Fibrogenesis in Human Mucosa and Muscularis Precision-Cut Intestinal Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Intestine
2.2. Human Intestinal Mucosa and Muscularis Slices
2.3. Incubation of Mucosa and Muscularis Slices
2.4. ATP/Protein Determination
2.5. Histochemical and Immunohistochemical Staining
2.6. mRNA Expression Analysis
2.7. Pro-Collagen I Alpha I ELISA
2.8. Biomarker Assays
2.9. Statistical Analysis
3. Results
3.1. Human Intestinal Mucosa and Muscularis Slices
3.2. hPCIS Viability
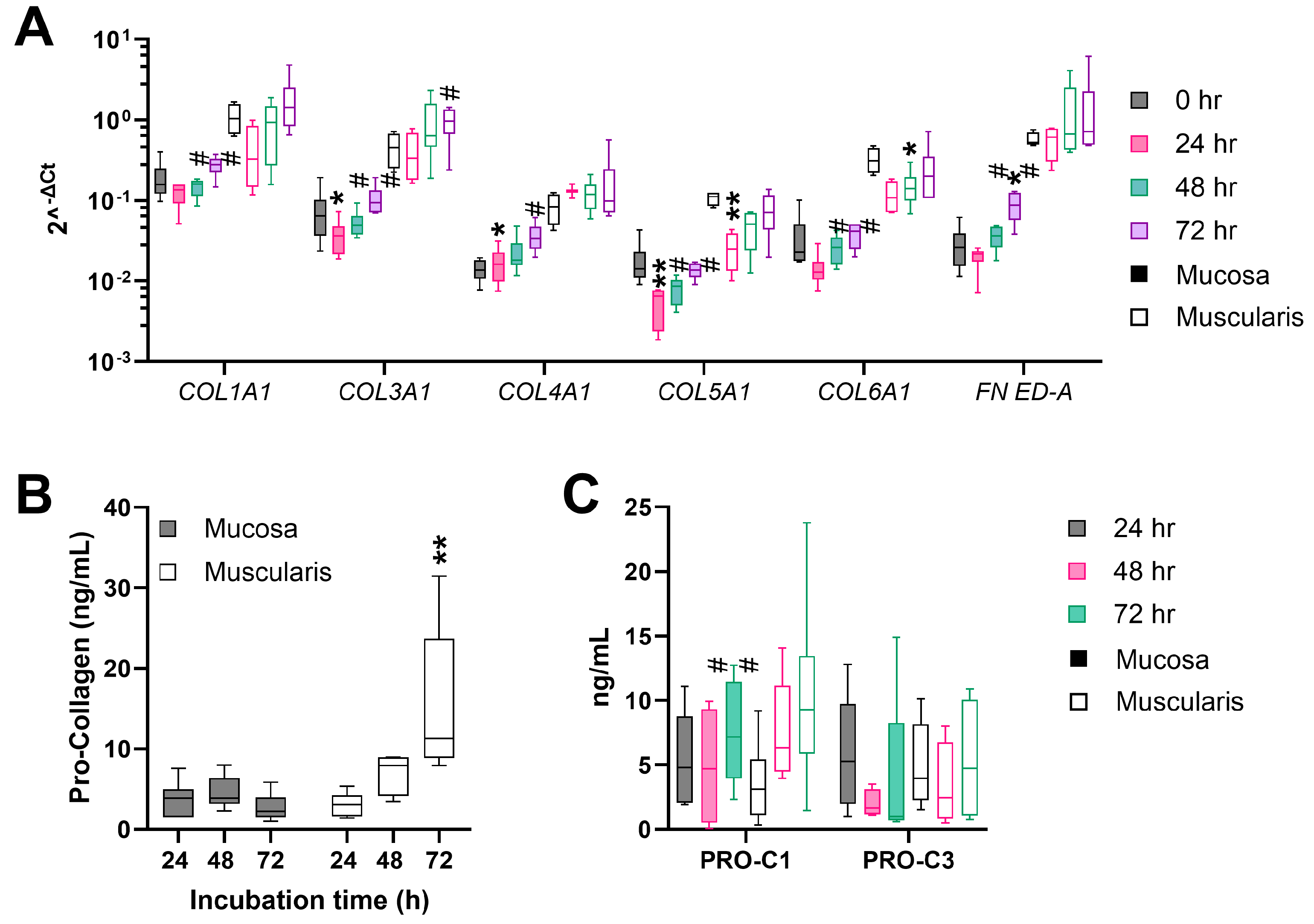
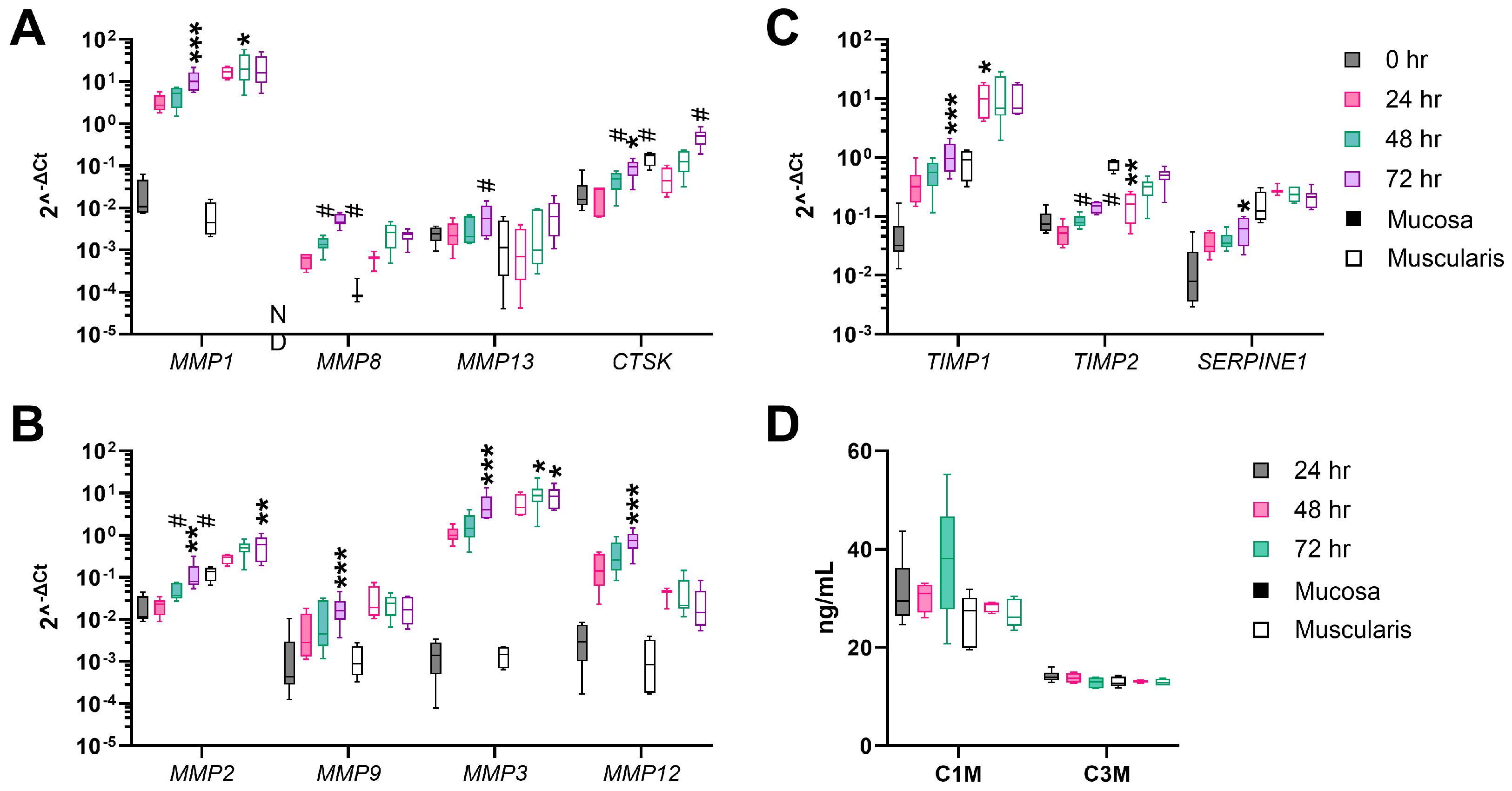
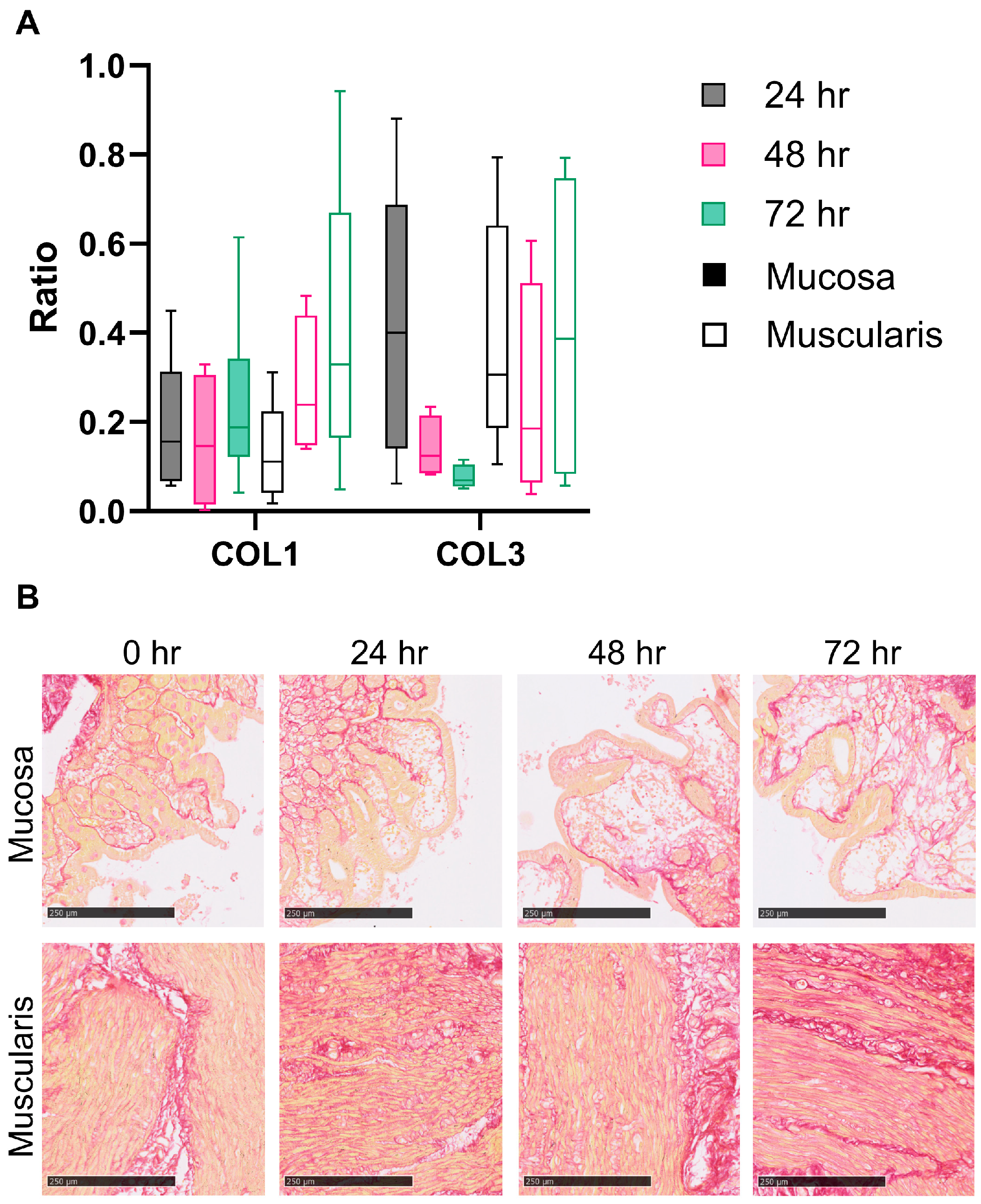
3.3. Fibrogenesis during the Incubation of hPCIS Is more Pronounced in Muscularis Compared to Mucosa
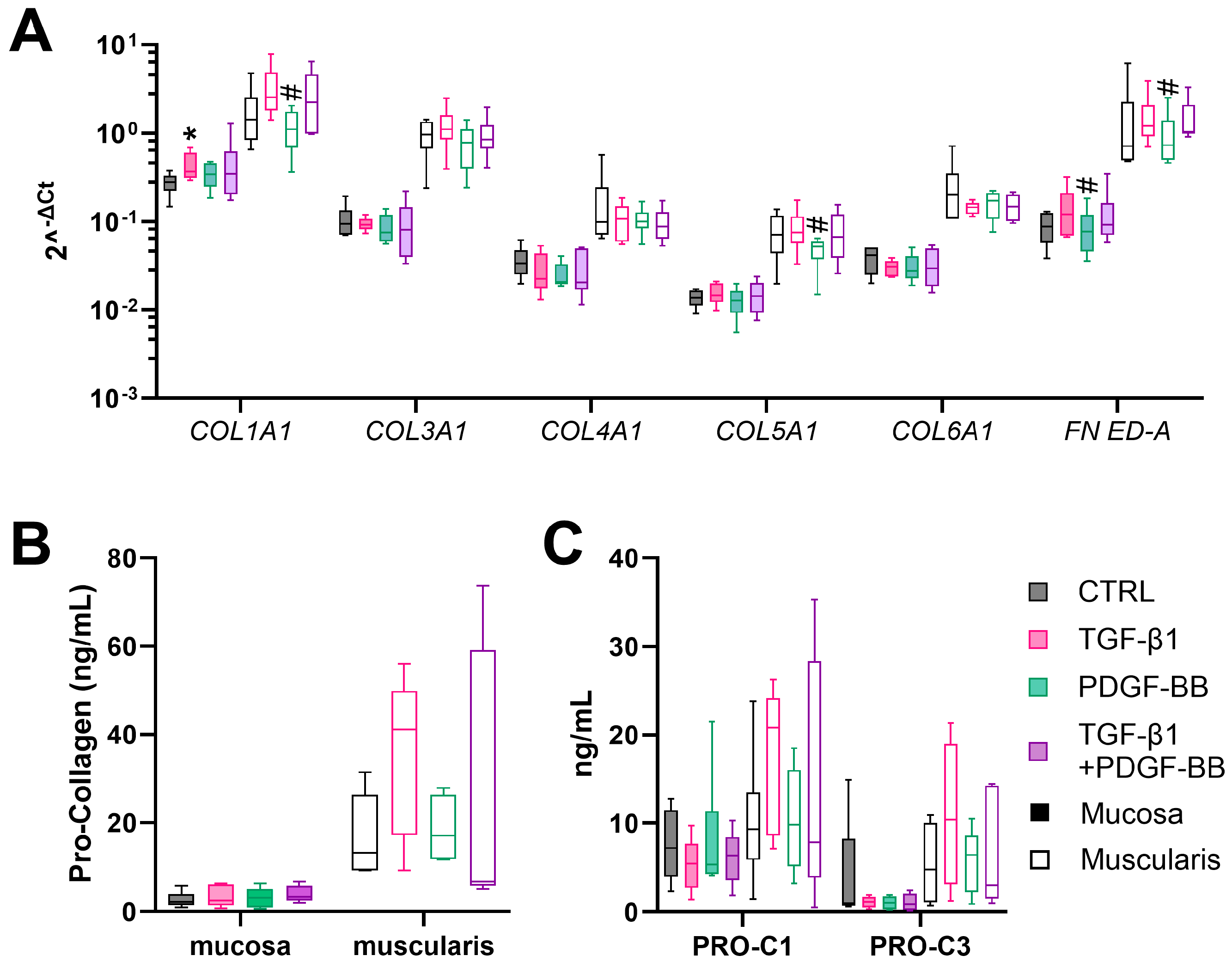
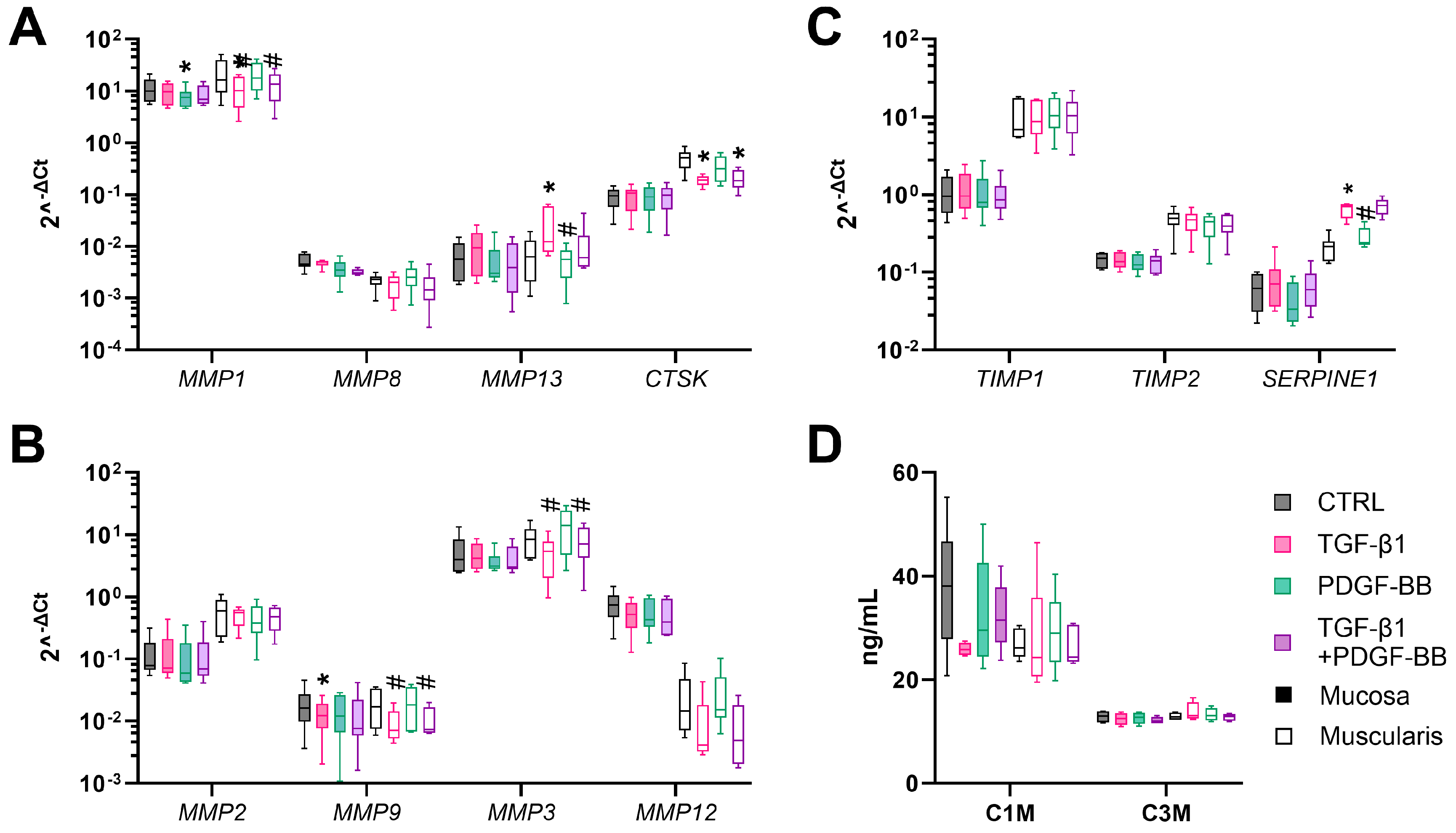
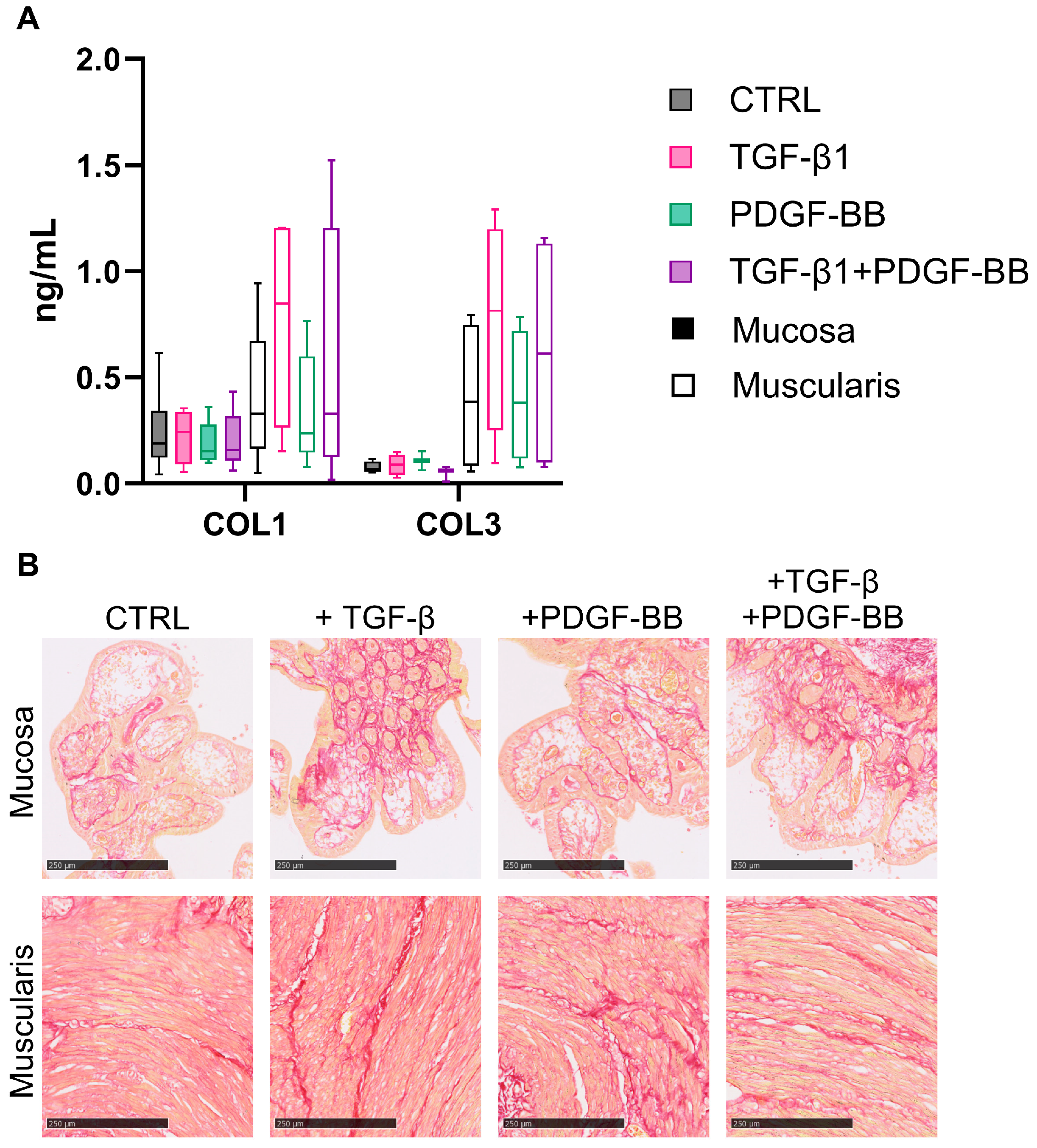
3.4. TGF-β1 Stimulates Fibrogenesis in Muscularis hPCIS but Not in Mucosal hPCIS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfredsson, J.; Wick, M.J. Mechanism of Fibrosis and Stricture Formation in Crohn’s Disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Latella, G.; Magro, F.; Yuksel, E.S.; Higgins, P.D.R.; Di Sabatino, A.; de Bruyn, J.R.; Rimola, J.; Brito, J.; Bettenworth, D.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Severi, C.; Sferra, R.; Scirocco, A.; Vetuschi, A.; Pallotta, N.; Pronio, A.; Caronna, R.; Di Rocco, G.; Gaudio, E.; Corazziari, E.; et al. Contribution of Intestinal Smooth Muscle to Crohn’s Disease Fibrogenesis. Eur. J. Histochem. 