Abstract
Over the past decade, the development of three-dimensional (3D) models has increased exponentially, facilitating the unravelling of fundamental and essential cellular mechanisms by which cells communicate with each other, assemble into tissues and organs and respond to biochemical and biophysical stimuli under both physiological and pathological conditions. This section presents a concise overview of the most recent updates on the significant contribution of different types of 3D cell cultures including spheroids, organoids and organ-on-chip and bio-printed tissues in advancing our understanding of cellular and molecular mechanisms. The case studies presented include the 3D cultures of breast cancer (BC), endometriosis, the liver microenvironment and infections. In BC, the establishment of 3D culture models has permitted the visualization of the role of cancer-associated fibroblasts in the delivery of exosomes, as well as the significance of the physical properties of the extracellular matrix in promoting cell proliferation and invasion. This approach has also become a valuable tool in gaining insight into general and specific mechanisms of drug resistance. Given the considerable heterogeneity of endometriosis, 3D models offer a more accurate representation of the in vivo microenvironment, thereby facilitating the identification and translation of novel targeted therapeutic strategies. The advantages provided by 3D models of the hepatic environment, in conjunction with the high throughput characterizing various platforms, have enabled the elucidation of complex molecular mechanisms underlying various threatening hepatic diseases. A limited number of 3D models for gut and skin infections have been developed. However, a more profound comprehension of the spatial and temporal interactions between microbes, the host and their environment may facilitate the advancement of in vitro, ex vivo and in vivo disease models. Additionally, it may pave the way for the development of novel therapeutic approaches in diverse research fields. The interested reader will also find concluding remarks on the challenges and prospects of using 3D cell cultures for discovering cellular and molecular mechanisms in the research areas covered in this review.
1. Introduction
In today’s fast-paced world of life science and biomedical research, a large body of experimental evidence has clearly demonstrated the enormous potential of three-dimensional (3D) cell cultures for improving our understanding of cell biology and the molecular mechanisms underlying disease [1,2,3], for drugs development and testing [4,5,6,7], in regenerative medicine [8,9] and also in tissue engineering [1]. Research has focused heavily on developing protocols and fine-tuning new technological approaches to developing diverse 3D in vitro models, also in agreement with the 3Rs (Replacement, Reduction, Refinement) principles of the European Union [10]. For years, two-dimensional (2D) cell culture systems have been used extensively in biomedical research to study cellular and molecular mechanisms in both physiological and pathological conditions and to develop new therapies and treatments. Despite their low cost and the possibility of high-throughput analysis, 2D cultures do not mimic typical tissue architecture, limiting cell–cell and cell–extracellular matrix (ECM) interactions or the correct oxygen and nutrient gradients that are responsible for the activation of specific cellular and molecular events involved in the underlying biological processes and responsible for the cellular phenotype [10,11]. 3D cell culture platforms can overcome the limits of both 2D cultures and animal models [12,13], providing the conditions for establishing the critical characteristics of the in vivo environment to reproduce the complexity typical of healthy or diseased tissues, thus enabling a better understanding of the underlying cellular and molecular mechanisms [14]. Reviewing the literature makes clear that the enormous and unprecedented potential of 3D cultures is helping to improve the biological relevance of cell lines, which are widely used in scientific research for their stability, reliability and degree of batch-to-batch standardization [15,16,17], by increasing their ability to behave in a manner similar to physiological behavior [18,19]. Similarly, the 3D system allows for the improved phenotypic stability of primary cells, long-term expansion and differentiation into multiple lineages of induced pluripotent stem cells (iPSCs), offering significant advantages over current approaches [20,21]. The ability to obtain a high degree of cell organization, cell–cell interactions and ECM components from all these cell types is closely linked to the use of methods and technologies developed to facilitate the formation of 3D cell models capable of mimicking the complexity of tissues and organs. Spheroids and organoids represent the two most important structures where cells are cultured in 3D [22]. The first is the simplest model of 3D organization, composed of cellular aggregates primarily formed via cell-to cell adhesion derived from cell lines, primary cells or tumor biopsies in mono- or co-cultures. Despite their lower complexity structurally, they have applications in drug and nanoparticles screening and disease modeling, such as for tumors [23]. They can be generated by methods that force the formation of cell aggregation with spherical cells, in the absence or presence of biomimetic and modulable scaffolds/hydrogels (natural or synthetic). The latter are able to provide biomimetic structures capable of recapitulating the physical and biochemical cues required for the cell to adhere and grow to form the desired tissue features [24]. Compared to spheroids, organoids are highly complex self-organized 3D structures derived from the self-organizing properties of stem cells (embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), adult stem cells (ASCs) and even tumor cells. These amazing 3D constructs harbor multifarious cell types of original organs and mimic the derived organs in both architecture and function to a great degree [25]. Until now, the generation of organoids from tissue-derived cells (TDCs) or induced pluripotent stem cells (iPSCs) has involved many optimized step-by-step protocols that typically take several months [26]. Patient/tissue-derived organoids or tumor organoids are obtained through optimized tissue dissociation methods (mechanical and enzymatic dissociation can be combined to generate better cell yields) to isolate starting cell populations (tissue–resident stem/progenitor cells or tumor cells), while iPSC-derived organoids are established from fully characterized iPSC lines. The maturation of organoids requires the seeding the cells onto a specific matrix (biologically derived, such as a Matrigel or synthetic hydrogel) and adding specific growth factor cocktails at each step [27,28,29]. Organoids can be also obtained by using spinning bioreactors (rotating vessel bioreactors, clinostat bioreactors and stirred-tank bioreactors), which partially resolve the inadequate nutrient and waste diffusion typically observed in 3D cell cultures [30]. Despite some limitations (e.g., the protocols are not standardized globally; microenvironment components are missing, particularly in organoids derived from adult stem cells; and they are difficult to adapt to microplates and high-throughput screening contexts), organoids are very promising tools for tissue engineering, regenerative medicine, cancer research, new drug screening and personalized therapies. Advances in the development of spheroids and organoids and their application in various fields of research, including clarifying cellular and molecular aspects, have been enabled by the rise of new technological approaches frameable within high-throughput experimental workflows, such as 3D bioprinting or microfluidic techniques [31,32]. 3D bioprinting uses bio-ink, comprising living organoids or spheroids encapsulated within tunable-biomaterial, to precisely create 3D biological geometries mimicking those of the native tissue in a layer-by-layer approach [33]. This technology has rapidly emerged as a promising tool for the creation of 3D cell models with a well-defined architecture, composition and high reproducibility, particularly useful for tumor–stroma investigation and drug screening applications. Microfluidic is another bioengineering approach that rapidly gained attention in cell cultures [34]. It is based on platforms in which living cells are cultured in small micrometer chambers and the medium is continuously infused inside the chambers. With the ability to manipulate flows in the order of a few μL/min, the nutrient supply, the oxygen exchange and the removal of waste products (e.g., cellular debris) can be regulated in a spatially controlled manner. This facilitates both the formation of uniformly sized spheroids (homotypic or heterotypic) and perfused organoids, even dispersed in natural/synthetic scaffolds, as well as the study of growth factors or drug effects and the mechanobiology of cell–cell and cell–matrix interactions, by exploiting the possibility of working in a biomimetic physiological environment [35,36]. Microfluidics have also opened up the development of miniaturized cell models, known as organ-on-a-chip models, which combine different organoids with the ultimate goal of better reflecting the physiology of the human organ [37]. The interested reader may consult the recent reviews in [22,38,39] describing the advantages and limits for obtaining 3D models and also the strategies for overcoming them.
The exciting power of 3D cell cultures is currently being exploited in so many different research fields that it is not possible to provide a complete and comprehensive review. For that reason, the review will emphasize some significant contributions of 3D models in expanding knowledge on the most common cancer in women, breast cancer (BC), and on a non-cancerous but emerging and disabling disease of the female reproductive system, endometriosis. New cellular and molecular aspects obtained by reproducing the complex of the liver in 3D vitro 3D cultures, an organ which plays a central role in metabolic functions, will also be highlighted. Finally, interesting insights that 3D technology has brought to light in the study of bacterial infections will be reported.
2. 3D Culture Models for Breast Cancer
In the modern industrialized world, cancer has become the most feared disease, representing one of the main causes of death after cardiovascular diseases [40]. Currently, there is an ever-growing plethora of scientific articles reporting on the use of 3D cultures in oncological research of both so-called ‘solid’ [14,41] and ‘liquid’ tumors [42,43]. The shift from 2D in vitro systems to 3D cultures is explained by the fact that it is possible to tune these to more closely mimic in vivo tumor characteristics, including the heterogeneity of the tumor microenvironment (TME) [44], cell–cell (i.e., tumor cell–immune cell) [45] and cell–extracellular matrix contacts [46], hypoxia [47], nutrient and pH gradients [48] and biomechanical properties (such as extracellular matrix (ECM) stiffness) [49] (Figure 1a). This cancer-mimicking approach facilitates the study of mechanisms of cancer initiation, progression, resistance recurrence and tumour–stroma interaction, which can identify markers for early diagnosis and therapies [41,50]. At the same time, 3D cultures provide a tool for predicting therapeutic responses, optimizing treatment strategies and exploring potential therapies in the battle against cancer [23].
In this section, we briefly focus on some recent findings using 3D cell cultures to understand the mechanisms underlying BC, the most common cancer in the world [51]. BC is a heterogeneous disease determined by both genetic and environmental factors and is categorized into different subtypes based on the levels of the receptors for estrogen, progesterone and human epidermal growth factor 2 (HER2) and the absence of the aforementioned receptors [52]. Since the inter- and intra-heterogeneity of breast tumours complicates their treatment, it is very relevant to have systems in place for identifying, clarifying, and defining changes in cellular properties for each type and stage of BC. Currently, xenografts and syngeneic in vivo breast cancer models are most used to test the in vivo efficacy of new treatments before entering clinical trials., but each has advantages and disadvantages [53]. As recently reviewed by Fröhlich et al. [39], to increase translation from in vitro findings to a clinical setting, many 3D models are available, including, e.g., spheroids, organoids and breast cancer on-a-chip and bio-printed tissues. The literature on the subject is vast. In this review, we have mainly focused on some recent articles that, by culturing immortalized breast cancer cell lines and other key TME cells, such as cancer-associated fibroblasts (CAFs) and adipocytes (ASCs), on 3D platforms, have contributed to shedding light on aspects related to the processes of BC proliferation, migration, invasion, tumour–stroma interaction and drug resistance (Table 1).

