Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments
Abstract
1. Introduction
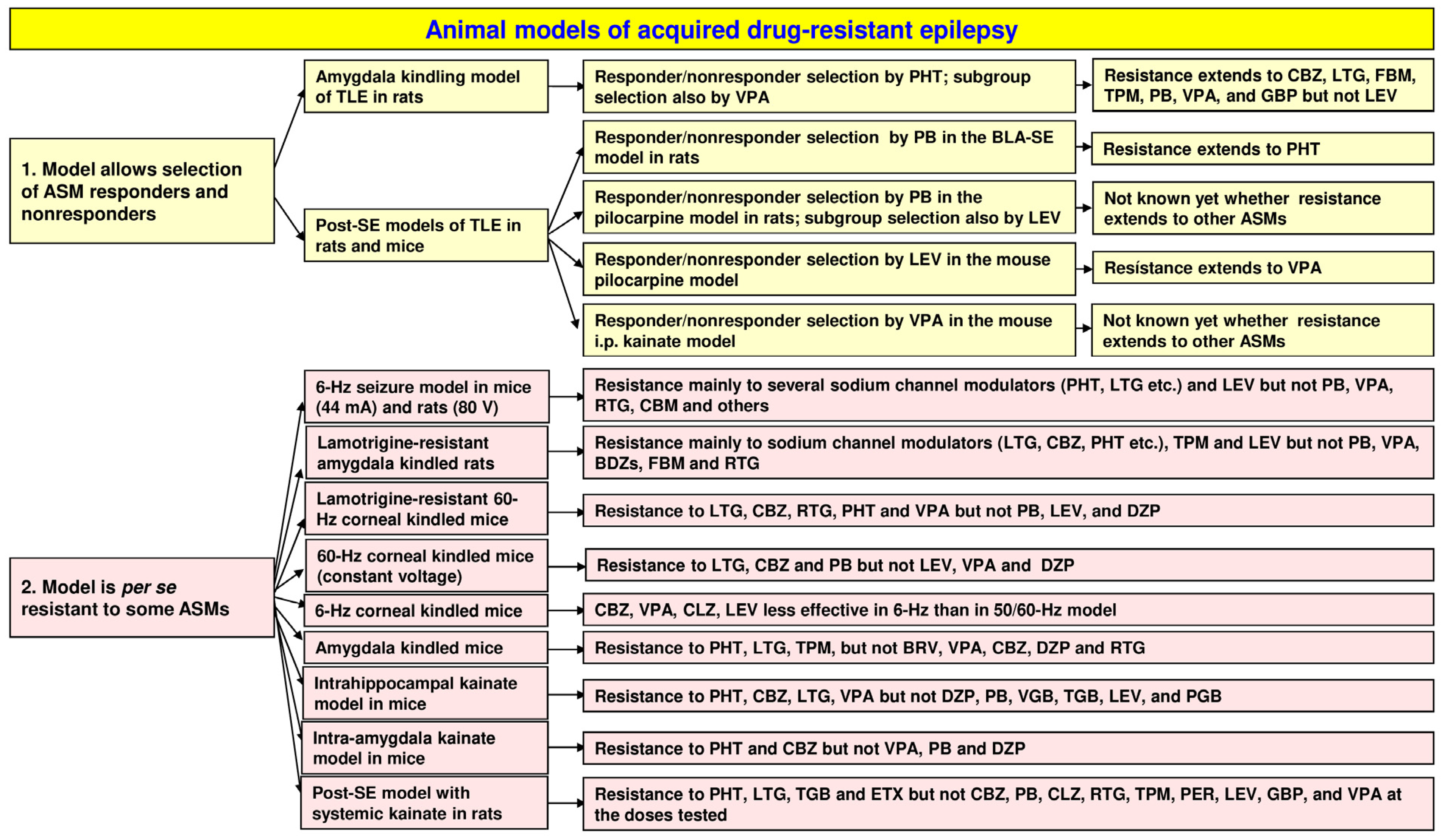
2. Chronic Animal Models That Allow Selecting Drug Responders and Nonresponders
2.1. The Amygdala Kindling Model of Temporal Lobe Epilepsy
2.2. Amygdala Kindling as a Model of Pharmacoresistant Seizures

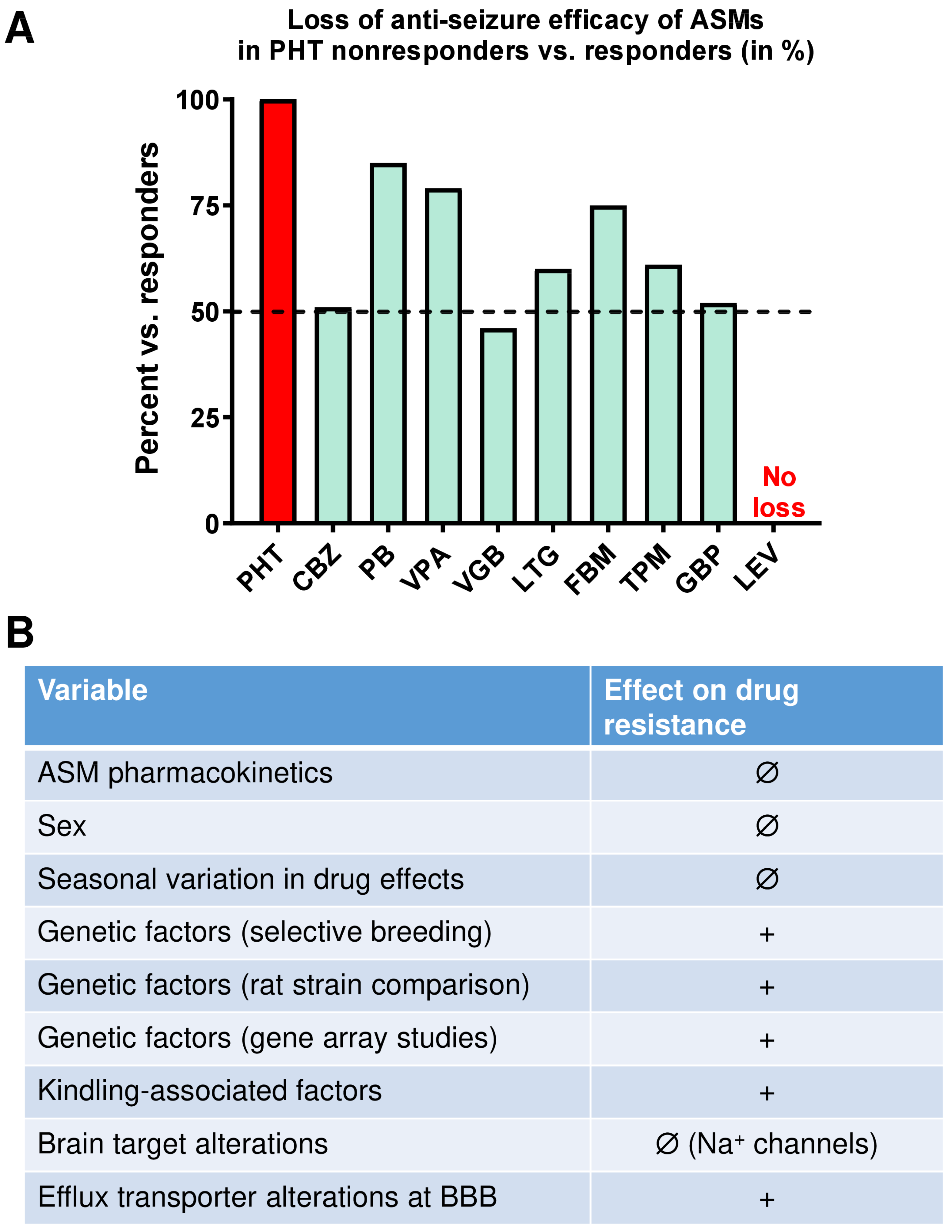
2.3. Selection of Phenytoin Responders and Nonresponders from Large Groups of Amygdala-Kindled Rats
2.3.1. The Resistance to Phenytoin Extends to Other ASMs
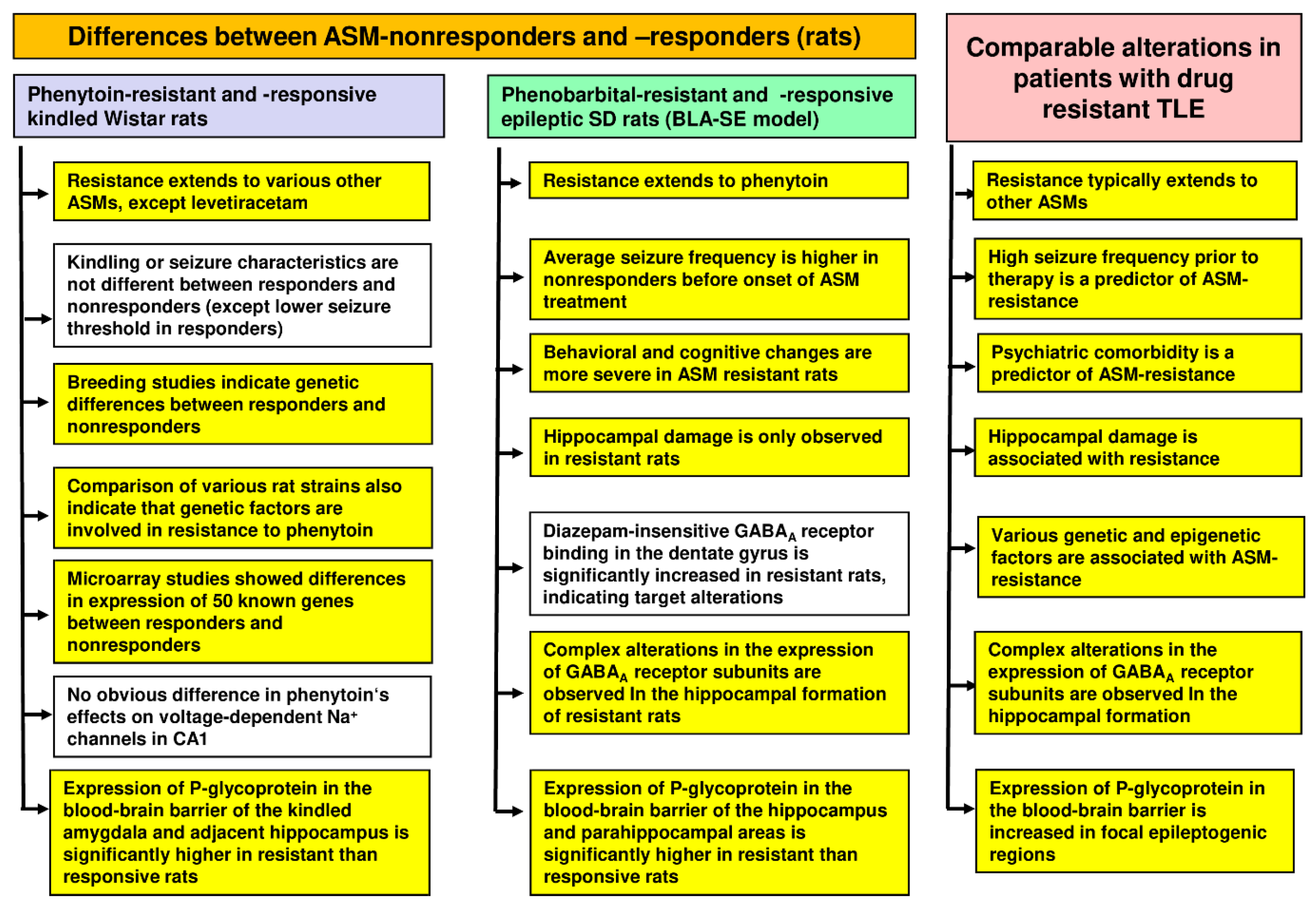
2.3.2. Cellular and Molecular Mechanisms of Pharmacoresistance in Amygdala-Kindled Rats—Studies from the Löscher Group
2.3.3. Cellular and Molecular Mechanisms of Pharmacoresistance in Amygdala-Kindled Rats—Studies from Other Groups
2.3.4. Advantages and Limitations of Phenytoin-Resistant Kindled Rats as a Model of Drug-Resistant Epilepsy
2.4. Post-Status Epilepticus Models of Mesial Temporal Lobe Epilepsy
2.5. Selection of Phenobarbital Responders and Nonresponders from Epileptic Rats in the BLA-SE Model of TLE
2.5.1. Extension of Resistance to Other ASMs
2.5.2. Mechanisms of Resistance
2.6. Selection of ASM Responders and Nonresponders from Epileptic Rats and Mice in the Pilocarpine Model of TLE
2.7. Selection of ASM Responders and Nonresponders from Epileptic Mice in the Kainate Model of TLE
2.8. Advantages and Limitations of ASM-Resistant Epileptic Rats and Mice as a Model of Drug-Resistant Epilepsy
3. Animal Models with Induced Seizures That Are Resistant to ASMs
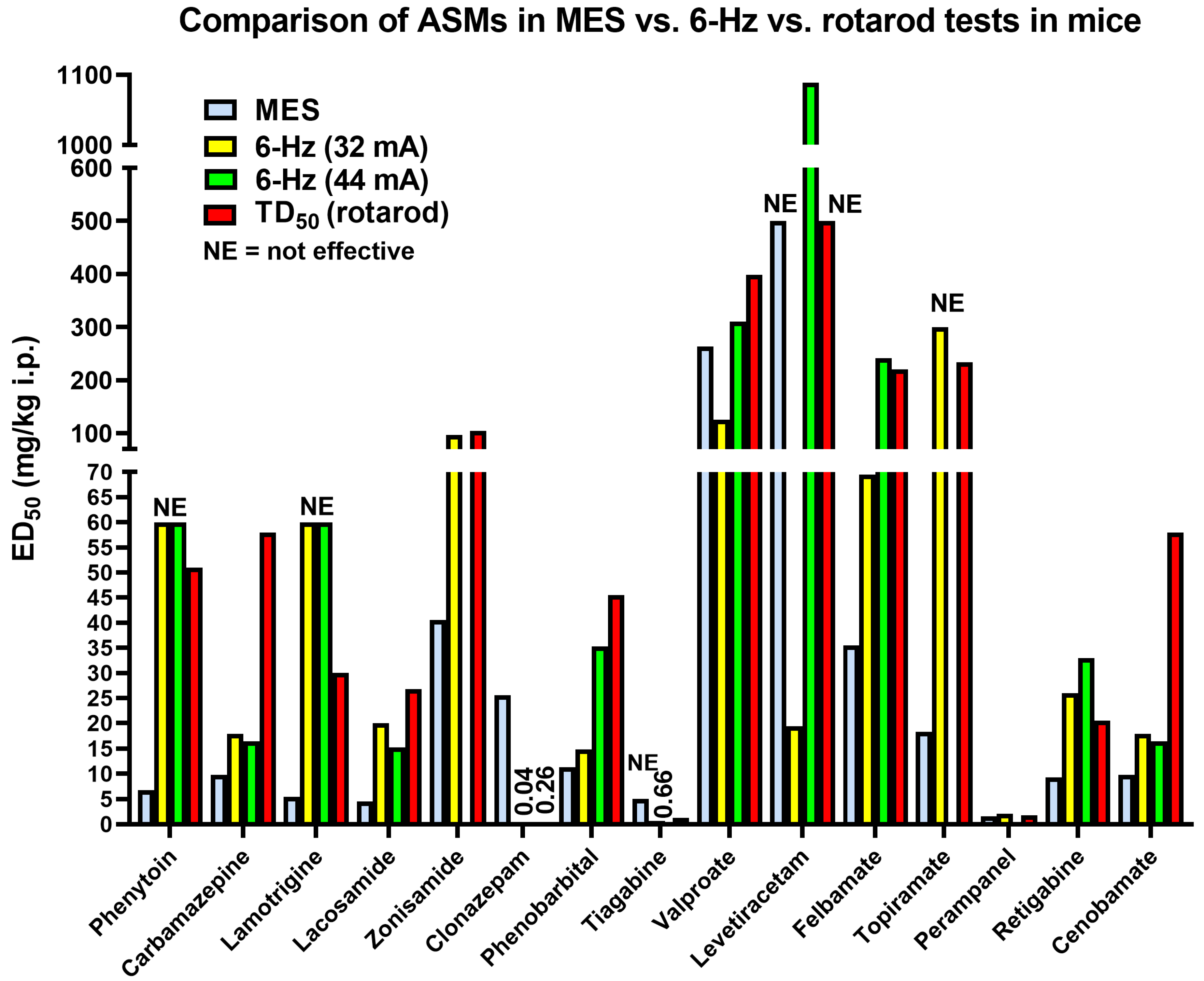
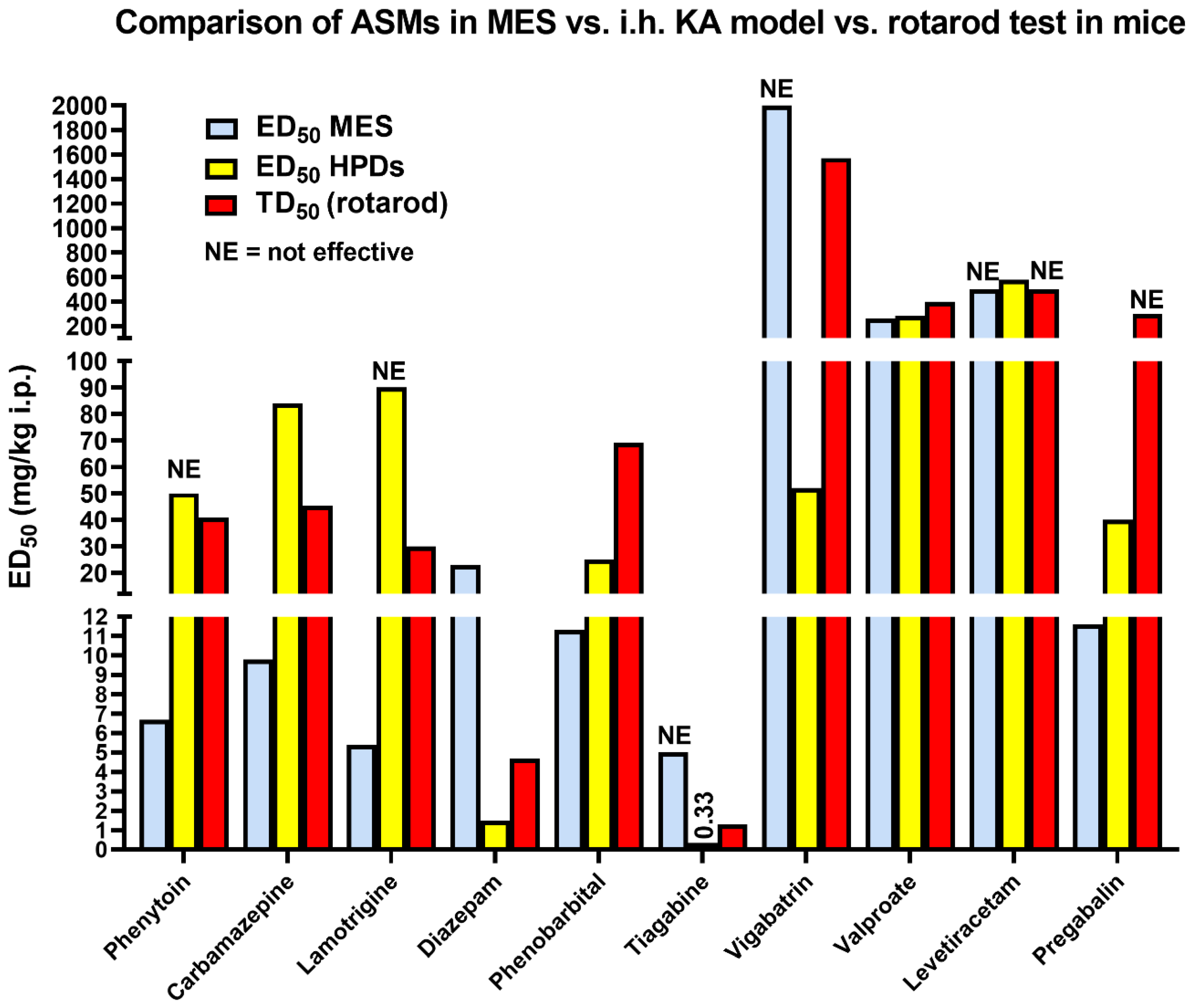
3.1. The 6 Hz Psychomotor Seizure Model
3.2. Induction of Acute Seizures in Epileptic Animals
3.3. The Lamotrigine-Resistant Kindled Rat Model
3.4. Corneal Kindling with 50 or 60 Hz in Mice and Rats
3.5. Corneal Kindling with 6 Hz in Mice
3.6. The Amygdala-Kindled Mouse Model of TLE
3.7. Advantages and Limitations of Animal Models with Induced Seizures That Are Resistant to ASMs
4. Animal Models with Spontaneous Seizures That Are Resistant to ASMs
4.1. Post-SE Models of TLE
4.1.1. The Intrahippocampal Kainate Mouse and Rat Models of TLE
4.1.2. The Intra-Amygdala Kainate Mouse Model of TLE
4.1.3. The Systemic Kainate Rat Model of TLE
4.1.4. The Systemic Pilocarpine Rat and Mouse Models of TLE
4.1.5. Advantages and Limitations of Animal Models of TLE with Spontaneous Seizures That Are Resistant to ASMs
4.2. Models of Focal Neocortical Epilepsy
4.3. Genetic Models
| ASM | Anti-Seizure Activity | |||
|---|---|---|---|---|
| DS Patients (Spontaneous Seizures; Chronic Treatment) * | Scn1a+/− Mice | Scn1labs552 Zebrafish (Spontaneous Seizures; Acute Treatment) | ||
| Spontaneous Seizures (Subchronic Treatment) | Hyperthermia- Induced Seizures (Single-Dose Treatment) | |||
| Valproate | Yes | No (no **) | Yes *** | Yes |
| Clobazam | Yes | No (yes **) | Yes | Yes |
| Stiripentol | Yes | No (no **) | Yes *** | Yes |
| Topiramate | Yes | No | No | Yes |
| Clonazepam | Yes | ? | Yes | Yes |
| Fenfluramine | Yes | ? | ? | Yes |
| Cannabidiol | Yes | Yes | Yes | Yes |
| Stiripentol plus clobazam | Yes | Yes ** | Yes | ? |
| Levetiracetam | No | No | Yes | No |
| Phenobarbital | No | No | Yes | No |
| Lamotrigine | No (worse) | No (worse) | No (worse) | No |
| Phenytoin | No (worse) | No | No | No |
| Carbamazepine | No (worse) | No | No | No (worse) |
| No. of ASMs predictive | 8/11 | 9/11 | 12/12 | |
5. Pharmacology of Induced vs. Spontaneous Seizures in Animal Models of Drug-Resistant Epilepsy
6. Evaluation of Drug Combinations vs. Single Drug Testing
7. Evaluation of Drug Potency vs. Efficacy
8. The Importance of Pharmacokinetics for Anti-Seizure Efficacy Testing in Animal Models
9. The Use of Animal Models as Tools for Developing Novel Non-Pharmacological Treatment Strategies
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshé, S.L.; Perucca, E.; Engel, J., Jr.; Löscher, W.; Noebels, J.L.; Pitkänen, A.; Stables, J.; et al. The American Epilepsy Society Basic Science Committee and The International League Against Epilepsy Working Group on Recommendations for Preclinical Epilepsy Drug Discovery. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [PubMed]
- Potschka, H. Animal models of drug-resistant epilepsy. Epileptic Disord. 2012, 14, 226–234. [Google Scholar] [CrossRef]
- Guignet, M.; White, H.S. Animal Models of Pharmacoresistant Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 5th ed.; Noebels, J., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford Medicine Online: London, UK, 2023; in press. [Google Scholar]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen, H.W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Stables, J.P.; Bertram, E.; Dudek, F.E.; Holmes, G.; Mathern, G.; Pitkänen, A.; White, H.S. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: Summary of the NIH/NINDS/AES models II workshop. Epilepsia 2003, 44, 1472–1478. [Google Scholar] [CrossRef]
- Regesta, G.; Tanganelli, P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999, 34, 109–122. [Google Scholar] [CrossRef]
- Schmidt, D.; Löscher, W. Drug Resistance in Epilepsy: Putative Neurobiologic and Clinical Mechanisms. Epilepsia 2005, 46, 858–877. [Google Scholar] [CrossRef]
- Nabbout, R.; Andrade, D.M.; Bahi-Buisson, N.; Cross, H.; Desquerre, I.; Dulac, O.; Granata, T.; Hirsch, E.; Navarro, V.; Ouss, L.; et al. Outcome of childhood-onset epilepsy from adolescence to adulthood: Transition issues. Epilepsy Behav. 2017, 69, 161–169. [Google Scholar] [CrossRef]
- Engel, J., Jr. What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology 2016, 87, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Janmohamed, M.; Brodie, M.J.; Kwan, P. Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020, 168, 107790. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for antiepileptic drug discovery and de-velopment. Nat. Rev. Drug Discov. 2013, 12, 757–776. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early Identification of Refractory Epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016, 126, 157–184. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of drug-refractory epilepsy. In Models of Seizures and Epilepsy, 2nd ed.; Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., Moshé, S.L., Eds.; Academic Press: London, UK, 2017; pp. 743–760. [Google Scholar] [CrossRef]
- Löscher, W. Experimental models for intractable epilepsy in nonprimate animal species. In Intractable Epilepsy: Experimental and Clinical Aspects; Schmidt, D., Morselli, P.L., Eds.; Raven Press: New York, NY, USA, 1986; pp. 25–37. [Google Scholar]
- Löscher, W.; Rundfeldt, C. Kindling as a model of drug-resistant partial epilepsy: Selection of phenytoin-resistant and nonresistant rats. Experiment 1991, 258, 483–489. [Google Scholar]
- Brandt, C.; Volk, H.A.; Löscher, W. Striking Differences in Individual Anticonvulsant Response to Phenobarbital in Rats with Spontaneous Seizures after Status Epilepticus. Epilepsia 2004, 45, 1488–1497. [Google Scholar] [CrossRef]
- Bethmann, K.; Brandt, C.; Löscher, W. Resistance to phenobarbital extends to phenytoin in a rat model of temporal lobe epilepsy. Epilepsia 2007, 48, 816–826. [Google Scholar] [CrossRef]
