Abstract
The genomic activity of 1,25(OH)2D3 is mediated by vitamin D receptor (VDR), whilst non-genomic is associated with protein disulfide isomerase family A member 3 (PDIA3). Interestingly, our recent studies documented that PDIA3 is also involved, directly or indirectly, in the modulation of genomic response to 1,25(OH)2D3. Moreover, PDIA3 was also shown to regulate cellular bioenergetics, possibly through the modulation of STAT signaling. Here, the role of VDR and PDIA3 proteins in membrane response to 1,25(OH)2D3 and calcium signaling was investigated in squamous cell carcinoma A431 cell line with or without the deletion of VDR and PDIA3 genes. Calcium influx was assayed by Fura-2AM or Fluo-4AM, while calcium-regulated element (NFAT) activation was measured using a dual luciferase assay. Further, the levels of proteins involved in membrane response to 1,25(OH)2D3 in A431 cell lines were analyzed via Western blot analysis. The deletion of either PDIA3 or VDR resulted in the decreased baseline levels of Ca2+ and its responsiveness to 1,25(OH)2D3; however, the effect was more pronounced in A431∆PDIA3. Furthermore, the knockout of either of these genes disrupted 1,25(OH)2D3-elicited membrane signaling. The data presented here indicated that the VDR is essential for the activation of calcium/calmodulin-dependent protein kinase II alpha (CAMK2A), while PDIA3 is required for 1,25(OH)2D3-induced calcium mobilization in A431 cells. Taken together, those results suggest that both VDR and PDIA3 are essential for non-genomic response to this powerful secosteroid.
1. Introduction
The classical signaling of 1,25(OH)2D3 is mediated by the nuclear vitamin D receptor (VDR). This nuclear receptor for vitamin D, together with its co-receptor protein, retinoid X receptor (RXR), forms heterodimers, forming a powerful transcription factor. The VDR-RXR dimer upon ligand binding is translocated into the nucleus, where it binds to vitamin D response elements (VDRE) in the regulatory region of the vitamin D target genes and modulates their expression [1,2]. One of the principal activities of 1,25(OH)2D3 is the regulation of calcium-phosphate homeostasis [3,4].
However, not all the effects of 1,25(OH)2D3 can be attributed to genomic response; thus, the idea of alternative signaling pathways with potential membrane-bound receptor for this secosteroid has emerged [5]. The presence of such a pathway, for example, explained the rapid influx of calcium ions induced with 1,25(OH)2D3 [6,7]. Eventually, Nemere and coworkers described a 1,25D3-membrane-associated, rapid response steroid-binding protein (1,25D3-MARRS), which was also known as protein disulfide isomerase family A member 3 (PDIA3) [8]. PDIA3 is an endoplasmic reticulum (ER) protein involved in protein folding, together with other chaperones like calnexin or calreticulin [9]. Outside of ER, PDIA3 was localized within the cell membrane, nucleus, cytoplasm, or mitochondria [10]. Importantly, this protein was proven to be involved in a rapid uptake of calcium and phosphate in intestine cells induced by 1,25(OH)2D3 [11,12]. Thus, PDIA3 is strongly linked to calcium homeostasis. Lately, it has been shown that PDIA3 knockout in squamous cell carcinoma alters the expression of the genes connected to the regulation of bone mineralization, phospholipase C activity, and calcium-dependent phospholipid binding [13]. Moreover, it was shown that the partial silencing of PDIA3 (PDIA3+/−) in mice model impaired skeletal development, while deletion was lethal [14,15,16].
PDIA3 was shown to interact with the phospholipase A2-activating protein (PLAA), subsequently leading to the activation of phospholipase A2 (PLA2) and the mediation of the non-genomic rapid response to 1,25(OH)2D3. As a result, calcium is released to the cytoplasm, followed by the activation of protein kinase C (PKC) or calcium/calmodulin-dependent protein kinase II (CaMKII). Thus, this leads to the induction of downstream signaling such as mitogen-activated protein kinases (MAPK) pathways and other transcription factors (STAT1-3, NF-kB). It has been shown that the disruption of PDIA3 protein attenuated PKA, PKC signaling, and calcium influx [11,14,17,18,19]. Recently, it has been shown that PDIA3 influences STAT3 expression in the C. elegans model, regulating cellular respiration [20]. Additionally, it has been shown that PDIA3 modulates STAT3 signaling induced by 1,25(OH)2D3 [21].
In our latest studies, we investigated the impact of deletion of either VDR or PDIA3 on gene expression profile and non-genomic effects of 1,25(OH)2D3 in A431 squamous cell carcinoma, showing that PDIA3 is involved in genomic responses to 1,25(OH)2D3 [13,22]. Moreover, we have shown that the deletion of PDIA3 abrogates the effects of 1,25(OH)2D3 on cellular bioenergetics, possibly through STAT3 signaling [21]. In this study, the effects of knockout of PDIA3 and VDR on the 1,25(OH)2D3 membrane’s non-genomic signaling were investigated.
2. Materials and Methods
2.1. 1,25(OH)2D3
The active form of vitamin D (1,25(OH)2D3) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). 1,25(OH)2D3 was dissolved in ethanol and stored at −20 °C. A 100 nM concentration was used in all experiments, as it was proven to have the most potent activity for proliferation as well as for photoprotection in other research [13,23,24].
2.2. Cell Cultures
Immortalized human basal cell carcinoma cell line (A431) was purchased from Synthego Corporation (Menlo Park, CA, USA). PDIA3 and VDR knock-out cell lines were obtained with CRISPR/Cas9 technology, as described previously [13]. For cell cultures, DMEM high glucose medium (4.5 g/L) was used. Additionally, the medium was supplemented with 10% fetal bovine serum (FBS), penicillin (10,000 units/mL), and streptomycin (10 mg/mL) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A431 cell lines were cultured in the incubator with 5% CO2 at 37 °C. For experimental conditions, the medium was changed to DMEM supplemented with 2% charcoal-stripped FBS.
2.3. Measurement of Intracellular Calcium Concentration
A431 cell lines were seeded onto 96-well plates in DMEM medium supplemented with charcoal-stripped FBS and antibiotics. To test the effects of VDR and PDIA3 deletion on calcium influx, cells were incubated with 1µM Fura-2AM solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in HBSS for 1 h. Afterwards, incubation cells were rinsed with medium. The fluorescence intensity of the cells was measured using a plate reader at λex 355 nm and λem 495 nm for 16 min.
A431 cell lines were seeded onto an 8-well chamber slide in DMEM medium supplemented with charcoal-stripped FBS and antibiotics. To test the effects of VDR and PDIA3 deletion on calcium influx, cells were incubated with 1 µM Fluo-4AM solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in Hank’s Balanced Salt solution (HBSS) (PAN Biotech, Aidenbach, Germany) for 30 min. Cells were rinsed with HBSS and left in medium. The intensity of fluorescence was observed with the use of live microscopy on Olympus Cell-Vivo IX 83 (Olympus, Tokyo, Japan).
2.4. Luciferase Reporter Assay
The human VDRE and NFAT firefly luciferase reporter constructs were purchased from Qiagen (Hilden, Germany). To test the effects of the deletion of either VDR or PDIA3 on VDRE and NFAT elements, A431 cells were seeded onto 96-well polystyrene, white/clear flat bottom plates (30,000 cells/per well) and transfected with the constructs using Lipofectamin 2000 (Thermo Fisher Scientific, Waltham, MA, USA). After 24 h transfected cells were treated with 100 nM 1,25(OH)2D3 for 4, 8, or 24 h. Then, cells were lysed using luciferase assay lysis buffer (Promega, Madison, WI, USA), and the firefly-Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol using GloMax-Multi + Detection System (Promega, Madison, WI, USA). Results were normalized to non-treated cells for all A431 sublines, separately.
