Centrosome Formation in the Bovine Early Embryo
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Embryo Production by Fertilization
2.2. Parthenogenetic Development of Embryos In Vitro
2.3. Transmission Electron Microscopy (TEM)
2.4. Gene Expression
2.5. Measuring the Length of Centrioles
3. Results
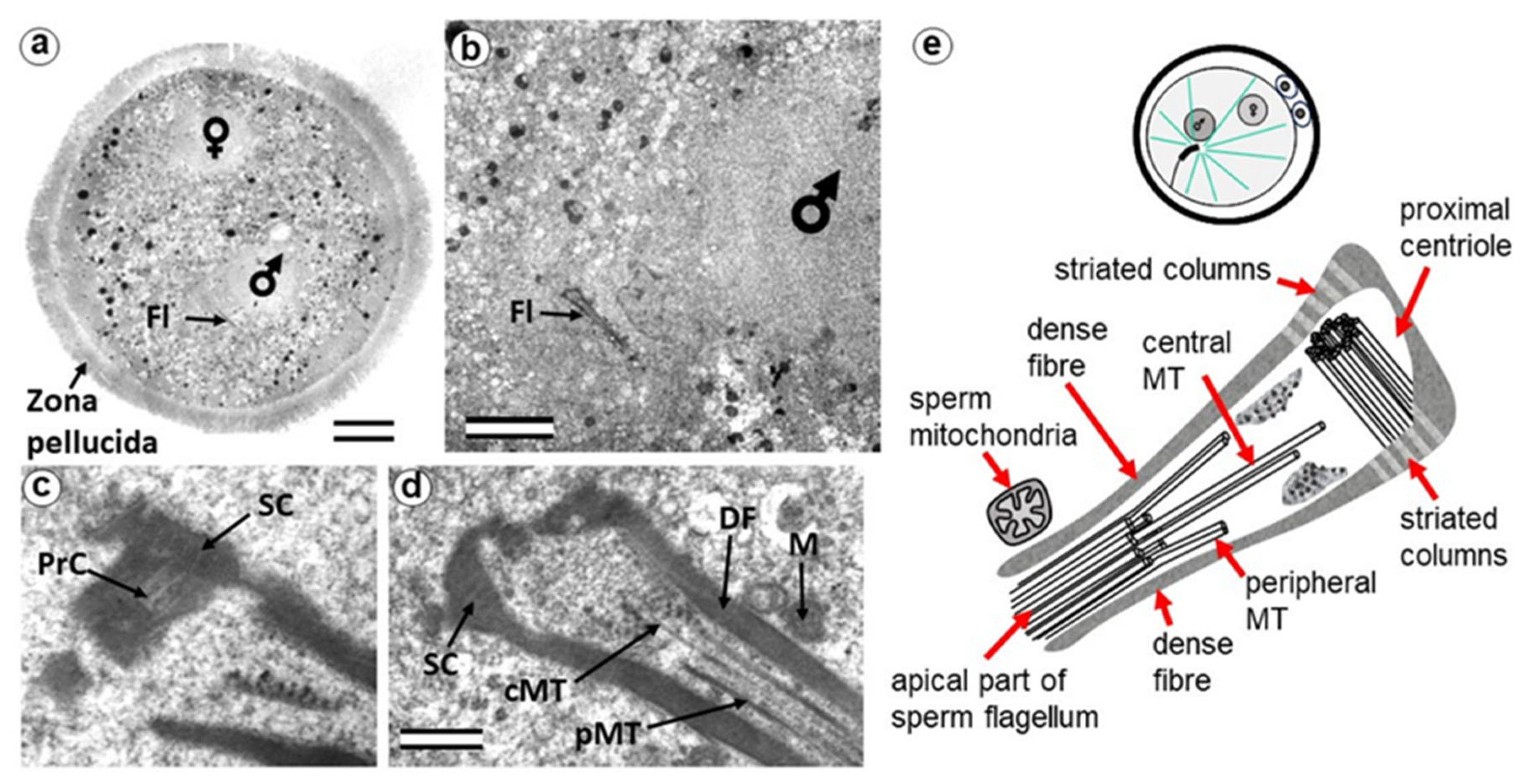
