Trisomy 21 with Maternally Inherited Balanced Translocation (15q;22q) in a Female Fetus: A Rare Case of Probable Interchromosomal Effect
Abstract
1. Introduction
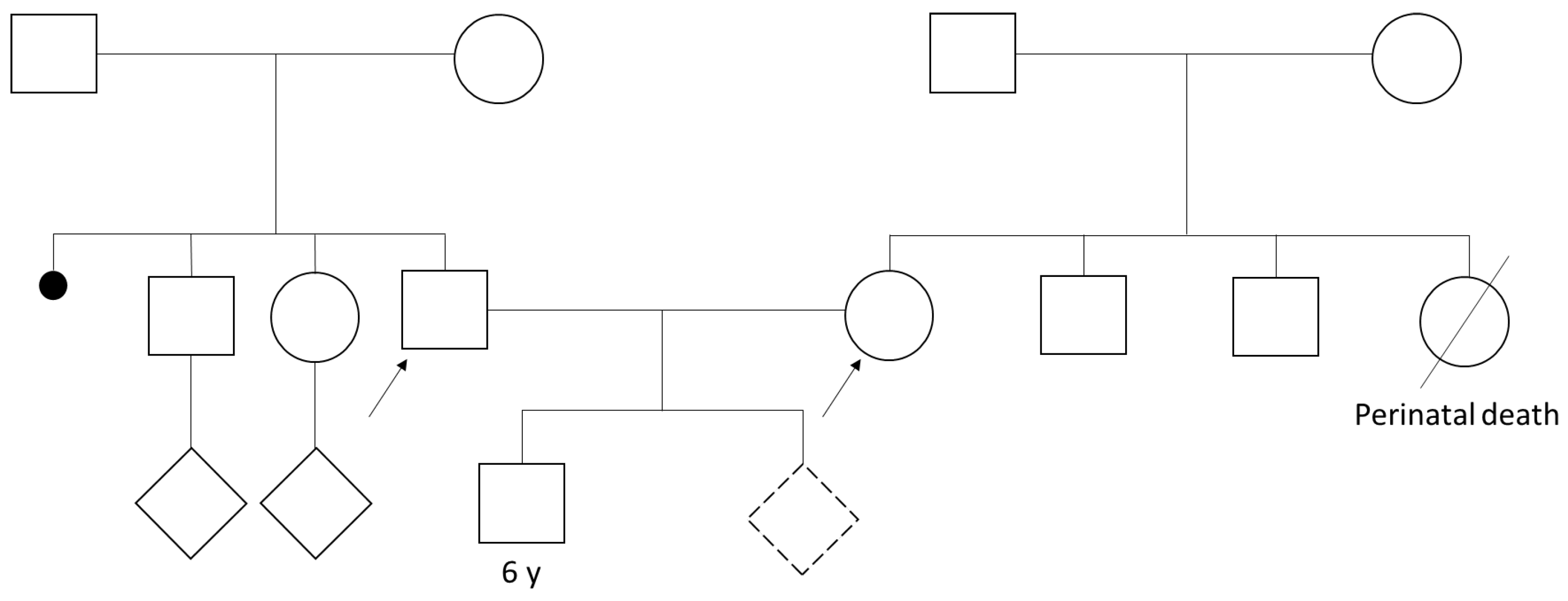
Clinical Description
2. Materials and Methods
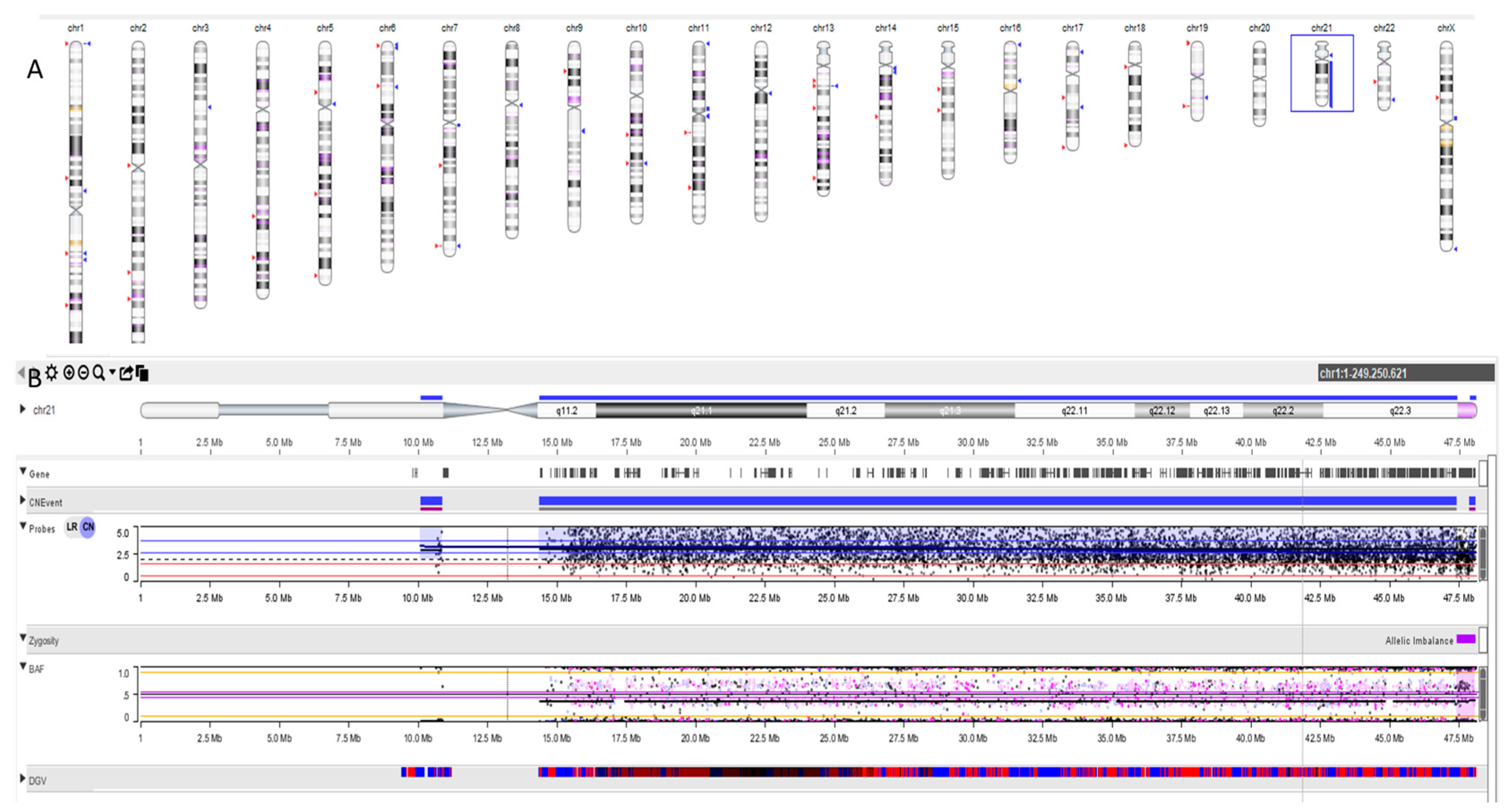
2.1. Molecular Cytogenetic Analysis
2.2. Ethical Consent
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochstenbach, R.; van Binsbergen, E.; Schuring-Blom, H.; Buijs, A.; Ploos van Amstel, H.K. A Survey of Undetected, Clinically Relevant Chromosome Abnormalities When Replacing Postnatal Karyotyping by Whole Genome Sequencing. Eur. J. Med. Genet. 2019, 62, 103543. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, R.; Heller, A.; Knox-DuBois, C.; McCaskill, C.; Berend, S.A.; Page, S.L.; Shaffer, L.G. Parental Origin and Timing of de Novo Robertsonian Translocation Formation. Am. J. Hum. Genet. 2002, 71, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Poot, M.; Hochstenbach, R. Prevalence and Phenotypic Impact of Robertsonian Translocations. Mol. Syndromol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Schoemaker, M.J.; Jones, M.E.; Higgins, C.D.; Wright, A.F.; United Kingdom Clinical Cytogenetics Group; Swerdlow, A.J. Mortality and Cancer Incidence in Carriers of Balanced Robertsonian Translocations: A National Cohort Study. Am. J. Epidemiol. 2019, 188, 500–508. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the Number of People with Down Syndrome in Europe. Eur. J. Hum. Genet. 2021, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Cuckle, H.S.; Wald, N.J.; Thompson, S.G. Estimating a Woman’s Risk of Having a Pregnancy Associated with Down’s Syndrome Using Her Age and Serum Alpha-Fetoprotein Level. Br. J. Obstet. Gynaecol. 1987, 94, 387–402. [Google Scholar] [CrossRef]
- Newberger, D.S. Down Syndrome: Prenatal Risk Assessment and Diagnosis. Am. Fam. Physician 2000, 62, 825–832, 837–838. [Google Scholar] [PubMed]
- Wald, N.J.; Cuckle, H.S.; Densem, J.W.; Nanchahal, K.; Royston, P.; Chard, T.; Haddow, J.E.; Knight, G.J.; Palomaki, G.E.; Canick, J.A. Maternal Serum Screening for Down’s Syndrome in Early Pregnancy. BMJ 1988, 297, 883–887. [Google Scholar] [CrossRef]
