The Therapeutic Potential of Non-Invasive and Invasive Cerebellar Stimulation Techniques in Hereditary Ataxias
Abstract
1. Introduction
2. Cerebellar tDCS in Hereditary Ataxias
2.1. General Background
2.2. Cellular Mechanisms and Network Effects of tDCS
2.2.1. Insights from Supratentorial Brain Regions
2.2.2. Evidence from Cerebellar tDCS
2.3. Clinical and Neurophysiological Effects of Cerebellar tDCS
2.3.1. Single-Session Cerebellar tDCS
2.3.2. Repeated Sessions of Cerebellar tDCS
2.4. Future Perspectives
3. Cerebellar TMS in Hereditary Ataxias
3.1. General Background
3.2. Cellular Mechanisms and Network Effects of TMS
3.3. Clinical and Neurophysiological Effects of Cerebellar TMS
3.4. Future Perspectives
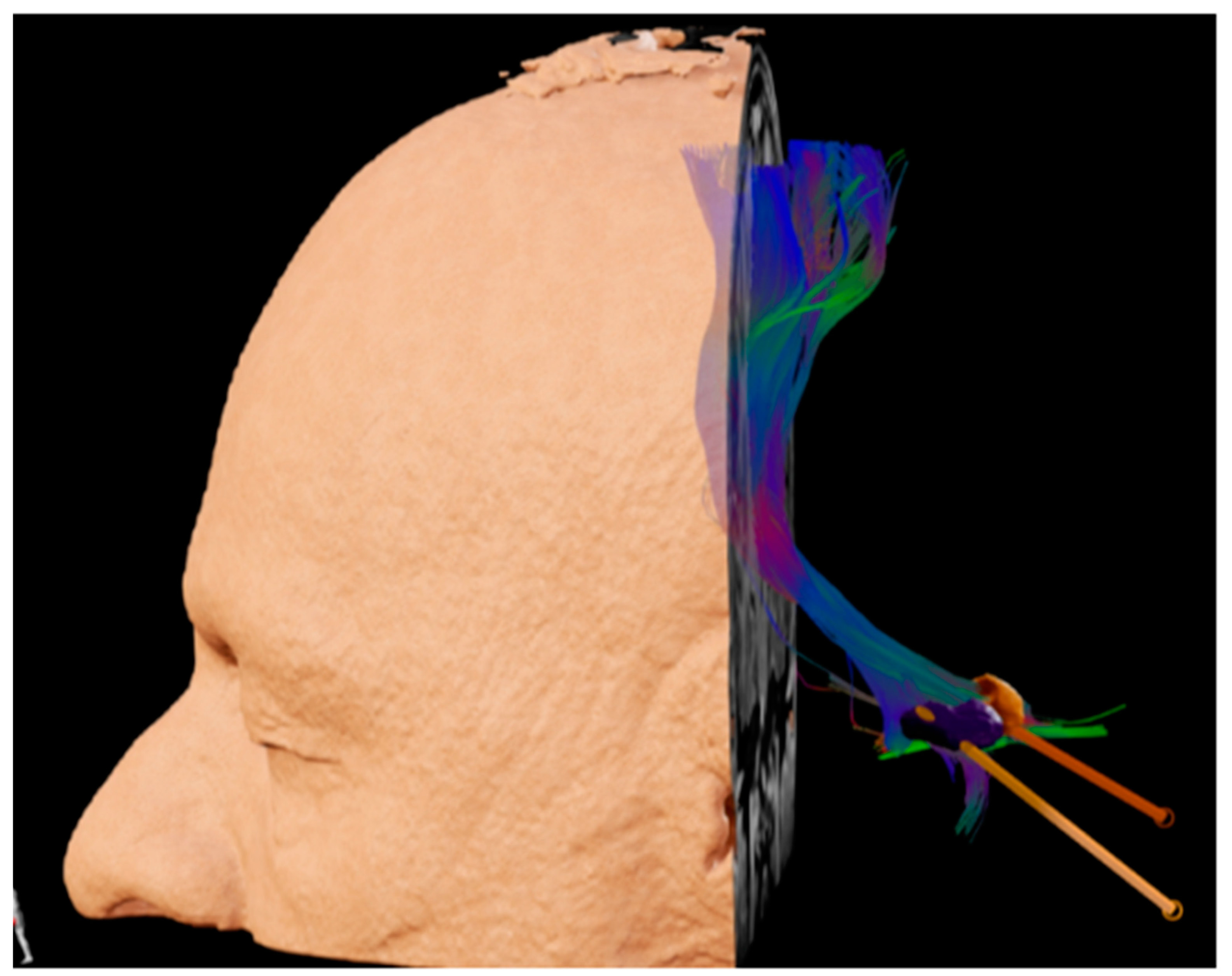
4. Cerebellar DBS in Hereditary Ataxias
4.1. General Background
4.2. Cellular Mechanisms and Network Effects of DBS
4.3. Clinical and Neurophysiological Effects of Cerebellar DBS
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuo, S.H. Ataxia. Continuum 2019, 25, 1036–1054. [Google Scholar] [CrossRef] [PubMed]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121 Pt 4, 561–579. [Google Scholar] [CrossRef]
- Salman, M.S. Epidemiology of Cerebellar Diseases and Therapeutic Approaches. Cerebellum 2018, 17, 4–11. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef]
- Synofzik, M.; Nemeth, A.H. Recessive ataxias. Handb. Clin. Neurol. 2018, 155, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Zanni, G.; Bertini, E. X-linked ataxias. Handb. Clin. Neurol. 2018, 155, 175–189. [Google Scholar] [CrossRef]
- Nagafuchi, S.; Yanagisawa, H.; Ohsaki, E.; Shirayama, T.; Tadokoro, K.; Inoue, T.; Yamada, M. Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat. Genet. 1994, 8, 177–182. [Google Scholar] [CrossRef]
- Jen, J.C.; Graves, T.D.; Hess, E.J.; Hanna, M.G.; Griggs, R.C.; Baloh, R.W.; CINCH Investigators. Primary episodic ataxias: Diagnosis, pathogenesis and treatment. Brain 2007, 130, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Durr, A.; Cossee, M.; Agid, Y.; Campuzano, V.; Mignard, C.; Penet, C.; Mandel, J.L.; Brice, A.; Koenig, M. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N. Engl. J. Med. 1996, 335, 1169–1175. [Google Scholar] [CrossRef]
- Gatti, R.A.; Berkel, I.; Boder, E.; Braedt, G.; Charmley, P.; Concannon, P.; Ersoy, F.; Foroud, T.; Jaspers, N.G.; Lange, K.; et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. Nature 1988, 336, 577–580. [Google Scholar] [CrossRef]
- Le Ber, I.; Bouslam, N.; Rivaud-Pechoux, S.; Guimaraes, J.; Benomar, A.; Chamayou, C.; Goizet, C.; Moreira, M.C.; Klur, S.; Yahyaoui, M.; et al. Frequency and phenotypic spectrum of ataxia with oculomotor apraxia 2: A clinical and genetic study in 18 patients. Brain 2004, 127, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Le Ber, I.; Moreira, M.C.; Rivaud-Pechoux, S.; Chamayou, C.; Ochsner, F.; Kuntzer, T.; Tardieu, M.; Said, G.; Habert, M.O.; Demarquay, G.; et al. Cerebellar ataxia with oculomotor apraxia type 1: Clinical and genetic studies. Brain 2003, 126, 2761–2772. [Google Scholar] [CrossRef]
- Ouahchi, K.; Arita, M.; Kayden, H.; Hentati, F.; Ben Hamida, M.; Sokol, R.; Arai, H.; Inoue, K.; Mandel, J.L.; Koenig, M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat. Genet. 1995, 9, 141–145. [Google Scholar] [CrossRef]
- Cortese, A.; Simone, R.; Sullivan, R.; Vandrovcova, J.; Tariq, H.; Yau, W.Y.; Humphrey, J.; Jaunmuktane, Z.; Sivakumar, P.; Polke, J.; et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat. Genet. 2019, 51, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Cali, J.J.; Hsieh, C.L.; Francke, U.; Russell, D.W. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 1991, 266, 7779–7783. [Google Scholar] [CrossRef]
- Hagerman, R.; Hagerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.J.; Bindoff, L.A. Mitochondrial ataxias. Handb. Clin. Neurol. 2018, 155, 129–141. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Wilmot, G.; Kuo, S.H.; Perlman, S.; Greenstein, P.E.; Ying, S.H.; Ashizawa, T.; Subramony, S.H.; Schmahmann, J.D.; Figueroa, K.P.; et al. Comprehensive systematic review summary: Treatment of cerebellar motor dysfunction and ataxia: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 464–471. [Google Scholar] [CrossRef]
- Ferrucci, R.; Bocci, T.; Cortese, F.; Ruggiero, F.; Priori, A. Cerebellar transcranial direct current stimulation in neurological disease. Cerebellum Ataxias 2016, 3, 16. [Google Scholar] [CrossRef]
- Grimaldi, G.; Argyropoulos, G.P.; Bastian, A.; Cortes, M.; Davis, N.J.; Edwards, D.J.; Ferrucci, R.; Fregni, F.; Galea, J.M.; Hamada, M.; et al. Cerebellar Transcranial Direct Current Stimulation (ctDCS): A Novel Approach to Understanding Cerebellar Function in Health and Disease. Neuroscientist 2016, 22, 83–97. [Google Scholar] [CrossRef]
