Progesterone: A Neuroprotective Steroid of the Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation for Cryosectioning
2.2. Laser Microdissection and RT-qPCR
2.3. Immunohistochemistry
2.4. Cell Culture
2.5. Preparation and Cultivation
2.6. Intoxication with Rotenone
2.7. Immunohistochemistry, Counting Method and Statistics
3. Results
3.1. Progesterone Receptor mRNA Expressions in the Myenteric Plexus of Rats
3.2. Progesterone Receptors Are Expressed on Protein Level
3.3. Reduction in Cell Death In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Dilution mix: 100 mL MEM-P/S: 99 mL MEM-Hepes (#M7278, Sigma-Aldrich); 1 mL PenStrep (PS) (#p4333, Sigma-Aldrich); store at 4 °C;
- Enzymatic digestion: 0.05% Trypsin + EDTA (#25300054, Gibco);
- Stop of enzymatic digestion: 44,5 mL Neurobasal Medium (#10888022, Thermo Fisher), 5 mL Fetal Bovine Serum (#F7524, Sigma-Aldrich), 1 mL Penicillin-Streptomycin (#P4333, Sigma-Aldrich), store at 4 °C;
- Proliferation medium: For 50 mL: 47.875 mL Neurobasal medium (#10888022, Thermo Fisher); 0.125 mL Glutamine (#G7513, Sigma-Aldrich); 0.5 mL FBS (#F7524, Sigma-Aldrich); 0.5 mL PS (#P4333, Sigma-Aldrich); 1 μL bFGF (#SRP4039-50UG, Sigma-Aldrich); 20 ng/mL 0.5 μL EGF (#SRP3238-100UG, Sigma-Aldrich); 10 ng/mL 1 mL Neuromix 2a without retinoic acid (#12587010, Thermo Fisher); store at 4 °C;
- Differentiation medium: For 50 mL: 47.375 mL Neurobasal medium (#10888022, Thermo Fisher); 0.125 mL Glutamine (G#7513, Sigma-Aldrich); 0.5 mL FBS (#F7524, Sigma-Aldrich); 0.5 mL PS (#P4333, Sigma-Aldrich); 1 mL Neuromix 2a plus retinoic acid (#17504044, Thermo Fisher); 0.5 mL Mitosis-inhibitor; store at 4 °C;
- Mitosis-inhibitor stock solution: into 10 mL aqua dest. (1 mM/1 mM/1 mM) 2.46 mg 5-Fluoro-2′-Deoxyuridine (#21555, Serva); 2.8 mg Cytosine-ßD-Arabinofuranoside (#C6645, Sigma-Aldrich); 2.4 mg Uridine (#u3003, Sigma-Aldrich); store at −20 °C;
- Poly-d-Lysin (500 μg/)mL (#P7280-5MG, Sigma-Aldrich); store at −20 °C;
- Rotenone (#45656, Sigma Aldrich): 1 nM, diluted in DMSO; store at −20 °C;
- Propidium-Iodide (#P4170, Sigma Aldrich); store at −4 °C;
- Progesterone (#P8783; Sigma Aldrich): 10 nM diluted in EtOH; store at −20 °C;
- AG205 (#A1487, Sigma-Aldrich) 5 nM: diluted in DMSO; store at −20 °C.