- Saller, D.N.; Canick, J.A. Maternal Serum Screening for Fetal Down Syndrome: Clinical Aspects. Clin. Obstet. Gynecol. 1996, 39, 783–792. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Knight, G.J.; McCarthy, J.E.; Haddow, J.E.; Donhowe, J.M. Maternal Serum Screening for Down Syndrome in the United States: A 1995 Survey. Am. J. Obstet. Gynecol. 1997, 176, 1046–1051. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Knight, G.J.; Williams, J.; Pulkkinen, A.; Canick, J.A.; Saller, D.N.; Bowers, G.B. Prenatal Screening for Down’s Syndrome with Use of Maternal Serum Markers. N. Engl. J. Med. 1992, 327, 588–593. [Google Scholar] [CrossRef]
- Benn, P.A.; Borgida, A.; Horne, D.; Briganti, S.; Collins, R.; Rodis, J.F. Down Syndrome and Neural Tube Defect Screening: The Value of Using Gestational Age by Ultrasonography. Am. J. Obstet. Gynecol. 1997, 176, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Chitty, L.S. Antenatal Screening for Aneuploidy. Curr. Opin. Obstet. Gynecol. 1998, 10, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of Fetal DNA in Maternal Plasma and Serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Alberry, M.; Maddocks, D.; Jones, M.; Abdel Hadi, M.; Abdel-Fattah, S.; Avent, N.; Soothill, P.W. Free Fetal DNA in Maternal Plasma in Anembryonic Pregnancies: Confirmation That the Origin Is the Trophoblast. Prenat. Diagn. 2007, 27, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Flori, E.; Doray, B.; Gautier, E.; Kohler, M.; Ernault, P.; Flori, J.; Costa, J.M. Circulating Cell-Free Fetal DNA in Maternal Serum Appears to Originate from Cyto- and Syncytio-Trophoblastic Cells. Case Report. Hum. Reprod. 2004, 19, 723–724. [Google Scholar] [CrossRef]
- Lo, Y.M.; Zhang, J.; Leung, T.N.; Lau, T.K.; Chang, A.M.; Hjelm, N.M. Rapid Clearance of Fetal DNA from Maternal Plasma. Am. J. Hum. Genet. 1999, 64, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, R.; Elferink, M.G.; van Zon, P.H.A.; Lichtenbelt, K.D.; van Harssel, J.; Schuring-Blom, H.; Page-Christiaens, G.C.M.L. Discordant NIPT Result in a Viable Trisomy-21 Pregnancy Due to Prolonged Contribution to CfDNA by a Demised Trisomy-14 Cotwin. Clin. Case Rep. 2018, 6, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Benn, P.; Cuckle, H. Overview of Noninvasive Prenatal Testing (NIPT) for the Detection of Fetal Chromosome Abnormalities; Differences in Laboratory Methods and Scope of Testing. Clin. Obstet. Gynecol. 2023, 66, 536–556. [Google Scholar] [CrossRef]
- Chitty, L.S.; Hudgins, L.; Norton, M.E. Current Controversies in Prenatal Diagnosis 2: Cell-Free DNA Prenatal Screening Should Be Used to Identify All Chromosome Abnormalities. Prenat. Diagn. 2018, 38, 160–165. [Google Scholar] [CrossRef]
