Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved?
Abstract
:1. Introduction
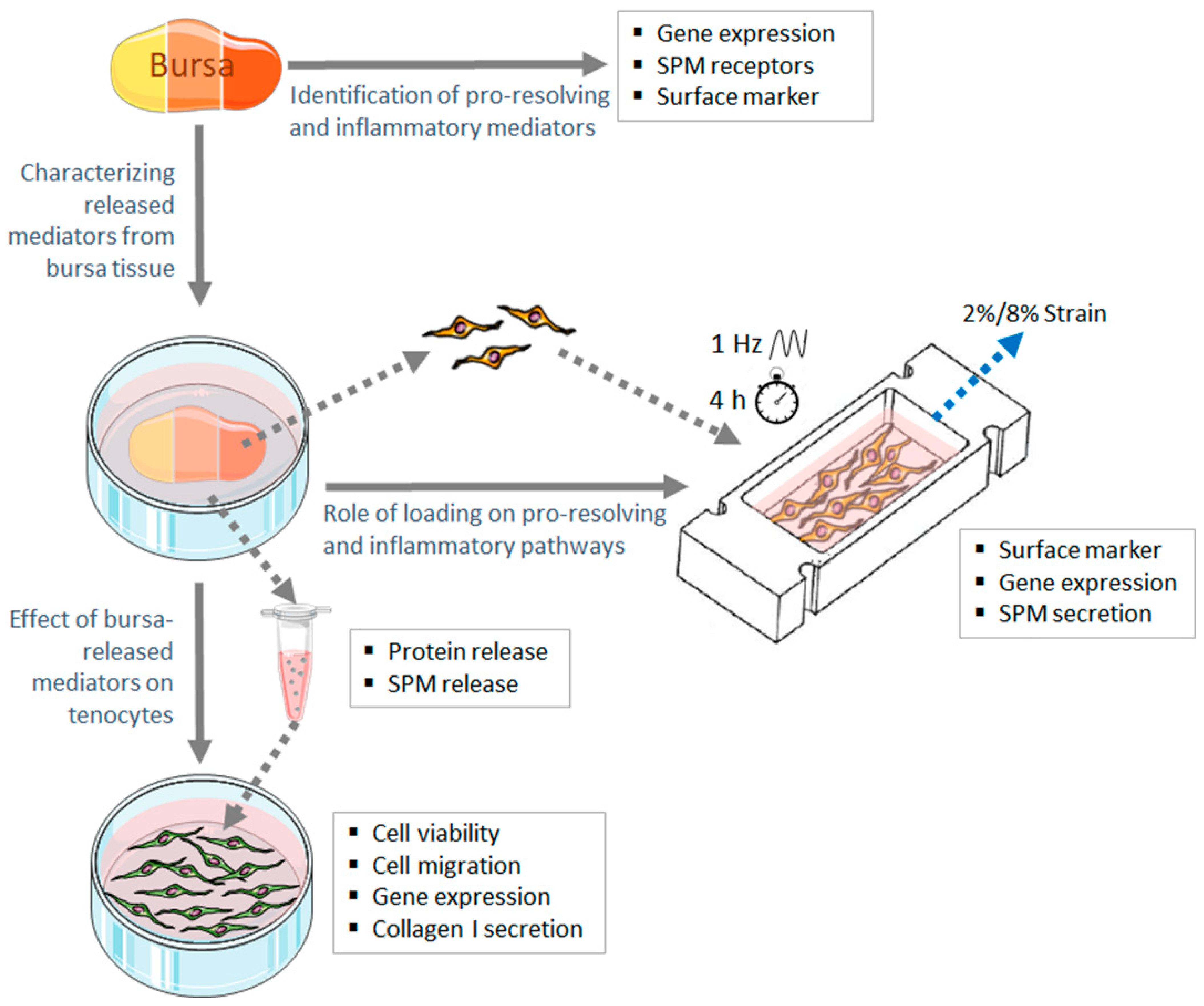
2. Materials and Methods
2.1. Tissue Harvesting
2.2. Identification of Pro-Resolving and Inflammatory Mediators in Bursa Tissue at RNA Level
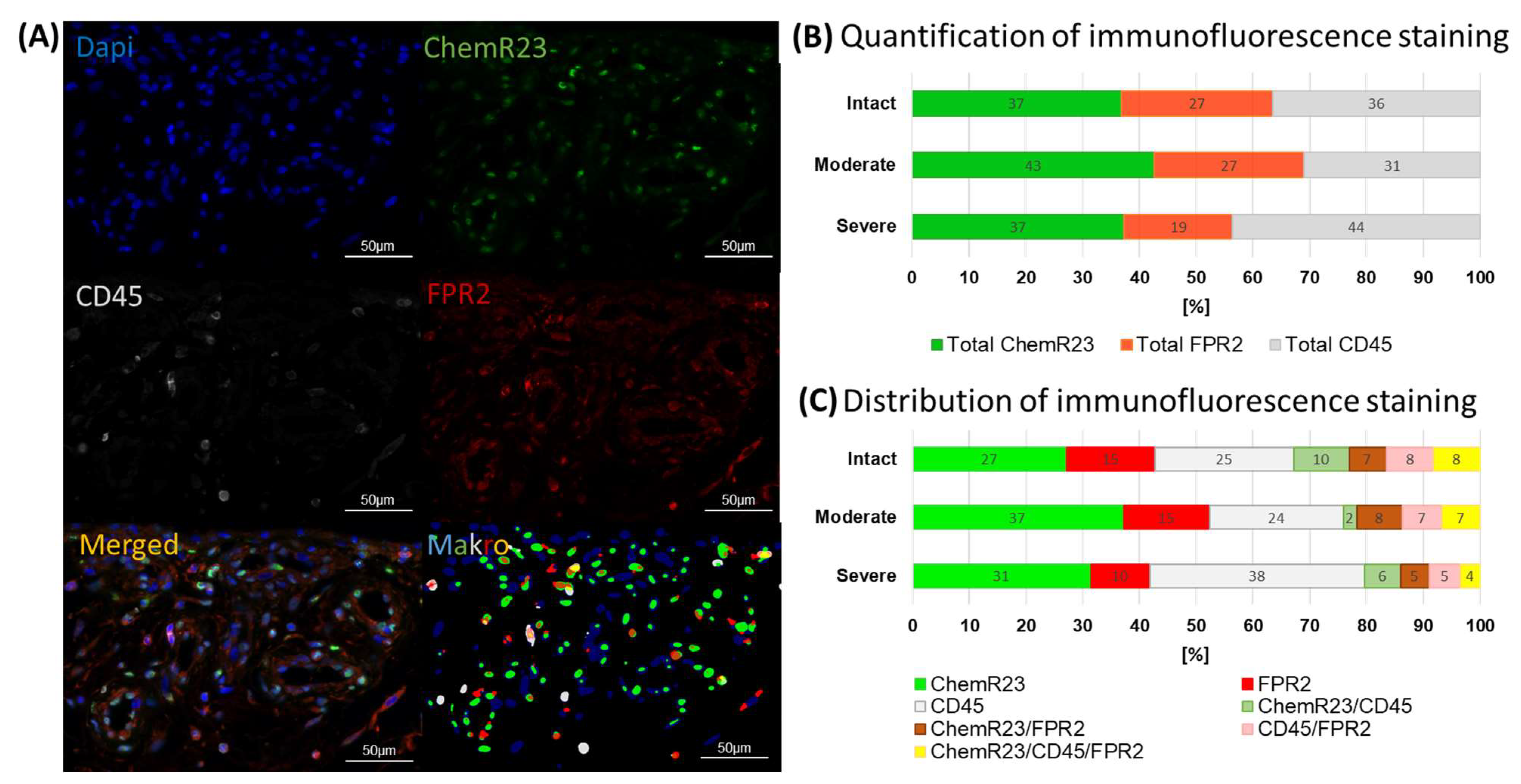
2.3. Multiplex Immunofluorescence Staining of SPM Receptors
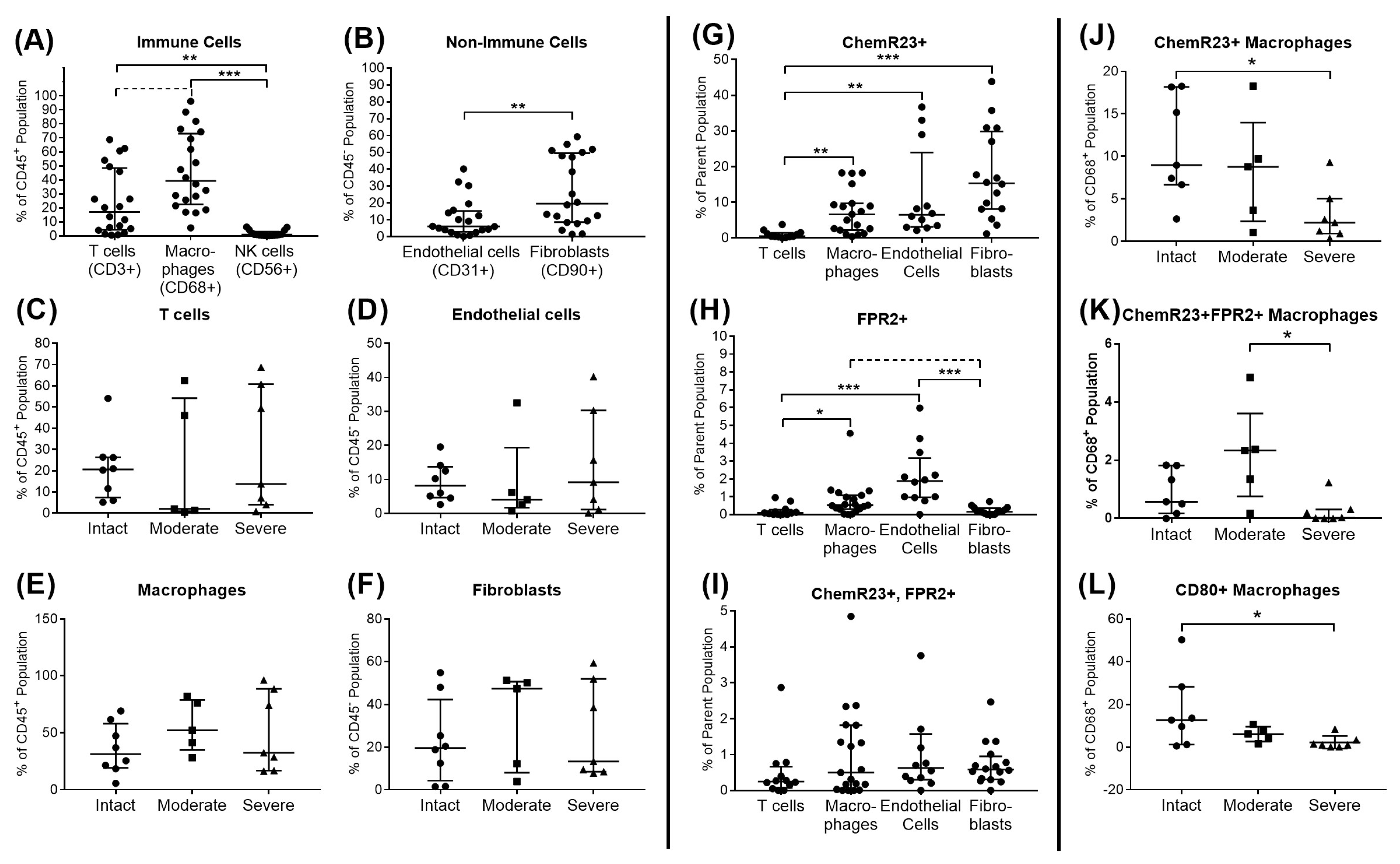
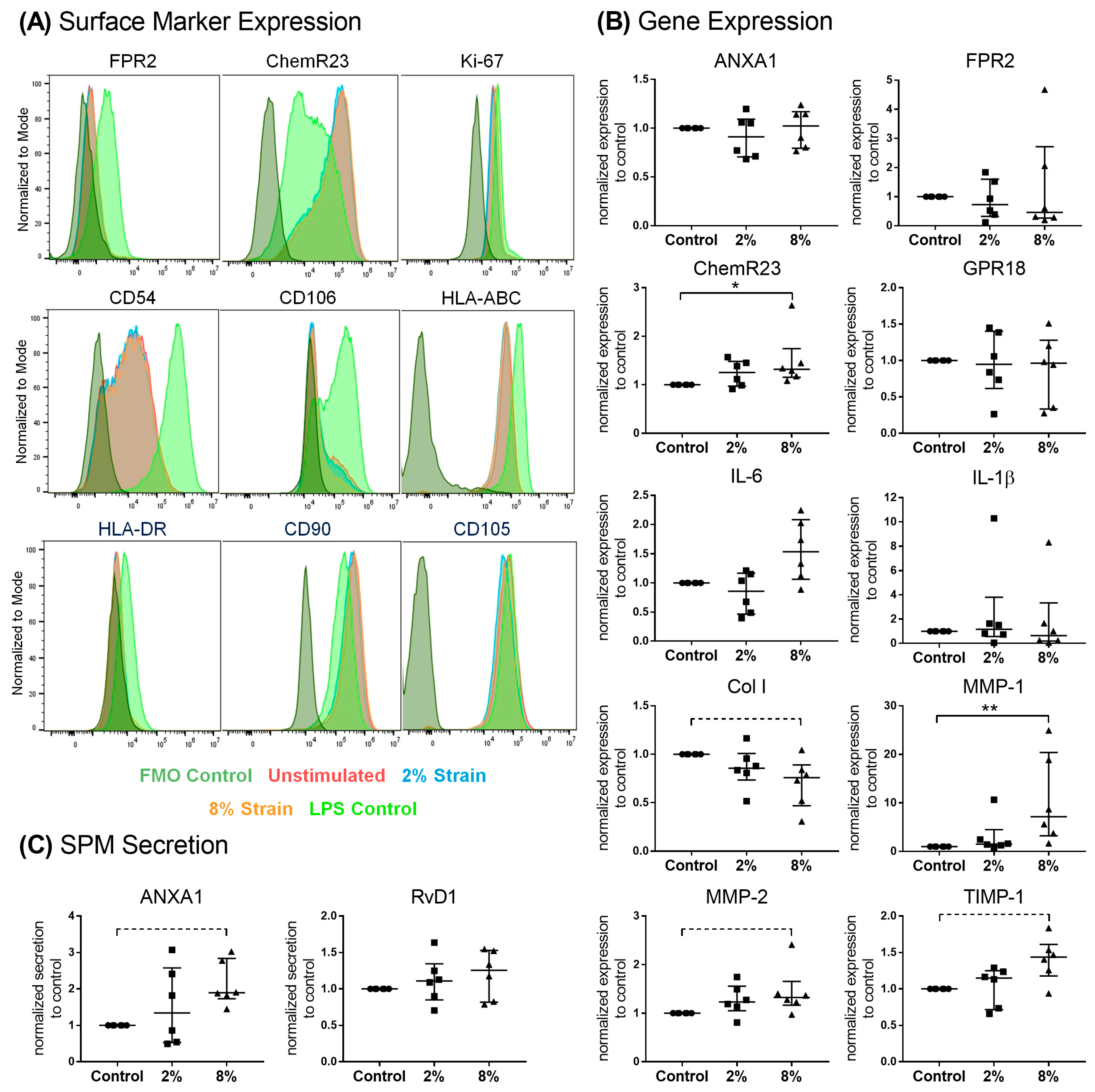
2.4. Flow Cytometric Analysis of SPM Receptors and Immune Cell Subsets in Bursa Tissue
2.5. Characterization of Factors Released from Bursa Tissue
2.6. Assay to Analyze the Effect of Bursa-Released Factors on Tenocytes
2.7. Analysis of the Role of Loading on Pro-Resolving and Inflammatory Pathways in Bursa Cells
2.8. Statistics
3. Results
3.1. Identification of Pro-Resolving and Inflammatory Mediators in Bursae from Patients with Rotator Cuff Disease
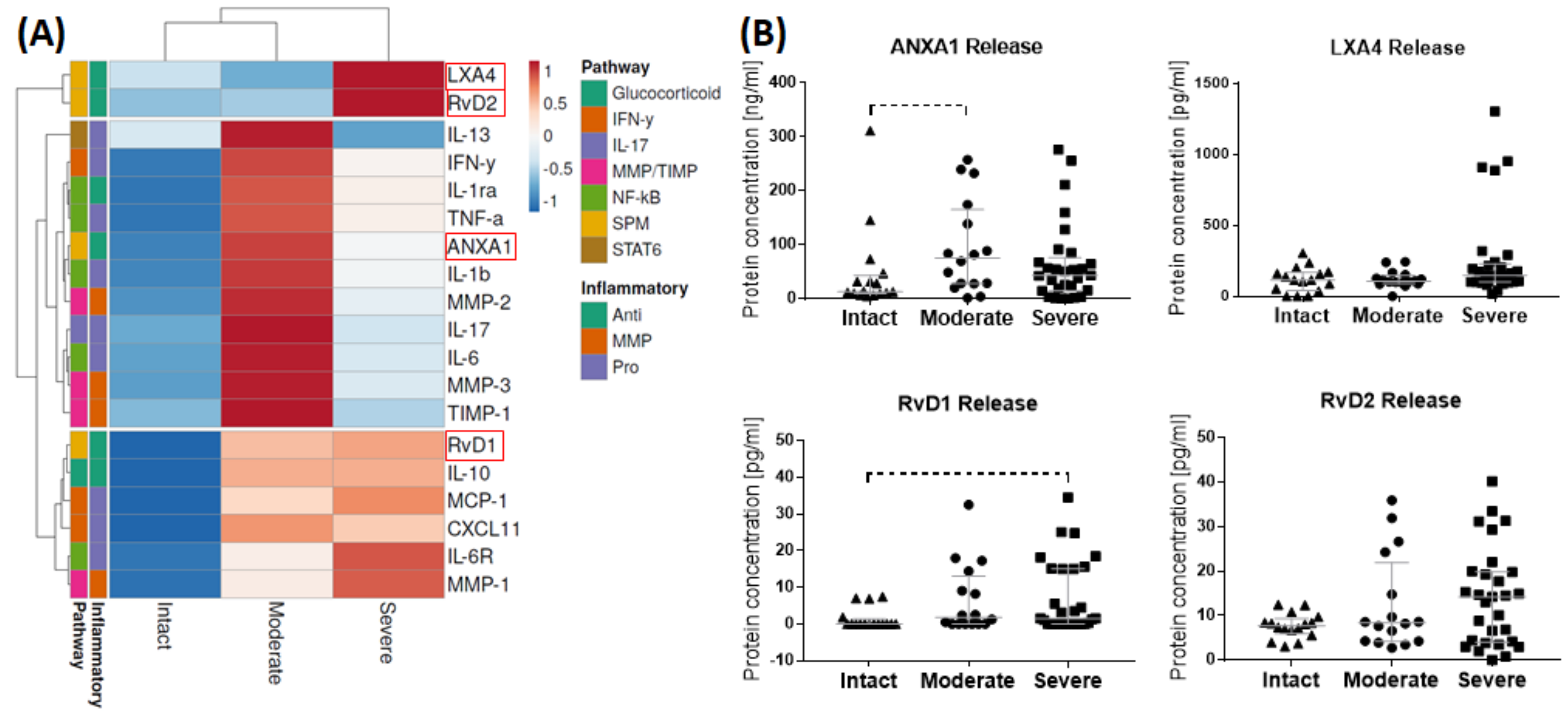
3.2. Release of SPMs and Inflammatory Mediators from Bursa Tissue
3.3. Influence of Bursa-Released Factors on Tenocytes
3.4. Role of Mechanical Loading on SPM Signaling Mediators in Bursa Cells
4. Discussion
4.1. Identification of Pro-Resolving and Inflammatory Mediators in Bursa Tissues
4.2. Effect of Bursa-Released Factors on IL-1β-Challenged Tenocytes
4.3. Effect of Loading on Pro-Resolving Processes in Bursa-Derived Cells
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klatte-Schulz, F.; Thiele, K.; Scheibel, M.; Duda, G.N.; Wildemann, B. Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research. Cells 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Gohlke, F. Editorial Commentary: Subacromial Bursa-Friend or Foe Within The Shoulder? An Old Debate with New Insights. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2989–2991. [Google Scholar] [CrossRef] [PubMed]
