Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Materials
2.3. Research-Grade Human Corneo-Scleral Tissues
2.4. Primary Corneal Endothelial Cells for CECI
2.4.1. Expanded Primary CECs for CECI
2.4.2. Primary ‘Simple Non-Expanded Endothelial Cells’ (SNECs) for CECI
2.5. Animal Surgeries
2.6. Corneal Endothelial Cell Injection
2.7. Post-Operative Care
2.8. Corneal Imaging and Intra-Ocular Pressure Measurement
2.9. Statistical Analysis
3. Results
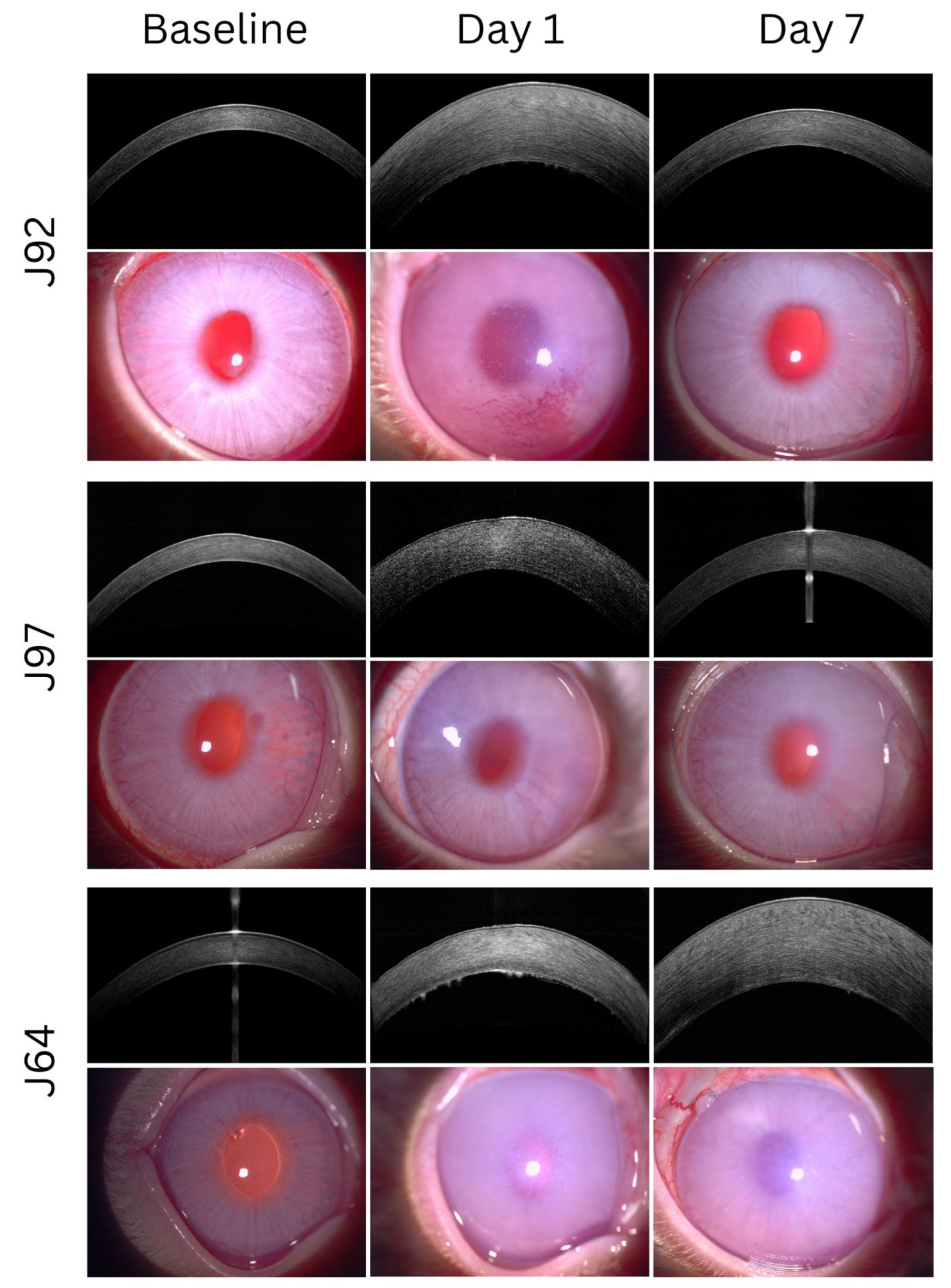
3.1. Pre-Operative Assessment of Rabbits Following Lens Extraction
3.2. Post-Operative Assessment of Clinical Outcomes in a Rabbit Model of Bullous Keratopathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol. 1972, 221, 43–54. [Google Scholar] [CrossRef]
- Bonanno, J.A. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M. The cornea and sclera. Collagen. In Structure and Mechanics; Springer: New York, NY, USA, 2008; pp. 359–396. [Google Scholar]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Le, R.; Yucel, N.; Khattak, S.; Yucel, Y.H.; Prud’homme, G.J.; Gupta, N. Current indications and surgical approaches to corneal transplants at the University of Toronto: A clinical-pathological study. Can. J. Ophthalmol. 2017, 52, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Coster, D.J.; Lowe, M.T.; Keane, M.C.; Williams, K.A. A comparison of lamellar and penetrating keratoplasty outcomes: A registry study. Ophthalmology 2014, 121, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Thomson, A.C.; Ronquillo, Y. Corneal Endothelial Transplantation; NCBI Bookshelf: Treasure Island, FL, USA, 2021; pp. 1–16. [Google Scholar]
- Tillett, C.W. Posterior lamellar keratoplasty. Am. J. Ophthalmol. 1956, 41, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Dapena, I.; Ham, L.; Melles, G.R.J. Endothelial keratoplasty: DSEK/DSAEK or DMEK—The thinner the better? Curr. Opin. Ophthalmol. 2009, 20, 299–307. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Beuerman, R.W.; Colman, A.; Tan, D.T.; Mehta, J.S. Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 2011, 91, 811–819. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs Population Division. World Population Ageing 2019. Internet. Vol. Highlights, World Population Ageing 2019. 2019. 64 p. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 3 March 2023).