- Dungan, J.S.; Klugman, S.; Darilek, S.; Malinowski, J.; Akkari, Y.M.N.; Monaghan, K.G.; Erwin, A.; Best, R.G.; ACMG Board of Directors. Noninvasive Prenatal Screening (NIPS) for Fetal Chromosome Abnormalities in a General-Risk Population: An Evidence-Based Clinical Guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100336. [Google Scholar] [CrossRef]
- Morris, J.K.; Mutton, D.E.; Alberman, E. Recurrences of Free Trisomy 21: Analysis of Data from the National Down Syndrome Cytogenetic Register. Prenat. Diagn. 2005, 25, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Stene, J.; Stene, E.; Mikkelsen, M. Risk for Chromosome Abnormality at Amniocentesis Following a Child with a Non-Inherited Chromosome Aberration. A European Collaborative Study on Prenatal Diagnoses 1981. Prenat. Diagn. 1984, 4, 81–95. [Google Scholar] [CrossRef]
- Gardner, R.J.M.; Sutherland, G.R. Chromosome Abnormalities and Genetic Counseling, 3rd ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Durban, M.; Benet, J.; Boada, M.; Fernández, E.; Calafell, J.M.; Lailla, J.M.; Sánchez-García, J.F.; Pujol, A.; Egozcue, J.; Navarro, J. PGD in Female Carriers of Balanced Robertsonian and Reciprocal Translocations by First Polar Body Analysis. Hum. Reprod. Update 2001, 7, 591–602. [Google Scholar] [CrossRef]
- Ogur, G.; Van Assche, E.; Vegetti, W.; Verheyen, G.; Tournaye, H.; Bonduelle, M.; Van Steirteghem, A.; Liebaers, I. Chromosomal Segregation in Spermatozoa of 14 Robertsonian Translocation Carriers. Mol. Hum. Reprod. 2006, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tamaren, J.; Spuhler, K.; Sujansky, E. Risk of Down Syndrome among Second- and Third-Degree Relatives of a Proband with Trisomy 21. Am. J. Med. Genet. 1983, 15, 393–403. [Google Scholar] [CrossRef]
- Papavassiliou, P.; Charalsawadi, C.; Rafferty, K.; Jackson-Cook, C. Mosaicism for Trisomy 21: A Review. Am. J. Med. Genet. A 2015, 167A, 26–39. [Google Scholar] [CrossRef]
- Mutton, D.; Alberman, E.; Hook, E.B. Cytogenetic and Epidemiological Findings in Down Syndrome, England and Wales 1989 to 1993. National Down Syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J. Med. Genet. 1996, 33, 387–394. [Google Scholar] [CrossRef] [PubMed]
- German, J. Bloom Syndrome: A Mendelian Prototype of Somatic Mutational Disease. Medicine 1993, 72, 393–406. [Google Scholar] [CrossRef]
- Anton, E.; Vidal, F.; Blanco, J. Interchromosomal Effect Analyses by Sperm FISH: Incidence and Distribution among Reorganization Carriers. Syst. Biol. Reprod. Med. 2011, 57, 268–278. [Google Scholar] [CrossRef]