- Bankstahl, M.; Bankstahl, J.P.; Löscher, W. Inter-individual variation in the anticonvulsant effect of phenobarbital in the pilocarpine rat model of temporal lobe epilepsy. Exp. Neurol. 2012, 234, 70–84. [Google Scholar] [CrossRef]
- Brandt, C.; Löscher, W. Antiepileptic efficacy of lamotrigine in phenobarbital-resistant and -responsive epileptic rats: A pilot study. Epilepsy Res. 2014, 108, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Bankstahl, M.; Löscher, W. Inter-individual variation in the effect of antiepileptic drugs in the in-trahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2015, 90, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Toman, J.E.P. Neuropharmacologic considerations in psychic seizures. Neurology 1951, 1, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Toman, J.E.P.; Everett, G.M.; Richards, R.K. The search for new drugs against epilepsy. Tex. Rep. Biol. Med. 1952, 10, 96–104. [Google Scholar]
- Srivastava, A.K.; White, H.S. Carbamazepine, but not valproate, displays pharmacoresistance in lamotrigi-ne-resistant amygdala kindled rats. Epilepsy Res. 2013, 104, 26–34. [Google Scholar] [CrossRef]
- Duveau, V.; Roucard, C. A Mesiotemporal Lobe Epilepsy Mouse Model. Neurochem. Res. 2017, 42, 1919–1925. [Google Scholar]
- Löscher, W. Animal models of intractable epilepsy. Prog. Neurobiol. 1997, 53, 239–258. [Google Scholar] [CrossRef]
- Löscher, W. Animal Models of Drug-Refractory Epilepsy. In Models of Seizures and Epilepsy; Pitkänen, A., Schwartzkroin, P.A., Moshé, S.L., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2006; pp. 551–567. [Google Scholar] [CrossRef]
- Goddard, G.V.; McIntyre, D.C.; Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef]
- Sato, M.; Racine, R.; McIntyre, D. Kindling: Basic mechanisms and clinical validity. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 459–472. [Google Scholar] [CrossRef]
- McIntyre, D.C.; O Poulter, M.; Gilby, K. Kindling: Some old and some new. Epilepsy Res. 2002, 50, 79–92. [Google Scholar] [CrossRef]
- Löscher, W.; Hönack, D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. Eur. J. Pharmacol. 1993, 232, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, H. Levetiracetam: The preclinical profile of a new class of antiepileptic drugs? Epilepsia 2001, 42, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Gillard, M.; Sands, Z.A.; Kaminski, R.M.; Klitgaard, H. Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond. CNS Drugs 2016, 30, 1055–1077. [Google Scholar] [CrossRef]
- Wilcox, K.S.; Dixon-Salazar, T.; Sills, G.J.; Ben Menachem, E.; White, H.S.; Porter, R.J.; Dichter, M.A.; Moshé, S.L.; Noebels, J.L.; Privitera, M.D.; et al. Issues related to development of new antiseizure treatments. Epilepsia 2013, 54, 24–34. [Google Scholar] [CrossRef]
- Bialer, M.; White, H.S. Key factors in the discovery and development of new antiepileptic drugs. Nat. Rev. Drug Discov. 2010, 9, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Jäckel, R.; Czuczwar, S.J. Is amygdala kindling in rats a model for drug-resistant partial epilepsy? Exp. Neurol. 1986, 93, 211–226. [Google Scholar] [CrossRef]
- Hönack, D.; Löscher, W. Kindling Increases the Sensitivity of Rats to Adverse Effects of Certain Antiepileptic Drugs. Epilepsia 1995, 36, 763–771. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988, 2, 145–181. [Google Scholar] [CrossRef]
- Löscher, W.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. IV. Protective indices. Epilepsy Res. 1991, 9, 1–10. [Google Scholar] [CrossRef]
- Rundfeldt, C.; Hönack, D.; Löscher, W. Phenytoin potently increases the threshold for focal seizures in amygdala-kindled rats. Neuropharmacology 1990, 29, 845–851. [Google Scholar] [CrossRef]
- Ashton, D.; Wauquier, A. Behavioral analysis of the effects of 15 anticonvulsants in the amygdaloid kindled rat. Psychopharmacology 1979, 65, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Albright, P.S.; Burnham, W.M. Development of a New Pharmacological Seizure Model: Effects of Anticonvulsants on Cortical-and Amygdala-Kindled Seizures in the Rat. Epilepsia 1980, 21, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Rigsbee, L.C.; Butler, L.S.; McNamara, J.O. Intravenous phenytoin blocks amygdaloid kindled seizures. Soc. Neurosci. Abstr. 1989, 15, 453. [Google Scholar]
- Babington, R.; Wedeking, P. The pharmacology of seizures induced by sensitization with low intensity brain stimulation. Pharmacol. Biochem. Behav. 1973, 1, 461–467. [Google Scholar] [CrossRef]
- Albertson, T.; Peterson, S.; Stark, L. Anticonvulsant drugs and their antagonism of kindled amygdaloid seizures in rats. Neuropharmacology 1980, 19, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Mace, J.; Burnham, W. The effect of repeated seizures on anticonvulsant drug response in the kindling model. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 171–175. [Google Scholar] [CrossRef]
- Renfrey, G.; Schlinger, H.; Jakubow, J.; Poling, A. Effects of phenytoin and phenobarbital on schedule-controlled responding and seizure activity in the amygdala-kindled rat. J. Pharmacol. Exp. Ther. 1989, 248, 967–973. [Google Scholar]
- Callaghan, D.; Schwark, W. Pharmacological modification of amygdaloid-kindled seizures. Neuropharmacology 1980, 19, 1131–1136. [Google Scholar] [CrossRef]
- Wise, R.A.; Chinerman, J. Effects of diazepam and phenobarbital on electrically-induced amygdaloid seizures and seizure development. Exp. Neurol. 1974, 45, 355–363. [Google Scholar] [CrossRef]
- Baimbridge, K.G.; Miller, J.J. Hippocampal calcium-binding protein during commissural kindling-induced epileptogenesis: Progressive decline and effects of anticonvulsants. Brain Res. 1984, 324, 85–90. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of drug resistant epilepsy. In Mechanisms of Drug Resistance in Epilepsy: Lessons from Oncology; Ling, V., Ed.; Wiley: Chichester, UK, 2002; pp. 149–159. [Google Scholar]
- Ebert, U.; Rundfeldt, C.; Löscher, W. Sex differences in the anticonvulsant efficacy of phenytoin in amygdala-kindled rats. Brain Res. 1994, 638, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H.; Baltes, S.; Löscher, W. Inhibition of multidrug transporters by verapamil or probenecid does not alter blood-brain barrier penetration of levetiracetam in rats. Epilepsy Res. 2004, 58, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ebert, U.; Rundfeldt, C.; Lehmann, H.; Löscher, W. Characterization of phenytoin-resistant kindled rats, a new model of drug-resistant partial epilepsy: Influence of experimental and environmental factors. Epilepsy Res. 1999, 33, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Ebert, U.; Löscher, W. Characterization of phenytoin-resistant kindled rats, a new model of drug-resistant partial epilepsy: Influence of genetic factors. Epilepsy Res. 1999, 33, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.; Ebert, U.; Löscher, W. Characterization of phenytoin-resistant kindled rats, a new model of drug-resistant partial epilepsy: Comparison of inbred strains. Epilepsia 1998, 39, 1046–1053. [Google Scholar] [CrossRef]
- Löscher, W.; Cramer, S.; Ebert, U. Differences in Kindling Development in Seven Outbred and Inbred Rat Strains. Exp. Neurol. 1998, 154, 551–559. [Google Scholar] [CrossRef]
