New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants
Abstract
1. Introduction
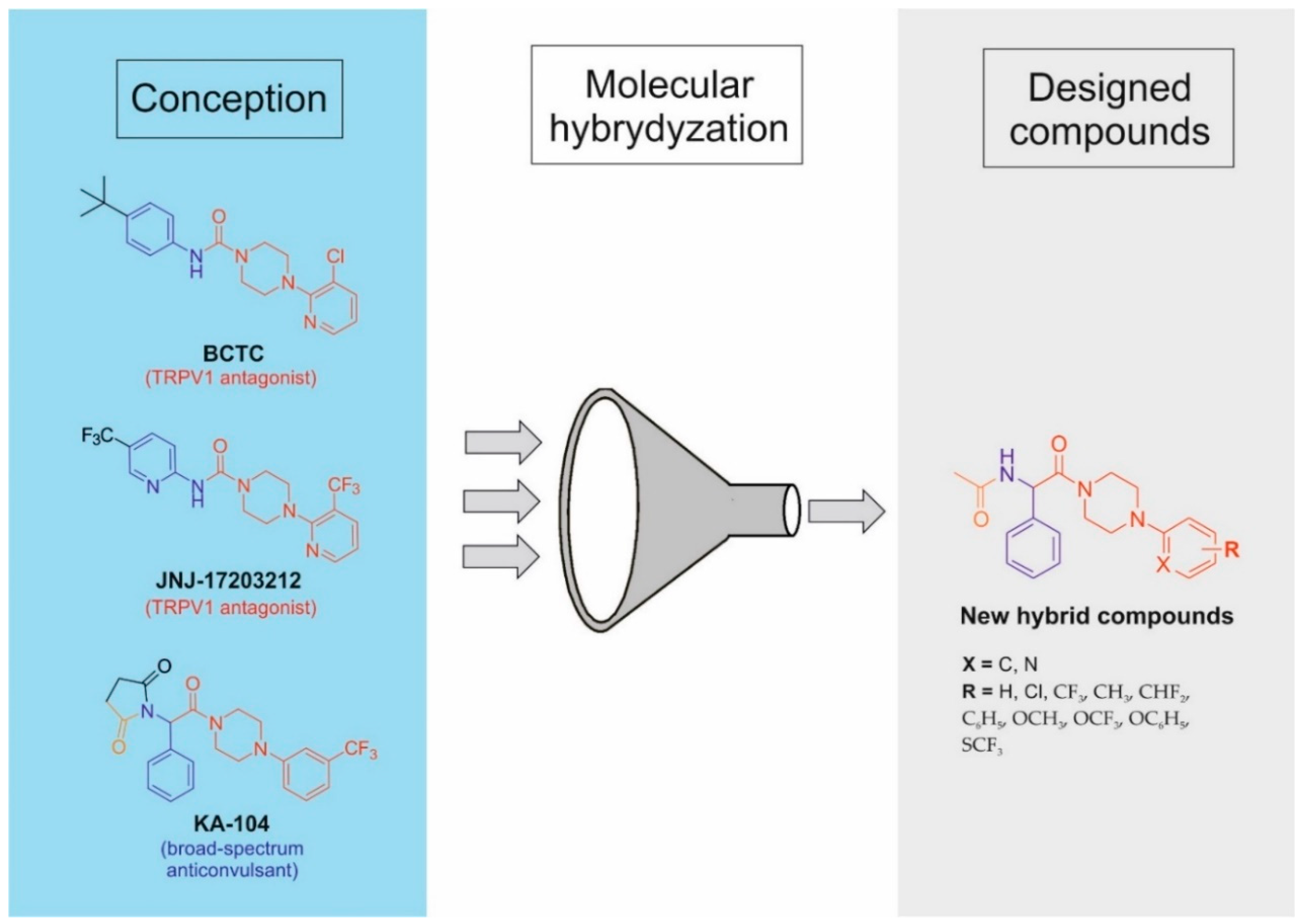
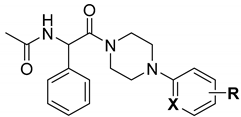
2. Materials and Methods
2.1. Chemistry
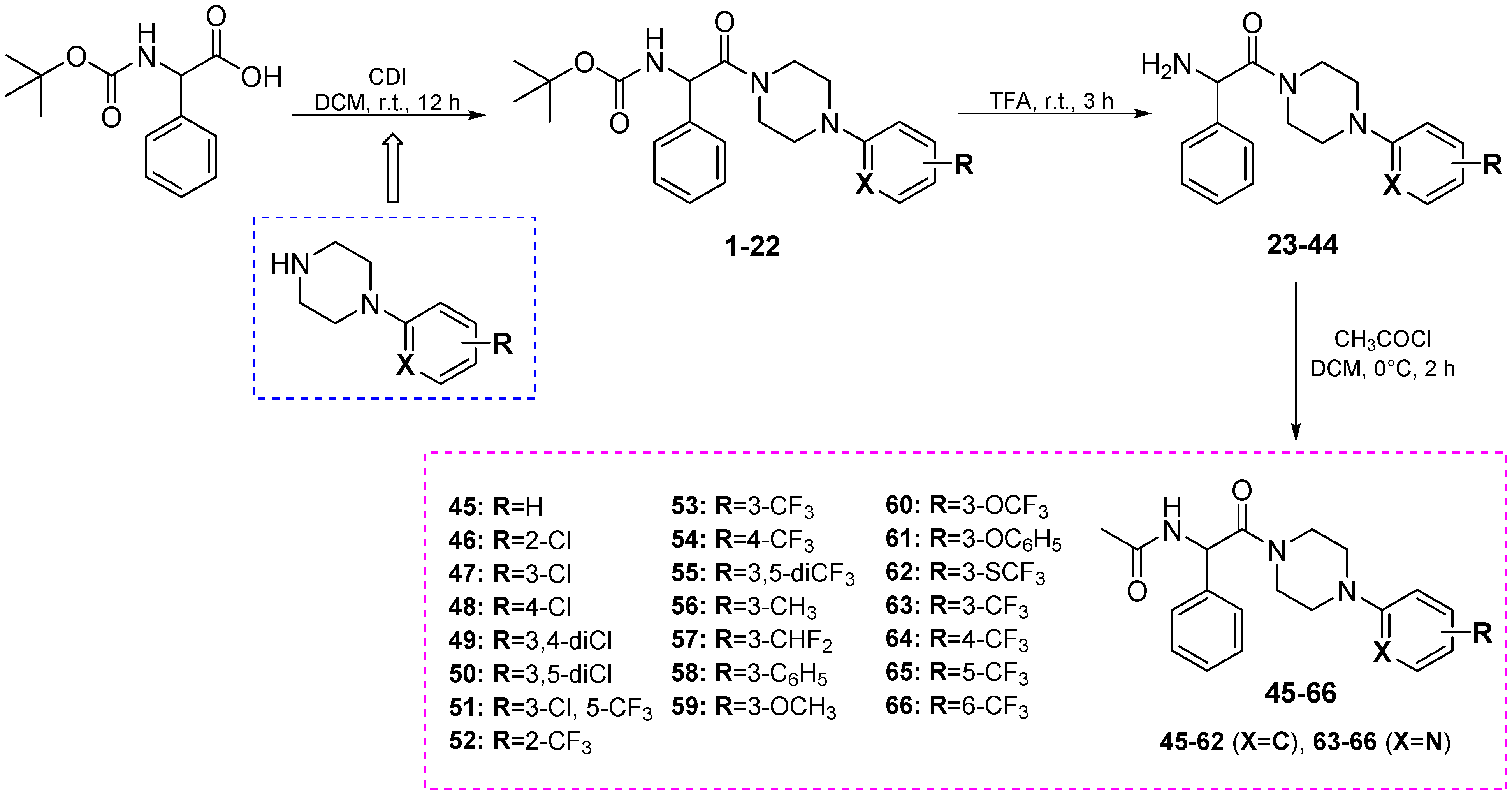
2.1.1. General Method for the Preparation of Intermediates A1–A12
2.1.2. General Method for the Preparation of Starting Amines A13–24
2.1.3. General Method for the Preparation of Intermediates 1–22
2.1.4. General Method for the Preparation of Intermediates 23–44
2.1.5. General Method for the Preparation of Final Compounds 45–66
2.2. In Silico Studies
2.3. In Vivo Studies
2.3.1. Animals
2.3.2. Anticonvulsant Activity and Acute Neurotoxicity
2.3.3. Timed ivPTZ Seizure Threshold and Grip Strength Tests
2.3.4. Capsaicin-Induced Hypothermia Model in Mice
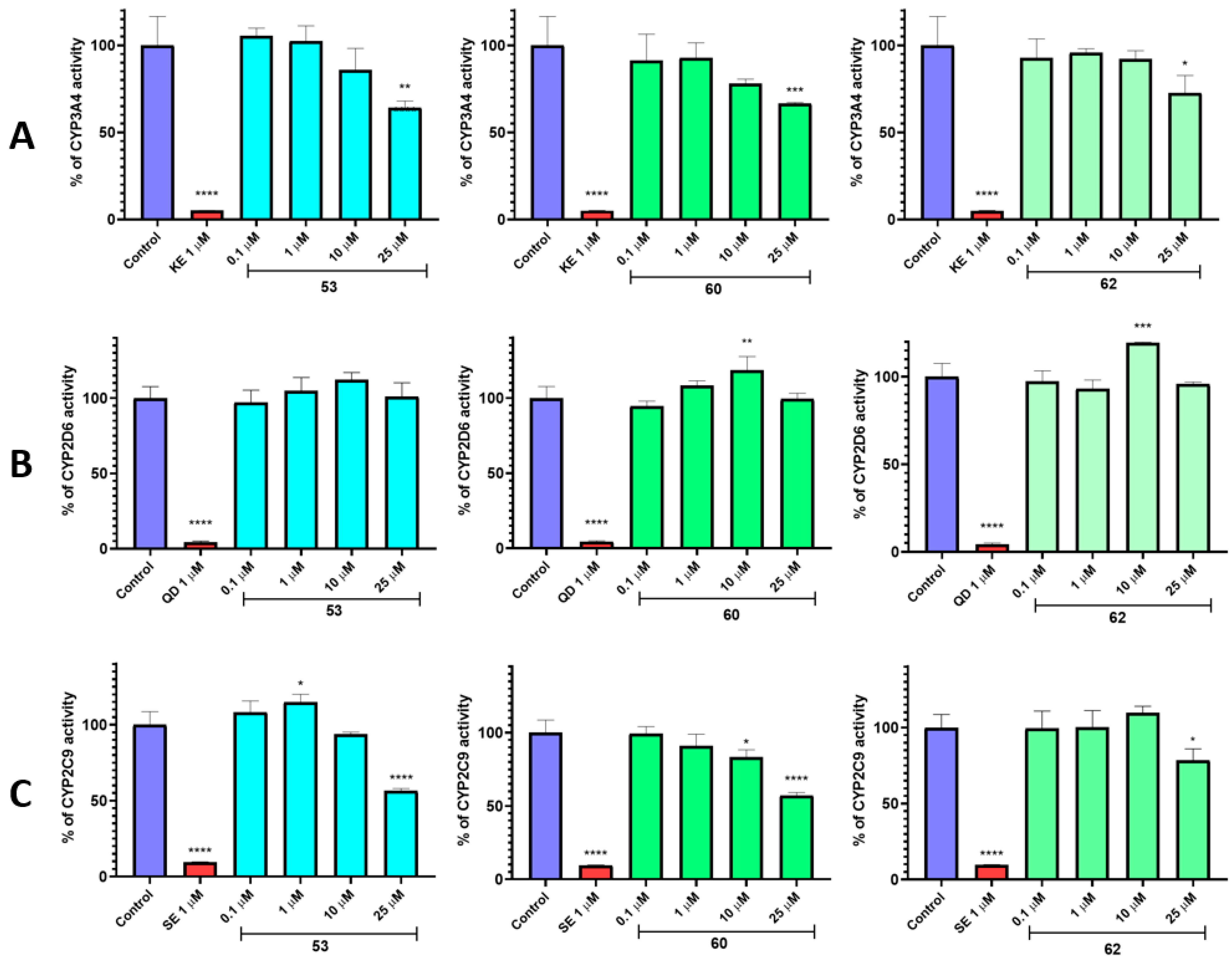
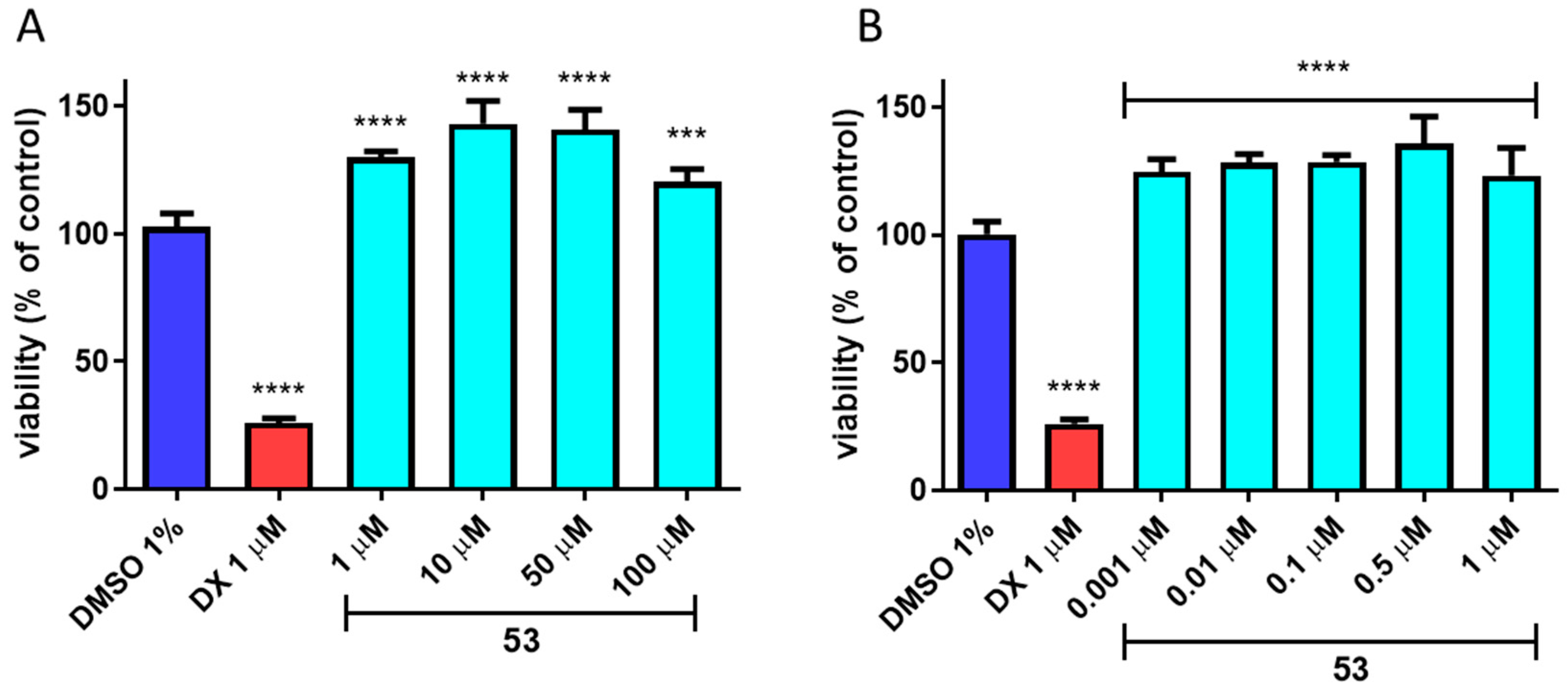
2.4. In Vitro ADME-Tox Studies
2.5. In Vitro Electrophysiological Studies
2.6. Binding/Functional Studies
3. Results and Discussion
3.1. In Silico Studies
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmpd | X | R | Lipinski Rule | Violations of Rules | Veber Rule | CNS MPO e | ||||
| MW ≤ 500 | Log P ≤ 5 | NHD a ≤ 5 | NHA b ≤ 10 | NBR c ≤ 10 | TPSA d ≤ 140 Å2 | |||||
| 45 | C | H | 337.42 | 2.04 | 1 | 2 | 0 | 6 | 52.65 | 5.8 |
| 46 | C | 2-Cl | 371.86 | 2.60 | 1 | 2 | 0 | 6 | 52.65 | 5.5 |
| 47 | C | 3-Cl | 371.86 | 2.56 | 1 | 2 | 0 | 6 | 52.65 | 5.5 |
| 48 | C | 4-Cl | 371.86 | 2.56 | 1 | 2 | 0 | 6 | 52.65 | 5.5 |
| 49 | C | 3,4-diCl | 406.31 | 3.05 | 1 | 2 | 0 | 6 | 52.65 | 4.8 |
| 50 | C | 3,5-diCl | 406.31 | 3.08 | 1 | 2 | 0 | 6 | 52.65 | 4.8 |
| 51 | C | 3-Cl,5- CF3 | 439.86 | 3.64 | 1 | 5 | 0 | 7 | 52.65 | 4.3 |
| 52 | C | 2-CF3 | 405.31 | 3.08 | 1 | 5 | 0 | 7 | 52.65 | 5.1 |
| 53 | C | 3-CF3 | 405.31 | 3.10 | 1 | 5 | 0 | 7 | 52.65 | 5.1 |
| 54 | C | 4-CF3 | 405.31 | 3.06 | 1 | 5 | 0 | 7 | 52.65 | 5.1 |
| 55 | C | 3,5-diCF3 | 473.41 | 4.10 | 1 | 8 | 0 | 8 | 52.65 | 3.8 |
| 56 | C | 3-CH3 | 351.44 | 2.36 | 1 | 2 | 0 | 6 | 52.65 | 5.6 |
| 57 | C | 3-CHF2 | 387.43 | 2.80 | 1 | 4 | 0 | 7 | 52.65 | 5.5 |
| 58 | C | 3-C6H5 | 413.51 | 3.35 | 1 | 2 | 0 | 7 | 52.65 | 4.3 |
| 59 | C | 3-OCH3 | 367.44 | 2.00 | 1 | 3 | 0 | 7 | 61.88 | 5.8 |
| 60 | C | 3-OCF3 | 421.41 | 2.91 | 1 | 6 | 0 | 8 | 61.88 | 4.5 |
| 61 | C | 3-OC6H5 | 429.51 | 3.18 | 1 | 3 | 0 | 8 | 61.88 | 4.4 |
| 62 | C | 3-SCF3 | 437.48 | 3.47 | 1 | 5 | 0 | 8 | 77.95 | 3.8 |
| 63 | N | 3-CF3 | 406.40 | 2.58 | 1 | 6 | 0 | 7 | 65.54 | 5.4 |
| 64 | N | 4-CF3 | 406.40 | 2.51 | 1 | 6 | 0 | 7 | 65.54 | 5.4 |
| 65 | N | 5-CF3 | 406.40 | 2.52 | 1 | 6 | 0 | 7 | 65.54 | 5.4 |
| 66 | N | 6-CF3 | 406.40 | 2.54 | 1 | 6 | 0 | 7 | 65.54 | 5.4 |
3.2. Chemistry

3.3. Anticonvulsant Activity
| Cmpd | PT (h) a | ED50 MES (mg/kg) | ED50 6 Hz (32 mA) (mg/kg) | TD50 (mg/kg) | PI (TD50/ED50) |
|---|---|---|---|---|---|
| 45 | 0.5 | 125.2 (105.3−148.9) | 81.6 (61.8−107.8) | 268.2 (242.0−297.1) * | 2.1 (MES) 3.3 (6 Hz) |
| 47 | 0.5 | >150 | 88.4 (73.2−106.8) | >500 * | >5.6 (6 Hz) |
| 48 | 0.5 | >150 | 60.1 (44.9−80.6) | 216.9 * (179.6−261.9) | 3.6 (6 Hz) |
| 53 | 0.5 | 89.7 (71.4−112.8) | 29.9 (20.1−44.4) | 179.7 * (161.0−200.5) | 2.0 (MES) 6.0 (6 Hz, 32 mA) |
| 60 | 0.5 | 73.6 (63.6−85.2) | 24.6 (12.2−49.5) | 166.8 * (109.6−253.8) | 2.3 (MES) 6.8 (6 Hz) |
| 62 | 0.5 | 76.1 (61.5−94.3) | 33.2 (21.2−52.0) | 156.2 * (137.7−177.1) | 2.1 (MES) 4.7 (6 Hz) |
| 65 | 0.5 | >100 | 64.9 (50.1−84.1) | 177.8 * (166.8−190.4) | 2.7 (6 Hz) |
| KA-104 b | 0.5 | 23.7 (18.4−31.2) | 22.4 (17.4−28.8) | 195.7 ** (132.7−288.6) | 8.2 (MES), 8.7 (6 Hz) |