References
- Theis, V.; Theiss, C. Progesterone Effects in the Nervous System. Anat. Rec. 2019, 302, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A.; Jazayeri, J.A.; Catalano, S.M.; Toyokuni, S.; Kovacevic, Z.; Richardson, D.R. The Emerging Role of Progesterone Receptor Membrane Component 1 (PGRMC1) in Cancer Biology. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2016, 1866, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.-E.; Robel, P. Neurosteroids: A New Brain Function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, V.; Meyer, L.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Progress in Dorsal Root Ganglion Neurosteroidogenic Activity: Basic Evidence and Pathophysiological Correlation. Prog. Neurobiol. 2010, 92, 33–41. [Google Scholar] [CrossRef]
- Wessel, L.; Balakrishnan-Renuka, A.; Henkel, C.; Meyer, H.E.; Meller, K.; Brand-Saberi, B.; Theiss, C. Long-Term Incubation with Mifepristone (MLTI) Increases the Spine Density in Developing Purkinje Cells: New Insights into Progesterone Receptor Mechanisms. Cell. Mol. Life Sci. 2014, 71, 1723–1740. [Google Scholar] [CrossRef]
- Singh, M.; Su, C. Progesterone and Neuroprotection. Horm. Behav. 2013, 63, 284–290. [Google Scholar] [CrossRef]
- Jarras, H.; Bourque, M.; Poirier, A.; Morissette, M.; Coulombe, K.; Di Paolo, T.; Soulet, D. Neuroprotection and Immunomodulation of Progesterone in the Gut of a Mouse Model of Parkinson’s Disease. J. Neuroendocrinol. 2020, 32, e12782. [Google Scholar] [CrossRef]
- Furness, J.B. Types of Neurons in the Enteric Nervous System. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Derkinderen, P.; Rouaud, T.; Lebouvier, T.; Des Varannes, S.B.; Neunlist, M.; De Giorgio, R. Parkinson Disease: The Enteric Nervous System Spills Its Guts. Neurology 2011, 77, 1761–1767. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and Clinical Implications of the Brain–Gut–Enteric Microbiota Axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric α-Synuclein Immunoreactive Inclusions in Meissner’s and Auerbach’s Plexuses in Cases Staged for Parkinson’s Disease-Related Brain Pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct Evidence of Parkinson Pathology Spread from the Gastrointestinal Tract to the Brain in Rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef]