- Grimaldi, G.; Argyropoulos, G.P.; Boehringer, A.; Celnik, P.; Edwards, M.J.; Ferrucci, R.; Galea, J.M.; Groiss, S.J.; Hiraoka, K.; Kassavetis, P.; et al. Non-invasive cerebellar stimulation—A consensus paper. Cerebellum 2014, 13, 121–138. [Google Scholar] [CrossRef]
- Maas, R.P.P.W.M.; Helmich, R.C.G.; van de Warrenburg, B.P.C. The role of the cerebellum in degenerative ataxias and essential tremor: Insights from noninvasive modulation of cerebellar activity. Mov. Disord. 2020, 35, 215–227. [Google Scholar] [CrossRef]
- Manto, M.; Argyropoulos, G.P.D.; Bocci, T.; Celnik, P.A.; Corben, L.A.; Guidetti, M.; Koch, G.; Priori, A.; Rothwell, J.C.; Sadnicka, A.; et al. Consensus Paper: Novel Directions and Next Steps of Non-invasive Brain Stimulation of the Cerebellum in Health and Disease. Cerebellum 2022, 21, 1092–1122. [Google Scholar] [CrossRef]
- Manto, M.; Ben Taib, N.O. A novel approach for treating cerebellar ataxias. Med. Hypotheses 2008, 71, 58–60. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M. The Era of Cerebellar Therapy. Curr. Neuropharmacol. 2019, 17, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.M.; Janssen, A.M.; Lucka, F.; Aydin, U.; Lanfer, B.; Lew, S.; Wolters, C.H.; Stegeman, D.F.; Oostendorp, T.F. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tames, J.; Asai, A.; Mikkonen, M.; Laakso, I.; Tanaka, S.; Uehara, S.; Otaka, Y.; Hirata, A. Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J. Neural Eng. 2019, 16, 036001. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, M.; Rossi, E.; Ferrucci, R.; Liorni, I.; Priori, A.; Ravazzani, P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin. Neurophysiol. 2014, 125, 577–584. [Google Scholar] [CrossRef]
- Ferrucci, R.; Cortese, F.; Priori, A. Cerebellar tDCS: How to do it. Cerebellum 2015, 14, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. J. Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef]
- Purpura, D.P.; McMurtry, J.G. Intracellular Activities and Evoked Potential Changes during Polarization of Motor Cortex. J. Neurophysiol. 1965, 28, 166–185. [Google Scholar] [CrossRef]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of Transcranial Direct Current Stimulation. J. ECT 2018, 34, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Jaussi, W.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology 2004, 29, 1573–1578. [Google Scholar] [CrossRef]
- Clark, V.P.; Coffman, B.A.; Trumbo, M.C.; Gasparovic, C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A (1)H magnetic resonance spectroscopy study. Neurosci. Lett. 2011, 500, 67–71. [Google Scholar] [CrossRef]
- Krause, B.; Marquez-Ruiz, J.; Cohen Kadosh, R. The effect of transcranial direct current stimulation: A role for cortical excitation/inhibition balance? Front. Hum. Neurosci. 2013, 7, 602. [Google Scholar] [CrossRef]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef]
- Antonenko, D.; Thielscher, A.; Saturnino, G.B.; Aydin, S.; Ittermann, B.; Grittner, U.; Floel, A. Towards precise brain stimulation: Is electric field simulation related to neuromodulation? Brain Stimul. 2019, 12, 1159–1168. [Google Scholar] [CrossRef]
- Nandi, T.; Puonti, O.; Clarke, W.T.; Nettekoven, C.; Barron, H.C.; Kolasinski, J.; Hanayik, T.; Hinson, E.L.; Berrington, A.; Bachtiar, V.; et al. tDCS induced GABA change is associated with the simulated electric field in M1, an effect mediated by grey matter volume in the MRS voxel. Brain Stimul. 2022, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Stephenson, M.C.; Morris, P.G.; Jackson, S.R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7 T magnetic resonance spectroscopy study. Neuroimage 2014, 99, 237–243. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage 2014, 86, 19–27. [Google Scholar] [CrossRef] [PubMed]
- McLaren, M.E.; Nissim, N.R.; Woods, A.J. The effects of medication use in transcranial direct current stimulation: A brief review. Brain Stimul. 2018, 11, 52–58. [Google Scholar] [CrossRef]
- Evans, R.C.; Blackwell, K.T. Calcium: Amplitude, duration, or location? Biol. Bull. 2015, 228, 75–83. [Google Scholar] [CrossRef]
- Yang, S.N.; Tang, Y.G.; Zucker, R.S. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 1999, 81, 781–787. [Google Scholar] [CrossRef]