| CBD c | 1.0 | 80.0 (65.5−96.0) | 144.0 (102.0−194.0) | 272.0 ** (241.0−303.0) | 3.4 (MES) 1.9 (6 Hz) |
| LEV d | 1.0 | >500 | 15.7 (11.2−18.4) | >500 ** | >31.8 (6 Hz) |
| LCS d | 0.5 | 9.2 (8.5−10.0) | 5.3 (3.5−7.8) | 46.2 ** (44.5−48.0) | 5.0 (MES) 8.8 (6 Hz) |
| VPA d | 0.5 | 252.7 (220.1−290.2) | 130.6 (117.6−145.2) | 430.7 ** (407.9−454.9) | 1.7 (MES) 3.3 (6 Hz) |
3.4. Effect on the Seizure Threshold in the ivPTZ Test in Mice

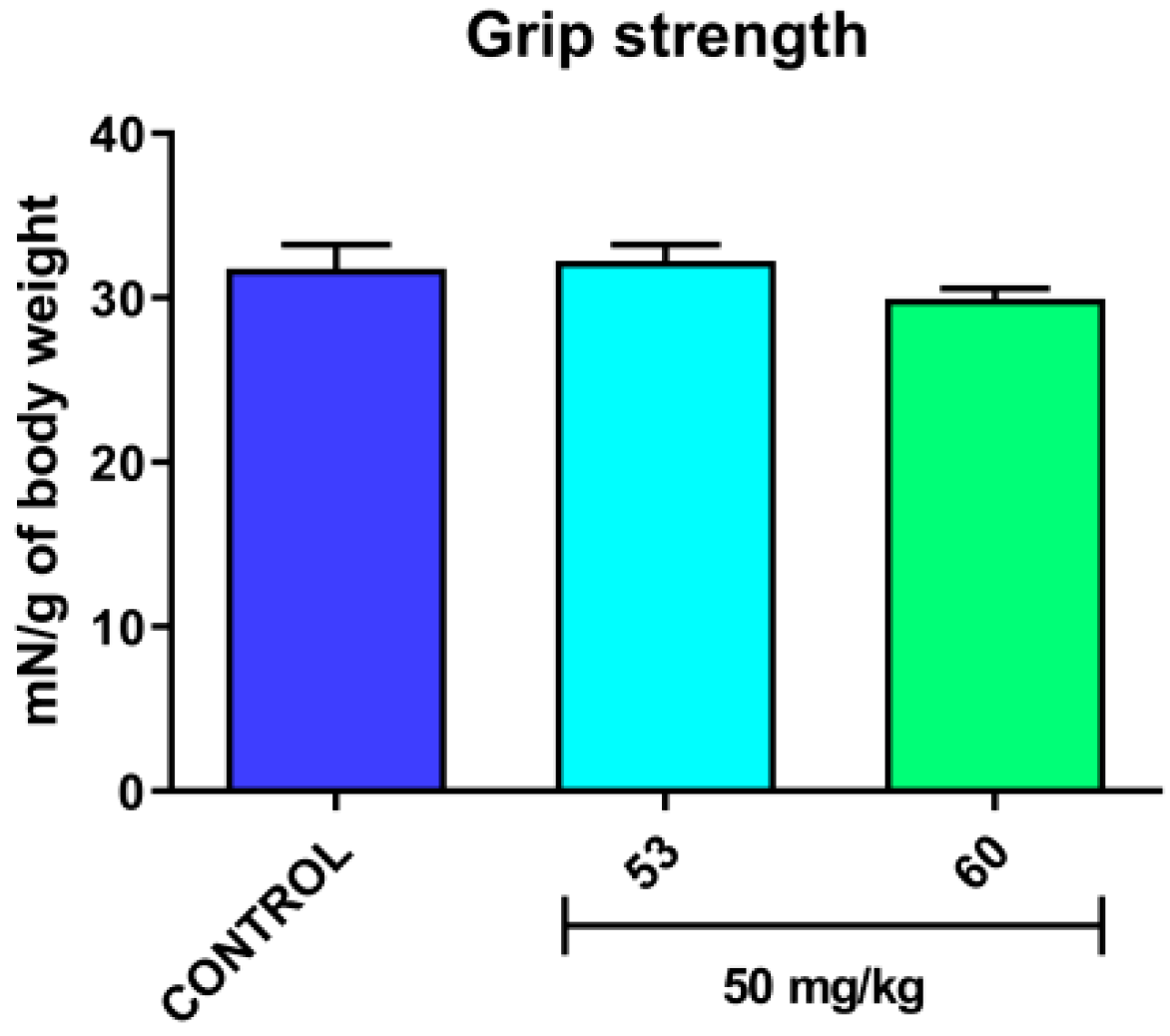
3.5. Acute Effect on the Neuromuscular Strength in Mice
3.6. Effect on the Capsaicin-Induced Hypothermia in Mice

3.7. In Vitro Radioligand Binding Studies and Functional Assays
| Binding Studies | Source | % Inhibition of Control Specific Binding (Concentration [µM]) a | |||
|---|---|---|---|---|---|
| 53 | 60 | 62 | CBD | ||
| Na+ channel (site 2) | Rat cerebral cortex | 75.5 (100) 17.4 (10) | 37.0 (10) | 57.0 (10) | 94.8 (100) |
| Calcium Cav1.2 channels (dihydropyridine site antagonist radioligand) | Human recombinant HEK-293 cell | 4.0 (10) | 7.0 (10) | 12.0 (10) | 58.0 (100) |
| Functional studies | Source | % Inhibition of control agonist response (concentration [µM]) a | |||
| 53 | 60 | 62 | CDB | ||
| Cav1.2 (h) calcium ion channel cell-based antagonist calcium flux assay | Human recombinant HEK-293 cell | 55.0 (10) | 31.0 (10) | 65.0 (10) | NT |
| TRPV1 (VR1) (h) (antagonist effect) | Human recombinant CHO cells | 128.5 (100) IC50 = 13 μM, KB = 1.7 μM | 109.7 (100) IC50 = 11 μM, KB = 1.5 μM | 135.1 (100) IC50 = 10 μM, KB = 1.4 μM | 48.0 (100) |
| Binding Studies | Source | % Inhibition of Control Specific Binding (Concentration [µM]) a |
|---|---|---|
| NMDA (antagonist radioligand) | Rat cerebral cortex | 9.0 (100) |
| N-type Ca2+ (antagonist radioligand) | Rat cerebral cortex | 1.5 (100) |
| GABA transporter (antagonist radioligand) | Rat cerebral cortex | 2.8 (100) |
| GABAA ion channel [3H]GABA (agonist radioligand) | Rat cerebral cortex | −1.1 (100) |
| Potassium channel (hERG) | Human recombinant HEK-293 cell | 24.0 (100) |
3.8. In Vitro Electrophysiological Studies
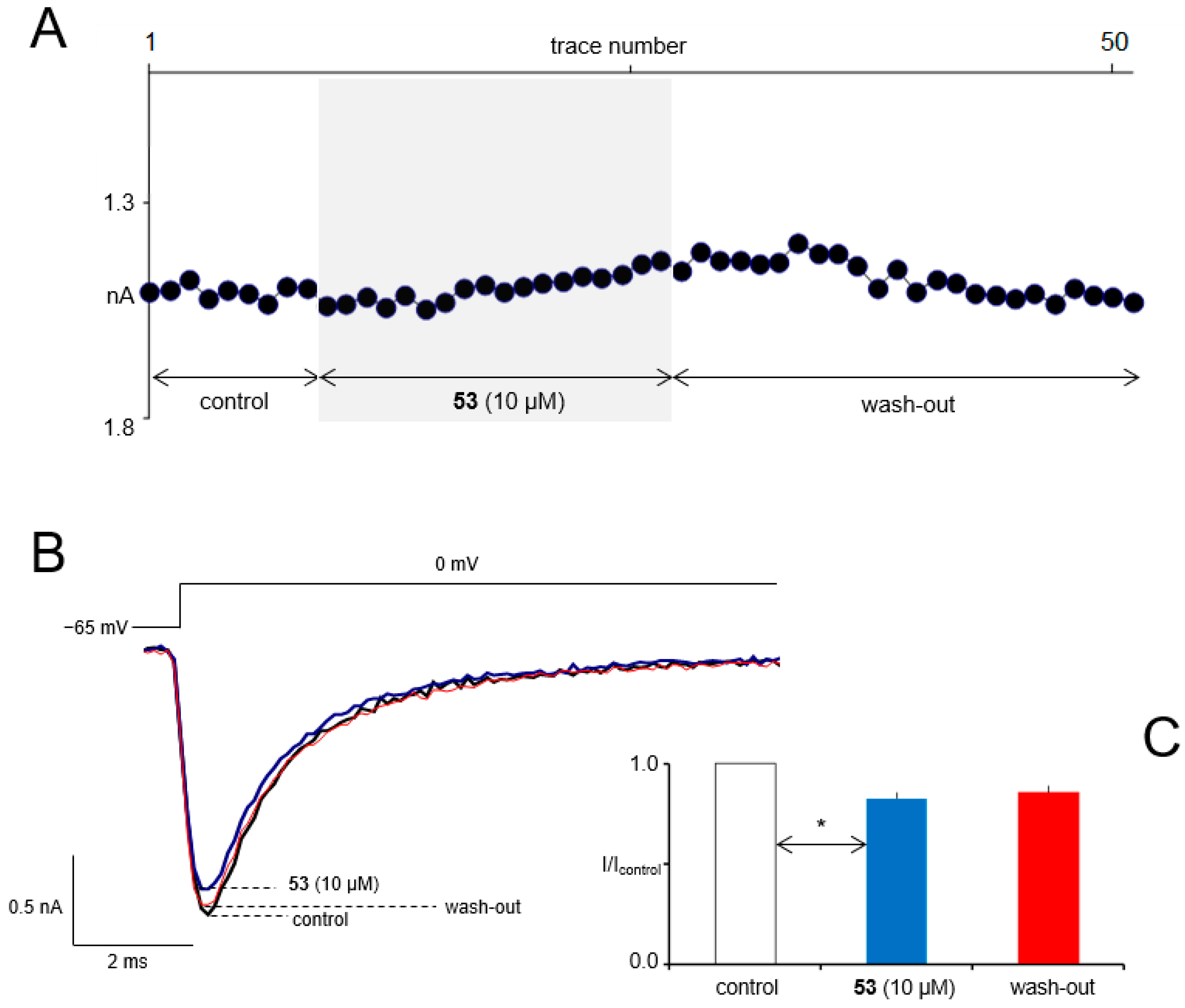
3.9. In Vitro ADME-Tox Assays

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Golyala, A.; Kwan, P. Drug Development for Refractory Epilepsy: The Past 25 Years and Beyond. Seizure 2017, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Talevi, A. Multi-Target Pharmacology: Possibilities and Limitations of the “Skeleton Key Approach” from a Medicinal Chemist Perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. Multifunctional Compounds: Smart Molecules for Multifactorial Diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs. 2021, 35, 935–963. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Kupershmidt, L.; Amit, T.; Weinreb, O. Promises of Novel Multi-Target Neuroprotective and Neurorestorative Drugs for Parkinson’s Disease. Parkinsonism Relat. Disord. 2014, 20, S132–S136. [Google Scholar] [CrossRef]
- Simone Tranches Dias, K.; Viegas, C. Multi-Target Directed Drugs: A Modern Approach for Design of New Drugs for the Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2014, 12, 239–255. [Google Scholar] [CrossRef]
- Millan, M.J. On ‘Polypharmacy’ and Multi-Target Agents, Complementary Strategies for Improving the Treatment of Depression: A Comparative Appraisal. Int. J. Neuropsychopharmacol. 2014, 17, 1009–1037. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules. 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Wecksler, A.T.; Wagner, K.; Hammock, B.D. Rationally Designed Multitarget Agents Against Inflammation and Pain. Curr. Med. Chem. 2013, 20, 1783–1799. [Google Scholar] [CrossRef]