- Lejeune, J. Autosomal Disorders. Pediatrics 1963, 32, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, N.V. Increased Risk of Tisomy 21 in Offspring of Carriers of Balanced Non-Contributing Autosomal Rearrangements Is Not Explained by Interchromosomal Effect. Genetika 2013, 49, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Murray, A.; Sampson, J. Harper’s Practical Genetic Counselling, 7th ed.; Harper, P.S., Ed.; Preceded by Practical genetic counselling, Eighth edition; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780367371944. [Google Scholar]
- Kleinfinger, P.; Lohmann, L.; Luscan, A.; Trost, D.; Bidat, L.; Debarge, V.; Castaigne, V.; Senat, M.-V.; Brechard, M.-P.; Guilbaud, L.; et al. Strategy for Use of Genome-Wide Non-Invasive Prenatal Testing for Rare Autosomal Aneuploidies and Unbalanced Structural Chromosomal Anomalies. J. Clin. Med. 2020, 9, 2466. [Google Scholar] [CrossRef]
- Pertile, M.D.; Flowers, N.; Vavrek, D.; Andrews, D.; Kalista, T.; Craig, A.; Deciu, C.; Duenwald, S.; Meier, K.; Bhatt, S. Performance of a Paired-End Sequencing-Based Noninvasive Prenatal Screening Test in the Detection of Genome-Wide Fetal Chromosomal Anomalies. Clin. Chem. 2021, 67, 1210–1219. [Google Scholar] [CrossRef]
- Mann, K.; Fox, S.P.; Abbs, S.J.; Yau, S.C.; Scriven, P.N.; Docherty, Z.; Ogilvie, C.M. Development and Implementation of a New Rapid Aneuploidy Diagnostic Service within the UK National Health Service and Implications for the Future of Prenatal Diagnosis. Lancet 2001, 358, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.F.; McKim, K.S. Meiotic Checkpoints and the Interchromosomal Effect on Crossing over in Drosophila Females. Fly 2011, 5, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.F.; McKim, K.S. Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing over during Drosophila Meiosis. PLoS Genet. 2010, 6, e1001059. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Bonet, M.; Benet, J.; Sun, F.; Navarro, J.; Abad, C.; Liehr, T.; Starke, H.; Greene, C.; Ko, E.; Martin, R.H. Meiotic Studies in Two Human Reciprocal Translocations and Their Association with Spermatogenic Failure. Hum. Reprod. 2005, 20, 683–688. [Google Scholar] [CrossRef]
- Cheng, E.Y.; Chen, Y.J.; Disteche, C.M.; Gartler, S.M. Analysis of a Paracentric Inversion in Human Oocytes: Nonhomologous Pairing in Pachytene. Hum. Genet. 1999, 105, 191–196. [Google Scholar] [CrossRef]