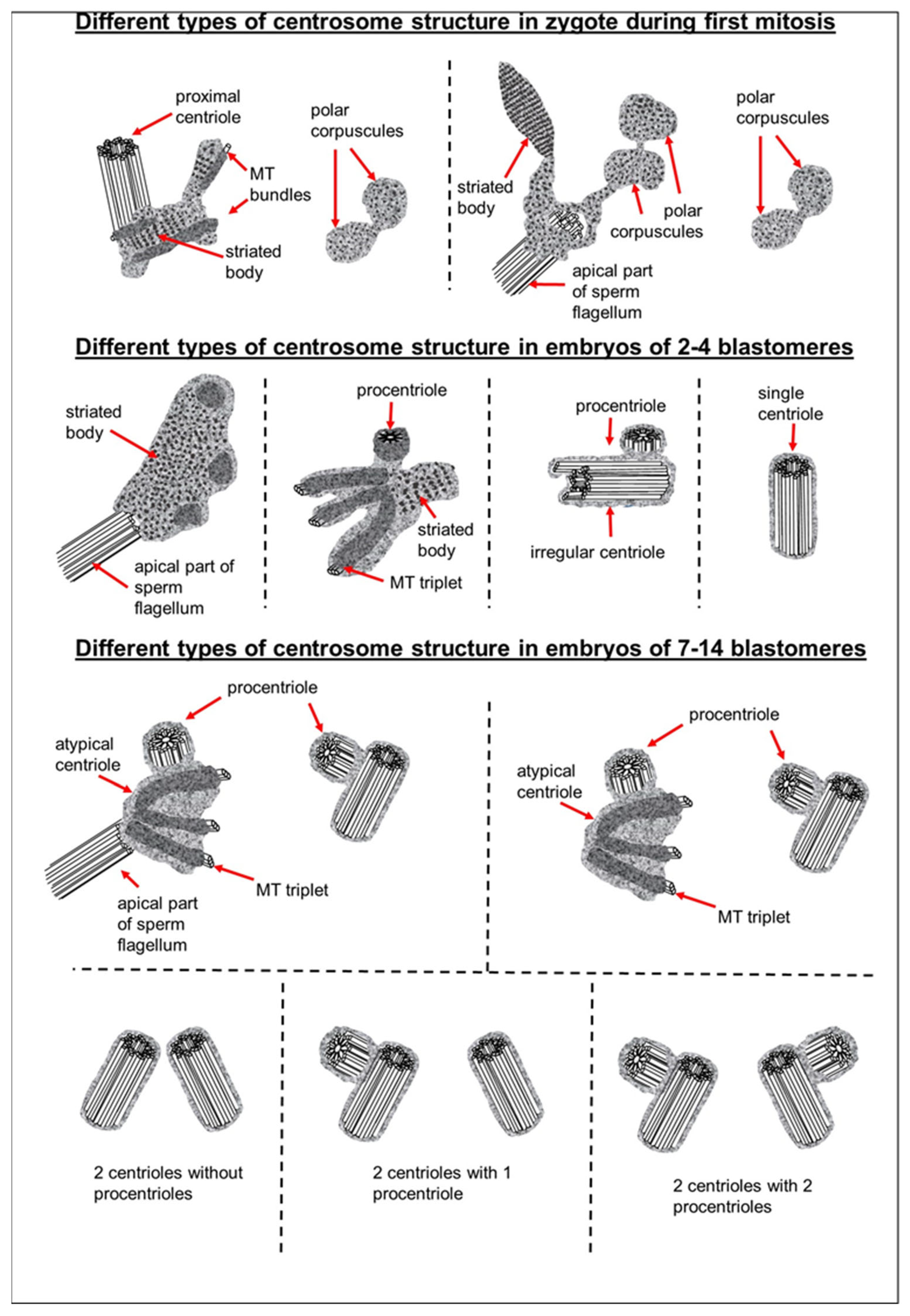
3.1. The Pre-Convergence, Two Pronuclei Zygote Has the Sperm-Typical PC and -Atypical DC
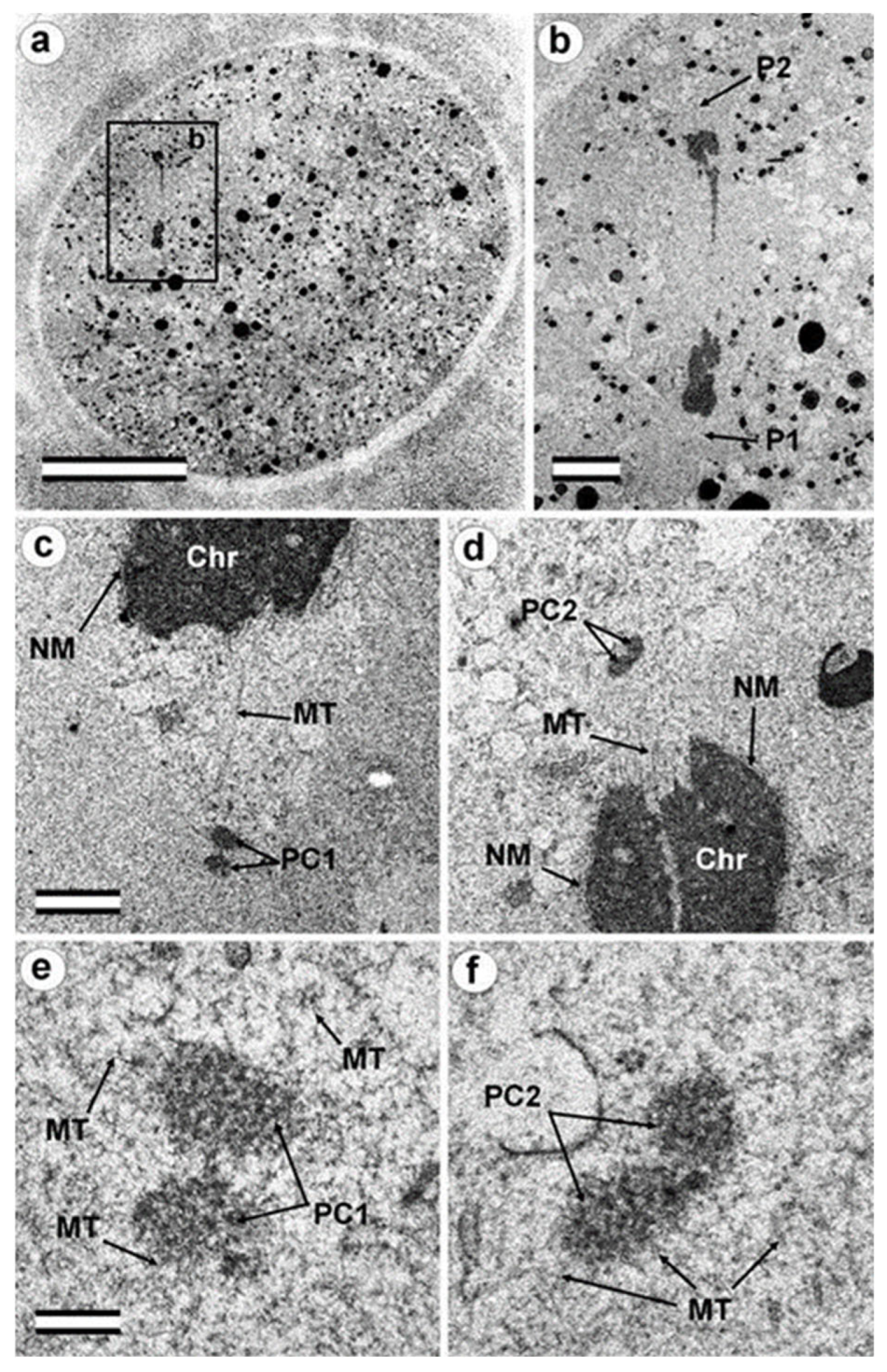
3.2. The Prophase Zygote Has a Typical PC and an Atypical DC
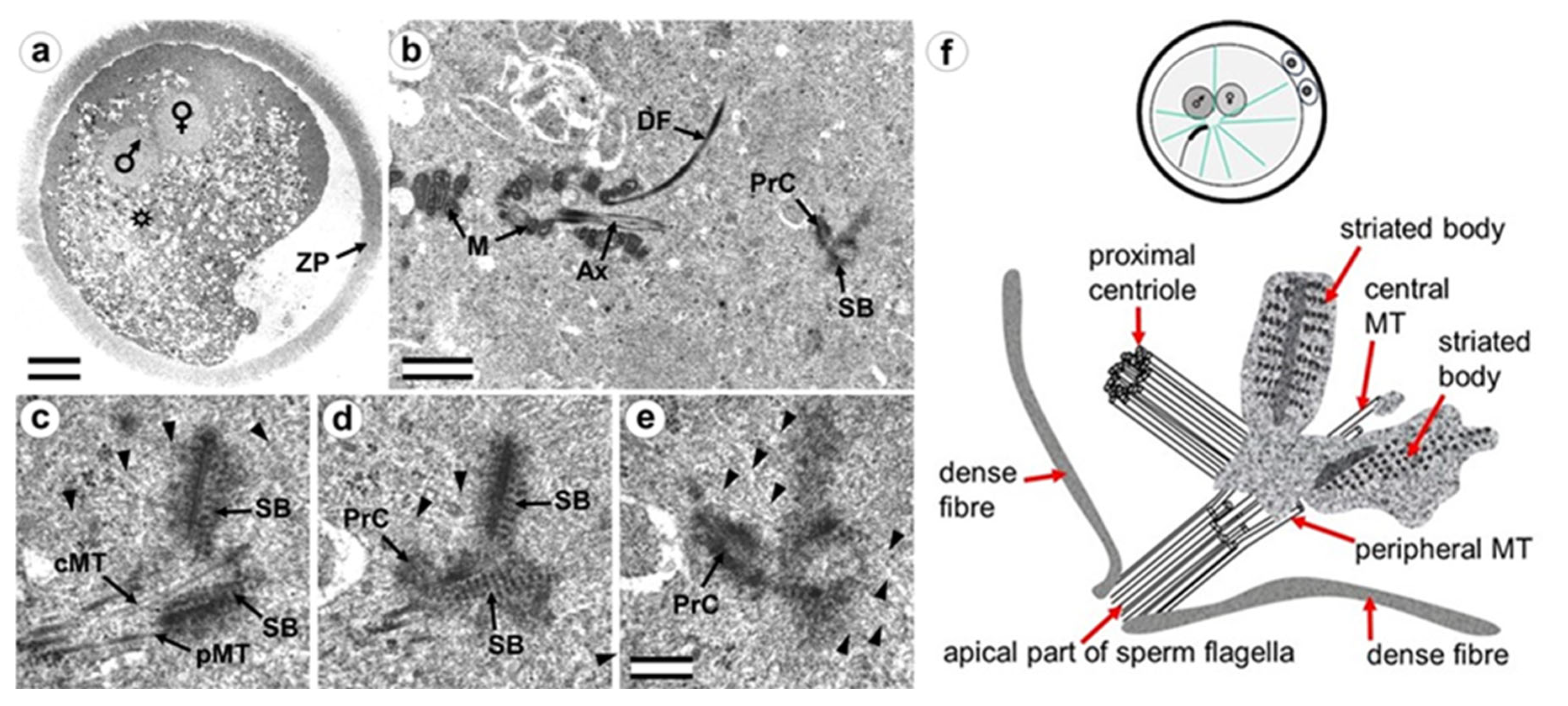
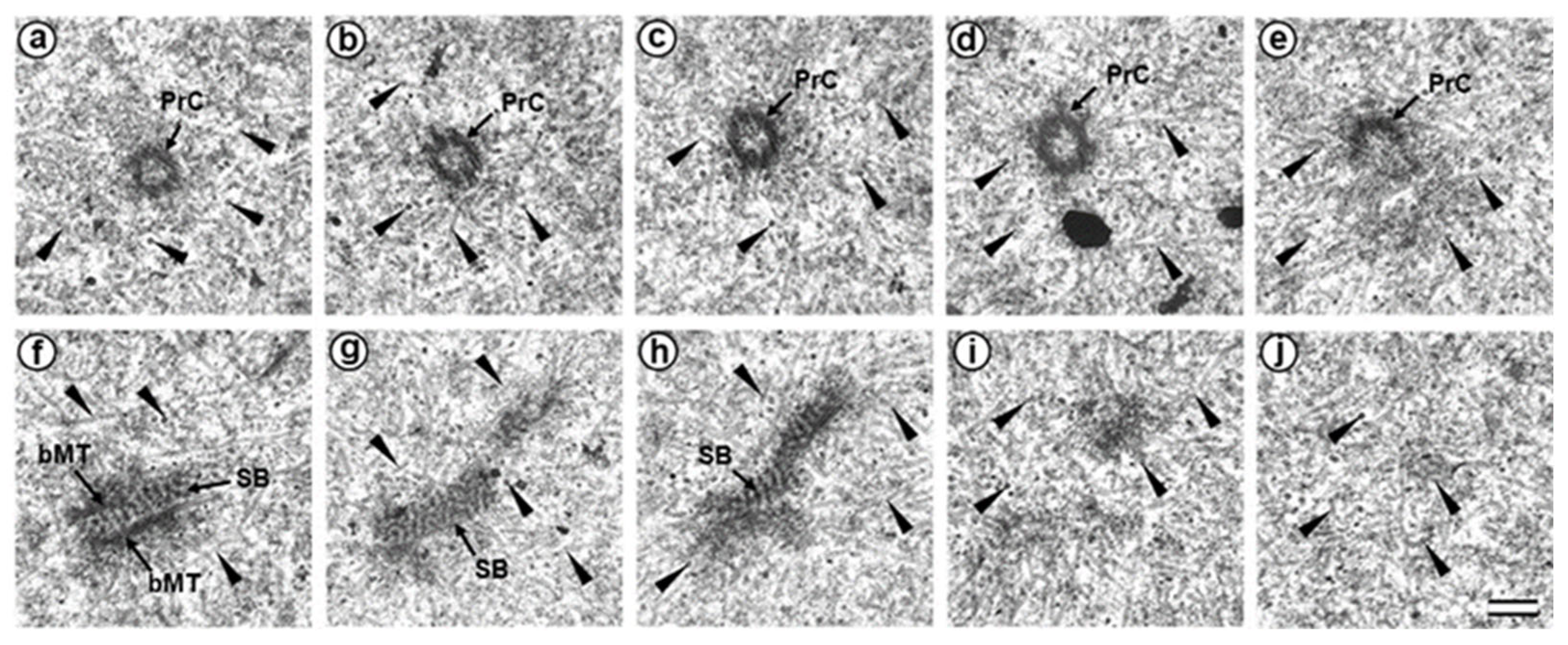