2.5. Western Blot
Squamous cell carcinoma cell lines were treated with 100 nM 1,25(OH)2D3 for 4, 8, and 24 h. After a given time (4 h, 8 h, and 24 h), the medium was removed and cells were washed twice with PBS. Next, A431 cells were scratched from the plate in cooled PBS, and the suspension was moved to an Eppendorf tube and centrifuged for 10 min at 16,000× g. The cell pellet was resuspended in RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) with the addition of phosphatase and protease inhibitors (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). To measure the concentration of lysates, modified Bradford Assay was used according to manufacturer protocol (Bio-Rad, Hercules, CA, USA). Amounts of 10% bottom gel and 5% upper gel were used for SDS-PAGE electrophoresis. An equal amount of lysates (20 µg) was loaded into each well, and gels were resolved at 90–110 V in the Bio-Rad apparatus (Bio-Rad, Hercules, CA, USA). A Trans-Blot Turbo system was used for protein transfer to PVDF membranes (Bio-Rad, Hercules, CA, USA). Then, membranes were blocked in 5% milk dissolved in TBS-T. The membranes were incubated with specific primary antibodies: anti-PLAA, anti-PLCγ, anti-PKCα, anti-ERK1/2, anti-phophoERK1/2 (Thr202/Tyr204), anti-Caveolin 1, anti-Caveolin 3, anti-CAMK2A, anti-phosphoCAMK2A (T286), and anti-TRPV6 (Abclonal, Woburn, MA, USA), overnight at 4 °C. Proper secondary fluorescent antibodies conjugated with AlexaFluor® 790 or AlexaFluor® 680 were used (Jackson ImmunoResearch, Cambridgeshire, UK). As a loading control, anti-β-actin antibodies (Abclonal, Woburn, MA, USA) were used. Results were visualized with the Odyssey Clx system and calculated with the use of Image Studio Software Ver 5.2 (both LI-COR Biosciences, Lincoln, NE, USA).
2.6. Bioinformatic Analysis
As described in a previous study [13,22], data quality and cell line disparity were checked. To identify differentially expressed genes, the absolute value of log2fold change ≥ 1.0 and adjusted p-value < 0.05 were used. To see whether the expression of selected genes was regulated by either the deletion of PDIA3 or 1,25(OH)2D3 treatment, the heat maps were prepared. Data showed the clustering of RNA-seq expression data and technical repeats within the groups. The RNA-seq data have been deposited in Sequence Read Archive (SRA) under accession number PRJNA926032.
2.7. Statistical Analysis
GraphPad Prism version 7.05 was used for the statistical analysis of obtained data (GraphPad Software, Inc., La Jolla, CA, USA). Results are presented as mean ± SD and were analyzed either with Student’s t-test or one-way ANOVA analysis of variance with appropriate post hoc tests. Statistical significance is illustrated as asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, or **** p < 0.0001.
3. Results
3.1. Deletion of PDIA3 Modulates the Expression of Calcium-Associated Genes in A431 Cells
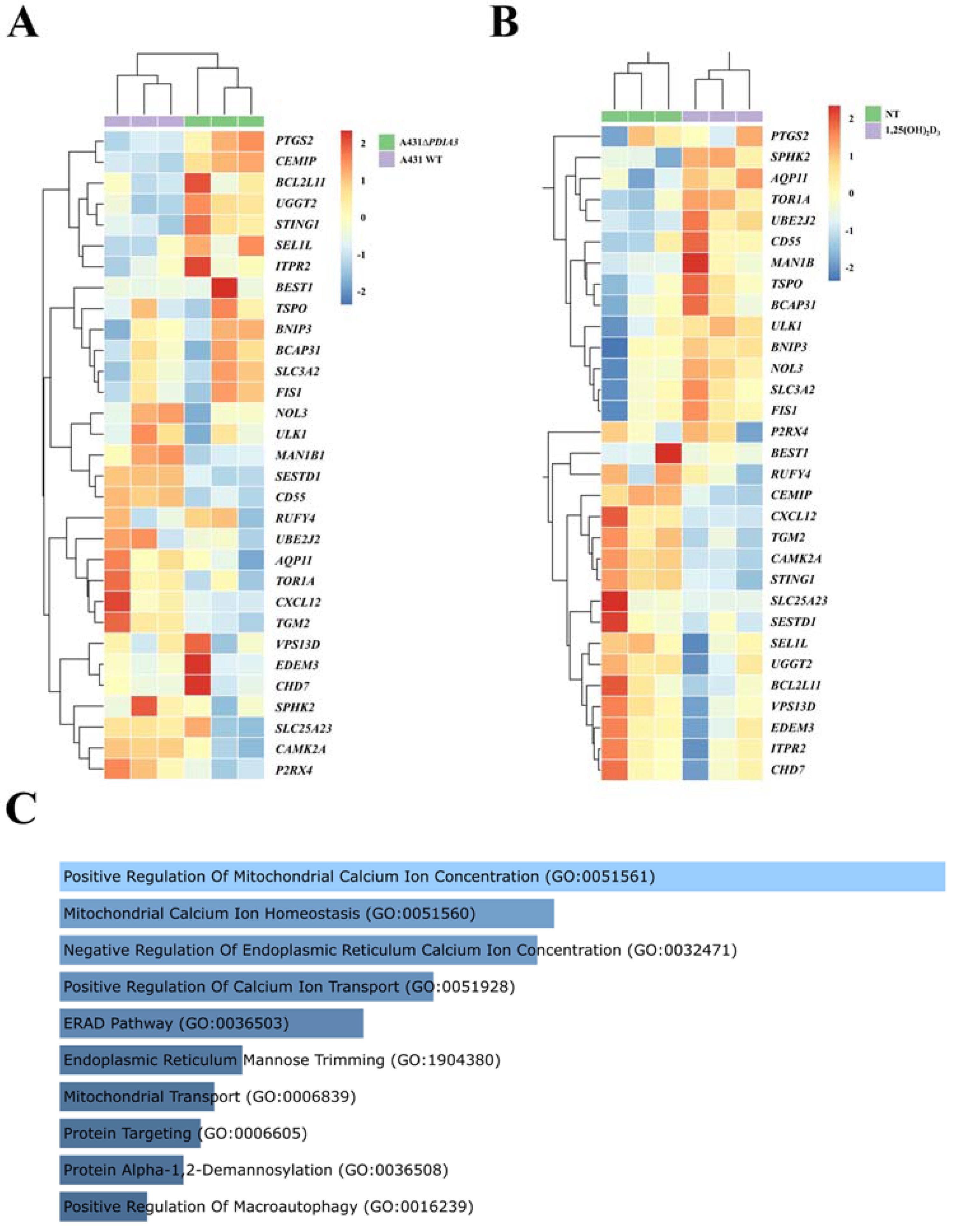
Previously, it was shown that deletion of the PDIA3 gene in the A431 squamous cell carcinoma line (A431ΔPDIA3) affected expression of more than 1800 genes, including genes modulated by 1,25(OH)2D3 treatment [13]. Here, the expression of calcium-associated genes and ER-related genes was evaluated. Thirty calcium-associated genes were found amongst differently expressed genes (DEGs) in the A431ΔPDIA3 cell line, including 12 upregulated and 18 downregulated genes (Figure 1A). All of those DEGs associated with calcium were further affected by 1,25(OH)2D3 treatment, and 14 were upregulated and 16 downregulated in the PDIA3 knockout cell line (Figure 1B). Further, those 30 calcium-associated DEGs disrupted by PDIA3 deletion were analyzed in terms of the biological process using gene ontology. The analysis revealed their involvement in biological processes related to the positive regulation of mitochondrial calcium ion concentration, mitochondrial calcium ion homeostasis, the negative regulation of endoplasmic reticulum calcium ion concentration, and the positive regulation of calcium ion transport (Figure 1C).
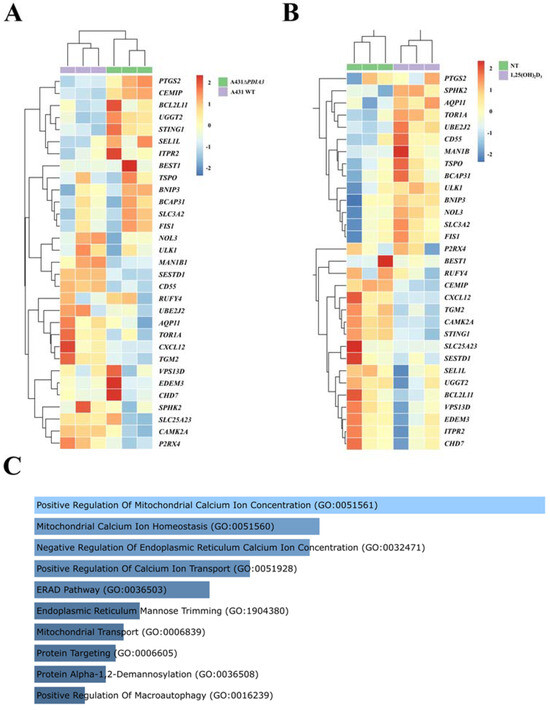
Figure 1.
