Differential Activation of Splenic cDC1 and cDC2 Cell Subsets following Poxvirus Infection of BALB/c and C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Animals
2.3. Preparation of Single-Cell Suspension of Splenocytes
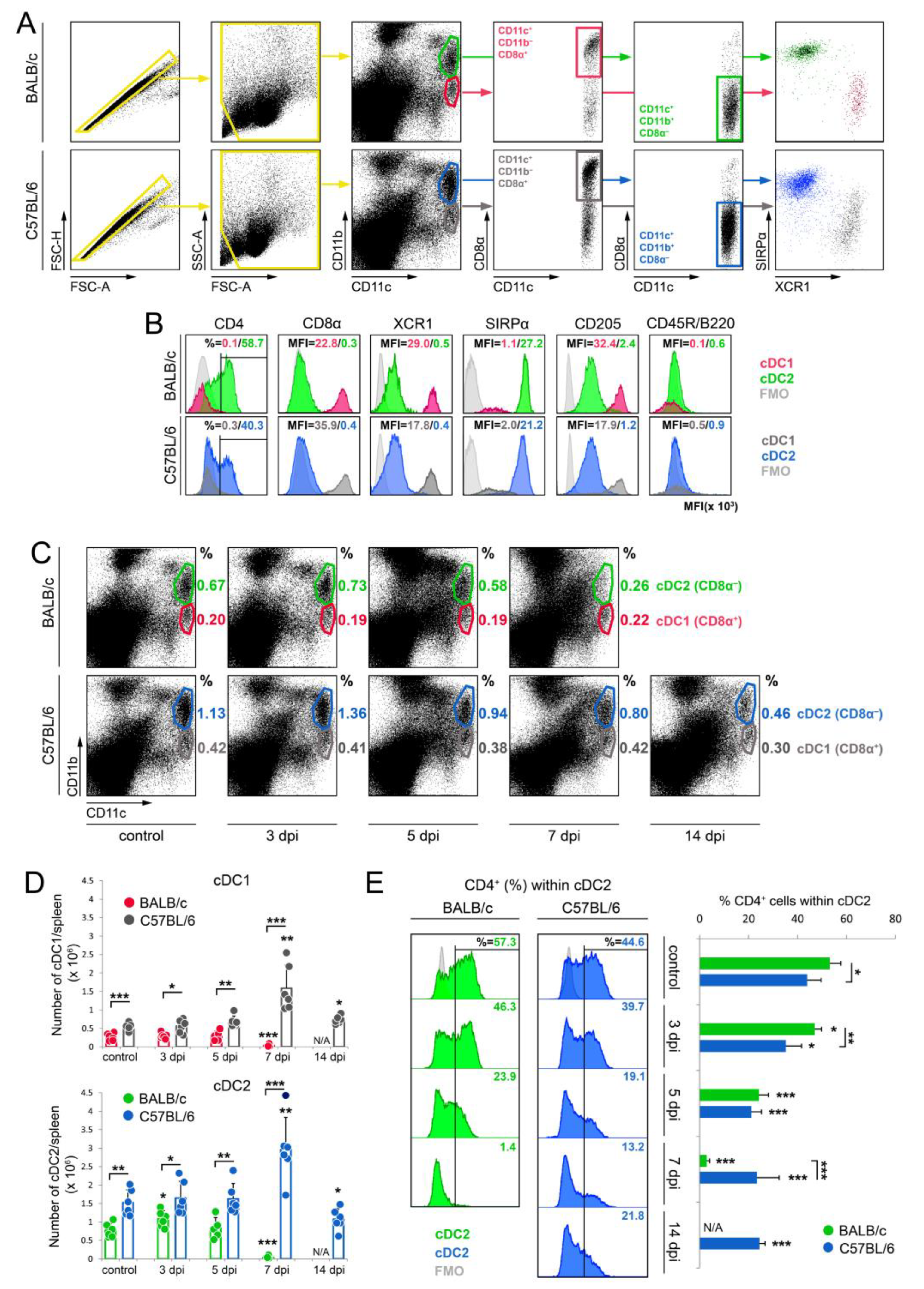
2.4. Multi-Color Immunophenotyping
2.5. Intracellular Cytokine Assay
2.6. Flow Cytometry Analysis
2.7. Isolation of Splenic cDC1 (CD8α+) and cDC2 (CD8α−) Subsets
2.8. Fluorescence Microscopy
2.9. Plaque Forming Assay
2.10. RNA Isolation
2.11. RT-qPCR
2.12. Ingenuity Pathway Analysis (IPA)
2.13. Mixed Leukocyte Reaction (MLR)
2.14. Cytokine ELISA
2.15. Statistical Analysis
3. Results
3.1. The Percentage and the Number of cDC1 and cDC2 Subsets in Spleens of Susceptible BALB/c Mice Decreases during the Acute Phase of Mousepox
3.2. cDC Subsets from BALB/c Mice Exhibit Higher Viral Load Than Those from C57BL/6 Mice during Early Stages of Mousepox
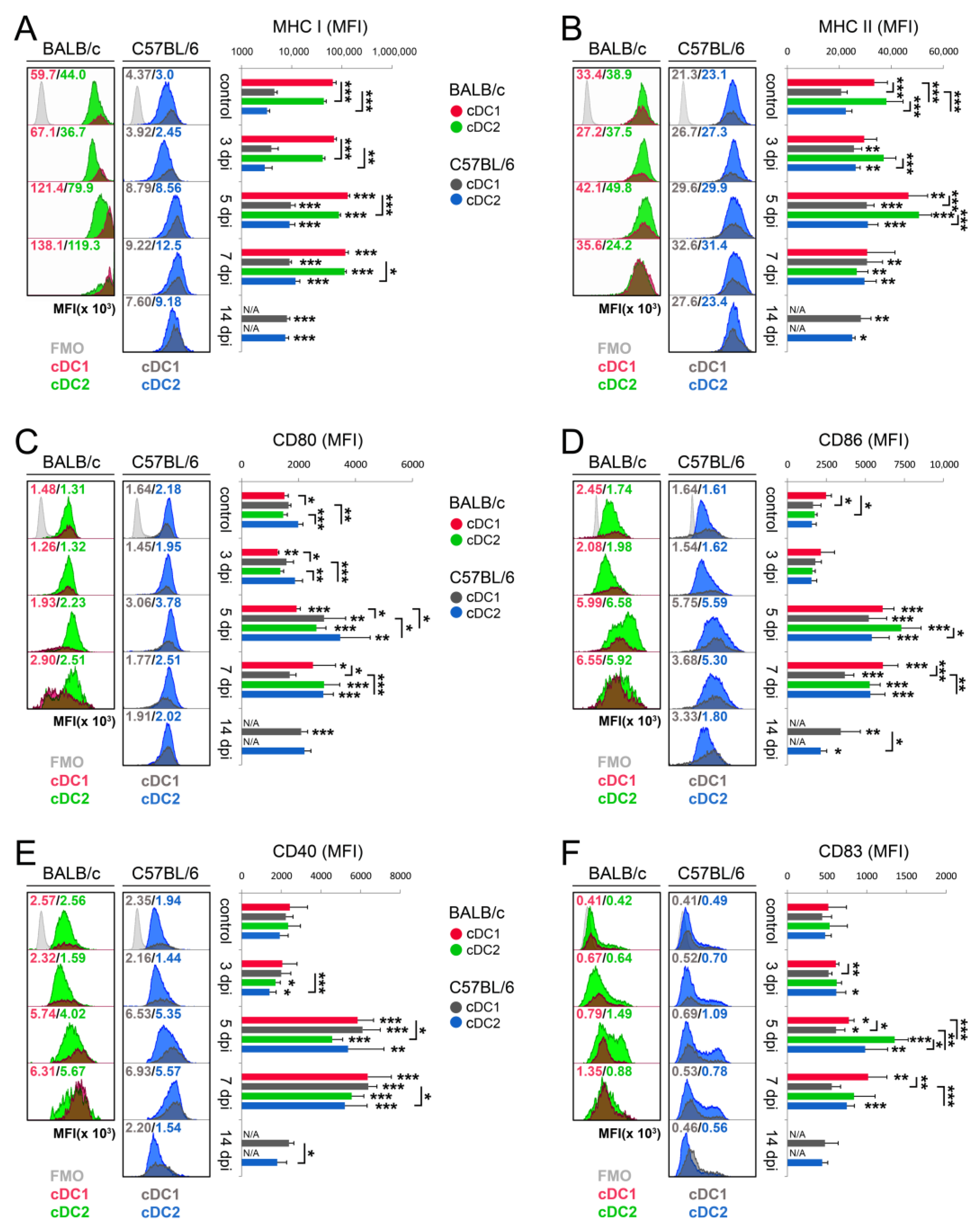
3.3. cDC Subsets from BALB/c Mice Show Higher Maturation Profile than Those from C57BL/6 Mice during Acute Phase of Mousepox
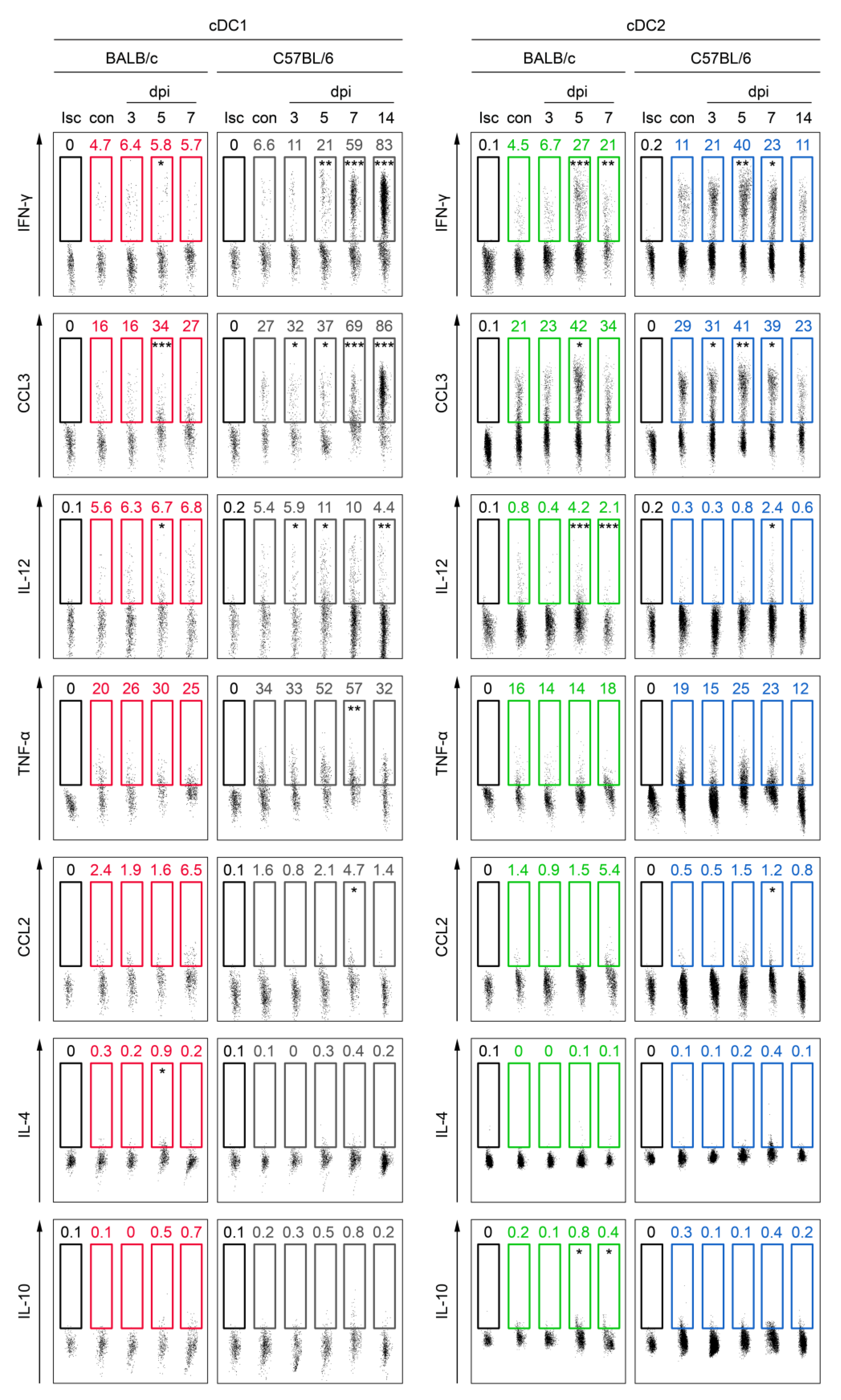
3.4. cDC Subsets from C57BL/6 Mice Secrete Higher Levels of Th1-Polarizing Cytokines Than Those from BALB/c Mice in Mousepox
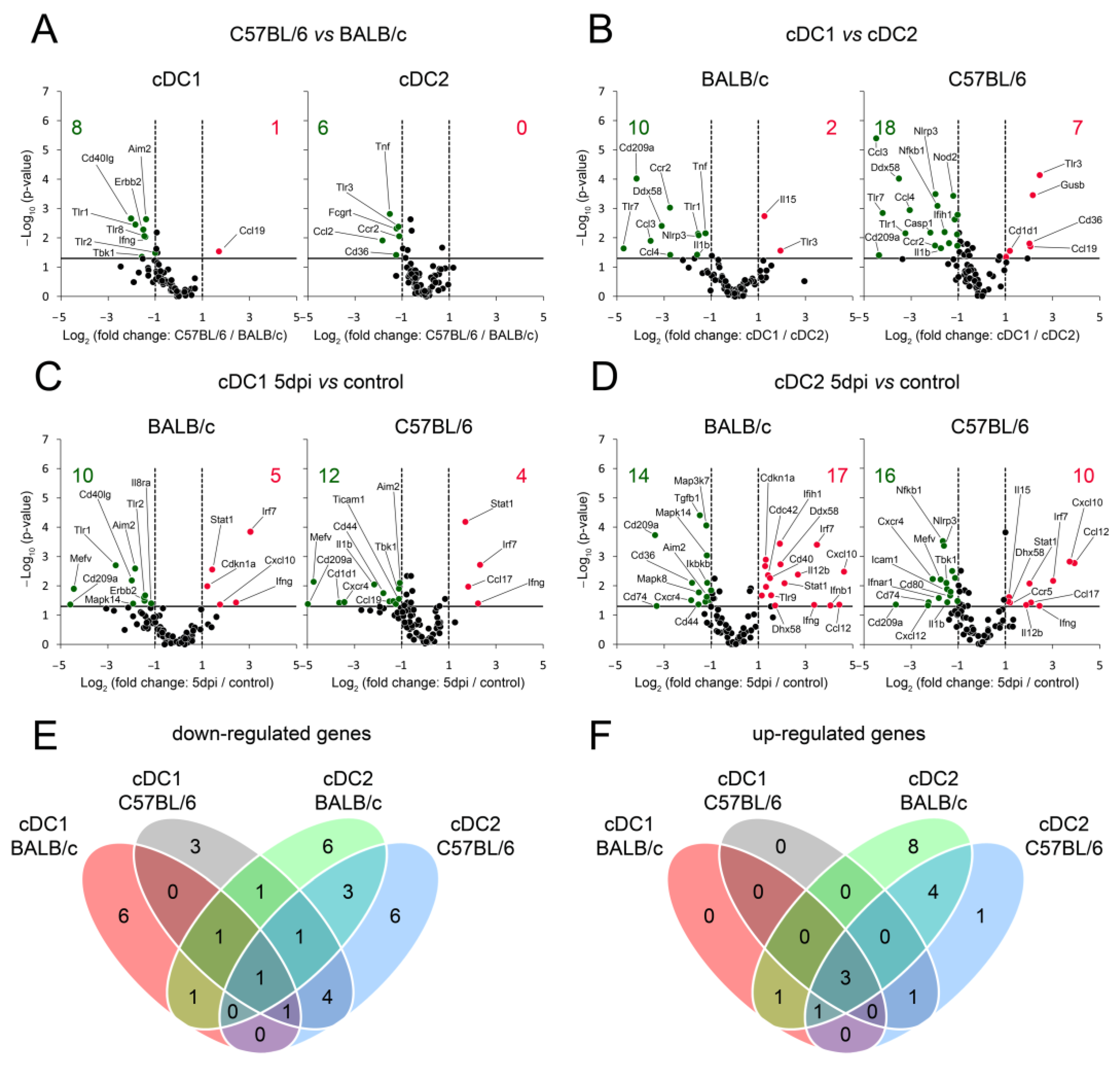
3.5. cDC Subsets, Especially cDC2, from BALB/c Mice Exhibit More Up-Regulated Genes Engaged in Cell Maturation and Activation Than C57BL/6 cDCs during Early Stages of Mousepox
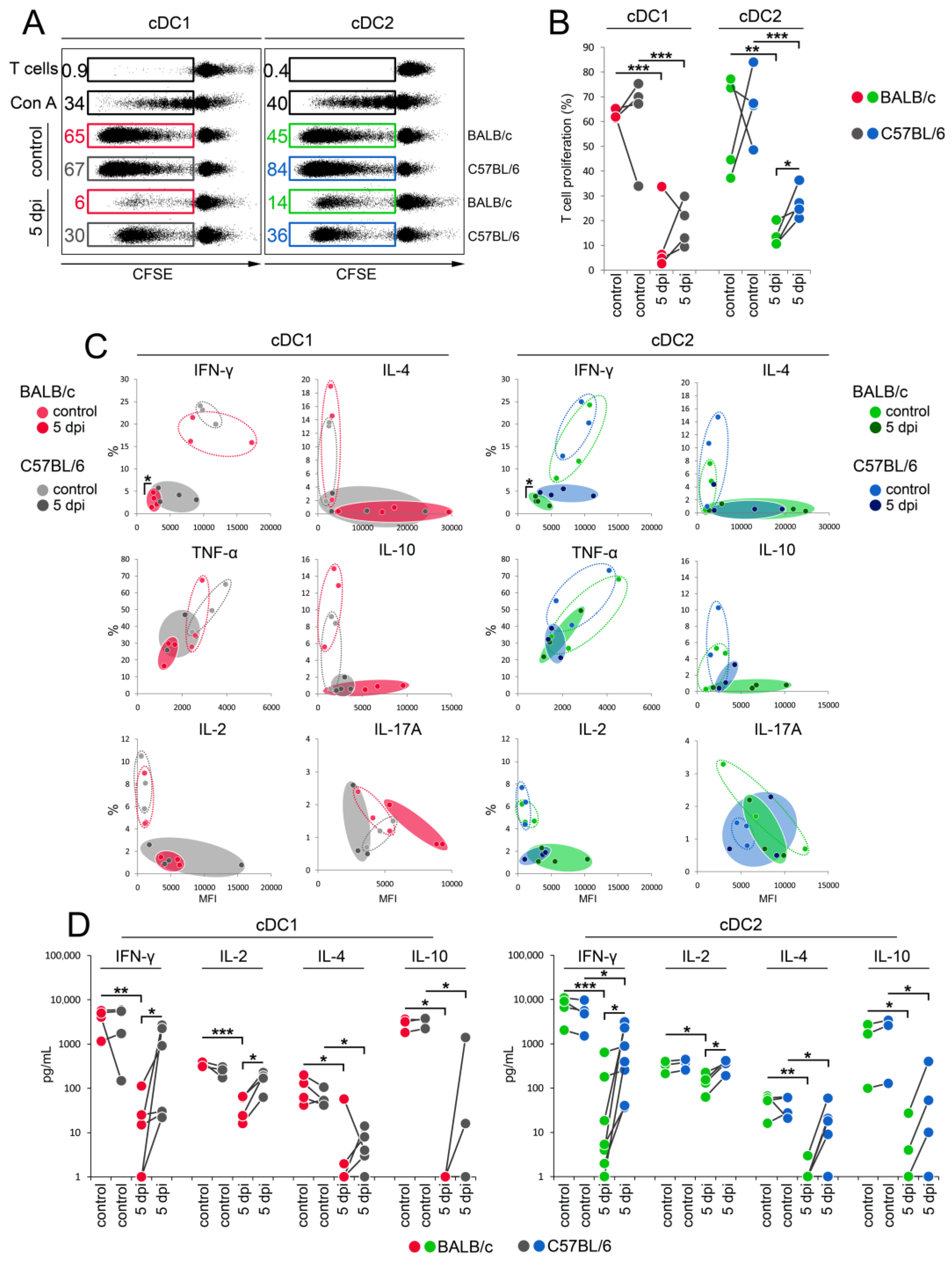
3.6. cDC1 and cDC2 from C57BL/6 Mice Are More Potent in Stimulating the Th1 Immune Response Than Those from BALB/c Mice during Early Stages of Mousepox
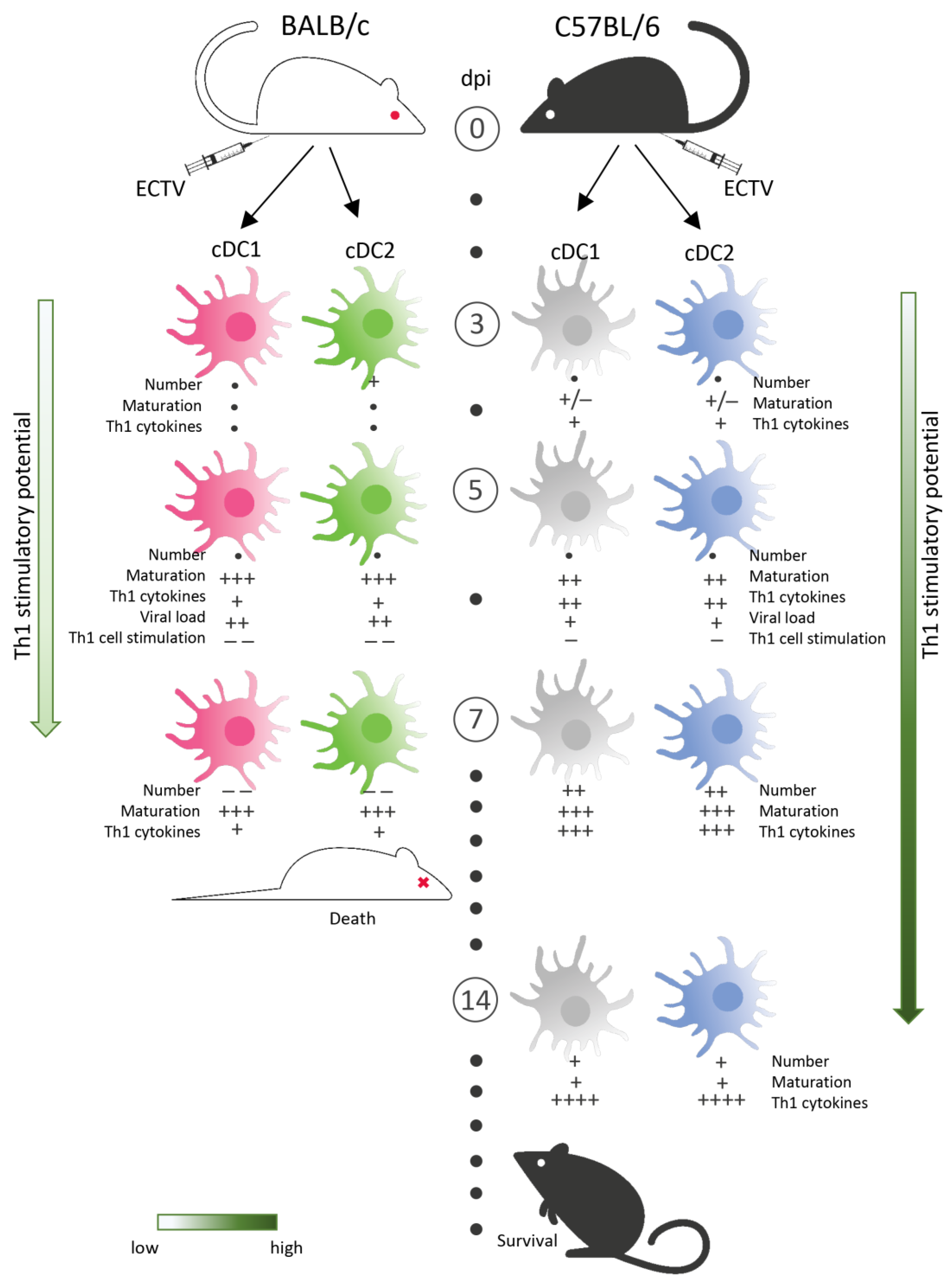
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Bayl. Univ. Med. Cent. Proc. 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, A.L. Comparative pathology of zoonotic orthopoxviruses. Pathogens 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Freer, G.; Matteucci, D. Influence of dendritic cells on viral pathogenicity. PLoS Pathog. 2009, 5, e1000384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans cells—The macrophage in dendritic cell clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, A.; Ginhoux, F. Organization of the mouse and human DC network. Curr. Opin. Immunol. 2014, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef]