- Löscher, W.; Reissmüller, E.; Ebert, U. Kindling alters the anticonvulsant efficacy of phenytoin in Wistar rats. Epilepsy Res. 2000, 39, 211–220. [Google Scholar] [CrossRef]
- Remy, S.; Beck, H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2005, 129, 18–35. [Google Scholar] [CrossRef]
- Jeub, M.; Beck, H.; Siep, E.; Ruschenschmidt, C.; Speckmann, E.J.; Ebert, U.; Potschka, H.; Freichel, C.; Reissmüller, E.; Löscher, W. Effect of phenytoin on sodium and calcium currents in hippocampal CA1 neurons of phenytoin-resistant kindled rats. Neuropharmacology 2002, 42, 107–116. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Löscher, W.; Luna-Tortos, C.; Romermann, K.; Fedrowitz, M. Do ATP-Binding Cassette Transporters Cause Pharmacoresistance in Epilepsy? Problems and Approaches in Determining which Antiepileptic Drugs are Affected. Curr. Pharm. Des. 2011, 17, 2808–2828. [Google Scholar] [CrossRef]
- Potschka, H.; Löscher, W. A comparison of extracellular levels of phenytoin in amygdala and hippocampus of kindled and non-kindled rats. Neuroreport 2002, 13, 167–171. [Google Scholar] [CrossRef]
- Potschka, H.; Volk, H.A.; Löscher, W. Pharmacoresistance and expression of multidrug transporter P-glycoprotein in kindled rats. Neuroreport 2004, 15, 1657–1661. [Google Scholar] [CrossRef]
- Töllner, K.; Wolf, S.; Löscher, W.; Gernert, M. The anticonvulsant response to valproate in kindled rats is corre-lated with its effect on neuronal firing in the substantia nigra pars reticulata: A new mechanism of pharmacoresistance. J. Neurosci. 2011, 31, 16423–16434. [Google Scholar] [CrossRef]
- Velíšková, J.; Moshé, S.L. Update on the Role of Substantia Nigra Pars Reticulata in the Regulation of Seizures. Epilepsy Curr. 2006, 6, 83–87. [Google Scholar] [CrossRef]
- Ma, A.; Wang, C.; Chen, Y.; Yuan, W. P-glycoprotein alters blood–brain barrier penetration of antiepileptic drugs in rats with medically intractable epilepsy. Drug Des. Dev. Ther. 2013, 7, 1447–1454. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, C.C.; Xiao, X.; Wei, L.; Xu, G. Multidrug resistance-associated protein 1 decreases the concentrations of antiepileptic drugs in cortical extracellular fluid in amygdale kindling rats. Acta Pharmacol. Sin. 2013, 34, 473–479. [Google Scholar] [CrossRef]
- Jiang, W.; Du, B.; Chi, Z.; Ma, L.; Wang, S.; Zhang, X.; Wu, W.; Wang, X.; Xu, G.; Guo, C. Preliminary explorations of the role of mitochondrial proteins in refractory epilepsy: Some findings from comparative proteomics. J. Neurosci. Res. 2007, 85, 3160–3170. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Wang, X.; Wang, Y.; Yan, Y. Enhanced Synaptic Vesicle Traffic in Hippocampus of Phenytoin-Resistant Kindled Rats. Neurochem. Res. 2008, 34, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Luna-Munguia, H.; Orozco-Suarez, S.; Rocha, L. Effects of high frequency electrical stimulation and R-verapamil on seizure susceptibility and glutamate and GABA release in a model of phenytoin-resistant seizures. Neuropharmacology 2011, 61, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, J.; Wu, G.; Zhou, F.; Hong, Z. Hippocampal low-frequency stimulation increased SV2A expression and inhibited the seizure degree in pharmacoresistant amygdala-kindling epileptic rats. Epilepsy Res. 2014, 108, 1483–1491. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, F.; Wang, L.-K.; Wu, G.-F. Synaptic vesicle protein2A decreases in amygdaloid-kindling pharmcoresistant epileptic rats. J. Huazhong Univ. Sci. Technol. 2015, 35, 716–722. [Google Scholar] [CrossRef]
- van Vliet, E.; Aronica, E.; Redeker, S.; Boer, K.; Gorter, J.A. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia 2009, 50, 422–433. [Google Scholar] [CrossRef]
- Crèvecoeur, J.; Kaminski, R.M.; Rogister, B.; Foerch, P.; Vandenplas, C.; Neveux, M.; Mazzuferi, M.; Kroonen, J.; Poulet, C.; Martin, D.; et al. Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol. Appl. Neurobiol. 2014, 40, 191–204. [Google Scholar] [CrossRef]
- Li, M.C.H.; Cook, M.J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia 2017, 59, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hong, Z.; Li, Y.; Zhou, F.; Shi, J. Effects of Low-Frequency Hippocampal Stimulation on Gamma-Amino Butyric Acid Type B Receptor Expression in Pharmacoresistant Amygdaloid Kindling Epileptic Rats. Neuromodul. Technol. Neural Interface 2012, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, L.; Hong, Z.; Ren, S.; Zhou, F. Hippocampal low-frequency stimulation inhibits afterdischarge and increases GABA (A) receptor expression in amygdala-kindled pharmacoresistant epileptic rats. Neurol. Res. 2017, 39, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Z.; Wang, L.; Wu, G.; Liu, T. Influence of hippocampal low-frequency stimulation on GABAA R α1, ICER and BNDF expression level in brain tissues of amygdala-kindled drug-resistant temporal lobe epileptic rats. Brain Res. 2018, 1698, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sutula, T.; He, X.X.; Cavazos, J.; Scott, G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science 1988, 239, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Ren, S.; Wu, G.; Wu, J. The Expression of ZnT3 and GFAP Is Potentiated in the Hippocampus of Drug-Resistant Epileptic Rats Induced by Amygdala Kindling. Neuroimmunomodulation 2020, 27, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, S.; Gong, Y.; Nao, J.; Shen, Y.; Tan, B.; Xu, S.; Cui, S.; Ruan, Y.; Wang, S.; et al. Subicular Caspase-1 Contributes to Pharmacoresistance in Temporal Lobe Epilepsy. Ann. Neurol. 2021, 90, 377–390. [Google Scholar] [CrossRef]
- Brandt, C.; Ebert, U.; Löscher, W. Epilepsy induced by extended amygdala-kindling in rats: Lack of clear association between development of spontaneous seizures and neuronal damage. Epilepsy Res. 2004, 62, 135–156. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002, 50, 105–123. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Avoli, M.; Bernard, C. Animal models of temporal lobe epilepsy following systemic chemocon-vulsant administration. J. Neurosci. Methods 2016, 260, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rusina, E.; Bernard, C.; Williamson, A. The Kainic Acid Models of Temporal Lobe Epilepsy. Eneuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Glien, M.; Potschka, H.; Volk, H.; Löscher, W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003, 55, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. The Pharmacokinetics of Antiepileptic Drugs in Rats: Consequences for Maintaining Effective Drug Levels during Prolonged Drug Administration in Rat Models of Epilepsy. Epilepsia 2007, 48, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Löscher, W.; Brandt, C. High seizure frequency prior to antiepileptic treatment is a predictor of pharmacoresistant epilepsy in a rat model of temporal lobe epilepsy. Epilepsia 2010, 51, 89–97. [Google Scholar] [CrossRef]
- Rogawski, M.A. The intrinsic severity hypothesis of pharmacoresistance to antiepileptic drugs. Epilepsia 2013, 54, 33–40. [Google Scholar] [CrossRef]