- Singaram, C.; Gaumnitz, E.; Torbey, C.; Ashraf, W.; Quigley, E.; Sengupta, A.; Pfeiffer, R. Dopaminergic Defect of Enteric Nervous System in Parkinson’s Disease Patients with Chronic Constipation. Lancet 1995, 346, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of Toxicity in Rotenone Models of Parkinson’s Disease. J. Neurochem. 2007, 100, 1469. [Google Scholar] [CrossRef]
- Ramalingam, M.; Huh, Y.-J.; Lee, Y.-I. The Impairments of α-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front. Neurosci. 2019, 13, 1028. [Google Scholar] [CrossRef]
- Hecking, I.; Stegemann, L.N.; Stahlke, S.; Theis, V.; Vorgerd, M.; Matschke, V.; Theiss, C. Methods to Study the Myenteric Plexus of Rat Small Intestine. Cell. Mol. Neurobiol. 2023, 43, 315–325. [Google Scholar] [CrossRef]
- Tapani, E.; Taavitsainen, M.; Lindros, K.; Vehmas, T.; Lehtonen, E. Toxicity of Ethanol in Low Concentrations: Experimental Evaluation in Cell Culture. Acta Radiol. 1996, 37, 923–926. [Google Scholar] [CrossRef]
- Cheng, L.; Pricolo, V.; Biancani, P.; Behar, J. Overexpression of Progesterone Receptor B Increases Sensitivity of Human Colon Muscle Cells to Progesterone. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G493–G502. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone Receptors: Form and Function in Brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Hirata, S.; Nozawa, A.; Yamada-Mouri, N. Gene Expression of Progesterone Receptor Isoforms in the Rat Brain. Horm. Behav. 1994, 28, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Roach, J.K.; del Cerro, M.C.R.; Guillamón, A.; Segovia, S.; Sheehan, T.P.; Numan, M.J. Expression of Intracellular Progesterone Receptors in Rat Brain during Different Reproductive States, and Involvement in Maternal Behavior. Brain Res. 1999, 830, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone Receptors, Their Isoforms and Progesterone Regulated Transcription. Mol. Cell. Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef]
- Guerra-Araiza, C.; Reyna-Neyra, A.; Salazar, A.M.; Cerbón, M.A.; Morimoto, S.; Camacho-Arroyo, I. Progesterone Receptor Isoforms Expression in the Prepuberal and Adult Male Rat Brain. Brain Res. Bull. 2001, 54, 13–17. [Google Scholar] [CrossRef]
- Sakamoto, H.; Shikimi, H.; Ukena, K.; Tsutsui, K. Neonatal Expression of Progesterone Receptor Isoforms in the Cerebellar Purkinje Cell in Rats. Neurosci. Lett. 2003, 343, 163–166. [Google Scholar] [CrossRef]