- Kucuksayan, E.; Ozben, T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Valabrega, G. Multitarget Drugs: The Present and the Future of Cancer Therapy. Expert Opin. Pharmacother. 2009, 10, 589–600. [Google Scholar] [CrossRef]
- Löscher, W. Single-Target Versus Multi-Target Drugs Versus Combinations of Drugs with Multiple Targets: Preclinical and Clinical Evidence for the Treatment or Prevention of Epilepsy. Front. Pharmacol. 2021, 12, 730257. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. Multitarget Approaches against Multiresistant Superbugs. ACS Infect. Dis. 2020, 6, 1346–1365. [Google Scholar] [CrossRef]
- Braga, S.S. Multi-Target Drugs Active against Leishmaniasis: A Paradigm of Drug Repurposing. Eur. J. Med. Chem. 2019, 183, 111660. [Google Scholar] [CrossRef]
- Li, K.; Schurig-Briccio, L.A.; Feng, X.; Upadhyay, A.; Pujari, V.; Lechartier, B.; Fontes, F.L.; Yang, H.; Rao, G.; Zhu, W.; et al. Multitarget Drug Discovery for Tuberculosis and Other Infectious Diseases. J. Med. Chem. 2014, 57, 3126–3139. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M. Spicy Science: David Julius and the Discovery of Temperature-Sensitive TRP Channels. Temperature 2015, 2, 135–141. [Google Scholar] [CrossRef][Green Version]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.-K. TRPV1-Expressing Primary Afferents Generate Behavioral Responses to Pruritogens via Multiple Mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef]
- Chen, R.; Coppes, M.; Urman, R. Receptor and Molecular Targets for the Development of Novel Opioid and Non-Opioid Analgesic Therapies. Pain Phys. 2021, 24, 153–163. [Google Scholar] [CrossRef]
- Wang, M.; Thyagarajan, B. Pain Pathways and Potential New Targets for Pain Relief. Biotechnol. Appl. Biochem. 2022, 69, 110–123. [Google Scholar] [CrossRef]
- Knotkova, H.; Pappagallo, M.; Szallasi, A. Capsaicin (TRPV1 Agonist) Therapy for Pain Relief: Farewell or Revival? Clin. J. Pain. 2008, 24, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cruz, F.; Geppetti, P. TRPV1: A Therapeutic Target for Novel Analgesic Drugs? Trends Mol. Med. 2006, 12, 545–554. [Google Scholar] [CrossRef]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef]
- Nazıroglu, M. TRPV1 Channel: A Potential Drug Target for Treating Epilepsy. Curr. Neuropharmacol. 2015, 13, 239–247. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.-L.; Kong, W.-L.; Zeng, M.-L.; Shao, L.; Jiang, G.-T.; Cheng, J.-J.; Kong, S.; He, X.-H.; Liu, W.-H.; et al. TRPV1 Translocated to Astrocytic Membrane to Promote Migration and Inflammatory Infiltration Thus Promotes Epilepsy after Hypoxic Ischemia in Immature Brain. J. Neuroinflamm. 2019, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Vaca, M.A.; Miranda, C.J.; N’Gouemo, P. Inhibition of Transient Potential Receptor Vanilloid Type 1 Suppresses Seizure Susceptibility in the Genetically Epilepsy-Prone Rat. CNS Neurosci. Ther. 2018, 24, 18–28. [Google Scholar] [CrossRef]