Table 1.
Summary of some recent findings on BC using 3D cell cultures.
The great potential of 3D models (like spheroids and organoids) and linked methods/technologies is to recreate important features of the tumor, such as the organization of the multicellular layer and the environment in which micro-metastases develop, as nutrients and oxygen are limited in these large structures [24,47]. In addition, they make it possible to represent and preserve the cellular heterogeneity present in tumors: combining different cell types in the same spheroid, i.e., tumor cells, monocytes and CAFs, allows for studying the role of these cells and the cell–cell interaction in tumour initiation and progression as well as all the variations in the signalling, gene expression and protein production pathways involved [14]. CAFs are among the central components of TME, and they promote tumor progression and metastasis [68]. Increasing evidence points to exosomes, small membranous vesicles containing lipids, proteins and nucleic acid (i.e., DNA, mRNA and non-coding RNA like microRNA), as critical cellular communicators involved in the interaction mechanism between CAF and cancer cells [2]. Using spheroid models, Cheng et al. [55] aimed to study in BC the role of miR-500a-5p, whose expression is known to be up-regulated in other cancer types [69]. In this work, the 3D cell culture allowed them to observe that CAFs promote breast cancer progression and metastasis through exosomal miR-500a-5p, which led the authors to hypothesize that CAF-derived miR-500a-5p inhibition could be an alternative modality for treating BC [55]. However, spheroid-based 3D models are often oversimplified and do not reproduce the correct dynamic tumour–stroma interactions at cellular and molecular levels. Microfluidic models in combination with hydrogel-based 3D matrices may help to establish the proper tumour–stroma architecture. Glycoprotein nonmetastatic melanoma protein B (GPNMB) is a transmembrane glycoprotein found to be highly expressed in many types of cancer, including breast cancer, with various roles in tumour invasion, angiogenesis, cell adhesion and immunosuppression [70]. Truong et al. [67] engineered a 3D organotypic microfluidic coculture system of tumor–stroma interactions. RNA-seq profiled the transcriptome of cancer cells in this 3D cell culture, which led to the identification, for the first time in breast carcinoma cells, of the involvement of GPNM in the invasion process promoted by CAFs by exploiting the 3D tumor microenvironment in which interactions with CAFs were present. Dysfunctional adipocyte metabolism also plays a very important role in BC progression, as ASCs interact with the mammary epithelium by secreting a variety of cytokines and hormones [71]. CCR1 is the C-C motif chemokine receptor ligand 5 (CCL5) and has been reported to be a chemokine that fosters metastasis and is expressed during crosstalk between breast cancer and stromal cells [72]. In 2023, Watzling et al. [56], utilizing an ASCs/BC spheroid model, observed a predominant chemokine/receptor interaction between CCL5-producing ASCs and its cognate receptor (CCR1) expressing BC cells in promoting the migration of triple-negative MDA-MB-231 breast cancer cells, proving the crucial role of 3D cell cultures in deciphering BC cells (Figure 1b,c). Regarding BC progression, in 2023, Itah el al., culturing human mammary epithelial MCF-10A.B2 cells in a 3D cell culture, established a tumour suppression function for the JNK signalling pathway in HER2+ BC [65].
The ECM of the BC has a subtype-specific composition that may also contribute to changes in the biophysical properties, such as stiffness, of the BC tissue [53,73,74,75]. Enhanced matrix deposition and realignment of collagen fibres are detected by tumor cells, triggering the epithelial–mesenchymal transition (EMT), which in turn stimulates cell motility and invasiveness. In contrast to conventional 2D cell cultures, in which the cell morphology is constrained in a ‘flat’ plain, spheroids can be surrounded by a matrix that can be shaped and stiffened. In this way, it is possible to study the role of these signals in directing the polarity of tumor cells and, thus, their ability to migrate and invade [76]. Very recently, a group of researchers embedded spheroids of HCC1954 human BC cells in 3D collagen scaffolds with increasing stiffness provided by different concentrations of ribose (0, 50 and 200 mM). They showed that the invasion of BC spheroids is driven by the activity of ERKs (extracellular signal-regulated kinases) and the transcriptional regulator YAP (Yes-associated protein). They observed that ERK activity is increased under high stiffness conditions: it was evident how ECM stiffness improves ERK nuclear localization. (Figure 1e). Similarly, YAP activity is affected by matrix stiffness: like ERK, at high ribose concentrations, spheroids showed more YAP activation and nuclear distribution compared to the citoplasmic compartment (Figure 1f). There is evidence that both YAP and ERK activity may play a key role in ECM remodeling to provide a favorable matrix for BC cell migration [57]. Although the underlying mechanism remains elusive, it is known that the stiffness of the ECM matrix influences cell behavior through cancer metabolism regulation [77,78]. Interestingly, applying a well-defined hybrid hydrogel system mimicking the heterogeneous local stiffness of TME, Liu et al. showed that BC cells proliferate in a soft core environment while migrating in a stiff peripheral environment. Furthermore, they observed that BC cells shift from glycolysis to OXPHOS and fatty acid metabolism, responding to a stiff matrix microenvironment [66].
3D cell cultures helped to investigate the BC lineage-specific resistance mechanisms [60,61,62,64]. In these systems, tumor cells display typical tumor characteristics (relative quiescent state, self-renewal capacity and growth in spheroid structures), live in hypoxic and nutrient-poor conditions and express specific ECM components [79]. All these aspects might influence the response to chemotherapy by limiting drug accessibility in the cancer cells [80]. In this way, the evaluation of drug resistance in 3D cell culture models can be valuable in gaining insight into the general and specific mechanisms of drug resistance, while providing more physiologically relevant systems for disease modelling and drug screening. Doxorubicin is considered a first-line anticancer drug in several types of cancer, but drug-induced cardiotoxicity and drug resistance are the main obstacles to its use [81]. In 2022, using a 3D model, Liverani et al. [64] attempted to explain the mechanisms behind these barriers. Specifically, they engineered a 3D model based on biomimetic collagen scaffolds, revealed the involvement of hypoxia in doxorubicin resistance in MDA-MB-231 and also made it possible to identify the most significantly altered pathways involved in drug resistance (Figure 1g). As mentioned above, there are numerous examples of the great potential of 3D models in the study of breast cancer.
The articles cited in this brief section help to demonstrate how 3D models can be effectively used to understand tumour cell–TME interactions and their impact on drug sensitivity through the ability to mimic cancer characteristics.
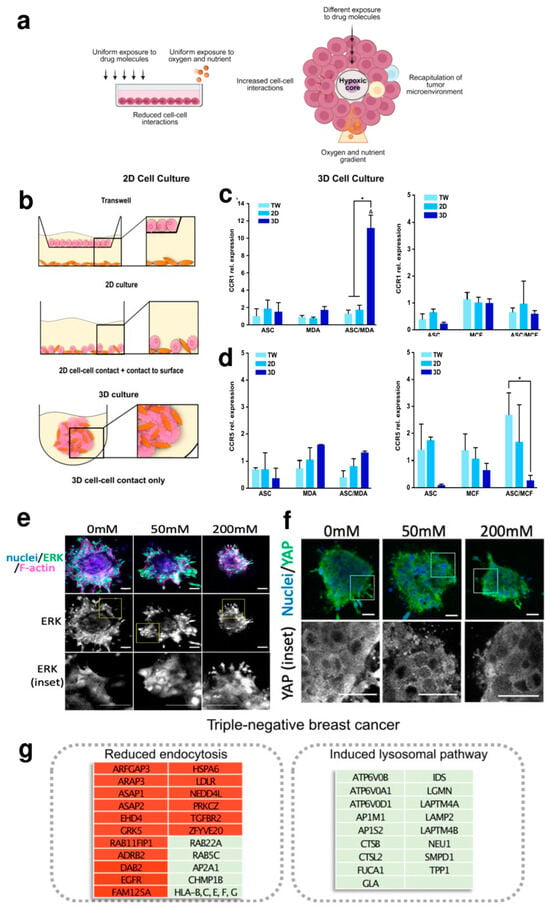
Figure 1.
(a) In 2D adherent cultures, cells grow as a monolayer on a flat surface, allowing unrestricted access to a similar number of nutrients and growth factors in the culture medium, resulting in homogeneous growth and proliferation. Cell–cell interactions and the extracellular environment are absent. The 3D model recapitulates the characteristics of the tumour microenvironment. Adequate cell–cell and extracellular environment interactions are allowed. A variable availability of oxygen, nutrients, metabolites and signalling molecules is established (adapted from [82] under the terms and conditions of the Creative Commons Attribution (CC-BY) license (CC-BY 4.0)). (b–d) Schematic representation of different culture conditions (b), expression of CCL5 receptors (CCR1 and CCR5) in mono- and co-culture spheroids of ASCs and MDA-MB-231 or MCF-7 compared to indirect and direct 2D cultures (c,d). * indicates statistically significant differences (p < 0.05) between culture systems; Δ indicates statistically significant differences (p < 0.05) to corresponding monocultures (adapted from [56] under the terms of the CC-BY 4.0 publishing license). (e,f) Images of HCC1954 spheroids stiffened by different concentrations of ribose (0.50 and 200 mM). (e) Fixed samples show the distribution of ERK (green) and F-actin (magenta), with counterstained nuclei in blue. Scale bars, 20 μm. (f) Spheroids embedded in a 3D collagen structure show the localization of YAP (green); nuclei are stained blue. Scale bars, 20 μm (adapted from [57] under the terms of the CC-BY 4.0 publishing license). (g) Box representation of doxorubicin effects in the MDA-MB-231 cell line cultured within the 3D biomimetic collagen scaffold, indicating the most significantly altered pathways implicated in DOX resistance (green = up-regulation; red = down-regulation) (adapted from [64] under the terms of the CC-BY 4.0 publishing license).
