Abstract
Human-relevant three-dimensional (3D) models of cerebral tissue can be invaluable tools to boost our understanding of the cellular mechanisms underlying brain pathophysiology. Nowadays, the accessibility, isolation and harvesting of human neural cells represents a bottleneck for obtaining reproducible and accurate models and gaining insights in the fields of oncology, neurodegenerative diseases and toxicology. In this scenario, given their low cost, ease of culture and reproducibility, neural cell lines constitute a key tool for developing usable and reliable models of the human brain. Here, we review the most recent advances in 3D constructs laden with neural cell lines, highlighting their advantages and limitations and their possible future applications.
1. Introduction
Despite considerable efforts, we still have a very limited understanding of how the brain works when it is healthy or sick. Non-invasive imaging methods performed in humans lack the spatial and temporal resolution to probe its microscopic anatomy and function. Thus, a simplified and accessible model of the human brain is urgently needed.
Although animal models have provided a significant boost to neuroscience research and are still widely used, their limits have been extensively demonstrated. In fact, besides the ethical issues, studies have shown that the results of animal experiments often fail to translate into human clinical trials [1]. Moreover, microscopic studies of post-mortem human brains have revealed neural structures, enhanced wiring, and forms of connectivity among nerve cells not found in other animals [2,3,4]. In this scenario, it is crucial to develop more reproducible models, exploiting human cells for facilitating the translatability of the results obtained to humans [5]. Transitioning to non-animal models is also in compliance with the 3R (Replacement, Reduction and Refinement) principles [6].
Human neural cells cultured in highly controllable and monitorable environments have been widely used for investigating neurotoxicity, neuroprotection, drug screening and therapeutic assessment for both brain tumors and neurodegenerative diseases, e.g., Parkinson’s and Alzheimer’s [7,8,9]. Possible sources of human cells are primary neurons, immortalized neural stem cells from embryonic stem cells and cell lines. Adult neural stem or progenitor cells have also been used to investigate neurodegenerative and neurological disorders, brain cancer and ischemia in vitro [10,11,12]. However, primary neurons from human brains are often prohibitively expensive, mainly because of a lack of accessibility and the costs associated with harvesting and isolation. Indeed, in many countries, brain tissue is practically unobtainable because of cultural issues [13], and there are challenges regarding where it can be collected, obtaining ethical approval and access to donors. Moreover, inter-individual variability among donors limits the standardization of procedures for their characterization and culture. Furthermore, primary neurons do not undergo cellular division, limiting the number of experiments that can be performed [14]. On the other hand, embryonic or induced pluripotent stem cells can be differentiated into neural cells. Although these cells offer an unprecedented opportunity for investigating the pathogenesis of neurodegenerative disorders, culturing and maintaining stem cells is, again, highly expensive and technically challenging. Indeed, culture and environmental conditions can alter their capacity for self-renewal and differentiation [15]. As an alternative to primary or stem cells, researchers can also exploit established cell lines, which have important benefits. They have lower costs, can be cultured more easily than primary neurons and they can expand almost indefinitely. Hence, an (almost) unlimited number of cells are available, as long as they are not induced to differentiate, allowing for experiments with several duplicates and many different conditions [14]. Furthermore, cell lines are not beset with the ethical issues associated with culturing human primary neurons and stem cells or with experiments involving animals [16,17]. However, human neural cell lines often have malignant origins, whose genetic drifts may hamper their physiology and integrity [14].
Since soma are unrealistically flattened and neuronal axonal and dendritic outgrowth cannot occur in all directions in traditional monolayers [18], advanced models have been developed where cellular protrusions arranged in space are characterized by more physiological neural dynamics [19]. In particular, brain or cerebral organoids, which are derived from human (usually pluripotent) stem cells, can mimic the 3D (three-dimensional) structure and salient functional features of the brain [20,21,22]. However, despite their use for exploring developmental diseases and neurodegenerative disorders, brain organoids still suffer from the so-called ‘batch syndrome’ (variability from batch to batch), thus they lack reproducibility in generating cellular diversity and producing mature traits [23]. Moreover, their physiological relevance and translational potential is often hindered by non-viable cores, probably due to limitations in nutrient diffusion [23,24,25].
Three-dimensional constructs have also been generated from neural cell lines and may provide a strategy for developing standardized models with better physiological relevance compared with traditional 2D (two-dimensional) cultures. In this context, after an overview of the neural cell lines commonly used in the literature, we describe recent approaches exploiting cell lines for generating 3D models of brain tissue. Given the legislative and public urge to reduce the use of animals in scientific experiments, we suggest their re-evaluation in humane and human-relevant research, particularly for regulatory applications where standardized and reproducible inter-laboratory outcomes are crucial.
2. Search Methodology
To identify articles dealing with three-dimensional in vitro human-relevant models of brain tissue involving cell lines, we first conducted an analysis of the existing scientific literature. Web of Science was used with the query: (((neur* OR brain OR cereb*) AND model*) AND (neurosphere* OR 3d OR three-dimension*) AND human AND “cell* line*”). Only studies published from 2000 to 2022 were selected, thus identifying 304 original articles and 37 review papers.
Oncology (18%) and science technology (18%) are the research areas where cellular models of human brain tissue are mainly involved (Figure 1). Scientific efforts are mostly focused on improving cell culture methods (i.e., protocols for scaffold-based or scaffold-free spheroid cultures, novel technologies for monitoring cell parameters, imaging techniques suitable for cell cultures and genetic analyses of in vitro cells) and on applying such technologies to drug testing, the analysis of signaling pathways and subcellular mechanisms crucial in cancer development.
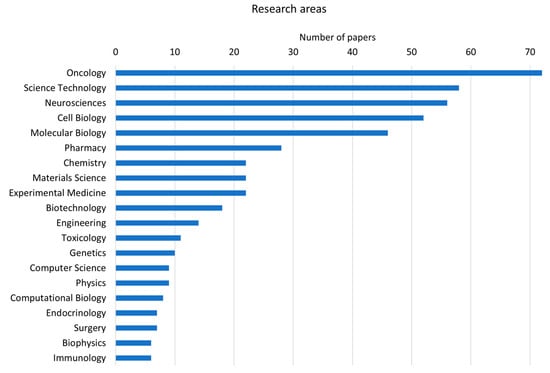
Figure 1.
Bar chart of the first 20 research areas to which the papers retrieved from the literature search belong (data derived from Clarivate Web of Science, Copyright Clarivate 2022. All rights reserved).
