Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cultivation of hiPSCs Derived from Human Renal Epithelial Cells (RECs)
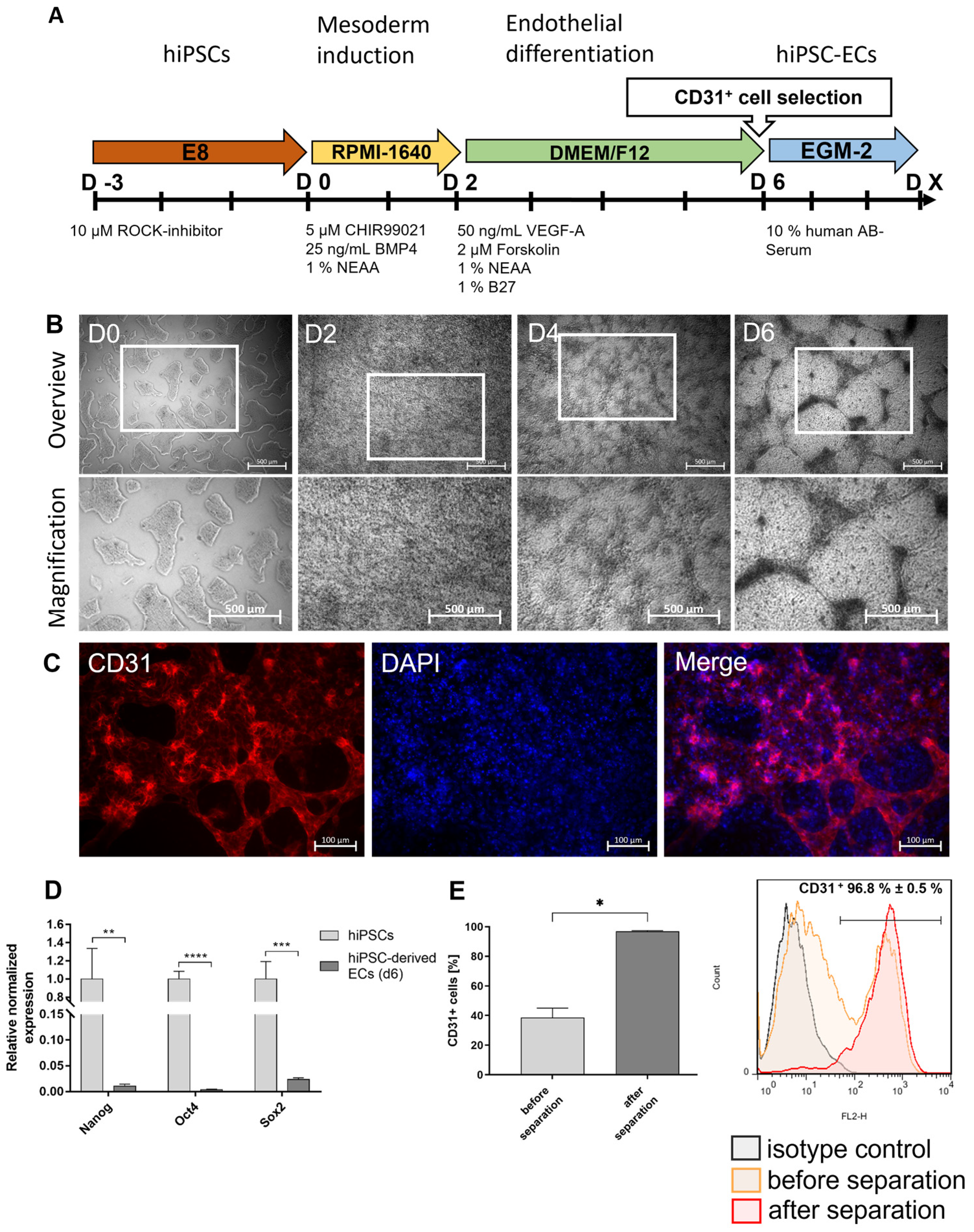
2.3. Differentiation of hiPSCs Towards ECs
2.4. Separation of CD31+ Cells after Endothelial Differentiation
2.5. Flow Cytometry
2.6. Immunocytochemistry
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Tube Formation Assay
2.9. TNF-α Stimulation of hiPSC-ECs
2.10. Cultivation of Human Umbilical Vein Endothelial Cells (HUVECs)
2.11. Isolation of Granulocytes
2.12. Calcein AM Labeling of the Isolated Granulocytes
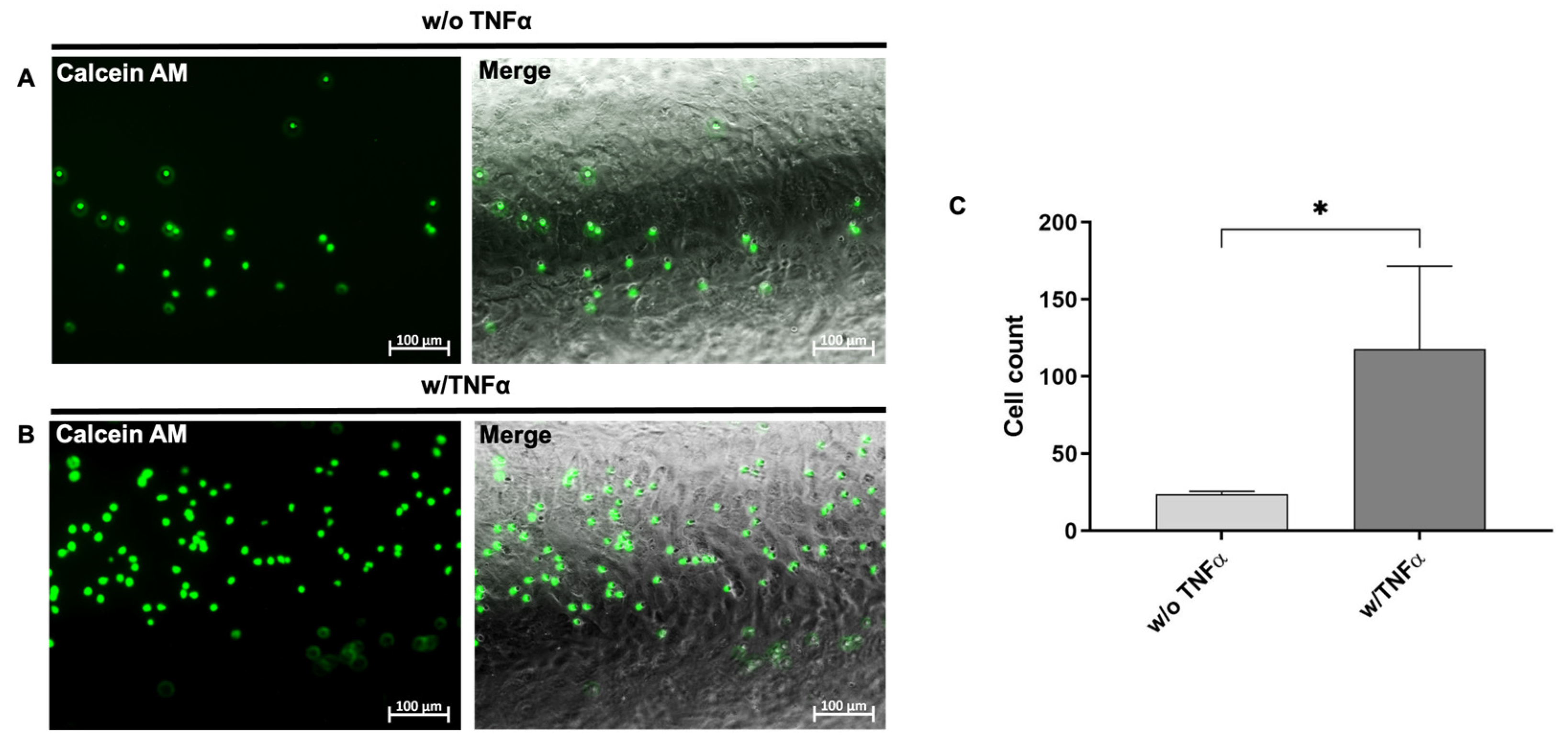
2.13. Static Interaction of Granulocytes with TNF-α Stimulated hiPSC-ECs
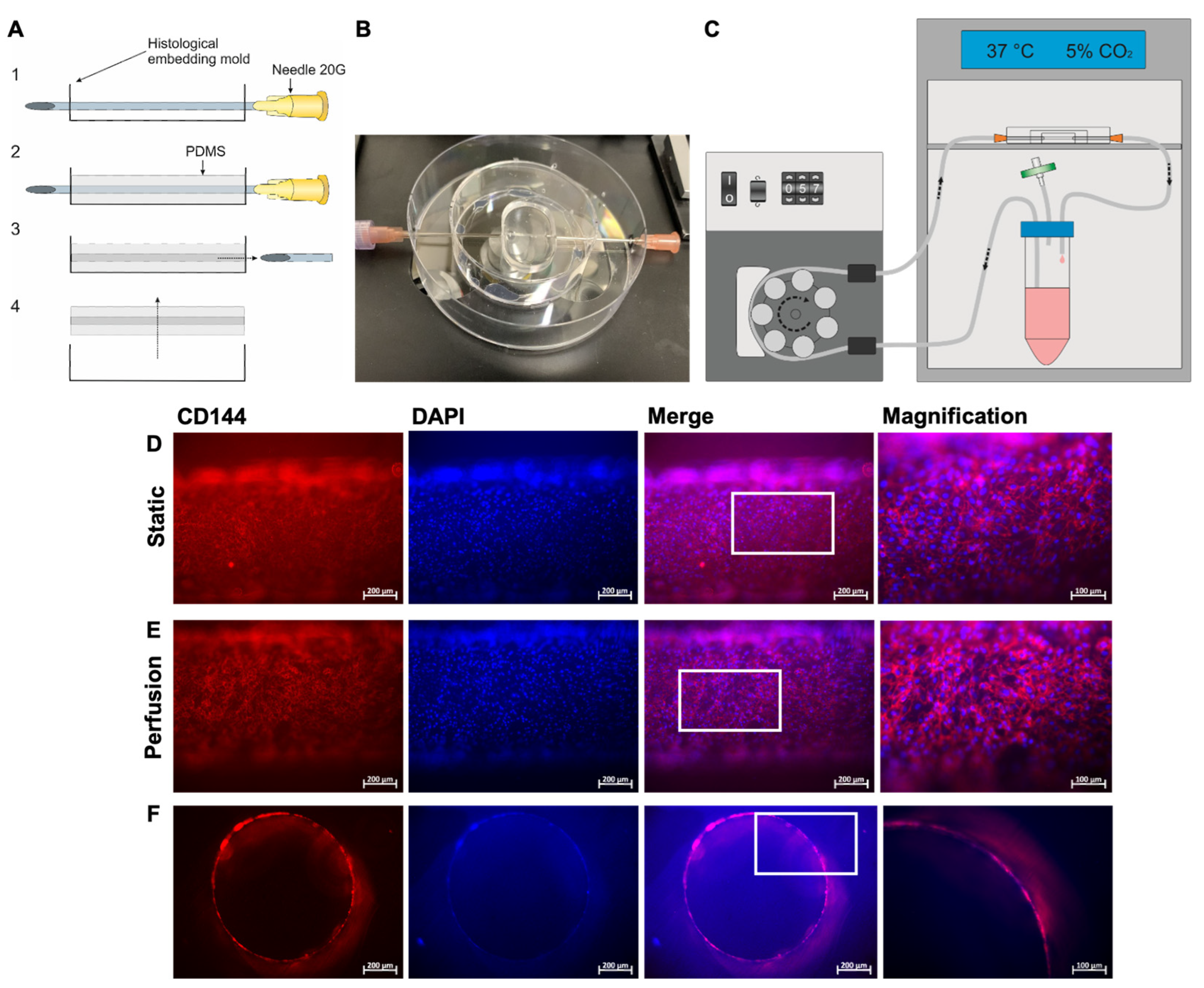
2.14. Fabrication of a Blood Vessel-Like Structure Embedded in Polydimethylsiloxane (PDMS)
2.15. Population of the Blood Vessel-Like Structure with hiPSC-ECs and Perfusion
2.16. Dynamic Interaction of Granulocytes with TNF-α Stimulated Endothelium in the PDMS Blood Vessel Model
2.17. Analysis of Stent Endothelialization in the PDMS Blood Vessel Model
2.18. Statistical Analyses
3. Results
3.1. Differentiation of Footprint-Free Generated hiPSCs into ECs
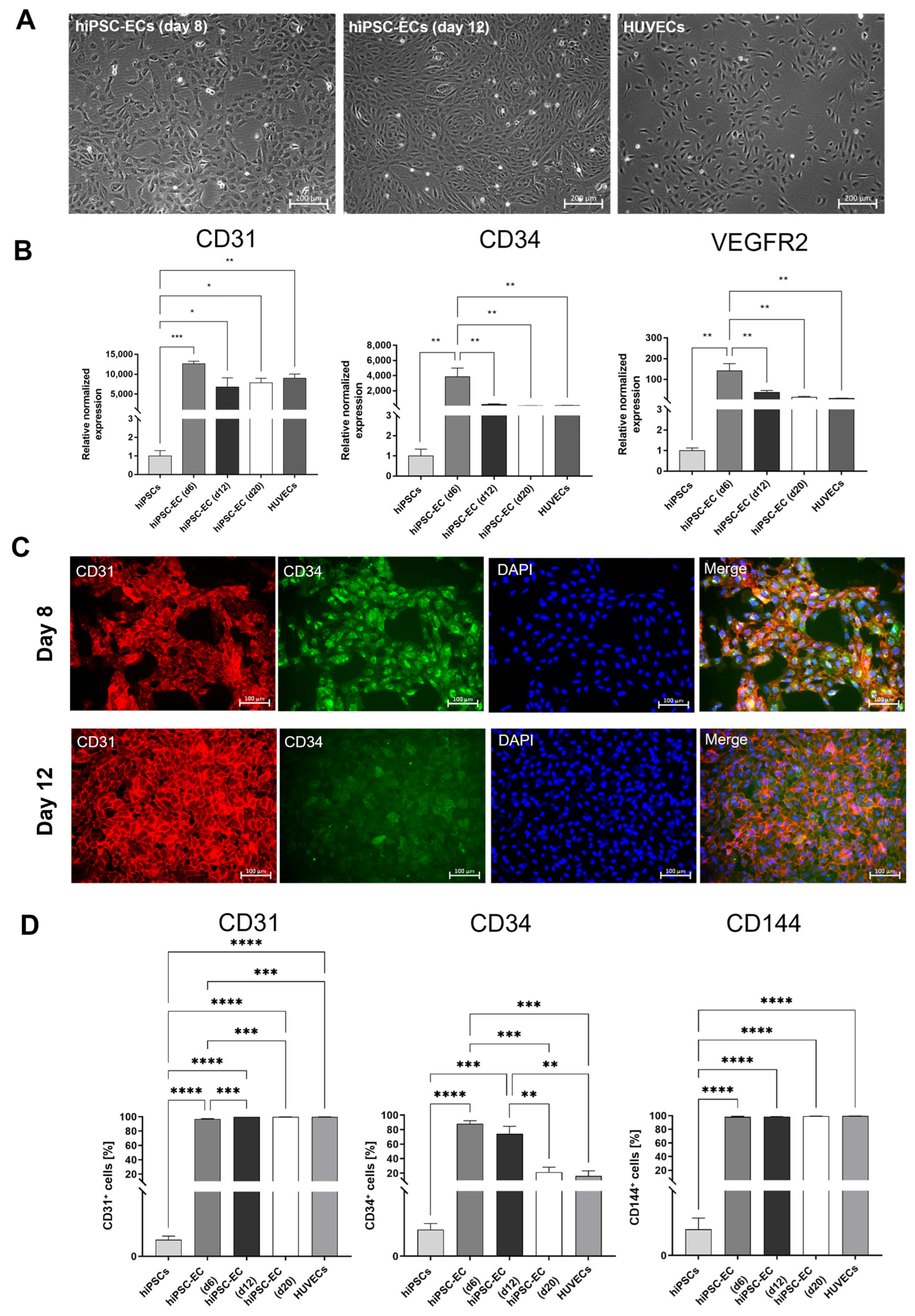
3.2. Characterization of hiPSC-ECs
3.3. Functional Analyses of hiPSC-Derived ECs
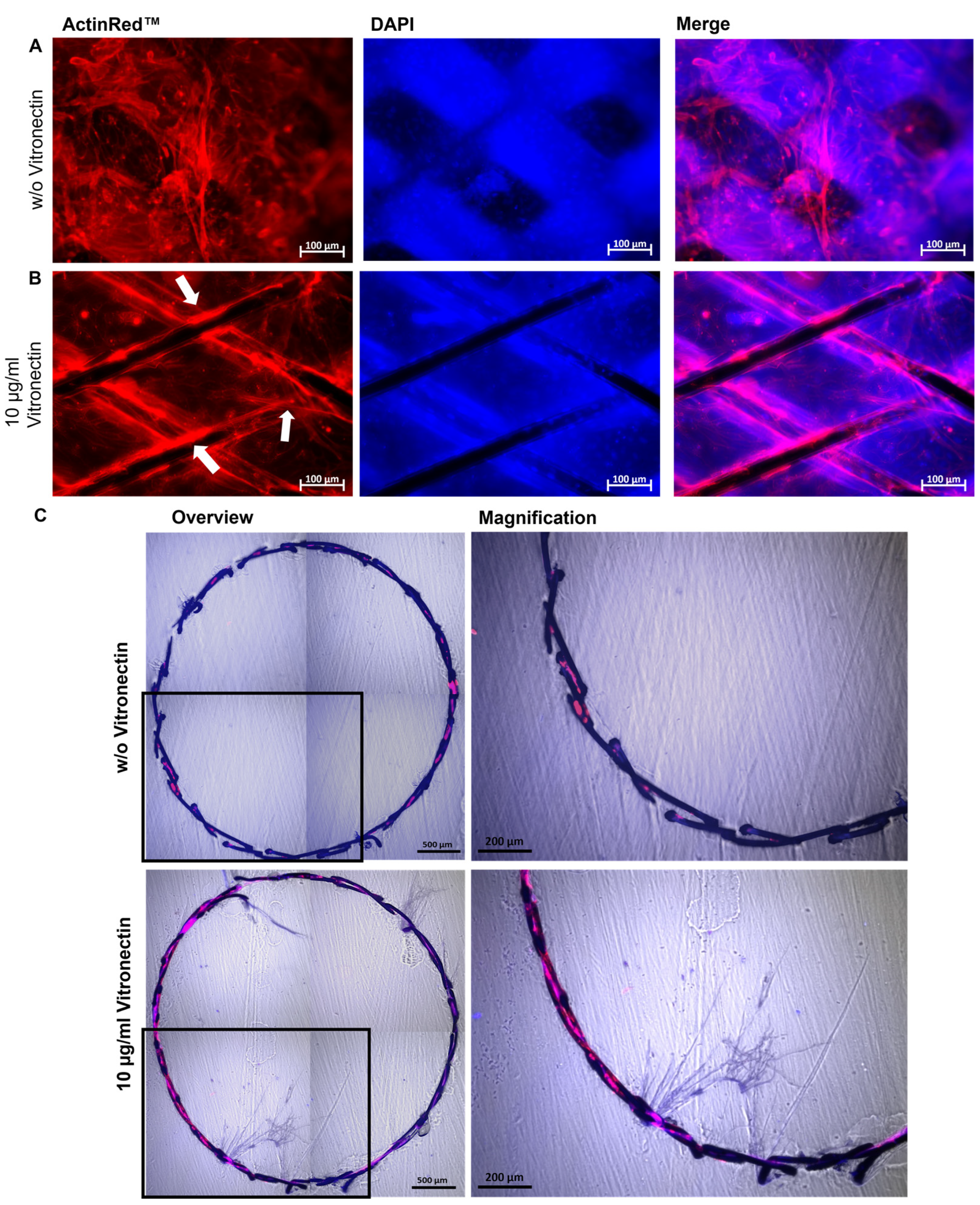
3.4. Fabrication of a Blood Vessel-Like PDMS Model and Analysis of Endothelialization with hiPSC-ECs