- Vremec, D.; Pooley, J.; Hochrein, H.; Wu, L.; Shortman, K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000, 164, 2978–2986. [Google Scholar] [CrossRef]
- Da Silva, C.; Wagner, C.; Bonnardel, J.; Gorvel, J.P.; Lelouard, H. The Peyer’s patch mononuclear phagocyte system at steady state and during infection. Front. Immunol. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Crozat, K.; Tamoutounour, S.; Vu Manh, T.P.; Fossum, E.; Luche, H.; Ardouin, L.; Guilliams, M.; Azukizawa, H.; Bogen, B.; Malissen, B.; et al. Cutting edge: Expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α+ type. J. Immunol. 2011, 187, 4411–4415. [Google Scholar] [CrossRef] [PubMed]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Hartung, E.; Güttler, S.; Mora, A.; Zhou, X.; Hegemann, A.; Plantinga, M.; Mazzini, E.; Stoitzner, P.; Gurka, S.; et al. Expression of XCR1 characterizes the Batf3-dependent lineage of dendritic cells capable of antigen cross-presentation. Front. Immunol. 2012, 3, 214. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic cell-mediated Th2 immunity and immune disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [PubMed]
- Esteban, D.; Parker, S.; Schriewer, J.; Hartzler, H.; Buller, R.M. Mousepox, a small animal model of smallpox. Methods Mol. Biol. 2012, 890, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, G.; Panchanathan, V.; Buller, R.M.; van den Eertwegh, A.J.; Claassen, E.; Zhou, J.; de Chazal, R.; Laman, J.D.; Karupiah, G. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl. Acad. Sci. USA 2004, 101, 9057–9062. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, I.B.; Bohn, E.; Beer, M.; Preger, S.; Heinze, G.; Heesemann, J. Role of T-helper-cell subtypes and cytokines in immunity to Yersinia enterocolitica in susceptible and resistant strains of mice. Contrib. Microbiol. Immunol. 1995, 13, 203–206. [Google Scholar] [PubMed]