After this general inspection, a more precise analysis was carried out. Each abstract was read and assessed for inclusion according to whether its focus was on 3D models of brain tissue or neural tissue generated from immortalized human cell lines. Thus, a consistent number of papers was discarded because they focused on non-human models (e.g., murine, canine or porcine models), in vivo models, models developed with induced pluripotent stem cells and primary cells, or papers describing models of tissues different from the brain (e.g., colorectal, breast and pancreatic cancer models). Thus, a total of 96 papers (85 original articles and 11 review papers) was selected.
3. Cell Lines in 3D Culture
Most of the cell lines used in 3D in vitro models in neuroscience research derive from tumor tissues, in particular glioblastoma and neuroblastoma. In the following paragraphs, we describe the studies identified with the literature search in which 3D in vitro models of brain tissue have been developed using cell lines derived from tumors and from healthy tissues.
3.1. Cancer Cell Lines
The cancer cell lines most exploited in cellular neuroscience research derive from glioblastoma (52% of all papers) and neuroblastoma (35%). However, 3D models of brain tissue have also been generated using cells from embryonal carcinoma and medulloblastoma.
3.1.1. Glioblastoma Cell Lines
Glioblastoma cell lines are used in 3D in vitro constructs for modeling gliomas, in particular glioblastoma multiforme (GBM), the most common malignant brain tumor with the poorest prognosis and survival [26,27]. Most of these 3D in vitro models are focused on the development of new therapies. However, cancer treatments and the possibility of unravelling cellular and subcellular mechanisms associated with cancer are also assessed by culturing 3D glioblastoma models. The most common glioblastoma cell lines used for 3D in vitro constructs are U-87MG [28], U-251MG, U-373MG [29,30], A 172 [28] and T-98G [31]. Table 1 summarizes their origin, gender, and the age of the sample from which the cell lines were derived, the cell morphology and the first ever citation, while a general overview of the applications employing glioblastoma cell lines in 3D in vitro models is reported in Table 2.

Table 1.
Characteristics of the most common glioblastoma cell lines and year first cited.

Table 2.
Three-dimensional models generated with glioblastoma cell lines.
As detailed in Table 2, glioblastoma cell lines have been widely used for generating spheroids, even in co-cultures, mainly for oncological applications, e.g., for evaluating the effects of chemotherapeutic agents at the microscale [33,34] and for better characterizing the effects of microenvironment on cell invasive behavior [37]. However, attempts at exploiting such cells in 3D environments for toxicological applications are also present in the literature [43].
3.1.2. Neuroblastoma Cell Lines
Neuroblastoma is the most common extracranial solid tumor observed in childhood (less than five years old) [44,45], originating from precursor cells of the neural crest. Table 3 summarizes the origin, gender and age of the sample from which cell lines were derived, as well as salient information about cell morphology and their first introduction, while Table 4 recaps the main findings for neuroblastoma-derived cell lines. Where 3D models are concerned, the most widely used neuroblastoma-derived cell line is the SH-SY5Y cell line (68%, versus IMR-32 (24%), HTLA-230 (4%) and Kelly (4%)).

Table 3.
Characteristics of neuroblastoma-derived cell lines and year first cited.

Table 4.
Three-dimensional models involving neuroblastoma cell lines.
As regards the applications, even for neuroblastoma cell lines, most of the efforts have been directed towards characterizing the effects of the microenvironment on cell behavior, e.g., in terms of proliferation, cell invasiveness and differentiation. However, it is interesting to note that SH-SY5Y cells have been successfully exploited for neurodegenerative studies, and in particular Parkinson’s and Alzheimer’s diseases, accounting for 14% of the articles. Indeed, they are usually chosen for their catecholaminergic (though not strictly dopaminergic) neuronal properties [71], which the cells manifest after treatment with retinoic acid (RA), a derivative of vitamin A and serum deprivation. It has also been demonstrated that SH-SY5Y cells possess two distinct populations, an N-type which differentiates to the neural lineage, and an S-type of substrate adherent cells which express characteristics of glial cells [72]. SH-SY5Y cells have been characterized as neurosteroid-producing cells expressing key steroidogenic enzymes, and were exploited for determining whether neurosteroidogenesis may be an endogenous mechanism involved in the protection against neurodegenerative processes [73]. Recently, Martin et al. [74] showed that this cell line can express glutaminergic markers when supplemented with B-27, which widens its field of application.
Once differentiated, SH-SY5Y cells show the formation of neural processes and functional synapses, as well as the production of neuron-specific enzymes, neurotransmitters and neurotransmitter receptors [68,75,76]. In addition, unlike other neural cell lines (e.g., the IMR-32), SH-SY5Y cells can develop a resting membrane potential and have been shown to possess voltage gated calcium channels upon differentiation. Moreover, the expression and localization of key molecules involved in the pathogenesis of Alzheimer’s disease has been shown to be dramatically altered in fully differentiated SH-SY5Y cells [77].
3.1.3. Other Cancer Cell Lines
In addition to glioblastoma and neuroblastoma cell lines, 3D human brain models have been generated using cells originating from embryonal carcinoma and medulloblastoma. Human pluripotent embryonal carcinoma NTera2 (NT2) cells are widely used for in vitro neurotoxicity studies thanks to their ability to differentiate into post-mitotic neurons after treatment with RA [78,79]. NT2-laden spheroids differentiated with RA are known to express neural markers, such as tubulin and synaptophysin [80]. NT2 cells were also bioprinted, demonstrating their adhesion to fibrin gels [81].
The UW228-3 cell line was established from human posterior fossa medulloblastoma [82]. This line has been used in combination with human neural stem cells in ultra-low cell attachment plates for generating spheroids, which are exploited as an assay to test the effects of the cytotoxic drug etoposide. The spheroids comprise both cancer and stem cells, allowing the optimization of drug delivery for brain tumors in a more physiologically relevant model [83,84]. The Daoy cell line also derives from human medulloblastoma. Neurospheres laden with Daoy cells were cultured and compared with 2D monolayers grown on soft agar, revealing a higher expression of a protein related to cancer development in the 3D constructs [85].
3.2. Cell Lines Derived from Healthy Tissues
Although the majority of cell lines identified by the literature search had tumor origins, cell lines derived from healthy tissue can be used in 3D in vitro models of the human brain. Among these, the Lund human mesencephalic (LUHMES) line, which originates from the mesencephalon of a human subject, was established in 1998. The LHUMES neuron-like immortalized cells have been extensively characterized as a robust neuronal model suitable for neurodevelopmental studies, neurotoxicity and the modeling of brain diseases [86,87]. Indeed, they can be differentiated into dopaminergic neurons and thus are particularly suitable for modeling Parkinson’s disease in vitro.