- Lanham, N.S.; Swindell, H.W.; Levine, W.N. The Subacromial Bursa: Current Concepts Review. JBJS Rev. 2021, 9, e21. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Sarkar, K. Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J. Bone Jt. Surg. Br. Vol. 1991, 73, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. Rotator cuff related shoulder pain: Assessment, management and uncertainties. Man. Ther. 2016, 23, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Urwin, M.; Symmons, D.; Allison, T.; Brammah, T.; Busby, H.; Roxby, M.; Simmons, A.; Williams, G. Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Skazalski, C.; Bahr, R.; Whiteley, R. Shoulder complaints more likely in volleyball players with a thickened bursa or supraspinatus tendon neovessels. Scand. J. Med. Sci. Sports 2021, 31, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef]
- Faruqi, T.; Rizvi, T.J. Subacromial Bursitis; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Morikawa, D.; Johnson, J.D.; Kia, C.; McCarthy, M.B.R.; Macken, C.; Bellas, N.; Baldino, J.B.; Cote, M.P.; Mazzocca, A.D. Examining the Potency of Subacromial Bursal Cells as a Potential Augmentation for Rotator Cuff Healing: An In Vitro Study. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2978–2988. [Google Scholar] [CrossRef]
- Lhee, S.H.; Jo, Y.H.; Kim, B.Y.; Nam, B.M.; Nemeno, J.G.; Lee, S.; Yang, W.; Lee, J.I. Novel supplier of mesenchymal stem cell: Subacromial bursa. Transplant. Proc. 2013, 45, 3118–3121. [Google Scholar] [CrossRef]
- Steinert, A.F.; Kunz, M.; Prager, P.; Gobel, S.; Klein-Hitpass, L.; Ebert, R.; Noth, U.; Jakob, F.; Gohlke, F. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Bormann, N.; Voss, I.; Melzer, J.; Schmock, A.; Bucher, C.H.; Thiele, K.; Moroder, P.; Haffner-Luntzer, M.; Ignatius, A.; et al. Bursa-Derived Cells Show a Distinct Mechano-Response to Physiological and Pathological Loading in vitro. Front. Cell Dev. Biol. 2021, 9, 657166. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Brunet, J.A.; Welsh, R.P.; Uhthoff, H.K. “Bursal reactions” in rotator cuff tearing, the impingement syndrome, and calcifying tendinitis. J. Shoulder Elb. Surg. 1997, 6, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Minkwitz, S.; Thiele, K.; Schmock, A.; Bormann, N.; Nguyen, T.H.; Moroder, P.; Scheibel, M.; Wildemann, B.; Plachel, F.; Klatte-Schulz, F. Histological and molecular features of the subacromial bursa of rotator cuff tears compared to non-tendon defects: A pilot study. BMC Musculoskelet. Disord. 2021, 22, 877. [Google Scholar] [CrossRef] [PubMed]