3.3. The Prometaphase Zygote Has One Centriole and a Striated Body in the Flagellum-Associated Pole and Polar Corpuscles in the Opposite Pole
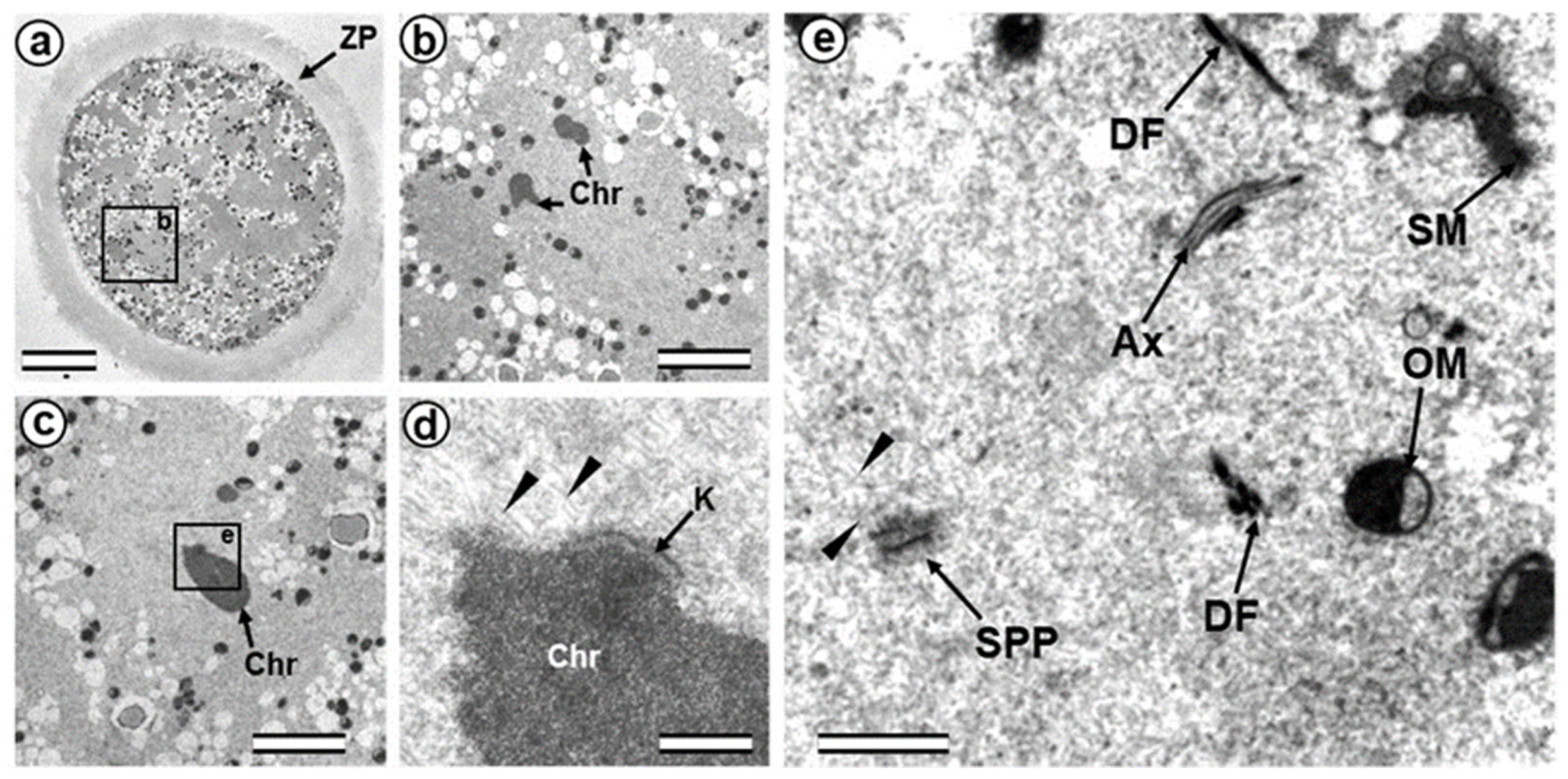
3.4. The Anaphase–Telophase Zygote Has Two Polar Corpuscles in Each Pole, One of Which Is Associated with the Apical Region of the Flagellar Axoneme
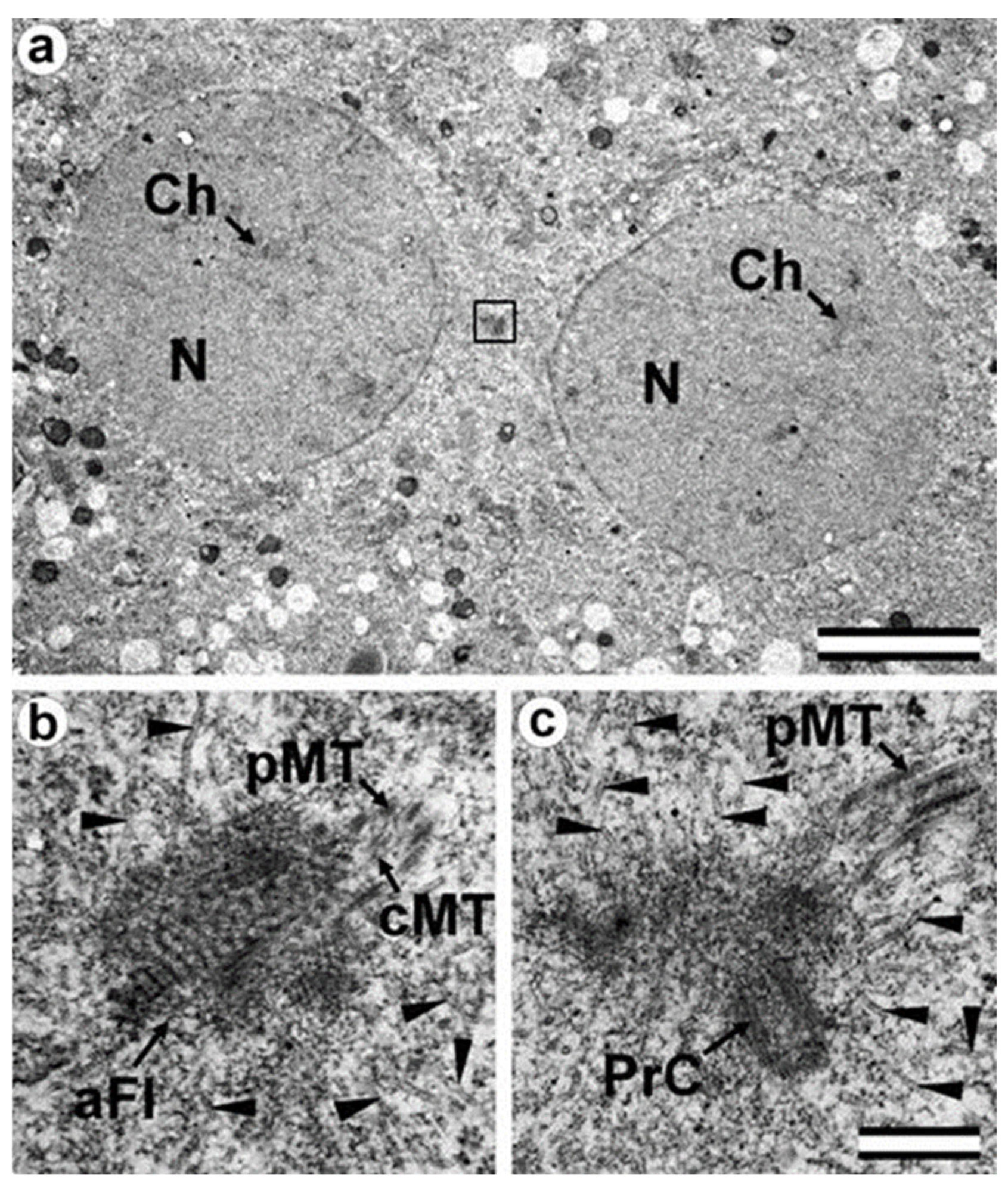