Neural Progenitor Cells (NPCs) have a self-renewal capability and can give rise to healthy neural cell lineages. They can be obtained from iPSCs or derived directly from brain tissues [88,89]. In the context of this review, we considered the immortalized NPC lines because their reproducibility and proliferative potential can be exploited for the standardization of culture protocols for regulatory or preclinical tests. Commercial immortalized NPC lines employed in 3D neural in vitro models are ReNcell VM and ReNcell CX, derived, respectively, from the ventral mesencephalic and cortical region of the developing human brain and capable of differentiating into neurons and glial cells after the administration of growth factors.
Table 5 summarizes the studies where the above-mentioned cell lines derived from healthy tissues were used for generating 3D in vitro models.

Table 5.
Three-dimensional models with cell lines derived from healthy neural tissues.
4. Discussion
Over the last few decades, numerous studies have highlighted the superiority of 3D cultures with respect to monolayers as they are able to better recapitulate the morphology and architecture of tissues and cells in their native environment, both in physiological and diseases conditions. 3D systems based on human neural cell lines exhibit specific cellular and molecular features which occur in vivo [71,97] and support the expression of typical neural phenotypes and markers. Given their higher reproducibility with respect to primary cells, neural cell lines are fundamental for the definition of a standard brain environment useful for regulatory applications.
Such 3D models can be realized using both scaffold-less and scaffold-based strategies [83,84]. The former typically exploits the predominance of cohesive forces with respect to adhesion forces when cells are cultured on low attachment plates or in suspension conditions (i.e., the hanging drop method or dynamic suspension culture with bioreactors and orbital shakers) [84]. On the other hand, the scaffold-based strategy is usually obtained through cell encapsulation in polymeric solutions which undergo gelation in cytocompatible conditions or through cell seeding and colonization of pre-formed scaffolds [98]. Scaffold-based constructs can be obtained either by casting or rapid prototyping methods (e.g., bioprinting) [99,100]. Among the materials used to replicate a tissue 3D matrix, alginate is widely used for its mild and rapid gelation in contact with aqueous solutions containing divalent ions [101,102], enabling the formation of spheroids with controlled shapes through the tuning of different bioprinting parameters, such as solution viscosity, extrusion speed and needle dimension [102,103].
Several examples of alginate-based spheroids laden with the cell lines described in this review can be found in the literature, in combination with different cell adhesive materials such as gelatin and collagen [36,57,61,64,70,93]. Interestingly, some tests have never been reported; for example there are no reports on SH-SY5Y differentiation in alginate-based spheroids, although studies have been carried out in other 3D gels fabricated with different methods and geometries. Some examples include collagen gels [68], collagen-coated nanocellulose [104] or silk scaffolds [53], hyaluronic acid scaffolds [66] and chitosan–graphene oxide nanocomposite hydrogels [65]. These methods, materials and geometries could enable the creation of more physiologically relevant models, because they allow cells to grow and connect to each other directly in 3D.
Although the results obtained with the generation of spheroids are promising, more systematic studies are still needed to exploit their versatility. For instance, 3D constructs may differ significantly in terms of mechanical and transport properties. This is mainly due to the high variability of the biomaterials used for fabricating them. Matrigel- and ECM-derived matrices are known to suffer from ‘batch syndrome’; on the other hand, alginate is more reproducible, but it is available in different molecular weights, and the protocols for obtaining spheroids involve different concentrations and crosslinking methods. Moreover, as alginate is a marine-plant-derived material, it lacks cell binding sites such as RGD motifs. For this reason, some attempts to combine alginate with cell adhesive materials, e.g., gelatin [58], can be found in the literature. The use of composite materials and biofabrication strategies is increasing as they enable finer control of the geometrical features of the scaffold and tuning of material mechanical properties [81], both of which are known to influence cell behavior [83,105,106]. This is even more important for brain tissue which has a low elastic modulus that increases with age (from around 110 Pa in neonate up to ≈1 kPa in adults [107]), and which changes significantly in some neuropathologies [108].
The applications of the 3D in vitro models described in Table 2, Table 4 and Table 5 can be divided into three main fields: oncology, neurodegenerative diseases, and neurotoxicity. Most of the studies described in this review employ 3D neural models for inspecting their responses against drugs, treatments, and chemotherapeutic agents (e.g., temozolomide, epigallocatechin gallate, natural killer cells, nanoparticles, and rotenone) [33,34,35,41,42,70,91,92]. For these studies, the 3D models represent an advanced tool more closely resembling the characteristics of in vivo tumor tissues. Indeed, the resistance of human tissues to anticancer drugs is a crucial factor which needs to be assessed and characterized for developing more efficient treatments. The delivery of such compounds should be investigated and optimized considering the whole microenvironment [109]. Furthermore, the possibility of tailoring some microenvironment features, e.g., matrix stiffness [37,38], for assessing the cell response to different conditions is supported by 3D models and represents one of their advantages over conventional monolayers. Finally, cell proliferation and invasion patterns can be studied in a more relevant context in 3D models [36,39,40,64,65,69].
The development of 3D in vitro models mimicking the in vivo cell environment and behavior is also essential to obtain relevant results in toxicity testing [68,110]. A number of studies compare cell responses to toxic compounds in 3D and 2D models developed with human neural cell lines. Differentiated neuroblastoma SH-SY5Y cells exhibit lower sensitivity to toxins when cultured in 3D constructs than in 2D ones [66,67]. Furthermore, 3D models allow the inspection of the influence of the micro-environment on the cell response and sensitivity to neurotoxins [84]. For example, matrix stiffness is responsible for regulating cell sensitivity to toxins, with softer matrices reducing cell viability [43]. Chemicals or materials used in therapy (e.g., gold nanoparticles) should also be assessed to reveal any adverse effects on cell physiology when administered in different concentrations. Three-dimensional models enable the assessment of cell responses and their capability for recovery after washing out the compound in a more physiological context than monolayers [91,92]. When generated from human neural cell lines, 3D models can be judiciously employed for the high throughput screening of neurotoxic compounds, to gain as much knowledge as possible about the potential adverse effects and risks of chemicals and drugs on cell viability [80,93,94].