- Theis, V.; Theiss, C. Progesterone: A Universal Stimulus for Neuronal Cells? Neural Regen. Res. 2015, 10, 547. [Google Scholar] [CrossRef]
- Toms, D.; Xu, S.; Pan, B.; Wu, D.; Li, J. Progesterone Receptor Expression in Granulosa Cells Is Suppressed by MicroRNA-378-3p. Mol. Cell. Endocrinol. 2015, 399, 95–102. [Google Scholar] [CrossRef]
- Rowan, B.G.; O’Malley, B.W. Progesterone Receptor Coactivators. Steroids 2000, 65, 545–549. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Yahn, S.L.; Pang, Y.; Quihuis, A.M.; Oyola, M.G.; Reyna, A.; Thomas, P.; Handa, R.J.; Mani, S.K. Distribution and Estrogen Regulation of Membrane Progesterone Receptor-β in the Female Rat Brain. Endocrinology 2012, 153, 4432–4443. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Petersen, S.L. Distribution of MRNAs Encoding Classical Progestin Receptor, Progesterone Membrane Components 1 and 2, Serpine MRNA Binding Protein 1, and Progestin and ADIPOQ Receptor Family Members 7 and 8 in Rat Forebrain. Neuroscience 2011, 172, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.L.; Deniselle, M.G.; Vinson, G.; Schumacher, M.; De Nicola, A.F.; Guennoun, R. Effects of Injury and Progesterone Treatment on Progesterone Receptor and Progesterone Binding Protein 25-Dx Expression in the Rat Spinal Cord. J. Neurochem. 2003, 87, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; De Nicola, A.F.; Stein, D.; Schumacher, M.; Guennoun, R. Distribution of Membrane Progesterone Receptor Alpha in the Male Mouse and Rat Brain and Its Regulation after Traumatic Brain Injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.-M.; Rodrigo-Angulo, M.; et al. Environmental Toxins Trigger PD-like Progression via Increased Alpha-Synuclein Release from Enteric Neurons in Mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef]
- Pan-Montojo, F.; Anichtchik, O.; Dening, Y.; Knells, L.; Pursche, S.; Jung, R.; Jackson, S.; Gille, G.; Spillantini, M.G.; Reichmann, H.; et al. Progression of Parkinson’s Disease Pathology Is Reproduced by Intragastric Administration of Rotenone in Mice. PLoS ONE 2010, 5, e8762. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Gawkowska, A.; Lodde, V.; Wu, C.A. Progesterone Inhibits Apoptosis in Part by PGRMC1-Regulated Gene Expression. Mol. Cell. Endocrinol. 2010, 320, 153–161. [Google Scholar] [CrossRef]