- Gray, R.A.; Whalley, B.J. The Proposed Mechanisms of Action of CBD in Epilepsy. Epileptic Disord. 2020, 22, S10–S15. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and Potential Therapeutic Role in Epilepsy and Other Neuropsychiatric Disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Klein, B.D.; Jacobson, C.A.; Metcalf, C.S.; Smith, M.D.; Wilcox, K.S.; Hampson, A.J.; Kehne, J.H. Evaluation of Cannabidiol in Animal Seizure Models by the Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Zagaja, M.; Łuszczki, J.J.; Rapacz, A.; Andres-Mach, M.; Latacz, G.; Kieć-Kononowicz, K. Design, Synthesis, and Anticonvulsant Activity of New Hybrid Compounds Derived from 2-(2,5-Dioxopyrrolidin-1-Yl)Propanamides and 2-(2,5-Dioxopyrrolidin-1-Yl)Butanamides. J. Med. Chem. 2015, 58, 5274–5286. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Zagaja, M.; Mogilski, S.; Andres-Mach, M.; Latacz, G.; Baś, S.; Łuszczki, J.J.; Kieć-Kononowicz, K.; Kamiński, K. Multifunctional Hybrid Compounds Derived from 2-(2,5-Dioxopyrrolidin-1-Yl)-3-Methoxypropanamides with Anticonvulsant and Antinociceptive Properties. J. Med. Chem. 2017, 60, 8565–8579. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Rapacz, A.; Mogilski, S.; Latacz, G.; Lubelska, A.; Kamiński, R.M.; Kamiński, K. Multitargeted Compounds Derived from (2,5-Dioxopyrrolidin-1-Yl)(Phenyl)-Acetamides as Candidates for Effective Anticonvulsant and Antinociceptive Agents. ACS Chem. Neurosci. 2020, 11, 1996–2008. [Google Scholar] [CrossRef]
- Abram, M.; Rapacz, A.; Latacz, G.; Szulczyk, B.; Kalinowska-Tłuścik, J.; Otto-Ślusarczyk, D.; Struga, M.; Kamiński, R.M.; Kamiński, K. Asymmetric Synthesis and in Vivo/in Vitro Characterization of New Hybrid Anticonvulsants Derived from (2,5-Dioxopyrrolidin-1-Yl)Phenylacetamides. Bioorg. Chem. 2021, 109, 104751. [Google Scholar] [CrossRef]
- Kamiński, K.; Mogilski, S.; Abram, M.; Rapacz, A.; Latacz, G.; Szulczyk, B.; Walczak, M.; Kuś, K.; Matyjaszczyk, K.; Kamiński, R.M. KA-104, a New Multitargeted Anticonvulsant with Potent Antinociceptive Activity in Preclinical Models. Epilepsia 2020, 61, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, D.; Jha, S.K.; Jha, N.K.; Ambasta, R.K. Chapter Three—Ion Channels in Neurological Disorders. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Ion Channels as Therapeutic Targets, Part A; Academic Press: Boston, MA, USA, 2016; Volume 103, pp. 97–136. [Google Scholar]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Roca-Lapirot, O.; Radwani, H.; Aby, F.; Nagy, F.; Landry, M.; Fossat, P. Calcium Signalling through L-Type Calcium Channels: Role in Pathophysiology of Spinal Nociceptive Transmission. Br. J. Pharmacol. 2018, 175, 2362–2374. [Google Scholar] [CrossRef]
- Radwani, H.; Lopez-Gonzalez, M.J.; Cattaert, D.; Roca-Lapirot, O.; Dobremez, E.; Bouali-Benazzouz, R.; Eiríksdóttir, E.; Langel, Ü.; Favereaux, A.; Errami, M.; et al. Cav1.2 and Cav1.3 L-Type Calcium Channels Independently Control Short- and Long-Term Sensitization to Pain. J. Physiol. 2016, 594, 6607–6626. [Google Scholar] [CrossRef]
- Berger, S.M.; Bartsch, D. The Role of L-Type Voltage-Gated Calcium Channels Cav1.2 and Cav1.3 in Normal and Pathological Brain Function. Cell. Tissue Res. 2014, 357, 463–476. [Google Scholar] [CrossRef]
- Ortner, N.J.; Striessnig, J. L-Type Calcium Channels as Drug Targets in CNS Disorders. Channels. 2016, 10, 7–13. [Google Scholar] [CrossRef]
- Schampel, A.; Kuerten, S. Danger: High Voltage—The Role of Voltage-Gated Calcium Channels in Central Nervous System Pathology. Cells. 2017, 6, 43. [Google Scholar] [CrossRef]
- Wiskur, B.J.; Tyler, K.; Campbell-Dittmeyer, K.; Chaplan, S.R.; Wickenden, A.D.; Greenwood-Van Meerveld, B. A Novel TRPV1 Receptor Antagonist JNJ-17203212 Attenuates Colonic Hypersensitivity in Rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Pomonis, J.D.; Harrison, J.E.; Mark, L.; Bristol, D.R.; Valenzano, K.J.; Walker, K. N-(4-Tertiarybutylphenyl)-4-(3-Cholorphyridin-2-Yl)Tetrahydropyrazine -1(2H)-Carbox-Amide (BCTC), a Novel, Orally Effective Vanilloid Receptor 1 Antagonist with Analgesic Properties: II. In Vivo Characterization in Rat Models of Inflammatory and Neuropathic Pain. J. Pharmacol. Exp. Ther. 2003, 306, 387–393. [Google Scholar] [CrossRef]