A wide class of pathologies is included under the definition of neurodegenerative diseases, all of them characterized by the degeneration and death of neural cells [111,112]. The development of models closely resembling in vivo neural tissues is fundamental to advance our understanding of the pathogenesis and progress of neurodegenerative diseases, as well as to assess treatment efficacy. Most of the studies analyzed in this review regarding neurodegenerative diseases employ the neuroblastoma SH-SY5Y cell line to develop 3D models where cells are differentiated into mature neurons. The efforts are directed at creating more in vivo-like models to better understand features of neurodegenerative diseases [50,51,52,54,57], pathogenesis [53] and treatment effects [60,61]. Some investigations are focused on the optimization of the differentiation protocols, and assess the capability for obtaining differentiated cells with electrically active behavior [58,59,62,63,90]. Three-dimensional models can recapitulate the salient features of neurodegenerative diseases at the microscale and, with respect to 2D models, they better resemble features of in vivo tissues [55,56]. The use of NPC lines in 3D models allows the generation of a greater level of physiological relevance since different types of neural cells can be represented [95,96].
5. Conclusions and Future Perspectives
The ability to produce in vitro models with neural cells has been fundamental to advancing the understanding of the central nervous system’s (CNS) function at the microscale, as well as of the disease mechanisms underlying neuropathologies and neurotoxicity [14,113]. We argue that some of the challenges associated with culturing primary neural cells or stem cells could be overcome with the use of neural cell lines. Indeed, since these cells express human-specific proteins and have a complete human genomic profile, they have successfully been used for different applications, as reported in this review.
From our literature search, the first thing that stands out is the heterogeneity of the studies reported and the fact that many of them do not carry on a systematic characterization of the cell lines or materials involved. Long-term culture often leads to the accumulation of mutations in such cells, resulting in outcomes which are difficult to reproduce in laboratories [114]. We also underline that most of the human cell lines used in 3D neural models originate from tumors; hence, they may not recapitulate the properties of neural cells in vivo [14]. For example, they usually show a higher sensitivity toward oxidative stress with respect to primary cells [115]. Unless characterized and quantified, these factors may limit the translatability of the results, particularly when considering clinical applications.
The regulatory testing of chemical substances to define their limits of safety for humans and the environment using in vitro methods requires a high degree of inter-lab reproducibility and throughput, with tightly defined experimental protocols. This is necessary for compliance with the Guidance on Good Cell Culture Practice (GCCP) standards [116]. Risk and hazard assessments are then carried out, using a tiered approach which considers the integration of data from different endpoints and routes, rates, and duration of exposure. Safe doses are always estimated conservatively with built in precautions, which does not account for experimental variability [117]. Most regulatory authorities do agree that animal tests should be minimized and encourage the development of alternative or non-animal methods for chemical safety assessment. Although very few in vitro methods for chemical safety assessment have been approved by the Organization for Economic Co-operation and Development (OECD), the USA’s Environmental Protection Agency (EPA) and the European Chemical Agency (ECHA) to date, several are based on cell lines. This is partly because of their accessibility and reproducibility, as well as a great deal of investment in characterization and protocol development.
We suggest that the 3D culture of neural cell lines should be improved to promote their use in safety and toxicity testing of chemicals at a regulatory level, leveraging the versatility of cells such as SH-SY5Y to exploit their full potential. Further investigations should be performed towards the standardization of protocols and towards the identification of supplements able to generate different classes of neurons in a controlled manner. Alternatively, since glial cells modulate neuron function and signaling, while neurons generate and propagate electrical and chemical signals [118], neuroblastoma and glioblastoma cell lines could be co-cultured with the aim of delivering a more physiologically relevant model of the human brain. Additionally, 3D models based on NPC lines should be exploited for their ability to differentiate into the cell types that constitute the brain. In this way, we will be able to deliver a cellular model where both the main actors in the CNS—neurons and glia—are present. The future perspectives that we suggest for improving the development of more physiologically relevant 3D in vitro models of the human brain, leveraging neuronal cell lines, are summarized in Figure 2. Once standardized and characterized [119], their full-blown reproducibility can be exploited for generating large-scale studies based on 3D spheroids for high-throughput chemical safety and toxicity testing, as well as for oncological or neurodegenerative pre-clinical screening applications [75,120,121,122].
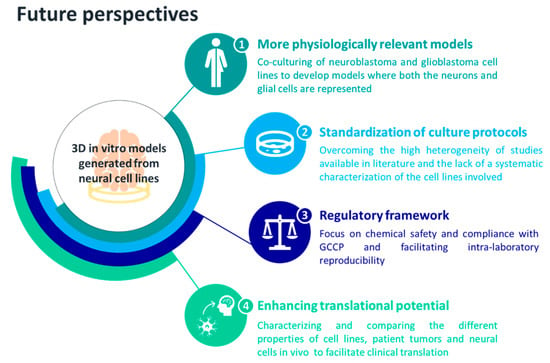
Figure 2.
Future perspectives proposed for exploiting neural cell lines in 3D culture systems.
