermatogonial stem number in the neonatal mouse testis, but excess retinoic acid results in no change. Dev. Biol. 2017, 432, 229–236. [Google Scholar] [CrossRef]
- Paik, J.; Haenisch, M.; Muller, C.H.; Goldstein, A.S.; Arnold, S.; Isoherranen, N.; Brabb, T.; Treuting, P.M.; Amory, J.K. Inhibition of retinoic acid biosynthesis by the bisdichloroacetyldiamine WIN 18,446 markedly suppresses spermatogenesis and alters retinoid metabolism in mice. J. Biol. Chem. 2014, 289, 15104–15117. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Filipponi, D.; Gori, M.; Barrios, F.; Lolicato, F.; Grimaldi, P.; Rossi, P.; Jannini, E.A.; Geremia, R.; Dolci, S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008, 7, 3878–3888. [Google Scholar] [CrossRef]
- Zhou, Q.; Nie, R.; Li, Y.; Friel, P.; Mitchell, D.; Hess, R.A.; Small, C.; Griswold, M.D. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: An in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 2008, 79, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Li, H.Y.; Li, X.Y.; Yu, K.; Deng, S.L.; Tian, L. Protective effects of melatonin on male fertility preservation and reproductive system. Cryobiology 2020, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Feng, X.; Liao, S.; Wang, X.; Gan, H.; Wang, L.; Lin, X.; Han, C. BMP4 Cooperates with Retinoic Acid to Induce the Expression of Differentiation Markers in Cultured Mouse Spermatogonia. Stem Cells Int. 2016, 2016, 9536192. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, Y.; Deng, S.L.; Liu, Y.X.; Lian, Z.X.; Yu, K. Recent Research Advances in Mitosis during Mammalian Gametogenesis. Cells 2019, 8, 567. [Google Scholar] [CrossRef]
- Zhong, X.; Li, N.; Liang, S.; Huang, Q.; Coukos, G.; Zhang, L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J. Biol. Chem. 2010, 285, 41961–41971. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.H.; Mitchell, D.; Evanoff, R.; Griswold, M.D. Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biol. Reprod. 2011, 85, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.H.; Mitchell, D.A.; McGowan, S.D.; Evanoff, R.; Griswold, M.D. Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol. Reprod. 2012, 86, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, R.; Xie, K.; Hu, C.; Zhao, Z.; Wu, Y.; Zuo, Q.; Li, B.; Zhang, Y. Analysis of the Promoter Regions of gga-miR-31 and Its Regulation by RA and C-jun in Chicken. Int. J. Mol. Sci. 2023, 24, 12516. [Google Scholar] [CrossRef] [PubMed]
- Dolci, S.; Pellegrini, M.; Di Agostino, S.; Geremia, R.; Rossi, P. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J. Biol. Chem. 2001, 276, 40225–40233. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Griswold, M.D. MEIOSIN: A New Watchman of Meiotic Initiation in Mammalian Germ Cells. Dev. Cell 2020, 52, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, W.; Zhou, Y.; Ma, L.; Miao, Y.; Mu, N.; Ren, H.; Cheng, Z. RAD51C-RAD51D interplays with MSH5 and regulates crossover maturation in rice meiosis. New Phytol. 2023, 239, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, C.; Álvarez-González, L.; Marín-Gual, L.; Casillas, S.; Picón, J.; Yam, K.; Garcias-Ramis, M.M.; Vara, C.; Ventura, J.; Ruiz-Herrera, A. Multiple Genomic Landscapes of Recombination and Genomic Divergence in Wild Populations of House Mice-The Role of Chromosomal Fusions and Prdm9. Mol. Biol. Evol. 2024, 41, msae063. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Kim, S.W.; Koo, J.S. Sp1 up-regulates cAMP-response-element-binding protein expression during retinoic acid-induced mucous differentiation of normal human bronchial epithelial cells. Biochem. J. 2008, 410, 49–61. [Google Scholar] [CrossRef]