- Blaine, T.A.; Cote, M.A.; Proto, A.; Mulcahey, M.; Lee, F.Y.; Bigliani, L.U. Interleukin-1beta stimulates stromal-derived factor-1alpha expression in human subacromial bursa. J. Orthop. Res. 2011, 29, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Lho, Y.M.; Ha, E.; Cho, C.H.; Song, K.S.; Min, B.W.; Bae, K.C.; Lee, K.J.; Hwang, I.; Park, H.B. Inflammatory cytokines are overexpressed in the subacromial bursa of frozen shoulder. J. Shoulder Elb. Surg. 2013, 22, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Fujita, K.; Sakai, Y.; Mizuno, K. Immunolocalization of cytokines and growth factors in subacromial bursa of rotator cuff tear patients. Kobe J. Med. Sci. 2001, 47, 25–34. [Google Scholar]
- Voloshin, I.; Gelinas, J.; Maloney, M.D.; O’Keefe, R.J.; Bigliani, L.U.; Blaine, T.A. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1076.e1–1076.e9. [Google Scholar] [CrossRef]
- Kim, Y.S.; Bigliani, L.U.; Fujisawa, M.; Murakami, K.; Chang, S.S.; Lee, H.J.; Lee, F.Y.; Blaine, T.A. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J. Orthop. Res. 2006, 24, 1756–1764. [Google Scholar] [CrossRef]
- Ko, J.Y.; Wang, F.S.; Huang, H.Y.; Wang, C.J.; Tseng, S.L.; Hsu, C. Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J. Orthop. Res. 2008, 26, 1090–1097. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Bena, S.; Brancaleone, V.; Wang, J.M.; Perretti, M.; Flower, R.J. Annexin A1 interaction with the FPR2/ALX receptor: Identification of distinct domains and downstream associated signaling. J. Biol. Chem. 2012, 287, 24690–24697. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N.; Hickey, M.J. Annexin A1 and the regulation of innate and adaptive immunity. Front. Immunol. 2012, 3, 354. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.; Smith, R.D.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Sugg, K.B.; Sarver, D.C.; Maddipati, K.R.; Brooks, S.V. Local shifts in inflammatory and resolving lipid mediators in response to tendon overuse. FASEB J. 2021, 35, e21655. [Google Scholar] [CrossRef]