- Lisman, J.E. Three Ca2+ levels affect plasticity differently: The LTP zone, the LTD zone and no man’s land. J. Physiol. 2001, 532, 285. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Aggleton, J.P.; Brown, M.W.; Bashir, Z.I. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J. Physiol. 2001, 532, 459–466. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Agboada, D.; Mosayebi Samani, M.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci. Rep. 2019, 9, 18185. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Rezaee, Z.; Chang, D.I.; Gerwig, M.; Herlitze, S.; Dutta, A.; Nitsche, M.A.; Timmann, D. Effects of cerebellar transcranial direct current stimulation on cerebellar-brain inhibition in humans: A systematic evaluation. Brain Stimul. 2019, 12, 1177–1186. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Boggio, P.S.; Rigonatti, S.P.; Ribeiro, R.B.; Myczkowski, M.L.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 2008, 11, 249–254. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanão, T.A.; de Oliveira, J.F.; Goulart, A.; Boggio, P.S.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F. The sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013, 70, 383–391. [Google Scholar] [CrossRef]
- Gartside, I.B. Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: Reverberating circuits or modification of synaptic conductance? Nature 1968, 220, 382–383. [Google Scholar] [CrossRef]
- Gartside, I.B. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: Role of protein synthesis. Nature 1968, 220, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J. Neurophysiol. 2010, 103, 1735–1740. [Google Scholar] [CrossRef]
- Mosayebi Samani, M.; Agboada, D.; Kuo, M.F.; Nitsche, M.A. Probing the relevance of repeated cathodal transcranial direct current stimulation over the primary motor cortex for prolongation of after-effects. J. Physiol. 2020, 598, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, J.; Muggleton, N.; Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008, 12, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Zhang, L.; Chan, S.S.M.; Lam, L.C.W. Dynamic changes of region-specific cortical features and scalp-to-cortex distance: Implications for transcranial current stimulation modeling. J. Neuroeng. Rehabil. 2021, 18, 2. [Google Scholar] [CrossRef]
- Rahman, A.; Toshev, P.K.; Bikson, M. Polarizing cerebellar neurons with transcranial Direct Current Stimulation. Clin. Neurophysiol. 2014, 125, 435–438. [Google Scholar] [CrossRef]
- Chan, C.Y.; Hounsgaard, J.; Nicholson, C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J. Physiol. 1988, 402, 751–771. [Google Scholar] [CrossRef]
- Chan, C.Y.; Nicholson, C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J. Physiol. 1986, 371, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hancock, R.; Santaniello, S. Transcranial direct current stimulation of cerebellum alters spiking precision in cerebellar cortex: A modeling study of cellular responses. PLoS Comput. Biol. 2021, 17, e1009609. [Google Scholar] [CrossRef]
- Ugawa, Y.; Uesaka, Y.; Terao, Y.; Hanajima, R.; Kanazawa, I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995, 37, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, Z.J.; Paradiso, G.O.; Christensen, B.K.; Fitzgerald, P.B.; Gunraj, C.; Chen, R. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004, 557, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.D.; Chen, R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp. Brain Res. 2001, 140, 505–510. [Google Scholar] [CrossRef]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef]
- Kuper, M.; Mallick, J.S.; Ernst, T.; Kraff, O.; Thurling, M.; Stefanescu, M.R.; Goricke, S.; Nitsche, M.A.; Timmann, D. Cerebellar transcranial direct current stimulation modulates the fMRI signal in the cerebellar nuclei in a simple motor task. Brain Stimul. 2019, 12, 1169–1176. [Google Scholar] [CrossRef]
- Pope, P.A.; Miall, R.C. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012, 5, 84–94. [Google Scholar] [CrossRef]
- Ferrucci, R.; Giannicola, G.; Rosa, M.; Fumagalli, M.; Boggio, P.S.; Hallett, M.; Zago, S.; Priori, A. Cerebellum and processing of negative facial emotions: Cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn. Emot. 2012, 26, 786–799. [Google Scholar] [CrossRef]
- D’Mello, A.M.; Turkeltaub, P.E.; Stoodley, C.J. Cerebellar tDCS Modulates Neural Circuits during Semantic Prediction: A Combined tDCS-fMRI Study. J. Neurosci. 2017, 37, 1604–1613. [Google Scholar] [CrossRef]
- Oldrati, V.; Schutter, D.J.L.G. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: A Meta-Analysis. Cerebellum 2018, 17, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, Z.; Ferrari, C.; Ciricugno, A.; Heleven, E.; Schutter, D.; Manto, M.; Van Overwalle, F. New Horizons on Non-invasive Brain Stimulation of the Social and Affective Cerebellum. Cerebellum 2022, 21, 482–496. [Google Scholar] [CrossRef]
- Grimaldi, G.; Manto, M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann. Biomed. Eng. 2013, 41, 2437–2447. [Google Scholar] [CrossRef]
- Grimaldi, G.; Oulad Ben Taib, N.; Manto, M.; Bodranghien, F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: Tremor reduction and re-programming of the timing of antagonist commands. Front. Syst. Neurosci. 2014, 8, 9. [Google Scholar] [CrossRef]