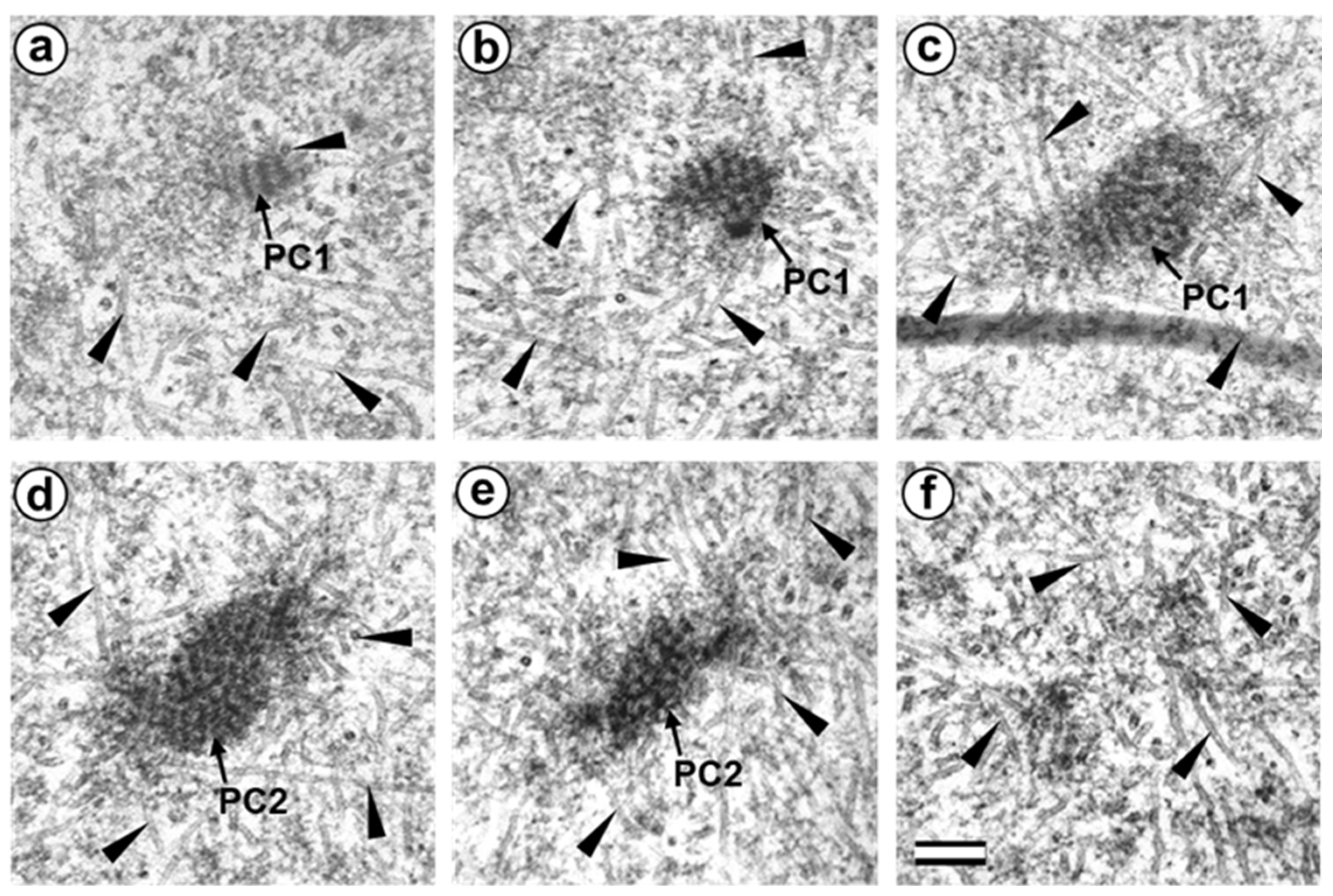
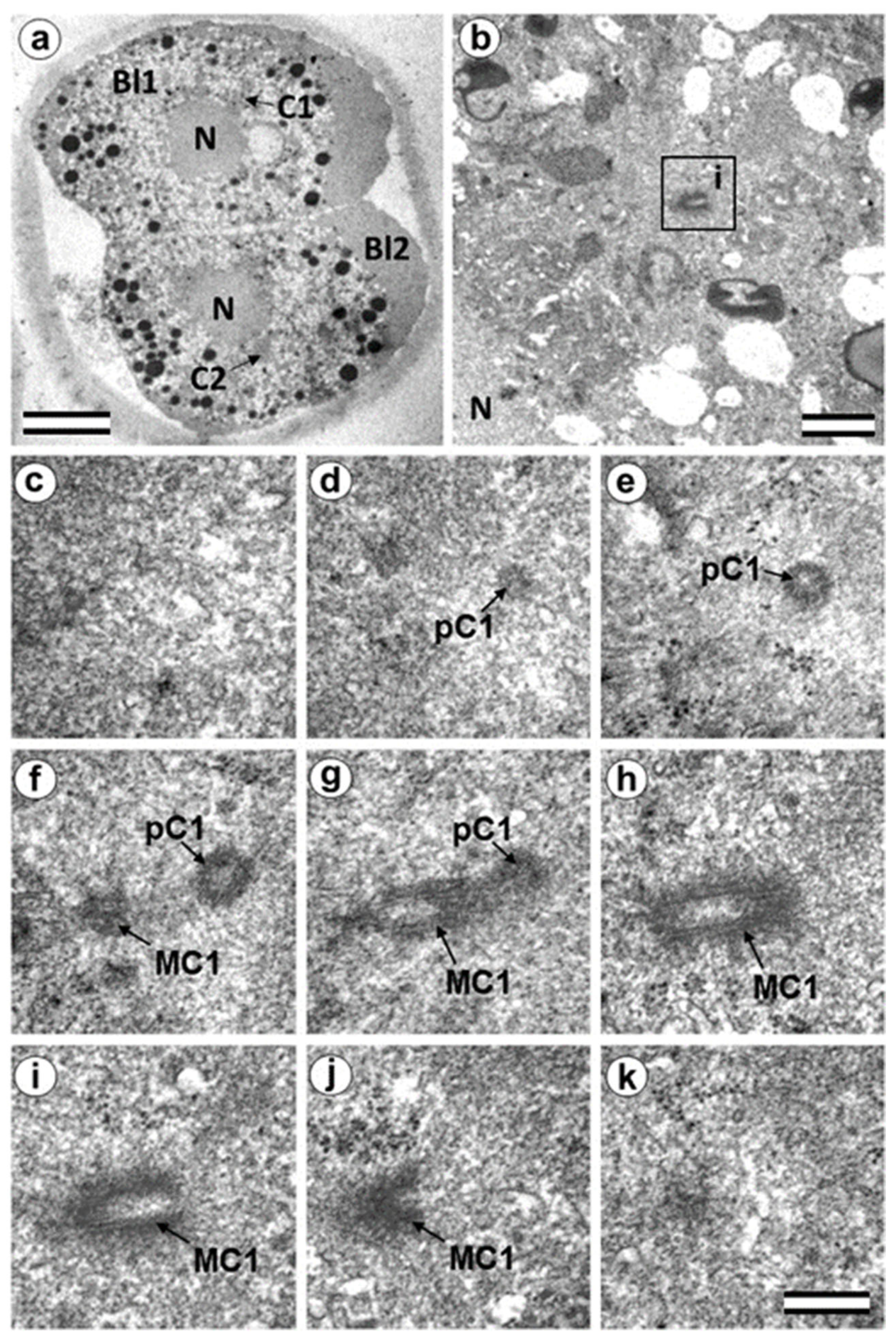
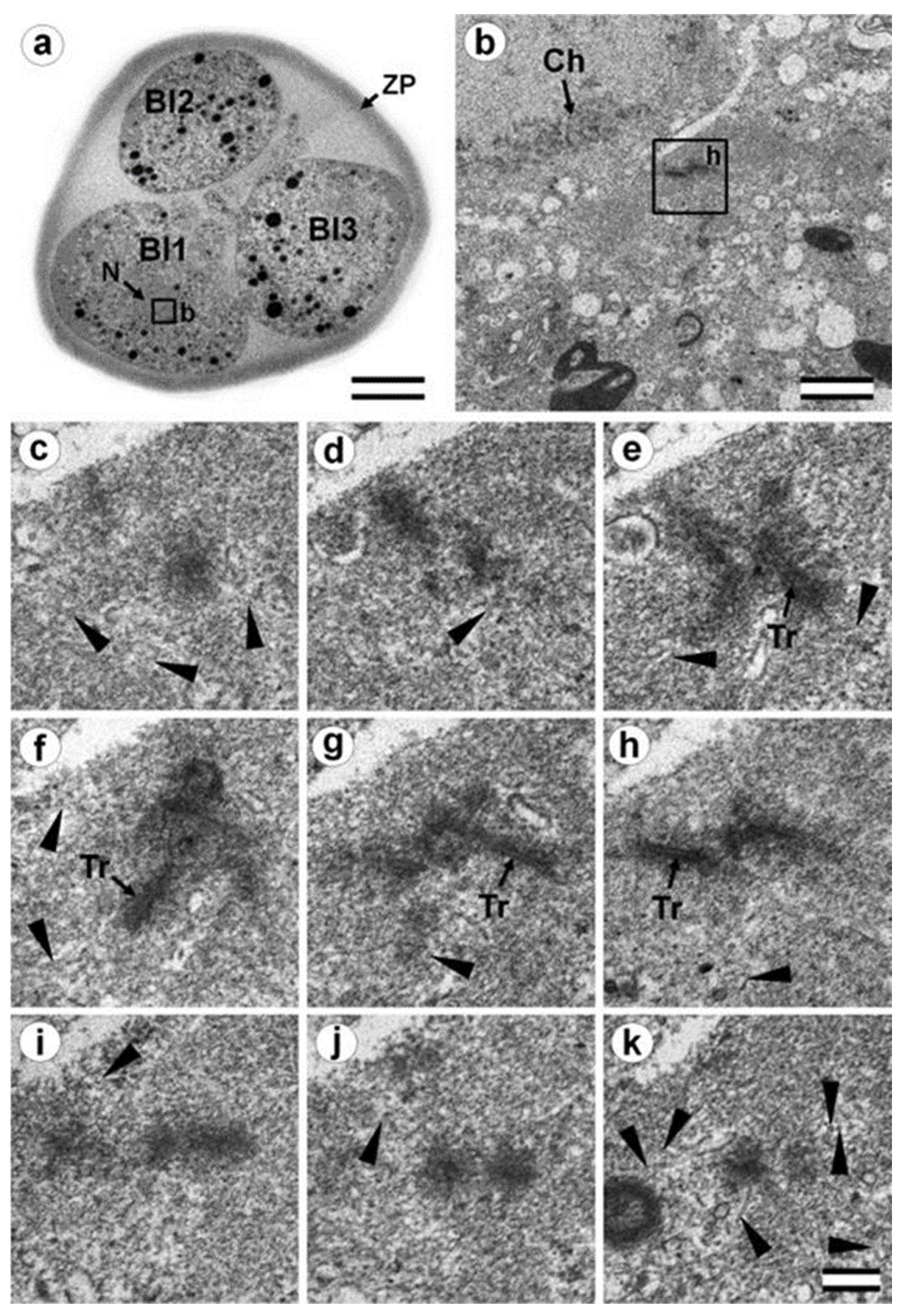
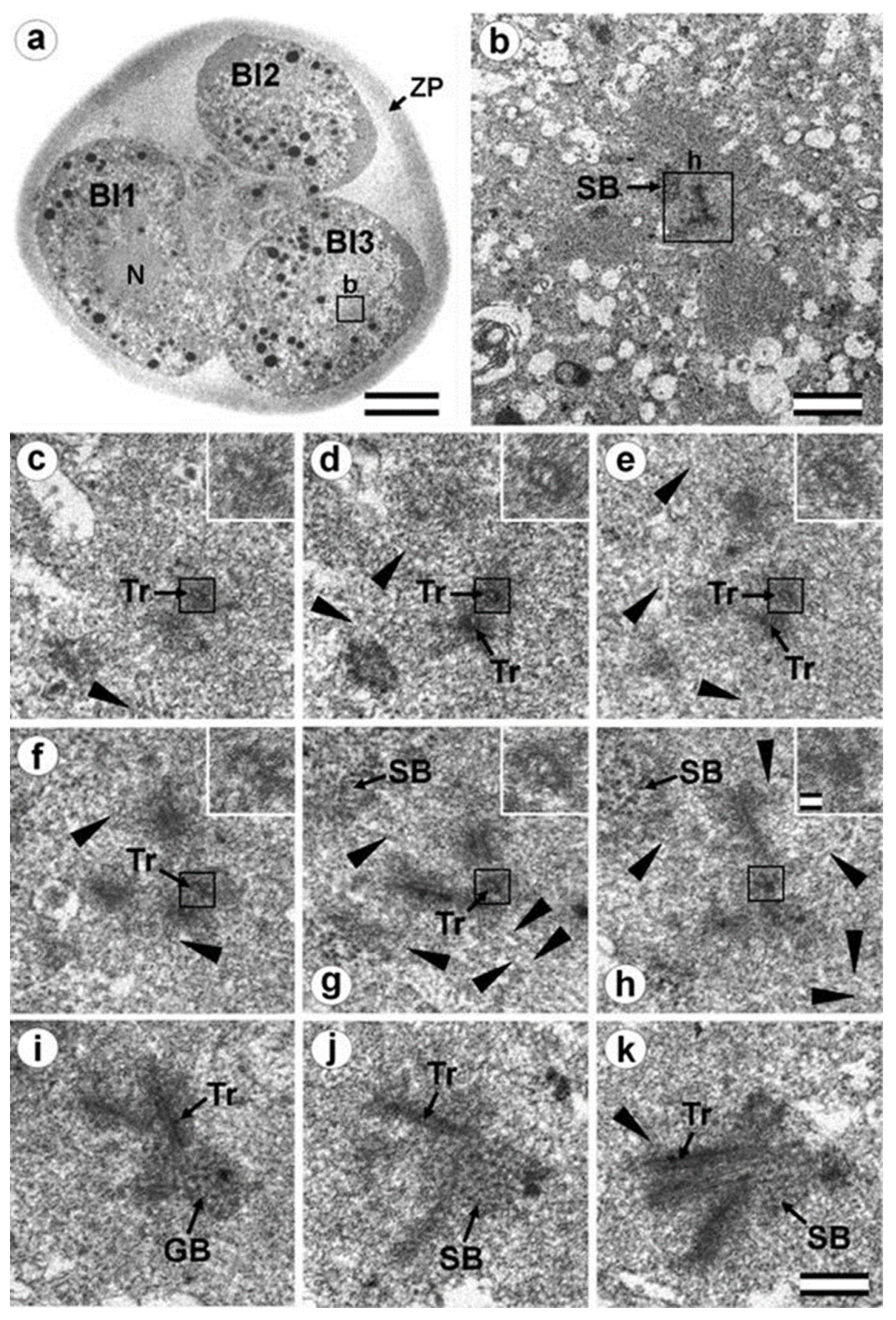
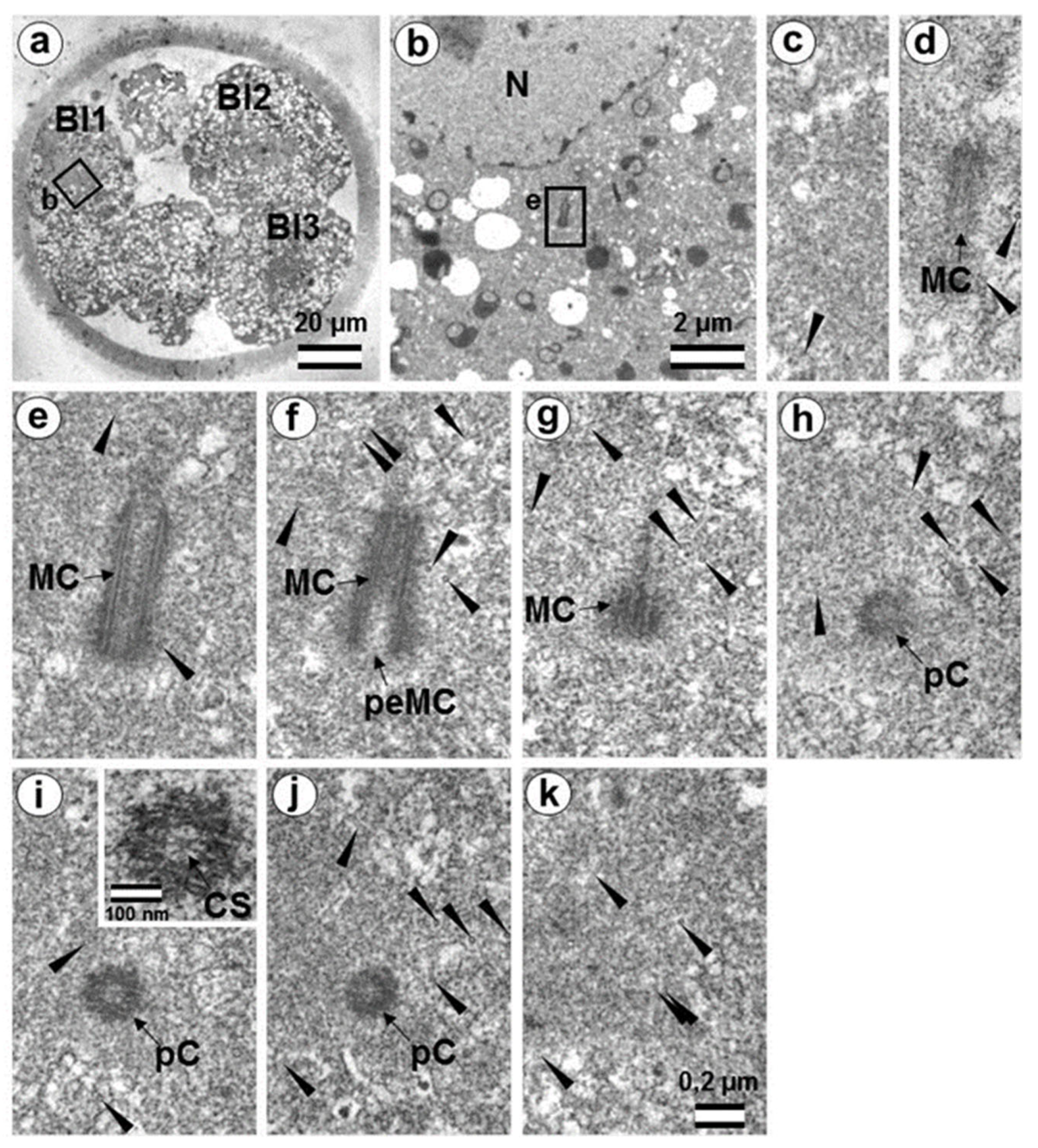
3.5. The Two-Cell Embryo Has Two Centrioles in Each Blastomere
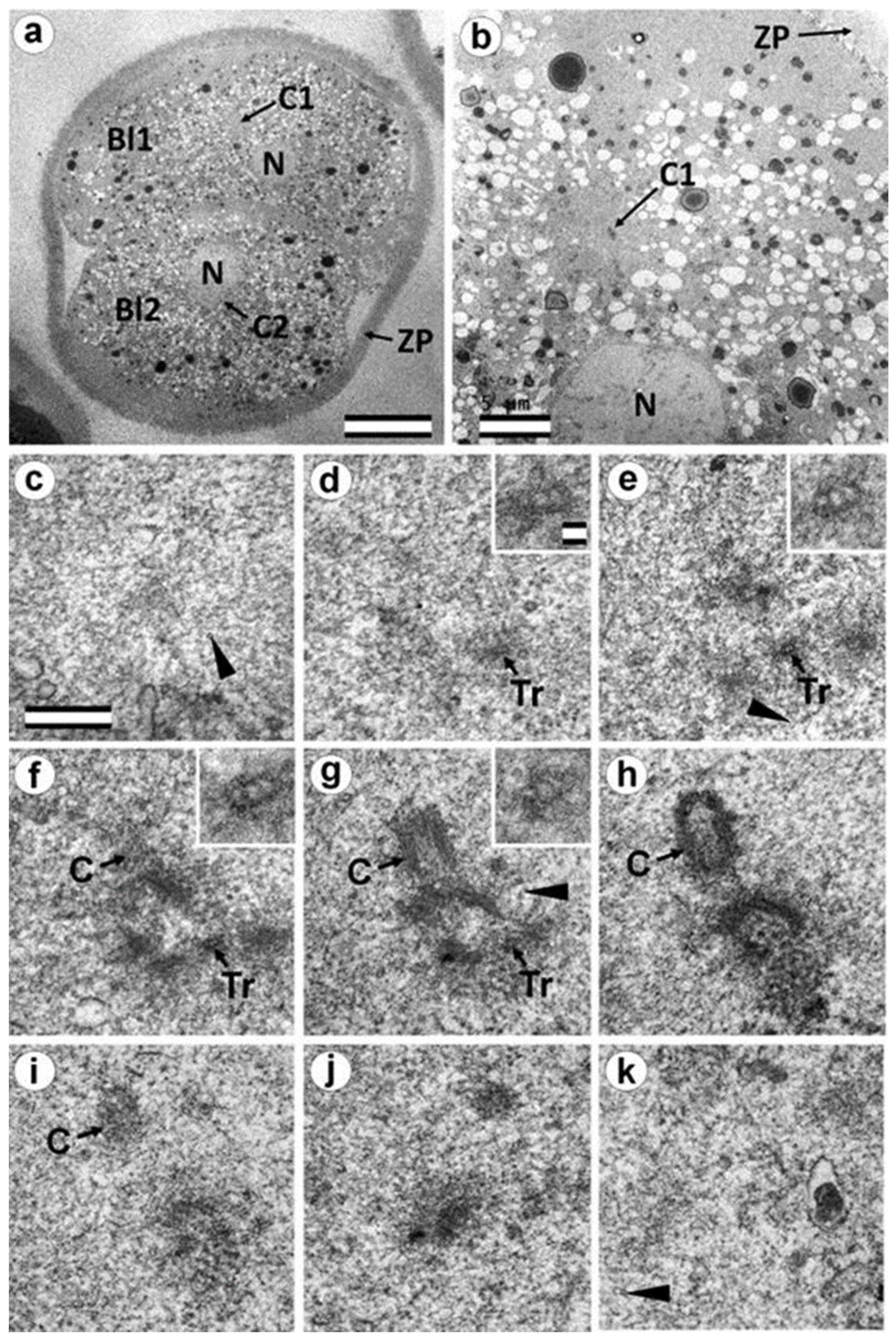
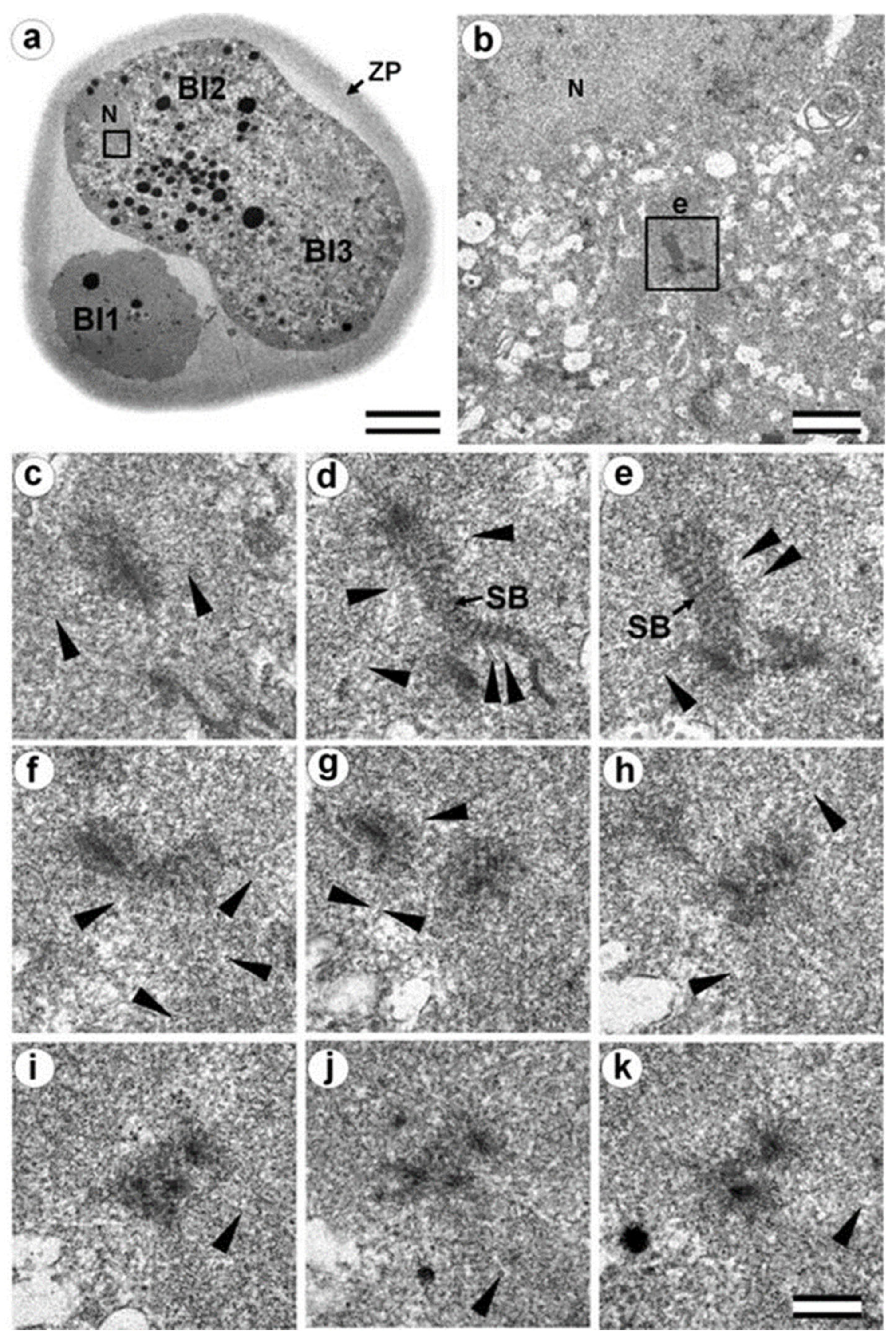