3.5. Analysis of the Interaction of Immune Cells with the Generated Endothelium in the Blood Vessel-Like PDMS Model
3.6. Evaluation of the Stent Endothelialization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751. [Google Scholar] [CrossRef]
- Dogne, S.; Flamion, B.; Caron, N. Endothelial Glycocalyx as a Shield against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases. Thromb. Vasc. Biol. 2018, 38, 1427–1439. [Google Scholar] [CrossRef]
- Kennedy, C.C.; Brown, E.E.; Abutaleb, N.O.; Truskey, G.A. Development and Application of Endothelial Cells Derived From Pluripotent Stem Cells in Microphysiological Systems Models. Front. Cardiovasc. Med. 2021, 8, 625016. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Maruyama, A.; Kang, M.I.; Kawatani, Y.; Shibata, T.; Uchida, K.; Itoh, K.; Yamamoto, M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem. 2005, 280, 27244–27250. [Google Scholar] [CrossRef][Green Version]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular Tissue Engineering: Polymers and Methodologies for Small Caliber Vascular Grafts. Front. Cardiovasc. Med. 2020, 7, 592361. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, J. Hypersensitivity and in-stent restenosis in coronary stent materials. Front. Bioeng. Biotechnol. 2022, 10, 1003322. [Google Scholar] [CrossRef] [PubMed]