Changes in gene expression connected to calcium metabolism and endoplasmic reticulum homeostasis in human squamous carcinoma cell lines (A431). Heatmaps of selected genes with a statistically significant change in expression level after (A) PDIA3 knockout and (B) after 1,25(OH)2D3 treatment solely in A431∆PDIA3 cells. The color of cells represents the Z-score of normalized gene expression values. (C) Gene ontology biological process analysis of calcium-associated genes disrupted after PDIA3 deletion. Color intensity and length correspond to the p-value of the GO biological process.
3.2. Deletion of PDIA3 Decreases Calcium Levels after 1,25(OH)2D3 Treatment and Consequently Disrupts the Activity of Calcium-Regulated Nuclear Factor of Activated T-Cells (NFAT) in A431 Cells
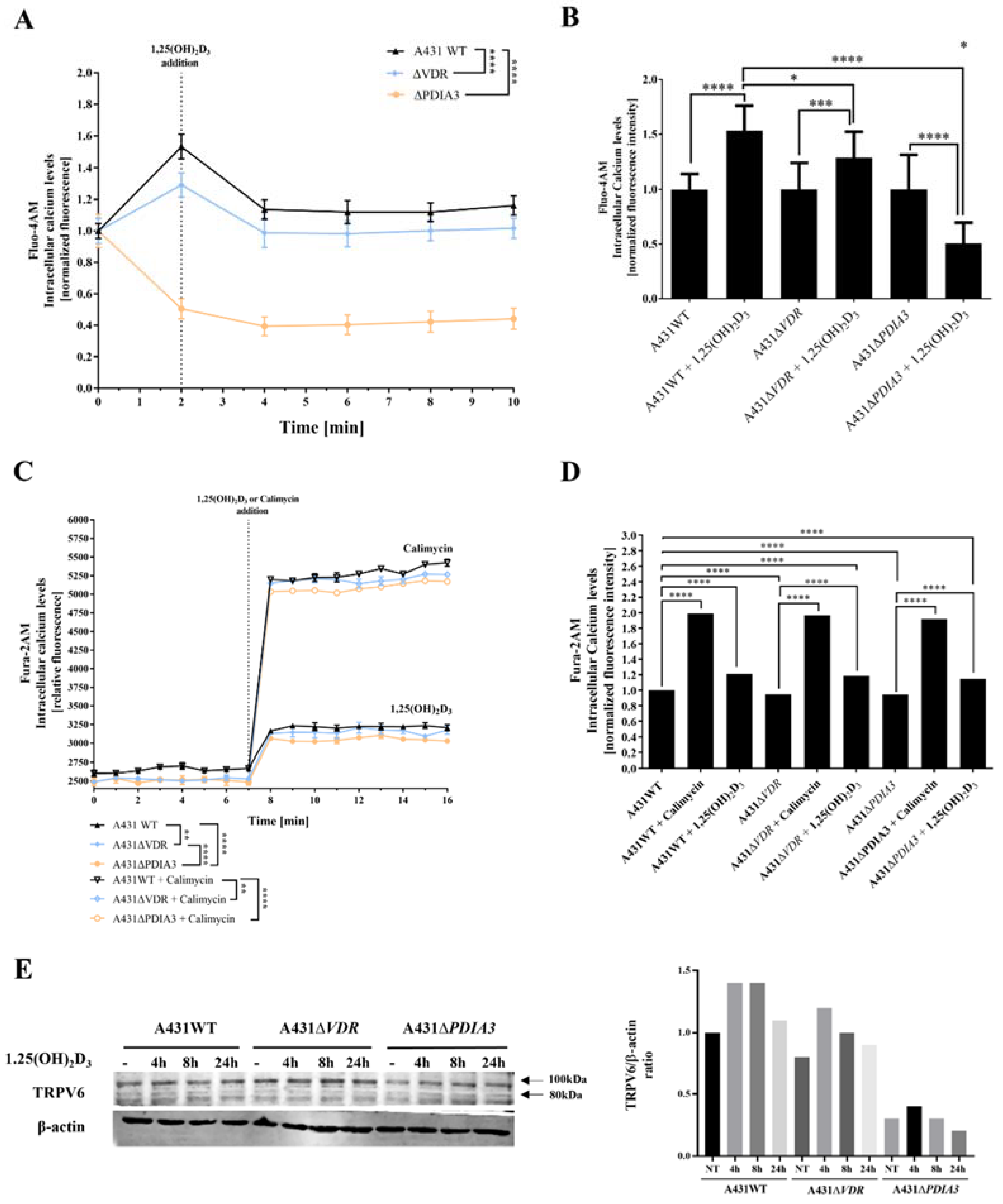
Subsequent experiments were focused on changes in levels of intracellular calcium induced by 1,25(OH)2D3. To verify an impact of PDIA3 on calcium homeostasis, intracellular levels of calcium were measured with the use of two fluorescence probes (Fura-2AM and Fluo-4AM) via fluorescent measurement using plate reader or live microscopy on Olympus Cell-Vivo IX 83, respectively (Figure 2). In A431 knockout cell lines, the baseline level of intracellular calcium measured with Fluo-4AM probe was decreased in comparison to A431WT cells. The effect was less pronounced for A431∆VDR cells. Curiously, in the case of A431∆PDIA3 cells, there was a decrease in fluorescence after 1,25(OH)2D3 addition (Figure 2A,B). Those results are further supported by data acquired with a Fura-2AM probe, indicating a role of PDIA3 in calcium mobilization and 1,25(OH)2D3-induced calcium influx. An addition of 100 nM 1,25(OH)2D3 elicited an influx of calcium ions into the A431 cells; however, the increase in both cell lines, A431∆VDR and A431∆PDIA3, was significantly smaller in comparison to A431WT cells. A431∆PDIA3 cells were characterized by the lowest baseline calcium level and 1,25(OH)2D3-induced calcium influx. Additionally, lower concentrations of 1,25(OH)2D3 (1 nM and 10 nM) were tested with a similar effect (Supplementary Figure S1), suggesting that even low concentrations of 1,25(OH)2D3 are sufficient for the activation of calcium influx in A431 cells. This is in agreement with previous studies where calcium uptake was triggered by even lower, picomolar concentrations of 1,25(OH)2D3 (0.13 nM [7] or 0.3 nM [25]). Calimycin (calcium ionophore) was used as a positive control of calcium influx. Interestingly, a calimycin-induced influx of calcium ions was also slightly affected by either VDR or PDIA3 deletion in A431 cells and the effect was more pronounced in ΔPDIA3 cells (Figure 2C,D). In our recent publication, it was shown that PDIA3 deletion impacts the expression of the well-known calcium channel TRPV6 [13]. Here, the level of the mentioned protein was checked after the deletion of VDR or PDIA3 and 1,25(OH)2D3 treatment. In A431WT cells 1,25(OH)2D3 treatment increased levels of TRPV6 protein. The same effect was observed for A431∆VDR cells, but the increase was slightly reduced in comparison to the A431WT cell line. Interestingly, the deletion of PDIA3 prevented the increase in TRPV6 protein (Figure 2E).
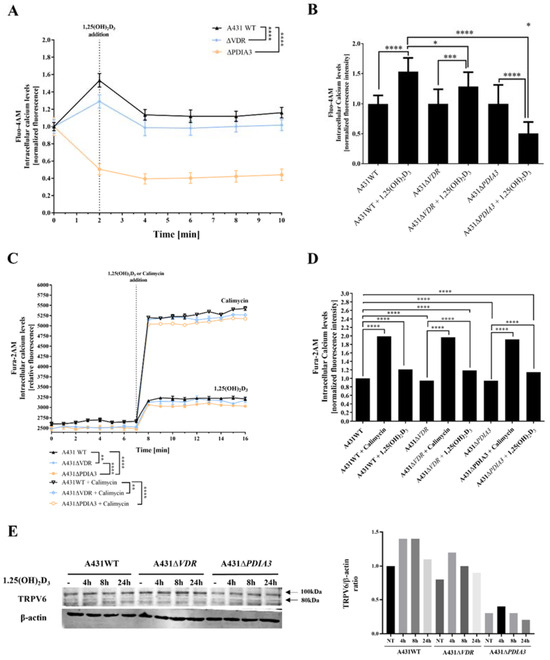
Figure 2.
