Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model
Abstract
1. Introduction
2. Cellular Contributors to Membrane Potential and Bioelectricity
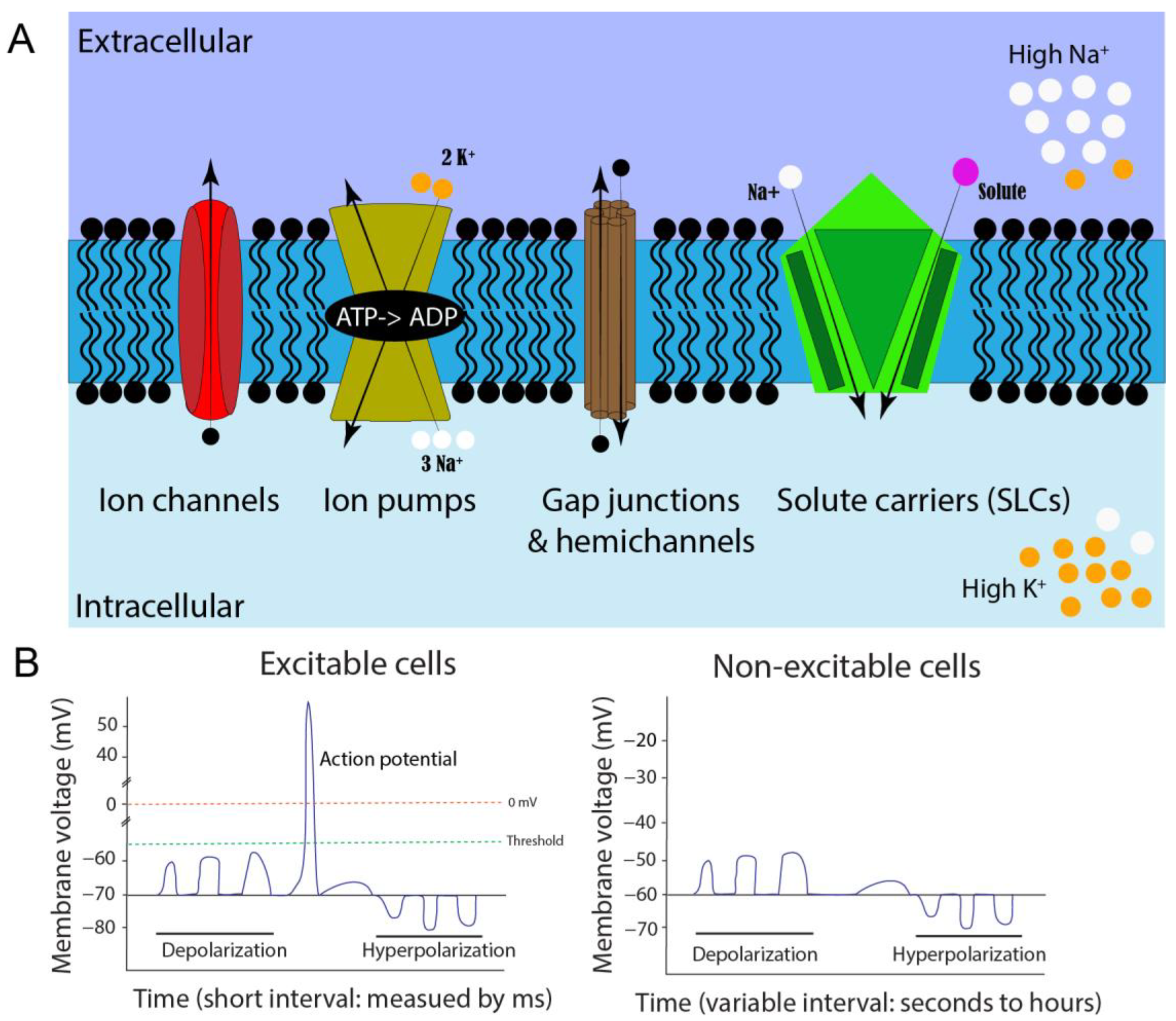
2.1. Cell Membrane Potential and Concentration Gradients
2.2. Membrane Potential Contributors: Ion Channels, Gap Junctions, and Solute Carriers
3. Bioelectricity Evidence from Zebrafish Genetics
3.1. Zebrafish as a Superior Model for Bioelectric Research
3.2. Zebrafish Mutants with Adult Fin-Size Change
| Mutant | Fin | Gene | Mutation Nature | Fin Ray Segment Length | Fin Ray Numbers | Dominant or Recessive | Somite or Local Fin | References |
|---|---|---|---|---|---|---|---|---|
| lof | Long | kcnh2a | Ectopic by cis-regulatory change | Normal | Increased | Dominant | Local | [105,106] |
| alf | Long | kcnk5b | GOF | Increased | Decreased | Dominant | Local | [107] |
| schleier | Long | slc12a7a/kcc4a | LOF or dominant negative? Dose dependent | Normal | Increased | Dominant | Local | [108] |
| Dhi2059 | Long | kcnj13 | Ectopic by cis-regulatory change, Dose dependent | Increased | Decreased | Dominant | Somite | [112] |
| sof | Short | cx43 | Hypomorphic | Decreased | Decreased | Dominant | Local | [109] |
| mau | Short | aqp3a | Neomorphic, Dose dependent | Normal | Decreased | Dominant | Local | [110] |
| nr21 | Short | slc43a2/lat4a | GOF | Decreased | Not reported | Dominant | Local | [111] |
3.3. Zebrafish Mutants with Adult Pigmentation Pattern Alterations
4. Genetically Encoded Tools That Can Be Used for Studying Developmental Bioelectricity
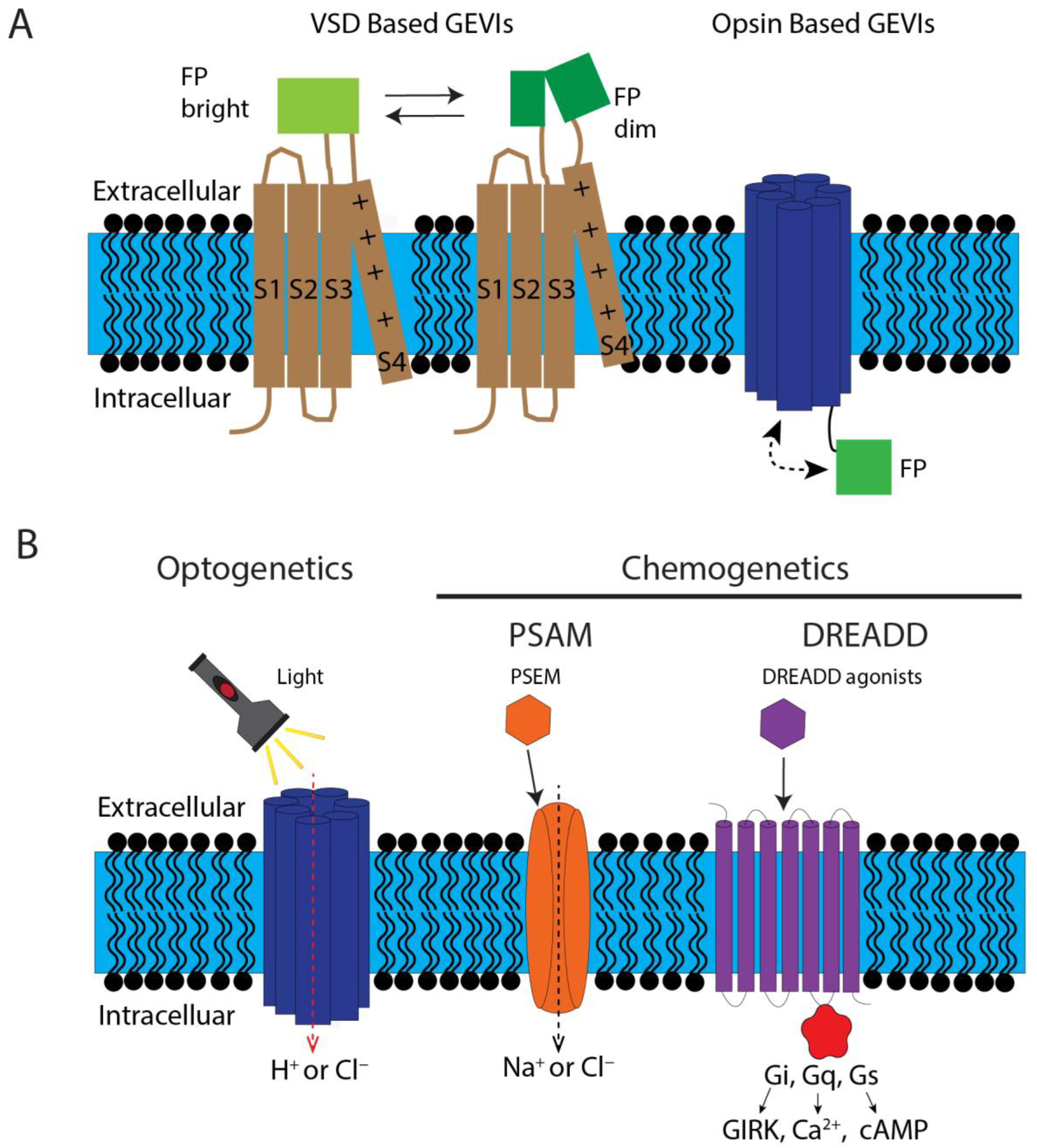
4.1. Measuring Cellular Bioelectricity: Genetically Encoded Voltage Indicators
| GEVIs | Fluorescence Indication | Fluorophore | Tested in Zebrafish | References |
|---|---|---|---|---|
| VSD-based | ||||
| ASAP1–3 | Hyperpolarize—brighter | GFP | Whole fish embryos and larva. Adult malignant nerve sheath tumors, larval fish cerebellum, spinal cord. | [166,167,168,169,170,171,172,173] |
| ASAP4 | Depolarize—brighter | GFP | [184,185] | |
| Marina | Depolarize—brighter | GFP | [186] | |