Author Contributions
R.F. data curation, figures and original draft preparation. All authors contributed to conceptualization, writing, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the HORIZON European Innovation Council (HORIZON-EIC-2022-PathfinderOpen) under grant agreement number 101099310 (NAP).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsilidis, K.K.; Panagiotou, O.A.; Sena, E.S.; Aretouli, E.; Evangelou, E. Evaluation of Excess Significance Bias in Animal Studies of Neurological Diseases. PLoS Biol. 2013, 11, 1001609. [Google Scholar] [CrossRef] [PubMed]
- Premack, D. Human and Animal Cognition: Continuity and Discontinuity. Proc. Natl. Acad. Sci. USA 2007, 104, 13861–13867. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Bunge, M.B.; Fawcett, J.W.; Grossman, R.G.; Kaas, J.H.; Lemon, R.; Maier, I.; Martin, J.; Nudo, R.J.; Ramon-Cueto, A.; et al. Can Experiments in Nonhuman Primates Expedite the Translation of Treatments for Spinal Cord Injury in Humans? Nat. Med. 2007, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Mattes, W.B. In Vitro to in Vivo Translation. Curr. Opin. Toxicol. 2020, 23–24, 114–118. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Koh, I.; Kim, P. In Vitro Reconstruction of Brain Tumor Microenvironment. Rev. Artic. BioChip J. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Coecke, S. Relevance of in Vitro Neurotoxicity Testing for Regulatory Requirements: Challenges to Be Considered. Neurotoxicol Teratol. 2008, 32, 36–41. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell. Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- da Silva Siqueira, L.; Majolo, F.; da Silva, A.P.B.; da Costa, J.C.; Marinowic, D.R. Neurospheres: A Potential in Vitro Model for the Study of Central Nervous System Disorders. Mol. Biol. Rep. 2021, 48, 3649–3663. [Google Scholar] [CrossRef]
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in Vitro Alzheimer Disease Neuronal Models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- van Veen, E.; van der Jagt, M.; Cnossen, M.C.; Maas, A.I.R.; de Beaufort, I.D.; Menon, D.K.; Citerio, G.; Stocchetti, N.; Rietdijk, W.J.R.; van Dijck, J.T.J.M.; et al. Brain Death and Postmortem Organ Donation: Report of a Questionnaire from the CENTER-TBI Study. Crit. Care 2018, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Amini, S. General Overview of Neuronal Cell Culture. Methods Mol. Biol. 2021, 2311, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zahumenska, R.; Nosal, V.; Smolar, M.; Okajcekova, T.; Skovierova, H.; Strnadel, J.; Halasova, E. Induced Pluripotency: A Powerful Tool for In Vitro Modeling. Int. J. Mol. Sci. 2020, 21, 8910. [Google Scholar] [CrossRef] [PubMed]
- Farahany, N.A.; Greely, H.T.; Hyman, S.; Koch, C.; Grady, C.; Pasca, S.P.; Sestan, N.; Arlotta, P.; Bernat, J.L.; Ting, J.; et al. The Ethics of Experimenting with Human Brain Tissue. Nature 2018, 556, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Rosser, A.E. Challenges for Taking Primary and Stem Cells into Clinical Neurotransplantation Trials for Neurodegenerative Disease. Neurobiol. Dis. 2014, 61, 79–89. [Google Scholar] [CrossRef]
- Cullen, D.K.; Pfister, B. State of the Art and Future Challenges in Neural Engineering: Neural Interfaces: Foreword/Editors’ Commentary (Volume 1). Crit. Rev. Biomed. Eng. 2011, 39, 1–3. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Madhavan, R.; Pine, J.; Potter, S.M. Controlling Bursting in Cortical Cultures with Closed-Loop Multi-Electrode Stimulation. J. Neurosci. 2005, 25, 680–688. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesisin a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell. Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Poli, D.; Magliaro, C.; Ahluwalia, A. Experimental and Computational Methods for the Study of Cerebral Organoids: A Review. Front. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef]
- Berger, E.; Magliaro, C.; Paczia, N.; Monzel, A.S.; Antony, P.; Linster, C.L.; Bolognin, S.; Ahluwalia, A.; Schwamborn, J.C. Millifluidic Culture Improves Human Midbrain Organoid Vitality and Differentiation. Lab. Chip 2018, 18, 3172–3183. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part. C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma Multiforme: Pathogenesis and Treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Pontén, J.; Macintyre, E.H. Long Term Culture of Normal and Neoplastic Human Glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef]
- Bigner, D.D.; Bigner, S.H.; Pontén, J.; Westermark, B.; Mahaley, M.S.; Ruoslahti, E.; Herschman, H.; Eng, L.F.; Wlkstrand, C.J. Heterogeneity of Genotypic and Phenotypic Characteristics of Fifteen Permanent Cell Lines Derived from Human Gliomas. J. Neuropathol. Exp. Neurol. 1981, 40, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kum, W.; Cockram, C.S.; Teoh, R.; Young, J.D. Functional Substance P Receptors on a Human Astrocytoma Cell Line (U-373 MG). Brain Res. 1989, 488, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.H. T98G: An Anchorage-Independent Human Tumor Cell Line That Exhibits Stationary Phase G1 Arrest in Vitro. J. Cell. Physiol. 1979, 99, 43–54. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors. JNCI J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yahyanejad, S.; Van Hoof, S.J.; Theys, J.; Barbeau, L.M.O.; Granton, P.V.; Paesmans, K.; Verhaegen, F.; Vooijs, M. An Image Guided Small Animal Radiation Therapy Platform (SmART) to Monitor Glioblastoma Progression and Therapy Response. Radiother. Oncol. 2015, 116, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yahyanejad, S.; King, H.; Iglesias, V.S.; Granton, P.V.; Barbeau, L.M.O.; van Hoof, S.J.; Groot, A.J.; Habets, R.; Prickaerts, J.; Chalmers, A.J.; et al. NOTCH Blockade Combined with Radiation Therapy and Temozolomide Prolongs Survival of Orthotopic Glioblastoma. Oncotarget 2016, 7, 41251. [Google Scholar] [CrossRef]
- Morimoto, T.; Nakazawa, T.; Matsuda, R.; Nishimura, F.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Park, Y.S.; Tsujimura, T.; Nakase, H. Evaluation of Comprehensive Gene Expression and Nk Cellmediated Killing in Glioblastoma Cell Line-Derived Spheroids. Cancers 2021, 13, 4896. [Google Scholar] [CrossRef]