3.6. Blastomeres of the Second Cleavage Have Atypical Centrioles
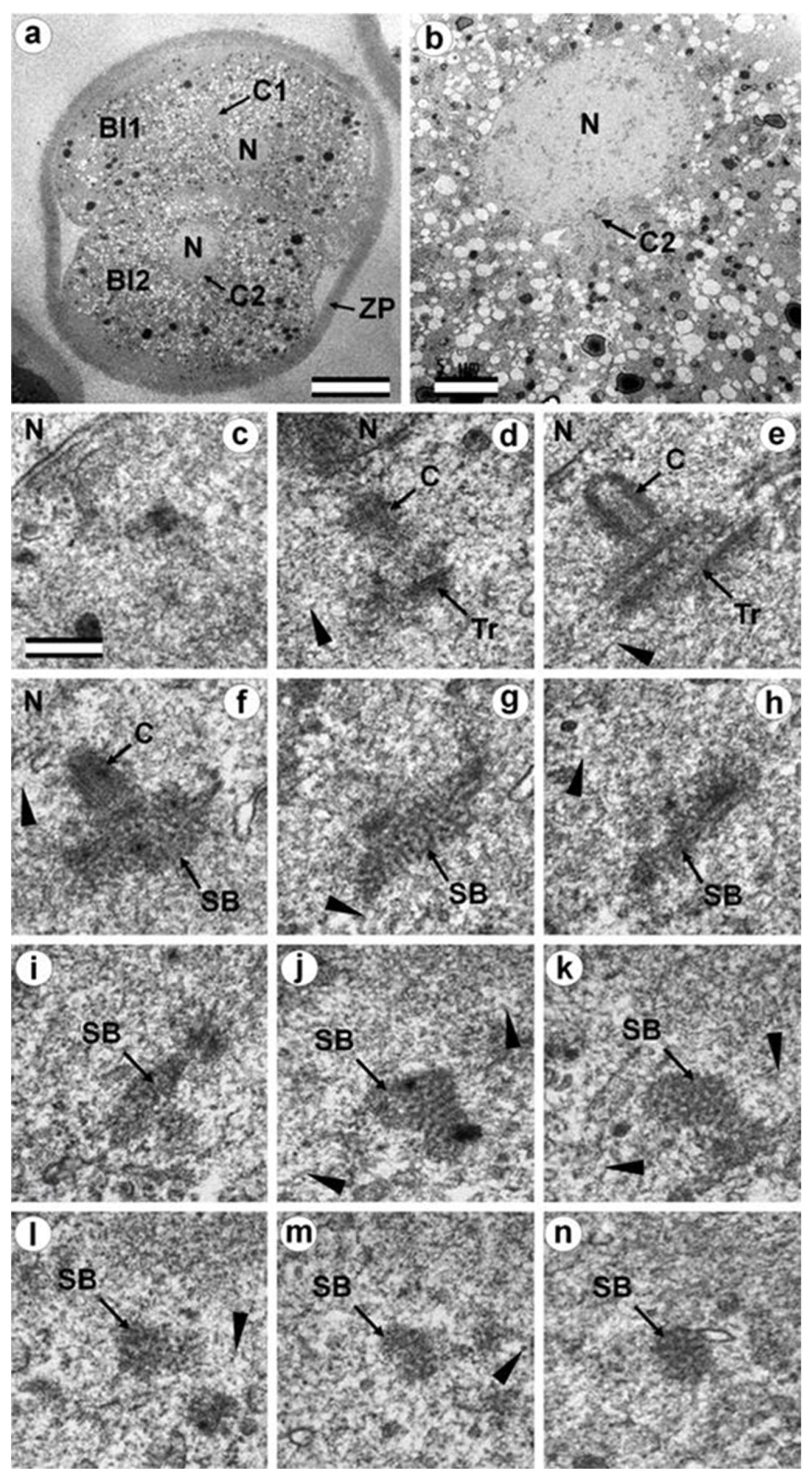
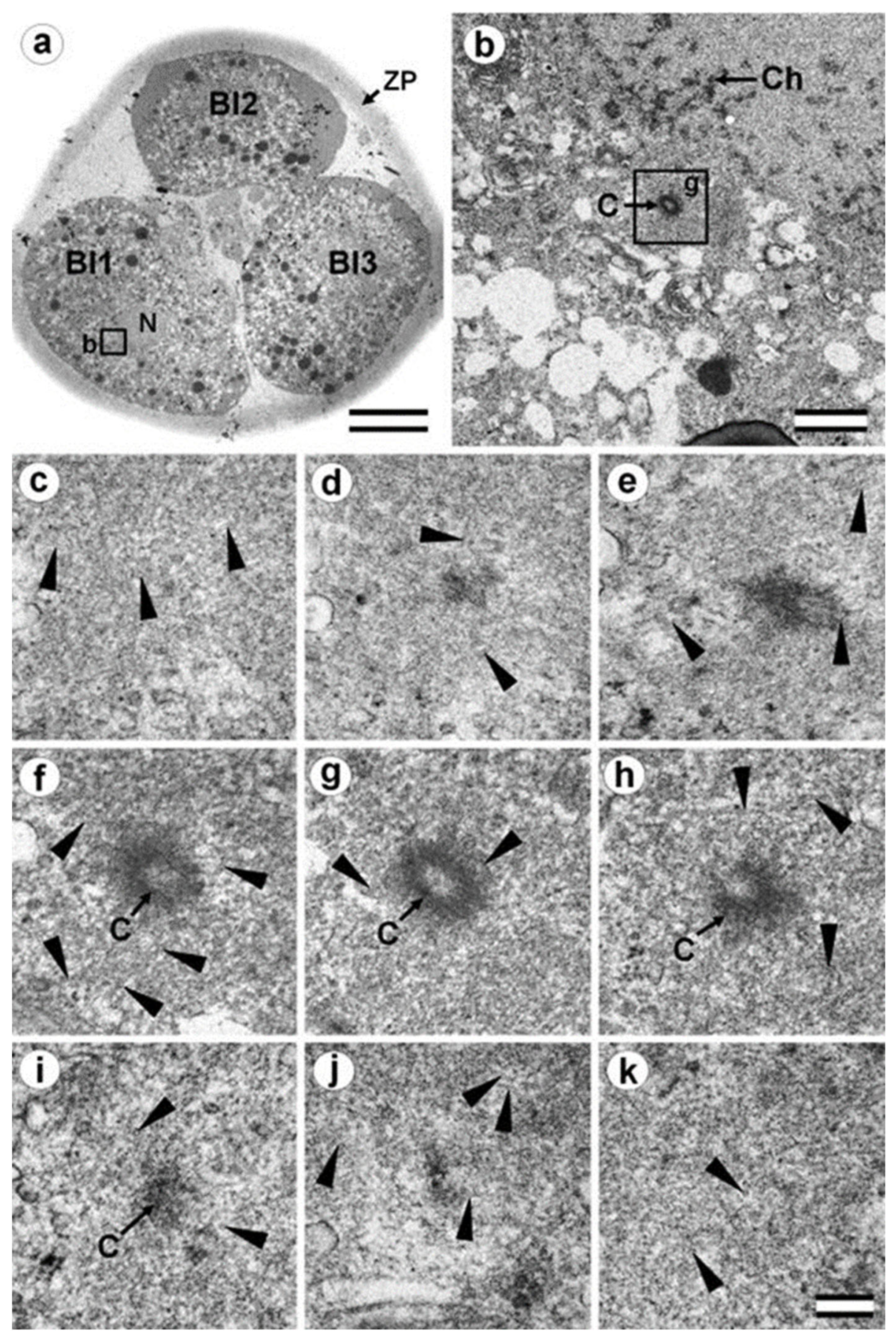
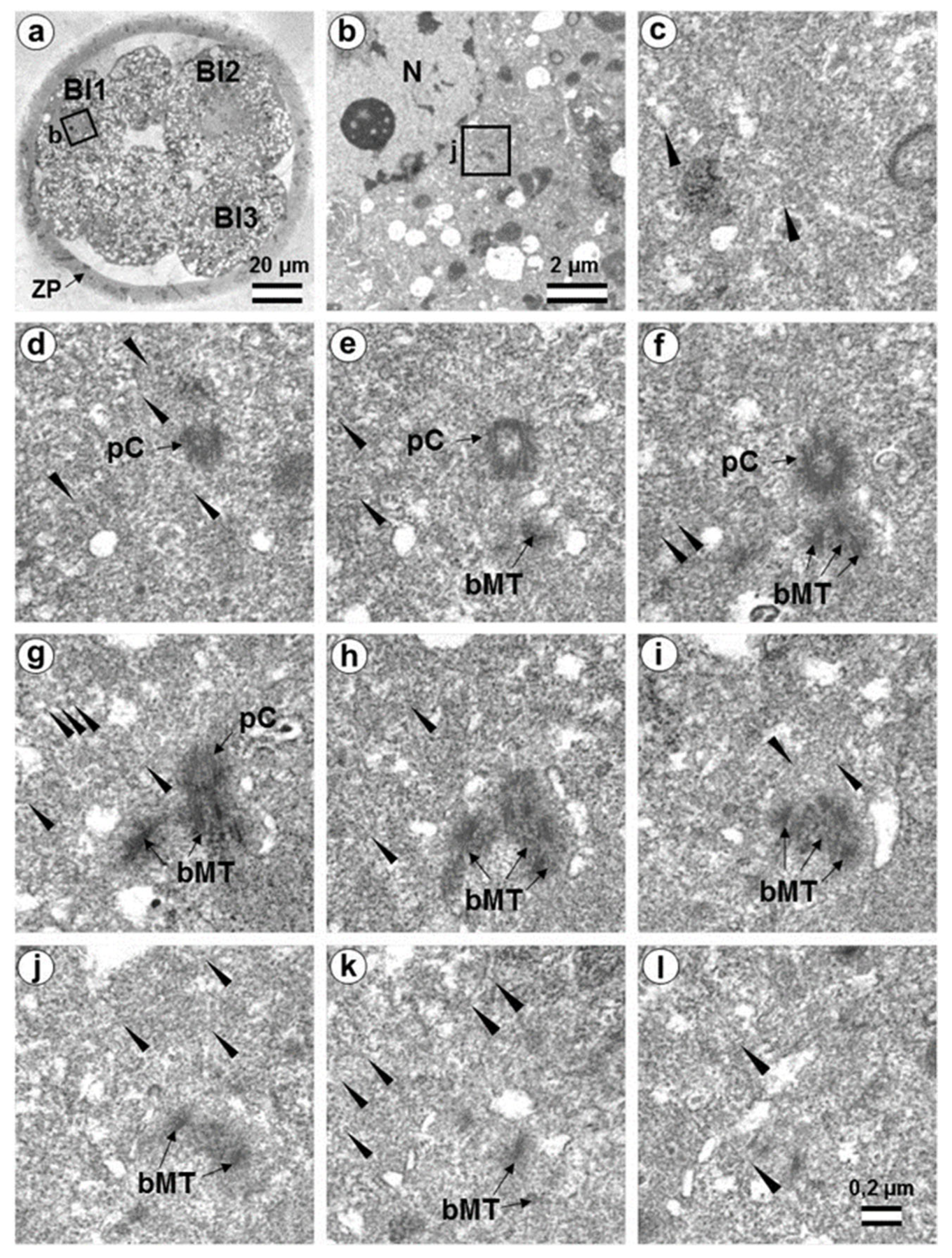
3.7. Typical Centrosomes Are Formed in Blastomeres after the Third Cleavage
3.8. Centriole Appearance in Parthenogenetic Embryos