| FlicR1 | Depolarize—brighter | RFP | [187] | |
| Arclight | Hyperpolarize—brighter | GFP | [188,189] | |
| Bongwoori | Hyperpolarize—brighter | GFP | Larval olfactory bulb | [190,191] |
| Aahn | Hyperpolarize—brighter (external) | GFP | [192] | |
| VSFP x | Depolarize—FRET increase | Multiple | Larval zebrafish heart | [193,194,195,196,197,198,199,200] |
| Mermaid | Depolarize—FRET increase | Multiple | [201] | |
| Nabi | Depolarize—FRET increase | UGK/mKO | [202] | |
| JEDI-2P | Hyperpolarize—brighter | GFP | [178] | |
| Opsin-based | ||||
| Arch | Depolarize—brighter | GFP | [203] | |
| QuasAr x | Hyperpolarize—brighter | Multiple | Larval zebrafish heart | [204,205,206,207,208] |
| Archon1 | Depolarize—brighter | GFP/RFP | Brain and spinal V3 interneurons | [209,210] |
| Ace x | Hyperpolarize—brighter | Green/RFP | [211,212] | |
| Ace-mNeon2 | Hyperpolarize—brighter | GFP | [182] | |
| VARNAM | Hyperpolarize—brighter | RFP | [213] | |
| VARNAM2 | Hyperpolarize—brighter | RFP | [182] | |
| pAce | Depolarize—brighter | GFP | [182] | |
| pAceR | Depolarize—brighter | RFP | [182] | |
| Dye- or bioluminescence-based | ||||
| Voltron | Hyperpolarize—brighter | Multiple dyes | Larval brain | [214] |
| Voltron2 | Hyperpolarize—brighter | Multiple dyes | Larval olfactory sensory neurons | [215] |
| Positron | Depolarize—brighter | Multiple dyes | Larval zebrafish brain | [216] |
| hVOS | Depolarize—brighter | Green dye | [217] | |
| Voltage spy | Depolarize—brighter | Green dye | [218] | |
| LOTUS | Depolarize—FRET increase | Blue/green bioluminescence | [219] | |
| AMBER | Depolarize—voltage-gated luciferase increase | Blue/green bioluminescence | [220] | |
4.2. Manipulate Cellular Bioelectricity: Optogenetic and Chemogenetic Tools
| Optogenetic Tools | Chemogenetic Tools | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Activation Method | Activation Result | Tested in Zebrafish | References | Name | Activation Method | Activation Result | Tested in Zebrafish | References |
| ChR2 | Blue light (470 nm) | Depolarization | Melanophores, hair-cells, neurons | [141,230,235,255] | hM4DGi | DREADD agonists | Hyperpolarization | Melanophore | [222,250] |