2014, 58, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Shelley-Fraser, G.; Borley, N.R.; Warren, B.F.; Shepherd, N.A. The Connective Tissue Changes of Crohn’s Disease. Histopathology 2012, 60, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth Muscle Hyperplasia/Hypertrophy Is the Most Prominent Histological Change in Crohn’s Fibrostenosing Bowel Strictures: A Semiquantitative Analysis by Using a Novel Histological Grading Scheme. J. Crohn’s Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef]
- Li, C.; Kuemmerle, J.F. The Fate of Myofibroblasts during the Development of Fibrosis in Crohn’s Disease. J. Dig. Dis. 2020, 21, 326–331. [Google Scholar] [CrossRef]
- Latella, G.; Sferra, R.; Speca, S.; Vetuschi, A.; Gaudio, E. Can We Prevent, Reduce or Reverse Intestinal Fibrosis in IBD? Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1283–1304. [Google Scholar]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- De Bruyn, J.R.; van den Brink, G.R.; Steenkamer, J.; Buskens, C.J.; Bemelman, W.A.; Meisner, S.; Muncan, V.; Te Velde, A.A.; D’haens, G.R.; Wildenberg, M.E.; et al. Fibrostenotic Phenotype of Myofibroblasts in Crohn’s Disease Is Dependent on Tissue Stiffness and Reversed by LOX Inhibition. J. Crohn’s Colitis 2018, 12, 849–859. [Google Scholar] [CrossRef]