Deletion of PDIA3 decreases calcium levels after 1,25(OH)2D3 treatment in squamous cell carcinoma. (A) Intracellular calcium levels monitored with Fluo-4AM fluorescence probe in A431 cell lines with live cell time-lapse microscopy. (B) Normalized fluorescence intensity at 0 and 2 min time points. (C) Time-resolved analysis of intracellular calcium levels measured with Fura-2AM probe on microplate reader in A431. Results were calculated as a mean ± SD of triplicates. Statistically significant differences are illustrated with asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, or **** p < 0.0001. (D) Alterations of normalized fluorescence intensity at the 7th and 8th minute of the experiment. Representative results were presented in the graph. (E) Analysis of TRPV6 protein levels in A431 cell lines with VDR or PDIA3 deletion. Two bands represent glycosylated (100 kDa) and non-glycosylated (80 kDa). The quantity of non-glycosylated TRPV6 (80 kDa) was calculated as a protein/β-actin ratio. Protein levels are calculated as means from three independent experiments. Representative pictures are shown.
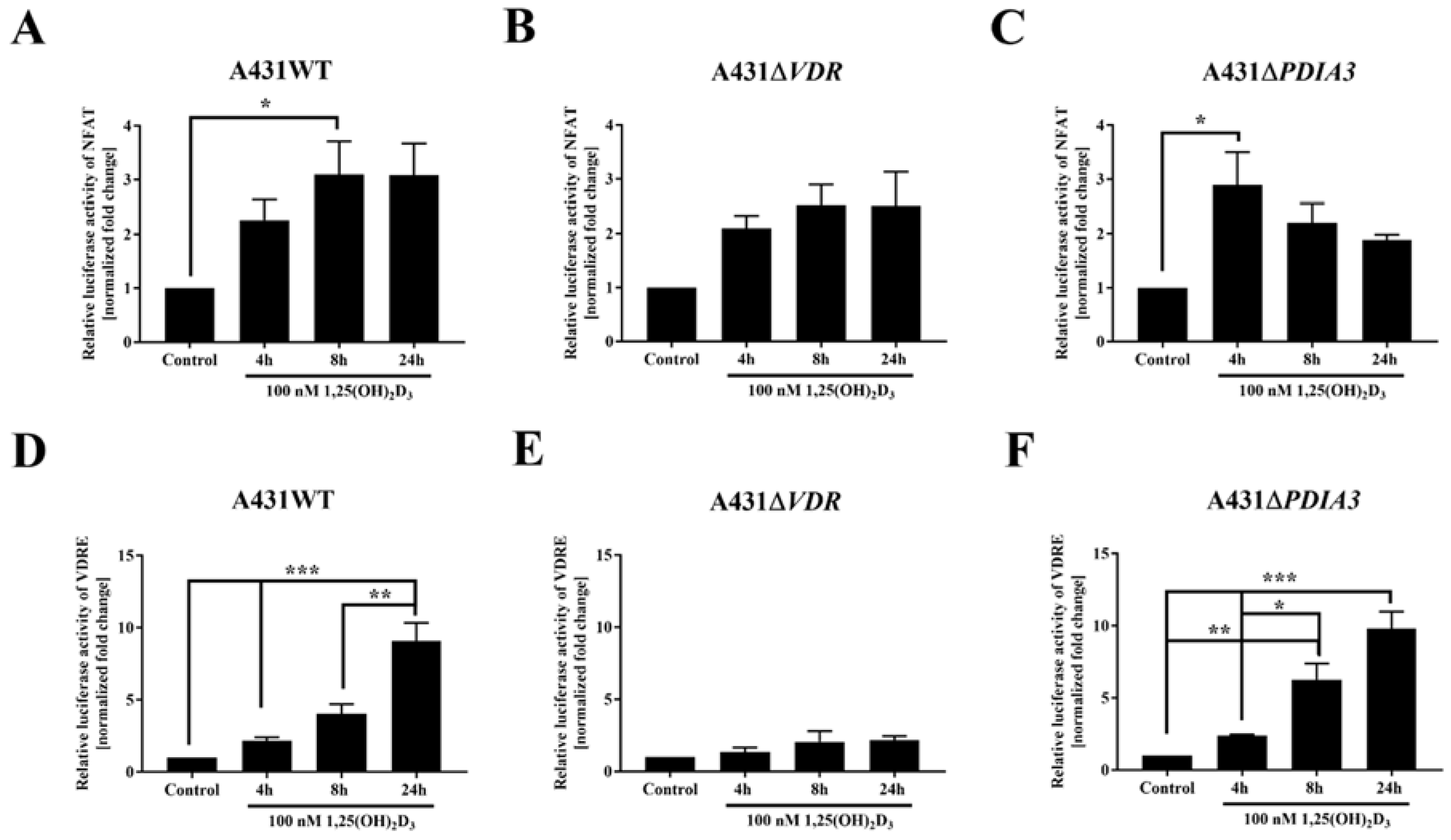
Since the transcriptome analysis revealed numerous changes in gene expression after PDIA3 deletion [13] and, most importantly, among calcium-associated genes, the effects of VDR and PDIA3 deletion on the activity of the calcium-associated nuclear factor of activated T-cells (NFAT) and vitamin D response elements (VDRE) were investigated. VDRE served as additional control. Briefly, after the transfection of A431WT, A431∆VDR, and A431∆PDIA3 with NFAT or VDRE luciferase reporter vectors, cells were treated with 100 nM 1,25(OH)2D3 and luciferase activity was measured (Figure 3). The NFAT is a transcription factor activated by calcium signaling and was previously linked to the immunosuppressive activity of 1,25(OH)2D3 [26,27]. The NFAT activity was increased almost threefold in A431WT cells after 8 h of 1,25(OH)2D3 treatment (Figure 3A). The deletion of VDR did not affect NFAT activity (Figure 3B). Interestingly, in A431∆PDIA3 cells, 1,25(OH)2D3 treatment elicited a rapid increase in NFAT activity after 4 h with further decrease in activity after 8 and 24 h (Figure 3C). In wild-type A431 cell line, 1,25(OH)2D3 treatment increased the activity of VDRE most efficiently after 24 h (Figure 3D). The deletion of VDR completely eliminated effect of 1,25(OH)2D3 treatment on vitamin D response element (Figure 3E). In contrast, PDIA3 deletion slightly enhanced VDRE activation by 1,25(OH)2D3 in comparison to A431WT cells, and the effect was noticeable after 4 or 8 h of incubation (Figure 3F).
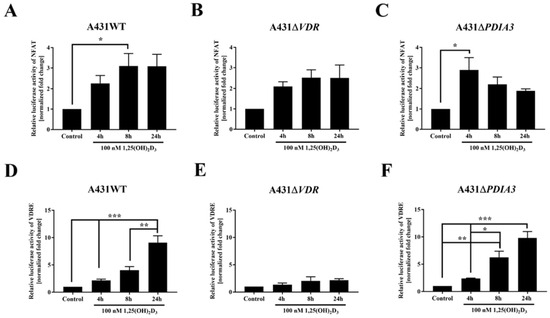
Figure 3.
Changes in the activity of VDRE and NFAT response element to 1,25(OH)2D3 in A431 cell lines. The bar graphs indicate the normalized fold change of the firefly/Renilla luciferase ratio of NFAT (A–C) and VDRE (D–F) in A431WT, ∆VDR, and ∆PDIA3. Dual luciferase reporter assay results are represented as means ± SEM of triplicate samples. Statistically significant differences are illustrated with asterisks: * p < 0.05, ** p < 0.01, or *** p < 0.001.