| eNpHR3.0 | Yellow light (590 nm) | Hyperpolarization | Neurons | [141,256] | hM3DGq | DREADD agonists | Depolarization | [222] | |
| CoChR | Blue light (470 nm) | Depolarization | Neurons | [141,257] | hM3DGs | DREADD agonists | Depolarization | [222] | |
| GtACR1 | Green light (515 nm) | Hyperpolarization | Neurons, heart | [141,258,259,260] | KORD | DREADD agonists | Hyperpolarization | [222] | |
| GtACR2 | Blue light (470 nm) | Hyperpolarization | Neurons, heart | [141,259,260,261] | PSAM-5HT3-HC | PSEM ligands | Depolarization | Horizontal cells | [251] |
| BLINK2 | Blue light (455 nm) | Hyperpolarization | Hair cells, lateral line neuromasts, neurons | [262] | PSAM-5HT3-LC | PSEM ligands | Depolarization | Horizontal cells | [251] |
| CheRiff | UV light (460 nm) | Depolarization | Neurons | [141,204] | PSAM-GlyR | PSEM ligands | Hyperpolarization | Horizontal cells | [251,253] |
| Chronos | Yellow light (500 nm) | Depolarization | Neurons | [141,263] | TRPV1 | Capsaicin | Depolarization | Neurons, neutrophils | [150,249] |
| eArchT3.0 | Yellow (570 nm) | Hyperpolarization | Neurons | [141,264] | TRPM8 | Menthol | Depolarization | Neurons | [249] |
| ChrimsonR | Red light (590 nm) | Depolarization | Neurons | [141,265] | TRPA1 | >28 °C | Depolarization | Neurons | [249] |
| GluCl v2.0 | Ivermectin | hyperpolarization | [266] | ||||||
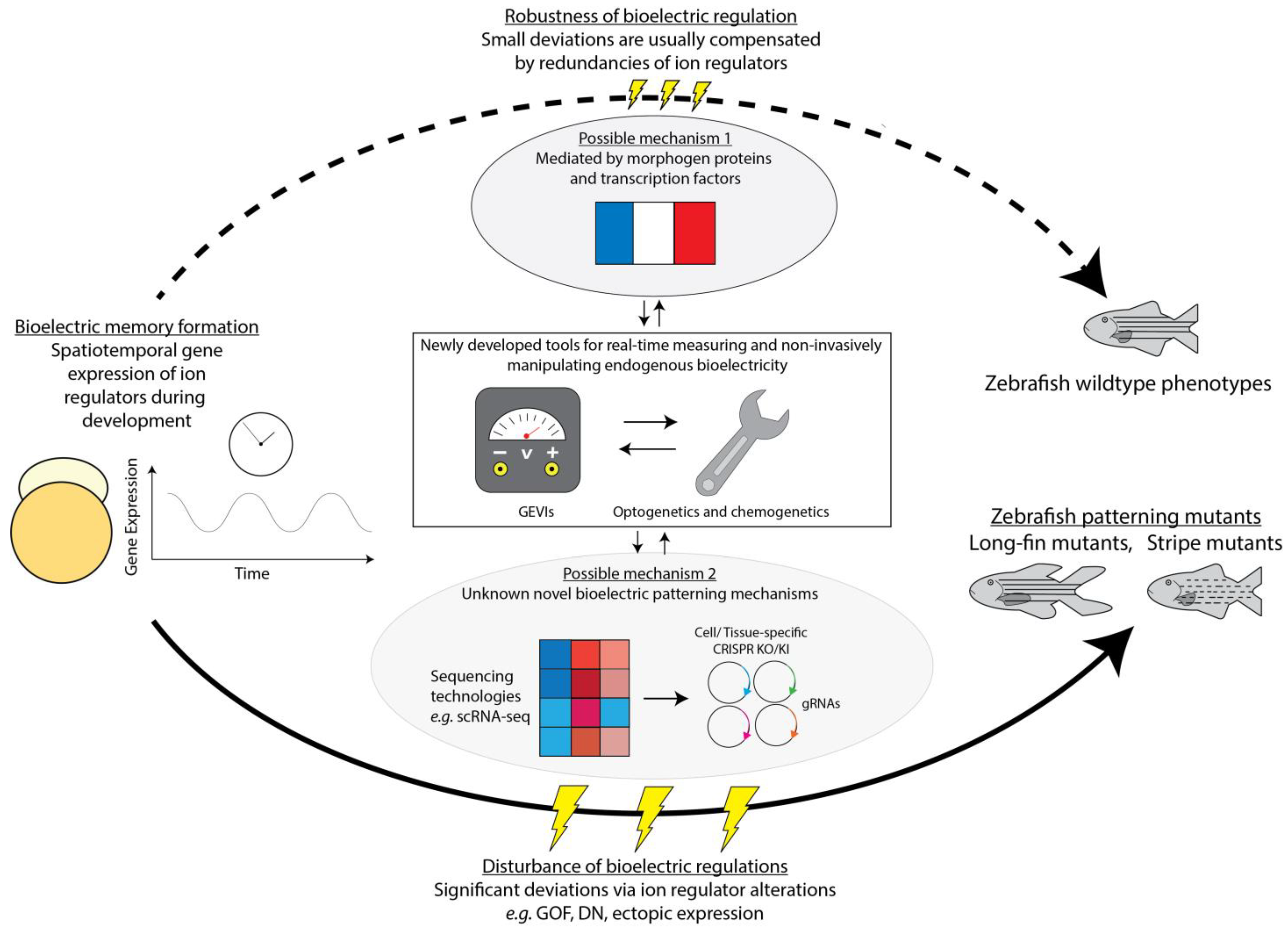
5. Prospects and Opportunities: Future Directions for Developmental Bioelectricity
5.1. Systematic Zebrafish Embryo Bioelectricity Characterization
5.2. Identifying Bioelectricity Contributing Genes and Redundancy of Ion Regulators
5.3. Developmental Patterning by Bioelectric Memory
5.4. Biological Pathways Downstream of Bioelectricity in Different Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lander, A.D. Pattern, growth, and control. Cell 2011, 144, 955–969. [Google Scholar] [CrossRef]
- Salazar-Ciudad, I.; Jernvall, J.; Newman, S.A. Mechanisms of pattern formation in development and evolution. Development 2003, 130, 2027–2037. [Google Scholar] [CrossRef]