- Gastens, A.M.; Brandt, C.; Bankstahl, J.P.; Löscher, W. Predictors of pharmacoresistant epilepsy: Pharma-coresistant rats differ from pharmacoresponsive rats in behavioral and cognitive abnormalities associated with experimentally induced epilepsy. Epilepsia 2008, 49, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.A.; Arabadzisz, D.; Fritschy, J.-M.; Brandt, C.; Bethmann, K.; Löscher, W. Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABAA receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol. Dis. 2006, 21, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Bethmann, K.; Fritschy, J.-M.; Brandt, C.; Löscher, W. Antiepileptic drug resistant rats differ from drug responsive rats in GABAA receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol. Dis. 2008, 31, 169–187. [Google Scholar] [CrossRef] [PubMed]
- French, J.A. Refractory Epilepsy: Clinical Overview. Epilepsia 2007, 48, 3–7. [Google Scholar] [CrossRef]
- Volk, H.A.; Löscher, W. Multidrug resistance in epilepsy: Rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain 2005, 128, 1358–1368. [Google Scholar] [CrossRef]
- Brandt, C.; Bethmann, K.; Gastens, A.M.; Löscher, W. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol. Dis. 2006, 24, 202–211. [Google Scholar] [CrossRef]
- Glien, M.; Brandt, C.; Potschka, H.; Löscher, W. Effects of the novel antiepileptic drug levetiracetam on sponta-neous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 2002, 43, 350–357. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.T.; Choi, J.; Jung, K.H.; Yang, H.; Khalid, A.; Kim, J.M.; Park, K.I.; Shin, J.W.; Ban, J.J.; et al. Unique behavioral characteristics and microRNA signatures in a drug resistant epilepsy model. PLoS ONE 2014, 9, e85617. [Google Scholar] [CrossRef]
- Bohosova, J.; Vajcner, J.; Jabandziev, P.; Oslejskova, H.; Slaby, O.; Aulicka, S. MicroRNAs in the development of resistance to antiseizure drugs and their potential as biomarkers in pharmacoresistant epilepsy. Epilepsia 2021, 62, 2573–2588. [Google Scholar] [CrossRef]
- Fu, M.; Zhu, Y.; Zhang, J.; Wu, W.; Sun, Y.; Zhang, X.; Tao, J.; Li, Z. MicroRNA-221-3p Suppresses the Microglia Activation and Seizures by Inhibiting of HIF-1α in Valproic Acid-Resistant Epilepsy. Front. Pharmacol. 2021, 12, 714556. [Google Scholar] [CrossRef] [PubMed]
- Bankstahl, M.; Klein, S.; Römermann, K.; Löscher, W. Knockout of P-glycoprotein does not alter antiepileptic drug efficacy in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology 2016, 109, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, U.; Löscher, W. Lack of changes in seizure susceptibility during the estrous cycle in kindled rats. Epilepsy Res. 1992, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rattka, M.; Brandt, C.; Löscher, W. Do proconvulsants modify or halt epileptogenesis? Pentylenetetrazole is ineffective in two rat models of temporal lobe epilepsy. Eur. J. Neurosci. 2012, 36, 2505–2520. [Google Scholar] [CrossRef]
- Brandt, C.; Bankstahl, M.; Töllner, K.; Klee, R.; Löscher, W. The pilocarpine model of temporal lobe epilepsy: Marked intrastrain differences in female Sprague–Dawley rats and the effect of estrous cycle. Epilepsy Behav. 2016, 61, 141–152. [Google Scholar] [CrossRef]
- Wilcox, K.S.; West, P.J.; Metcalf, C.S. The Current Approach of the Epilepsy Therapy Screening Program Contract Site for Identifying Improved Therapies for the Treatment of Pharmacoresistant Seizures in Epilepsy. Neuropharmacology 2020, 166, 107811. [Google Scholar] [CrossRef]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Metcalf, C.S.; West, P.J.; Thomson, K.E.; Edwards, S.F.; Smith, M.D.; White, H.S.; Wilcox, K.S. Development and pharmacologic characterization of the rat 6 Hz model of partial seizures. Epilepsia 2017, 58, 1073–1084. [Google Scholar] [CrossRef]
- Brown, W.C.; Schiffman, D.O.; Swinyard, E.A.; Goodman, L.S. Comparative assay of antiepileptic drugs by “pychomotor” seizure test and minimal electroshock threshold test. J. Pharmacol. Exp. Ther. 1953, 107, 273–283. [Google Scholar]
- Guignet, M.; Campbell, A.; White, H.S. Cenobamate (XCOPRI®): Can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia 2020, 61, 2329–2339. [Google Scholar] [CrossRef]
- French, J.A. Cenobamate for focal seizures—A game changer? Nat. Rev. Neurol. 2020, 16, 133–134. [Google Scholar] [CrossRef]
- Klitgaard, H.; Matagne, A.; Gobert, J.; Wülfert, E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998, 353, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, K.; Kaminski, R.M. Genetic background of mice strongly influences treatment resistance in the 6 Hz seizure model. Epilepsia 2015, 56, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Ferland, R.J.; Ferraro, T.N. The relevance of inter- and intrastrain differences in mice and rats and their implications for models of seizures and epilepsy. Epilepsy Behav. 2017, 73, 214–235. [Google Scholar] [CrossRef]
- Blanco, M.M.; Dos, S.J., Jr.; Perez-Mendes, P.; Kohek, S.R.; Cavarsan, C.F.; Hummel, M.; Albuquerque, C.; Mello, L.E. Assessment of seizure susceptibility in pilocarpine epileptic and nonepileptic Wistar rats and of seizure reinduction with pentylenetetrazole and electroshock models. Epilepsia 2009, 50, 824–831. [Google Scholar] [CrossRef]
- Töllner, K.; Twele, F.; Löscher, W. Evaluation of the pentylenetetrazole seizure threshold test in epileptic mice as surrogate model for drug testing against pharmacoresistant seizures. Epilepsy Behav. 2016, 57, 95–104. [Google Scholar] [CrossRef]
- Bankstahl, M.; Bankstahl, J.P.; Löscher, W. Pilocarpine-induced epilepsy in mice alters seizure thresholds and the efficacy of antiepileptic drugs in the 6-Hertz psychomotor seizure model. Epilepsy Res. 2013, 107, 205–216. [Google Scholar] [CrossRef]
- Leclercq, K.; Kaminski, R.M. Status epilepticus induction has prolonged effects on the efficacy of antiepileptic drugs in the 6-Hz seizure model. Epilepsy Behav. 2015, 49, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hampel, P.; Römermann, K.; Gailus, B.; Johne, M.; Gericke, B.; Kaczmarek, E.; Löscher, W. Effects of the NKCC1 inhibitors bumetanide, azosemide, and torasemide alone or in combination with phenobarbital on seizure threshold in epileptic and nonepileptic mice. Neuropharmacology 2021, 185, 108449. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach? Neurochem. Res. 2017, 42, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, M.; Holsinger, D.; Ikeda-Douglas, C.; Cammisuli, S.; Ferbinteanu, J.; DeSouza, C.; DeSouza, S.; Fecteau, J.; Racine, R.J.; Milgram, N.W. Development of spontaneous seizures over extended electrical kindling. I. Electro-graphic, behavioral, and transfer kindling correlates. Brain Res. 1998, 793, 197–211. [Google Scholar] [PubMed]
- Postma, T.; Krupp, E.; Li, X.L.; Post, R.M.; Weiss, S.R. Lamotrigine Treatment During Amygdala-Kindled Seizure Development Fails to Inhibit Seizures and Diminishes Subsequent Anticonvulsant Efficacy. Epilepsia 2000, 41, 1514–1521. [Google Scholar] [CrossRef]
- Singh, E.; Pillai, K.K.; Mehndiratta, M. Characterization of a lamotrigine-resistant kindled model of epilepsy in mice: Evaluation of drug resistance mechanisms. Basic Clin. Pharmacol. Toxicol. 2014, 115, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.S.; Huff, J.; Thomson, K.E.; Johnson, K.; Edwards, S.F.; Wilcox, K.S. Evaluation of antiseizure drug efficacy and tolerability in the rat lamotrigine-resistant amygdala kindling model. Epilepsia Open 2019, 4, 452–463. [Google Scholar] [CrossRef]
