Dysfunction of Small-Conductance Ca2+-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Neuropathic Pain Model
2.3. Behavior
2.3.1. Mechanosensitivity
2.3.2. Vocalizations
2.4. Electrophysiology
2.4.1. Brain Slice Preparation
2.4.2. Patch-Clamp Recording of CeA Neurons in the Right Amygdala
2.5. Drugs and Drug Application
2.5.1. Behavioral Experiments
2.5.2. Brain Slice Experiments
2.6. Histology
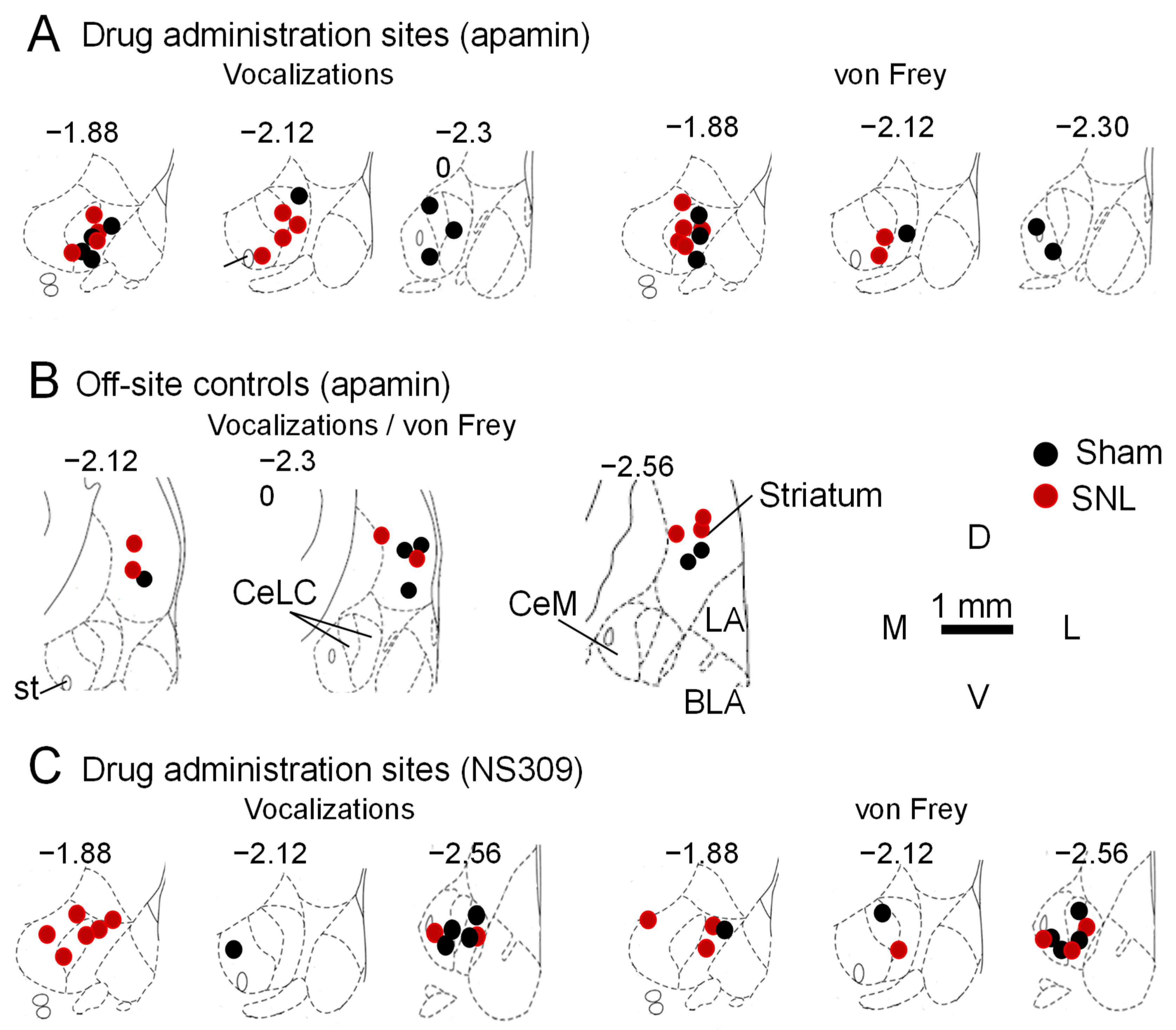
2.6.1. Verification of Microdialysis Probe Locations
2.6.2. Immunohistochemistry for SK2 Protein Detection in the Right CeLC
2.7. Molecular Biology Experiments
2.7.1. Tissue Preparation
2.7.2. Western Blotting
2.7.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.7.4. Bisulfite Conversion of DNA and Methylation-Specific PCR
2.7.5. Bisulfite Sequencing Specific PCR and Cloning
2.7.6. Bisulfite DNA Sequencing and Analysis
2.8. Statistical Analysis and Scientific Rigor
3. Results
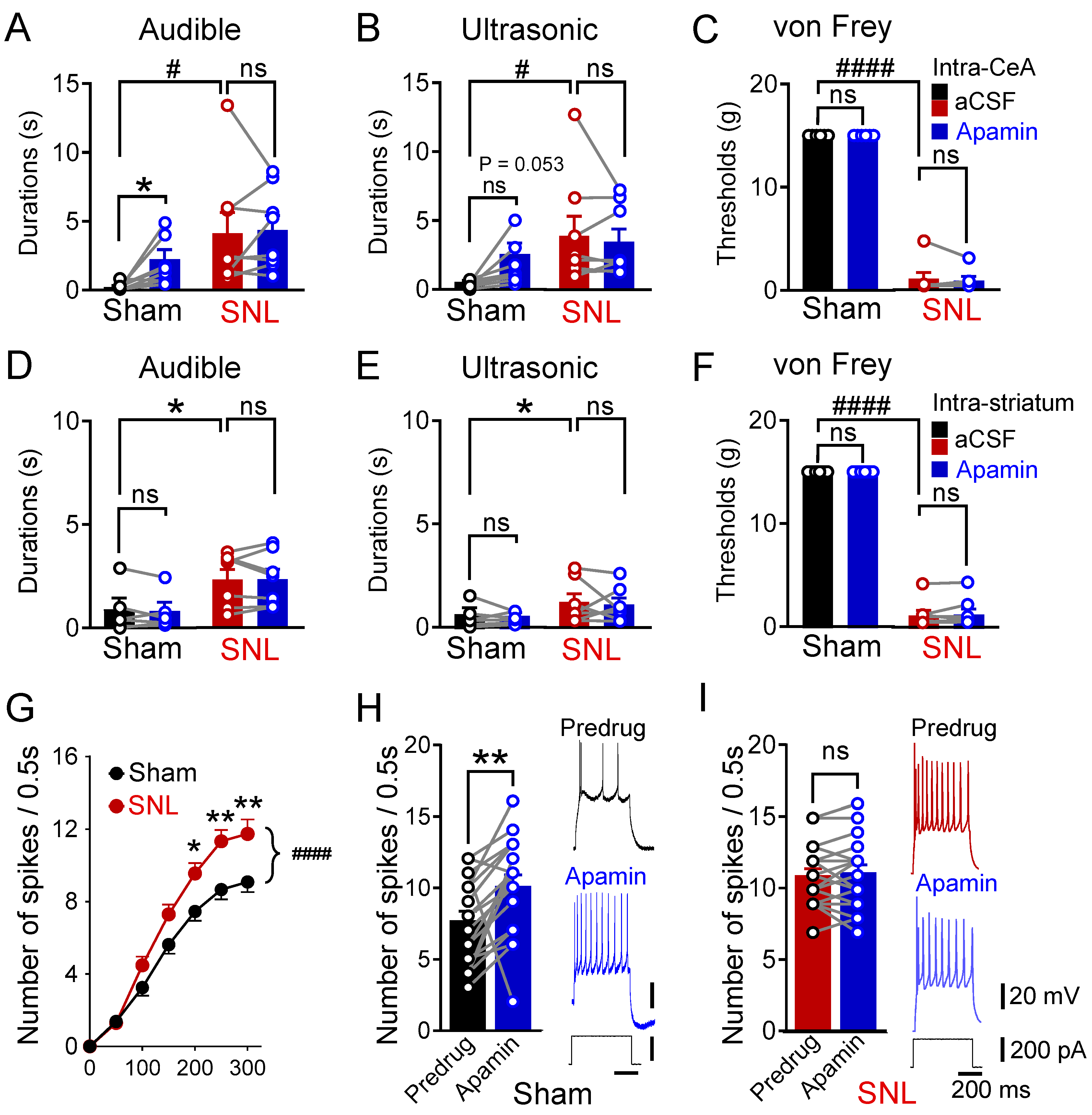
3.1. Loss of SK Channel-Mediated Control of Pain Behaviors and Neuronal Excitability in Neuropathic Pain
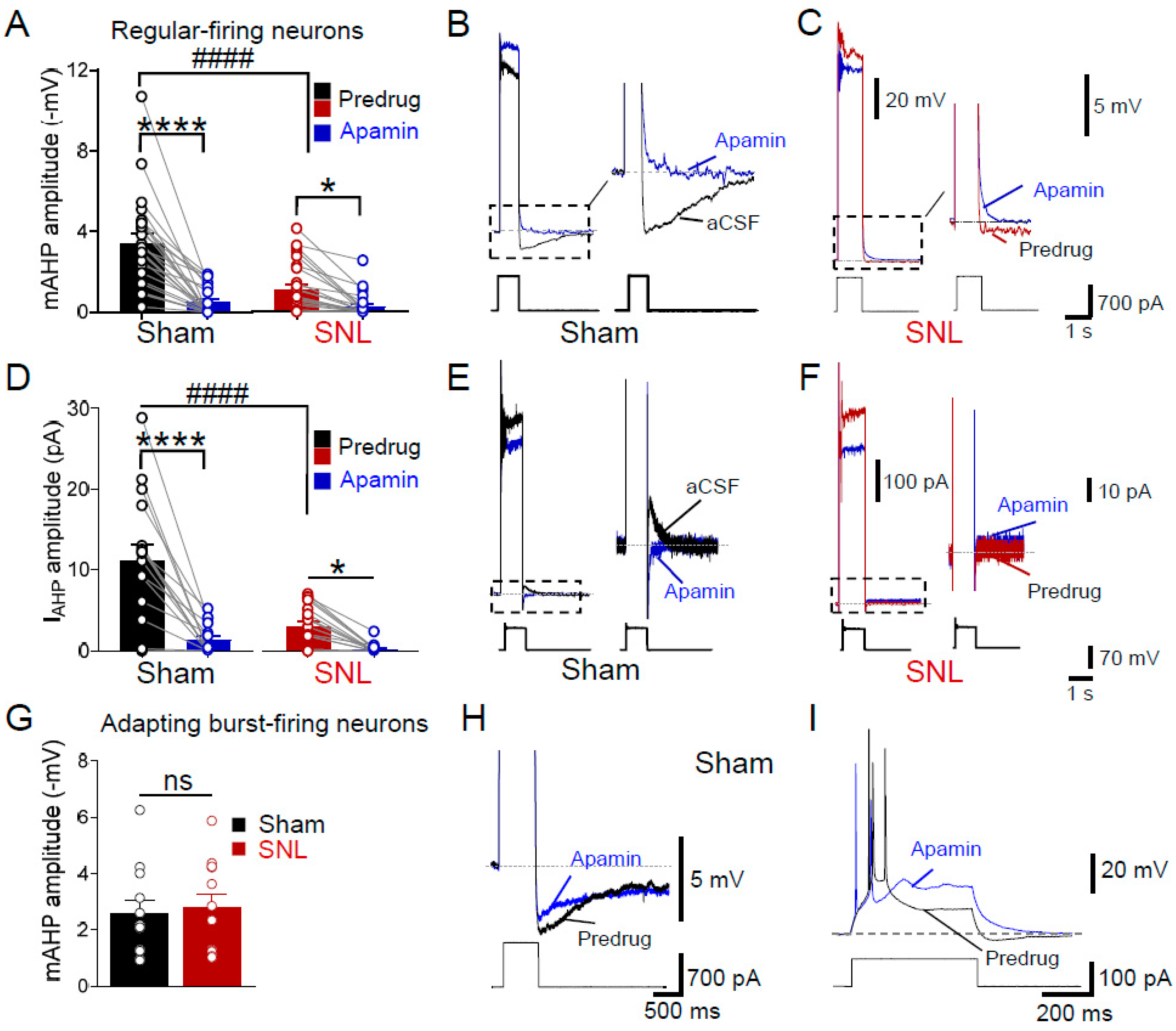
3.2. Loss of SK Channel Function (mAHP) in Regular-Firing Amygdala Neurons in Neuropathic Pain
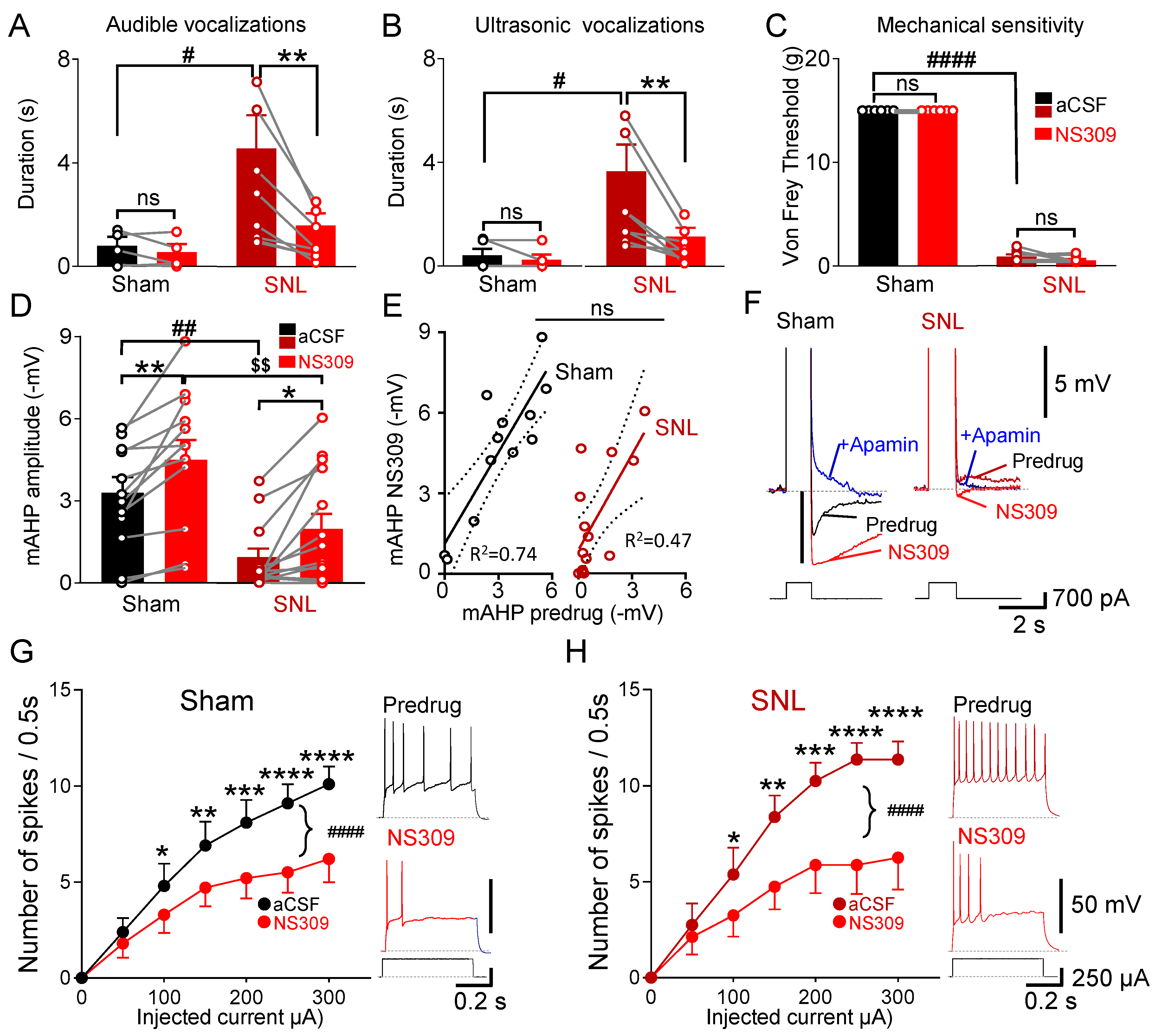
3.3. Selective SK Channel Activation Inhibits Neuropathic Pain Behaviors Due to Partial Rescue of mAHP
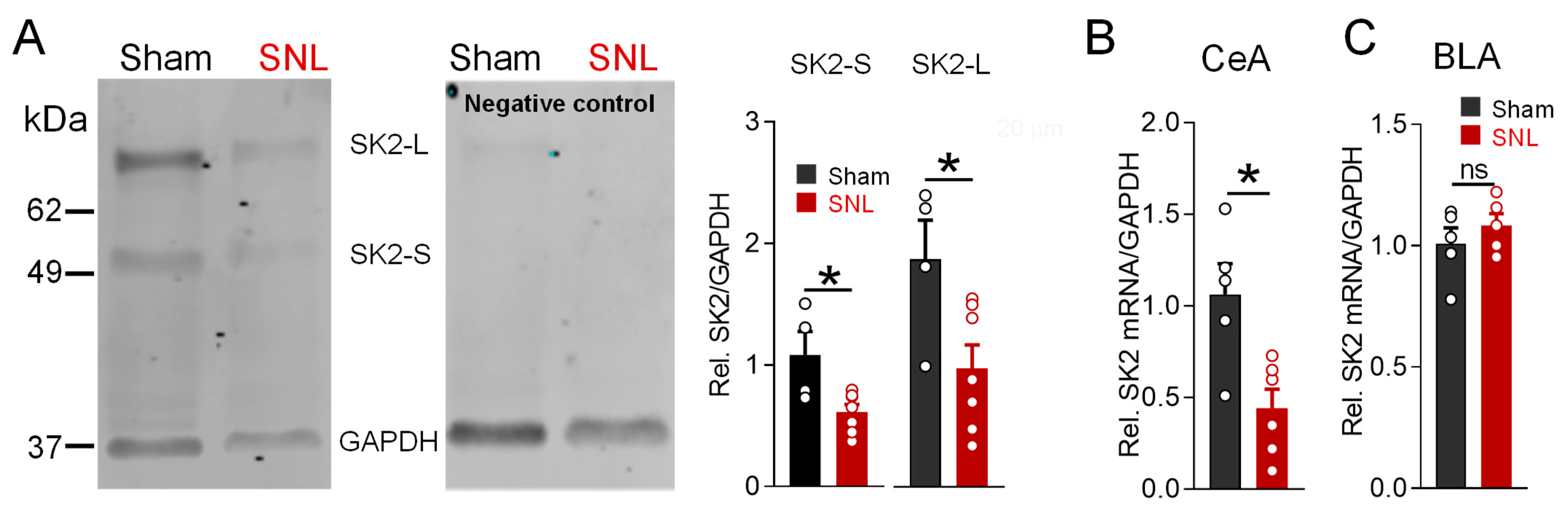
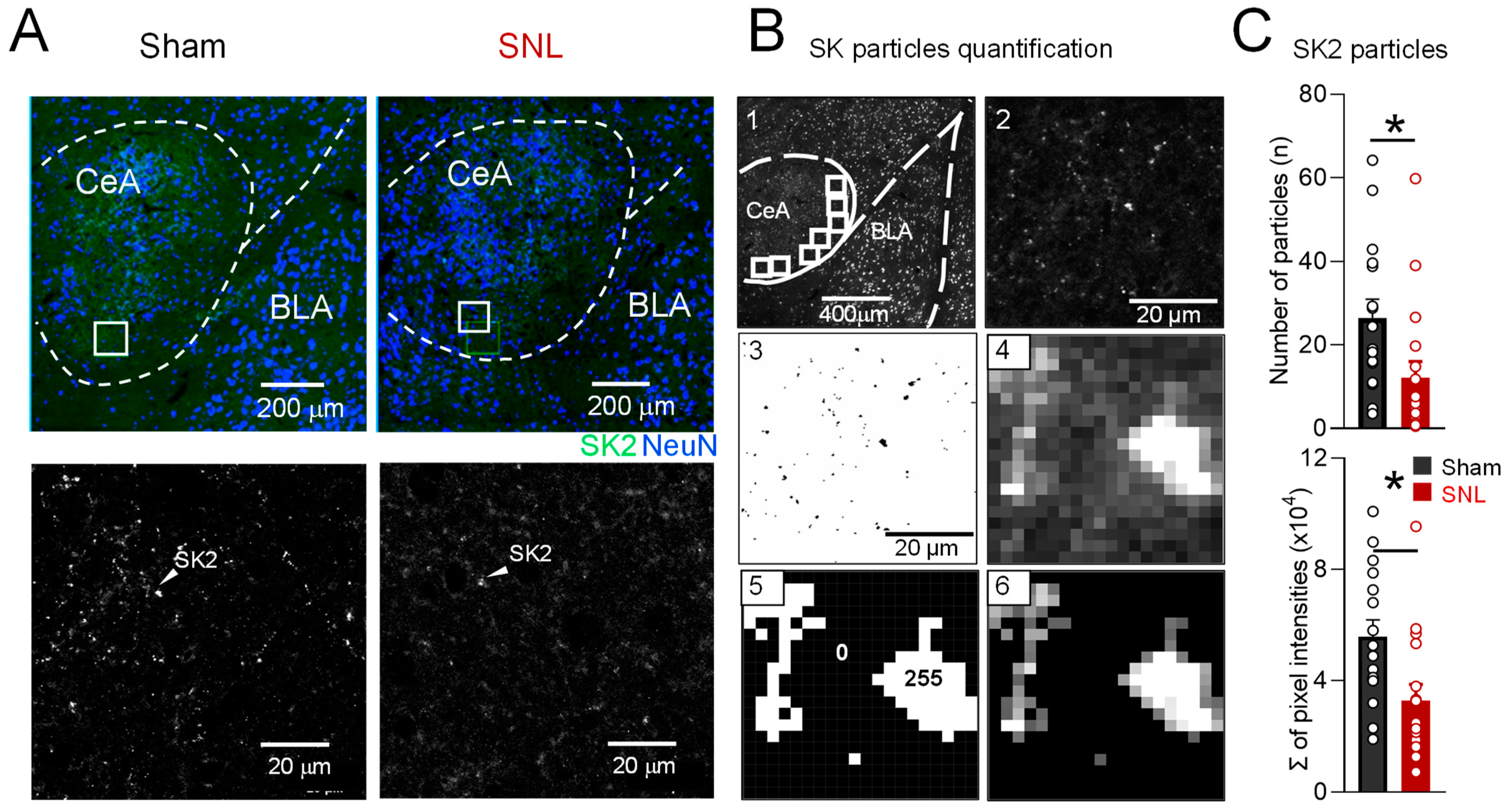
3.4. Decreased SK2 Subunit Expression in Neuropathic Pain State Is Localized to CeA
3.5. DNA Methylation Profile of CpG Island in the SK2 Gene Promoter Region in CeA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neugebauer, V. Amygdala physiology in pain. Handb. Behav. Neurosci. 2020, 26, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The amygdala and persistent pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef]
- Bernard, J.F.; Alden, M.; Besson, J.M. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J. Comp. Neurol. 1993, 329, 201–229. [Google Scholar] [CrossRef]
- Gauriau, C.; Bernard, J.F. Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 2002, 87, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kissiwaa, S.A.; Bagley, E.E. Central sensitization of the spino-parabrachial-amygdala pathway that outlasts a brief nociceptive stimulus. J. Physiol. 2018, 596, 4457–4473. [Google Scholar] [CrossRef]
- Matsumoto, N.; Bester, H.; Menendez, L.; Besson, J.M.; Bernard, J.F. Changes in the responsiveness of parabrachial neurons in the arthritic rat: An electrophysiological study. J. Neurophysiol. 1996, 76, 4113–4126. [Google Scholar] [CrossRef]
- Uddin, O.; Studlack, P.; Akintola, T.; Raver, C.; Castro, A.; Masri, R.; Keller, A. Amplified parabrachial nucleus activity in a rat model of trigeminal neuropathic pain. Neurobiol. Pain 2018, 3, 22–30. [Google Scholar] [CrossRef]