- Takahashi, Y.; Osumi, N.; Patel, N.H. Body patterning. Proc. Natl. Acad. Sci. USA 2001, 98, 12338–12339. [Google Scholar] [CrossRef]
- Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Peter, I.S.; Davidson, E.H. Assessing regulatory information in developmental gene regulatory networks. Proc. Natl. Acad. Sci. USA 2017, 114, 5862–5869. [Google Scholar] [CrossRef]
- Negrete, J., Jr.; Oates, A.C. Towards a physical understanding of developmental patterning. Nat. Rev. Genet. 2021, 22, 518–531. [Google Scholar] [CrossRef]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef]
- Chang, F.; Minc, N. Electrochemical control of cell and tissue polarity. Annu. Rev. Cell Dev. Biol. 2014, 30, 317–336. [Google Scholar] [CrossRef]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef]
- Mathews, J.; Levin, M. The body electric 2.0: Recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin. Biotechnol. 2018, 52, 134–144. [Google Scholar] [CrossRef]
- Levin, M. Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays 2012, 34, 205–217. [Google Scholar] [CrossRef]
- Kulbacka, J.; Choromanska, A.; Rossowska, J.; Wezgowiec, J.; Saczko, J.; Rols, M.P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef]
- Catterall, W.A.; Wisedchaisri, G.; Zheng, N. The chemical basis for electrical signaling. Nat. Chem. Biol. 2017, 13, 455–463. [Google Scholar] [CrossRef]
- Stratford, J.P.; Edwards, C.L.A.; Ghanshyam, M.J.; Malyshev, D.; Delise, M.A.; Hayashi, Y.; Asally, M. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl. Acad. Sci. USA 2019, 116, 9552–9557. [Google Scholar] [CrossRef]
- Chimerel, C.; Field, C.M.; Pinero-Fernandez, S.; Keyser, U.F.; Summers, D.K. Indole prevents Escherichia coli cell division by modulating membrane potential. Biochim. Biophys. Acta 2012, 1818, 1590–1594. [Google Scholar] [CrossRef]
- Wayne, R. The excitability of plant cells: With a special emphasis on characean internodal cells. Bot. Rev. 1994, 60, 265–367. [Google Scholar] [CrossRef]
- Martinac, B.; Saimi, Y.; Kung, C. Ion channels in microbes. Physiol. Rev. 2008, 88, 1449–1490. [Google Scholar] [CrossRef]