- Stein, A.M.; Demuth, T.; Mobley, D.; Berens, M.; Sander, L.M. A Mathematical Model of Glioblastoma Tumor Spheroid Invasion in a Three-Dimensional In Vitro Experiment. Biophys. J. 2007, 92, 356–365. [Google Scholar] [CrossRef]
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3D Brain Tumor Model to Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using Peg-Based Hydrogels. Mol. Pharm. 2014, 11, 2115–2125. [Google Scholar] [CrossRef]
- Hermida, M.A.; Kumar, J.D.; Schwarz, D.; Laverty, K.G.; Di Bartolo, A.; Ardron, M.; Bogomolnijs, M.; Clavreul, A.; Brennan, P.M.; Wiegand, U.K.; et al. Three Dimensional in Vitro Models of Cancer: Bioprinting Multilineage Glioblastoma Models. Adv. Biol. Regul. 2020, 75, 100658. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G.; Marino, B.A.; Battaglini, M.; Ciofani, G.; et al. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Lee, C.; Abelseth, E.; de la Vega, L.; Willerth, S.M. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bioink for Drug Screening. Mater. Today Chem. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.X.; Ma, J.W.; Li, H.Y.; Ye, J.C.; Xie, S.M.; Du, B.; Zhong, X.Y. EGCG Inhibits Properties of Glioma Stem-like Cells and Synergizes with Temozolomide through Downregulation of P-Glycoprotein Inhibition. J. Neurooncol. 2015, 121, 41–52. [Google Scholar] [CrossRef]
- Calori, I.R.; Alves, S.R.; Bi, H.; Tedesco, A.C. Type-I Collagen/Collagenase Modulates the 3D Structure and Behavior of Glioblastoma Spheroid Models. ACS Appl. Bio. Mater. 2022, 5, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Hara, J.; Ito, C.E.; Asuri, P. Role of Three-Dimensional Matrix Stiffness in Regulating the Response of Human Neural Cells to Toxins. Cell. Mol. Bioeng. 2014, 7, 278–284. [Google Scholar] [CrossRef]
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68, e28473. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. NIH Public Acess 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and Growth, Tumorigenicity, and Cytogenetics of Human Neuroblastoma Cells in Continuous Culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Tumilowicz, J.J.; Nichols, W.W.; Cholon, J.J.; Greene Departments, A.E.; Biology, C. Definition of a Continuous Human Cell Line Derived from Neuroblastoma1. Cancer Res. 1970, 30, 2110–2118. [Google Scholar]
- Matsushima, H.; Bogenmann, E. BI-Modal Differentiation Pattern in a New Human Neuroblastoma Cell Line in Vitro. Int. J. Cancer 1992, 51, 250–258. [Google Scholar] [CrossRef]
- Gilbert, F.; Balaban, G.; Moorhead, P.; Bianchi, D.; Schlesinger, H. Abnormalities of Chromosome 1p in Human Neuroblastoma Tumors and Cell Lines. Cancer Genet. Cytogenet. 1982, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Cui, L.; Jin, M.M.; Hu, D.Y.; Hou, X.G.; Liu, S.S.; Zhang, X.; Zhu, J.H. A Matrigel-Based 3D Construct of SH-SY5Y Cells Models the α-Synuclein Pathologies of Parkinson’s Disease. DMM Dis. Model. Mech. 2022, 15. [Google Scholar] [CrossRef]
- Taylor-Whiteley, T.R.; Le Maitre, C.L.; Duce, J.A.; Dalton, C.F.; Smith, D.P. Recapitulating Parkinson’s Disease Pathology in a Three-Dimensional Human Neural Cell Culture Model. DMM Dis. Model. Mech. 2019, 12, dmm038042. [Google Scholar] [CrossRef]
- Domert, J.; Sackmann, C.; Agholme, L.; Bergström, J.; Ingelsson, M.; Hallbeck, M.; Severinsson, E. Aggregated Alpha-Synuclein Transfer Efficiently between Cultured Human Neuron- like Cells and Localize to Lysosomes. PLoS ONE 2016, 11, e0168700. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.J.; Tamer-Mahoney, J.D.; Beheshti, A.; Nieland, T.J.F.; Kaplan, D.L. 3D Biocomposite Culture Enhances Differentiation of Dopamine-like Neurons from SH-SY5Y Cells: A Model for Studying Parkinson’s Disease Phenotypes. Biomaterials 2022, 290, 121858. [Google Scholar] [CrossRef] [PubMed]
- Chemmarappally, J.M.; Pegram, H.C.N.; Abeywickrama, N.; Fornari, E.; Hargreaves, A.J.; De Girolamo, L.A.; Stevens, B. A Co-Culture Nanofibre Scaffold Model of Neural Cell Degeneration in Relevance to Parkinson’s Disease. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krinke, D.; Jahnke, H.G.; Mack, T.G.A.; Hirche, A.; Striggow, F.; Robitzki, A.A. A Novel Organotypic Tauopathy Model on a New Microcavity Chip for Bioelectronic Label-Free and Real Time Monitoring. Biosens. Bioelectron. 2010, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Krinke, D.; Jahnke, H.G.; Hirche, A.; Kloß, D.; Mack, T.G.A.; Striggow, F.; Robitzki, A. Induced Tauopathy in a Novel 3D-Culture Model Mediates Neurodegenerative Processes: A Real-Time Study on Biochips. PLoS ONE 2012, 7, e49150. [Google Scholar] [CrossRef] [PubMed]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef]
- Dominguez-Alfaro, A.; Alegret, N.; Arnaiz, B.; Salsamendi, M.; Mecerreyes, D.; Prato, M. Toward Spontaneous Neuronal Differentiation of SH-SY5Y Cells Using Novel Three-Dimensional Electropolymerized Conductive Scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 57330–57342. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Nan, F.; Xiao, J.; Lian, J.C.; He, X.; Guo, X.; Sun, G.W.; Ma, X.J. Microcapsule Co-Culture System Enhances Neural Differentiation of Mesenchymal Stem Cells. J. Hard Tissue Biol. 2013, 22, 241–248. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined Treatment with Three Natural Antioxidants Enhances Neuroprotection in a SH-SY5Y 3D Culture Model. Antioxidants 2019, 8, 420. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Evans, R.E.; Atkinson, E.; Abdalla, A.; Gavins, F.K.H.; Boyd, A.S.; Williams, G.R.; Knowles, J.C.; Roberton, V.H.; Phillips, J.B. An Alginate-Based Encapsulation System for Delivery of Therapeutic Cells to the CNS. RSC Adv. 2022, 12, 4005–4015. [Google Scholar] [CrossRef]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, X.; Chen, X.Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.H.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Liaudanskaya, V.; Migliaresi, C.; Motta, A. Homeostasis Maintenance of Encapsulated Cells. J. Tissue Eng. Regen. Med. 2018, 12, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Geetha Marapureddy, S.; Hivare, P.; Sharma, A.; Chakraborty, J.; Ghosh, S.; Gupta, S.; Thareja, P. Rheology and Direct Write Printing of Chitosan-Graphene Oxide Nanocomposite Hydrogels for Differentiation of Neuroblastoma Cells. Carbohydr. Polym. 2021, 269, 118254. [Google Scholar] [CrossRef]