- Bodranghien, F.; Oulad Ben Taib, N.; Van Maldergem, L.; Manto, M. A Postural Tremor Highly Responsive to Transcranial Cerebello-Cerebral DCS in ARCA3. Front. Neurol. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Koch, G.; Cotelli, M.; Padovani, A.; Borroni, B. Cerebellar transcranial direct current stimulation in patients with ataxia: A double-blind, randomized, sham-controlled study. Mov. Disord. 2015, 30, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.P.P.W.M.; Teerenstra, S.; Toni, I.; Klockgether, T.; Schutter, D.; van de Warrenburg, B.P.C. Cerebellar Transcranial Direct Current Stimulation in Spinocerebellar Ataxia Type 3: A Randomized, Double-Blind, Sham-Controlled Trial. Neurotherapeutics 2022, 19, 1259–1272. [Google Scholar] [CrossRef]
- Galea, J.M.; Vazquez, A.; Pasricha, N.; de Xivry, J.J.; Celnik, P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cereb. Cortex 2011, 21, 1761–1770. [Google Scholar] [CrossRef]
- Zuchowski, M.L.; Timmann, D.; Gerwig, M. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 2014, 7, 525–531. [Google Scholar] [CrossRef]
- Cantarero, G.; Spampinato, D.; Reis, J.; Ajagbe, L.; Thompson, T.; Kulkarni, K.; Celnik, P. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J. Neurosci. 2015, 35, 3285–3290. [Google Scholar] [CrossRef]
- Hulst, T.; John, L.; Kuper, M.; van der Geest, J.N.; Goricke, S.L.; Donchin, O.; Timmann, D. Cerebellar patients do not benefit from cerebellar or M1 transcranial direct current stimulation during force-field reaching adaptation. J. Neurophysiol. 2017, 118, 732–748. [Google Scholar] [CrossRef]
- John, L.; Kuper, M.; Hulst, T.; Timmann, D.; Hermsdorfer, J. Effects of transcranial direct current stimulation on grip force control in patients with cerebellar degeneration. Cerebellum Ataxias 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Dell’Era, V.; Cotelli, M.S.; Turla, M.; Casali, C.; Padovani, A.; Borroni, B. Long term clinical and neurophysiological effects of cerebellar transcranial direct current stimulation in patients with neurodegenerative ataxia. Brain Stimul. 2017, 10, 242–250. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Bonetta, E.; Grasso, R.; Manenti, R.; Cotelli, M.; Padovani, A.; Borroni, B. Cerebello-spinal tDCS in ataxia: A randomized, double-blind, sham-controlled, crossover trial. Neurology 2018, 91, e1090–e1101. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Cantoni, V.; Manes, M.; Libri, I.; Dell’Era, V.; Datta, A.; Thomas, C.; Ferrari, C.; Di Fonzo, A.; Fancellu, R.; et al. Motor and cognitive outcomes of cerebello-spinal stimulation in neurodegenerative ataxia. Brain 2021, 144, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.P.P.W.M.; van de Warrenburg, B.P.C. Therapeutic Misestimation in Patients with Degenerative Ataxia: Lessons from a Randomized Controlled Trial. Mov. Disord. 2023, 38, 133–137. [Google Scholar] [CrossRef]
- Maas, R.P.P.W.M.; Schutter, D.J.L.G.; Toni, I.; Timmann, D.; van de Warrenburg, B.P.C. Cerebellar transcranial direct current stimulation modulates timing but not acquisition of conditioned eyeblink responses in SCA3 patients. Brain Stimul. 2022, 15, 806–813. [Google Scholar] [CrossRef]
- Pilloni, G.; Shaw, M.; Feinberg, C.; Clayton, A.; Palmeri, M.; Datta, A.; Charvet, L.E. Long term at-home treatment with transcranial direct current stimulation (tDCS) improves symptoms of cerebellar ataxia: A case report. J. Neuroeng. Rehabil. 2019, 16, 41. [Google Scholar] [CrossRef]
- Portaro, S.; Russo, M.; Bramanti, A.; Leo, A.; Billeri, L.; Manuli, A.; La Rosa, G.; Naro, A.; Calabro, R.S. The role of robotic gait training and tDCS in Friedrich ataxia rehabilitation: A case report. Medicine 2019, 98, e14447. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef]
- Pilloni, G.; Vogel-Eyny, A.; Lustberg, M.; Best, P.; Malik, M.; Walton-Masters, L.; George, A.; Mirza, I.; Zhovtis, L.; Datta, A.; et al. Tolerability and feasibility of at-home remotely supervised transcranial direct current stimulation (RS-tDCS): Single-center evidence from 6779 sessions. Brain Stimul. 2022, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Fregni, F.; Pascual-Leone, A. Technology insight: Noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 2007, 3, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Valls-Sole, J.; Wassermann, E.M.; Hallett, M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2000, 111, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Rodriguez-Labrada, R.; Velazquez-Perez, L.; Ziemann, U. Transcranial magnetic stimulation in hereditary ataxias: Diagnostic utility, pathophysiological insight and treatment. Clin. Neurophysiol. 2018, 129, 1688–1698. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Turrone, R.; Pilotto, A.; Alberici, A.; Cotelli, M.S.; Rizzetti, C.; Padovani, A.; Borroni, B. Stimulation over the cerebellum with a regular figure-of-eight coil induces reduced motor cortex inhibition in patients with progressive supranuclear palsy. Brain Stimul. 2019, 12, 1290–1297. [Google Scholar] [CrossRef]