- Allen, H.N.; Bobnar, H.J.; Kolber, B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021, 196, 101891. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Takahashi, Y.; Inoue, K.; Kato, F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 2007, 127, 161–172. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, D.; Kong, L.Y.; Jin, Z.R.; Cai, J.; Kang, X.J.; Wan, Y.; Xing, G.G. Sensitization of neurons in the central nucleus of the amygdala via the decreased GABAergic inhibition contributes to the development of neuropathic pain-related anxiety-like behaviors in rats. Mol. Brain 2014, 7, 72. [Google Scholar] [CrossRef]
- Kato, F.; Sugimura, Y.K.; Takahashi, Y. Pain-Associated Neural Plasticity in the Parabrachial to Central Amygdala Circuit: Pain Changes the Brain, and the Brain Changes the Pain. Adv. Exp. Med. Biol. 2018, 1099, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.T.; Maylie, J.; Adelman, J.P. SK channels in excitability, pacemaking and synaptic integration. Curr. Opin. Neurobiol. 2005, 15, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Faber, E.S.; Sah, P. Functions of SK channels in central neurons. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Matschke, L.A.; Rinne, S.; Snutch, T.P.; Oertel, W.H.; Dolga, A.M.; Decher, N. Calcium-activated SK potassium channels are key modulators of the pacemaker frequency in locus coeruleus neurons. Mol. Cell Neurosci. 2018, 88, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M. Ca2+-activated K+ channels: Molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004, 5, 758–770. [Google Scholar] [CrossRef]
- Storm, J.F. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J. Physiol. 1989, 409, 171–190. [Google Scholar] [CrossRef]
- Sailer, C.A.; Kaufmann, W.A.; Marksteiner, J.; Knaus, H.G. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol. Cell Neurosci. 2004, 26, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; Pedarzani, P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell Neurosci. 2000, 15, 476–493. [Google Scholar] [CrossRef]
- Ngo-Anh, T.J.; Bloodgood, B.L.; Lin, M.; Sabatini, B.L.; Maylie, J.; Adelman, J.P. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 2005, 8, 642–649. [Google Scholar] [CrossRef]
- Faber, E.S.; Delaney, A.J.; Sah, P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005, 8, 635–641. [Google Scholar] [CrossRef]
- Stackman, R.W.; Hammond, R.S.; Linardatos, E.; Gerlach, A.; Maylie, J.; Adelman, J.P.; Tzounopoulos, T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002, 22, 10163–10171. [Google Scholar] [CrossRef]
- Lamy, C.; Goodchild, S.J.; Weatherall, K.L.; Jane, D.E.; Liegeois, J.F.; Seutin, V.; Marrion, N.V. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 2010, 285, 27067–27077. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, K.L.; Goodchild, S.J.; Jane, D.E.; Marrion, N.V. Small conductance calcium-activated potassium channels: From structure to function. Prog. Neurobiol. 2010, 91, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Armentia, M.; Sah, P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J. Neurophysiol. 