- Komada, M.; McLean, D.J.; Griswold, M.D.; Russell, L.D.; Soriano, P. E-MAP-115, encoding a microtubule-associated protein, is a retinoic acid-inducible gene required for spermatogenesis. Genes Dev. 2000, 14, 1332. [Google Scholar] [CrossRef]
- Li, M.; Yu, M.; Zhu, H.; Song, W.; Hua, J. The effects of Nanos2 on Boule and Stra8 in male germline stem cells (mGSCs). Mol. Biol. Rep. 2013, 40, 4383–4389. [Google Scholar] [CrossRef]
- Kirsanov, O.; Johnson, T.A.; Niedenberger, B.A.; Malachowski, T.N.; Hale, B.J.; Chen, Q.; Lackford, B.; Wang, J.; Singh, A.; Schindler, K.; et al. Retinoic acid is dispensable for meiotic initiation but required for spermiogenesis in the mammalian testis. Development 2023, 150, dev201638. [Google Scholar] [CrossRef]
- Manku, G.; Culty, M. Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells. Int. J. Mol. Sci. 2016, 17, 1486. [Google Scholar] [CrossRef] [PubMed]
- Saflund, M.; Ozata, D.M. The MYBL1/TCFL5 transcription network: Two collaborative factors with central role in male meiosis. Biochem. Soc. Trans. 2023, 51, 2163–2172. [Google Scholar] [CrossRef]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; de Rooij, D.G.; Page, D.C. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef] [PubMed]
- Don, J.; Nir, U.; Breitbart, H. DMRT1 at the border between mitosis. and meiosis. Asian J. Androl. 2011, 13, 189–190. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Dann, C.T.; Alvarado, A.L.; Molyneux, L.A.; Denard, B.S.; Garbers, D.L.; Porteus, M.H. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells 2008, 26, 2928–2937. [Google Scholar] [CrossRef]
- Aravindan, G.R.; Gopalakrishnan, K.; Ravindranath, N.; Moudgal, N.R. Effect of altering endogenous gonadotrophin concentrations on the kinetics of testicular germ cell turnover in the bonnet monkey (Macaca radiata). J. Endocrinol. 1993, 137, 485–495. [Google Scholar] [CrossRef]
- Simorangkir, D.R.; Ramaswamy, S.; Marshall, G.R.; Pohl, C.R.; Plant, T.M. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta). Hum. Reprod. 2009, 24, 1584–1595. [Google Scholar] [CrossRef]
- Amory, J.K.; Arnold, S.; Lardone, M.C.; Piottante, A.; Ebensperger, M.; Isoherranen, N.; Muller, C.H.; Walsh, T.; Castro, A. Levels of the retinoic acid synthesizing enzyme aldehyde dehydrogenase-1A2 are lower in testicular tissue from men with infertility. Fertil. Steril. 2014, 101, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Kim, K.H. Activity of retinoic acid receptor-alpha is directly regulated at its protein kinase A sites in response to follicle-stimulating hormone signaling. Endocrinology 2010, 151, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Ma, L.; Lin, X.; Zhang, D.; Li, Z.; Wu, Y.; Zheng, C.; Feng, X.; Liao, S.; et al. Retinoic Acid Is Sufficient for the In Vitro Induction of Mouse Spermatocytes. Stem Cell Rep. 2016, 7, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Chen, S.R.; Wang, Z.P.; Zhang, Y.; Tang, J.X.; Li, J.; Wang, X.X.; Cheng, J.M.; Jin, C.; Li, X.Y.; et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition. J. Pineal. Res. 2016, 60, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Zhang, Y.; Yu, K.; Wang, X.X.; Chen, S.R.; Han, D.P.; Cheng, C.Y.; Lian, Z.X.; Liu, Y.X. Melatonin up-regulates the expression of the GATA-4 transcription factor and increases testosterone secretion from Leydig cells through RORalpha signaling in an in vitro goat spermatogonial stem cell differentiation culture system. Oncotarget 2017, 8, 110592–110605. [Google Scholar] [CrossRef] [PubMed]