- Valenzano, K.J.; Grant, E.R.; Wu, G.; Hachicha, M.; Schmid, L.; Tafesse, L.; Sun, Q.; Rotshteyn, Y.; Francis, J.; Limberis, J.; et al. N-(4-Tertiarybutylphenyl)-4-(3-Chloropyridin-2-Yl)Tetrahydropyrazine -1(2H)-Carbox-Amide (BCTC), a Novel, Orally Effective Vanilloid Receptor 1 Antagonist with Analgesic Properties: I. In Vitro Characterization and Pharmacokinetic Properties. J. Pharmacol. Exp. Ther. 2003, 306, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Jakubiec, M.; Rapacz, A.; Mogilski, S.; Latacz, G.; Szulczyk, B.; Szafarz, M.; Socała, K.; Nieoczym, D.; Wyska, E.; et al. Identification of New Compounds with Anticonvulsant and Antinociceptive Properties in a Group of 3-Substituted (2,5-Dioxo-Pyrrolidin-1-Yl)(Phenyl)-Acetamides. Int. J. Mol. Sci. 2021, 22, 13092. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Toman, J.E.P.; Swinyard, E.A.; Goodman, L.S. Properties of maximal seizures, and their alteration by anticonvulsant drugs and other agents. J. Neurophysiol. 1946, 9, 231–239. [Google Scholar] [CrossRef]
- Ferreri, G.; Chimirri, A.; Russo, E.; Gitto, R.; Gareri, P.; De Sarro, A.; De Sarro, G. Comparative Anticonvulsant Activity of N-Acetyl-1-Aryl-6,7-Dimethoxy-1,2,3,4-Tetrahydroisoquinoline Derivatives in Rodents. Pharmacol. Biochem. Behav. 2004, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Florek-Luszczki, M.; Wlaz, A.; Kondrat-Wrobel, M.W.; Tutka, P.; Luszczki, J.J. Effects of WIN 55,212-2 (a Non-Selective Cannabinoid CB1 and CB2 Receptor Agonist) on the Protective Action of Various Classical Antiepileptic Drugs in the Mouse 6 Hz Psychomotor Seizure Model. J. Neural. Transm. 2014, 121, 707–715. [Google Scholar] [CrossRef]
- Boissier, J.-R.; Tardy, J.; Diverres, J.-C. Une Nouvelle Méthode Simple Pour Explorer l’action «tranquillisante»: Le Test de La Cheminée. Public Health Action 1960, 3, 81–84. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, D.; Pieróg, M.; Wlaź, P. α-Spinasterol, a TRPV1 Receptor Antagonist, Elevates the Seizure Threshold in Three Acute Seizure Tests in Mice. J. Neural. Transm. 2015, 122, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Jakubiec, M.; Rapacz, A.; Mogilski, S.; Latacz, G.; Kamiński, R.M.; Kamiński, K. The Search for New Anticonvulsants in a Group of (2,5-Dioxopyrrolidin-1-Yl)(Phenyl)Acetamides with Hybrid Structure—Synthesis and In Vivo/In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 8780. [Google Scholar] [CrossRef]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Marć, M.A.; Satała, G.; Wilczyńska, D.; Kotańska, M.; Więcek, M.; Kamińska, K.; et al. The 1,3,5-Triazine Derivatives as Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 3420. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Więcek, M.; Łażewska, D.; Kurczab, R.; Jastrzębska-Więsek, M.; Satała, G.; Kucwaj-Brysz, K.; Lubelska, A.; Głuch-Lutwin, M.; Mordyl, B.; et al. Synthesis and Computer-Aided SAR Studies for Derivatives of Phenoxyalkyl-1,3,5-Triazine as the New Potent Ligands for Serotonin Receptors 5-HT6. Eur. J. Med. Chem. 2019, 178, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Szulczyk, B.; Spyrka, A. Menthol Exerts TRPM8-Independent Antiepileptic Effects in Prefrontal Cortex Pyramidal Neurons. Brain Res. 2022, 1783, 147847. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the Chemical Beauty of Drugs. Nature Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Wagaw, S.; Buchwald, S.L. An Improved Catalyst System for Aromatic Carbon−Nitrogen Bond Formation: The Possible Involvement of Bis(Phosphine) Palladium Complexes as Key Intermediates. J. Am. Chem. Soc. 1996, 118, 7215–7216. [Google Scholar] [CrossRef]