- Konomi, K.; Zhu, C.; Harris, D.; Joyce, N.C. Comparison of the proliferative capacity of human corneal endothelial cells from the central and peripheral areas. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4086–4091. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Chng, Z.; Ang, H.P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.-P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.; Ueno, M.; Hagyia, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef]
- Bartakova, A.; Kuzmenko, O.; Alvarez-Delfin, K.; Kunzevitzky, N.J.; Goldberg, J.L. A cell culture approach to optimized human corneal endothelial cell function. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1617–1629. [Google Scholar] [CrossRef]
- He, Z.; Okumura, N.; Sato, M.; Komori, Y.; Nakahara, M.; Gain, P.; Koizumi, N.; Thuret, G. Corneal endothelial cell therapy: Feasibility of cell culture from corneas stored in organ culture. Cell Tissue Bank. 2021, 22, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Zongyi, L.; Haoyun, D.; Yanni, J.; Can, Z.; Wenjing, L.; Xin, W.; Yajie, G.; Chunxiao, D.; Bochao, M.; Sengqian, D.; et al. Long-term corneal recovery by simultaneous delivery of hPSC-derived corneal endothelial precursors and nicotinamide. J. Clin. Investig. 2022, 132, e146658. [Google Scholar]
- Ali, M.; Khan, S.Y.; Gottsch, J.D.; Hutchinson, E.K.; Khan, A.; Riazuddin, S.A. Pluripotent stem cell–derived corneal endothelial cells as an alternative to donor corneal endothelium in keratoplasty. Stem Cell Rep. 2021, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators and Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The right to sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Santerre, K.; Xu, I.; Theriault, M.; Proulx, S. In vitro expansion of corneal endothelial cells for transplantation. In Corneal Regeneration. Methods in Molecular Biology; Ahearne, M., Ed.; Humana: New York, NY, USA, 2020; Vol 2145, pp. 17–28. [Google Scholar]
- Hatou, S.; Sayano, T.; Higa, K.; Inagaki, E.; Okano, Y.; Sato, Y.; Okano, H.; Tsubota, K.; Shimmura, S. Transplantation of iPSC-derived corneal endothelial substitutes in a monkey corneal edema model. Stem Cell Res. 2021, 55, 102497. [Google Scholar] [CrossRef]