3. 3D Culture Models for Endometriosis
A large body of current literature showed that the 3D microenvironment can be exploited to better understand cell–cell interactions in endometriosis [83]. Endometriosis is an inflammatory gynaecological disease that seriously affects the quality of a woman’s life. The pathogenesis of endometriosis is still unknown, but several leading theories include retrograde menstruation, altered immunity, coelomic metaplasia and metastatic cell spread [84]. The heterogeneity and differences among the three main classes of endometriosis presentation may suggest different multiple pathogenetic pathways [85]. Over the years, the vitro 3D culture models of the human endometrium have been gradually developed (Figure 2) as an alternative to classical 2D culture models [83]. Currently, several cell types are used in these models, including epithelial, stromal, endothelial and immune cells, as described in Table 2.

Table 2.
Summary of key 3D cell culture models for human endometriosis.
Both the immortalized cell lines and primary cells from healthy women or women with endometriosis were tested to construct the 3D model. In the endometriosis 3D model, organoids, chicken chorioallantoic membranes (cams), amniotic membranes, spheroids and organs-on-a-chip were used (Figure 2a) [83]. Due to the heterogeneity of pathogenesis, the main molecular mechanisms examined by 3D models concern the immunological aspects [86,89], the hormonal signaling [86,90] and the angiogenesis process related to endometriosis [91] and the endometrial stromal cells’ migration and invasion [87]. The models shown in Table 2 demonstrate how genes involved in the above mechanisms are over-expressed in the 3D model compared to those in the 2D model. More in detail, a 3D model based on spheroids has been established to mimic endometriosis using the endometriotic cell line, EEC16 and 12Z [86]. In this example, molecules related to the immune response (including IL6, IL8, CXCL12 and CXCR4), micro-environmental interactions (such as MMP2 and hepatocyte growth factor (HGF)) and hormonal signaling (including prostaglandin-endoperoxide synthase 2 (PTGS2) and cytochrome P450 family 19 subfamily A member 1 (CYP19A1)) were significantly up-regulated compared with the 2D models (Figure 2b–d) [86]. Furthermore, Stejskalová et al. observed the ability of the immortalized eutopic stromal cell line St-T1b to form spheroids (Figure 2e) [87]. Compared with 2D models, the obtained spheroids showed an increased expression of two matrix metalloproteinases (MMPs), MMP2 and MMP14, known to be highly expressed in the early stage of endometriosis [93]. Exploiting 3D cell cultures, the same authors also observed an altered expression pattern of microRNAs miR-200b and miR-145, previously shown to be dysregulated in endometriosis [94], suggesting their involvement in the invasive behavior of the endometriotic epithelial cell line 12Z. Wendel et al. proposed spheroids using 12Z cells to represent the inflammatory (IL6, IL8 and MCP1) and estrogen-related gene (CYP19A1, HSD17β1 and ESR1) expression of endometriosis [88]. These genes were up-regulated in cells grown as spheroids compared to monolayer cultures. Seeking the expression of the same markers described above involved in inflammation (Tumor necrosis factor-TNF), immune responses (interleukins-ILs) and invasion (MMPs), Song et al. created spheroids with endometriotic epithelial and stromal cell lines [89]. Moreover, Muruganandan et al. developed a 3D culture system with an endometrial tissue slice cultured by incorporating an air–liquid interface into a 3D matrix scaffold of type I collagen gel (Figure 2f) [90]. This long-term slice culture method provides a unique in vivo-like microenvironment for studies of human endometrial repair, regeneration and remodeling.

Figure 2.
3D culture models of the human endometrium. (a) Available in vitro experimental systems used in endometriotic studies, which reflect the multifactorial nature of the endometriotic lesion (adapted from [83] under the terms of a CC-BY 4.0 publishing license). (b,c) After 7 days of the 3D culture, EEC12Z and EEC16 both form dense, smooth and symmetrical spheroids. (b) Phase contrast and H&E images, (c) Cytokeratin expression is increased in 3D models versus that in 2D models and (d) Expression of genes relevant in endometriosis in EEC16 and EEC12Z after being cultures in 3D for 7 days. * p > 0.05. (adapted from [86] under the terms of a CC-BY 4.0 publishing license). (e) Spheroids’ shape and dimension characterization: bright-field images (Scale bars 250 µm), Cell Tracker staining (Red: St-T1b; green: 12Z. Scale bar 200 µm) and relative quantitative analysis. ** p < 0.01; *** p < 0.001, **** p < 0.0001; (adapted from ref. [87] under the terms of a CC-BY 4.0 publishing license). (f) Representative images showing a 3D cell culture model system for endometriosis based on a slice from a full-thickness human endometrium (adapted from ref. [90] under the terms of a CC-BY 4.0 publishing license).
Another example described a microengineered vascularized endometrium-on-a-chip (MVEOC) [91]. The model reconstitutes an endometrial environment including three distinct layers of the epithelium, stroma and blood vessels, and it has demonstrated its appropriate responsiveness to pro-angiogenic factors and hormonal stimulation. Finally, the last example proposed in Table 2 demonstrated that endometriosis also maintains the epigenetic changes in vitro, exploiting organoids as 3D in vitro platforms compared to endometriosis tissue biopsy specimens [92]. These are the most recent examples in the literature describing the applicable 3D model for endometriosis. The ongoing 3D in vitro development will lead to models that accurately reflect the disease phenotype and heterogeneity of this condition. Since endometriosis 3D models more closely resemble the in vivo microenvironment, the potential for identifying and translating novel targeted therapeutic strategies will be greatly enhanced by using these models.
4. 3D Culture Models for the Liver
A notable example of the transition from 2D to 3D models is 3D cultures that mimic the liver environment using both cell lines and iPSCs (Figure 3a,c). Regarding cell lines, HepG2 cells are particularly used. This hepatocyte-like cell line is derived from human hepatocellular carcinoma [15,95]. Different from physiological primary hepatocytes, HepG2 is characterized by an almost null metabolism [96]. This aspect greatly limited its application when modeling the hepatic activity with traditional monolayer cell cultures. However, it was first noticed that when grown within tridimensional environments, the production of albumin and urea was significantly enhanced [97]. The expression of these molecules is a key marker of hepatic metabolism. Usually, HepG2 is characterized by extremely low secretion rates for both molecules [98]. The awareness that their secretion can be boosted by adopting a 3D culture ignited a spark and trailblazed the development of subsequent 3D systems, aiming to understand if the metabolic activity of HepG2 could have been further pushed toward a physiological-like situation. Further studies exploited both scaffold-based cultures and spheroids to investigate to what extent the tridimensionality was responsible for increasing the functionality of HepG2 cells. Particularly, this approach allowed for understanding both qualitatively (immunofluorescence) and quantitatively (RT-qPCR) the relevance of a 3D environment in boosting the expression of relevant metabolic markers such as the secretion of albumin (ALB) and enzymes related to the processing of drugs, such as cytochromes from the cytochrome-P450 family (e.g., CYP 3A4) (Figure 3b) [99,100,101,102,103]. HepG2 cells usually lack the expression of these proteins, and such a behavior might open the door to produce predictive models based on this cell line. Similar findings, resulting from cells being grown in a 3D environment, were also observed for other hepatocyte-like lines, including HepaRG and HUH7 [104,105,106]. Additionally, the strategies currently adopted for fabricating 3D cultures enabled the production of multicellular cocultures. These tools allowed for exploring the impact providing cultured cells with relevant cell–cell interactions and underlined a further boost of the metabolic activity of hepatocyte-like cells when co-cultured with other non-parenchymal cell types (e.g., fibroblasts, stellate cells, endothelial cells) [107,108,109,110,111]. 3D cell cultures emerged as a powerful tool for enhancing the phenotypic stability of both primary hepatocytes and iPSC-derived hepatocytes (Figure 3c). This achievement was possible by providing a culture environment capable of mimicking the native tissue, thus overcoming the lack of compliance that characterizes polystyrene substrates used for monolayer cultures [112,113,114]. Additionally, the possibility of taking advantage of 3D environments to recapitulate cell–matrix interactions was shown to be pivotal for inducing the differentiation of iPSC and other multi-/pluripotent cells into hepatocytes [115,116,117] (Figure 3d). These findings and other relevant contributions (Table 3) are shedding new light on the possibility of successfully growing primary cells in vitro, thus opening pathways that might culminate in highly relevant personalized medicine.

Figure 3.
Beneficial impact of growing hepatic cells within a three-dimensional environment. (a) When cell lines are cultured in a 3D environment, the systematic up-regulation of in vivo-like functions has been observed. Scheme created with BioRender.com. (b) The increase in albumin secretion, as well as the up-regulation of relevant cytochromes, were observed for HepG2 cells grown within a 3D-bioprinted hydrogel matrix, the scale bars are all equal to 200 μm (adapted from [101] under the terms of a CC-BY 4.0 publishing license). (c) 3D culture environments positively impact both the establishment and the maintenance of physiological-like hepatic functions in primary hepatocytes, as well as in iPSC-derived hepatocytes. Scheme created with BioRender.com. (d) 3D cultures have also shown their potential in guiding the differentiation of iPSC-derived hepatocytes, as the coculture of the iPSC spheroid and primary hepatocytes spheroid led to the establishment of multiple physiological-like hepatic functions. Statistical significance is expressed as ns: p > 0.05, **: 0.01 ≤ p < 0.001, ***: p ≤ 0.001 (adapted from [115] under the terms of a CC-BY 4.0 publishing license).

Table 3.