3.3. VDR and PDIA3 Deletion Disrupts Membrane Response to 1,25(OH)2D3 in Squamous Cell Carcinoma
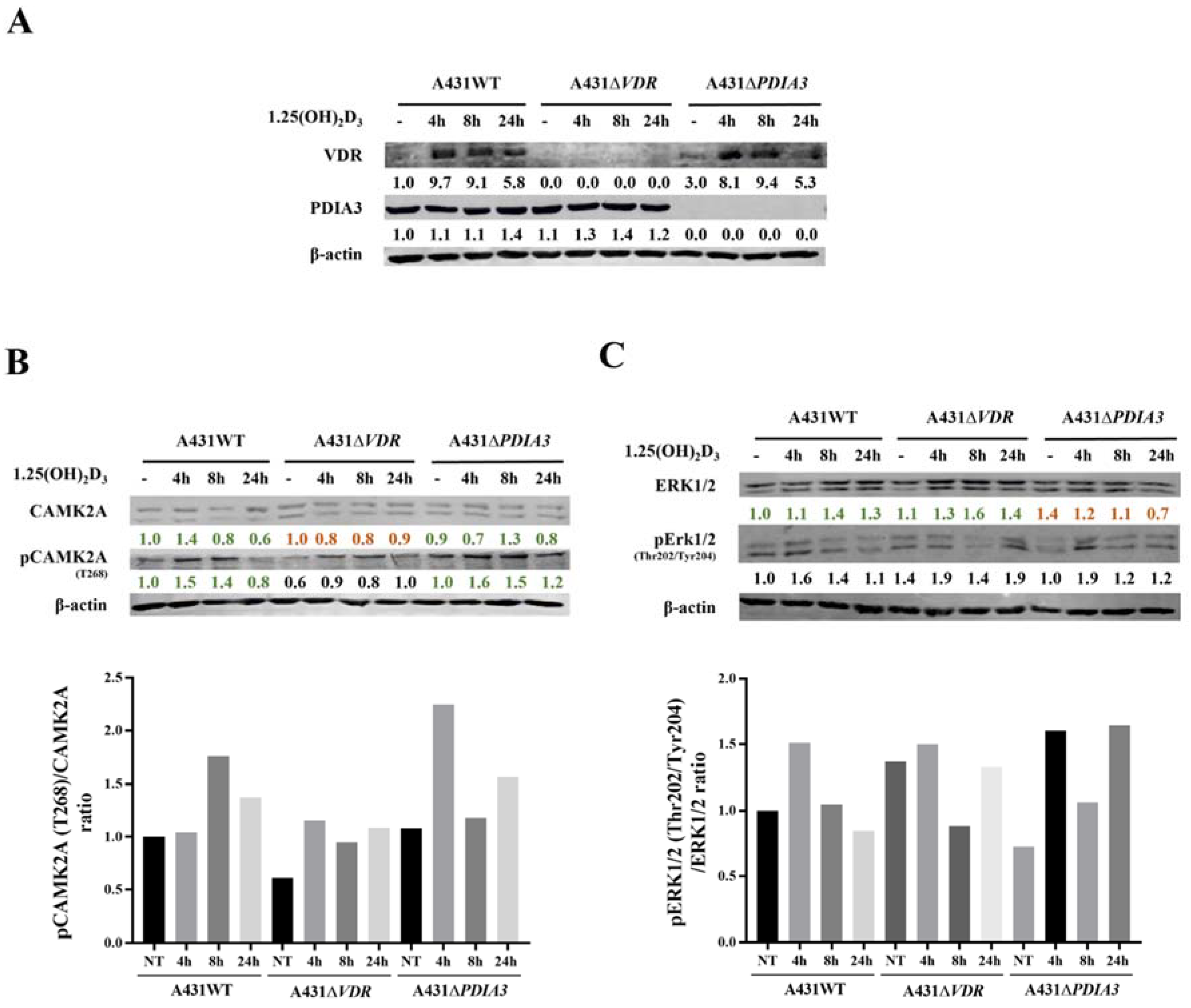
Calcium is a known secondary messenger, which activates several downstream targets such as PKC or CAMKII. Thus, we decided to investigate the downstream targets of calcium signaling as a part of membrane response to 1,25(OH)2D3 and the role of VDR and PDIA3 proteins in this process. Thus, time-resolved analysis of the levels of calcium-related protein in A431 cell with deletion of either VDR or PDIA3 was performed (Figure 4A). It was observed that VDR deletion alone increased baseline levels of the phosphorylated form of extracellular signal-regulated kinase 1/2 (pERK1/2; Thr202/Tyr204) while decreasing the phosphorylated form of calcium/calmodulin-dependent protein kinase II alpha (pCAMKIIα; T268) (Figure 4B,C). Interestingly, the deletion of PDIA3 increased baseline levels Erk1/2. The total amount of CAMKIIα was not affected by either VDR or PDIA3 deletion.
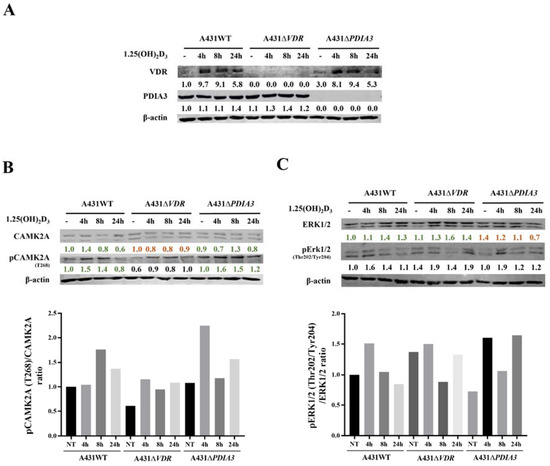
Figure 4.
Effects of PDIA3 and VDR deletion on 1,25(OH)2D3 signaling pathways in human squamous carcinoma cells (A431). (A) Validation of VDR and PDIA3 knockout in A431 cells. Ratio of phosphorylated forms of (B) CAMK2A (T286) and (C) Erk1/2 (Thr202/Tyr204) proteins. The red color illustrates a decrease in protein level, while green marks an increase. The quantity of each protein was calculated as a protein/β-actin ratio. Protein levels are calculated as means from three independent experiments. Representative pictures are shown for each protein. The red color illustrates a decrease in protein level, while green marks the increase.
Doroudi M and coworkers have shown that PDIA3 is essential for the activation of PLAA, PLCγ, and PKCα signaling cascade by 1,25(OH)2D3 [18]. Although we observed that the baseline total levels of PLAA, PLCγ, and PKCα were increased by PDIA3 deletion (Supplementary Figure S2), the change in PLA2 and PKCα activity measured by ELISA assays after 1,25(OH)2D3 stimulation was not observed on our cellular model (not shown). Finally, the knockout of the PDIA3 gene disrupted the response of Erk1/2 and its phosphorylation after 1,25(OH)2D3 treatment (Figure 4C). VDR knockout also abolished the response of CAMKIIα and its phosphorylation (Figure 4B). Interestingly, 1,25(OH)2D3 treatment induced a slight increase in PDIA3 level after 24 h incubation, and VDR deletion enhanced this effect.
4. Discussion
Complementary to our previous research on the topic of non-genomic responses to 1,25(OH)2D3 in squamous cell carcinoma [13,21], we further explored the role of PDIA3 in calcium homeostasis and membrane signaling after 1,25(OH)2D3 treatment.
Recently, it has been shown that PDIA3 is strongly involved in the regulation of mitochondrial bioenergetics [20,21]. Here, we have shown that the deletion of the PDIA3 gene affected the expression of calcium-associated genes and modulated their responses to 1,25(OH)2D3. Those genes were mainly involved in calcium-related processes within mitochondria and endoplasmic reticulum. Interestingly, the expression of some of those genes (TGM2, BNIP3, FIS1) has been linked as prognostic markers in squamous cell carcinomas [28,29]. The silencing of PDIA3 and 1,25(OH)2D3 treatment reversed the expression of those cancer-related genes in A431 cells. In accordance with our previous study, we observed that deletion of VDR completely eliminates 1,25(OH)2D3-dependent gene expression [22]. Furthermore, we observed that in cells lacking VDR, the activation of the vitamin D response element was completely eliminated, while the deletion of PDIA3 modulated the activity of VDRE to some extent, especially after 8 h of 1,25(OH)2D3 treatment.