- Mao, R.; Doyon, G.; Gordon, I.O.; Li, J.; Lin, S.; Wang, J.; Le, T.H.N.; Elias, M.; Kurada, S.; Southern, B.; et al. Activated Intestinal Muscle Cells Promote Preadipocyte Migration: A Novel Mechanism for Creeping Fat Formation in Crohn’s Disease. Gut 2022, 71, 55–67. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Cell-Matrix and Cell-Cell Contacts of Myofibroblasts: Role in Connective Tissue Remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Pozzer, D.; Invernizzi, R.W.; Blaauw, B.; Cantoni, O.; Zito, E. Ascorbic Acid Route to the Endoplasmic Reticulum: Function and Role in Disease. Antioxid. Redox Signal. 2021, 34, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, T.T.; Stappenbeck, T.S.; Rieder, F.; Rosen, M.J.; Colombel, J.-F.; Donowitz, M.; Towne, J.; Mazmanian, S.K.; Faith, J.J.; Hodin, R.A.; et al. Challenges in IBD Research: Preclinical Human IBD Mechanisms. Inflamm. Bowel Dis. 2019, 25, S5–S12. [Google Scholar] [CrossRef]
- Di Mola, F.F.; Friess, H.; Scheuren, A.; Di Sebastiano, P.; Graber, H.; Egger, B.; Zimmermann, A.; Korc, M.; Büchler, M.W. Transforming Growth Factor-Βs and Their Signaling Receptors Are Coexpressed in Crohn’s Disease. Ann. Surg. 1999, 229, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Jackson, C.L.; Pickard, K.M.; Buckley, M.; Rovedatti, L.; Leakey, N.A.B.; Picariello, L.; Cazzola, P.; Monteleone, G.; Tonelli, F.; et al. Transforming Growth