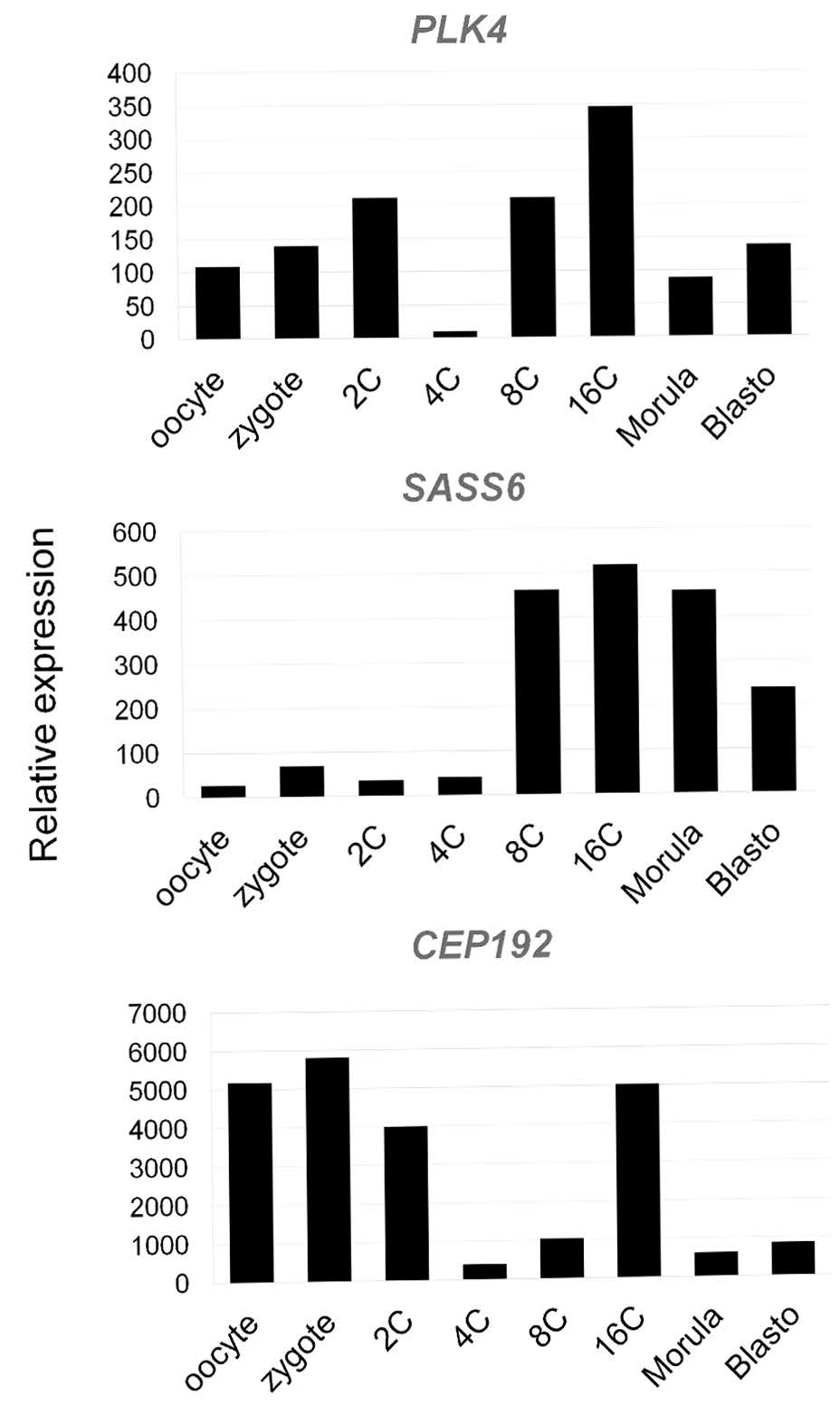
3.9. Centrosome-Associated Protein Gene Expression in Bovine Embryos
4. Discussion
4.1. Centrioles in Parthenogenetic Embryos
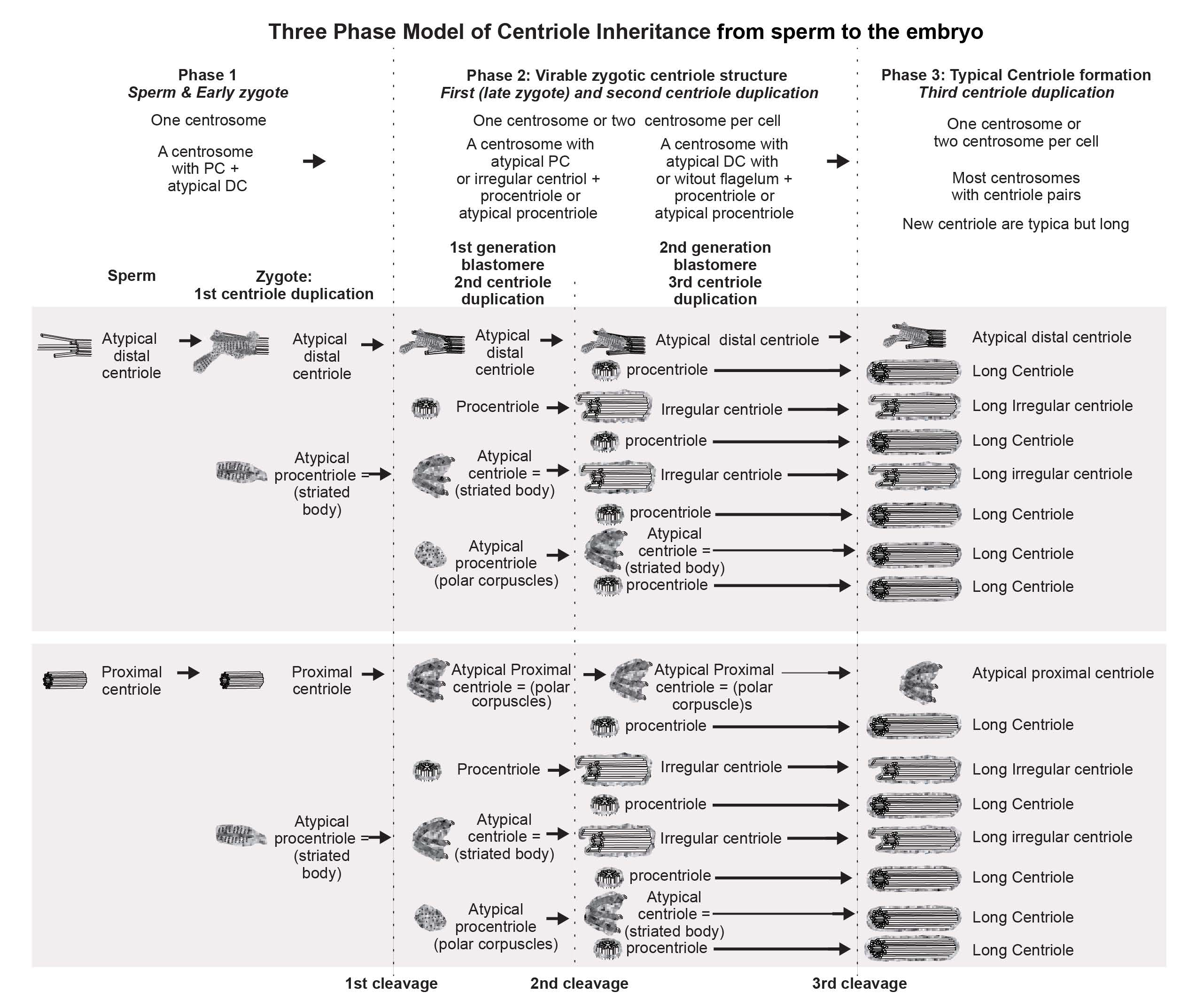
4.2. The Disappearance of the Proximal Sperm Centriole in the Zygote and Appearance of New Typical or Atypical Centrioles in Two- to Four-Cell Embryos