Calcium acts as a second messenger molecule and is critical for proper cell physiology and signal transduction [30,31]. Here, we showed the deletion of either VDR or PDIA3 decreased baseline calcium levels in squamous cell carcinoma A431 cells and further impaired calcium influx induced by 1,25(OH)2D3. Those findings were in accordance with our other study on PDIA3’s role in calcium signaling. He and coworkers showed that the knockdown of PDIA3 inhibited mitochondrial calcium uptake within HeLa cells, possibly through the regulation of mitochondrial calcium uniporter (MCU) expression [32]. Additionally, it was shown that the reduction in VDR levels in the intestine blunts the 1,25(OH)2D3-regulated absorption of calcium [33]. Furthermore, it was shown in mice models that the transient receptor potential vanilloid type 6 (TRPV6) is essential for vitamin D-induced active calcium transport in the intestine [34]. TRPV6 is a well-known classical target for 1,25(OH)2D3 action and plays a vital role in the transcellular transport of calcium ions and uptake [35]. TRPV6 occurs in two forms, glycosylated (gTRPV6, 100 kDa) and non-glycosylated (TRPV6, 80 kDa). It was suggested that glycosylation determines the stability and assembly of TRPV6 [36]. In our previous research, it was shown that PDIA3 deletion significantly impaired the expression of the TRPV6 gene and its responsiveness to 1,25(OH)2D3 in A431∆PDIA3 cells [13]. Bianco et al. have shown that the deletion of the TRPV6 calcium channel resulted in no response to PTH or 1,25(OH)2D3 treatment on mice models [37]. Here, we have shown that the expression of both forms of TRVP6 was also impaired by PDIA3 deletion in A431 cells. Decreased levels of TRPV6 in A431∆PDIA3 may explain the partial impairment of calcium influx observed with a Fura-2AM probe. Thus, our results indicate that PDIA3 plays a major role in the regulation of calcium homeostasis, including the vitamin D-induced uptake of Ca2+ or intracellular storage and trafficking with the possible involvement of epithelial channel TRPV6 and the regulation of its stability.
PDIA3 is a protein necessary to maintain normal cellular physiology, and its dysfunction may lead to numerous diseases, including cancers, neurodegenerative diseases, or respiratory pathologies [38,39,40,41,42]. Multiple studies linked PDIA3 to the rapid response of cells to 1,25(OH)2D3 [17,18] but the exact mechanism of its action remains unclear. In our previous report, we showed that the deletion of the PDIA3 gene strongly modulates the effect of 1,25(OH)2D3 on the gene expression profile of A431 cells, suggesting that it can directly (as a transcription factor or modulator) or indirectly (through activation of other signaling pathways and/or transcription factors [20,43,44]) affect the genomic activity of vitamin D. Among those genes, PDIA3 deletion increased the expression of PKCα [45]. Here, the total amount of PKCα was also disrupted and the effect of 1,25(OH)2D3 was reduced. This is in accordance with the study of Wang et al., where the deletion of PDIA3 impaired the 1,25(OH)2D3-induced activity of PKC [14]. Our results presented here demonstrate that both VDR and PDIA3 proteins are indeed involved in the 1,25(OH)2D3 membrane response of A431 cells [46]. Nevertheless, it seems that the cooperation of both is essential for membrane response. PDIA3 was co-localized with VDR in caveolae, and it was proven that both proteins can interact with caveolin-1 [17]. Another study by Doroudi and coworkers showed that CAMKIIA is required for mediating the rapid actions of 1,25(OH)2D3 [47]. However, in our study, only the deletion of VDR attenuated the impact of 1,25(OH)2D3 on levels of CAMKIIA. It was shown recently that the activation of PLCγ-mediated signaling results in the release of calcium from intracellular stores and induces an influx of ions across the plasma membrane [48]. Here, we observed that the deletion of PDIA3 had the most prominent effect on the expression of PLCγ; however, a change in its activity was not observed, and thus, the involvement of PLCγ in 1,25(OH)2D3 signaling in the A431 squamous cell carcinoma cell line requires further investigation. Decreased levels of PKCα to 1,25(OH)2D3 in A431∆PDIA3 cells are in accordance with the observations of Boyan and colleagues, who showed that the partial deletion of PDIA3 impaired the activation of PKC through PLAA induction due to lack of interaction between PDIA3/Cav1/PLAA [49].
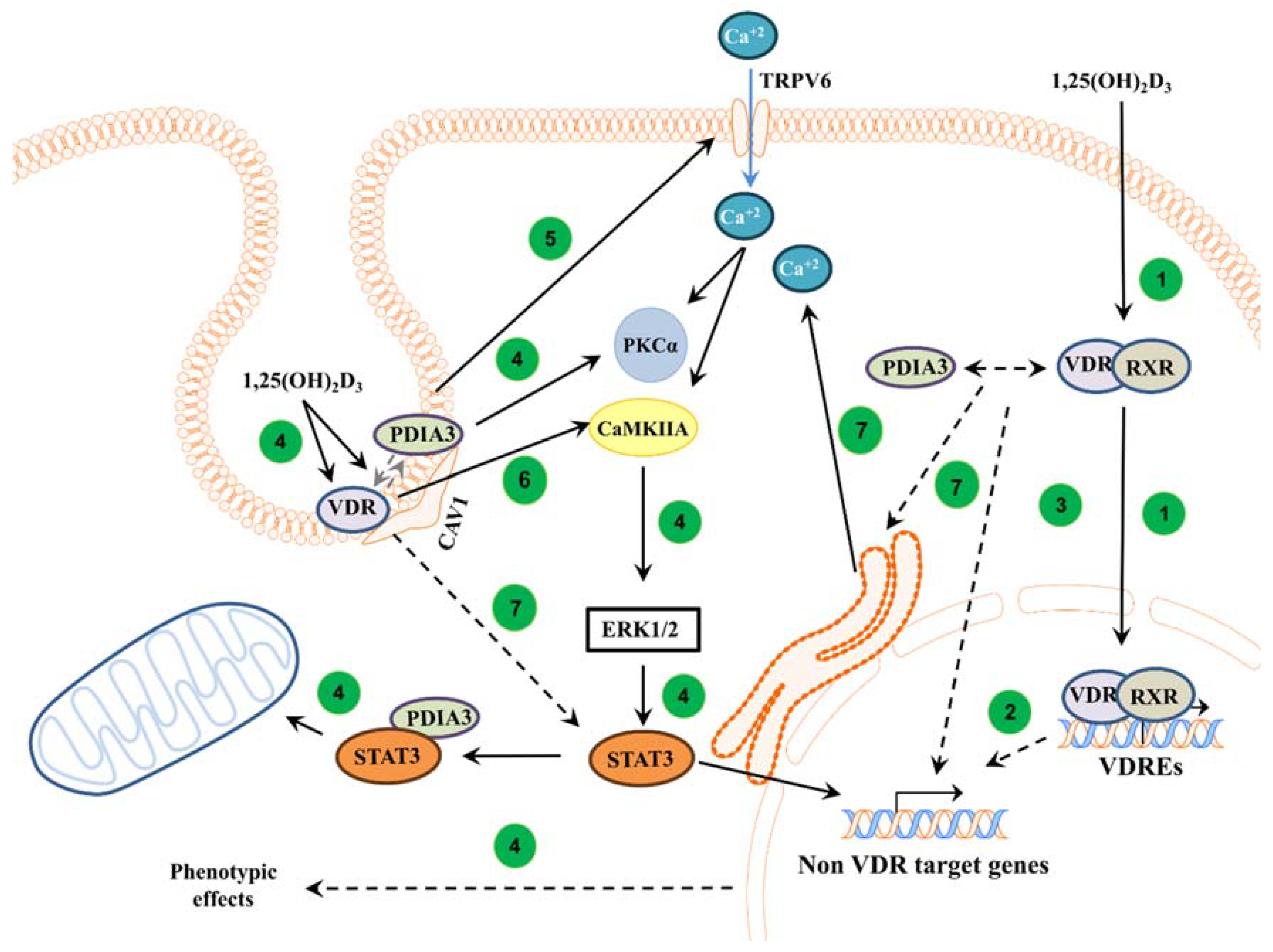
Here, we showed a major role of PDIA3 in membrane response to an active form of vitamin D. Our results indicate that PDIA3 is not solely responsible for the activation of the non-genomic pathway of 1,25(OH)2D3, but that VDR is also required for this action. However, it seems that VDR and PDIA3 affect different targets of the 1,25(OH)2D3 membrane response. Our results are further supported by a previous study in which we stated that PDIA3 is a modulator of the genomic actions of vitamin D [13]. Moreover, it seems that VDR and PDIA3 are required for the regulation of calcium influx induced by 1,25(OH)2D3 in squamous cell carcinoma. The proposed involvement of PDIA3 and VDR in 1,25(OH)2D3 action is shown in Figure 5.