- Adamatzky, A. Language of fungi derived from their electrical spiking activity. R. Soc. Open Sci. 2022, 9, 211926. [Google Scholar] [CrossRef]
- Dehshibi, M.M.; Adamatzky, A. Electrical activity of fungi: Spikes detection and complexity analysis. Biosystems 2021, 203, 104373. [Google Scholar] [CrossRef]
- Babikova, Z.; Gilbert, L.; Bruce, T.J.A.; Birkett, M.; Caulfield, J.C.; Woodcock, C.; Pickett, J.A.; Johnson, D. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 2013, 16, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Shtessel, Y.B. Underground electrotonic signal transmission between plants. Commun. Integr. Biol. 2020, 13, 54–58. [Google Scholar] [CrossRef]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef]
- Eckert, D.; Schulze, T.; Stahl, J.; Rauh, O.; Van Etten, J.L.; Hertel, B.; Schroeder, I.; Moroni, A.; Thiel, G. A small viral potassium ion channel with an inherent inward rectification. Channels 2019, 13, 124–135. [Google Scholar] [CrossRef]
- Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M. The Discovery of the Action-Potential. Trends Neurosci. 1983, 6, 164–168. [Google Scholar] [CrossRef]
- Carmeliet, E. From Bernstein’s rheotome to Neher-Sakmann’s patch electrode. The action potential. Physiol. Rep. 2019, 7, e13861. [Google Scholar] [CrossRef]
- Kamada, T. Some Observations on Potential Differences across the Ectoplasm Membrane of Paramecium. J. Exp. Biol. 1934, 11, 94–102. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef]
- Schwiening, C.J. A brief historical perspective: Hodgkin and Huxley. J. Physiol. 2012, 590, 2571–2575. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Maqoud, F.; Curci, A.; Scala, R.; Pannunzio, A.; Campanella, F.; Coluccia, M.; Passantino, G.; Zizzo, N.; Tricarico, D. Cell Cycle Regulation by Ca2+-Activated K+ (BK) Channels Modulators in SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2442. [Google Scholar] [CrossRef]
- Tolstykh, G.P.; Cantu, J.C.; Tarango, M.; Ibey, B.L. Receptor- and store-operated mechanisms of calcium entry during the nanosecond electric pulse-induced cellular response. Biochim. Biophys. Acta BBA-Biomembr. 2019, 1861, 685–696. [Google Scholar] [CrossRef]