2004, 92, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Royer, S.; Pare, D. Physiological properties of central medial and central lateral amygdala neurons. J. Neurophysiol. 1999, 82, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.C.; Martina, M.; Samson, R.D.; Drolet, G.; Pare, D. Physiological properties of central amygdala neurons: Species differences. Eur. J. Neurosci. 2002, 15, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Sheets, P.L. Spared nerve injury differentially alters parabrachial monosynaptic excitatory inputs to molecularly specific neurons in distinct subregions of the central amygdala. Pain 2020, 161, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Adke, A.P.; Khan, A.; Ahn, H.S.; Becker, J.J.; Wilson, T.D.; Valdivia, S.; Sugimura, Y.K.; Martinez Gonzalez, S.; Carrasquillo, Y. Cell-Type Specificity of Neuronal Excitability and Morphology in the Central Amygdala. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.D.; Valdivia, S.; Khan, A.; Ahn, H.S.; Adke, A.P.; Martinez Gonzalez, S.; Sugimura, Y.K.; Carrasquillo, Y. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep. 2019, 29, 332–346 e335. [Google Scholar] [CrossRef]
- Amano, T.; Amir, A.; Goswami, S.; Pare, D. Morphology, PKCdelta expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons. J. Neurophysiol. 2012, 108, 3196–3205. [Google Scholar] [CrossRef]
- Schiess, M.C.; Asprodini, E.K.; Rainnie, D.G.; Shinnick-Gallagher, P. The central nucleus of the rat amygdala: In vitro intracellular recordings. Brain Res. 1993, 604, 283–297. [Google Scholar] [CrossRef]
- Hunt, S.; Sun, Y.; Kucukdereli, H.; Klein, R.; Sah, P. Intrinsic Circuits in the Lateral Central Amygdala. eNeuro 2017, 4, e0367. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Sheets, P.L. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J. Physiol. 2018, 596, 6289–6305. [Google Scholar] [CrossRef]
- Hammond, R.S.; Bond, C.T.; Strassmaier, T.; Ngo-Anh, T.J.; Adelman, J.P.; Maylie, J.; Stackman, R.W. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J. Neurosci. 2006, 26, 1844–1853. [Google Scholar] [CrossRef]
- Kuiper, E.F.; Nelemans, A.; Luiten, P.; Nijholt, I.; Dolga, A.; Eisel, U. KCa2 and kCa3 channels in learning and memory processes, and neurodegeneration. Front. Pharmacol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Santini, E.; Porter, J.T. Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Front. Behav. Neurosci. 2014, 8, 96. [Google Scholar] [CrossRef]
- Zhang, W.H.; Liu, W.Z.; He, Y.; You, W.J.; Zhang, J.Y.; Xu, H.; Tian, X.L.; Li, B.M.; Mei, L.; Holmes, A.; et al. Chronic Stress Causes Projection-Specific Adaptation of Amygdala Neurons via Small-Conductance Calcium-Activated Potassium Channel Downregulation. Biol. Psychiatry 2019, 85, 812–828. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Wang, G.; Wang, Y. SK channels participate in the formation of after burst hyperpolarization and partly inhibit the burst strength of epileptic ictal discharges. Mol. Med. Rep. 2018, 17, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Bahia, P.K.; Suzuki, R.; Benton, D.C.; Jowett, A.J.; Chen, M.X.; Trezise, D.J.; Dickenson, A.H.; Moss, G.W. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J. Neurosci. 2005, 25, 3489–3498. [Google Scholar] [CrossRef][Green Version]