- Bushnell, C.D.; Chaturvedi, S.; Gage, K.R.; Herson, P.S.; Hurn, P.D.; Jimenez, M.C.; Kittner, S.J.; Madsen, T.E.; McCullough, L.D.; McDermott, M.; et al. Sex Differences in Stroke: Challenges and Opportunities. J. Cereb. Blood Flow Metab. 2018, 38, 2179–2191. [Google Scholar] [CrossRef]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. Traumatic Brain Injury: Sex, Gender and Intersecting Vulnerabilities. Nat. Rev. Neurol. 2018, 14, 711–722. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinson’S Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Peluso, J.J.; Romak, J.; Liu, X. Progesterone Receptor Membrane Component-1 (PGRMC1) Is the Mediator of Progesterone’s Antiapoptotic Action in Spontaneously Immortalized Granulosa Cells as Revealed by PGRMC1 Small Interfering Ribonucleic Acid Treatment and Functional Analysis of PGRMC1 Mutations. Endocrinology 2008, 149, 534–543. [Google Scholar] [PubMed]
- Peluso, J.J.; Pappalardo, A.; Losel, R.; Wehling, M. Expression and Function of PAIRBP1 within Gonadotropin-Primed Immature Rat Ovaries: PAIRBP1 Regulation of Granulosa and Luteal Cell Viability. Biol. Reprod. 2005, 73, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Peluso, J.J.; Yuan, A.; Liu, X.; Lodde, V. Plasminogen Activator Inhibitor 1 RNA-Binding Protein Interacts with Progesterone Receptor Membrane Component 1 to Regulate Progesterone’s Ability to Maintain the Viability of Spontaneously Immortalized Granulosa Cells and Rat Granulosa Cells. Biol. Reprod. 2013, 88, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A. Progesterone Receptor Membrane Component 1: An Integrative Review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.K.; Roemer, S.C.; Churchill, M.E.; Edwards, D.P. Structural and Functional Analysis of Domains of the Progesterone Receptor. Mol. Cell. Endocrinol. 2012, 348, 418–429. [Google Scholar] [CrossRef]
- DeMayo, F.J.; Lydon, J.P. New Insights into Progesterone Receptor Signaling in the Endometrium Required for Embryo Implantation. J. Mol. Endocrinol. 2020, 65, T1. [Google Scholar] [CrossRef]
- O’Malley, B.; Sherman, M.; Toft, D. Progesterone “Receptors” in the Cytoplasm and Nucleus of Chick Oviduct Target Tissue. Proc. Natl. Acad. Sci. USA 1970, 67, 501–508. [Google Scholar] [CrossRef]