- Maisel, W.H.; Laskey, W.K. Cardiology patient page. Drug-eluting stents. Circulation 2007, 115, e426–e427. [Google Scholar] [CrossRef][Green Version]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, R.; Chen, S.Y. Smooth muscle-specific drug targets for next-generation drug-eluting stent. Expert Rev. Cardiovasc. Ther. 2014, 12, 21–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avci-Adali, M.; Ziemer, G.; Wendel, H.P. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—A review of current strategies. Biotechnol. Adv. 2010, 28, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Wu, W.; Zhao, S.; Khan, B.; Sharzehee, M.; Panagopoulos, A.; Makadia, J.; Mickley, T.; Bicek, A.; Boismier, D.; et al. Computational and experimental mechanical performance of a new everolimus-eluting stent purpose-built for left main interventions. Sci. Rep. 2021, 11, 8728. [Google Scholar] [CrossRef]
- Gijsen, F.J.; Migliavacca, F.; Schievano, S.; Socci, L.; Petrini, L.; Thury, A.; Wentzel, J.J.; Van Der Steen, A.F.; Serruys, P.W.; Dubini, G. Simulation of stent deployment in a realistic human coronary artery. Biomed. Eng. Online 2008, 7, 23. [Google Scholar] [CrossRef][Green Version]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; von Ohle, C.; Popov, A.-F.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol. Ther.-Nucleic Acids 2019, 17, 907–921. [Google Scholar] [CrossRef][Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef][Green Version]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112373. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017. 6, 1700264. [CrossRef]