- Ferreira, B.L.; Ferreira, É.R.; de Brito, M.V.; Salu, B.R.; Oliva, M.L.V.; Mortara, R.A.; Orikaza, C.M. BALB/c and C57BL/6 mice cytokine responses to Trypanosoma cruzi infection are independent of parasite strain infectivity. Front. Microbiol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Byrne, A.B.; García, A.G.; Brahamian, J.M.; Mauri, A.; Ferretti, A.; Polack, F.P.; Talarico, L.B. A murine model of dengue virus infection in suckling C57BL/6 and BALB/c mice. Anim. Model. Exp. Med. 2020, 4, 16–26. [Google Scholar] [CrossRef]
- Okano, M.; Nishizaki, K.; Abe, M.; Wang, M.M.; Yoshino, T.; Satoskar, A.R.; Masuda, Y.; Harn, D.A., Jr. Strain-dependent induction of allergic rhinitis without adjuvant in mice. Allergy 1999, 54, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Yamaguchi, T.; Ishida, W.; Fukata, K.; Taniguchi, T.; Liu, F.T.; Ueno, H. Genetic background determines susceptibility to experimental immune-mediated blepharoconjunctivitis: Comparison of Balb/c and C57BL/6 mice. Exp. Eye Res. 2006, 82, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Filippi, C.; Hugues, S.; Cazareth, J.; Julia, V.; Glaichenhaus, N.; Ugolini, S. CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex-independent differences within dendritic cells. J. Exp. Med. 2003, 198, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Nishimura, H.; Matsuguchi, T.; Yoshikai, Y. Differences in interleukin-12 and-15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57 BL/6 mice. Cell. Immunol. 2000, 202, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Ismail, N.; Soong, L.; Popov, V.L.; Whitworth, T.; Bouyer, D.H.; Walker, D.H. Differential interaction of dendritic cells with Rickettsia conorii: Impact on host susceptibility to murine spotted fever rickettsiosis. Infect. Immun. 2007, 75, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; So, E.Y.; Kim, B.S. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 2007, 3, e124. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Sei, J.J.; Parekh, N.J.; Davies, M.L.; Reider, I.E.; Krouse, T.E.; Norbury, C.C. Redundant function of plasmacytoid and conventional dendritic cells is required to survive a natural virus infection. J. Virol. 2015, 89, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Xu, R.H.; Rubio, D.; Lev, A.; Stotesbury, C.; Fang, M.; Sigal, L.J. Migratory dendritic cells, group 1 innate lymphoid cells, and inflammatory monocytes collaborate to recruit NK cells to the virus-infected lymph node. Cell Rep. 2018, 24, 142–154. [Google Scholar] [CrossRef]