- Pagadala, P.; Park, C.K.; Bang, S.; Xu, Z.Z.; Xie, R.G.; Liu, T.; Han, B.X.; Tracey, W.D., Jr.; Wang, F.; Ji, R.R. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: Involvement of Ca2+-activated small conductance potassium channels. J. Neurosci. 2013, 33, 13425–13430. [Google Scholar] [CrossRef]
- Tsantoulas, C.; McMahon, S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, K. Regulation of excitability in tonic firing substantia gelatinosa neurons of the spinal cord by small-conductance Ca2+-activated K+ channels. Neuropharmacology 2016, 105, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Dreixler, J.C.; Couey, J.J.; Houamed, K.M. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur. J. Pharmacol. 2002, 449, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Yakhnitsa, V.; Ji, G.; Neugebauer, V. Small conductance calcium activated potassium (SK) channel dependent and independent effects of riluzole on neuropathic pain-related amygdala activity and behaviors in rats. Neuropharmacology 2018, 138, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Yao, R.A.; Chen, L.Y.; Renn, T.Y.; Klimenkov, I.V.; Sudakov, N.P.; Mai, F.D.; Chen, Y.T.; Chang, H.M. Olfactory Stimulation Successfully Modulates the Neurochemical, Biochemical and Behavioral Phenotypes of the Visceral Pain. Molecules 2022, 27, 7659. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.N.; Du, L.; Wang, Y.; Wu, K.; Chen, Z.Y.; Hua, R.; Zhang, Y.M. Small-Conductance Ca2+-Activated K+ Channels 2 in the Hypothalamic Paraventricular Nucleus Precipitates Visceral Hypersensitivity Induced by Neonatal Colorectal Distension in Rats. Front. Pharmacol. 2021, 11, 605618. [Google Scholar] [CrossRef] [PubMed]
- Presto, P.; Mazzitelli, M.; Junell, R.; Griffin, Z.; Neugebauer, V. Sex differences in pain along the neuraxis. Neuropharmacology 2022, 210, 109030. [Google Scholar] [CrossRef] [PubMed]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Simola, N.; Granon, S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology 2018, 159, 107420. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine-dopamine interaction and acoustic coding. Behav. Brain Res. 2007, 182, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Bird, G.C.; Li, W.; Jones, J.; Neugebauer, V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Methods 2005, 141, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Yakhnitsa, V.; Ji, G.; Hein, M.; Presto, P.; Griffin, Z.; Ponomareva, O.; Navratilova, E.; Porreca, F.; Neugebauer, V. Kappa Opioid Receptor Blockade in the Amygdala Mitigates Pain Like-Behaviors by Inhibiting Corticotropin Releasing Factor Neurons in a Rat Model of Functional Pain. Front. Pharmacol. 2022, 13, 903978. [Google Scholar] [CrossRef] [PubMed]