Factor Signalling and Matrix Metalloproteinases in the Mucosa Overlying Crohn’s Disease Strictures. Gut 2009, 58, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Ohtani, H.; Nagai, T.; Funa, K.; Hiwatashi, N.O.; Shimosegawa; Nagura, H. Platelet-Derived Growth Factor and Its Receptors Are Expressed in Areas of Both Active Inflammation and Active Fibrosis in Inflammatory Bowel Disease. Tohoku J. Exp. Med. 2001, 195, 21–33. [Google Scholar] [CrossRef]
- de Graaf, I.A.M.; Olinga, P.; de Jager, M.H.; Merema, M.T.; de Kanter, R.; van de Kerkhof, E.G.; Groothuis, G.M.M. Preparation and Incubation of Precision-Cut Liver and Intestinal Slices for Application in Drug Metabolism and Toxicity Studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef]
- Biel, C.; Martinec, O.; Sibering, B.; van Summeren, K.; Wessels, A.M.A.; Touw, D.J.; de Jong, K.P.; de Meijer, V.E.; Faber, K.N.; ten Klooster, J.P.; et al. Extending the Viability of Human Precision-Cut Intestinal Slice Model for Drug Metabolism Studies. Arch. Toxicol. 2022, 96, 1815–1827. [Google Scholar] [CrossRef]
- Leeming, D.J.; He, Y.; Veidal, S.S.; Nguyen, Q.H.T.; Larsen, D.V.; Koizumi, M.; Segovia-Silvestre, T.; Zhang, C.; Zheng, Q.; Sun, S.; et al. A Novel Marker for Assessment of Liver Matrix Remodeling: An Enzyme-Linked Immunosorbent Assay (ELISA) Detecting a MMP Generated Type I Collagen Neo-Epitope (C1M). Biomarkers 2011, 16, 616–628. [Google Scholar] [CrossRef]