Summary of recently developed 3D hepatic cultures based on cell lines and primary cells.
The advantages provided by 3D models of the hepatic environment, together with the high throughput characterizing these platforms, allowed for elucidating the complex molecular mechanisms behind different threatening hepatic diseases. Particularly, it was recently possible to unveil new aspects featuring the development and progression of hepatocellular carcinoma, including the interactions between the ubiquitin E3 ligase CHIP and transferrin receptor, causing the inhibition of ferroptosis and culminating in enhanced cell proliferation [118]. Similarly, the implications of SIRT7 in promoting the hippo/YAP pathways, thus promoting cancer progression, were recently discovered by exploiting 3D cell cultures [119]. Coherently, 3D in vitro models are playing a crucial role in the identification of novel therapeutic targets. To this end, recent research highlighted that inhibiting macroscopic DNA damages repair by targeting AP-2α with LEI110 leads to the eradication of hepatocellular carcinoma [120]. Research in this sense was not only focused on neoplastic disorders but also on other concerning diseases like hepatic fibrosis. In this sense, it was shown that by promoting PPARγ expression, it was possible to deactivate hepatic stellate cells (which are primarily involved in the production of fibrotic tissue) by inhibiting EZH2-mediated histone H3K27 trimethylation [121].
5. 3D Culture Models for Bacterial Infections
Bacterial infections are a leading cause of death worldwide, primarily due to the emergence of antibiotic-resistant bacteria. This poses a significant threat to public health. According to the World Health Organization, deaths caused by antibiotic-resistant strains are projected to surpass those caused by cancer by 2050. Therefore, scientists are endeavoring to enhance their comprehension of infection mechanisms, as host–microbe interactions significantly impact human physiology [122]. At present, many 2D models used to study bacterial infections are deficient due to several factors, including a failure to accurately mimic the in vivo bacterial environment, the lack of pathogen-specific cell types and receptors [123], the absence of cross-talking networks due to the human tissue structure [124] and other factors [125]. Recent discoveries have shown a growing interest in 3D culture models due to their ability to provide more accurate biochemical and biomechanical microenvironments [126]. The development of 3D organotypic models, such as organ-on-a-chip systems, provides a promising platform for modeling physiological and pathological functions of tissues and organs in vitro. These models have been applied in various fields, including the investigation of in vitro bacterial and viral infections. Various 3D models used in the study of bacterial infections are presented in Table 4.

Table 4.
Examples of 3D in vitro models for the study of host–microbe interactions.
Cheng et al. [127], in their study, bioprinted a gelatin methacryloyl channel with Caco-2 to explore the inflammatory pathways triggered by co-cultures with Salmonella enterica and Lactobacillus reuteri in aerobic and anaerobic environments. The 3D sacrificially printed gut model has been able to capture the key interactions between the host and the microbes, identifying the enrichments of pathways associated with the inflammatory response. In a separate study, Gardnerella, Prevotella, Atopobium vaginae and Sneathia amnii were co-cultured with a representative health-associated commensal, Lactobacillus crispatus, using a 3D cell model of the cervix, demonstrating that the four pathogens caused the synthesis of numerous pro-inflammatory chemicals [128]. Furthermore, S. amnii strains exhibit potential oncogenic mechanisms based on the altered immunemetabolic microenvironment. Calatayud et al. [129] employed the compartmentalization properties of transwell chambers, reproducing a 3D model of the small intestine and exposing it to synthetic microbiota composed of eight Gram-positive and -negative commensal bacterial strains. The authors studied the different responses in the model due to either the presence or absence of lipopolysaccharide (LPS), a component of Gram-negative bacterial cell walls. They observed that the presence of LPS caused a decrease in epithelial barrier function and an increase in the production of IL-6. Calatayud et al. discovered an increase in the mRNA expression of dual oxidase 2 (DUOX2) and toll-like receptors 2 and 4 (TLR-2, TLR-4) in eukaryotic cells when exposed to LPS and microbiota. DUOX2 is a protein belonging to the NADPH oxidase family and is involved in regulating reactive oxygen species (ROS) in eukaryotic cells, whereas TLRs are membrane receptors involved in host immune defense. The described approach can be utilized as a baseline for subsequent applications, such as the utilization of primary cells or organoids in a coculture with synthetic or natural complex microbial communities, to enhance host–microbiome in vitro research. Recently, organoids have emerged as a promising model for replicating 3D environments in vitro. Gut organoids are currently being used to study bacterial pathogenicity, including Listeria monocytogenes [130], which can cross the epithelial barrier and induce an inflammatory response in the epithelium.
A study conducted by Koestler et al. [131] reported human intestinal enteroids (HIE) monolayers infected with the virulent Shigella flexneri and an avirulent strain (CSF100). In the work, the expression (as fold change) of human host genes involved in inflammation, apoptosis and autophagy was analyzed (Figure 4a). The authors discovered, after the three hours of the infection of HIE with the wild-type (WT) Shigella, that, compared to the avirulent CFS100 control strain, there was a significant increase in host inflammation gene expression such as: nuclear factor kappaB (NF-κB), IL-8, interferon beta (INF-β), tumor necrosis factor alpha (TNF-α) and tumor necrosis factor alpha-induced protein 3 (TNFAIP3). The latter gene was significantly increased after infection with the avirulent Shigella, indicating that the control can induce changes in the host response. Furthermore, B-cell lymphoma 2 (BCL-2) and solute carrier family 7 member 5 (SLC7A5) genes, involved in apoptosis and autophagy, respectively, have been discovered to be up-regulated after infection with the wild type of a virulent strain (Figure 4a). HIEs can be powerful 3D models for future investigations of previously unknown features of Shigella pathogenesis, allowing researchers to explore bacterial interactions with the mucin, host immune cells and innate immune responses. A recent study on 3D models of the intestine simulated the initial phase of Salmonella enterica (serovar Typhimurium) infection using a low fluid shear culture system designed to mimic microgravity conditions (LSMMG—Low Shear Modeled Microgravity) [132]. In this study, wild-type and delta-hfq mutant Salmonella were cultured in LSMMG and in a control culture in order to investigate the role of the Hfq, an RNA-binding chaperone, in regulating the transcriptional stress response of microorganisms to the LSMMG culture. The authors, Barrila et al. [132], showed that a lack of gravity in LSMMG significantly up-regulated Salmonella virulence proteins involved in host adhesion and invasion. Although the mutant delta-hfq strains were defective in invasion genes, they were expressed in LSMMG. Panel B of Figure 4 reports the different gene expressions (as mean log2 fold change) of the WT and delta-hfq Salmonella strains in LSMMG culture conditions compared to control conditions. In particular, the analyzed genes were associated with the Salmonella Pathogenicity Island (SPI)—1 and 2, motility and chemotaxis [132]. Genes belonging to SPI-1 that are responsible for host colonization, such as invA, invG, prgI, sipC and other genes involved either in motility (flgA, flgB, flgC) or in chemotaxis (cheB, cheM), were up-regulated in WT cultured in LSMMG. These genes were divided into classes 2 and 3 according to either the middle or late assembly stages. Other genes included in SPI-2 such as ssaL, ssaM, sifA steC and the Salmonella anti-inflammatory response activator stm2585 gene were down-regulated in LSMMG. However, some of the delta-hfq expressed genes were oppositely regulated in LSMMG with respect to the WT, such as the D-galactonate transport dgoT gene, up-regulated in WT and down-regulated in the mutant. Other genes like SPI-1 iagB, responsible for the type III secretion system protein were down-regulated in WT and up-regulated in mutant Salmonella in LSMMG. Motility and chemotaxis genes were mostly up-regulated in the mutant strain, as shown in Figure 4b. Among SPI-2 genes, those involved in the Salmonella secretion system, such as ssaB and ssaK, and those responsible for the maintenance of Salmonella vacuolar internalization in hosts, like sifA and sifB, were down-regulated in the delta-hfq strain in response to LSMMG conditions. Bacterial infection can induce a different gene expression in a host. Indeed, Barrila et al. reported in Figure 4c the up- and down-regulation of host genes at 24 h post-infection (hpi) caused by WT and delta-hfq strains in LSMMG, compared to control culture conditions. Expression was represented as the FC (fold change) as a function of the FDR (false discovery rate). The latter represents the rate at which features considered significant are truly null and was <0.05. In the plots, values > 0.05 were significant. After Salmonella infection, mRNAs encoding for CXCL8 (IL-8) were up-regulated in host cells infected by both WT and delta-hfq strains in LSMMG conditions (Figure 4c). Other host genes involved in encoding for histone proteins (HIST1H3J, HIST1H1E, HIST1H2AG) were down-regulated in cells infected by WT in LSMMG. On the other hand, gene expression is associated with tumor and disease development, including MMP13 (matrix metallopeptidase 13), SERPINB4 (serine protease inhibitor serpin family B member 4), SOCS3 (suppressor of cytokine signaling 3) and SLC26A4 (Solute Carrier Family 26 Member 4), which were up-regulated after the infection with either the WT Salmonella or mutant strain, as shown in panel C. Finally, keratinocyte-fibroblast co-cultures are another promising 3D model for studying skin infections caused by various pathogens, including Staphylococcus aureus MRSA bacteria [133]. Panel D of Figure 4, proposed by Barua et al. [133], provides a better understanding of the interactions between MRSA strains and human skin after 48 h of infection. Each line, composed of i, ii, iii and iv, represents a strain of MRSA (ST8, ST30, ST59, ST22, ST45, ST239). The authors marked (i) HaCaT keratinocytes nuclei present in the strata basale and spinosum and fibroblasts localized in collagen gel. They moreover observed the dissemination of various MRSA strains (ii) through the layers of 3D skin models thanks to the interaction between bacterial FnBPs (fibronectin-binding proteins) and the epithelial cell HS60 protein (heat-shock protein 60). The staphylococcal colonization of human tissue is due to MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) surface proteins, including FnBPs [134,135], that interact with human extracellular matrix proteins, causing the infections.