- Velumian, A.A.; Zhang, L.; Pennefather, P.; Carlen, P.L. Reversible inhibition of IK, IAHP, Ih and ICa currents by internally applied gluconate in rat hippocampal pyramidal neurones. Pflugers Arch. 1997, 433, 343–350. [Google Scholar] [CrossRef]
- Zhang, L.; Weiner, J.L.; Valiante, T.A.; Velumian, A.A.; Watson, P.L.; Jahromi, S.S.; Schertzer, S.; Pennefather, P.; Carlen, P.L. Whole-cell recording of the Ca2+-dependent slow afterhyperpolarization in hippocampal neurones: Effects of internally applied anions. Pflugers Arch. 1994, 426, 247–253. [Google Scholar] [CrossRef]
- Yamamoto, S.; Takahashi, Y.; Kato, F. Input-dependent synaptic suppression by pregabalin in the central amygdala in male mice with inflammatory pain. Neurobiol. Pain 2021, 10, 100078. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, K.L.; Seutin, V.; Liegeois, J.F.; Marrion, N.V. Crucial role of a shared extracellular loop in apamin sensitivity and maintenance of pore shape of small-conductance calcium-activated potassium (SK) channels. Proc. Natl. Acad. Sci. USA 2011, 108, 18494–18499. [Google Scholar] [CrossRef]
- Nam, Y.W.; Orfali, R.; Liu, T.; Yu, K.; Cui, M.; Wulff, H.; Zhang, M. Structural insights into the potency of SK channel positive modulators. Sci. Rep. 2017, 7, 17178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pascal, J.M.; Zhang, J.F. Unstructured to structured transition of an intrinsically disordered protein peptide in coupling Ca2+-sensing and SK channel activation. Proc. Natl. Acad. Sci. USA 2013, 110, 4828–4833. [Google Scholar] [CrossRef]
- Strobaek, D.; Teuber, L.; Jorgensen, T.D.; Ahring, P.K.; Kjaer, K.; Hansen, R.S.; Olesen, S.P.; Christophersen, P.; Skaaning-Jensen, B. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim. Biophys. Acta 2004, 1665, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Neugebauer, V. Hemispheric lateralization of pain processing by amygdala neurons. J. Neurophysiol. 2009, 102, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Nation, K.M.; De Felice, M.; Hernandez, P.I.; Dodick, D.W.; Neugebauer, V.; Navratilova, E.; Porreca, F. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain 2018, 159, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.E.; Navratilova, E.; Dickenson, A.H.; Porreca, F.; Bannister, K. Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain 2019, 160, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Ji, G.; Neugebauer, V. Small-conductance calcium-activated potassium (SK) channels in the amygdala mediate pain-inhibiting effects of clinically available riluzole in a rat model of arthritis pain. Mol. Pain 2015, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Neugebauer, V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. 2008, 28, 3861–3876. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.M.; Oh, M.M.; Galvez, R.; Burgdorf, J.; Kroes, R.A.; Weiss, C.; Adelman, J.P.; Moskal, J.R.; Disterhoft, J.F. Increasing SK2 channel activity impairs associative learning. J. Neurophysiol. 2012, 108, 863–870. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Cambridge, MA, USA, 1998.