- Dakin, S.G.; Colas, R.A.; Wheway, K.; Watkins, B.; Appleton, L.; Rees, J.; Gwilym, S.; Little, C.; Dalli, J.; Carr, A.J. Proresolving Mediators LXB4 and RvE1 Regulate Inflammation in Stromal Cells from Patients with Shoulder Tendon Tears. Am. J. Pathol. 2019, 189, 2258–2268. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef]
- Patte, D. Classification of rotator cuff lesions. Clin. Orthop. Relat. Res. 1990, 254, 81–86. [Google Scholar] [CrossRef]
- Bayne, O.; Bateman, J.E.; Welsch, R.P. Long term results of surgical repair of full thickness rotator cuff tears. In Surgery of the Shoulder; Bateman, J.W.R., Ed.; Mosby: Philadelphia, PA, USA, 1984; pp. 167–171. [Google Scholar]
- Snyder, S.J.; Karzel, R.P.; Del Pizzo, W.; Ferkel, R.D.; Friedman, M.J. SLAP lesions of the shoulder. Arthrosc. J. Arthrosc. Relat. Surg. 1990, 6, 274–279. [Google Scholar] [CrossRef]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-Dependent Alterations of MMPs, TIMPs and Tendon Structure in Human Achilles Tendons after Acute Rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef] [PubMed]
- Brophy, R.H.; Rai, M.F.; Zhang, Z.; Torgomyan, A.; Sandell, L.J. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J. Bone Jt. Surg. Am. Vol. 2012, 94, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Klatte, F.; Strobel, C.; Schmidmaier, G.; Greiner, S.; Scheibel, M.; Wildemann, B. Characterization of tendon cell cultures of the human rotator cuff. Eur. Cells Mater. 2010, 20, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Wilke, H.J.; Claes, L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: A new experimental method and its application. J. Orthop. Res. 1994, 12, 70–78. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Lavagnino, M.; Egerbacher, M. The mechanobiological aetiopathogenesis of tendinopathy: Is it the over-stimulation or the under-stimulation of tendon cells? Int. J. Exp. Pathol. 2007, 88, 217–226. [Google Scholar] [CrossRef]