- Rorsman, P.; Ashcroft, F.M. Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Zhou, Y.; Wong, C.O.; Cho, K.J.; van der Hoeven, D.; Liang, H.; Thakur, D.P.; Luo, J.; Babic, M.; Zinsmaier, K.E.; Zhu, M.X.; et al. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science 2015, 349, 873–876. [Google Scholar] [CrossRef]
- Harris, M.P. Bioelectric signaling as a unique regulator of development and regeneration. Development 2021, 148, dev180794. [Google Scholar] [CrossRef]
- George, L.F.; Bates, E.A. Mechanisms Underlying Influence of Bioelectricity in Development. Front. Cell. Dev. Biol. 2022, 10, 772230. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Voltage-gated potassium channels and the diversity of electrical signalling. J. Physiol. 2012, 590, 2591–2599. [Google Scholar] [CrossRef]
- Fillafer, C.; Paeger, A.; Schneider, M.F. Collision of two action potentials in a single excitable cell. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3282–3286. [Google Scholar] [CrossRef]
- Cervera, J.; Meseguer, S.; Mafe, S. Intercellular Connectivity and Multicellular Bioelectric Oscillations in Nonexcitable Cells: A Biophysical Model. ACS Omega 2018, 3, 13567–13575. [Google Scholar] [CrossRef]
- Forsberg, A.M.; Bergström, J.; Lindholm, B.; Hultman, E. Resting Membrane Potential of Skeletal Muscle Calculated from Plasma and Muscle Electrolyte and Water Contents. Clin. Sci. 1997, 92, 391–396. [Google Scholar] [CrossRef]
- Varro, A.; Tomek, J.; Nagy, N.; Virag, L.; Passini, E.; Rodriguez, B.; Baczko, I. Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef]
- Kuriyama, H.; Kitamura, K.; Itoh, T.; Inoue, R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998, 78, 811–920. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Spruston, N.; Johnston, D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J. Neurophysiol. 1992, 67, 508–529. [Google Scholar] [CrossRef]
- Staley, K.J.; Otis, T.S.; Mody, I. Membrane properties of dentate gyrus granule cells: Comparison of sharp microelectrode and whole-cell recordings. J. Neurophysiol. 1992, 67, 1346–1358. [Google Scholar] [CrossRef]