- Kosir, R.; Juvan, P.; Perse, M.; Budefeld, T.; Majdic, G.; Fink, M.; Sassone-Corsi, P.; Rozman, D. Novel insights into the downstream pathways and targets controlled by transcription factors CREM in the testis. PLoS ONE 2012, 7, e31798. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.Y.; Shen, J.L.; Li, Q.M.; Wang, Y.; Huang, X.Y.; Guo, T.Y.; Liu, H.W.; Lei, L.; Jin, L.H. pCREB is involved in neural induction of mouse embryonic stem cells by RA. Anat. Rec. 2008, 291, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Shiguro, K.I.; Matsuura, K.; Tani, N.; Takeda, N.; Usuki, S.; Yamane, M.; Sugimoto, M.; Fujimura, S.; Hosokawa, M.; Chuma, S.; et al. MEIOSIN Directs the Switch from Mitosis to Meiosis in Mammalian Germ Cells. Dev. Cell 2020, 52, 429–445.e10. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Evanoff, R.; Snyder, E.; Kent, T.; Mitchell, D.; Small, C.; Amory, J.K.; Griswold, M.D. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol. Reprod. 2011, 84, 957–965. [Google Scholar] [CrossRef]
- Amory, J.K.; Muller, C.H.; Shimshoni, J.A.; Isoherranen, N.; Paik, J.; Moreb, J.S.; Amory DWSr Evanoff, R.; Goldstein, A.S.; Griswold, M.D. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J. Androl. 2011, 32, 111–119. [Google Scholar] [CrossRef]
- Huang, H.F.; Marshall, G.R. Failure of spermatid release under various vitamin A states—An indication of delayed spermiation. Biol. Reprod. 1983, 28, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.B.; Vernet, N.; Dennefeld, C.; Giese, N.; Nau, H.; Chambon, P.; Viville, S.; Mark, M. Retinoids and spermatogenesis: Lessons from mutant mice lacking the plasma retinol binding protein. Dev. Dyn. 2006, 235, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Dennefeld, C.; Rochette-Egly, C.; Oulad-Abdelghani, M.; Chambon, P.; Ghyselinck, N.B.; Mark, M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006, 147, 96–110. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Sosa, F.; Gunewardena, S.; Crawford, P.A.; Zielen, A.C.; Orwig, K.E.; Wang, N. Transcriptional metabolic reprogramming implements meiotic fate decision in mouse testicular germ cells. Cell Rep. 2023, 42, 112749. [Google Scholar] [CrossRef]
- Santi, C.M.; Santos, T.; Hernández-Cruz, A.; Darszon, A. Properties of a novel pH-dependent Ca2+ permeation pathway present in male germ cells with possible roles in spermatogenesis and mature sperm function. J. Gen. Physiol. 1998, 112, 33–53. [Google Scholar] [CrossRef]
- Akmal, K.M.; Dufour, J.M.; Kim, K.H. Retinoic acid receptor alpha gene expression in the rat testis: Potential role during the prophase of meiosis and in the transition from round to elongating spermatids. Biol. Reprod. 1997, 56, 549–556. [Google Scholar] [CrossRef]
- Chung, S.S.; Sung, W.; Wang, X.; Wolgemuth, D.J. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev. Dyn. 2004, 230, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wang, X.; Roberts, S.S.; Griffey, S.M.; Reczek, P.R.; Wolgemuth, D.J. Oral administration of a retinoic Acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology 2011, 152, 2492–2502. [Google Scholar] [CrossRef]
- Wang, G.S.; Liang, A.; Dai, Y.B.; Wu, X.L.; Sun, F. Expression and localization of retinoid receptors in the testis of normal and infertile men. Mol. Reprod. Dev. 2020, 87, 978–985. [Google Scholar] [CrossRef]