- Mahimainathan, L.; Ghosh-Choudhury, N.; Venkatesan, B.; Das, F.; Mandal, C.C.; Dey, N.; Habib, S.L.; Kasinath, B.S.; Abboud, H.E.; Ghosh Choudhury, G. TSC2 deficiency increases PTEN via HIF1alpha. J. Biol. Chem. 2009, 284, 27790–27798. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, W.; Mahimainathan, L.; Narasimhan, M.; Kiritoshi, T.; Fan, X.; Wang, J.; Green, T.A.; Neugebauer, V. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci. 2017, 37, 1378–1393. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Yakhnitsa, V.; Neugebauer, B.; Neugebauer, V. Optogenetic manipulations of CeA-CRF neurons modulate pain- and anxiety-like behaviors in neuropathic pain and control rats. Neuropharmacology 2022, 210, 109031. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.Y.; Cui, M.; Pascal, J.M.; Logothetis, D.E.; Zhang, J.F. Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat. Chem. Biol. 2014, 10, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Strassmaier, T.; Bond, C.T.; Sailer, C.A.; Knaus, H.G.; Maylie, J.; Adelman, J.P. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J. Biol. Chem. 2005, 280, 21231–21236. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.; Trieb, M.; Leitner, I.; Wietzorrek, G.; Marksteiner, J.; Knaus, H.G. Small-conductance calcium-activated potassium type 2 channels (SK2, KCa2.2) in human brain. Brain Struct. Funct. 2017, 222, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Li, W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol. 2003, 89, 716–727. [Google Scholar] [CrossRef]
- Adedoyin, M.O.; Vicini, S.; Neale, J.H. Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Mol. Pain 2010, 6, 60. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takahashi, Y.; Sugimura, Y.K.; Tokunaga, R.; Yajima, M.; Kato, F. Active role of the central amygdala in widespread mechanical sensitization in rats with facial inflammatory pain. Pain 2021, 162, 2273–2286. [Google Scholar] [CrossRef]
- Han, J.S.; Neugebauer, V. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci. Lett. 2004, 361, 254–257. [Google Scholar] [CrossRef]
- Feldman, S.; Conforti, N.; Weidenfeld, J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci. Biobehav. Rev. 1995, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, J.A.; Venheim, E.R.; Padival, M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 2010, 67, 1128–1136. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nguyen, E.K.; Canto-Bustos, M.; Papale, A.E.; Oswald, A.M.; Ross, S.E. Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron 2020, 106, 927–939.e5. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci. 2018, 41, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.K.; Takahashi, Y.; Watabe, A.M.; Kato, F. Synaptic and network consequences of monosynaptic nociceptive inputs of parabrachial nucleus origin in the central amygdala. J. Neurophysiol. 2016, 115, 2721–2739. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Veinante, P. Cell-type specific parallel circuits in the bed nucleus of the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct. Funct. 2019, 224, 1067–1095. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Presto, P.; Yakhnitsa, V.; Antenucci, N.; Mendoza, B.; Ji, G. Pain-related cortico-limbic plasticity and opioid signaling. Neuropharmacology 2023, 231, 109510. [Google Scholar] [CrossRef] [PubMed]
- Haubensak, W.; Kunwar, P.S.; Cai, H.; Ciocchi, S.; Wall, N.R.; Ponnusamy, R.; Biag, J.; Dong, H.W.; Deisseroth, K.; Callaway, E.M.; et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010, 468, 270–276. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.M.; Morrison, F.G.; Hartmann, J.; Carlezon, W.A., Jr.; Ressler, K.J. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eNeuro 2018, 5, 12. [Google Scholar] [CrossRef]
- Mork, B.E.; Lamerand, S.R.; Zhou, S.; Taylor, B.K.; Sheets, P.L. Sphingosine-1-phosphate receptor 1 agonist SEW2871 alters membrane properties of late-firing somatostatin expressing neurons in the central lateral amygdala. Neuropharmacology 2022, 203, 108885. [Google Scholar] [CrossRef]
- Yu, K.; Garcia da Silva, P.; Albeanu, D.F.; Li, B. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J. Neurosci. 2016, 36, 6488–6496. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Bond, C.T.; Lujan, R.; Ballesteros-Merino, C.; Lin, M.T.; Wang, K.; Klett, N.; Watanabe, M.; Shigemoto, R.; Stackman, R.W., Jr.; et al. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nat. Neurosci. 2011, 14, 744–749. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Kim, Y.H.; Bang, S.; Zhang, Y.; Berta, T.; Wang, F.; Oh, S.B.; Ji, R.R. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 2015, 21, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Lai, J.; Malan, T.P., Jr.; Porreca, F. Spinal and supraspinal mechanisms of neuropathic pain. Ann. N. Y. Acad. Sci. 2000, 909, 12–24. [Google Scholar] [CrossRef]
- Ma, C.; Shu, Y.; Zheng, Z.; Chen, Y.; Yao, H.; Greenquist, K.W.; White, F.A.; LaMotte, R.H. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 2003, 89, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Vargas-Parada, A.; Duran, P.; Sandoval, A.; Delgado-Lezama, R.; Khanna, R.; Felix, R. L5-6 Spinal Nerve Ligation-induced Neuropathy Changes the Location and Function of Ca2+ Channels and Cdk5 and Affects the Compound Action Potential in Adjacent Intact L4 Afferent Fibers. Neuroscience 2021, 471, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Ardid, D.; Chapuy, E.; Alloui, A.; Jourdan, D.; Eschalier, A. Evidence for an involvement of supraspinal delta- and spinal mu-opioid receptors in the antihyperalgesic effect of chronically administered clomipramine in mononeuropathic rats. J. Pharmacol. Exp. Ther. 2003, 307, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, D.; Ardid, D.; Chapuy, E.; Eschalier, A.; Le Bars, D. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain 1995, 63, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Calvino, B.; Besson, J.M.; Boehrer, A.; Depaulis, A. Ultrasonic vocalization (22–28 kHz) in a model of chronic pain, the arthritic rat: Effects of analgesic drugs. Neuroreport 1996, 7, 581–584. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Liu, M.G.; Chen, J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: Challenges and perspectives. Prog. Neurobiol. 2014, 116, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Marshall, K.; Pham, A.; Ji, G.; Neugebauer, V. Optogenetic Manipulations of Amygdala Neurons Modulate Spinal Nociceptive Processing and Behavior Under Normal Conditions and in an Arthritis Pain Model. Front. Pharmacol. 2021, 12, 668337. [Google Scholar] [CrossRef] [PubMed]