- Löscher, W.; Schmidt, D. Experimental and Clinical Evidence for Loss of Effect (Tolerance) during Prolonged Treatment with Antiepileptic Drugs. Epilepsia 2006, 47, 1253–1284. [Google Scholar] [CrossRef]
- Koneval, Z.; Knox, K.M.; White, H.S.; Barker-Haliski, M. Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia 2018, 59, 1245–1256. [Google Scholar] [CrossRef]
- Matagne, A.; Klitgaard, H. Validation of corneally kindled mice: A sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998, 31, 59–71. [Google Scholar] [CrossRef]
- Rowley, N.M.; White, H.S. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy Res. 2010, 92, 163–169. [Google Scholar] [CrossRef]
- Potschka, H.; Löscher, W. Corneal kindling in mice: Behavioral and pharmacological differences to conventional kindling. Epilepsy Res. 1999, 37, 109–120. [Google Scholar] [CrossRef]
- Koneval, Z.; Knox, K.M.; Memon, A.; Zierath, D.K.; White, H.S.; Barker-Haliski, M. Antiseizure drug efficacy and tolerability in established and novel drug discovery seizure models in outbred vs inbred mice. Epilepsia 2020, 61, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Swinyard, E.A.; Wolf, H.H.; White, H.S.; Skeen, G.A.; Stark, L.G.; Albertson, T.; Pong, S.F.; Drust, E.G. Characterization of the anticonvulsant properties of F-721. Epilepsy Res. 1993, 15, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Isoherranen, N.; Woodhead, J.H.; White, H.S.; Bialer, M. Anticonvulsant Profile of Valrocemide (TV1901): A New Antiepileptic Drug. Epilepsia 2001, 42, 831–836. [Google Scholar] [CrossRef]
- Leclercq, K.; Matagne, A.; Kaminski, R. Low potency and limited efficacy of antiepileptic drugs in the mouse 6 Hz corneal kindling model. Epilepsy Res. 2014, 108, 675–683. [Google Scholar] [CrossRef]
- Leclercq, K.; Matagne, A.; Provins, L.; Klitgaard, H.; Kaminski, R.M. Pharmacological Profile of the Novel Antiepileptic Drug Candidate Padsevonil: Characterization in Rodent Seizure and Epilepsy Models. J. Pharmacol. Exp. Pharmacol. 2020, 372, 11–20. [Google Scholar] [CrossRef]
- Rademacher, M.; Toledo, M.; Van Paesschen, W.; Liow, K.K.; Milanov, I.G.; Esch, M.L.; Wang, N.; MacPherson, M.; Byrnes, W.J.; Minh, T.D.C.; et al. Efficacy and safety of adjunctive padsevonil in adults with drug-resistant focal epilepsy: Results from two double-blind, randomized, placebo-controlled trials. Epilepsia Open 2022, 7, 758–770. [Google Scholar] [CrossRef]
- Pitkänen, A.; Kharatishvili, I.; Karhunen, H.; Lukasiuk, K.; Immonen, R.; Nairismagi, J.; Grohn, O.; Nissinen, J. Epileptogenesis in experimental models. Epilepsia 2007, 48, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Kyyriäinen, J.; Andrade, P.; Pasanen, L.; Ndode-Ekane, X.E. Epilepsy After Traumatic Brain Injury. In Models of Seizures and Epilepsy, 2nd ed.; Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., Moshé, S.L., Eds.; Academic Press: London, UK, 2017; pp. 661–681. [Google Scholar] [CrossRef]
- Löscher, W.; Howe, C.L. Molecular Mechanisms in the Genesis of Seizures and Epilepsy Associated with Viral Infection. Front. Mol. Neurosci. 2022, 15. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Fairbanks, J.P.; Fender, J.S.; Born, D.E.; Doyle, D.L.; Miller, J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 2004, 127, 304–314. [Google Scholar] [CrossRef]
- Eastman, C.L.; Fender, J.S.; Temkin, N.R.; D’Ambrosio, R. Optimized methods for epilepsy therapy development using an etiologically realistic model of focal epilepsy in the rat. Exp. Neurol. 2015, 264, 150–162. [Google Scholar] [CrossRef]
- Eastman, C.L.; Verley, D.R.; Fender, J.S.; Temkin, N.R.; D’Ambrosio, R. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp. Neurol. 2010, 224, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.L.; Verley, D.R.; Fender, J.S.; Stewart, T.H.; Nov, E.; Curia, G.; D’Ambrosio, R. Antiepileptic and an-tiepileptogenic performance of carisbamate after head injury in the rat: Blind and randomized studies. J. Pharmacol. Exp. Ther. 2011, 336, 779–790. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Eastman, C.L.; Darvas, F.; Fender, J.S.; Verley, D.R.; Farin, F.M.; Wilkerson, H.W.; Temkin, N.R.; Miller, J.W.; Ojemann, J.; et al. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann. Neurol. 2013, 73, 199–209. [Google Scholar] [CrossRef]
- Eastman, C.L.; Fender, J.S.; Klein, P.; D’ambrosio, R. Therapeutic Effects of Time-Limited Treatment with Brivaracetam on Posttraumatic Epilepsy after Fluid Percussion Injury in the Rat. Experiment 2021, 379, 310–323. [Google Scholar] [CrossRef]
- Dudek, F.E.; Bertram, E.H. Counterpoint to “what is an epileptic seizure?” by D’Ambrosio and Miller. Epilepsy Curr. 2010, 10, 91–94. [Google Scholar] [CrossRef]
- Rodgers, K.M.; Dudek, F.E.; Barth, D.S. Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy. J. Neurosci. 2015, 35, 9194–9204. [Google Scholar] [CrossRef] [PubMed]
- Tatum, S.; Smith, Z.Z.; Taylor, J.A.; Poulsen, D.J.; Dudek, F.E.; Barth, D.S. Sensitivity of unilateral-versus bilateral-onset spike-wave discharges to ethosuximide and carbamazepine in the fluid percussion injury rat model of traumatic brain injury. J. Neurophysiol. 2021, 125, 2166–2177. [Google Scholar] [CrossRef]
- Reid, A.Y.; Bragin, A.; Giza, C.C.; Staba, R.J.; Engel, J. The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia 2016, 57, 1558–1567. [Google Scholar] [CrossRef]
- Smith, D.; Rau, T.; Poulsen, A.; MacWilliams, Z.; Patterson, D.; Kelly, W.; Poulsen, D. Convulsive seizures and EEG spikes after lateral fluid-percussion injury in the rat. Epilepsy Res. 2018, 147, 87–94. [Google Scholar] [CrossRef]
- Löscher, W. Genetic animal models of epilepsy. In Genetically Defined Animal Models of Neurobehavioral Dysfunctions; Driscoll, P., Ed.; Birkhäuser: Boston, MA, USA, 1992; pp. 111–135. [Google Scholar]
- White, H.S.; Löscher, W. Searching for the Ideal Antiepileptogenic Agent in Experimental Models: Single Treatment Versus Combinatorial Treatment Strategies. Neurotherapeutics 2014, 11, 373–384. [Google Scholar] [CrossRef]
- Jefferys, J.; Steinhäuser, C.; Bedner, P. Chemically-induced TLE models: Topical application. J. Neurosci. Methods 2016, 260, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C. Poststatus epilepticus models: Focal kainic acid. In Models of Seizures and Epilepsy, 2nd ed.; Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., Moshé, S.L., Eds.; Academic Press: London, UK, 2017; pp. 611–624. [Google Scholar]
- Fonnum, F.; Walaas, I. The effect of intrahippocampal kainic acid injections and surgical lesions on neuro-transmitters in hippocampus and septum. J. Neurochem. 1978, 31, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Zaczek, R.; Coyle, J.T. Microinjection of kainic acid into the rat hippocampus. Eur. J. Pharmacol. 1978, 50, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, E.; Riche, D.; Salle, G.L.G.L. Long-term effects of intrahippocampal kainic acid injection in rats: A method for inducing spontaneous recurrent seizures. Electroencephalogr. Clin. Neurophysiol. 1982, 53, 581–589. [Google Scholar] [CrossRef]
- Ferkany, J.W.; Slevin, J.T.; Zaczek, R.; Coyle, J.T. Failure of folic acid derivatives to mimic the actions of kainic acid in brain in vitro or in vivo. Neurobehav. Toxicol. Teratol. 1982, 4, 573–579. [Google Scholar]