- De Conto, V.; Cheung, V.; Maubon, G.; Souguir, Z.; Maubon, N.; Vandenhaute, E.; Bérézowski, V. In Vitro Differentiation Modifies the Neurotoxic Response of SH-SY5Y Cells. Toxicol. Vitr. 2021, 77, 105235. [Google Scholar] [CrossRef]
- Ko, K.R.; Tam, N.W.; Teixeira, A.G.; Frampton, J.P. SH-SY5Y and LUHMES Cells Display Differential Sensitivity to MPP+, Tunicamycin, and Epoxomicin in 2D and 3D Cell Culture. Biotechnol. Prog. 2020, 36, e2942. [Google Scholar] [CrossRef]
- Desai, A.; Kisaalita, W.S.; Keith, C.; Wu, Z.Z. Human Neuroblastoma (SH-SY5Y) Cell Culture and Differentiation in 3-D Collagen Hydrogels for Cell-Based Biosensing. Biosens. Bioelectron. 2006, 21, 1483–1492. [Google Scholar] [CrossRef]
- Gallagher, C.; Murphy, C.; Kelly, G.; O’brien, F.J.; Piskareva, O. Three-Dimensional In Vitro Biomimetic Model of Neuroblastoma Using Collagen-Based Scaffolds. J. Vis. Exp. 2021, 173, e62627. [Google Scholar] [CrossRef]
- Marrella, A.; Dondero, A.; Aiello, M.; Casu, B.; Olive, D.; Regis, S.; Bottino, C.; Pende, D.; Meazza, R.; Caluori, G.; et al. Cell-Laden Hydrogel as a Clinical-Relevant 3D Model for Analyzing Neuroblastoma Growth, Immunophenotype, and Susceptibility to Therapies. Front. Immunol. 2019, 10, 1876. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Whitworth, C.L.; Redfern, C.P.F.; Cheek, T.R. Differentiation-Induced Remodelling of Store-Operated Calcium Entry Is Independent of Neuronal or Glial Phenotype but Modulated by Cellular Context. Mol. Neurobiol. 2019, 56, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Patte-Mensah, C.; Meyer, L.; Schaeffer, V.; Eckert, A.; Mensah-Nyagan, A.G. Transfection of Human Neuroblastoma Cells with Alzheimer’s Disease Brain Hallmarks as a Promising Strategy to Investigate the Role of Neurosteroidogenesis in Neuroprotection. Genet. Modif. Org. Genet. Eng. Res. Ther. 2012, 3, 50–59. [Google Scholar] [CrossRef]
- Martin, E.R.; Gandawijaya, J.; Oguro-Ando, A. A Novel Method for Generating Glutamatergic SH-SY5Y Neuron-like Cells Utilizing B-27 Supplement. Front. Pharm. 2022, 13, 4042. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant in Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef]
- Datta, P.K. Neuronal Cell Culture. Neuronal Cell. Cult. Methods Protoc. 2013, 1078, 35–44. [Google Scholar] [CrossRef]
- Riegerová, P.; Brejcha, J.; Bezděková, D.; Chum, T.; Mašínová, E.; Čermáková, N.; Ovsepian, S.V.; Cebecauer, M.; Štefl, M. Expression and Localization of AβPP in SH-SY5Y Cells Depends on Differentiation State. J. Alzheimer’s Dis. 2021, 82, 485. [Google Scholar] [CrossRef]
- Abolpour Mofrad, S.; Kuenzel, K.; Friedrich, O.; Gilbert, D.F. Optimizing Neuronal Differentiation of Human Pluripotent NT2 Stem Cells in Monolayer Cultures. Dev. Growth Differ. 2016, 58, 664–676. [Google Scholar] [CrossRef]
- Pleasure, S.J.; Lee, V.Y. NTera 2 Cells: A Human Cell Line Which Displays Characteristics Expected of a Human Committed Neuronal Progenitor Cell. J. Neurosci. Res. 1993, 35, 585–602. [Google Scholar] [CrossRef]
- Terrasso, A.P.; Pinto, C.; Serra, M.; Filipe, A.; Almeida, S.; Ferreira, A.L.; Pedroso, P.; Brito, C.; Alves, P.M. Novel Scalable 3D Cell Based Model for in Vitro Neurotoxicity Testing: Combining Human Differentiated Neurospheres with Gene Expression and Functional Endpoints. J. Biotechnol. 2015, 205, 82–92. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Keles, G.E.; Berger, M.S.; Srinivasan, J.; Kolstoe, D.D.; Bobola, M.S.; Silber, J.R. Establishment and Characterization of Four Human Medulloblastoma-Derived Cell Lines. Oncol. Res. 1995, 7, 493–503. [Google Scholar] [PubMed]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D Neural Tissue Models: From Spheroids to Bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. In Vitro Co-Culture Model of Medulloblastoma and Human Neural Stem Cells for Drug Delivery Assessment. J. Biotechnol. 2015, 205, 3–13. [Google Scholar] [CrossRef]
- Sanchez-Diaz, P.C.; Burton, T.L.; Burns, S.C.; Hung, J.Y.; Penalva, L.O.F. Musashi 1 Modulates Cell Proliferation Genes in the Medulloblastoma Cell Line Daoy. BMC Cancer 2008, 8, 1–12. [Google Scholar] [CrossRef]
- Lotharius, J.; Falsig, J.; Van Beek, J.; Payne, S.; Dringen, R.; Brundin, P.; Leist, M. Progressive Degeneration of Human Mesencephalic Neuron-Derived Cells Triggered by Dopamine-Dependent Oxidative Stress Is Dependent on the Mixed-Lineage Kinase Pathway. J. Neurosci. 2005, 25, 6329–6342. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Pöltl, D.; Genewsky, A.; Weng, M.; Waldmann, T.; Schildknecht, S.; Leist, M. Rapid, Complete and Large-Scale Generation of Post-Mitotic Neurons from the Human LUHMES Cell Line. J. Neurochem. 2011, 119, 957–971. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, W.; Qiu, B.; Zhao, J.; Yu, Y.; Guan, W.; Wang, M.; Yang, W.; Pei, G. Generation of Neural Progenitor Cells by Chemical Cocktails and Hypoxia. Cell. Res. 2014, 24, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Harris, G.; Delp, J.; Valadares, M.; Pamies, D.; Hogberg, H.T.; Waldmann, T.; Leist, M.; Hartung, T. A LUHMES 3D Dopaminergic Neuronal Model for Neurotoxicity Testing Allowing Long-Term Exposure and Cellular Resilience Analysis. Arch. Toxicol. 2016, 3, 2725–2743. [Google Scholar] [CrossRef]
- Leite, P.E.C.; Pereira, M.R.; Harris, G.; Pamies, D.; Dos Santos, L.M.G.; Granjeiro, J.M.; Hogberg, H.T.; Hartung, T.; Smirnova, L. Suitability of 3D Human Brain Spheroid Models to Distinguish Toxic Effects of Gold and Poly-Lactic Acid Nanoparticles to Assess Biocompatibility for Brain Drug Delivery. Part. Fibre Toxicol. 2019, 16, 1–20. [Google Scholar] [CrossRef]