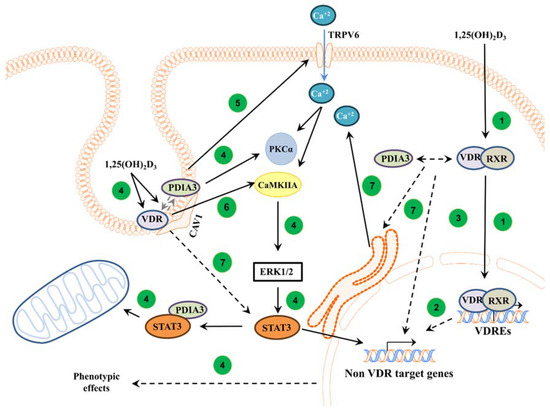
Figure 5.
Proposed mechanism of action of VDR and PDIA3 to 1,25(OH)2D3 membrane response in squamous cell carcinoma. In the classical pathway, 1,25(OH)2D3 is bound by a heterodimer of VDR/RXR proteins and, subsequently, the complex is translocated into the nucleus where it regulates the transcription of vitamin D target genes [1] (1). Further, primary VDR target transcription factors can regulate secondary non-vitamin D target genes [50] (2). It was also postulated that PDIA3 can modulate genomic response to 1,25(OH)2D3 [13] (3). In non-genomic pathways, VDR and PDIA3 were shown to interact with caveolin-1 [17]. PDIA3 was shown to be essential to activate PKC after 1,25(OH)2D3 treatment [14] (4). Moreover, PDIA3 affects TRPV6 levels within SCC cells, possibly disrupting calcium response (5). Either PDIA3 or VDR are needed to activate STAT3, possibly regulating mitochondrial bioenergetics and non-VDR target genes [13,21] (4). Interestingly, it seems that VDR is required to activate CAMK2IIA kinase (6), while either protein is essential for valid calcium signaling (7).
Taken together, our results presented here emphasize the importance of PDIA3 in 1,25(OH)2D3 signaling in squamous cell carcinoma cell line A431. The deletion of PDIA3 not only affected membrane signaling but also genomic responses to vitamin D. Moreover, it seems that both VDR and PDIA3 are required for the regulation of calcium signaling induced by 1,25(OH)2D3 in A431 squamous cell carcinoma.
5. Conclusions
In conclusion, this study demonstrated that both VDR and PDIA3 are needed to regulate membrane response to active forms of vitamin D in A431 squamous cell carcinoma, possibly through CAMKIIα and impaired calcium influx, respectively. The proposed roles of PDIA3 and VDR in the regulation of intracellular response to 1,25(OH)2D3 are summarized in Figure 5.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells13010011/s1, Figure S1: Time-resolved analysis of intracellular calcium levels measured with Fura-2AM probe on a mi-croplate reader. A431 sublines were stimulated with (A) 1 µM, (B) 100 nM or (C) 10 nM 1,25(OH)2D3. Please, note that panel C was taken from Figure 2C for comparison. Results were calculated as a mean ± SD of triplicates. Statistically significant differences are illustrated with asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, or **** p < 0.0001. Figure S2: Analysis of PLAA, PLCγ, PKCα protein levels in A431 sublines after 1,25(OH)2D3 treatment. The red color illustrates a decrease in protein level, while green marks the increase. The quantity of each protein was calculated as a protein/β-actin ratio. Protein levels are calculated as a mean from three independent experiments. Representative pictures were shown for each protein.
Author Contributions
Conceptualization, M.A.Ż. and R.B.; methodology, J.I.N., M.G., J.M.W. and A.M.O.; software, J.I.N. and M.G.; validation, M.A.Ż., J.I.N. and R.B.; investigation, J.I.N., M.G. and A.M.O.; resources, M.A.Ż.; data curation, J.I.N. and M.A.Ż.; writing—original draft preparation, J.I.N.; writing—review and editing, M.A.Ż.; visualization, J.I.N., M.G.; supervision, M.A.Ż.; funding acquisition, M.A.Ż. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a National Science Center OPUS Program under contract 2017/25/B/NZ3/00431.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
| 1,25-MARRS | Membrane-Associated, Rapid Response Steroid-binding |
| BNIP3 | BCL2 Interacting Protein 3 |
| CAMK2A | Calcium/Calmodulin-Dependent Protein Kinase II Alpha |
| CAV-1 | Caveolin 1 |
| CAV-3 | Caveolin 3 |
| Erk1/2 | Extracellular Signal-Regulated Kinase ½ |
| ERp57 | Endoplasmic Reticulum Resident Protein 57 |
| FIS1 | Fission, Mitochondrial 1 |
| NFAT | Nuclear Factor of Activated T-cells |
| PDIA3 | Protein Disulfide Isomerase Family A Member 3 |
| PKCα | Protein Kinase C-alpha |
| PLAA | Phospholipase A2-Activating Protein |
| PLCγ | Phospholipase C Gamma 1 |
| RXR | Retinoid X Receptor |
| A431 | Squamous Cell Carcinoma cell line |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TGM2 | Transglutaminase 2 |
| TRPV6 | Transient Receptor Potential Cation Channel Subfamily V Member 6 |
| VDR | Vitamin D Receptor |
| VDRE | Vitamin D Response Elements |
References
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)₂vitamin D₃: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.K.; Jurutka, P.W.; Haussler, C.A.; Hsieh, J.C.; Barthel, T.K.; Jacobs, E.T.; Dominguez, C.E.; Thatcher, M.L.; Haussler, M.R. CHAPTER 13—Nuclear Vitamin D Receptor: Structure-Function, Molecular Control of Gene Transcription, and Novel Bioactions. In Vitamin D (Second Edition); David, F., Ed.; Academic Press: Burlington, NJ, USA, 2005; pp. 219–261. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Biophys. Acta 2016, 1863, 2289–2298. [Google Scholar] [CrossRef]
- Civitelli, R.; Kim, Y.S.; Gunsten, S.L.; Fujimori, A.; Huskey, M.; Avioli, L.V.; Hruska, K.A. Nongenomic activation of the calcium message system by vitamin D metabolites in osteoblast-like cells. Endocrinology 1990, 127, 2253–2262. [Google Scholar] [CrossRef]
- Sterling, T.M.; Nemere, I. 1,25-dihydroxyvitamin D3 stimulates vesicular transport within 5 s in polarized intestinal epithelial cells. J. Endocrinol. 2005, 185, 81–91. [Google Scholar] [CrossRef][Green Version]
- Nemere, I.; Schwartz, Z.; Pedrozo, H.; Sylvia, V.L.; Dean, D.D.; Boyan, B.D. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J. Bone Miner. Res. 1998, 13, 1353–1359. [Google Scholar] [CrossRef]
- Nemere, I.; Safford, S.E.; Rohe, B.; DeSouza, M.M.; Farach-Carson, M.C. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3-MARRS) binding protein. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 281–285. [Google Scholar] [CrossRef]
- Hettinghouse, A.; Liu, R.; Liu, C.J. Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacol. Ther. 2018, 181, 34–48. [Google Scholar] [CrossRef]
- Nemere, I.; Garbi, N.; Hämmerling, G.J.; Khanal, R.C. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/PDIA3/Erp57. J. Biol. Chem. 2010, 285, 31859–31866. [Google Scholar] [CrossRef]
- Nemere, I.; Farach-Carson, M.C.; Rohe, B.; Sterling, T.M.; Norman, A.W.; Boyan, B.D.; Safford, S.E. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7392–7397. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.I.; Olszewska, A.M.; Piotrowska, A.; Myszczyński, K.; Domżalski, P.; Żmijewski, M.A. PDIA3 modulates genomic response to 1,25-dihydroxyvitamin D(3) in squamous cell carcinoma of the skin. Steroids 2023, 199, 109288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Lee, C.S.; Nizkorodov, A.; Riemenschneider, K.; Martin, D.; Hyzy, S.; Schwartz, Z.; Boyan, B.D. Disruption of Pdia3 gene results in bone abnormality and affects 1alpha,25-dihydroxy-vitamin D3-induced rapid activation of PKC. J. Steroid Biochem. Mol. Biol. 2010, 121, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Garbi, N.; Tanaka, S.; Momburg, F.; Hämmerling, G.J. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat. Immunol. 2006, 7, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nizkorodov, A.; Riemenschneider, K.; Lee, C.S.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Impaired bone formation in Pdia3 deficient mice. PLoS ONE 2014, 9, e112708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Doroudi, M.; Cheung, J.; Grozier, A.L.; Schwartz, Z.; Boyan, B.D. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1α,25(OH)(2)D(3). Cell. Signal. 2013, 25, 2362–2373. [Google Scholar] [CrossRef] [PubMed]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: A review of the roles of phospholipase A2 activating protein and Ca(2+)/calmodulin-dependent protein kinase II. J. Steroid Biochem. Mol. Biol. 2015, 147, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Yu, H.; Kim, J.J.; Lee, M.J.; Park, S.K. Vitamin D-induced ectodomain shedding of TNF receptor 1 as a nongenomic action: D3 vs D2 derivatives. J. Steroid Biochem. Mol. Biol. 2016, 155, 18–25. [Google Scholar] [CrossRef]