- Kolon, T.F.; Herndon, C.D.; Baker, L.A.; Baskin, L.S.; Baxter, C.G.; Cheng, E.Y.; Diaz, M.; Lee, P.A.; Seashore, C.J.; Tasian, G.E.; et al. Evaluation and treatment of cryptorchidism: AUA guideline. J. Urol. 2014, 192, 337–345. [Google Scholar] [CrossRef]
- Yang, S.; Ping, P.; Ma, M.; Li, P.; Tian, R.; Yang, H.; Liu, Y.; Gong, Y.; Zhang, Z.; Li, Z.; et al. Generation of haploid spermatids with fertilization and development capacity from human spermatogonial stem cells of cryptorchid patients. Stem Cell Rep. 2014, 3, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Shen, L.; Chen, J.; Cao, X.; Zhou, Y.; Weng, H.; Long, C.; Zhang, D.; Tu, S.; Zhang, Y.; et al. New discovery of cryptorchidism: Decreased retinoic acid in testicle. Saudi. Pharm. J. 2016, 24, 279–285. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Y.; Zhang, B.L.; Wu, H.Y.; Jiang, W.Q.; Wang, S.T.; Han, D.P.; Liu, Y.X.; Lian, Z.X.; Deng, S.L. In-Vitro differentiation of early pig spermatogenic cells to haploid germ cells. Mol. Hum. Reprod. 2019, 25, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Errotta, I.; Perri, M.; Santoro, M.; Panza, S.; Caroleo, M.C.; Guido, C.; Mete, A.; Cione, E.; Aquila, S. Expression and Subcellular Localization of Retinoic Acid Receptor-alpha (RARalpha) in Healthy and Varicocele Human Spermatozoa: Its Possible Regulatory Role in Capacitation and Survival. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 374–381. [Google Scholar] [CrossRef]
- Malivindi, R.; Rago, V.; De Rose, D.; Gervasi, M.C.; Cione, E.; Russo, G.; Santoro, M.; Aquila, S. Influence of all-trans retinoic acid on sperm metabolism and oxidative stress: Its involvement in the physiopathology of varicocele-associated male infertility. J. Cell Physiol. 2018, 233, 9526–9537. [Google Scholar] [CrossRef]
- Amory, J.K.; Ostrowski, K.A.; Gannon, J.R.; Berkseth, K.; Stevison, F.; Isoherranen, N.; Muller, C.H.; Walsh, T. Isotretinoin administration improves sperm production in men with infertility from oligoasthenozoospermia: A pilot study. Andrology 2017, 5, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Esposito, T.; Cerbo, G.; Angelini, F.; Varriale, B.; Cardone, A. Impairment of spermatogenesis and enhancement of testicular germ cell apoptosis induced by exogenous all-trans-retinoic acid in adult lizard Podarcis sicula. J. Exp. Zool. Part A Comp. Exp. Biol. 2006, 305, 288–298. [Google Scholar] [CrossRef]
- Jørgensen, A.; Nielsen, J.E.; Perlman, S.; Lundvall, L.; Mitchell, R.T.; Juul, A.; Rajpert-De Meyts, E. Ex vivo culture of human fetal gonads: Manipulation of meiosis signalling by retinoic acid treatment disrupts testis development. Hum. Reprod. 2015, 30, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Pederzoli, F.; Locatelli, I.; Ippolito, S.; Longhi, E.; Zerbi, P.; Ferrari, M.; Brendolan, A.; Montorsi, F.; Drago, D.; et al. Impaired testicular signaling of vitamin A and vitamin K contributes to the aberrant composition of the extracellular matrix in idiopathic germ cell aplasia. Fertil. Steril. 2019, 111, 687–698. [Google Scholar] [CrossRef]
- Roth, M.Y.; Amory, J.K. Beyond the Condom: Frontiers in Male Contraception. Semin. Reprod. Med. 2016, 34, 183–190. [Google Scholar] [CrossRef]
- Hong, S.H.; Castro, G.; Wang, D.; Nofsinger, R.; Kane, M.; Folias, A.; Atkins, A.R.; Yu, R.T.; Napoli, J.L.; Sassone-Corsi, P.; et al. Targeting nuclear receptor corepressors for reversible male contraception. Proc. Natl. Acad. Sci. USA 2024, 121, e2320129121. [Google Scholar] [CrossRef]