- Bouilleret, V.; Ridoux, V.; Depaulis, A.; Marescaux, C.; Nehlig, A.; Lasalle, G.L. Recurrent seizures and hippo-campal sclerosis following intrahippocampal kainate injection in adult mice: Electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 1999, 89, 717–729. [Google Scholar] [CrossRef]
- Riban, V.; Bouilleret, V.; Pham-Lê, B.; Fritschy, J.-M.; Marescaux, C.; Depaulis, A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience 2002, 112, 101–111. [Google Scholar] [CrossRef]
- Twele, F.; Töllner, K.; Bankstahl, M.; Löscher, W. The effects of carbamazepine in the intrahippocampal kainate model of temporal lobe epilepsy depend on seizure definition and mouse strain. Epilepsia Open 2016, 1, 45–60. [Google Scholar] [CrossRef]
- Duveau, V.; Pouyatos, B.; Bressand, K.; Bouyssieres, C.; Chabrol, T.; Roche, Y.; Depaulis, A.; Roucard, C. Differential Effects of Antiepileptic Drugs on Focal Seizures in the Intrahippocampal Kainate Mouse Model of Mesial Temporal Lobe Epilepsy. CNS Neurosci. Ther. 2016, 22, 497–506. [Google Scholar] [CrossRef]
- Klee, R.; Brandt, C.; Töllner, K.; Löscher, W. Various modifications of the intrahippocampal kainate model of mesial temporal lobe epilepsy in rats fail to resolve the marked rat-to-mouse differences in type and frequency of spontaneous seizures in this model. Epilepsy Behav. 2017, 68, 129–140. [Google Scholar] [CrossRef]
- Rattka, M.; Brandt, C.; Löscher, W. The intrahippocampal kainate model of temporal lobe epilepsy revisited: Epileptogenesis, behavioral and cognitive alterations, pharmacological response, and hippoccampal damage in epileptic rats. Epilepsy Res. 2013, 103, 135–152. [Google Scholar] [CrossRef]
- Welzel, L.; Schidlitzki, A.; Twele, F.; Anjum, M.; Löscher, W. A face-to-face comparison of the intra-amygdala and intrahippocampal kainate mouse models of mesial temporal lobe epilepsy and their utility for testing novel therapies. Epilepsia 2019, 61, 157–170. [Google Scholar] [CrossRef]
- West, P.J.; Thomson, K.; Billingsley, P.; Pruess, T.; Rueda, C.; Saunders, G.W.; Smith, M.D.; Metcalf, C.S.; Wilcox, K.S. Spontaneous recurrent seizures in an intra-amygdala kainate microinjection model of temporal lobe epilepsy are differentially sensitive to antiseizure drugs. Exp. Neurol. 2022, 349, 113954. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Ferraro, D.J.; Williams, P.A.; Chapman, P.L.; Dudek, F.E. Use of Chronic Epilepsy Models in Antiepileptic Drug Discovery: The Effect of Topiramate on Spontaneous Motor Seizures in Rats with Kainate-induced Epilepsy. Epilepsia 2005, 46, 8–14. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Clark, S.; Dudek, F.E. Anticonvulsant Effects of Carbamazepine on Spontaneous Seizures in Rats with Kainate-induced Epilepsy: Comparison of Intraperitoneal Injections with Drug-in-food Protocols. Epilepsia 2007, 48, 2287–2295. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Dudek, F.E. Effect of carbamazepine on spontaneous recurrent seizures recorded from the dentate gyrus in rats with kainate-induced epilepsy. Epilepsia 2019, 60, 636–647. [Google Scholar] [CrossRef]
- Thomson, K.E.; White, H.S. A novel open-source drug-delivery system that allows for first-of-kind simulation of nonadherence to pharmacological interventions in animal disease models. J. Neurosci. Methods 2014, 238, 105–111. [Google Scholar] [CrossRef]
- Hill, A.C.; Rower, J.E.; White, H.S. The pharmacokinetics of oral carbamazepine in rats dosed using an auto-mated drug delivery system. Epilepsia 2019, 60, 1829–1837. [Google Scholar] [CrossRef]
- Hill, A.C.; Thomson, K.E.; Newell, T.G.; White, H.S. Correction of medication nonadherence results in better seizure outcomes than dose escalation in a novel preclinical epilepsy model of adherence. Epilepsia 2019, 60, 475–484. [Google Scholar] [CrossRef]
- Thomson, K.E.; Metcalf, C.S.; Newell, T.G.; Huff, J.; Edwards, S.F.; West, P.J.; Wilcox, K.S. Evaluation of subchronic administration of antiseizure drugs in spontaneously seizing rats. Epilepsia 2020, 61, 1301–1311. [Google Scholar] [CrossRef]
- Leite, J.P.; Cavalheiro, E.A. Effects of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res. 1995, 20, 93–104. [Google Scholar] [CrossRef]
- Chakir, A.; Fabene, P.; Ouazzani, R.; Bentivoglio, M. Drug resistance and hippocampal damage after delayed treatment of pilocarpine-induced epilepsy in the rat. Brain Res. Bull. 2006, 71, 127–138. [Google Scholar] [CrossRef]
- Cascino, G.D.; Brinkmann, B.H. Advances in the Surgical Management of Epilepsy: Drug-Resistant Focal Epilepsy in the Adult Patient. Neurol. Clin. 2021, 39, 181–196. [Google Scholar] [CrossRef]
- Timofeev, I. Pathophysiology of Neocortical Epileptic Seizures; Springer: London, UK, 2010; pp. 203–212. [Google Scholar] [CrossRef]
- Purpura, D.P.; Penry, J.K.; Tower, D.; Woodbury, D.M.; Walter, R. Experimental Models of Epilepsy—A Manual for the Laboratory Worker; Raven Press: New York, NY, USA, 1972. [Google Scholar]
- Fisher, R.S. Animal models of the epilepsies. Brain Res. Rev. 1989, 14, 245–278. [Google Scholar] [CrossRef] [PubMed]
- Chauvette, S.; Soltani, S.; Seigneur, J.; Timofeev, I. In vivo models of cortical acquired epilepsy. J. Neurosci. Methods 2016, 260, 185–201. [Google Scholar] [CrossRef]
- Pitkänen, A.; Buckmaster, P.S.; Galanopoulou, A.S.; Moshé, S.L. Models of Seizures and Epilepsy, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Walker, M.C.; Jefferys, J.G.R.; Wykes, R.C. Tetanus toxin. In Models of Seizures and Epilepsy, 2nd ed.; Pitkänen, A., Buckmaster, P.S., Galanopoulou, A.S., Moshé, S.L., Eds.; Academic Press: London, UK, 2017; pp. 589–598. [Google Scholar]
- Nilsen, K.E.; Walker, M.C.; Cock, H. Characterization of the Tetanus Toxin Model of Refractory Focal Neocortical Epilepsy in the Rat. Epilepsia 2005, 46, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, K.E.; Kelso, A.R.C.; Cock, H.R. Antiepileptic Effect of Gap-junction Blockers in a Rat Model of Refractory Focal Cortical Epilepsy. Epilepsia 2006, 47, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Wykes, R.C.; Heeroma, J.H.; Mantoan, L.; Zheng, K.; MacDonald, D.C.; Deisseroth, K.; Hashemi, K.S.; Walker, M.C.; Schorge, S.; Kullmann, D.M. Optogenetic and potassium channel gene therapy in a rodent model of focal neo-cortical epilepsy. Sci. Transl. Med. 2012, 4, 161ra152. [Google Scholar] [CrossRef]
- Rassner, M.P.; Hebel, J.M.; Altenmüller, D.-M.; Volz, S.; Herrmann, L.S.; Feuerstein, T.J.; Freiman, T.M. Reduction of epileptiform activity through local valproate-implants in a rat neocortical epilepsy model. Seizure 2015, 30, 6–13. [Google Scholar] [CrossRef]
- De Sarro, G.; Russo, E.; Citraro, R.; Meldrum, B.S. Genetically epilepsy-prone rats (GEPRs) and DBA/2 mice: Two animal models of audiogenic reflex epilepsy for the evaluation of new generation AEDs. Epilepsy Behav. 2017, 71, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lerche, H.; Petrou, S. Genetic Animal Models of Epileptic Seizures. In Atlas of Epilepsies; Panayiotopoulos, C.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 295–308. [Google Scholar] [CrossRef]
- Gu, B.; Dalton, K.A. Models and detection of spontaneous recurrent seizures in laboratory rodents. Zool. Res. 2017, 38, 171–179. [Google Scholar] [PubMed]