- Iwata, N.K.; Ugawa, Y. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: A review. Cerebellum 2005, 4, 218–223. [Google Scholar] [CrossRef]
- Maas, R.P.P.W.M.; van de Warrenburg, B.P.C.; Schutter, D.J.L.G. Inverse associations between cerebellar inhibition and motor impairment in spinocerebellar ataxia type 3. Brain Stimul. 2021, 14, 351–357. [Google Scholar] [CrossRef]
- Ugawa, Y.; Genba-Shimizu, K.; Rothwell, J.C.; Iwata, M.; Kanazawa, I. Suppression of motor cortical excitability by electrical stimulation over the cerebellum in ataxia. Ann. Neurol. 1994, 36, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, Y.; Hanajima, R.; Kanazawa, I. Motor cortex inhibition in patients with ataxia. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, Y.; Terao, Y.; Hanajima, R.; Sakai, K.; Furubayashi, T.; Machii, K.; Kanazawa, I. Magnetic stimulation over the cerebellum in patients with ataxia. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Major, B.P.; Teo, W.P.; Byrne, L.K.; Enticott, P.G. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): A systematic review. Neurosci. Biobehav. Rev. 2018, 86, 176–206. [Google Scholar] [CrossRef]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.; Rinaldi, L.; Cristea, I.; Vecchi, T. Probing cerebellar involvement in cognition through a meta-analysis of TMS evidence. Sci. Rep. 2021, 11, 14777. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Zhu, Y. The Effect of Cerebellar rTMS on Modulating Motor Dysfunction in Neurological Disorders: A Systematic Review. Cerebellum 2022, in press. [CrossRef]
- Shimizu, H.; Tsuda, T.; Shiga, Y.; Miyazawa, K.; Onodera, Y.; Matsuzaki, M.; Nakashima, I.; Furukawa, K.; Aoki, M.; Kato, H.; et al. Therapeutic efficacy of transcranial magnetic stimulation for hereditary spinocerebellar degeneration. Tohoku J. Exp. Med. 1999, 189, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Tsuda, T.; Itoyama, Y.; Shimizu, H.; Miyazawa, K.I.; Jin, K.; Yamazaki, T. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J. Neurol. Neurosurg. Psychiatry 2002, 72, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Takata, H.; Tanabe, Y.; Nobukuni, K.; Hayabara, T. Influence of repetitive transcranial magnetic stimulation on disease severity and oxidative stress markers in the cerebrospinal fluid of patients with spinocerebellar degeneration. Neurol. Res. 2005, 27, 310–313. [Google Scholar] [CrossRef]
- Schulz, J.B.; Dehmer, T.; Schols, L.; Mende, H.; Hardt, C.; Vorgerd, M.; Burk, K.; Matson, W.; Dichgans, J.; Beal, M.F.; et al. Oxidative stress in patients with Friedreich ataxia. Neurology 2000, 55, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ando, Y.; Obayashi, K.; Terazaki, H.; Sakashita, N.; Uchida, K.; Ohama, E.; Ando, M.; Uchino, M. Oxidative injury is present in Purkinje cells in patients with olivopontocerebellar atrophy. J. Neurol. Sci. 2000, 175, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Muller, M.B.; Engelmann, M.; Keck, M.E. Repetitive transcranial magnetic stimulation in rats: Evidence for a neuroprotective effect in vitro and in vivo. Eur. J. Neurosci. 1999, 11, 3247–3254. [Google Scholar] [CrossRef]
- Manor, B.; Greenstein, P.E.; Davila-Perez, P.; Wakefield, S.; Zhou, J.; Pascual-Leone, A. Repetitive Transcranial Magnetic Stimulation in Spinocerebellar Ataxia: A Pilot Randomized Controlled Trial. Front. Neurol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Franca, C.; de Andrade, D.C.; Silva, V.; Galhardoni, R.; Barbosa, E.R.; Teixeira, M.J.; Cury, R.G. Effects of cerebellar transcranial magnetic stimulation on ataxias: A randomized trial. Park. Relat. Disord. 2020, 80, 1–6. [Google Scholar] [CrossRef]
- Chen, X.Y.; Lian, Y.H.; Liu, X.H.; Sikandar, A.; Li, M.C.; Xu, H.L.; Hu, J.P.; Chen, Q.L.; Gan, S.R. Effects of Repetitive Transcranial Magnetic Stimulation on Cerebellar Metabolism in Patients with Spinocerebellar Ataxia Type 3. Front. Aging Neurosci. 2022, 14, 827993. [Google Scholar] [CrossRef]