- Barascuk, N.; Veidal, S.S.; Larsen, L.; Larsen, D.V.; Larsen, M.R.; Wang, J.; Zheng, Q.; Xing, R.; Cao, Y.; Rasmussen, L.M.; et al. A Novel Assay for Extracellular Matrix Remodeling Associated with Liver Fibrosis: An Enzyme-Linked Immunosorbent Assay (ELISA) for a MMP-9 Proteolytically Revealed Neo-Epitope of Type III Collagen. Clin. Biochem. 2010, 43, 899–904. [Google Scholar] [CrossRef]
- Leeming, D.J.; Larsen, D.V.; Zhang, C.; Hi, Y.; Veidal, S.S.; Nielsen, R.H.; Henriksen, K.; Zheng, Q.; Barkholt, V.; Riis, B.J.; et al. Enzyme-Linked Immunosorbent Serum Assays (ELISAs) for Rat and Human N-Terminal pro-Peptide of Collagen Type I (PINP)—Assessment of Corresponding Epitopes. Clin. Biochem. 2010, 43, 1249–1256. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The Neo-Epitope Specific PRO-C3 ELISA Measures True Formation of Type III Collagen Associated with Liver and Muscle Parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar]
- Pham, B.T.; van Haaften, W.T.; Oosterhuis, D.; Nieken, J.; de Graaf, I.A.M.; Olinga, P. Precision-Cut Rat, Mouse, and Human Intestinal Slices as Novel Models for the Early-Onset of Intestinal Fibrosis. Physiol. Rep. 2015, 3, e12323. [Google Scholar] [CrossRef]
- Pucilowska, J.B.; McNaughton, K.K.; Mohapatra, N.K.; Hoyt, E.C.; Zimmermann, E.M.; Sartor, R.B.; Lund, P.K. IGF-I and Procollagen A1(I) Are Coexpressed in a Subset of Mesenchymal Cells in Active Crohn’s Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1307–G1322. [Google Scholar] [CrossRef]