- Guichaoua, M.R.; Quack, B.; Speed, R.M.; Noel, B.; Chandley, A.C.; Luciani, J.M. Infertility in Human Males with Autosomal Translocations: Meiotic Study of a 14;22 Robertsonian Translocation. Hum. Genet. 1990, 86, 162–166. [Google Scholar] [CrossRef]
- Codina-Pascual, M.; Navarro, J.; Oliver-Bonet, M.; Kraus, J.; Speicher, M.R.; Arango, O.; Egozcue, J.; Benet, J. Behaviour of Human Heterochromatic Regions during the Synapsis of Homologous Chromosomes. Hum. Reprod. 2006, 21, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Cremer, M.; Dietzel, S.; Müller, S.; Solovei, I.; Fakan, S. Chromosome Territories—A Functional Nuclear Landscape. Curr. Opin. Cell Biol. 2006, 18, 307–316. [Google Scholar] [CrossRef]
- Branco, M.R.; Pombo, A. Intermingling of Chromosome Territories in Interphase Suggests Role in Translocations and Transcription-Dependent Associations. PLoS Biol. 2006, 4, e138. [Google Scholar] [CrossRef]
- Gabriel-Robez, O.; Ratomponirina, C.; Dutrillaux, B.; Carré-Pigeon, F.; Rumpler, Y. Meiotic Association between the XY Chromosomes and the Autosomal Quadrivalent of a Reciprocal Translocation in Two Infertile Men, 46,XY,t(19;22) and 46,XY,t(17;21). Cytogenet. Genome Res. 1986, 43, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, R.; Löhrs, U.; Wolff, H.H.; Schwinger, E. Two Different XY-Quadrivalent Associations and Impairment of Fertility in Men. Cytogenet. Cell Genet. 1987, 45, 222–230. [Google Scholar] [CrossRef]
- De Braekeleer, M.; Dao, T.N. Cytogenetic Studies in Male Infertility: A Review. Hum. Reprod. 1991, 6, 245–250. [Google Scholar] [CrossRef]
- Chandley, A.C.; Speed, R.M.; McBeath, S.; Hargreave, T.B. A Human 9;20 Reciprocal Translocation Associated with Male Infertility Analyzed at Prophase and Metaphase I of Meiosis. Cytogenet. Cell Genet. 1986, 41, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Vidal, F.; Benet, J.; Templado, C.; Marina, S.; Egozcue, J. XY-Trivalent Association and Synaptic Anomalies in a Male Carrier of a Robertsonian t(13;14) Translocation. Hum. Reprod. 1991, 6, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Alfarawati, S.; Fragouli, E.; Colls, P.; Wells, D. Embryos of Robertsonian Translocation Carriers Exhibit a Mitotic Interchromosomal Effect That Enhances Genetic Instability during Early Development. PLoS Genet. 2012, 8, e1003025. [Google Scholar] [CrossRef]
- Snijders, R.J.; Sundberg, K.; Holzgreve, W.; Henry, G.; Nicolaides, K.H. Maternal Age- and Gestation-Specific Risk for Trisomy 21. Ultrasound Obstet. Gynecol. 1999, 13, 167–170. [Google Scholar] [CrossRef]