- Wolbach, S.B.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Long, J.E.; Lee, M.S.; Blithe, D.L. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin. Chem. 2019, 65, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Nya-Ngatchou, J.J.; Amory, J.K. New approaches to male non-hormonal contraception. Contraception 2013, 87, 296–299. [Google Scholar] [CrossRef]
- Heller, C.G.; Moore, D.J.; Paulsen, C.A. Suppression of spermatogenesis and chronic toxicity in men by a new series of bis(dichloroacetyl) diamines. Toxicol. Appl. Pharmacol. 1961, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.Y.; Hong, K.H.; Mikles, D.C.; Georg, G.I.; Goldstein, A.S.; Amory, J.K.; Schönbrunn, E. Structural Basis of ALDH1A2 Inhibition by Irreversible and Reversible Small Molecule Inhibitors. ACS Chem. Biol. 2018, 13, 582–590. [Google Scholar] [CrossRef]
- Schulze, G.E.; Clay, R.J.; Mezza, L.E.; Bregman, C.L.; Buroker, R.A.; Frantz, J.D. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol. Sci. 2001, 59, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wang, X.; Wolgemuth, D.J. Prolonged Oral Administration of a Pan-Retinoic Acid Receptor Antagonist Inhibits Spermatogenesis in Mice With a Rapid Recovery and Changes in the Expression of Influx and Efflux Transporters. Endocrinology 2016, 157, 1601–1612. [Google Scholar] [CrossRef]
- American Chemical Society. A Non-Hormonal Pill Could Soon Expand Men’s Birth Control Options. Available online: https://www.acs.org/content/acs/en/pressroom/newsreleases/2022/march/non-hormonal-pill-could-soon-expand-mens-birth-control-options.html (accessed on 3 August 2022).
- Teng, M.; Duong, T.T.; Johnson, A.T.; Klein, E.S.; Wang, L.; Khalifa, B.; Chandraratna, R.A. Identification of highly potent retinoic acid receptor alpha-selective antagonists. J. Med. Chem. 1997, 40, 2445–2451. [Google Scholar] [CrossRef]
- Chung, S.S.; Cuellar, R.A.; Wang, X.; Reczek, P.R.; Georg, G.I.; Wolgemuth, D.J. Pharmacological activity of retinoic acid receptor alpha-selective antagonists in vitro and in vivo. ACS Med. Chem. Lett. 2013, 4, 446–450. [Google Scholar] [CrossRef]
- Kyzer, J.L.; Noman, M.A.A.; Cuellar, R.A.D.; Chung, S.S.W.; Maitra, S.; Naqvi, T.; Hawkinson, J.E.; Wolgemuth, D.J.; Georg, G.I. Investigation of selective retinoic acid receptor alpha antagonist ER-50891 and related analogs for male contraception. Arch. Pharm. 2023, 356, e2300031. [Google Scholar] [CrossRef] [PubMed]
- Al Noman, M.A.; Cuellar, R.A.D.; Kyzer, J.L.; Chung, S.S.W.; Cheryala, N.; Holth, T.A.D.; Maitra, S.; Naqvi, T.; Wong, H.L.; Schönbrunn, E.; et al. Strategies for developing retinoic acid receptor alpha-selective antagonists as novel agents for male contraception. Eur. J. Med. Chem. 2023, 261, 115821. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Deng, S.; Li, C.; Cao, J.; Wu, A.; Chen, M.; Ma, X.; Wu, S.; Lian, Z. The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells 2024, 13, 1092. https://doi.org/10.3390/cells13131092
Zhao Y, Deng S, Li C, Cao J, Wu A, Chen M, Ma X, Wu S, Lian Z. The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells. 2024; 13(13):1092. https://doi.org/10.3390/cells13131092
Chicago/Turabian StyleZhao, Yue, Shoulong Deng, Chongyang Li, Jingchao Cao, Aowu Wu, Mingming Chen, Xuehai Ma, Sen Wu, and Zhengxing Lian. 2024. "The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction" Cells 13, no. 13: 1092. https://doi.org/10.3390/cells13131092
APA StyleZhao, Y., Deng, S., Li, C., Cao, J., Wu, A., Chen, M., Ma, X., Wu, S., & Lian, Z. (2024). The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells, 13(13), 1092. https://doi.org/10.3390/cells13131092