- Wong, E.; Montoya, B.; Stotesbury, C.; Ferez, M.; Xu, R.H.; Sigal, L.J. Langerhans cells orchestrate the protective antiviral innate immune response in the lymph node. Cell Rep. 2019, 29, 3047–3059.e3. [Google Scholar] [CrossRef]
- Melo-Silva, C.R.; Alves-Peixoto, P.; Heath, N.; Tang, L.; Montoya, B.; Knudson, C.J.; Stotesbury, C.; Ferez, M.; Wong, E.; Sigal, L.J. Resistance to lethal ectromelia virus infection requires Type I interferon receptor in natural killer cells and monocytes but not in adaptive immune or parenchymal cells. PLoS Pathog. 2021, 17, e1009593. [Google Scholar] [CrossRef]
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Szulc-Dąbrowska, L.; Struzik, J.; Cymerys, J.; Winnicka, A.; Nowak, Z.; Toka, F.N.; Gieryńska, M. The in vitro inhibitory effect of ectromelia virus infection on innate and adaptive immune properties of GM-CSF-derived bone marrow cells is mouse strain-independent. Front. Microbiol. 2017, 8, 2539. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Swiggard, W.J.; Steinman, R.M.; Romani, N.; Schuler, G.; Brinster, C. Isolation of dendritic cells. Curr. Protoc. Immunol. 2009, 86, 3.7.1–3.7.19. [Google Scholar] [CrossRef] [PubMed]
- Szulc-Dąbrowska, L.; Struzik, J.; Ostrowska, A.; Guzera, M.; Toka, F.N.; Bossowska-Nowicka, M.; Gieryńska, M.M.; Winnicka, A.; Nowak, Z.; Niemiałtowski, M.G. Functional paralysis of GM-CSF-derived bone marrow cells productively infected with ectromelia virus. PLoS ONE 2017, 12, e0179166. [Google Scholar] [CrossRef] [PubMed]
- Loevenich, K.; Ueffing, K.; Abel, S.; Hose, M.; Matuschewski, K.; Westendorf, A.M.; Buer, J.; Hansen, W. DC-derived IL-10 modulates pro-inflammatory cytokine production and promotes induction of CD4+IL-10+ regulatory T cells during Plasmodium yoelii infection. Front. Immunol. 