| Traditional Screening | NIPT |
|---|---|
| First and/or second trimester | Available from the 11th week of gestation |
| High DR when both first and second-trimester tests are performed and combined | Laboratory test performed only once |
| Ultrasound is a component of many algorithms | No ultrasound required |
| Includes screening for neural tube defects (second trimester) | No screening for neural tube defects |
| Rare indeterminate result | Approximately 1% inconclusive result |
| Decreased DR in twin pregnancies | DR in twin pregnancies equivalent to single pregnancies |
| Non-specific screening for CNVs | Targeted or genome-wide screening for CNVs available |
| Analyte | CBC of the Father | CBC of the Mother | Normal Range |
|---|---|---|---|
| RBC | 4.72 × 1012/L | 3.56 × 1012/L | 4.5–5.7 × 1012/L |
| WBC | 5.5 × 109/L | 5.2 × 109/L | 4.0–10.0 × 109/L |
| HGB | 140 g/L | 111 g/L | 133–167 g/L |
| HCT | 0.41 | 0.33 | 0.35–0.53 |
| MCV | 87 fL | 94 fL | 77–98 fL |
| MCH | 29 pg | 31 pg | 26–33 pg |
| MCHC | 33 g/L | 33 g/L | 330–370 g/L |
| RDW | 14% | 13% | 10.3–15.3% |
| Hb A1 | 97.2% | 97.5% | 96.5–98.5% |
| Hb A2 * | 2.8% | 2.5% | 2.0–3.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Falco, A.; Gambale, A.; Pinelli, M.; Suero, T.; De Falco, L.; Iolascon, A.; Martone, S. Trisomy 21 with Maternally Inherited Balanced Translocation (15q;22q) in a Female Fetus: A Rare Case of Probable Interchromosomal Effect. Cells 2024, 13, 1078. https://doi.org/10.3390/cells13131078
De Falco A, Gambale A, Pinelli M, Suero T, De Falco L, Iolascon A, Martone S. Trisomy 21 with Maternally Inherited Balanced Translocation (15q;22q) in a Female Fetus: A Rare Case of Probable Interchromosomal Effect. Cells. 2024; 13(13):1078. https://doi.org/10.3390/cells13131078
Chicago/Turabian StyleDe Falco, Alessandro, Antonella Gambale, Michele Pinelli, Teresa Suero, Luigia De Falco, Achille Iolascon, and Stefania Martone. 2024. "Trisomy 21 with Maternally Inherited Balanced Translocation (15q;22q) in a Female Fetus: A Rare Case of Probable Interchromosomal Effect" Cells 13, no. 13: 1078. https://doi.org/10.3390/cells13131078
APA StyleDe Falco, A., Gambale, A., Pinelli, M., Suero, T., De Falco, L., Iolascon, A., & Martone, S. (2024). Trisomy 21 with Maternally Inherited Balanced Translocation (15q;22q) in a Female Fetus: A Rare Case of Probable Interchromosomal Effect. Cells, 13(13), 1078. https://doi.org/10.3390/cells13131078