- Mishan, M.A.; Balagholi, S.; Chamani, T.; Feizi, S.; Soheili, Z.S.; Kanavi, M.R. Potential effect of human platelet lysate on in vitro expansion of human corneal endothelial cells compared with Y-27632 ROCK inhibitor. J. Ophthalmic Vis. Res. 2021, 16, 349–356. [Google Scholar] [CrossRef]
- So, S.; Park, Y.; Kang, S.S.; Han, J.; Sunwoo, J.H.; Lee, W.; Kim, J.; Ye, E.A.; Kim, J.Y.; Tchah, H.; et al. Therapeutic potency of induced pluripotent stem-cell-derived corneal endothelial-like cells for corneal endothelial dysfunction. Int. J. Mol. Sci. 2022, 24, 701. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Peh, G.; Jin Hui Neo, D.; Ang, H.-P.; Adnan, K.; Lwin Nyein, C.; Morales-Wong, F.; Bhogal, M.; Kocaba, V.; Mehta, J.S. A novel approach of harvesting viable single cells from donor corneal endothelium for cell-injection therapy. Cells 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.S.; Kocaba, V.; Soh, Y.Q. The future of keratoplasty: Cell-based therapy, regenerative medicine, bioengineering keratoplasty, gene therapy. Curr. Opin. Ophthalmol. 2019, 30, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Sun, P.; Zhang, C.; Yang, L.; Du, L.; Wu, X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci. Rep. 2017, 7, 13400. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Lim, L.S.; Aung, H.T.; Aung, T.; Tan, D.T.H. Corneal imaging with anterior segment optical coherence tomography for lamellar keratoplasty procedures. Am. J. Ophthalmol. 2008, 145, 81–90. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Kalina, A.; Im, A.; Davis, A.R.; Eslani, M.; Hogge, R.L.; Yeu, E. Region of interest densitometry analysis of Descemet membrane endothelial keratoplasty dehiscence on anterior segment optical coherence tomography. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef]
- Heslinga, F.G.; Lucassen, R.T.; van den Berg, M.A.; van der Hoek, L.; Pluim, J.P.W.; Cabrerizo, J.; Alberti, M.; Veta, M. Corneal pachymetry by AS-OCT after Descemet’s membrane endothelial keratoplasty. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Karadag, R.; Hammersmith, K.M.; Nagra, P.K.; Rapuano, C.J. Anterior chamber characteristics, endothelial parameters, and corneal densitometry after Descemet stripping automated endothelial keratoplasty in patients with Fuchs dystrophy. J. Ophthalmic Vis. Res. 2021, 16, 158–164. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ang, H.P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.Y.; Lin, S.-J.; Bhogal, M.; Liu, Y.-C.; Tan, D.T.; et al. Regulatory compliant tissue-engineered human corneal endothelial grafts restore corneal function of rabbits with bullous keratopathy. Sci. Rep. 2017, 7, 14149. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.-J.; Mehta, J.S. Functional evaluation of two corneal endothelial cell-based therapies: Tissue-engineered construct and cell injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef]
- Nanji, A.A.; Sayyad, F.E.; Galor, A.; Dubovy, S.; Karp, C.L. High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology. Ocul. Surf. 2015, 13, 226–235. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Kuo, J.; Anderson, D.; Hossain, P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am. J. Ophthalmol. 2008, 146, 4. [Google Scholar] [CrossRef]
- Fuentes, E.; Sandali, O.; El Sanharawi, M.; Basli, E.; Hamiche, T.; Goemaere, I.; Borderie, V.; Borheraoua, N.; Laroche, L. Anatomic predictive factors of acute corneal hydrops in keratoconus an optical coherence tomography study. Ophthalmology 2015, 122, 1653–1659. [Google Scholar] [CrossRef]
- Siebelmann, S.; Scholz, P.; Sonnenschein, S.; Bachmann, B.; Matthaei, M.; Cursiefen, C.; Heindl, L.M. Anterior segment optical coherence tomography for the diagnosis of corneal dystrophies according to the IC3D classification. Surv. Ophthalmol. 2018, 63, 365–380. [Google Scholar] [CrossRef]
- Szalai, E.; Németh, G.; Hassan, Z.; Módis, L. Noncontact evaluation of corneal grafts: Swept-source fourier domain oct versus high-resolution scheimpflug imaging. Cornea 2017, 36, 434–439. [Google Scholar] [CrossRef]
- Wong, E.N.; Mehta, J.S. Cell therapy in corneal endothelial disease. Curr. Opin. Ophthalmol. 2022, 33, 275–281. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S. Spectral domain optical coherence tomography for glaucoma (An AOS thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 426–458. [Google Scholar]