2017, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Draheim, M.; Wlodarczyk, M.F.; Crozat, K.; Saliou, J.M.; Alayi, T.D.; Tomavo, S.; Hassan, A.; Salvioni, A.; Demarta-Gatsi, C.; Sidney, J.; et al. Profiling MHC II immunopeptidome of blood-stage malaria reveals that cDC1 control the functionality of parasite-specific CD4 T cells. EMBO Mol. Med. 2017, 9, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B. Induction of CD4(+) regulatory and polarized effector/helper T cells by dendritic cells. Immune Netw. 2016, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Pollara, G.; Kwan, A.; Newton, P.J.; Handley, M.E.; Chain, B.M.; Katz, D.R. Dendritic cells in viral pathogenesis: Protective or defective? Int. J. Exp. Pathol. 2005, 86, 187–204. [Google Scholar] [CrossRef]
- Buller, R.M.; Palumbo, G.J. Poxvirus pathogenesis. Microbiol. Rev. 1991, 55, 80–122. [Google Scholar] [CrossRef]
- Ma, X.; Xu, R.H.; Roscoe, F.; Whitbeck, J.C.; Eisenberg, R.J.; Cohen, G.H.; Sigal, L.J. The mature virion of ectromelia virus, a pathogenic poxvirus, is capable of intrahepatic spread and can serve as a target for delayed therapy. J. Virol. 2013, 87, 7046–7053. [Google Scholar] [CrossRef]
- Parker, A.K.; Parker, S.; Yokoyama, W.M.; Corbett, J.A.; Buller, R.M. Induction of natural killer cell responses by ectromelia virus controls infection. J. Virol. 2007, 81, 4070–4079. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Lanier, L.L.; Sigal, L.J. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008, 4, e30. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, P.; Singh, P.; Thiele, A.T.; Wilkes, D.S.; Renukaradhya, G.J.; Brutkiewicz, R.R.; Travers, J.B.; Luker, G.D.; Hong, S.C.; et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell. Immunol. 2007, 246, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Pardecka, M.; Cymerys, J.; Krzyzowska, M. Dendritic cells during mousepox: The role of delayed apoptosis in the pathogenesis of infection. Microb. Pathog. 2017, 109, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hochrein, H.; Shortman, K.; Vremec, D.; Scott, B.; Hertzog, P.; O’Keeffe, M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J. Immunol. 2001, 166, 5448–5455. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The role of dendritic cells during infections caused by highly prevalent viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Pietilä, T.E.; Veckman, V.; Kyllönen, P.; Lähteenmäki, K.; Korhonen, T.K.; Julkunen, I. Activation, cytokine production, and intracellular survival of bacteria in Salmonella-infected human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 2005, 78, 909–920. [Google Scholar] [CrossRef]
- Fricke, I.; Mitchell, D.; Mittelstädt, J.; Lehan, N.; Heine, H.; Goldmann, T.; Böhle, A.; Brandau, S. Mycobacteria induce IFN-γ production in human dendritic cells via triggering of TLR2. J. Immunol. 2006, 176, 5173–5182. [Google Scholar] [CrossRef]
- Goodyear, A.; Troyer, R.; Bielefeldt-Ohmann, H.; Dow, S. MyD88-dependent recruitment of monocytes and dendritic cells required for protection from pulmonary Burkholderia mallei infection. Infect. Immun. 2012, 80, 110–120. [Google Scholar] [CrossRef]
- De Pascalis, R.; Rossi, A.P.; Mittereder, L.; Takeda, K.; Akue, A.; Kurtz, S.L.; Elkins, K.L. Production of IFN-γ by splenic dendritic cells during innate immune responses against Francisella tularensis LVS depends on MyD88, but not TLR2, TLR4, or TLR9. PLoS ONE 2020, 15, e0237034. [Google Scholar] [CrossRef]
- Das, G.; Sheridan, S.; Janeway, C.A., Jr. The source of early IFN-γ that plays a role in Th1 priming. J. Immunol. 2001, 167, 2004–2010. [Google Scholar] [CrossRef]
- Allan, R.S.; Smith, C.M.; Belz, G.T.; van Lint, A.L.; Wakim, L.M.; Heath, W.R.; Carbone, F.R. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 2003, 301, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Bordet, E.; Blanc, F.; Tiret, M.; Crisci, E.; Bouguyon, E.; Renson, P.; Maisonnasse, P.; Bourge, M.; Leplat, J.J.; Giuffra, E.; et al. Porcine reproductive and respiratory syndrome virus type 1.3 Lena triggers conventional dendritic cells 1 activation and T helper 1 immune response without infecting dendritic cells. Front. Immunol. 2018, 9, 2299. [Google Scholar] [CrossRef] [PubMed]