4.3. What Was Found at Division Poles of Bovine Blastomeres?
4.4. Retrospective Analysis of Centriolar and Centrosomal Formation during Early Embryonic Development According to Morphological Analysis of Different Blastomeres in Bovine Embryos
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.B. The Cell in Development and Inheritance, 2nd ed.; The Macmillan Company: New York, NY, USA, 1900. [Google Scholar]
- Boveri, T. Zellen-Studien: Ueber die Nature der Centrosomen; IV; Fischer: Jena, Germany, 1901. [Google Scholar]
- Mazia, D.; Harris, P.J.; Bibring, T. The Multiplicity of the Mitotic Centers and the Time-Course of Their Duplication and Separation. J. Biophys. Biochem. Cytol. 1960, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mazia, D. The Cell; Brachet, J., Mirsky, A., Eds.; Academic Press: London, UK, 1961; Volume III. [Google Scholar]
- Paweletz, N.; Mazia, D.; Finze, E.-M. Fine structural studies of the bipolarization of the mitotic apparatus in the fertilized sea urchin egg. I. The structure and behavior of centrosomes before fusion of the pronuclei. Eur. J. Cell Biol. 1987, 44, 195–204. [Google Scholar] [PubMed]
- Longo, F.J.; Anderson, E. A cytological study of the relation of the cortical reaction to subsequent events of fertilization in urethane-treated eggs of the sea urchin, Arbacia punctulata. J. Cell Biol. 1970, 47, 646–665. [Google Scholar] [CrossRef] [PubMed]
- Szöllõsi, D.; Calarco, P.; Donahue, R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972, 3, 207–230. [Google Scholar]
- Krioutchkova, M.M.; Onishchenko, G.E. Structural and functional characteristics of the centrosome in gametogenesis and early embryogenesis of animals. Int. Rev. Cytol. 1999, 185, 107–156. [Google Scholar] [PubMed]
- Sutovsky, P.; Manandhar, G.; Schatten, G. Biogenesis of the centrosome during mammalian gametogenesis and fertilization. Protoplasma 1999, 206, 249–262. [Google Scholar] [CrossRef]
- Sathananthan, A.H.; Ratnasooriya, W.D.; de Silva, P.K.; Menezes, J. Characterization of human gamete centrosomes for assisted reproduction. Ital. J. Anat. Embryol. 2001, 106 (Suppl. 2), 61–73. [Google Scholar]
- Manandhar, G.; Sutovsky, P.; Joshi, H.C.; Stearns, T.; Schatten, G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998, 203, 424–434. [Google Scholar] [CrossRef]
- Schatten, H.; Walter, M.; Biessmann, H.; Schatten, G. Microtubules are required for centrosome separation in sea urchin eggs during fertilization and mitosis. Cell Motil. Cytoskel. 1988, 11, 248–259. [Google Scholar] [CrossRef]
- Maro, B.; Gueth-Hallonet, C.; Aghion, J.; Antony, C. Cell polarity and microtubule organisation during mouse early embryogenesis. Development 1991, 113 (Suppl. 1), 17–25. [Google Scholar] [CrossRef]
- Gueth-Hallonet, C.; Antony, C.; Aghion, J.; Santa Maria, A.; Lajoie Mazenc, I.; Wright, M.; Maro, B. Gamma-Tubulin is present in acentriolar MTOCs during early mouse development. J. Cell Sci. 1993, 105, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Abumuslimov, S.S.; Nadezhdina, E.S.; Chentsov, I.S. An electron microscopic study of centriole and centrosome morphogenesis in the early development of the mouse. Tsitologiia 1994, 36, 1054–1061. [Google Scholar] [PubMed]
- Schatten, G. The centrosome and its mode of inheritance: The reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 1994, 165, 299–335. [Google Scholar] [CrossRef]
- Flach, G.; Johnson, M.H.; Braude, P.R.; Taylor, R.A.S.; Bolton, V.N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982, 1, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Bangs, F.K.; Schroder, N.; Hadjantonakis, A.-K.; Anderson, K.V. Lineage specificity of primary cilia in the mouse embryo. Nat. Cell Biol. 2015, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, E.R. The presence of centrioles in artificially activated sea urchin eggs. J. Biophys. Biochim. Cytol. 1961, 11, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Miki-Noumura, T. Studies on the de novo formation of centrioles: Aster formation in the activated eggs of sea urchin. J. Cell Sci. 1977, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Kalienbach, R.J.; Mazia, D. Origin and maturation of centrioles in association with the nuclear envelope in hypertonic-stressed sea urchin eggs. Eur. J. Cell Biol. 1982, 128, 68–76. [Google Scholar]
- Crozet, N.; Dahirel, M.; Chesne, P. Centrosome inheritance in sheep zygotes: Centrioles are contributed by the sperm. Microsc. Res. Tech. 2000, 49, 445–450. [Google Scholar] [CrossRef]
- Navara, C.S.; First, N.L.; Schatten, G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis and nuclear transfer: The role of the sperm aster. Dev. Biol. 1994, 162, 29–40. [Google Scholar] [CrossRef]
- Szöllõsi, D.; Ozil, J.P. De novo formation of centrioles in parthenogenetically activated, diploidized rabbit embryos. Biol. Cell 1991, 72, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H.; Schatten, G.; Mazia, D.; Balczon, R.; Simerly, C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl. Acad. Sci. USA 1986, 83, 105–109. [Google Scholar] [CrossRef]
- Garanina, A.S.; Alieva, I.B.; Bragina, E.E.; Blanchard, E.; Arbeille, B.; Guerif, F.; Uzbekova, S.; Uzbekov, R.E. The Centriolar Adjunct—Appearance and Disassembly in Spermiogenesis and the Potential Impact on Fertility. Cells 2019, 8, E180. [Google Scholar] [CrossRef] [PubMed]
- Paweletz, N.; Mazia, D.; Finze, E.M. Fine structural studies of the bipolarization of the mitotic apparatus in the fertilized sea urchin egg. II. Bipolarization before the first mitosis. Eur. J. Cell Biol. 1987, 44, 205–213. [Google Scholar] [PubMed]
- Alieva, I.; Staub, C.; Uzbekova, S.; Uzbekov, R. A Question of flagella origin for spermatids—Mother or daughter centriole? In Flagella and Cilia: Types, Structure and Functions; Uzbekov, R., Ed.; Nova Science Publisher, Inc.: New York, NY, USA, 2018; Chapter 5; pp. 109–126. [Google Scholar]
- Zamboni, L.; Stefanini, M. The fine structure of the neck of mammalian spermatozoa. Anat. Rec. 1971, 169, 155–172. [Google Scholar] [CrossRef]
- Sato, N.; Oura, C. The fine structure of the neck region of cat spermatozoa. Okajimas Folia Anat. Jpn. 1984, 61, 267–285. [Google Scholar] [CrossRef]
- Manandhar, G.; Simerly, C.; Schatten, G. Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum. Reprod. 2000, 15, 256–263. [Google Scholar] [CrossRef]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210, Erratum in Nat. Commun. 2018, 9, 2800. [Google Scholar] [CrossRef]
- Sathananthan, A.H.; Ratnam, S.S.; Ng, S.C.; Tarin, J.J.; Gianaroli, L.; Trounson, A. The sperm centriole: Its inheritance, replicationand perpetuation in early human embryos. Hum. Reprod. 1996, 1, 345–356. [Google Scholar] [CrossRef]
- Sathananthan, A.H.; Kola, I.; Osborne, J.; Trounson, A.; Ng, S.C.; Bongso, A.; Ratnam, S.S. Centrioles in the beginning of human development. Proc. Natl. Acad. Sci. USA 1991, 88, 4806–4810. [Google Scholar] [CrossRef]
- Crozet, N. Behavior of the sperm centriole during sheep oocyte fertilization. Eur. J. Cell Biol. 1990, 53, 326–332. [Google Scholar]
- Schatten, H.; Sun, Q.Y. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol. Hum. Reprod. 2009, 5, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Booth, P.J.; Schmidt, M.H.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Singina, G.N.; Shedova, E.N.; Lopukhov, A.V.; Mityashova, O.S.; Lebedeva, I.Y. Delaying effects of prolactin and growth hormone on aging processes in bovine oocytes matured in vitro. Pharmaceuticals 2021, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrans, C.F., Jr.; First, N.L. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J. Anim. Sci. 1994, 72, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Van Beneden, E. Recherches sur les Dicyémides, survivants actuels d’un embranchement des mésozoaires. Bull. Acad. Roy. Méd. Belg. 1876, 41, 1160–1205. [Google Scholar]