- Feng, H.; He, Z.; Twomey, K.; Ilaltdinov, A.W.; Leong, D.; Wang, Y.; Li, J.; Gonzalez, D.; Sun, H.B. Epigallocatechin-3-gallate suppresses pain-related and proinflammatory mediators in the subacromial bursa in rotator cuff tendinopathy. Discov. Med. 2019, 27, 63–77. [Google Scholar]
- Shih, C.A.; Wu, K.C.; Shao, C.J.; Chern, T.C.; Su, W.R.; Wu, P.T.; Jou, I.M. Synovial fluid biomarkers: Association with chronic rotator cuff tear severity and pain. J. Shoulder Elb. Surg. 2018, 27, 545–552. [Google Scholar] [CrossRef]
- Millar, N.L.; Wei, A.Q.; Molloy, T.J.; Bonar, F.; Murrell, G.A. Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Jt. Surg. Br. Vol. 2009, 91, 417–424. [Google Scholar] [CrossRef]
- Stolk, M.; Klatte-Schulz, F.; Schmock, A.; Minkwitz, S.; Wildemann, B.; Seifert, M. New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Sci. Rep. 2017, 7, 9801. [Google Scholar] [CrossRef]
- Zhang, K.; Asai, S.; Yu, B.; Enomoto-Iwamoto, M. IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun. 2015, 463, 667–672. [Google Scholar] [CrossRef]
- Manning, C.N.; Havlioglu, N.; Knutsen, E.; Sakiyama-Elbert, S.E.; Silva, M.J.; Thomopoulos, S.; Gelberman, R.H. The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J. Orthop. Res. 2014, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Tsuzaki, M.; Guyton, G.; Garrett, W.; Archambault, J.M.; Herzog, W.; Almekinders, L.; Bynum, D.; Yang, X.; Banes, A.J. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003, 21, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.; Tolar, M.; Peters, O.A. Lipoxin A(4) Attenuates the Inflammatory Response in Stem Cells of the Apical Papilla via ALX/FPR2. Sci. Rep. 2018, 8, 8921. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Pequera, M.; Vilarino, A.; Moreno, J.J. Specialized pro-resolvin mediators induce cell growth and improve wound repair in intestinal epithelial Caco-2 cell cultures. Prostaglandins Leukot. Essent. Fat. Acids 2022, 187, 102520. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Che, X.D.; Li, H.Z.; Gao, Y.Y.; Wei, X.C.; Li, P.C. Annexin A1 involved in the regulation of inflammation and cell signaling pathways. Chin. J. Traumatol. 2020, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, C.; Petrozza, V.; Garro, L.; Sardella, B.; Diotallevi, R.; Ferrara, A.; Gigante, A.; Di Cristofano, C.; Castagna, A.; Della Rocca, C. Rotator cuff re-tear or non-healing: Histopathological aspects and predictive factors. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1588–1596. [Google Scholar] [CrossRef]
- Marshall, B.P.; Ferrer, X.E.; Kunes, J.A.; Innis, A.C.; Luzzi, A.J.; Forrester, L.A.; Burt, K.G.; Lee, A.J.; Song, L.; Hung, C.T.; et al. The subacromial bursa is a key regulator of the rotator cuff and a new therapeutic target for improving repair. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dakin, S.G.; Colas, R.A.; Newton, J.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Appleton, L.; Wheway, K.; Watkins, B.; et al. 15-Epi-LXA(4) and MaR1 counter inflammation in stromal cells from patients with Achilles tendinopathy and rupture. FASEB J. 2019, 33, 8043–8054. [Google Scholar] [CrossRef]
- Hammerman, M.; Blomgran, P.; Ramstedt, S.; Aspenberg, P. COX-2 inhibition impairs mechanical stimulation of early tendon healing in rats by reducing the response to microdamage. J. Appl. Physiol. 2015, 119, 534–540. [Google Scholar] [CrossRef]
- Blomgran, P.; Blomgran, R.; Ernerudh, J.; Aspenberg, P. A possible link between loading, inflammation and healing: Immune cell populations during tendon healing in the rat. Sci. Rep. 2016, 6, 29824. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Chaudhry, S.; Lei, I.I.; Varone, A.; Riley, G.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand. J. Med. Sci. Sports 2015, 25, e381–e391. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, K.; Morawietz, L.; Moroder, P.; Scheibel, M. Synovitis as a concomitant disease in shoulder pathologies. Arch. Orthop. Trauma Surg. 2019, 139, 1111–1116. [Google Scholar] [CrossRef] [PubMed]