Figure 4.
Infection in 3D culture models. (a) Human intestinal enteroids (HIE) monolayers were infected with wild-type (WT) Shigella and the avirulent CSF100 strain. The panel shows host gene expression (as log2-fold change) after three hours of Shigella infection (adapted from [131] with permission from Copyright American Society for Microbiology-License number 1494825-1). Statistical significance is expressed as p < 0.05, *. (b,c) Salmonella infection in LSMMG and in control culture conditions (adapted from [132] under the terms of a CC-BY 4.0 publishing license). The analyzed strains were the wild-type (WT) and the mutant delta-hfq strains. (b) Bacterial genes that were up- and down-regulated, in red and blue, respectively, were associated with Salmonella Pathogenic Islands (SPI) 1, 2, motility and chemotaxis. The expression was reported as the mean log2 fold change. (c) Plots illustrating the up- (red dots) and down-regulation (blue dots) of genes expressed by host cells infected at 24 h post-infection (hpi) by WT (left panel) and delta-hfq (right panel) strains in LSMMG conditions. Expression reported as the logFC (logged fold change) as a function of the FDR (false discovery rate) < 0.05. (d) Label of a section of a skin 3D model after 48 h of infection with MRSA bacterial strains (ST8, ST30, ST59, ST22, ST45, ST239). Each line composed of i, ii, iii and iv represents a bacterial strain. The white dashed line marks the dermal epidermal barrier between the stratum basale and the collagen gel containing fibroblasts. Specifically, (i) shows HaCaT keratinocytes nuclei, at the strata basale and spinosum (yellow line), marked in blue with Hoechst stain. (ii) shows MRSA bacteria labeled with an anti-S. aureus antibody and Alexa Fluor® 568 conjugated secondary antibody. Those indicated by yellow arrows are in the collagen gel. (iii) shows the Click-iT® TUNEL Alexa Fluor® 488 cell for the detection of damaged DNA. Finally, (iv) is an overlay where bacteria and apoptosis/DNA damage are co-localized in keratinocytes in the stratum spinosum. The yellow circles in (iv) depict the model’s skin being exfoliated. Scale Bar (i–iv) of 50 µm (adapted from [133] under the terms of a CC-BY 4.0 publishing license).
Barua et al. [133] evaluated not only the staphylococcal internalization from epithelial cells but also the eukaryotic cell apoptosis (iii) induced by the different MRSA strains. Panel D (iv) shows a merger of the various kinds of staining used in the study. The present model used in this study is able to more accurately mimic physiological responses and provides reliable information for observing differences in the stimulation of cell death up to 48 h, information that could not be properly retrieved from a 2D model.
New culture methods are constantly being developed, while old methods are being adapted to meet the challenges of today. A deeper understanding of the spatial and temporal interactions between microbes, hosts and their environment may aid in the development of in vitro, ex vivo and in vivo disease models and may also lead to new therapeutic approaches in different research fields.
6. Discussion and Concluding Remarks
As is documented in this review, there is no doubt that 3D cultures can be a formidable tool for research aimed not only at better elucidating cellular and molecular mechanisms but also at identifying therapeutic targets and testing potential therapeutic strategies. A wide variety of 3D cultures have been used, ranging from the simplest and least expensive systems, such as spheroids, to those that allow for the spatial control of cells and the modulation of certain characteristics of the cellular microenvironment (microfluidic and bioprinting techniques-based). In general, it can be argued that the 3D cell cultures mentioned in this review provided new experimental evidence on cellular and molecular mechanisms, such as those related to proliferation, differentiation, migration, gene expression signatures, microRNA profiling and growth factor signaling in support of or in combination with 2D cultures.
It is worth emphasizing that developing accurate 3D models may lead to the evaluation of a certain association between the different research areas considered here. For example, 3D cell cultures could be established with cellular and matrix components to mimic metastatic sites of breast cancer, such the liver, bone, lungs and brains [75]. Some studies hypothesized the association between endometriosis and breast cancer [136]: 3D models could help us understand the signaling interaction between cancer cells and those involved in endometriosis and whether there are possible pathogenetic pathways linking the two diseases. It is estimated that infections may contribute to up to 20% of all human tumors [137,138]): the utilization of 3D models may prove to be a valuable approach in studying the correlation between bacterial infections and cancer, as well as identifying the adaptation strategies employed by bacteria to evade antibiotic/antimicrobial therapy. In addition, the CF-Mu3Gel hydrogel, which contains mucin, has recently been developed by our research group [139]. This hydrogel could serve as a valuable 3D model for evaluating the effectiveness of new antimicrobial drugs.
Another potential benefit of using 3D models is in the field of nanomedicine, which involves the application of nanotechnology in medicine. The similarity between spheroids or 3D models and diseased tissue can allow for screening and exploring the biological effects of different nanotherapeutics for different uses, such as chemotherapeutics, chemo-immunotherapeutics, radiotherapeutics, photothermal therapies, photodynamic therapies and gene delivery [4].
Despite the enormous potential, 3D cell cultures face many challenges, including the difficulty of replicating all the chemical and biophysical characteristics of the tissue microenvironment, the frequent use of the cell lines, the poor standardization of protocols and the higher costs compared to those of 2D systems [126]. Furthermore, the reproducibility of the results may be reduced by technical drawbacks along the quantification methods based on biochemical analysis or microscope imaging due to the complex nature of 3D cell cultures themselves. (e.g., the collection of cells or secreted factors for biochemical tests may be more difficult in some ECM-based 3D cell cultures) [140]. We believe that the publication of detailed protocols will help the research community standardize the way 3D cell cultures are set up and the way cellular and molecular effects are analyzed. Similarly, integrated analysis of the transcriptome and proteome could help determine the most appropriate model in terms of clinical translation, cost and efficiency. Beyond these considerations, in our view, the choice of the type of 3D platform/technology will undoubtedly need to be evaluated based on the scientific questions to be explored.
Author Contributions
Conceptualization, N.B. and L.V.; writing—original draft preparation, N.B., M.G., G.G., E.P., E.R., S.S., C.V., P.P. and L.V.; review and editing, N.B. and L.V.; supervision, N.B. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pavia University’s Crowdfunding (2021, https://universitiamo.eu/en/campaigns/tumore-al-seno-sconfiggerlo-con-nanosfere-doro-intelligenti-nuove-sfide/ accessed on 19 February 2024), a grant of the Italian Ministry of University and Research (MUR) to the Department of Molecular Medicine of the University of Pavia under the initiative “Dipartimenti di Eccellenza (2023–2027)” and “Progetti di Ricerca di Rilevante Interesse Nazionale (PRIN2022)” cod. 20227YWZSZ to L.V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Bianca Abbadessa (IULM University, Milan, Italy) for the linguistic proofreading.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pillai, S.; Munguia-Lopez, J.G.; Tran, S.D. Bioengineered Salivary Gland Microtissues—A Review of 3D Cellular Models and Their Applications. ACS Appl. Bio Mater. 2024, 7, 2620–2636. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, N.A.; El Khalki, L.; Wang, W.; Szpendyk, J.; Sossey-Alaoui, K. Advances in 3D Culture Models to Study Exosomes in Triple-Negative Breast Cancer. Cancers 2024, 16, 883. [Google Scholar] [CrossRef] [PubMed]
- Atat, O.E.; Farzaneh, Z.; Pourhamzeh, M.; Taki, F.; Abi-Habib, R.; Vosough, M.; El-Sibai, M. 3D Modeling in Cancer Studies. Hum. Cell 2022, 35, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Tofani, L.B.; Luiz, M.T.; Paes Dutra, J.A.; Abriata, J.P.; Chorilli, M. Three-Dimensional Culture Models: Emerging Platforms for Screening the Antitumoral Efficacy of Nanomedicines. Nanomedicine 2023, 18, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Esparza, A.; Jimenez, N.; Borrego, E.A.; Browne, S.; Natividad-Diaz, S.L. Review: Human Stem Cell-Based 3D in Vitro Angiogenesis Models for Preclinical Drug Screening Applications. Mol. Biol. Rep. 2024, 51, 260. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Huang, S. Advances in the Application of 3D Tumor Models in Precision Oncology and Drug Screening. Front. Bioeng. Biotechnol. 2022, 10, 1021966. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, J.F.; Sousa, F.L.; Monteiro, M.V.; Gaspar, V.M.; Silva, N.J.O.; Mano, J.F. Advances in Screening Hyperthermic Nanomedicines in 3D Tumor Models. Nanoscale Horiz. 2024, 9, 334–364. [Google Scholar] [CrossRef] [PubMed]