- Bostan, C.; Thériault, M.; Forget, K.J.; Doyon, C.; Cameron, J.D.; Proulx, S.; Brunette, I. In vivo functionality of a corneal endothelium transplanted by cell-injection therapy in a feline model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1620–1634. [Google Scholar] [CrossRef] [PubMed]
- Colby, K. Descemet stripping only for Fuchs endothelial corneal dystrophy: Will it become the gold standard? Cornea 2022, 41, 269–271. [Google Scholar] [CrossRef]
- Auffarth, G.U.; Son, H.S.; Koch, M.; Weindler, J.; Merz, P.; Daphna, O.; Marcovich, A.L.; Augustin, V.A. Implantation of an artificial endothelial layer for treatment of chronic corneal edema. Cornea 2021, 40, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; Lathrop, K.L.; Funderburgh, J.L.; Feinberg, A.W. In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci. Rep. 2015, 5, 7955. [Google Scholar] [CrossRef]
- Faye, P.A.; Poumeaud, F.; Chazelas, P.; Duchesne, M.; Rassat, M.; Miressi, F.; Lia, A.S.; Sturtz, F.; Robert, P.-Y.; Favreau, F.; et al. Focus on cell therapy to treat corneal endothelial diseases. Exp. Eye Res. 2021, 204, 108462. [Google Scholar] [CrossRef]
- Khalili, M.; Asadi, M.; Kahroba, H.; Soleyman, M.R.; Andre, H.; Alizadeh, E. Corneal endothelium tissue engineering: An evolution of signaling molecules, cells, and scaffolds toward 3D bioprinting and cell sheets. J. Cell Physiol. 2021, 236, 3275–3303. [Google Scholar] [CrossRef]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef]
- Park, S.; Leonard, B.C.; Raghunathan, V.K.; Kim, S.; Li, J.Y.; Mannis, M.J.; Murphy, C.J.; Thomasy, S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Trans. Med. 2021, 9, 1271–1288. [Google Scholar] [CrossRef]

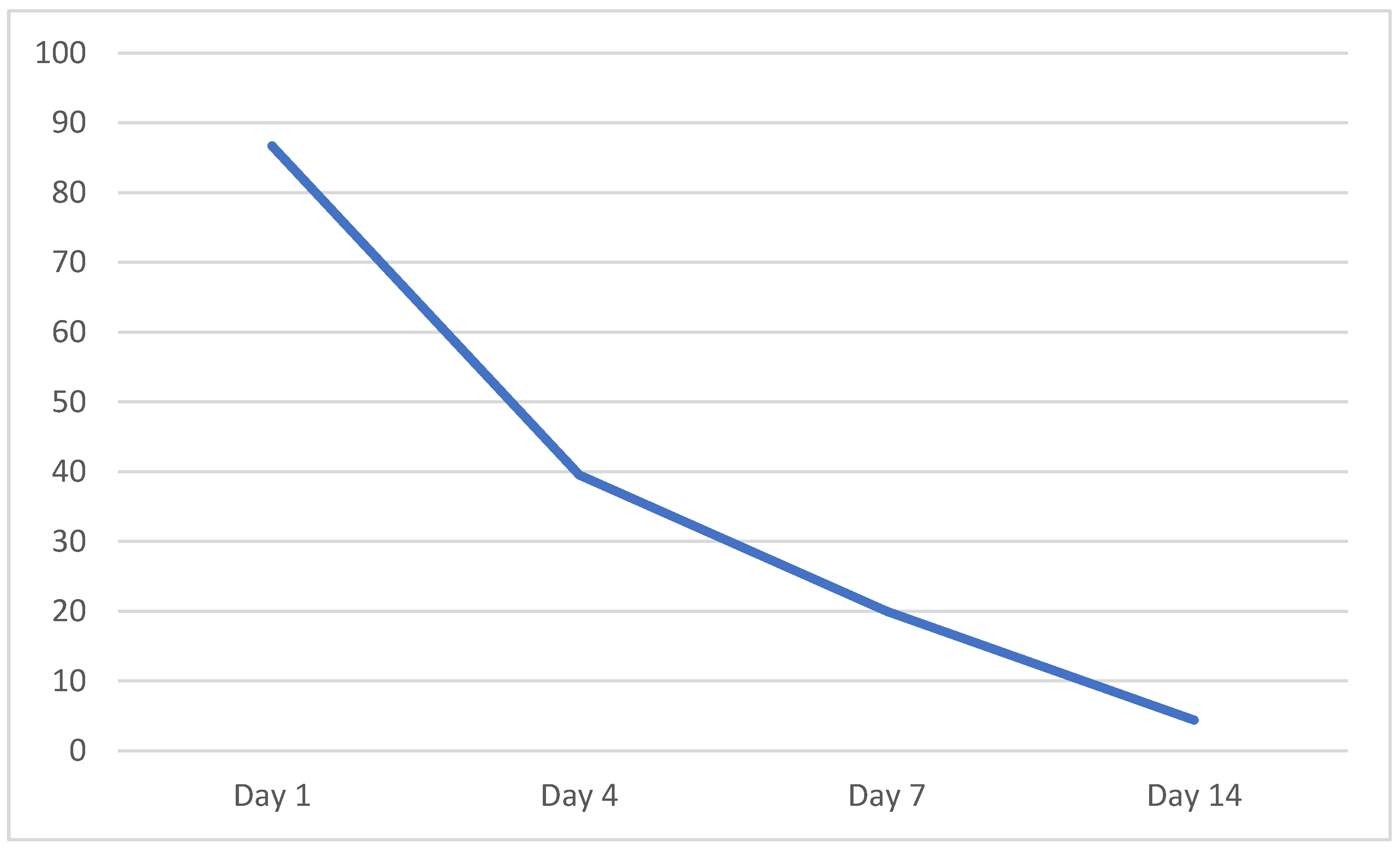
| Day 1 | Day 4 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Sensitivity | 0.903 | 0.367 | 0.194 | 0.065 |
| Specificity | 0.214 | 0.538 | 0.786 | 1.000 |
| Positive predictive value | 0.718 | 0.647 | 0.667 | 1.000 |
| Negative predictive value | 0.500 | 0.269 | 0.306 | 0.326 |
| Positive likelihood ratio | 1.150 | 0.794 | 0.903 | NA |
| Negative likelihood ratio | 0.452 | 1.176 | 1.026 | 0.935 |
| Cell Visibility | Chi-Square | df | p-Value |
|---|---|---|---|
| Day 1 | 1.080 | 1 | 0.299 |
| Day 4 | 0.339 | 1 | 0.561 |
| Day 7 | 0.260 | 1 | 0.873 |
| Day 14 | 1.532 | 1 | 0.216 |
| Cell visibility and CCT | |||