- Sikandar, A.; Liu, X.H.; Xu, H.L.; Li, Y.; Lin, Y.Q.; Chen, X.Y.; Li, G.H.; Lin, M.T.; Wang, N.; Chen, W.J.; et al. Short-term efficacy of repetitive transcranial magnetic stimulation in SCA3: A prospective, randomized, double-blind, sham-controlled study. Park. Relat. Disord. 2023, 106, 105236. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Follesa, P.; Tacconi, P.; Serra, M.; Pisu, M.G.; Cocco, V.; Figorilli, M.; Defazio, G.; Puligheddu, M. Therapeutic Use of Cerebellar Intermittent Theta Burst Stimulation (iTBS) in a Sardinian Family Affected by Spinocerebellar Ataxia 38 (SCA 38). Cerebellum 2022, 21, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.; Su, X.; Zhou, Z.; Che, S.; Zeng, S.; Chen, S.; Guo, Y. Beneficial effects of cerebellar rTMS stimulation on a patient with spinocerebellar ataxia type 6. Brain Stimul. 2019, 12, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Etoh, S.; Shimodozono, M. Transcranial magnetic stimulation for diplopia in a patient with spinocerebellar ataxia type 6: A case report. Cerebellum Ataxias 2018, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Chang, Y.; Liu, P.; Jones, J.A.; Chen, X.; Peng, D.; Chen, M.; Wu, C.; Liu, H. Cerebellar Continuous Theta Burst Stimulation Facilitates Auditory-Vocal Integration in Spinocerebellar Ataxia. Cereb. Cortex 2022, 32, 455–466. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, C.; Liang, J.; Hu, M.; Chen, Y.; Huang, Z.; Yang, Z.; Zhao, J.; Chu, J. Structural alterations of spinocerebellar ataxias type 3: From pre-symptomatic to symptomatic stage. Eur. Radiol. 2023, 33, 2881–2894. [Google Scholar] [CrossRef]
- Lee, S.; Jang, K.I.; Yoon, S.; Chae, J.H. The Efficacy of Miniaturized Repetitive Transcranial Magnetic Stimulation in Patients with Depression. Clin. Psychopharmacol. Neurosci. 2019, 17, 409–414. [Google Scholar] [CrossRef]
- Fasano, A.; Aquino, C.C.; Krauss, J.K.; Honey, C.R.; Bloem, B.R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol. 2015, 11, 98–110. [Google Scholar] [CrossRef]
- Moro, E.; LeReun, C.; Krauss, J.K.; Albanese, A.; Lin, J.P.; Walleser Autiero, S.; Brionne, T.C.; Vidailhet, M. Efficacy of pallidal stimulation in isolated dystonia: A systematic review and meta-analysis. Eur. J. Neurol. 2017, 24, 552–560. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Fontaine, D.; Vandersteen, C.; Magis, D.; Lanteri-Minet, M. Neuromodulation in Cluster Headache. In Advances and Technical Standards in Neurosurgery; Schramm, J., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 42, pp. 3–21. [Google Scholar]
- Pereira, E.A.C.; Aziz, T.Z. Neuropathic Pain and Deep Brain Stimulation. Neurotherapeutics 2014, 11, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lozano, C.S.; Dallapiazza, R.F.; Lozano, A.M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. 2019, 131, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, I.E.; Elias, G.J.B.; Beyn, M.E.; Boutet, A.; Pancholi, A.; Germann, J.; Mansouri, A.; Lozano, C.S.; Lozano, A.M. Clinical trials for deep brain stimulation: Current state of affairs. Brain Stimul. 2020, 13, 378–385. [Google Scholar] [CrossRef]
- Cernera, S.; Eisinger, R.S.; Wong, J.K.; Ho, K.W.D.; Lopes, J.L.; To, K.; Carbunaru, S.; Ramirez-Zamora, A.; Almeida, L.; Foote, K.D.; et al. Long-term Parkinson’s disease quality of life after staged DBS: STN vs GPi and first vs second lead. NPJ Park. Dis. 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.B.; Gale, J.T. Mechanisms of action of deep brain stimulation (DBS). Neurosci. Biobehav. Rev. 2008, 32, 388–407. [Google Scholar] [CrossRef]
- Montgomery, E. Effects of GPi stimulation on human thalamic neuronal activity. Clin. Neurophysiol. 2006, 117, 2691–2702. [Google Scholar] [CrossRef]
- Anderson, M.E.; Postupna, N.; Ruffo, M. Effects of High-Frequency Stimulation in the Internal Globus Pallidus on the Activity of Thalamic Neurons in the Awake Monkey. J. Neurophysiol. 2003, 89, 1150–1160. [Google Scholar] [CrossRef]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef]
- Hashimoto, T.; Elder, C.M.; Okun, M.S.; Patrick, S.K.; Vitek, J.L. Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons. J. Neurosci. 2003, 23, 1916–1923. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Grill, W.M.; Sherman, D.L.; Thakor, N.V. Cellular Effects of Deep Brain Stimulation: Model-Based Analysis of Activation and Inhibition. J. Neurophysiol. 2004, 91, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Bar-Gad, I. Complex Locking Rather Than Complete Cessation of Neuronal Activity in the Globus Pallidus of a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Primate in Response to Pallidal Microstimulation. J. Neurosci. 2004, 24, 7410–7419. [Google Scholar] [CrossRef] [PubMed]
- Ruge, D.; Tisch, S.; Hariz, M.I.; Zrinzo, L.; Bhatia, K.P.; Quinn, N.P.; Jahanshahi, M.; Limousin, P.; Rothwell, J.C. Deep brain stimulation effects in dystonia: Time course of electrophysiological changes in early treatment. Mov. Disord. 2011, 26, 1913–1921. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Kuhn, A.A.; Brown, P. Beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp. Neurol. 2012, 236, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; de Hemptinne, C.; Miocinovic, S.; Qasim, S.E.; Miller, A.M.; Ostrem, J.L.; Galifianakis, N.B.; San Luciano, M.; Starr, P.A. Subthalamic local field potentials in Parkinson’s disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol. Dis. 2016, 89, 213–222. [Google Scholar] [CrossRef]