- Guller, A.; Igrunkova, A. Engineered Microenvironments for 3D Cell Culture and Regenerative Medicine: Challenges, Advances, and Trends. Bioengineering 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, P.E.; Abrahamse, H.; Crous, A. Insights on Three Dimensional Organoid Studies for Stem Cell Therapy in Regenerative Medicine. Stem Cell Rev. Rep. 2024, 20, 509–523. [Google Scholar] [CrossRef]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- d’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Guagliano, G.; Volpini, C.; Briatico-Vangosa, F.; Cornaglia, A.I.; Visai, L.; Petrini, P. Toward 3D-Bioprinted Models of the Liver to Boost Drug Development. Macromol. Biosci. 2022, 22, e2200264. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell Lines: Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, G.M. Advantages and Disadvantages of Using Human Cells for Pharmacological and Toxicological Studies. Hum. Exp. Toxicol. 1994, 13, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Ramesh, A.; Pattabhi, A. Contributions of 3D Cell Cultures for Cancer Research. J. Cell. Physiol. 2017, 232, 2679–2697. [Google Scholar] [CrossRef]
- Gultian, K.A.; Gandhi, R.; Sarin, K.; Sladkova-Faure, M.; Zimmer, M.; de Peppo, G.M.; Vega, S.L. Human Induced Mesenchymal Stem Cells Display Increased Sensitivity to Matrix Stiffness. Sci. Rep. 2022, 12, 8483. [Google Scholar] [CrossRef]
- Septiana, W.L.; Noviantari, A.; Antarianto, R.D. Induced Pluripotent Stem Cells (Ipscs) Based Liver Organoid: The Benefits and Challenges. Cell Physiol. Biochem. 2023, 57, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Sumbalova Koledova, Z. 3D Cell Culture: Techniques for and Beyond Organoid Applications. In 3D Cell Culture: Methods and Protocols; Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2764, pp. 1–12. [Google Scholar] [CrossRef]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-Generation Cancer Organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Kalyani, F.S.; Liu, L.; Cheng, T.; Chen, L. Tumor Organoids: Synergistic Applications, Current Challenges, and Future Prospects in Cancer Therapy. Cancer Commun. 2021, 41, 1331–1353. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, L.; Ding, Y.; Dong, Y.; Ma, M. Advances in Biomimetic Hydrogels for Organoid Culture. Chem. Commun. 2023, 59, 9675–9686. [Google Scholar] [CrossRef] [PubMed]
- Rassomakhina, N.V.; Ryazanova, A.Y.; Likhov, A.R.; Bruskin, S.A.; Maloshenok, L.G.; Zherdeva, V.V. Tumor Organoids: The Era of Personalized Medicine. Biochemistry 2024, 89, S127–S147. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.-L. Generation of Human Brain Region-Specific Organoids Using a Miniaturized Spinning Bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Trossbach, M.; Åkerlund, E.; Langer, K.; Seashore-Ludlow, B.; Joensson, H.N. High-Throughput Cell Spheroid Production and Assembly Analysis by Microfluidics and Deep Learning. SLAS Technol. 2023, 28, 423–432. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Maharjan, S.; Ma, C.; Singh, B.; Kang, H.; Orive, G.; Yao, J.; Shrike Zhang, Y. Advanced 3D Imaging and Organoid Bioprinting for Biomedical Research and Therapeutic Applications. Adv. Drug Deliv. Rev. 2024, 208, 115237. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Guzzi, F.; Parrotta, E.; Candeloro, P.; Scalise, S.; Lucchino, V.; Gentile, F.; Tirinato, L.; Coluccio, M.L.; Torre, B.; et al. Microfluidics for 3D Cell and Tissue Cultures: Microfabricative and Ethical Aspects Updates. Cells 2022, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lam, P.Y.; Jayaraman, A.; Han, A. Uniform Sized Cancer Spheroids Production Using Hydrogel-Based Droplet Microfluidics: A Review. Biomed. Microdevices 2024, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, M.S.; Hammer, J.A. Using Biosensors to Study Organoids, Spheroids and Organs-on-a-Chip: A Mechanobiology Perspective. Biosensors 2023, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Żuchowska, A.; Baranowska, P.; Flont, M.; Brzózka, Z.; Jastrzębska, E. Review: 3D Cell Models for Organ-on-a-Chip Applications. Anal. Chim. Acta 2024, 1301, 342413. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The Variety of 3D Breast Cancer Models for the Study of Tumor Physiology and Drug Screening. Int. J. Mol. Sci. 2023, 24, 7116. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D Cancer Models: One Step Closer to in Vitro Human Studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef]
- Lu, X.; Lodi, A.; Konopleva, M.; Tiziani, S. Three-Dimensional Leukemia Co-Culture System for In Vitro High-Content Metabolomics Screening. SLAS Discov. 2019, 24, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaabneh, B.; Frisch, B.; Aljitawi, O.S. The Potential Role of 3D In Vitro Acute Myeloid Leukemia Culture Models in Understanding Drug Resistance in Leukemia Stem Cells. Cancers 2022, 14, 5252. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, D.P.; Matias, A.T.; Braga, S.; Jacinto, A.; Cabral, M.G. Establishment of a 3D Co-Culture With MDA-MB-231 Breast Cancer Cell Line and Patient-Derived Immune Cells for Application in the Development of Immunotherapies. Front. Oncol. 2020, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Tevlek, A. The Role of Decellularized Cell Derived Extracellular Matrix in the Establishment and Culture Ofin Vitrobreast Cancer Tumor Model. Biomed. Mater. 2024, 19, 025037. [Google Scholar] [CrossRef] [PubMed]
- Kumano, K.; Nakahashi, H.; Louphrasitthiphol, P.; Kuroda, Y.; Miyazaki, Y.; Shimomura, O.; Hashimoto, S.; Akashi, Y.; Mathis, B.J.; Kim, J.; et al. Hypoxia at 3D Organoid Establishment Selects Essential Subclones within Heterogenous Pancreatic Cancer. Front. Cell Dev. Biol. 2024, 12, 1327772. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-Munoz, M.; McMinn, P.H.; Rehman, S.; Gomez, I.; Karim, M.R.; Trusttchel, R.; Wisinski, K.B.; Beebe, D.J.; Skala, M.C. Tumor-on-a-Chip: A Microfluidic Model to Study Cell Response to Environmental Gradients. Lab Chip 2019, 19, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Ahn, M.; Cho, W.-W.; Santos, M.; Wise, J.; Phillips, E.; Wise, S.G.; Jang, J.; Rnjak-Kovacina, J.; Woodfield, T.; et al. Programming Temporal Stiffness Cues within Extracellular Matrix Hydrogels for Modelling Cancer Niches. Mater. Today Bio 2024, 25, 101004. [Google Scholar] [CrossRef]
- Briem, E.; Ingthorsson, S.; Traustadottir, G.A.; Hilmarsdottir, B.; Gudjonsson, T. Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture. J. Mammary Gland. Biol. Neoplasia 2019, 24, 139–147. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast Cancer Models: Engineering the Tumor Microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sang, Y.; Song, X.; Zhang, D.; Wang, L.; Zhao, W.; Liang, Y.; Zhang, N.; Yang, Q. Exosomal miR-500a-5p Derived from Cancer-Associated Fibroblasts Promotes Breast Cancer Cell Proliferation and Metastasis through Targeting USP28. Theranostics 2021, 11, 3932–3947. [Google Scholar] [CrossRef] [PubMed]
- Watzling, M.; Klaus, L.; Weidemeier, T.; Horder, H.; Ebert, R.; Blunk, T.; Bauer-Kreisel, P. Three-Dimensional Breast Cancer Model to Investigate CCL5/CCR1 Expression Mediated by Direct Contact between Breast Cancer Cells and Adipose-Derived Stromal Cells or Adipocytes. Cancers 2023, 15, 3501. [Google Scholar] [CrossRef] [PubMed]
- Jahin, I.; Phillips, T.; Marcotti, S.; Gorey, M.-A.; Cox, S.; Parsons, M. Extracellular Matrix Stiffness Activates Mechanosensitive Signals but Limits Breast Cancer Cell Spheroid Proliferation and Invasion. Front. Cell Dev. Biol. 2023, 11, 1292775. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, S.; Ahmadi, S.; Mokarian, F.; Sharifi, M.; Ghobakhloo, S.; Yazdi, M.; Nedaeinia, R.; Salehi, R. MSC-Derived Exosomes Enhance the Anticancer Activity of Drugs in 3D Spheroid of Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2024, 92, 105375. [Google Scholar] [CrossRef]
- Shibuya, N.; Kakeji, Y.; Shimono, Y. MicroRNA-93 Targets WASF3 and Functions as a Metastasis Suppressor in Breast Cancer. Cancer Sci. 2020, 111, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhong, F.; Sun, S.; Ou, X.; Yuan, J.; Zhu, J.; Zeng, Z. Tamoxifen Induces Ferroptosis in MCF-7 Organoid. J. Cancer Res. Ther. 2023, 19, 1627–1635. [Google Scholar] [CrossRef]
- Engel, M.; Belfiore, L.; Aghaei, B.; Sutija, M. Enabling High Throughput Drug Discovery in 3D Cell Cultures through a Novel Bioprinting Workflow. SLAS Technol. 2022, 27, 32–38. [Google Scholar] [CrossRef]
- Jaiswal, C.; Mandal, B.B. A 3D In Vitro Triculture Hybrid Model Recapitulating Tumor Stromal Interaction of Triple-Negative Breast Cancer as a High Throughput Anticancer Drug Screening Platform. Adv. Ther. 2024, 2300450. [Google Scholar] [CrossRef]