- Keasey, M.P.; Razskazovskiy, V.; Jia, C.; Peterknecht, E.D.; Bradshaw, P.C.; Hagg, T. PDIA3 inhibits mitochondrial respiratory function in brain endothelial cells and C. elegans through STAT3 signaling and decreases survival after OGD. Cell Commun. Signal. 2021, 19, 119. [Google Scholar] [CrossRef]
- Nowak, J.I.; Olszewska, A.M.; Król, O.; Żmijewski, M.A. Protein Disulfide Isomerase Family A Member 3 Knockout Abrogate Effects of Vitamin D on Cellular Respiration and Glycolysis in Squamous Cell Carcinoma. Nutrients 2023, 15, 4529. [Google Scholar] [CrossRef]
- Olszewska, A.M.; Nowak, J.I.; Myszczynski, K.; Slominski, A.; Zmijewski, M.A. Dissection of an Impact of Vdr and Rxra on Genomic Activity of 1,25(Oh)2d3 in A431 Squamous Cell Carcinoma. Mol. Cell Endocrinol. 2023, in press. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D(3) and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Li, W.; Shehabi, H.Z.; Janjetovic, Z.; Nguyen, M.N.; Kim, T.K.; Chen, J.; Howell, D.E.; Benson, H.A.; Sweatman, T.; et al. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab. Dispos. 2011, 39, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Tunsophon, S.; Nemere, I. Protein kinase C isotypes in signal transduction for the 1,25D3-MARRS receptor (ERp57/PDIA3) in steroid hormone-stimulated phosphate uptake. Steroids 2010, 75, 307–313. [Google Scholar] [CrossRef]
- Takeuchi, A.; Reddy, G.S.; Kobayashi, T.; Okano, T.; Park, J.; Sharma, S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J. Immunol. 1998, 160, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium-Calcineurin-NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lin, H.X.; Lin, H.; Guo, L.B.; Ge, N.; Cai, X.Y.; Sun, R.; Chen, W.K.; Li, Q.L.; Hu, W.H. Expression TGM2 and BNIP3 have prognostic significance in laryngeal cancer patients receiving surgery and postoperative radiotherapy: A retrospective study. J. Transl. Med. 2012, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Chen, W.X.; Lv, X.B.; Tang, Q.L.; Sun, L.J.; Liu, B.D.; Zhong, J.L.; Lin, Z.Y.; Wang, Y.Y.; Li, Q.X.; et al. miR-483-5p determines mitochondrial fission and cisplatin sensitivity in tongue squamous cell carcinoma by targeting FIS1. Cancer Lett. 2015, 362, 183–191. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nascimento Da Conceicao, V.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium Signaling Regulates Autophagy and Apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- He, J.; Shi, W.; Guo, Y.; Chai, Z. ERp57 modulates mitochondrial calcium uptake through the MCU. FEBS Lett. 2014, 588, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fleet, J.C. Intestinal resistance to 1,25 dihydroxyvitamin D in mice heterozygous for the vitamin D receptor knockout allele. Endocrinology 2007, 148, 1396–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benn, B.S.; Ajibade, D.; Porta, A.; Dhawan, P.; Hediger, M.; Peng, J.B.; Jiang, Y.; Oh, G.T.; Jeung, E.B.; Lieben, L.; et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 2008, 149, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Khattar, V.; Wang, L.; Peng, J.B. Calcium selective channel TRPV6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Voets, T.; Hoefs, S.; Weidema, F.; Prenen, J.; Nilius, B.; Bindels, R.J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. Embo J. 2003, 22, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.; Peng, J.B.; Takanaga, H.; Suzuki, Y.; Crescenzi, A.; Kos, C.H.; Zhuang, L.; Freeman, M.R.; Gouveia, C.H.; Wu, J.; et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007, 22, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.M.; Chapman, D.G.; Lahue, K.G.; Cahoon, J.M.; Rattu, G.K.; Daphtary, N.; Aliyeva, M.; Fortner, K.A.; Erzurum, S.C.; Comhair, S.A.; et al. Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness. J. Allergy Clin. Immunol. 2016, 137, 822–832.e827. [Google Scholar] [CrossRef]
- Hoffman, S.M.; Tully, J.E.; Nolin, J.D.; Lahue, K.G.; Goldman, D.H.; Daphtary, N.; Aliyeva, M.; Irvin, C.G.; Dixon, A.E.; Poynter, M.E.; et al. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir. Res. 2013, 14, 141. [Google Scholar] [CrossRef]
- Diaz Cruz, M.A.; Karlsson, S.; Szekeres, F.; Faresjö, M.; Lund, D.; Larsson, D. Differential expression of protein disulfide-isomerase A3 isoforms, PDIA3 and PDIA3N, in human prostate cancer cell lines representing different stages of prostate cancer. Mol. Biol. Rep. 2021, 48, 2429–2436. [Google Scholar] [CrossRef]
- Ramos, F.S.; Serino, L.T.; Carvalho, C.M.; Lima, R.S.; Urban, C.A.; Cavalli, I.J.; Ribeiro, E.M. PDIA3 and PDIA6 gene expression as an aggressiveness marker in primary ductal breast cancer. Genet. Mol. Res. 2015, 14, 6960–6967. [Google Scholar] [CrossRef]
- Gonzalez-Perez, P.; Woehlbier, U.; Chian, R.J.; Sapp, P.; Rouleau, G.A.; Leblond, C.S.; Daoud, H.; Dion, P.A.; Landers, J.E.; Hetz, C.; et al. Identification of rare protein disulfide isomerase gene variants in amyotrophic lateral sclerosis patients. Gene 2015, 566, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Beilhartz, G.; Roy, Y.; Richard, C.L.; Curtin, M.; Brown, L.; Cadieux, D.; Coppolino, M.; Farach-Carson, M.C.; Nemere, I.; et al. Nuclear translocation of the 1,25D3-MARRS (membrane associated rapid response to steroids) receptor protein and NFkappaB in differentiating NB4 leukemia cells. Exp. Cell Res. 2010, 316, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Żmijewski, M.A. Nongenomic Activities of Vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Doroudi, M.; Chen, J.; Boyan, B.D.; Schwartz, Z. New insights on membrane mediated effects of 1α,25-dihydroxy vitamin D3 signaling in the musculoskeletal system. Steroids 2014, 81, 81–87. [Google Scholar] [CrossRef]
- Gusev, K.; Glouchankova, L.; Zubov, A.; Kaznacheyeva, E.; Wang, Z.; Bezprozvanny, I.; Mozhayeva, G.N. The store-operated calcium entry pathways in human carcinoma A431 cells: Functional properties and activation mechanisms. J. Gen. Physiol. 2003, 122, 81–94. [Google Scholar] [CrossRef]
- Boyan, B.D.; Chen, J.; Schwartz, Z. Mechanism of Pdia3-dependent 1α,25-dihydroxy vitamin D3 signaling in musculoskeletal cells. Steroids 2012, 77, 892–896. [Google Scholar] [CrossRef]
- Warwick, T.; Schulz, M.H.; Günther, S.; Gilsbach, R.; Neme, A.; Carlberg, C.; Brandes, R.P.; Seuter, S. A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci. Rep. 2021, 11, 6518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).