- Schaner, P.J.; Martin, N.; Tulenko, T.N.; Shapiro, I.M.; Tarola, N.A.; Leichter, R.F.; Carabasi, R.; DiMuzio, P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. 2004, 40, 146–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Tanyeri, M.; Tay, S. Viable cell culture in PDMS-based microfluidic devices. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 148, pp. 3–33. [Google Scholar]
- Lau, S.; Gossen, M.; Lendlein, A.; Jung, F. Venous and Arterial Endothelial Cells from Human Umbilical Cords: Potential Cell Sources for Cardiovascular Research. Int. J. Mol. Sci. 2021, 22, 978. [Google Scholar] [CrossRef]
- Majewska, A.; Wilkus, K.; Brodaczewska, K.; Kieda, C. Endothelial Cells as Tools to Model Tissue Microenvironment in Hypoxia-Dependent Pathologies. Int. J. Mol. Sci. 2021, 22, 520. [Google Scholar] [CrossRef]
- Cerella, C.; Grandjenette, C.; Dicato, M.; Diederich, M. Roles of Apoptosis and Cellular Senescence in Cancer and Aging. Curr. Drug Targets 2016, 17, 405–415. [Google Scholar] [CrossRef][Green Version]
- Williams, I.M.; Wu, J.C. Generation of Endothelial Cells from Human Pluripotent Stem Cells. Arter. Thromb. Vasc. Biol. 2019, 39, 1317–1329. [Google Scholar] [CrossRef]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Generation of iPSCs by Nonintegrative RNA-Based Reprogramming Techniques: Benefits of Self-Replicating RNA versus Synthetic mRNA. Stem Cells Int. 2019, 2019, 7641767. [Google Scholar] [CrossRef][Green Version]