- Brancato, V.; Kundu, B.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Tumor-Stroma Interactions Alter the Sensitivity of Drug in Breast Cancer. Front. Mater. 2020, 7, 116. [Google Scholar] [CrossRef]
- Liverani, C.; De Vita, A.; Spadazzi, C.; Miserocchi, G.; Cocchi, C.; Bongiovanni, A.; De Lucia, A.; La Manna, F.; Fabbri, F.; Tebaldi, M.; et al. Lineage-Specific Mechanisms and Drivers of Breast Cancer Chemoresistance Revealed by 3D Biomimetic Culture. Mol. Oncol. 2022, 16, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Itah, Z.; Chaudhry, S.; Raju Ponny, S.; Aydemir, O.; Lee, A.; Cavanagh-Kyros, J.; Tournier, C.; Muller, W.J.; Davis, R.J. HER2-Driven Breast Cancer Suppression by the JNK Signaling Pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2218373120. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Dong, Z.-X.; Jiang, D.; Li, X.; Lin, S.; Chen, D.; Zou, X.; Zhang, X.-D.; Luker, G.D. Heterogeneous Microenvironmental Stiffness Regulates Pro-Metastatic Functions of Breast Cancer Cells. Acta Biomater. 2021, 131, 326–340. [Google Scholar] [CrossRef]
- Truong, D.D.; Kratz, A.; Park, J.G.; Barrientos, E.S.; Saini, H.; Nguyen, T.; Pockaj, B.; Mouneimne, G.; LaBaer, J.; Nikkhah, M. A Human Organotypic Microfluidic Tumor Model Permits Investigation of the Interplay between Patient-Derived Fibroblasts and Breast Cancer Cells. Cancer Res. 2019, 79, 3139–3151. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-Associated Fibroblasts: From Basic Science to Anticancer Therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]
- Zheng, G.-D.; Xu, Z.-Y.; Hu, C.; Lv, H.; Xie, H.-X.; Huang, T.; Zhang, Y.-Q.; Chen, G.-P.; Fu, Y.-F.; Cheng, X.-D. Exosomal miR-590-5p in Serum as a Biomarker for the Diagnosis and Prognosis of Gastric Cancer. Front. Mol. Biosci. 2021, 8, 636566. [Google Scholar] [CrossRef]
- Manevich, L.; Okita, Y.; Okano, Y.; Sugasawa, T.; Kawanishi, K.; Poullikkas, T.; Dang Cao, L.T.L.; Zheng, L.; Nakayama, M.; Matsumoto, S.; et al. Glycoprotein NMB Promotes Tumor Formation and Malignant Progression of Laryngeal Squamous Cell Carcinoma. Cancer Sci. 2022, 113, 3244–3254. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S. Mechanistic Insights of Adipocyte Metabolism in Regulating Breast Cancer Progression. Pharmacol. Res. 2020, 155, 104741. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takagi, K.; Narita, K.; Miki, Y.; Onodera, Y.; Miyashita, M.; Sasano, H.; Suzuki, T. Stromal CCL5 Promotes Breast Cancer Progression by Interacting with CCR3 in Tumor Cells. Int. J. Mol. Sci. 2021, 22, 1918. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM Stiffness-Tuned Exosomes Drive Breast Cancer Motility through Thrombospondin-1. Biomaterials 2021, 279, 121185. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Wong, B.S.; Shriver, M.; Phillip, J.M.; Wirtz, D.; Kontrogianni-Konstantopoulos, A.; Konstantopoulos, K. Loss of Giant Obscurins Alters Breast Epithelial Cell Mechanosensing of Matrix Stiffness. Oncotarget 2017, 8, 54004–54020. [Google Scholar] [CrossRef] [PubMed]
- Balachander, G.M.; Kotcherlakota, R.; Nayak, B.; Kedaria, D.; Rangarajan, A.; Chatterjee, K. 3D Tumor Models for Breast Cancer: Whither We Are and What We Need. ACS Biomater. Sci. Eng. 2021, 7, 3470–3486. [Google Scholar] [CrossRef] [PubMed]
- Micalet, A.; Moeendarbary, E.; Cheema, U. 3D In Vitro Models for Investigating the Role of Stiffness in Cancer Invasion. ACS Biomater. Sci. Eng. 2021, 9, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 631991. [Google Scholar] [CrossRef] [PubMed]
- Ponce, I.; Garrido, N.; Tobar, N.; Melo, F.; Smith, P.C.; Martínez, J. Matrix Stiffness Modulates Metabolic Interaction between Human Stromal and Breast Cancer Cells to Stimulate Epithelial Motility. Metabolites 2021, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Nikdouz, A.; Orso, F. Emerging Roles of 3D-Culture Systems in Tackling Tumor Drug Resistance. Cancer Drug Resist. 2023, 6, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug Resistance Evaluation in Novel 3D in Vitro Model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef]
- Al-Malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major Obstacles to Doxorubicin Therapy: Cardiotoxicity and Drug Resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. Three-Dimensional Cell Cultures as an In Vitro Tool for Prostate Cancer Modeling and Drug Discovery. Int. J. Mol. Sci. 2020, 21, 6806. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek-Grenda, A.; Olejnik, A. In Vitro Modeling of Endometriosis and Endometriotic Microenvironment-Challenges and Recent Advances. Cell Signal. 2022, 97, 110375. [Google Scholar] [CrossRef] [PubMed]
- Volpini, C.; Bloise, N.; Dominoni, M.; Barra, F.; Vellone, V.G.; Minzioni, P.; Gardella, B.; Ferrero, S.; Visai, L. The Nano-Revolution in the Diagnosis and Treatment of Endometriosis. Nanoscale 2023, 15, 17313–17325. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Brueggmann, D.; Templeman, C.; Starzinski-Powitz, A.; Rao, N.P.; Gayther, S.A.; Lawrenson, K. Novel Three-Dimensional in Vitro Models of Ovarian Endometriosis. J. Ovarian Res. 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.-K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I Triggers Directional Migration, Invasion and Matrix Remodeling of Stroma Cells in a 3D Spheroid Model of Endometriosis. Sci. Rep. 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.R.H.; Wang, X.; Smith, L.J.; Hawkins, S.M. Three-Dimensional Biofabrication Models of Endometriosis and the Endometriotic Microenvironment. Biomedicines 2020, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Burns, G.W.; Joshi, N.R.; Arora, R.; Kim, J.J.; Fazleabas, A.T. Spheroids as a Model for Endometriotic Lesions. JCI Insight 2023, 8, e160815. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Fan, X.; Dhal, S.; Nayak, N.R. Development of A 3D Tissue Slice Culture Model for the Study of Human Endometrial Repair and Regeneration. Biomolecules 2020, 10, 136. [Google Scholar] [CrossRef]
- Ahn, J.; Yoon, M.-J.; Hong, S.-H.; Cha, H.; Lee, D.; Koo, H.S.; Ko, J.-E.; Lee, J.; Oh, S.; Jeon, N.L.; et al. Three-Dimensional Microengineered Vascularised Endometrium-on-a-Chip. Hum. Reprod. 2021, 36, 2720–2731. [Google Scholar] [CrossRef]
- Esfandiari, F.; Favaedi, R.; Heidari-Khoei, H.; Chitsazian, F.; Yari, S.; Piryaei, A.; Ghafari, F.; Baharvand, H.; Shahhoseini, M. Insight into Epigenetics of Human Endometriosis Organoids: DNA Methylation Analysis of HOX Genes and Their Cofactors. Fertil. Steril. 2021, 115, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Nap, A.W.; Dunselman, G.A.J.; de Goeij, A.F.P.M.; Evers, J.L.H.; Groothuis, P.G. Inhibiting MMP Activity Prevents the Development of Endometriosis in the Chicken Chorioallantoic Membrane Model. Hum. Reprod. 2004, 19, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson Teague, E.M.C.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-Regulated Pathways Associated with Endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Guagliano, G.; Volpini, C.; Sardelli, L.; Bloise, N.; Briatico-Vangosa, F.; Cornaglia, A.I.; Dotti, S.; Villa, R.; Visai, L.; Petrini, P. Hep3Gel: A Shape-Shifting Extracellular Matrix-Based, Three-Dimensional Liver Model Adaptable to Different Culture Systems. ACS Biomater. Sci. Eng. 2023, 9, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Becattini, B.; Solinas, G. Insulin Signaling and Glucose Metabolism in Different Hepatoma Cell Lines Deviate from Hepatocyte Physiology toward a Convergent Aberrant Phenotype. Sci. Rep. 2020, 10, 12031. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Han, R.; Chang, S.; Ni, J.; Hunziker, W.; Goryachev, A.B.; Ong, S.H.; Yu, H. Improved Hepatocyte Excretory Function by Immediate Presentation of Polarity Cues. Tissue Eng. 2006, 12, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.A.; Gaudenzi, G.; Russom, A. 3D Bioprinting of Multi-Material Decellularized Liver Matrix Hydrogel at Physiological Temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Khati, V.; Turkki, J.A.; Ramachandraiah, H.; Pati, F.; Gaudenzi, G.; Russom, A. Indirect 3D Bioprinting of a Robust Trilobular Hepatic Construct with Decellularized Liver Matrix Hydrogel. Bioengineering 2022, 9, 603. [Google Scholar] [CrossRef]
- Sun, L.; Yang, H.; Wang, Y.; Zhang, X.; Jin, B.; Xie, F.; Jin, Y.; Pang, Y.; Zhao, H.; Lu, X.; et al. Application of a 3D Bioprinted Hepatocellular Carcinoma Cell Model in Antitumor Drug Research. Front. Oncol. 2020, 10, 878. [Google Scholar] [CrossRef]
- Ramaiahgari, S.C.; den Braver, M.W.; Herpers, B.; Terpstra, V.; Commandeur, J.N.M.; van de Water, B.; Price, L.S. A 3D in Vitro Model of Differentiated HepG2 Cell Spheroids with Improved Liver-like Properties for Repeated Dose High-Throughput Toxicity Studies. Arch. Toxicol. 2014, 88, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Miyamoto, H.; Tokunaga, A.; Fumoto, S.; Tanaka, T.; Nishida, K. Evaluation of mRNA Expression of Drug-Metabolizing Enzymes in Acetaminophen-Induced Hepatotoxicity Using a Three-Dimensional Hepatocyte Culture System. Xenobiotica 2020, 50, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Cuvellier, M.; Ezan, F.; Oliveira, H.; Rose, S.; Fricain, J.-C.; Langouët, S.; Legagneux, V.; Baffet, G. 3D Culture of HepaRG Cells in GelMa and Its Application to Bioprinting of a Multicellular Hepatic Model. Biomaterials 2021, 269, 120611. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hori, Y.; Yamamoto, T.; Urashima, T.; Ohara, Y.; Tanaka, H. 3D Spheroid Cultures Improve the Metabolic Gene Expression Profiles of HepaRG Cells. Biosci. Rep. 2015, 35, e00208. [Google Scholar] [CrossRef] [PubMed]