- Harpur, C.M.; Kato, Y.; Dewi, S.T.; Stankovic, S.; Johnson, D.N.; Bedoui, S.; Whitney, P.G.; Lahoud, M.H.; Caminschi, I.; Heath, W.R.; et al. Classical type 1 dendritic cells dominate priming of Th1 responses to herpes simplex virus type 1 skin infection. J. Immunol. 2019, 202, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Denniston, A.K.; Hirani, S.; Hannes, S.; Nussenblatt, R.B. Role of dendritic cell subsets in immunity and their contribution to noninfectious uveitis. Surv. Ophthalmol. 2015, 60, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Trifilo, M.J.; Lane, T.E. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8α− dendritic cell maturation and activation following viral infection of the central nervous system: Implications for a role in T cell activation. Virology 2004, 327, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.A.; Diener, N.; Clausen, B.E. Langerin+CD8+ dendritic cells in the splenic marginal zone: Not so marginal after all. Front. Immunol. 2019, 10, 741. [Google Scholar] [CrossRef] [PubMed]
- Bošnjak, B.; Do, K.T.H.; Förster, R.; Hammerschmidt, S.I. Imaging dendritic cell functions. Immunol. Rev. 2022, 306, 137–163. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Liu, D.; Duan, L.; Cyster, J.G. Chemo- and mechanosensing by dendritic cells facilitate antigen surveillance in the spleen. Immunol. Rev. 2022, 306, 25–42. [Google Scholar] [CrossRef]
- Liu, D.; Duan, L.; Rodda, L.B.; Lu, E.; Xu, Y.; An, J.; Qiu, L.; Liu, F.; Looney, M.R.; Yang, Z.; et al. CD97 promotes spleen dendritic cell homeostasis through the mechanosensing of red blood cells. Science 2022, 375, eabi5965. [Google Scholar] [CrossRef]
- Oda, M. Vaccinia virus-HeLa cell interaction. II. The effect of mitomycin C on the production of infective virus, complement-fixing antigen, and hemagglutinin. Virology 1963, 21, 533–539. [Google Scholar] [CrossRef]
- Baxby, D.; Rondle, C.J. The inhibition of growth of vaccinia and cowpox viruses in RK 13 cells. J. Hyg. 1968, 66, 191–205. [Google Scholar] [CrossRef]


| Feature | cDC1 | cDC2 |
|---|---|---|
| location | marginal zone | T-cell areas of PALS |
| phenotype | CD8α+CD11b− | CD8α−CD11b+ |
| XCR1+ | SIRPα+ | |
| DNGR-1+ | CD4+/− | |
| CD205+ | ||
| CD207+ | ||