- Bigaeva, E.; Gore, E.; Simon, E.; Zwick, M.; Oldenburger, A.; de Jong, K.P.; Hofker, H.S.; Schlepütz, M.; Nicklin, P.; Boersema, M.; et al. Transcriptomic Characterization of Culture-Associated Changes in Murine and Human Precision-Cut Tissue Slices. Arch. Toxicol. 2019, 93, 3549–3583. [Google Scholar] [CrossRef]
- Ortega, M.; Ríos-Navarro, C.; Gavara, J.; de Dios, E.; Perez-Solé, N.; Marcos-Garcés, V.; Ferrández-Izquierdo, A.; Bodí, V.; Ruiz-Saurí, A. Meta-Analysis of Extracellular Matrix Dynamics after Myocardial Infarction Using RNA-Sequencing Transcriptomic Database. Int. J. Mol. Sci. 2022, 23, 15615. [Google Scholar] [CrossRef]
- van Haaften, W.T.; Blokzijl, T.; Hofker, H.S.; Olinga, P.; Dijkstra, G.; Bank, R.A.; Boersema, M. Intestinal Stenosis in Crohn’s Disease Shows a Generalized Upregulation of Genes Involved in Collagen Metabolism and Recognition That Could Serve as Novel Anti-Fibrotic Drug Targets. Ther. Adv. Gastroenterol. 2020, 13, 175628482095257. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Rønnow, S.; Sand, J.M.; et al. The Good and the Bad Collagens of Fibrosis—Their Role in Signaling and Organ Function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef]