- Galanopoulou, A.S.; Kokaia, M.; Loeb, J.A.; Nehlig, A.; Pitkänen, A.; Rogawski, M.A.; Staley, K.J.; Whittemore, V.H.; Dudek, F.E. Epilepsy Therapy Development: Technical and Methodologic Issues in Studies with Animal Models. Epilepsia. 2013, 54, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; Steve White, H. Pharmacological Characterization of the 6 Hz Psychomotor Seizure Model of Partial Epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Löscher, W. Critical Review of Current Animal Models of Seizures and Epilepsy Used in the Discovery and Development of New Antiepileptic Drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Taner, A.N.; Balbay, E.; Çiğ, B. Inhibitions of Anandamide Transport and FAAH Synthesis Decrease Apoptosis and Oxidative Stress through Inhibition of TRPV1 Channel in an in Vitro Seizure Model. Mol. Cell. Biochem. 2019, 453, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F.A. Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Patra, P.H.; Barker-Haliski, M.; White, H.S.; Whalley, B.J.; Glyn, S.; Sandhu, H.; Jones, N.; Bazelot, M.; Williams, C.M.; McNeish, A.J. Cannabidiol Reduces Seizures and Associated Behavioral Comorbidities in a Range of Animal Seizure and Epilepsy Models. Epilepsia 2019, 60, 303–314. [Google Scholar] [CrossRef]
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Current Neuropharmacol. 2019, 17, 926–946. [Google Scholar] [CrossRef]
- Kehne, J.H.; Klein, B.D.; Raeissi, S.; Sharma, S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem. Res. 2017, 42, 1894–1903. [Google Scholar] [CrossRef]
- Peterson, S.L.; Albertson, T.E. Neuropharmacology Methods in Epilepsy Research; CRC Press Methods in the Life Sciences Cellular and Molecular Neuropharmacology; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-0-8493-3362-0. [Google Scholar]
- Löscher, W. Preclinical Assessment of Proconvulsant Drug Activity and Its Relevance for Predicting Adverse Events in Humans. Eur. J. Pharmacol. 2009, 610, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.R.; Truong, D.D.; Nguyen, K.D.; Dang, A.T.; Hoang, T.T.; Vo, P.Q.; Sandroni, P. Involvement of GABAA Receptors in Myoclonus. Mov. Disord. 2000, 15, 47–52. [Google Scholar] [CrossRef]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 Antagonists That Cause Hypothermia, Instead of Hyperthermia, in Rodents: Compounds’ Pharmacological Profiles, in Vivo Targets, Thermoeffectors Recruited and Implications for Drug Development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia Induced by Transient Receptor Potential Vanilloid-1 (TRPV1) Antagonists in Human Clinical Trials: Insights from Mathematical Modeling and Meta-Analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef]
- Gomtsyan, A.; McDonald, H.A.; Schmidt, R.G.; Daanen, J.F.; Voight, E.A.; Segreti, J.A.; Puttfarcken, P.S.; Reilly, R.M.; Kort, M.E.; Dart, M.J.; et al. TRPV1 Ligands with Hyperthermic, Hypothermic and No Temperature Effects in Rats. Temperature 2015, 2, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J.; Rogawski, M.A. Mechanisms of Action of Currently Used Antiseizure Drugs. Neuropharmacol. 2020, 168, 107966. [Google Scholar] [CrossRef]