| Intact Rotator Cuff | Moderate Rotator Cuff Disease | Severe Rotator Cuff Disease | |
|---|---|---|---|
| N-number total | 28 | 24 | 45 |
| Age (Mean ± StD) | 32.8 ± 11 | 53.1 ± 11.8 | 59.9 ± 8.7 |
| BMI (Mean ± StD) | 24.3 ± 3.3 | 26.6 ± 4.1 | 27.9 ± 4.9 |
| Female/Male (N) | 4/24 | 6/18 | 16/29 |
| Disease | Primary shoulder instability: 8 Recurrent shoulder instability: 3 AC joint luxation: 17 | Impingement: 2 Partial SSP tear: 22 Intratendinous: 2 Articular side: 7 Bursa side: 13 | Full-thickness SSP tear: 45 |
| Classification | - | Snyder: A1: 3, A2: 2, A3: 2 B1: 8, B2: 3, B3: 2 | Patte: 1.5 ± 0.7 Bateman: 2.3 ± 0.6 |
| Abbreviation | Full Name | Function | |
|---|---|---|---|
| SPM signaling | ANXA1 | Annexin A1 (Target for FPR2) | SPM |
| ALOX5 | Arachidonat-5-Lipoxygenase | Enzyme for SPM synthesis | |
| FPR2/ALX | Formyl peptide receptor 2 (Ligands: LXA4, ANXA1, RvD1, RvD2) | Receptors for SPMs | |
| ChemR23/CMKLR1 | Chemerin Receptor 23/Chemokine-like receptor 1 (ligands: RvE1, Chemerin) | ||
| GPR32 | G-protein-coupled receptor 32 (ligand: RvD1) | ||
| GPR18 | G-protein-coupled receptor 18 (ligand: RvD2) | ||
| Pro-/anti-inflammatory anti-inflammatory | CXCL11 | C-X-C motif chemokine ligand 11 | IFN pathway targets |
| IRF1 | Interferon regulatory factor 1 | ||
| WARS | Tryptophanyl-tRNA synthetase | ||
| VAMP5 | Vesicle-associated membrane protein 5 | ||
| SRRM2 | Serine/arginine repetitive matrix 2 | ||
| APOL3 | Apolipoprotein L 3 | ||
| IL-6 | Interleukin 6 | NF-κB pathway targets | |
| TNF-α | Tumor necrosis factor α | ||
| CCL20 | C-C motif chemokine ligand 20 | ||
| MCP1 | Monocyte chemoattractant protein 1 | ||
| IL-8 | Interleukin 8 | ||
| IDO1 | Indoleamine 2,3-dioxygenase 1 | ||
| IL-1β | Interleukin 1β | ||
| TGM2 | Transglutaminase 2 | STAT-6 pathway targets | |
| CD206 | Mannose receptor | ||
| CISH | Cytokine inducible SH2-containing protein | ||
| FGL2 | Fibrinogen-like protein 2 | ||
| IL-13 | Interleukin 13 | ||
| CD163 | CD163 molecule | Glucocorticoid receptor pathway targets | |