- Afratis, N.A.; Selman, M.; Pardo, A.; Sagi, I. Emerging Insights into the Role of Matrix Metalloproteases as Therapeutic Targets in Fibrosis. Matrix Biol. 2018, 68–69, 167–179. [Google Scholar] [CrossRef]
- Walraven, M.; Hinz, B. Therapeutic Approaches to Control Tissue Repair and Fibrosis: Extracellular Matrix as a Game Changer. Matrix Biol. 2018, 71–72, 205–224. [Google Scholar] [CrossRef]
- van Haaften, W.T.; Mortensen, J.H.; Karsdal, M.A.; Bay-Jensen, A.C.; Dijkstra, G.; Olinga, P. Misbalance in Type III Collagen Formation/Degradation as a Novel Serological Biomarker for Penetrating (Montreal B3) Crohn’s Disease. Aliment. Pharmacol. Ther. 2017, 46, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Alexdottir, M.S.; Otten, A.T.; Loveikyte, R.; Bay-Jensen, A.-C.; Pehrsson, M.; van Dullemen, H.M.; Visschedijk, M.C.; M Festen, E.A.; Weersma, R.K.; et al. Serological Biomarkers of Type I, III and IV Collagen Turnover Are Associated with the Presence and Future Progression of Stricturing and Penetrating Crohnʼs Disease. Aliment. Pharmacol. Ther. 2022, 56, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Latella, G.; Di Gregorio, J.; Flati, V.; Rieder, F.; Lawrance, I.C. Mechanisms of Initiation and Progression of Intestinal Fibrosis in IBD. Scand. J. Gastroenterol. 2015, 50, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Iswandana, R.; Irianti, M.I.; Oosterhuis, D.; Hofker, H.S.; Merema, M.T.; de Jager, M.H.; Mutsaers, H.A.M.; Olinga, P. Regional Differences in Human Intestinal Drug Metabolism. Drug Metab. Dispos. 2018, 46, 1879–1885. [Google Scholar] [CrossRef]