- Nicita, F.; Spalice, A.; Raucci, U.; Iannetti, P.; Parisi, P. The Possible Use of the L-Type Calcium Channel Antagonist Verapamil in Drug-Resistant Epilepsy. Expert Rev. Neurother. 2016, 16, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Weiergräber, M.; Stephani, U.; Köhling, R. Voltage-Gated Calcium Channels in the Etiopathogenesis and Treatment of Absence Epilepsy. Brain Res. Rev. 2010, 62, 245–271. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Boyaji, S.; Merkow, J.; Elman, R.N.M.; Kaye, A.D.; Yong, R.J.; Urman, R.D. The Role of Cannabidiol (CBD) in Chronic Pain Management: An Assessment of Current Evidence. Curr. Pain Headache Rep. 2020, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Barakat, K.H. Development of Safe Drugs: The HERG Challenge. Med. Res. Rev. 2018, 38, 525–555. [Google Scholar] [CrossRef]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.L.; Balimane, P.V. A Novel Design of Artificial Membrane for Improving the PAMPA Model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S. Prediction of Human Clearance of Twenty-Nine Drugs from Hepatic Microsomal Intrinsic Clearance Data: An Examination of In Vitro Half-Life Approach and Nonspecific Binding to Microsomes. Drug Metab. Dispos. 1999, 27, 1350–1359. [Google Scholar]
- Pauli-Magnus, C.; von Richter, O.; Burk, O.; Ziegler, A.; Mettang, T.; Eichelbaum, M.; Fromm, M.F. Characterization of the Major Metabolites of Verapamil as Substrates and Inhibitors of P-Glycoprotein. J. Pharmacol. Exp. Ther. 2000, 293, 376–382. [Google Scholar]
- Więckowska, A.; Wichur, T.; Godyń, J.; Bucki, A.; Marcinkowska, M.; Siwek, A.; Więckowski, K.; Zaręba, P.; Knez, D.; Głuch-Lutwin, M.; et al. Novel Multitarget-Directed Ligands Aiming at Symptoms and Causes of Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Mogilski, S.; Pieróg, M.; Nieoczym, D.; Abram, M.; Szulczyk, B.; Lubelska, A.; Latacz, G.; Doboszewska, U.; Wlaź, P.; et al. KA-11, a Novel Pyrrolidine-2,5-Dione Derived Broad-Spectrum Anticonvulsant: Its Antiepileptogenic, Antinociceptive Properties and in Vitro Characterization. ACS Chem. Neurosci. 2019, 10, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Szala-Rycaj, J.; Lemieszek, M.K.; Maj, M.; Abram, M.; Kaminski, K. Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice. Int. J. Mol. Sci. 2021, 22, 3240. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.B. 3H-Batrachotoxinin-A Benzoate Binding to Voltage-Sensitive Sodium Channels: Inhibition by the Channel Blockers Tetrodotoxin and Saxitoxin. J. Neurosci. 1986, 6, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.J.; Murphy, K.M.; Snyder, S.H. [3H]Nitrendipine-Labeled Calcium Channels Discriminate Inorganic Calcium Agonists and Antagonists. Proc. Natl. Acad. Sci. USA. 1982, 79, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Sills, M.A.; Fagg, G.; Pozza, M.; Angst, C.; Brundish, D.E.; Hurt, S.D.; Jay Wilusz, E.; Williams, M. [3H]CGP 39653: A New N-Methyl-D-Aspartate Antagonist Radioligand with Low Nanomolar Affinity in Rat Brain. Eur. J. Pharmacol. 1991, 192, 19–24. [Google Scholar] [CrossRef]
- Wagner, J.A.; Snowman, A.M.; Biswas, A.; Olivera, B.M.; Snyder, S.H. Omega-Conotoxin GVIA Binding to a High-Affinity Receptor in Brain: Characterization, Calcium Sensitivity, and Solubilization. J. Neurosci. 1988, 8, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Shank, R.P.; Baldy, W.J.; Mattucci, L.C.; Villani Jr., F.J. Ion and Temperature Effects on the Binding of γ-Aminobutyrate to Its Receptors and the High-Affinity Transport System. J. Neurochem. 1990, 54, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Eurofins Discovery. Available online: Https://www.Eurofinsdiscoveryservices.Com/Catalogmanagement/ViewItem/Non-Selective-RatGABAA-Ion-Channel-3H-Muscimol-Binding-Agonist-Radioligand-Assay-Panlabs/226500 (accessed on 15 December 2021).