| IL-10 | Interleukin 10 | ||
| CD1D | Cluster of Differentiation 1D | ||
| PTX3 | Pentraxin 3, long |
| Target | Fluorochrome | Clone | Manufacturer | Dilution |
|---|---|---|---|---|
| Live/Dead | Zombi UV | - | BioLegend | 1:100 |
| CD3 | BV650 | OKT3 | BioLegend | 1:20 |
| CD31 | BV605 | WM59 | BioLegend | 1:100 |
| CD45 | Pac. Blue | J33 | Beckman Coulter | 1:40 |
| CD54 | PE/Dazzle 594 | HA58 | BioLegend | 1:10 |
| CD56 | APC-F700 | HCD56 | BioLegend | 1:20 |
| CD68 | PE/CF594 | Y1/82A | BD Horizon * | 1:50 |
| CD80 | BV785 | 2D10 | BioLegend | 1:10 |
| CD90 | FITC | 5E10 | BioLegend | 1:200 |
| CD105 | PE/Cy7 | 43A3 | BioLegend | 1:200 |
| CD106 | BV421 | STA | BioLegend | 1:20 |
| CD206 | APC-F750 | 15-2 | BioLegend | 1:20 |
| ChemR23 | APC | 15-2 | R&D Systems ** | 1:15 |
| Ki-67 | eFluor506 | SolA15 | eBiosciences *** | 1:40 |
| FPR2 | PE | 304405 | R&D Systems | 1:15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klatte-Schulz, F.; Bormann, N.; Bonell, A.; Al-Michref, J.; Nguyen, H.L.; Klöckner, P.; Thiele, K.; Moroder, P.; Seifert, M.; Sawitzki, B.; et al. Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved? Cells 2024, 13, 17. https://doi.org/10.3390/cells13010017
Klatte-Schulz F, Bormann N, Bonell A, Al-Michref J, Nguyen HL, Klöckner P, Thiele K, Moroder P, Seifert M, Sawitzki B, et al. Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved? Cells. 2024; 13(1):17. https://doi.org/10.3390/cells13010017
Chicago/Turabian StyleKlatte-Schulz, Franka, Nicole Bormann, Aysha Bonell, Jasmin Al-Michref, Hoang Le Nguyen, Pascal Klöckner, Kathi Thiele, Philipp Moroder, Martina Seifert, Birgit Sawitzki, and et al. 2024. "Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved?" Cells 13, no. 1: 17. https://doi.org/10.3390/cells13010017
APA StyleKlatte-Schulz, F., Bormann, N., Bonell, A., Al-Michref, J., Nguyen, H. L., Klöckner, P., Thiele, K., Moroder, P., Seifert, M., Sawitzki, B., Wildemann, B., & Duda, G. N. (2024). Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved? Cells, 13(1), 17. https://doi.org/10.3390/cells13010017