| Th cell differentiation | Th1 | Th2 |
| human homologues | CD141+ DCs | CD1+ DCs |
| Pathogen | Type of DCs | Resistance | Susceptibility | Ref. |
|---|---|---|---|---|
| Leishmania major | CD11b+ DCs ex vivo | Th1 response in B10.D2 mice | Th2 response in BALB/c mice | [23] |
| BMDCs in vitro | Th1 response in B10.D2 mice | Th2 response in BALB/c mice | [23] | |
| Listeria monocytogenes | cDC in vivo | Higher expression of IL-12p40 in cDCs of C57BL/6 mice | Higher expression of CCL2 in cDCs of BALB/c mice | [24] |
| Rickettsia conorii | BMDCs | High ability to stimulate IFN-γ-producing Th1 cells from C57BL/6 mice | Low ability to stimulate IFN-γ-producing Th1 cells from C3H mice | [25] |
| Theiler’s murine encephalomyelitis virus (TMEV) | DCs in vitro and in vivo | Lack of conversion from Th1 into Th2 in C57BL/6 mice | Conversion from Th1 into Th2 in SJL mice | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc-Dąbrowska, L.; Biernacka, Z.; Koper, M.; Struzik, J.; Gieryńska, M.; Schollenberger, A.; Lasocka, I.; Toka, F.N. Differential Activation of Splenic cDC1 and cDC2 Cell Subsets following Poxvirus Infection of BALB/c and C57BL/6 Mice. Cells 2024, 13, 13. https://doi.org/10.3390/cells13010013
Szulc-Dąbrowska L, Biernacka Z, Koper M, Struzik J, Gieryńska M, Schollenberger A, Lasocka I, Toka FN. Differential Activation of Splenic cDC1 and cDC2 Cell Subsets following Poxvirus Infection of BALB/c and C57BL/6 Mice. Cells. 2024; 13(1):13. https://doi.org/10.3390/cells13010013
Chicago/Turabian StyleSzulc-Dąbrowska, Lidia, Zuzanna Biernacka, Michał Koper, Justyna Struzik, Małgorzata Gieryńska, Ada Schollenberger, Iwona Lasocka, and Felix N. Toka. 2024. "Differential Activation of Splenic cDC1 and cDC2 Cell Subsets following Poxvirus Infection of BALB/c and C57BL/6 Mice" Cells 13, no. 1: 13. https://doi.org/10.3390/cells13010013
APA StyleSzulc-Dąbrowska, L., Biernacka, Z., Koper, M., Struzik, J., Gieryńska, M., Schollenberger, A., Lasocka, I., & Toka, F. N. (2024). Differential Activation of Splenic cDC1 and cDC2 Cell Subsets following Poxvirus Infection of BALB/c and C57BL/6 Mice. Cells, 13(1), 13. https://doi.org/10.3390/cells13010013