- Seamon, K.B.; Padgett, W.; Daly, J.W. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3363–3367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamizu, K.; Kawasaki, K.; Katayama, S.; Watabe, T.; Yamashita, J.K. Enhancement of vascular progenitor potential by protein kinase A through dual induction of Flk-1 and Neuropilin-1. Blood 2009, 114, 3707–3716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.G.; Economides, C.; Mayeda, G.S.; Burstein, S.; Kloner, R.A. The endothelial cell in health and disease: Its function, dysfunction, measurement and therapy. Int. J. Impot. Res. 2010, 22, 77–90. [Google Scholar] [CrossRef][Green Version]
- Zilla, P.; Bezuidenhout, D.; Human, P. Human, Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef]
- Antoniadis, A.P.; Mortier, P.; Kassab, G.; Dubini, G.; Foin, N.; Murasato, Y.; Giannopoulos, A.A.; Tu, S.; Iwasaki, K.; Hikichi, Y.; et al. Biomechanical Modeling to Improve Coronary Artery Bifurcation Stenting: Expert Review Document on Techniques and Clinical Implementation. JACC Cardiovasc. Interv. 2015, 8, 1281–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genuardi, L.; Chatzizisis, Y.S.; Chiastra, C.; Sgueglia, G.; Samady, H.; Kassab, G.S.; Migliavacca, F.; Trani, C.; Burzotta, F. Local fluid dynamics in patients with bifurcated coronary lesions undergoing percutaneous coronary interventions. Cardiol. J. 2021, 28, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, H.