- Kiritoshi, T.; Neugebauer, V. Pathway-Specific Alterations of Cortico-Amygdala Transmission in an Arthritis Pain Model. ACS Chem. Neurosci. 2018, 9, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Zhang, R.L.; Chen, L.; Zhao, H.Y.; Cai, J.; Wang, J.K.; Guo, D.Q.; Cui, Y.J.; Xing, G.G. Chronic stress increases pain sensitivity via activation of the rACC-BLA pathway in rats. Exp. Neurol. 2019, 313, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. Amygdala pain mechanisms. Handb. Exp. Pharmacol. 2015, 227, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Liang, S.H.; Ge, J.; Lu, Y.C.; Li, J.N.; Chen, Y.B.; Luo, D.S.; Li, H.; Li, Y.Q. Involvement of the Ventrolateral Periaqueductal Gray Matter-Central Medial Thalamic Nucleus-Basolateral Amygdala Pathway in Neuropathic Pain Regulation of Rats. Front. Neuroanat. 2020, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Pishva, E.; Drukker, M.; Viechtbauer, W.; Decoster, J.; Collip, D.; van Winkel, R.; Wichers, M.; Jacobs, N.; Thiery, E.; Derom, C.; et al. Epigenetic genes and emotional reactivity to daily life events: A multi-step gene-environment interaction study. PLoS ONE 2014, 9, e100935. [Google Scholar] [CrossRef]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Vachon, P.; Crosby, C.; Bushnell, M.C.; Szyf, M.; Stone, L.S. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS ONE 2013, 8, e55259. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Ausio, J.; Georgel, P.T. MeCP2 and CTCF: Enhancing the cross-talk of silencers. Biochem. Cell Biol. 2017, 95, 593–608. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Garriga, J.; Laumet, G.; Chen, S.R.; Zhang, Y.; Madzo, J.; Issa, J.J.; Pan, H.L.; Jelinek, J. Nerve Injury-Induced Chronic Pain Is Associated with Persistent DNA Methylation Reprogramming in Dorsal Root Ganglion. J. Neurosci. 2018, 38, 6090–6101. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Millecamps, M.; Naso, L.; Do Carmo, S.; Cuello, A.C.; Szyf, M.; Stone, L.S. Therapeutic benefits of the methyl donor S-adenosylmethionine on nerve injury-induced mechanical hypersensitivity and cognitive impairment in mice. Pain 2017, 158, 802–810. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Liang, L.; Gu, X.; Li, Z.; Wu, S.; Sun, L.; Atianjoh, F.E.; Feng, J.; Mo, K.; Jia, S.; et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat. Commun. 2017, 8, 14712. [Google Scholar] [CrossRef]
- Jiang, W.; Tan, X.Y.; Li, J.M.; Yu, P.; Dong, M. DNA Methylation: A Target in Neuropathic Pain. Front. Med. 2022, 9, 879902. [Google Scholar] [CrossRef]
- Borck, G.; Hog, F.; Dentici, M.L.; Tan, P.L.; Sowada, N.; Medeira, A.; Gueneau, L.; Thiele, H.; Kousi, M.; Lepri, F.; et al. BRF1 mutations alter RNA polymerase III-dependent transcription and cause neurodevelopmental anomalies. Genome Res. 2015, 25, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Damberg, M. Transcription factor AP-2 and monoaminergic functions in the central nervous system. J. Neural. Transm. 2005, 112, 1281–1296. [Google Scholar] [CrossRef]
- Kaneko, K.J.; Kohn, M.J.; Liu, C.; DePamphilis, M.L. Transcription factor TEAD2 is involved in neural tube closure. Genesis 2007, 45, 577–587. [Google Scholar] [CrossRef]
- Verheul, T.C.J.; van Hijfte, L.; Perenthaler, E.; Barakat, T.S. The Why of YY1: Mechanisms of Transcriptional Regulation by Yin Yang 1. Front. Cell Dev. Biol. 2020, 8, 592164. [Google Scholar] [CrossRef]
- Ross, S.; Tienhaara, A.; Lee, M.S.; Tsai, L.H.; Gill, G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J. Biol. Chem. 2002, 277, 4455–4464. [Google Scholar] [CrossRef]
- Mariottini, C.; Munari, L.; Gunzel, E.; Seco, J.M.; Tzavaras, N.; Hansen, J.; Stern, S.A.; Gao, V.; Aleyasin, H.; Sharma, A.; et al. Wilm’s tumor 1 promotes memory flexibility. Nat. Commun. 2019, 10, 3756. [Google Scholar] [CrossRef] [PubMed]
- Gronostajski, R.M. Roles of the NFI/CTF gene family in transcription and development. Gene 2000, 249, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A.; Olhava, E.J.; Scott, M.P. Targeting epigenetic enzymes for drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wu, S.; Gu, X.; Du, S.; Mo, K.; Sun, L.; Cao, J.; Bekker, A.; Chen, L.; Tao, Y.X. DNMT3a-triggered downregulation of K(2p) 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int. J. Cancer 2019, 145, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gu, X.; Pan, Z.; Guo, X.; Liu, J.; Atianjoh, F.E.; Wu, S.; Mo, K.; Xu, B.; Liang, L.; et al. Contribution of DNMT1 to Neuropathic Pain Genesis Partially through Epigenetically Repressing Kcna2 in Primary Afferent Neurons. J. Neurosci. 2019, 39, 6595–6607. [Google Scholar] [CrossRef]
- Liu, L.; Xu, D.; Wang, T.; Zhang, Y.; Yang, X.; Wang, X.; Tang, Y. Epigenetic reduction of miR-214-3p upregulates astrocytic colony-stimulating factor-1 and contributes to neuropathic pain induced by nerve injury. Pain 2020, 161, 96–108. [Google Scholar] [CrossRef]
- Fox, A.J.G.; Zuke, J.; Tomasch, M.; Naess, E.; Kennedy, A.J.; Burman, M.A. Epigenetic changes in DNA methylation are involved in the lasting changes in pain sensitivity following neonatal intensive care unit (NICU)-like treatment in rats. In Sosciety for Neuroscience; Washington, DC, USA, 2023.