- Zhao, H.; Sapolsky, R.M.; Steinberg, G.K. Phosphoinositide-3-Kinase/Akt Survival Signal Pathways Are Implicated in Neuronal Survival after Stroke. Mol. Neurobiol. 2006, 34, 249–269. [Google Scholar] [CrossRef]
- Ishrat, T.; Sayeed, I.; Atif, F.; Hua, F.; Stein, D.G. Progesterone Is Neuroprotective against Ischemic Brain Injury through Its Effects on the Phosphoinositide 3-Kinase/Protein Kinase B Signaling Pathway. Neuroscience 2012, 210, 442–450. [Google Scholar] [CrossRef]
- Zhang, M.; He, Q.; Chen, G.; Li, P.A. Suppression of NLRP3 Inflammasome, Pyroptosis, and Cell Death by NIM811 in Rotenone-Exposed Cells as an in Vitro Model of Parkinson’s Disease. Neurodegener. Dis. 2020, 20, 73–83. [Google Scholar] [CrossRef]
- Singh, M. Ovarian Hormones Elicit Phosphorylation of Akt and Extracellular-Signal Regulated Kinase in Explants of the Cerebral Cortex. Endocrine 2001, 14, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Intlekofer, K.; Moura-Conlon, P.; Brewer, D.; Del Pino Sans, J.; Lopez, J. Nonclassical Progesterone Signalling Molecules in the Nervous System. J. Neuroendocrinol. 2013, 25, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Rausch, W.-D.; Gille, G. Rotenone Induces Cell Death in Primary Dopaminergic Culture by Increasing ROS Production and Inhibiting Mitochondrial Respiration. Neurochem. Int. 2006, 49, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Roof, R.L.; Hoffman, S.W.; Stein, D.G. Progesterone Protects against Lipid Peroxidation Following Traumatic Brain Injury in Rats. Mol. Chem. Neuropathol. 1997, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Crudden, G.; Loesel, R.; Craven, R.J. Overexpression of the Cytochrome P450 Activator Hpr6 (Heme-1 Domain Protein/Human Progesterone Receptor) in Tumors. Tumor Biol. 2005, 26, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Hand, R.A.; Craven, R.J. Hpr6. 6 Protein Mediates Cell Death from Oxidative Damage in MCF-7 Human Breast Cancer Cells. J. Cell. Biochem. 2003, 90, 534–547. [Google Scholar] [CrossRef]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Ann. Emerg. Med. 2007, 49, 391–402. [Google Scholar] [CrossRef]