- Harris, G.; Eschment, M.; Orozco, S.P.; Mccaffery, J.M.; Maclennan, R.; Severin, D.; Leist, M.; Kleensang, A.; Pamies, D.; Maertens, A.; et al. Toxicity, Recovery, and Resilience in a 3D Dopaminergic Neuronal in Vitro Model Exposed to Rotenone. Arch. Toxicol. 2018, 92, 2587–2606. [Google Scholar] [CrossRef]
- Joshi, P.; Yu, K.N.; Kang, S.Y.; Kwon, S.J.; Kwon, P.S.; Dordick, J.S.; Kothapalli, C.R.; Lee, M.Y. 3D-Cultured Neural Stem Cell Microarrays on a Micropillar Chip for High-Throughput Developmental Neurotoxicology. Exp. Cell. Res. 2018, 370, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Nierode, G.J.; Gopal, S.; Kwon, P.; Clark, D.S.; Schaffer, D.V.; Dordick, J.S. High-Throughput Identification of Factors Promoting Neuronal Differentiation of Human Neural Progenitor Cells in Microscale 3D Cell Culture. Biotechnol. Bioeng. 2019, 116, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Tomaskovic-Crook, E.; Zhang, P.; Ahtiainen, A.; Kaisvuo, H.; Lee, C.-Y.; Beirne, S.; Aqrawe, Z.; Svirskis, D.; Hyttinen, J.; Wallace, G.G.; et al. Human Neural Tissues from Neural Stem Cells Using Conductive Biogel and Printed Polymer Microelectrode Arrays for 3D Electrical Stimulation. Adv. Heal. Mater. 2019, 8, 1900425. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Heal. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Agholme, L.; Lindström, T.; Kgedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Cacopardo, L.; Guazzelli, N.; Ahluwalia, A. Characterizing and Engineering Biomimetic Materials for Viscoelastic Mechanotransduction Studies. Tissue Eng. Part. B Rev. 2022, 28, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Mattei, G.; Magliaro, C.; Giusti, S.; Ramachandran, S.D.; Heinz, S.; Braspenning, J.; Ahluwalia, A. On the Adhesion-Cohesion Balance and Oxygen Consumption Characteristics of Liver Organoids. PLoS ONE 2017, 12, e0173206. [Google Scholar] [CrossRef]
- Serpeloni, M.; Ilce Mara, C.; Gania, Z.; Tiara Noorintan, S.; Putu Diah Pradnya Septiari, N.; Sandra Fitriany, D.; Gandhi Torizal, F. Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy. Future Pharmacol. 2022, 2, 360–376. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Cacopardo, L.; Lamanna, D.; Temerario, L.; Brunetti, G.; Carluccio, A.; Robbe, D.; Dell’aquila, M.E. Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Magliaro, C.; Penta, M.; Troncone, M.; Pimentel, R.; Ahluwalia, A. Sphyga: A Multiparameter Open Source Tool for Fabricating Smart and Tunable Hydrogel Microbeads. Biofabrication 2014, 6, 025009. [Google Scholar] [CrossRef] [PubMed]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and Differentiation of SH-SY5Y Neuroblastoma Cells on Bacterial Nanocellulose Scaffolds 3D Culturing and Differentiation of SH-SY5Y Neuroblastoma Cells on Bacterial Nanocellulose Scaffolds. Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Frimat, J.-P.; Xie, S.; Bastiaens, A.; Schurink, B.; Wolbers, F.; Toonder, J.D.; Luttge, R. Advances in 3D Neuronal Cell Culture. J. Vac. Sci. Technology. 2015, 33, 06F902. [Google Scholar] [CrossRef]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in Vitro Modeling of the Central Nervous System. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318. [Google Scholar] [CrossRef]
- Park, K.; Lonsberry, G.E.; Gearing, M.; Levey, A.I.; Desai, J.P. Viscoelastic Properties of Human Autopsy Brain Tissues as Biomarkers for Alzheimer’s Diseases. IEEE Trans. Biomed. Eng. 2019, 66, 1705–1713. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Lenas, P.; van Vliet, E.; Hartung, T. In Vitro Developmental Neurotoxicity (DNT) Testing: Relevant Models and Endpoints. Neurotoxicology 2010, 31, 545–554. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Alexander, H.P.; Ironside, J.W. Molecular Pathology in Neurodegenerative Diseases. Curr. Drug. Targets 2012, 13, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- McComish, S.F.; MacMahon Copas, A.N.; Caldwell, M.A. Human Brain-Based Models Provide a Powerful Tool for the Advancement of Parkinson’s Disease Research and Therapeutic Development. Front. Neurosci. 2022, 16, 851058. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Marshall, D.; Reid, Y.; Parkes, H.; Gelber, C. The Costs of Using Unauthenticated, over-Passaged Cell Lines: How Much More Data Do We Need? Biotechniques 2007, 43, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Moors, M.; Rockel, T.D.; Abel, J.; Cline, J.E.; Gassmann, K.; Schreiber, T.; Shuwald, J.; Weinmann, N.; Fritsche, E. Human Neurospheres as Three-Dimensional Cellular Systems for Developmental Neurotoxicity Testing. Env. Health Perspect. 2009, 117, 1131–1138. [Google Scholar] [CrossRef]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Gstraunthaler, G.; Hartung, T.; Hay, R.; Merten, O.W.; Price, A.; Schechtman, L.; et al. Guidance on Good Cell Culture Practice: A Report of the Second ECVAM Task Force on Good Cell Culture Practice. ATLA Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef]
- Ball, N.; Bars, R.; Botham, P.A.; Cuciureanu, A.; Cronin, M.T.D.; Doe, J.E.; Dudzina, T.; Gant, T.W.; Leist, M.; van Ravenzwaay, B. A Framework for Chemical Safety Assessment Incorporating New Approach Methodologies within REACH. Arch. Toxicol. 2022, 96, 743–766. [Google Scholar] [CrossRef]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell. Proteom. 2016, 15, 355. [Google Scholar] [CrossRef]
- Hirsch, C.; Schildknecht, S. In Vitro Research Reproducibility: Keeping up High Standards. Front. Pharm. 2019, 10, 1484. [Google Scholar] [CrossRef]
- Zingales, V.; Torriero, N.; Zanella, L.; Fernández-Franzón, M.; Ruiz, M.J.; Esposito, M.R.; Cimetta, E. Development of an in Vitro Neuroblastoma 3D Model and Its Application for Sterigmatocystin-Induced Cytotoxicity Testing. Food Chem. Toxicol. 2021, 157, 112605. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Lo, S.S.; Hung, S.W.; Wang, C.J.; Lin, C.H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 11678. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.; Donato, M.; Lahoz, A.; Castell, J. Cell Lines: A Tool for In Vitro Drug Metabolism Studies. Curr. Drug. Metab. 2008, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).