- Xie, D.; Chen, C.C.; Ptaszek, L.M.; Xiao, S.; Cao, X.; Fang, F.; Ng, H.H.; Lewin, H.A.; Cowan, C.; Zhong, S. Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res. 2010, 20, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Khire, A.; Fishman, E.L.; Jo, K.H. Atypical centrioles during sexual reproduction. Front. Cell Dev. Biol. 2015, 3, 21. [Google Scholar] [CrossRef]
- Khanal, S.; Leung, M.R.; Royfman, A.; Fishman, E.L.; Saltzman, B.; Bloomfield-Gadêlha, H.; Zeev-Ben-Mordehai, T.; Avidor-Reiss, T. A dynamic basal complex modulates mammalian sperm movement. Nat. Commun. 2021, 12, 3808. [Google Scholar] [CrossRef]
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevičius, J.; Lukinavičius, G.; Elder, K.; et al. Parental genome unification is highly error-prone in mammalian embryos. Cell 2021, 184, 2860–2877. [Google Scholar] [CrossRef]
- Kai, Y.; Kawano, H.; Yamashita, N. First mitotic spindle formation is led by sperm centrosome-dependent MTOCs in humans. Reproduction 2021, 161, V19–V22. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; de Ruijter-Villani, M.; Julius Hossain, M.; Stout, T.A.E.; Ellenberg, J. Dual spindles assemble in bovine zygotes despite the presence of paternal centrosomes. J. Cell Biol. 2021, 220, e202010106. [Google Scholar] [CrossRef]
- Wang, W.-J.; Acehan, D.; Kao, C.-H.; Jane, W.-N.; Uryu, K.; Tsou, M.-F.B. De novo centriole formation in human cells is error-prone and does not require SAS-6 self-assembly. eLife 2015, 4, e10586. [Google Scholar] [CrossRef]
- Uzbekov, R.E. Centriole duplication in PE (SPEV) cells starts before beginning of DNA replication. Biol. Membranes 2007, 24, 292–298. (In Russian); Biochemistry (Moscow) Suppl. Series A Membr. Cell Biol. 2007, 1, 206–211. (In English) [Google Scholar] [CrossRef]
- Uzbekov, R.E.; Alieva, I.B. Centriole duplication or DNA replication—What starts earlier? In Cytoskeleton: Cell Movement, Cytokinesis and Organelles Organization; Lansing, S., Rousseau, T., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; Chapter 5; pp. 127–137. [Google Scholar]
- Uzbekov, R.E.; Alieva, I.B. Centrosome—History of Study and New Discoveries. From Cytoplasmic Granule to the Center of Intracellular Regulation; Nadezhdina, E.S., Ed.; Moscow University Press: Moscow, Russia, 2013; pp. 1–320. [Google Scholar]
- Dvorak, M. The differentiation of rat ova during cleavage. Adv. Anat. Embryol. Cell Biol. 1978, 55, 5–81. [Google Scholar]
- Abumuslimov, S.S.; Nadezhdina, E.S.; Chentsov, I.S. Centrosome morphogenesis in the early development of mice: An immunofluorescence study using antibodies to the centriole antigen. Tsitologiia 1996, 38, 5–13. [Google Scholar]
- Brueckner, M. Cilia propel the embryo in the right direction. Am. J. Med. Genet. 2001, 101, 339–344. [Google Scholar] [CrossRef]
- Essner, J.J.; Vogan, K.J.; Wagner, M.K.; Tabin, C.J.; Yost, H.J.; Brueckner, M. Conserved function for embryonic nodal cilia. Nature 2002, 418, 37–38. [Google Scholar] [CrossRef]
- Uzbekov, R.; Garanina, A.; Bressac, C. Centrioles without microtubules: A new morphological type of centriole. Biol. Open. 2018, 7, bio036012. [Google Scholar] [CrossRef]
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. The Drosophila centriole—Conversion of doublets into triplets within the stem cell niche. J. Cell Sci. 2015, 128, 2437–2442. [Google Scholar] [CrossRef]
- Grzonka, M.; Bazzi, H. Mouse SAS-6 is required for centriole formation in embryos and integrity in embryonic stem cells. BioRxiv 2022. [Google Scholar] [CrossRef]
- Telford, N.A.; Watson, A.J.; Schultz, G.A. Transition from maternal to embryonic control in early mammalian development: A comparison of several species. Mol. Reprod. Dev. 1990, 26, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Vakonakis, I.; Olieric, N.; Hilbert, M.; Keller, D.; Olieric, V.; Bortfeld, M.; Erat, M.C.; Flückiger, I.; Gönczy, P.; et al. Structural basis of the 9-fold symmetry of centrioles. Cell 2011, 144, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Van Breugel, M.; Hirono, M.; Andreeva, A.; Yanagisawa, H.A.; Yamaguchi, S.; Nakazawa, Y.; Morgner, N.; Petrovich, M.; Ebong, I.O.; Robinson, C.V.; et al. Structures of SAS-6 suggest its organization in centrioles. Science 2011, 331, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Indjeian, V.B.; McManus, M.; Wang, L.; Dynlacht, B.D. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell. 2002, 3, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kleylein-Sohn, J.; Westendorf, J.; Le Clech, M.; Habedanck, R.; Stierhof, Y.D.; Nigg, E.A. Plk4-induced centriole biogenesis in human cells. Dev. Cell 2007, 13, 190–202. [Google Scholar] [CrossRef]
- Schmidt, T.I.; Kleylein-Sohn, J.; Westendorf, J.; Le Clech, M.; Lavoie, S.B.; Stierhof, Y.D.; Nigg, E.A. Control of centriole length by CPAP and CP110. Curr. Biol. 2009, 19, 1005–1011. [Google Scholar] [CrossRef]
- Tang, C.J.; Fu, R.H.; Wu, K.S.; Hsu, W.B.; Tang, T.K. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 2009, 11, 825–831. [Google Scholar] [CrossRef]
- Kohlmaier, G.; Loncarek, J.; Meng, X.; McEwen, B.F.; Mogensen, M.M.; Spektor, A.; Dynlacht, B.D.; Khodjakov, A.; Gonczy, P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 2009, 19, 1012–1018. [Google Scholar] [CrossRef]
- Cunningham, N.H.J.; Bouhlel, I.B.; Conduit, P.T. Daughter centrioles assemble preferentially towards the nuclear envelope in Drosophila syncytial embryos. Open Biol. 2022, 12, 210343. [Google Scholar] [CrossRef]
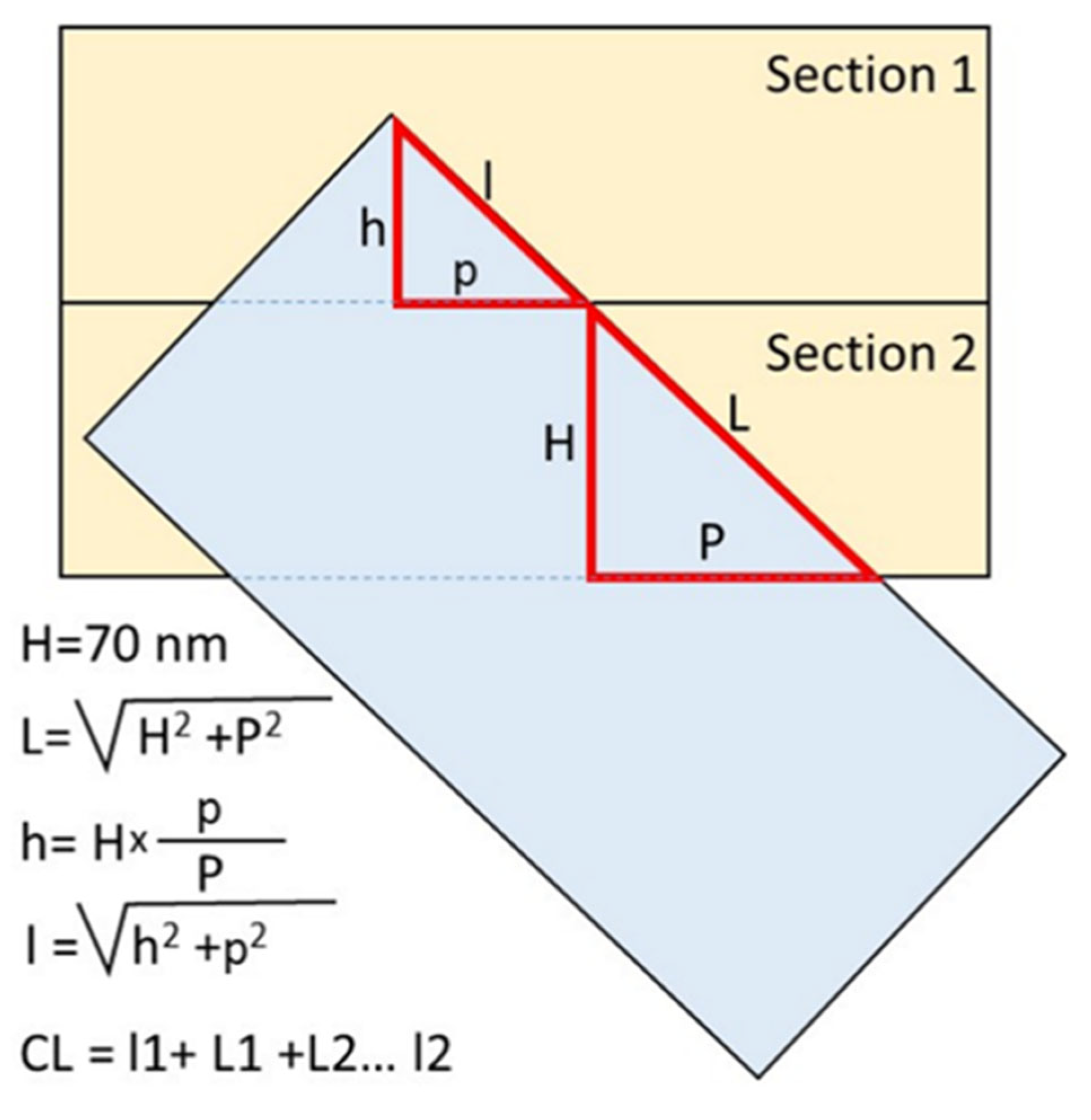




| Blastomere | Mother Centriole (Length in nm) | Procentriole of Mother Centriole (Length in nm) | Daughter Centriole (Length in nm) | Procentriole of Daughter Centriole (Length in nm) |
|---|---|---|---|---|
| Cell 1 | 700 | 333 | 623 | 407 |
| Cell 2 | 652 | 488 | 584 | 357 |
| Cell 3 (flagellum in cytoplasm) | Atypical centriole | 312 | 607 | 280 |
| Cell 4 | 812 | 405 | 593 | 378 |
| Cell 5 | 1059 | 518 | 607 | 280 |
| Cell 6 | 732 | 292 | 574 | 312 |
| Cell 7 | Atypical centriole | 474 | 754 | 311 |
| Average | * 791 ± 161 | 403 ± 92 | 620 ± 61 | 332 ± 49 |
| Blastomere | Mother Centriole (Length in nm) | Procentriole of Mother Centriole (Length in nm) | Daughter Centriole (Length in nm) | Procentriole of Daughter Centriole (Length in nm) |
|---|---|---|---|---|
| Cell 1 (flagellum in cytoplasm) | Atypical centriole | 366 | 644 | 194 |
| Cell 2 | 786 | No procentriole | 645 | No procentriole |
| Cell 3 | 870 | No procentriole | 755 | No procentriole |
| Cell 4 | 1024 | 443 | 640 | 410 |
| Cell 5 | 984 | 397 | 797 | 138 |
| Cell 6 | 814 | No procentriole | 644 | No procentriole |
| Cell 7 | Atypical centriole | 394 | 735 | 259 |
| Cell 8 | 892 | No procentriole | 625 | No procentriole |
| Cell 9 | 778 | No procentriole | 740 | No procentriole |
| Cell 10 | 848 | 319 | 691 | 163 |
| Cell 11 | 897 | No procentriole | 644 | No procentriole |
| Cell 12 | 729 | 452 | 724 | 392 |
| Cell 13 | 897 | 238 | 677 | 148 |
| Cell 14 | 844 | 350 | 666 | No procentriole |
| Average | * 864 ± 84 | 370 ± 69 (** 211 ± 197) | 688 ± 53 | 243 ± 115 (** 122 ± 148) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzbekov, R.; Singina, G.N.; Shedova, E.N.; Banliat, C.; Avidor-Reiss, T.; Uzbekova, S. Centrosome Formation in the Bovine Early Embryo. Cells 2023, 12, 1335. https://doi.org/10.3390/cells12091335
Uzbekov R, Singina GN, Shedova EN, Banliat C, Avidor-Reiss T, Uzbekova S. Centrosome Formation in the Bovine Early Embryo. Cells. 2023; 12(9):1335. https://doi.org/10.3390/cells12091335
Chicago/Turabian StyleUzbekov, Rustem, Galina N. Singina, Ekaterina N. Shedova, Charles Banliat, Tomer Avidor-Reiss, and Svetlana Uzbekova. 2023. "Centrosome Formation in the Bovine Early Embryo" Cells 12, no. 9: 1335. https://doi.org/10.3390/cells12091335
APA StyleUzbekov, R., Singina, G. N., Shedova, E. N., Banliat, C., Avidor-Reiss, T., & Uzbekova, S. (2023). Centrosome Formation in the Bovine Early Embryo. Cells, 12(9), 1335. https://doi.org/10.3390/cells12091335