- Xiao, G.; Wei, J.; Yan, W.; Wang, W.; Lu, Z. Improved Outcomes from the Administration of Progesterone for Patients with Acute Severe Traumatic Brain Injury: A Randomized Controlled Trial. Crit. Care 2008, 12, R61. [Google Scholar] [CrossRef]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R.; Palesch, Y.Y.; Hertzberg, V.S.; Frankel, M.; Goldstein, F.C.; Caveney, A.F.; Howlett-Smith, H.; Bengelink, E.M.; et al. Very Early Administration of Progesterone for Acute Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; Van Der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, I.; Stein, D.G. Response to Korley et al.: Progesterone Treatment Does Not Decrease Serum Levels of Biomarkers of Glial and Neuronal Cell Injury in Moderate and Severe TBI Subjects: A Secondary Analysis of the Progesterone for Traumatic Brain Injury, Experimental Clinical Treatment (ProTECT) III Trial (DOI: 10.1089/Neu. 2020.7072). J. Neurotrauma 2021, 38, 2923–2926. [Google Scholar] [PubMed]

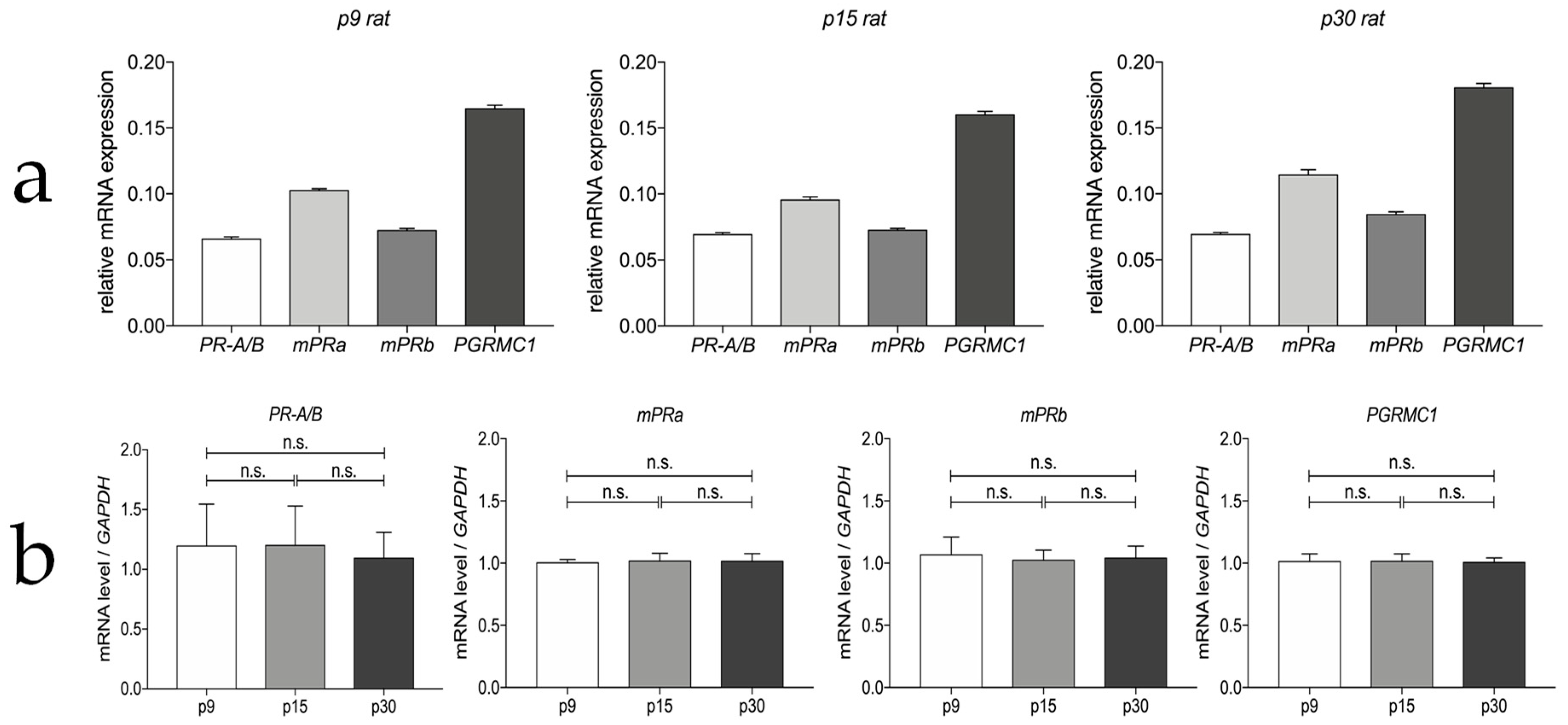
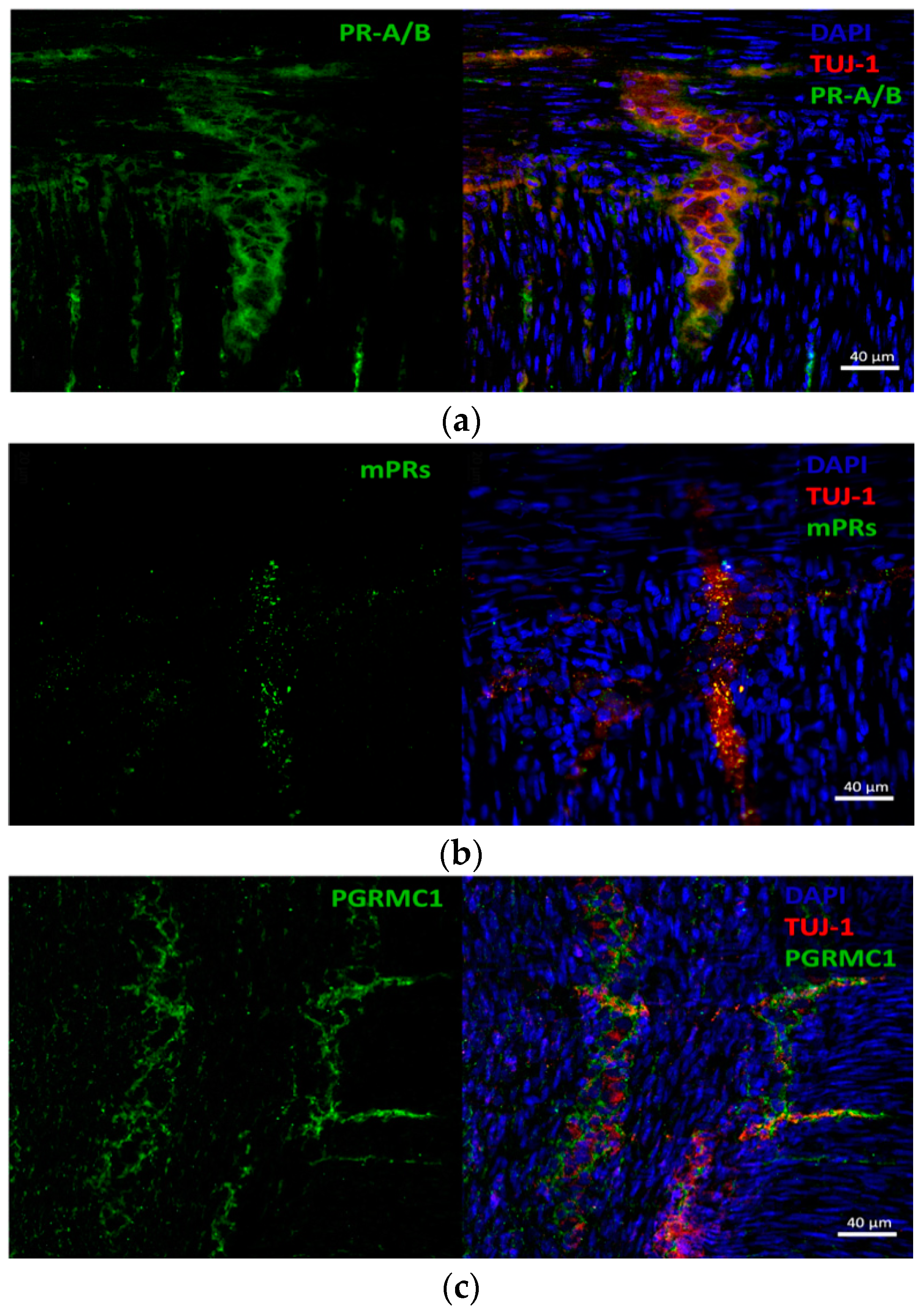
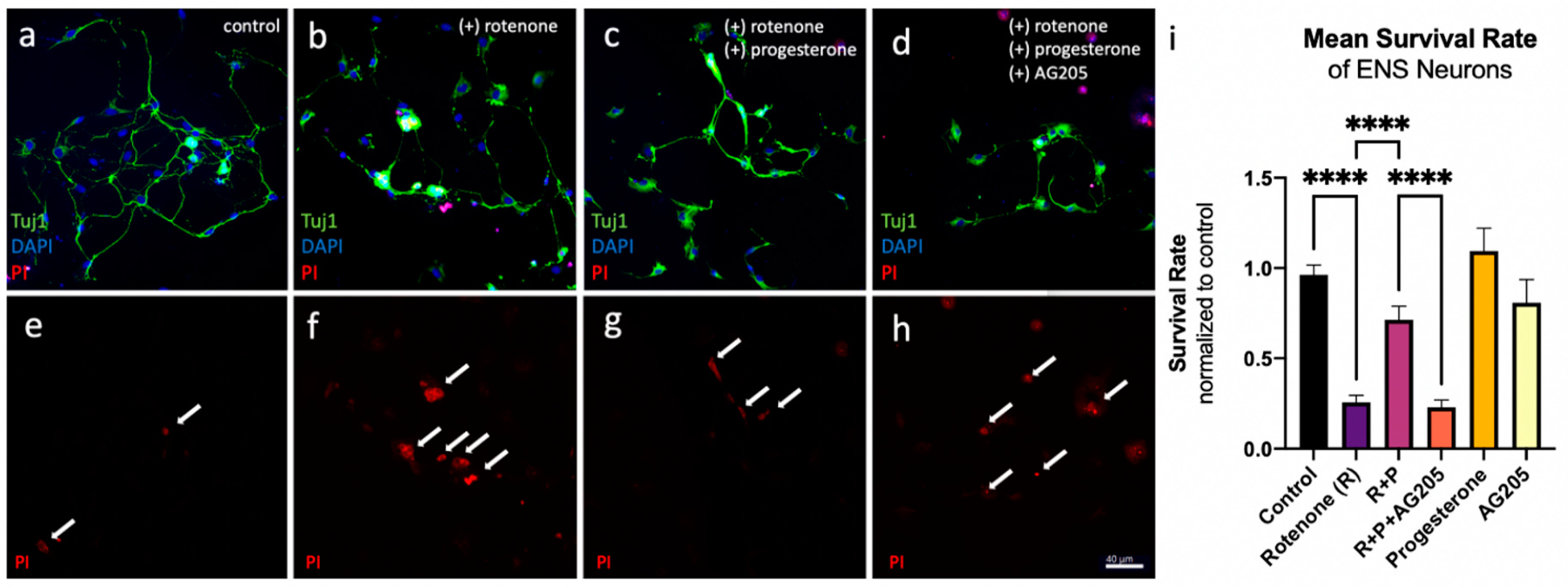
| GAPDH | 5′-ACT CCC ATT CTT CCA CCT TTG-3′, 3′-CCC TGT TGC TGT AGC CAT ATT-5′ (Microsynth) |
| NR3C3 (PR-A/B) | 5′-AGC ATG TCA GTG GAC AGA TG-3′, 3′-TAA GGC ACA GCG AGT AGA ATG-5′ (Microsynth) |
| PAQR7 (mPRa) | 5′-CCA CGG TTA TGC CTG AGA GT-3′, 3′-TAG TCC AGC GTC ACA GCT TC-5′ (Microsynth) |
| PAQR8 (mPRb) | 5′-AGA AGG GCT TCC CAA GAT GC-3′, 3′-AGT AGT AACGCC ACT CGT GC-5′ (Microsynth) |
| PGRMC1 | 5′-GAT GAC CTT TCT GAC CTCA CTC-3′, 3′-TTC CCA CGT GAT GGT ACT TG-5′ (Microsynth) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stegemann, L.N.; Neufeld, P.M.; Hecking, I.; Vorgerd, M.; Matschke, V.; Stahlke, S.; Theiss, C. Progesterone: A Neuroprotective Steroid of the Intestine. Cells 2023, 12, 1206. https://doi.org/10.3390/cells12081206
Stegemann LN, Neufeld PM, Hecking I, Vorgerd M, Matschke V, Stahlke S, Theiss C. Progesterone: A Neuroprotective Steroid of the Intestine. Cells. 2023; 12(8):1206. https://doi.org/10.3390/cells12081206
Chicago/Turabian StyleStegemann, Lennart Norman, Paula Maria Neufeld, Ines Hecking, Matthias Vorgerd, Veronika Matschke, Sarah Stahlke, and Carsten Theiss. 2023. "Progesterone: A Neuroprotective Steroid of the Intestine" Cells 12, no. 8: 1206. https://doi.org/10.3390/cells12081206
APA StyleStegemann, L. N., Neufeld, P. M., Hecking, I., Vorgerd, M., Matschke, V., Stahlke, S., & Theiss, C. (2023). Progesterone: A Neuroprotective Steroid of the Intestine. Cells, 12(8), 1206. https://doi.org/10.3390/cells12081206