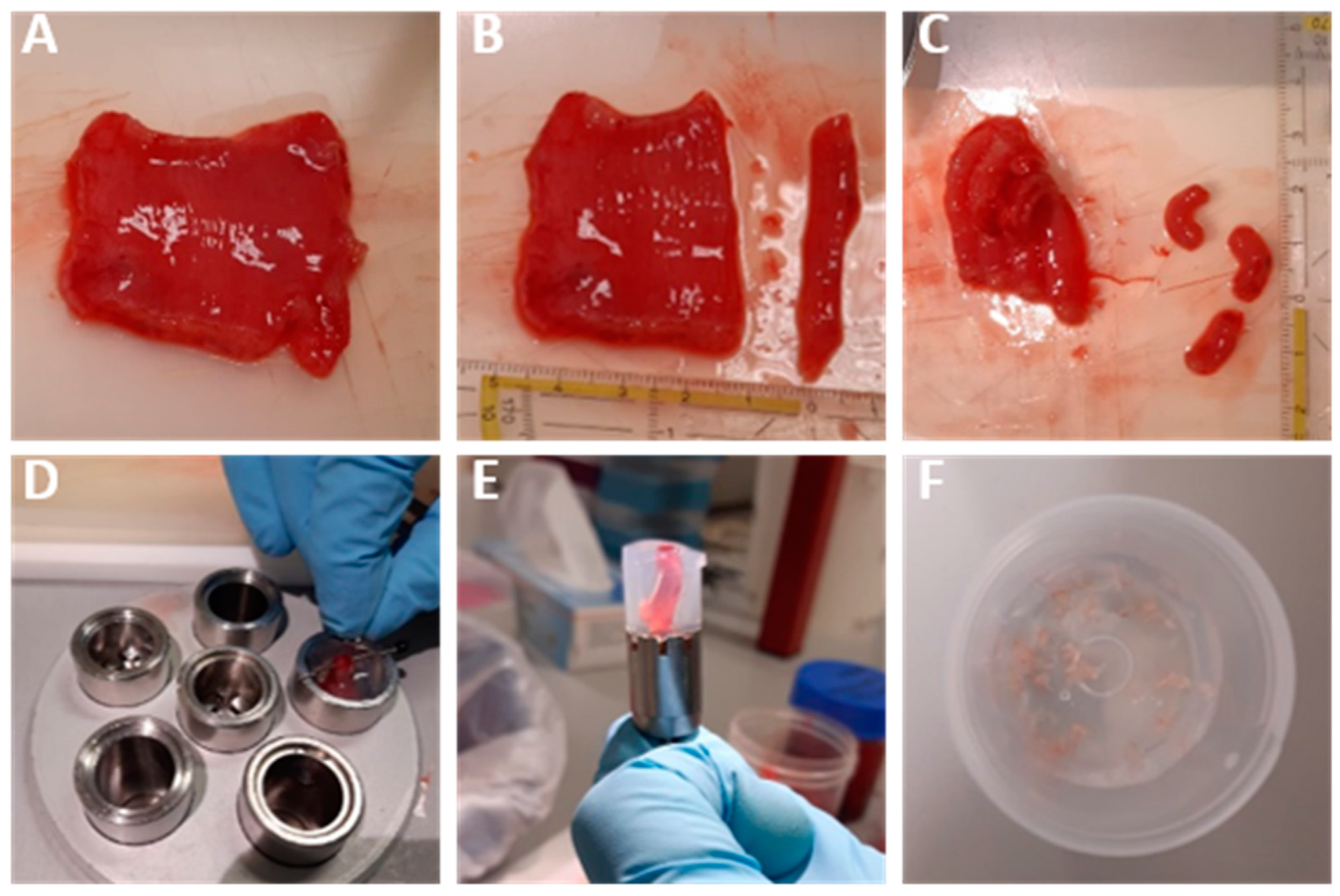

| Experiment # | Sex | Age |
|---|---|---|
| 1 | M | 74 |
| 2 | F | 68 |
| 3 | F | 71 |
| 4 | F | 63 |
| 5 | M | 57 |
| 6 | F | 73 |
| 7 | F | 68 |
| 8 | F | 51 |
| 9 | M | 46 |
| 10 | F | 68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biel, C.; Kastermans, A.; Heidema, J.; Pehrsson, M.; Henstra, C.; Mortensen, J.; Faber, K.N.; Olinga, P. Fibrogenesis in Human Mucosa and Muscularis Precision-Cut Intestinal Slices. Cells 2024, 13, 1084. https://doi.org/10.3390/cells13131084
Biel C, Kastermans A, Heidema J, Pehrsson M, Henstra C, Mortensen J, Faber KN, Olinga P. Fibrogenesis in Human Mucosa and Muscularis Precision-Cut Intestinal Slices. Cells. 2024; 13(13):1084. https://doi.org/10.3390/cells13131084
Chicago/Turabian StyleBiel, Carin, Anniek Kastermans, Janneke Heidema, Martin Pehrsson, Charlotte Henstra, Joachim Mortensen, Klaas Nico Faber, and Peter Olinga. 2024. "Fibrogenesis in Human Mucosa and Muscularis Precision-Cut Intestinal Slices" Cells 13, no. 13: 1084. https://doi.org/10.3390/cells13131084
APA StyleBiel, C., Kastermans, A., Heidema, J., Pehrsson, M., Henstra, C., Mortensen, J., Faber, K. N., & Olinga, P. (2024). Fibrogenesis in Human Mucosa and Muscularis Precision-Cut Intestinal Slices. Cells, 13(13), 1084. https://doi.org/10.3390/cells13131084