- Machado, A.G.; Baker, K.B.; Schuster, D.; Butler, R.S.; Rezai, A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009, 1280, 107–116. [Google Scholar] [CrossRef]
- Baker, K.B.; Schuster, D.; Cooperrider, J.; Machado, A.G. Deep brain stimulation of the lateral cerebellar nucleus produces frequency-specific alterations in motor evoked potentials in the rat in vivo. Exp. Neurol. 2010, 226, 259–264. [Google Scholar] [CrossRef]
- Chan, H.H.; Cooperrider, J.; Chen, Z.; Gale, J.T.; Baker, K.B.; Wathen, C.A.; Modic, C.R.; Park, H.-J.; Machado, A.G. Lateral Cerebellar Nucleus Stimulation has Selective Effects on Glutamatergic and GABAergic Perilesional Neurogenesis After Cortical Ischemia in the Rodent Model. Neurosurgery 2018, 83, 1057–1067. [Google Scholar] [CrossRef]
- Machado, A.G.; Cooperrider, J.; Furmaga, H.T.; Baker, K.B.; Park, H.-J.; Chen, Z.; Gale, J.T. Chronic 30-Hz Deep Cerebellar Stimulation Coupled with Training Enhances Post-ischemia Motor Recovery and Peri-infarct Synaptophysin Expression in Rodents. Neurosurgery 2013, 73, 344–353. [Google Scholar] [CrossRef]
- Teixeira, M.J.; Cury, R.G.; Galhardoni, R.; Barboza, V.R.; Brunoni, A.R.; Alho, E.; Lepski, G.; Ciampi de Andrade, D. Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology 2015, 85, 2075–2076. [Google Scholar] [CrossRef]
- Cury, R.G.; França, C.; Barbosa, E.R.; Galhardoni, R.; Lepski, G.; Teixeira, M.J.; Ciampi de Andrade, D. Dentate nucleus stimulation in a patient with cerebellar ataxia and tremor after cerebellar stroke: A long-term follow-up. Park. Relat. Disord. 2019, 60, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; França, C.; Duarte, K.P.; Paraguay, I.; Diniz, J.M.; Cunha, P.; Galhardoni, R.; Silva, V.; Iglesio, R.; Bissoli, A.B.; et al. Safety and Outcomes of Dentate Nucleus Deep Brain Stimulation for Cerebellar Ataxia. Cerebellum 2021, 21, 861–865. [Google Scholar] [CrossRef]
- Miterko, L.N.; Lin, T.; Zhou, J.; van der Heijden, M.E.; Beckinghausen, J.; White, J.J.; Sillitoe, R.V. Neuromodulation of the cerebellum rescues movement in a mouse model of ataxia. Nat. Commun. 2021, 12, 1295. [Google Scholar] [CrossRef]
- Anderson, C.J.; Figueroa, K.P.; Dorval, A.D.; Pulst, S.M. Deep cerebellar stimulation reduces ataxic motor symptoms in the shaker rat. Ann. Neurol. 2019, 85, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Asthana, P.; Yung, W.H.; Kwan, K.M.; Tin, C.; Ma, C.H.E. Deep Brain Stimulation of the Interposed Nucleus Reverses Motor Deficits and Stimulates Production of Anti-inflammatory Cytokines in Ataxia Mice. Mol. Neurobiol. 2022, 59, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Hekman, K.E.; Gomez, C.M. The autosomal dominant spinocerebellar ataxias: Emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J. Neurol. Neurosurg. Psychiatry 2015, 86, 554–561. [Google Scholar] [CrossRef]
- Diniz, J.M.; Cury, R.G.; Iglesio, R.F.; Lepski, G.A.; França, C.C.; Barbosa, E.R.; de Andrade, D.C.; Teixeira, M.J.; Duarte, K.P. Dentate nucleus deep brain stimulation: Technical note of a novel methodology assisted by tractography. Surg. Neurol. Int. 2021, 12, 400. [Google Scholar] [CrossRef]
- Cury, R.G.; França, C.; Silva, V.; Barbosa, E.R.; Capato, T.T.C.; Lepski, G.; Duarte, K.P.; Teixeira, M.J.; Ciampi de Andrade, D. Effects of dentate nucleus stimulation in spinocerebellar ataxia type 3. Park. Relat. Disord. 2019, 69, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Chen, H.; Hu, W.; Liu, Y.; Wang, Z.; Wang, X.; Liu, G.; Ma, H.; Zhou, J.; Feng, T. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson’s disease: A meta-analysis of controlled trials. Sci. Rep. 2018, 8, 14456. [Google Scholar] [CrossRef]
- França, C.; Carra, R.B.; Diniz, J.M.; Munhoz, R.P.; Cury, R.G. Deep brain stimulation in Parkinson’s disease: State of the art and future perspectives. Arq. Neuro-Psiquiatr. 2022, 80, 105–115. [Google Scholar] [CrossRef]
- Ushe, M.; Mink, J.W.; Revilla, F.J.; Wernle, A.; Schneider Gibson, P.; McGee-Minnich, L.; Hong, M.; Rich, K.M.; Lyons, K.E.; Pahwa, R.; et al. Effect of stimulation frequency on tremor suppression in essential tremor. Mov. Disord. 2004, 19, 1163–1168. [Google Scholar] [CrossRef]
- Constantoyannis, C.; Kumar, A.; Stoessl, A.J.; Honey, C.R. Tremor induced by thalamic deep brain stimulation in patients with complex regional facial pain. Mov. Disord. 2004, 19, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Grill, W.M.; Snyder, A.N.; Miocinovic, S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. NeuroReport 2004, 15, 1137–1140. [Google Scholar] [CrossRef]