-K.; Kim, P.-H.; Kim, D.W.; Cho, H.-M.; Jeong, M.J.; Kim, D.H.; Joung, Y.K.; Lim, K.S.; Kim, H.B.; Lim, H.C.; et al. Coronary stents with inducible VEGF/HGF-secreting UCB-MSCs reduced restenosis and increased re-endothelialization in a swine model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Rodriguez, S.; Rasser, C.; Mesnier, J.; Chevallier, P.; Gallet, R.; Choqueux, C.; Even, G.; Sayah, N.; Chaubet, F.; Nicoletti, A.; et al. Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo. Eur. Heart J. 2021, 42, 1760–1769. [Google Scholar] [CrossRef]
- Paul, A.; Elias, C.B.; Shum-Tim, D.; Prakash, S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci. Rep. 2013, 3, 2366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsen, K.; Cheng, C.; Tempel, D.; Parker, S.; Yazdani, S.; Dekker, W.K.D.; Houtgraaf, J.H.; De Jong, R.; Hoor, S.S.-T.; Ligtenberg, E.; et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur. Heart J. 2012, 33, 120–128. [Google Scholar] [CrossRef][Green Version]
- Ortega, M.A.; Saez, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Pekarek, L.; Bravo, C.; Coca, S.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; et al. Increased Angiogenesis and Lymphangiogenesis in the Placental Villi of Women with Chronic Venous Disease during Pregnancy. Int. J. Mol. Sci. 2020, 21, 2487. [Google Scholar] [CrossRef][Green Version]

| Gene | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| CD31 | GAACGGAAGGCTCCCTTGA | AGGGCAGGTTCATAAATAAGTGC |