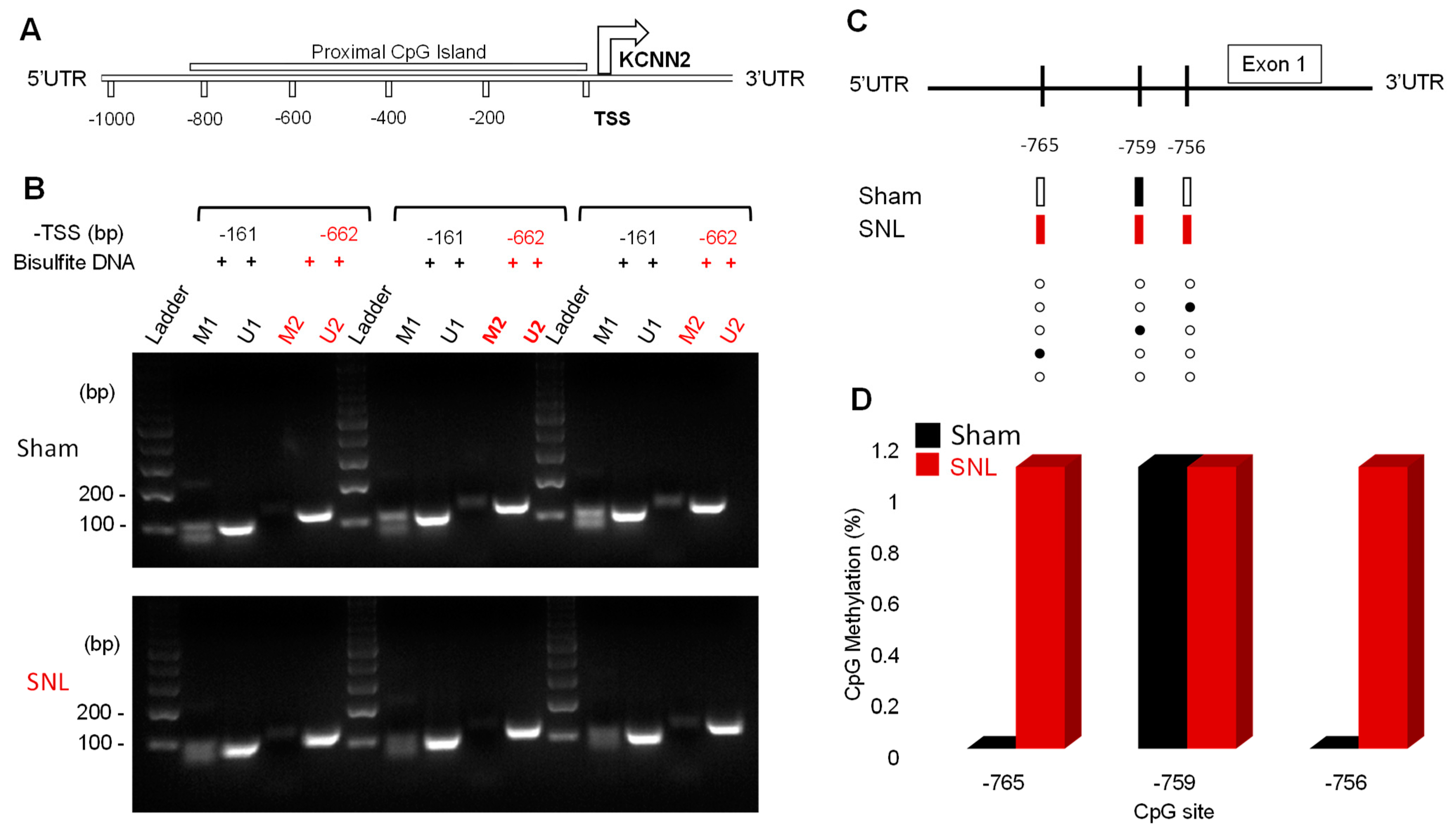

| Methylation-Specific Primers | Sequence | Amplicon Size |
| M1-left | GGGTAGTTAGTTTAATGTGAGCGA | 102 bp |
| M1-right | TAATAATACAAAAAAACGAACGCG | |
| U1-left | GGGGTAGTTAGTTTAATGTGAGTGA | 102 bp |
| U1-right | AATAATACAAAAAAACAAACACAAA | |
| M2-left | GTGCGTTTAATTAATCGGATTC | 120 bp |
| M2-right | GATAATACACCCTACGCAATACGTT | |
| U2-left | TTGGTGTGTTTAATTAATTGGATTT | 124 bp |
| U2-right | CAATAATACACCCTACACAATACATT | |
| Bisulfite-Specific Primers | ||
| BSP-F1 | AGGGGTTTTTATTTTGTAGG | 285 bp |
| BSP-R1 | ACAATAAAATTTCCATATCAAACCTAT | |
| BSP-F2 | AATAGGTTTGATATGGAAATTTTA | 287 bp |
| BSP-R2 | AACAACAAAAACAAATTATCCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakhnitsa, V.; Thompson, J.; Ponomareva, O.; Ji, G.; Kiritoshi, T.; Mahimainathan, L.; Molehin, D.; Pruitt, K.; Neugebauer, V. Dysfunction of Small-Conductance Ca2+-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms. Cells 2024, 13, 1055. https://doi.org/10.3390/cells13121055
Yakhnitsa V, Thompson J, Ponomareva O, Ji G, Kiritoshi T, Mahimainathan L, Molehin D, Pruitt K, Neugebauer V. Dysfunction of Small-Conductance Ca2+-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms. Cells. 2024; 13(12):1055. https://doi.org/10.3390/cells13121055
Chicago/Turabian StyleYakhnitsa, Vadim, Jeremy Thompson, Olga Ponomareva, Guangchen Ji, Takaki Kiritoshi, Lenin Mahimainathan, Deborah Molehin, Kevin Pruitt, and Volker Neugebauer. 2024. "Dysfunction of Small-Conductance Ca2+-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms" Cells 13, no. 12: 1055. https://doi.org/10.3390/cells13121055
APA StyleYakhnitsa, V., Thompson, J., Ponomareva, O., Ji, G., Kiritoshi, T., Mahimainathan, L., Molehin, D., Pruitt, K., & Neugebauer, V. (2024). Dysfunction of Small-Conductance Ca2+-Activated Potassium (SK) Channels Drives Amygdala Hyperexcitability and Neuropathic Pain Behaviors: Involvement of Epigenetic Mechanisms. Cells, 13(12), 1055. https://doi.org/10.3390/cells13121055