- Marshall, G.F.; Gonzalez-Sulser, A.; Abbott, C.M. Modelling epilepsy in the mouse: Challenges and solutions. Dis. Model. Mech. 2021, 14, dmm047449. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.K.; Helbig, I.; Metcalf, C.S.; Lubbers, L.S.; Isom, L.L.; Demarest, S.; Goldberg, E.M.; George, A.L., Jr.; Lerche, H.; Weckhuysen, S.; et al. Precision medicine for genetic epilepsy on the horizon: Recent advances, present challenges, and suggestions for continued progress. Epilepsia 2022, 63, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Frankel, W.N. Overlaps, gaps, and complexities of mouse models of Developmental and Epileptic Encephalopathy. Neurobiol. Dis. 2020, 148, 105220. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical Animal Models for Dravet Syndrome: Seizure Phenotypes, Comorbidities and Drug Screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef]
- Baraban, S.C.; Löscher, W. What new modeling approaches will help us identify promising drug treatments? Adv. Exp. Med. Biol. 2014, 813, 283–294. [Google Scholar]
- Chilcott, E.; Díaz, J.A.; Bertram, C.; Berti, M.; Karda, R. Genetic therapeutic advancements for Dravet Syndrome. Epilepsy Behav. 2022, 132, 108741. [Google Scholar] [CrossRef]
- National Guideline Alliance (UK). Effectiveness of Antiseizure Therapies in the Treatment of Dravet Syndrome: Epilepsies in Children, Young People and Adults; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [Google Scholar]
- Vasquez, A.; Buraniqi, E.; Wirrell, E.C. New and emerging pharmacologic treatments for developmental and epileptic encephalopathies. Curr. Opin. Neurol. 2022, 35, 145–154. [Google Scholar] [CrossRef]
- Oakley, J.C.; Cho, A.R.; Cheah, C.S.; Scheuer, T.; Catterall, W.A. Synergistic GABA-Enhancing Therapy against Seizures in a Mouse Model of Dravet Syndrome. Experiment 2013, 345, 215–224. [Google Scholar] [CrossRef]
- Hawkins, N.A.; Anderson, L.L.; Gertler, T.S.; Laux, L.; George, A.L., Jr.; Kearney, J.A. Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Ann. Clin. Transl. Neurol. 2017, 4, 326–339. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef]
- Thornton, C.; Dickson, K.E.; Carty, D.R.; Ashpole, N.M.; Willett, K.L. Cannabis constituents reduce seizure behavior in chemically-induced and scn1a-mutant zebrafish. Epilepsy Behav. 2020, 110, 107152. [Google Scholar] [CrossRef]
- Pernici, C.D.; Mensah, J.A.; Dahle, E.J.; Johnson, K.J.; Handy, L.; Buxton, L.; Smith, M.D.; West, P.J.; Metcalf, C.S.; Wilcox, K.S. Development of an antiseizure drug screening platform for Dravet syndrome at the NINDS contract site for the Epilepsy Therapy Screening Program. Epilepsia 2021, 62, 1665–1676. [Google Scholar] [CrossRef]
- Grone, B.P.; Baraban, S.C. Animal models in epilepsy research: Legacies and new directions. Nat. Neurosci. 2015, 18, 339–343. [Google Scholar] [CrossRef]
- Crouzier, L.; Richard, E.M.; Sourbron, J.; Lagae, L.; Maurice, T.; Delprat, B. Use of Zebrafish Models to Boost Re-search in Rare Genetic Diseases. Int. J. Mol. Sci. 2021, 22, 13356. [Google Scholar] [CrossRef]
- Vela, J.M.; Maldonado, R.; Hamon, M. In Vivo Models for Drug Discovery; Wiley-VCH: Weinheim, Germany, 2014; Volume 62. [Google Scholar]
- Baraban, S.C.; Dinday, M.T.; Hortopan, G.A. Drug screening and transcriptomic analysis in Scn1a zebrafish mutants identifies potential lead compound for Dravet Syndrome. Nat. Comm. 2013, 4, 2410. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Knupp, K.; Hong, S.; Lee, L.P.; Baraban, S.C. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 2017, 140, 669–683. [Google Scholar] [CrossRef]
- Perucca, E.; Brodie, M.J.; Kwan, P.; Tomson, T. 30 years of second-generation antiseizure medications: Impact and future perspectives. Lancet Neurol. 2020, 19, 544–556. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Combination therapy in epilepsy: When and what to use. Drugs 2006, 66, 1817–1829. [Google Scholar] [CrossRef]
- Brodie, M.J.; Sills, G.J. Combining antiepileptic drugs—Rational polytherapy? Seizure 2011, 20, 369–375. [Google Scholar] [CrossRef]
- Löscher, W. Single-Target Versus Multi-Target Drugs Versus Combinations of Drugs with Multiple Targets: Pre-clinical and Clinical Evidence for the Treatment or Prevention of Epilepsy. Front. Pharmacol. 2021, 12, 730257. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef]
- Brodie, M.J.; Yuen, A.W.C. Lamotrigine substitution study: Evidence for synergism with sodium valproate? Epilepsy Res. 1997, 26, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Deckers, C.L.P.; Czuczwar, S.J.; Hekster, Y.A.; Kewser, A.; Kubova, H.; Meinardi, H.; Patsalos, P.N.; Renier, W.O.; Van Rijn, C.M. Selection of Antiepileptic Drug Polytherapy Based on Mechanisms of Action: The Evidence Reviewed. Epilepsia 2000, 41, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Lattanzi, S.; Brigo, F.; Zaccara, G. Pharmacodynamic interactions of antiepileptic drugs: From bench to clinical practice. Epilepsy Behav. 2020, 104, 106939. [Google Scholar] [CrossRef]
- Czuczwar, S.J.; Kaplanski, J.; Swiderska-Dziewit, G.; Gergont, A.; Kroczka, S.; Kaciński, M. Pharmacodynamic interactions between antiepileptic drugs: Preclinical data based on isobolography. Expert Opin. Drug Metab. Toxicol. 2009, 5, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, B.; Miziak, B.; Czuczwar, P.; Wierzchowska-Cioch, E.; Pluta, R.; Czuczwar, S.J. A viewpoint on rational and irrational fixed-drug combinations. Expert. Rev. Clin. Pharmacol. 2018, 11, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Panasiuk, A.; Zagaja, M.; Karwan, S.; Bojar, H.; Plewa, Z.; Florek-Łuszczki, M. Polygonogram and isobolographic analysis of interactions between various novel antiepileptic drugs in the 6-Hz corneal stimulation-induced seizure model in mice. PLoS ONE 2020, 15, e0234070. [Google Scholar] [CrossRef]
- Wojda, E.; Wlaź, A.; Patsalos, P.N.; Luszczki, J.J. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6Hz psychomotor seizure model. Epilepsy Res. 2009, 86, 163–174. [Google Scholar] [CrossRef]
- Waldman, S.A. Does potency predict clinical efficacy? Illustration through an antihistamine model. Ann. Allergy Asthma Immunol. 2002, 89, 7–12. [Google Scholar] [CrossRef]
- Rundfeldt, C.; Löscher, W. The Pharmacology of Imepitoin: The First Partial Benzodiazepine Receptor Agonist Developed for the Treatment of Epilepsy. CNS Drugs 2013, 28, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Schuck, E.; Bohnert, T.; Chakravarty, A.; Damian-Iordache, V.; Gibson, C.; Hsu, C.P.; Heimbach, T.; Krishnatry, A.S.; Liederer, B.M.; Lin, J.; et al. Preclinical pharmacokinetic/pharmacodynamic modeling and simulation in the pharmaceutical industry: An IQ consortium survey examining the current landscape. AAPS J. 2015, 17, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Sakei, C.; Iwano, S.; Yamazaki, Y.; Ando, A.; Nakane, F.; Kouno, M.; Yamazaki, H.; Miyamoto, Y. Species Differences in the Pharmacokinetic Parameters of Cytochrome P450 Probe Substrates between Experimental Animals, such as Mice, Rats, Dogs, Monkeys, and Microminipigs, and Humans. J. Drug Metab. Toxicol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Brodie, B.B.; Reid, W.D. Is man a unique animal in response to drugs? Am. J. Pharm. Sci. Support Public Health 1969, 141, 21–27. [Google Scholar]