| CD34 | GATTGCACTGGTCACCTCGG | TCCGTGTAATAAGGGTCTTCGC |
| CD62E | GCCCAGAGCCTTCAGTGTACC | GGAATGGCTGCACCTCACAG |
| GAPDH | TCAACAGCGACACCCACTCC | TGAGGTCCACCACCCTGTTG |
| ICAM-1 | CTTGAGGGCACCTACCTCTGTC | CGGCTGCTACCACAGTGATG |
| Lin28 | CTTCTTCTCCGAACCAACC | CAGCCACCTGCAAACTG |
| Nanog | TGAACCTCAGCTACAAACAG | TGGTGGTAGGAGAGTAAAG |
| Oct4 | AGCGAACCAGTATCGAGAAC | TTACAGAACCACACTCGGAC |
| Sox2 | AGCTACAGCATGATGCAGGA | GGTCATGGAGTTGTACTGCA |
| VEGFR2 | TCACAATTCCAAAAGTGATCGG | GGTCACTAACAGAAGCAATAAATGG |
| VCAM-1 | ACACTTTATGTCAATGTTGCCCC | GAGGCTGTAGCTCCCCGTTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, J.; Weber, M.; Feile, A.; Schlensak, C.; Avci-Adali, M. Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants. Cells 2023, 12, 1217. https://doi.org/10.3390/cells12091217
Weber J, Weber M, Feile A, Schlensak C, Avci-Adali M. Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants. Cells. 2023; 12(9):1217. https://doi.org/10.3390/cells12091217
Chicago/Turabian StyleWeber, Josefin, Marbod Weber, Adrian Feile, Christian Schlensak, and Meltem Avci-Adali. 2023. "Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants" Cells 12, no. 9: 1217. https://doi.org/10.3390/cells12091217
APA StyleWeber, J., Weber, M., Feile, A., Schlensak, C., & Avci-Adali, M. (2023). Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants. Cells, 12(9), 1217. https://doi.org/10.3390/cells12091217