| Day 1 | 5.761 | 1 | 0.560 |
| Day 4 | 14.656 | 1 | 0.001 |
| Day 7 | 22.702 | 1 | 0.000 |
| Day 14 | 22.257 | 1 | 0.000 |
| CCT | Odds ratio | 95% CI | |
| Day 1 | 0.996 | 0.993–1.000 | |
| Day 4 | 0.996 | 0.993–0.999 | |
| Day 7 | 0.998 | 0.991–0.998 | |
| Day 14 | 0.996 | 0.994–0.998 | |
| Cell Visibility | |||||
|---|---|---|---|---|---|
| SNECi CECs | Primary Expanded CECs | Exact Sig. (2-Sided) p-Value | |||
| Day 1 | N | 0 (0%) | 6 (16.2%) | 0.572 | |
| Y | 8 (100%) | 31 (83.8%) | |||
| Day 4 | N | 2 (25%) | 24 (68.6%) | 0.042 | |
| Y | 6 (75%) | 11 (31.4%) | |||
| Day 7 | N | 4 (50%) | 32 (86.5%) | 0.039 | |
| Y | 4 (50%) | 5 (13.5%) | |||
| Day 14 | N | 8 (100%) | 35 (94.6%) | 1.000 | |
| Y | 0 (0%) | 2 (5.4%) | |||
| Pachymetry | |||||
| SNECi CECs | Primary Expanded CECs | ||||
| N | Median (IQR) | N | Median (IQR) | Asymp. Sig. (2-Tailed) p-Value | |
| Baseline | 8 | 351 (322–375) | 34 | 374 (352–395) | 0.065 |
| Day 1 | 8 | 842 (625–1012) | 37 | 720 (641–866) | 0.467 |
| Day 4 | 8 | 884 (726–1360) | 37 | 652 (460–987) | 0.085 |
| Day 7 | 8 | 785 (538–1020) | 37 | 517 (461–873) | 0.109 |
| Day 14 | 8 | 571 (486–1525) | 37 | 635 (489–1225) | 0.941 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, E.N.; Foo, V.H.X.; Peh, G.S.L.; Htoon, H.M.; Ang, H.-P.; Tan, B.Y.L.; Ong, H.-S.; Mehta, J.S. Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy. Cells 2023, 12, 1167. https://doi.org/10.3390/cells12081167
Wong EN, Foo VHX, Peh GSL, Htoon HM, Ang H-P, Tan BYL, Ong H-S, Mehta JS. Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy. Cells. 2023; 12(8):1167. https://doi.org/10.3390/cells12081167
Chicago/Turabian StyleWong, Evan N., Valencia H. X. Foo, Gary S. L. Peh, Hla M. Htoon, Heng-Pei Ang, Belinda Y. L. Tan, Hon-Shing Ong, and Jodhbir S. Mehta. 2023. "Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy" Cells 12, no. 8: 1167. https://doi.org/10.3390/cells12081167
APA StyleWong, E. N., Foo, V. H. X., Peh, G. S. L., Htoon, H. M., Ang, H.-P., Tan, B. Y. L., Ong, H.-S., & Mehta, J. S. (2023). Early Visibility of Cellular Aggregates and Changes in Central Corneal Thickness as Predictors of Successful Corneal Endothelial Cell Injection Therapy. Cells, 12(8), 1167. https://doi.org/10.3390/cells12081167