- Woodbury, D.M. Experimental models of status epilepticus and mechanisms of drug action. In Advances in Neurology, Vol. 34: Status Epilepticus; Delgado-Escueta, A.V., Wasterlain, C.G., Treiman, D.M., Eds.; Raven Press: New York, NY, USA, 1983; pp. 149–160. [Google Scholar]
- Nau, H.; Löscher, W. Valproic acid: Brain and plasma levels of the drug and its metabolites, anticonvulsant effects and GABA metabolism in the mouse. J. Pharmacol. Exp. Ther. 1982, 220, 654–659. [Google Scholar] [PubMed]
- Frey, H.-H.; Löscher, W. Anticonvulsant potency of unmetabolized diazepam. Pharmacology 1982, 25, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.; Abernethy, D.R.; Greenblatt, D.J.; Shader, R.I. The pharmacokinetics of diazepam and desmethyldiazepam in rat brain and plasma. Psychopharmacology 1986, 88, 267–270. [Google Scholar] [CrossRef]
- Treiman, D.M. Pharmacokinetics and clinical use of benzodiazepines in the management of status epilepticus. Epilepsia 1989, 30 (Suppl. S2), S4–S10. [Google Scholar] [CrossRef]
- Marcucci, F.; Guaitani, A.; Kvetina, J.; Mussini, E.; Garattini, S. Species difference in diazepam metabolism and anticonvulsant effect. Eur. J Pharmacol. 1968, 4, 467–470. [Google Scholar] [CrossRef]
- Mensah, J.A.; Johnson, K.; Reilly, C.A.; Wilcox, K.S.; Rower, J.E.; Metcalf, C.S. Evaluating the efficacy of prototype antiseizure drugs using a preclinical pharmacokinetic approach. Epilepsia 2022, 63, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Schmidt, D. New horizons in the development of antiepileptic drugs: The search for new targets. Epilepsy Res. 2004, 60, 77–159. [Google Scholar]
- Riban, V.; Fitzsimons, H.L.; During, M.J. Gene therapy in epilepsy. Epilepsia 2009, 50, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Carvill, G.L.; Dulla, C.G.; Lowenstein, D.H.; Brooks-Kayal, A.R. The path from scientific discovery to cures for epilepsy. Neuropharmacology 2010, 167, 107702. [Google Scholar] [CrossRef]
- Lybrand, Z.R.; Goswami, S.; Hsieh, J. Stem cells: A path towards improved epilepsy therapies. Neuropharmacology 2020, 168, 107781. [Google Scholar] [CrossRef]
- Lange, J.; Zhou, H.; McTague, A. Cerebral Organoids and Antisense Oligonucleotide Therapeutics: Challenges and Opportunities. Front. Mol. Neurosci. 2022, 15, 941528. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; O’Neill, N.; Maffei, B.; Zourray, C.; Almacellas-Barbanoj, A.; Carpenter, J.C.; Jones, S.P.; Leite, M.; Turner, T.J.; Moreira, F.C.; et al. On-demand cell-autonomous gene therapy for brain circuit disorders. Science 2022, 378, 523–532. [Google Scholar] [CrossRef]
- Staley, K. Gene therapy for epilepsy. Science 2022, 378, 471–472. [Google Scholar] [CrossRef]
- Walker, M.C.; Kullmann, D.M. Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology 2020, 168, 107751. [Google Scholar] [CrossRef]
- Löscher, W. Dogs as a natural animal model of epilepsy. Front. Vet. Sci. 2022, 9, 928009. [Google Scholar] [CrossRef]
- Krauss, G.L.; Klein, P.; Brandt, C.; Lee, S.K.; Milanov, I.; Milovanovic, M.; Steinhoff, B.J.; Kamin, M. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: A multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020, 19, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.R.; Klein, P.; Aboumatar, S.; Gelfand, M.; Halford, J.J.; Krauss, G.L.; Rosenfeld, W.E.; Vossler, D.G.; Wechsler, R.; Borchert, L.; et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia 2020, 61, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; French, J.A.; Kowalski, J.; Krauss, G.L.; Lee, S.K.; Maciejowski, M.; Rosenfeld, W.E.; Sperling, M.R.; Mizne, S.; Kamin, M. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology 2020, 94, e2311–e2322. [Google Scholar] [CrossRef]
- Cereghino, J.J.; Biton, V.; Abou-Khalil, B.; Dreifuss, F.; Gauer, L.J.; Leppik, I. Levetiracetam for partial seizures: Results of a double-blind, randomized clinical trial. Neurology 2000, 55, 236–242. [Google Scholar] [CrossRef]
- Grant, R.; Shorvon, S. Efficacy and tolerability of 1000–4000 mg per day of levetiracetam as add-on therapy in patients with refractory epilepsy. Epilepsy Res. 2000, 42, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients with Newly Diagnosed Epilepsy Treated with Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]




| Medication | Elimination Half-Life (h) | Comments | ||
|---|---|---|---|---|
| Humans | Rats | Mice | ||
| Acetazolamide | 10–15 | 0.33 | ? | |
| Brivaracetam | 7–8 | 2.8 | ? | |
| Cannabidiol | 18–32 | 7.8 | 4.7 | |
| Carbamazepine | 25–50 | 1.2–3.5 | 3.4 | Active metabolite = carbamazepine-10,11-epoxide; reduction in half-life during chronic treatment (autoinduction) |
| Cenobamate | 50–60 | 2.9 | ? | |
| Clobazam | 10–30 | 1 | 0.25 | Active metabolite = norclobazam |
| Clonazepam | 17–56 | ? | 2.1 | |
| Diazepam | 30–56 (nordazepam 36–200) | 0.88 (nordazepam 1.1) | 0.67 (nordazepam > 4 h) | Active metabolites = nordazepam (main metabolite), oxazepam, and temazepam |
| Eslicarbazepine acetate | 10–20 | ? | 5.2 | Half-lives refer to active metabolite = (S)-licarbazepine (eslicarbazepine) |
| Ethosuximide | 40–60 | 10–16 | ? | |
| Everolimus | ~30 | 20 | 4.3 | Long persistence in the brain |
| Felbamate | 16–22 | 2–17 | ? | In rodents, non-linear kinetics (half-life increases with increasing doses) |
| Fenfluramine | 13–30 | 2.6 | 4.3 | Active metabolite = norfenfluramine |
| Gabapentin | 5–9 | 2–3 | ? | |
| Lacosamide | 13 | 3 | ? | |
| Lamotrigine | 15–35 | 12–30 | ||
| Levetiracetam | 6–8 | 2–3 | 1.5 | |
| Oxcarbazepine | 8–15 | 0.7–4 | 6.8 | Half-lives refer to active metabolite = (S)-licarbazepine (eslicarbazepine) |
| Perampanel | 70 | 2 | ? | |
| Phenobarbital | 70–140 | 9–20 | 4–7.5 | Reduction in half-life during chronic treatment (autoinduction) |
| Phenytoin | 15–20 | ~2 | 5–16 | Non-linear kinetics (half-life increases with increasing doses); autoinduction |
| Pregabalin | 5–7 | ~2 | ~2 | |
| Primidone | 6–12 | 5 | 2.2 | Active metabolite = phenobarbital; autoinduction |
| Retigabine (ezogabine) | 6–8 | ~2 | ? | |
| Rufinamide | 6–10 | ~8 | ? | |
| Stiripentol | 4.5–13 | 13 | ? | |
| Sulthiame | 2–16 | ? | ? | |
| Tiagabine | 5–9 | 1 | ? | |
| Topiramate | 20–30 | 2.5 | ? | |
| Valproate | 8–15 | ~1.5 | 0.8 | In rodents, non-linear kinetics (half-life increases with increasing doses) |
| Vigabatrin | 5–8 | ~1 | ? | Duration of action independent of half-life because of irreversible inhibition of GABA degradation |
| Zonisamide | 50–70 | 8 | ? | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löscher, W.; White, H.S. Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells 2023, 12, 1233. https://doi.org/10.3390/cells12091233
Löscher W, White HS. Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells. 2023; 12(9):1233. https://doi.org/10.3390/cells12091233
Chicago/Turabian StyleLöscher, Wolfgang, and H. Steve White. 2023. "Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments" Cells 12, no. 9: 1233. https://doi.org/10.3390/cells12091233
APA StyleLöscher, W., & White, H. S. (2023). Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells, 12(9), 1233. https://doi.org/10.3390/cells12091233