- Anderson, C.J.; Sheppard, D.T.; Huynh, R.; Anderson, D.N.; Polar, C.A.; Dorval, A.D. Subthalamic deep brain stimulation reduces pathological information transmission to the thalamus in a rat model of parkinsonism. Front. Neural Circuits 2015, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Agnesi, F.; Connolly, A.T.; Baker, K.B.; Vitek, J.L.; Johnson, M.D. Deep Brain Stimulation Imposes Complex Informational Lesions. PLoS ONE 2013, 8, e74462. [Google Scholar] [CrossRef]
- Birdno, M.J.; Grill, W.M. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics 2008, 5, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kvernmo, N.; Konglund, A.E.; Reich, M.M.; Roothans, J.; Pripp, A.H.; Dietrichs, E.; Volkmann, J.; Skogseid, I.M. Deep Brain Stimulation for Arm Tremor: A Randomized Trial Comparing Two Targets. Ann. Neurol. 2022, 91, 585–601. [Google Scholar] [CrossRef]
- Neudorfer, C.; Butenko, K.; Oxenford, S.; Rajamani, N.; Achtzehn, J.; Goede, L.; Hollunder, B.; Ríos, A.S.; Hart, L.; Tasserie, J.; et al. Lead-DBS v3.0: Mapping deep brain stimulation effects to local anatomy and global networks. NeuroImage 2023, 268, 119862. [Google Scholar] [CrossRef]
- Béreau, M.; Kibleur, A.; Bouthour, W.; Tomkova Chaoui, E.; Maling, N.; Nguyen, T.A.K.; Momjian, S.; Vargas Gomez, M.I.; Zacharia, A.; Bally, J.F.; et al. Modeling of Electric Fields in Individual Imaging Atlas for Capsular Threshold Prediction of Deep Brain Stimulation in Parkinson’s Disease: A Pilot Study. Front. Neurol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Krüger, M.T.; Kurtev-Rittstieg, R.; Kägi, G.; Naseri, Y.; Hägele-Link, S.; Brugger, F. Evaluation of Automatic Segmentation of Thalamic Nuclei through Clinical Effects Using Directional Deep Brain Stimulation Leads: A Technical Note. Brain Sci. 2020, 10, 642. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Bonnì, S.; Ponzo, V.; Caltagirone, C.; Koch, G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct. Neurol. 2014, 29, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonni, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients with Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 170–178. [Google Scholar] [CrossRef]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, S.; Otsuru, N.; Kojima, S.; Saito, K.; Inukai, Y.; Masaki, M.; Onishi, H. Transcranial Alternating Current Stimulation with Gamma Oscillations Over the Primary Motor Cortex and Cerebellar Hemisphere Improved Visuomotor Performance. Front. Behav. Neurosci. 2018, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, S.; Otsuru, N.; Kojima, S.; Yokota, H.; Saito, K.; Inukai, Y.; Onishi, H. The effect of gamma tACS over the M1 region and cerebellar hemisphere does not depend on current intensity. J. Clin. Neurosci. 2019, 65, 54–58. [Google Scholar] [CrossRef]
- Miyaguchi, S.; Otsuru, N.; Kojima, S.; Yokota, H.; Saito, K.; Inukai, Y.; Onishi, H. Gamma tACS over M1 and cerebellar hemisphere improves motor performance in a phase-specific manner. Neurosci. Lett. 2019, 694, 64–68. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, A.; Leo, A.; Manuli, A.; Sciarrone, F.; Russo, M.; Bramanti, P.; Calabro, R.S. Effects of cerebellar transcranial alternating current stimulation on motor cortex excitability and motor function. Brain Struct. Funct. 2017, 222, 2891–2906. [Google Scholar] [CrossRef]
- Naro, A.; Leo, A.; Russo, M.; Cannavo, A.; Milardi, D.; Bramanti, P.; Calabro, R.S. Does Transcranial Alternating Current Stimulation Induce Cerebellum Plasticity? Feasibility, Safety and Efficacy of a Novel Electrophysiological Approach. Brain Stimul. 2016, 9, 388–395. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benussi, A.; Batsikadze, G.; França, C.; Cury, R.G.; Maas, R.P.P.W.M. The Therapeutic Potential of Non-Invasive and Invasive Cerebellar Stimulation Techniques in Hereditary Ataxias. Cells 2023, 12, 1193. https://doi.org/10.3390/cells12081193
Benussi A, Batsikadze G, França C, Cury RG, Maas RPPWM. The Therapeutic Potential of Non-Invasive and Invasive Cerebellar Stimulation Techniques in Hereditary Ataxias. Cells. 2023; 12(8):1193. https://doi.org/10.3390/cells12081193
Chicago/Turabian StyleBenussi, Alberto, Giorgi Batsikadze, Carina França, Rubens G. Cury, and Roderick P. P. W. M. Maas. 2023. "The Therapeutic Potential of Non-Invasive and Invasive Cerebellar Stimulation Techniques in Hereditary Ataxias" Cells 12, no. 8: 1193. https://doi.org/10.3390/cells12081193
APA StyleBenussi, A., Batsikadze, G., França, C., Cury, R. G., & Maas, R. P. P. W. M. (2023). The Therapeutic Potential of Non-Invasive and Invasive Cerebellar Stimulation Techniques in Hereditary Ataxias. Cells, 12(8), 1193. https://doi.org/10.3390/cells12081193