- Huang, X.-P.; Mangano, T.; Hufeisen, S.; Setola, V.; Roth, B.L. Identification of Human Ether-à-Go-Go Related Gene Modulators by Three Screening Platforms in an Academic Drug-Discovery Setting. ASSAY Drug Dev. Technol. 2010, 8, 727–742. [Google Scholar] [CrossRef]
- Phelps, P.T.; Anthes, J.C.; Correll, C.C. Cloning and Functional Characterization of Dog Transient Receptor Potential Vanilloid Receptor-1 (TRPV1). Eur. J. Pharmacol. 2005, 513, 57–66. [Google Scholar] [CrossRef]
- Sirenko, O.; Crittenden, C.; Callamaras, N.; Hesley, J.; Chen, Y.-W.; Funes, C.; Rusyn, I.; Anson, B.; Cromwell, E.F. Multiparameter In Vitro Assessment of Compound Effects on Cardiomyocyte Physiology Using IPSC Cells. J. Biomol. Screen. 2013, 18, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Imredy, J.P.; Koblan, K.S.; Bennett, P.; Connolly, T.M. State-Dependent Inhibition of L-Type Calcium Channels: Cell-Based Assay in High-Throughput Format. Anal. Biochem. 2004, 327, 74–81. [Google Scholar] [CrossRef] [PubMed]
| Cmpd | PT (h) a | ED50 6 Hz (44 mA) (mg/kg) | TD50 (mg/kg) | PI (TD50/ED50) |
|---|---|---|---|---|
| 53 | 0.5 | 68.0 (57.2–80.9) | 179.7 * (161.0−200.5) | 2.6 |
| 60 | 0.5 | 56.3 (46.8–67.7) | 156.2 * (137.7−177.1) | 2.8 |
| KA-104 b | 0.5 | 73.2 (57.4–93.4) | 195.7 ** (132.7–288.6) | 2.7 |
| CBD c | 1 | 173.0 (136–213) | 272.0 ** (241.0−303.0) | 1.6 |
| LEV d | 0.5 | 204.0 (154.5–269.5) | >500 ** | >2.5 |
| LCS d | 0.5 | 6.9 (5.4–8.6) | 46.2 ** (44.5–48.0) | 6.7 |
| VPA d | 0.5 | 183.1 (143.5–233.7) | 430.7 ** (407.9−454.9) | 2.3 |
| Comp | Pe * (10−6 cm/s) ± SD |
|---|---|
| CFN | 12.22 ± 0.94 |
| NFX | 0.056 ± 0.01 |
| 60 | 9.15 ± 0.78 |
| Substrate | Molecular Mass (m/z) | % Remaining | Molecular Mass of the Metabolite (m/z) | Metabolic Pathway * |
|---|---|---|---|---|
| 53 | 406.28 | 95.59 | 422.30 (M1) | hydroxylation |
| 60 | 422.30 | 88.80 | 438.25 (M1) | hydroxylation |
| 420.24 (M2) | dehydrogenation | |||
| 62 | 438.25 | 84.01 | 454.26 (M1) | hydroxylation |
| 454.26 (M2) | hydroxylation | |||
| Verapamil ** | 455.31 | 30.84 | 441.35 (M1) | demethylation |
| 291.328 (M2) | defragmentation | |||
| 165.09 (M3) | defragmentation | |||
| 441.29 (M4) | demethylation | |||
| 427.33 (M5) | double-demethylation | |||
| 277.26 (M6) | defragmentation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubiec, M.; Abram, M.; Zagaja, M.; Andres-Mach, M.; Szewczyk, A.; Latacz, G.; Szulczyk, B.; Socała, K.; Nieoczym, D.; Wlaź, P.; et al. New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants. Cells 2022, 11, 1862. https://doi.org/10.3390/cells11121862
Jakubiec M, Abram M, Zagaja M, Andres-Mach M, Szewczyk A, Latacz G, Szulczyk B, Socała K, Nieoczym D, Wlaź P, et al. New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants. Cells. 2022; 11(12):1862. https://doi.org/10.3390/cells11121862
Chicago/Turabian StyleJakubiec, Marcin, Michał Abram, Mirosław Zagaja, Marta Andres-Mach, Aleksandra Szewczyk, Gniewomir Latacz, Bartłomiej Szulczyk, Katarzyna Socała, Dorota Nieoczym, Piotr Wlaź, and et al. 2022. "New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants" Cells 11, no. 12: 1862. https://doi.org/10.3390/cells11121862
APA StyleJakubiec, M., Abram, M., Zagaja, M., Andres-Mach, M., Szewczyk, A., Latacz, G., Szulczyk, B., Socała, K., Nieoczym, D., Wlaź, P., Metcalf, C. S., Wilcox, K., Kamiński, R. M., & Kamiński, K. (2022). New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants. Cells, 11(12), 1862. https://doi.org/10.3390/cells11121862