- Pihl, A.F.; Offersgaard, A.F.; Mathiesen, C.K.; Prentoe, J.; Fahnøe, U.; Krarup, H.; Bukh, J.; Gottwein, J.M. High Density Huh7.5 Cell Hollow Fiber Bioreactor Culture for High-Yield Production of Hepatitis C Virus and Studies of Antivirals. Sci. Rep. 2018, 8, 17505. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, Y.; Wang, Y.; Cai, Z.; Liao, N.; Liu, J.; Zhang, W. Co-Culture System of Hepatocytes and Endothelial Cells: Two in Vitro Approaches for Enhancing Liver-Specific Functions of Hepatocytes. Cytotechnology 2018, 70, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Taymour, R.; Kilian, D.; Ahlfeld, T.; Gelinsky, M.; Lode, A. 3D Bioprinting of Hepatocytes: Core-Shell Structured Co-Cultures with Fibroblasts for Enhanced Functionality. Sci. Rep. 2021, 11, 5130. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Johnson, B.; Seirup, M.; Ardalani, H.; Duffin, B.; Barrett-Wilt, G.A.; Stewart, R.; Thomson, J.A. Co-Culture with Mouse Embryonic Fibroblasts Improves Maintenance of Metabolic Function of Human Small Hepatocyte Progenitor Cells. Curr. Res. Toxicol. 2020, 1, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ware, B.R.; Liu, J.S.; Monckton, C.P.; Ballinger, K.R.; Khetani, S.R. Micropatterned Coculture With 3T3-J2 Fibroblasts Enhances Hepatic Functions and Drug Screening Utility of HepaRG Cells. Toxicol. Sci. 2021, 181, 90–104. [Google Scholar] [CrossRef]
- Lee, H.W.; Kook, Y.-M.; Lee, H.J.; Park, H.; Koh, W.-G. A Three-Dimensional Co-Culture of HepG2 Spheroids and Fibroblasts Using Double-Layered Fibrous Scaffolds Incorporated with Hydrogel Micropatterns. RSC Adv. 2014, 4, 61005–61011. [Google Scholar] [CrossRef]
- Kiamehr, M.; Heiskanen, L.; Laufer, T.; Düsterloh, A.; Kahraman, M.; Käkelä, R.; Laaksonen, R.; Aalto-Setälä, K. Dedifferentiation of Primary Hepatocytes Is Accompanied with Reorganization of Lipid Metabolism Indicated by Altered Molecular Lipid and miRNA Profiles. Int. J. Mol. Sci. 2019, 20, 2910. [Google Scholar] [CrossRef] [PubMed]
- Handin, N.; Mickols, E.; Ölander, M.; Rudfeldt, J.; Blom, K.; Nyberg, F.; Senkowski, W.; Urdzik, J.; Maturi, V.; Fryknäs, M.; et al. Conditions for Maintenance of Hepatocyte Differentiation and Function in 3D Cultures. iScience 2021, 24, 103235. [Google Scholar] [CrossRef]
- Nagata, S.; Ozawa, F.; Nie, M.; Takeuchi, S. 3D Culture of Functional Human iPSC-Derived Hepatocytes Using a Core-Shell Microfiber. PLoS ONE 2020, 15, e0234441. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, P.; de Hoyos-Vega, J.M.; Choi, J.H.; Duffy, C.D.; Gonzalez-Suarez, A.M.; Ishida, Y.; Nguyen, K.M.; Gwon, K.; Peterson, Q.P.; Saito, T.; et al. Guiding Hepatic Differentiation of Pluripotent Stem Cells Using 3D Microfluidic Co-Cultures with Human Hepatocytes. Cells 2023, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Freyer, N.; Brzeszczynska, J.; Knöspel, F.; Armstrong, L.; Lako, M.; Greuel, S.; Damm, G.; Ludwig-Schwellinger, E.; Deschl, U.; et al. Hepatic Differentiation of Human iPSCs in Different 3D Models: A Comparative Study. Int. J. Mol. Med. 2017, 40, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Ramanathan, R.; Fisher, R.A.; Mangino, M.J.; Zhang, N.; Wen, X. Scalable Differentiation of Human iPSCs in a Multicellular Spheroid-Based 3D Culture into Hepatocyte-like Cells through Direct Wnt/β-Catenin Pathway Inhibition. Sci. Rep. 2016, 6, 32888. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Qi, K.; Wang, L.; Yu, X.; Zhang, Q.; Yu, L.; Wang, L.; Yang, C.; Fan, L. E3 Ubiquitin Ligase CHIP Interacts with Transferrin Receptor 1 for Degradation and Promotes Cell Proliferation through Inhibiting Ferroptosis in Hepatocellular Carcinoma. Cell Signal. 2024, 118, 111148. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ding, C.; Yu, T.; Liu, B.; Tang, W.; Wang, Z.; Tang, X.; Liang, G.; Peng, J.; Zhang, X.; et al. SIRT7 Promotes Hippo/YAP Activation and Cancer Cell Proliferation in Hepatocellular Carcinoma via Suppressing MST1. Cancer Sci. 2024, 115, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Z.; Zhao, Y.; Zhao, J.; Xia, L.; Xia, Q. Macroscopic Inhibition of DNA Damage Repair Pathways by Targeting AP-2α with LEI110 Eradicates Hepatocellular Carcinoma. Commun. Biol. 2024, 7, 342. [Google Scholar] [CrossRef]
- Lan, T.; Li, P.; Zhang, S.-J.; Liu, S.-Y.; Zeng, X.-X.; Chai, F.; Tong, Y.-H.; Mao, Z.-J.; Wang, S.-W. Paeoniflorin Promotes PPARγ Expression to Suppress HSCs Activation by Inhibiting EZH2-Mediated Histone H3K27 Trimethylation. Phytomedicine 2024, 128, 155477. [Google Scholar] [CrossRef]
- Henneke, P.; Golenbock, D.T. Phagocytosis, Innate Immunity, and Host-Pathogen Specificity. J. Exp. Med. 2004, 199, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fasciano, A.C.; Mecsas, J.; Isberg, R.R. New Age Strategies to Reconstruct Mucosal Tissue Colonization and Growth in Cell Culture Systems. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.D.; Raghani, N.R.; Chorawala, M.R.; Singh, S.; Prajapati, B.G. Harnessing Three-Dimensional (3D) Cell Culture Models for Pulmonary Infections: State of the Art and Future Directions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.; Gaston, K.A.; McCracken, C.E.; Erdman, D.D.; Anderson, L.J. Response to Rhinovirus Infection by Human Airway Epithelial Cells and Peripheral Blood Mononuclear Cells in an in Vitro Two-Chamber Tissue Culture System. PLoS ONE 2013, 8, e66600. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, T.; Liu, Q.; Lian, L.; Tang, G.; Mille, L.S.; García, F.R.; Engstrand, L.; Zhang, Y.S.; Du, J. A 3D Bioprinted Gut Anaerobic Model for Studying Bacteria-Host Interactions. Research 2023, 6, 0058. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial Vaginosis and Health-Associated Bacteria Modulate the Immunometabolic Landscape in 3D Model of Human Cervix. NPJ Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Dezutter, O.; Hernandez-Sanabria, E.; Hidalgo-Martinez, S.; Meysman, F.J.R.; Van De Wiele, T. Development of a Host-Microbiome Model of the Small Intestine. FASEB J. 2019, 33, 3985–3996. [Google Scholar] [CrossRef] [PubMed]
- Roodsant, T.; Navis, M.; Aknouch, I.; Renes, I.B.; van Elburg, R.M.; Pajkrt, D.; Wolthers, K.C.; Schultsz, C.; van der Ark, K.C.H.; Sridhar, A.; et al. A Human 2D Primary Organoid-Derived Epithelial Monolayer Model to Study Host-Pathogen Interaction in the Small Intestine. Front. Cell. Infect. Microbiol. 2020, 10, 272. [Google Scholar] [CrossRef]
- Koestler, B.J.; Ward, C.M.; Fisher, C.R.; Rajan, A.; Maresso, A.W.; Payne, S.M. Human Intestinal Enteroids as a Model System of Shigella Pathogenesis. Infect. Immun. 2019, 87, e00733-18. [Google Scholar] [CrossRef]
- Barrila, J.; Yang, J.; Franco Meléndez, K.P.; Yang, S.; Buss, K.; Davis, T.J.; Aronow, B.J.; Bean, H.D.; Davis, R.R.; Forsyth, R.J.; et al. Spaceflight Analogue Culture Enhances the Host-Pathogen Interaction Between Salmonella and a 3-D Biomimetic Intestinal Co-Culture Model. Front. Cell. Infect. Microbiol. 2022, 12, 705647. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.; Huang, L.; Li, C.; Yang, Y.; Luo, M.; Wei, W.I.; Wong, K.T.; Lo, N.W.S.; Kwok, K.O.; Ip, M. Comparative Study of Two-Dimensional (2D) vs. Three-Dimensional (3D) Organotypic Kertatinocyte-Fibroblast Skin Models for Staphylococcus Aureus (MRSA) Infection. Int. J. Mol. Sci. 2022, 23, 299. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus Aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Linek, U.; Höök, M.; Potts, J.R. Fibronectin-Binding Proteins of Gram-Positive Cocci. Microbes Infect. 2006, 8, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Anifantaki, F.; Boutas, I.; Kalampokas, T.; Kalampokas, E.; Sofoudis, C.; Salakos, N. Association of Endometriosis and Breast Cancer: Mini Review of the Literature. Arch. Gynecol. Obstet. 2016, 293, 5–10. [Google Scholar] [CrossRef] [PubMed]
- van Elsland, D.; Neefjes, J. Bacterial Infections and Cancer. EMBO Rep. 2018, 19, e46632. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association and Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef]
- Peneda Pacheco, D.; Bertoglio, F.; Butnarasu, C.; Suarez Vargas, N.; Guagliano, G.; Ziccarelli, A.; Briatico-Vangosa, F.; Ruzzi, V.; Buzzaccaro, S.; Piazza, R.; et al. Heterogeneity Governs 3D-Cultures of Clinically Relevant Microbial Communities. Adv. Funct. Mater. 2023, 33, 2306116. [Google Scholar] [CrossRef]
- Temple, J.; Velliou, E.; Shehata, M.; Lévy, R.; Gupta, P. Current Strategies with Implementation of Three-Dimensional Cell Culture: The Challenge of Quantification. Interface Focus 2022, 12, 20220019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).