- Matthews, E.K. Membrane potential measurement in cells of the adrenal gland. J. Physiol. 1967, 189, 139–148. [Google Scholar] [CrossRef]
- Grinstein, S.; Dixon, S.J. Ion transport, membrane potential, and cytoplasmic pH in lymphocytes: Changes during activation. Physiol. Rev. 1989, 69, 417–481. [Google Scholar] [CrossRef]
- Green, S.T.; Petersen, O.H. Thyroid follicular cells: The resting membrane potential and the communication network. Pflug. Arch. 1981, 391, 119–124. [Google Scholar] [CrossRef]
- Lewis, R.; Asplin, K.E.; Bruce, G.; Dart, C.; Mobasheri, A.; Barrett-Jolley, R. The role of the membrane potential in chondrocyte volume regulation. J. Cell. Physiol. 2011, 226, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Leijh, P.C.; Meijer, J.; Van Bavel, E.; Ypey, D.L. Oscillatory hyperpolarizations and resting membrane potentials of mouse fibroblast and macrophage cell lines. J. Physiol. 1984, 352, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Lammerich, A.; Bohm, J.; Persson, P.B.; Gunther, J. Mechanically induced potentials in fibroblasts from human right atrium. Exp. Physiol. 1999, 84, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.E.; Ghimire, S.; Hepler, C.; Miller, K.E.; Bruggink, S.M.; Kentch, K.P.; Higgins, M.R.; Banek, C.T.; Yoshino, J.; Klein, S.; et al. Hepatocyte membrane potential regulates serum insulin and insulin sensitivity by altering hepatic GABA release. Cell Rep. 2021, 35, 109298. [Google Scholar] [CrossRef]
- Yada, T.; Okada, Y. Electrical activity of an intestinal epithelial cell line: Hyperpolarizing responses to intestinal secretagogues. J. Membr. Biol. 1984, 77, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Schwarz, G.; Droogmans, G. Control of intracellular calcium by membrane potential in human melanoma cells. Am. Physiol. Soc. 1993, 265, C1501–C1510. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, H.; Stark, A.; Kilch, T.; Schwarz, E.C.; Müller, C.S.L.; Peinelt, C.; Hoth, M.; Niemeyer, B.A.; Vogt, T.; Bogeski, I. ORAI1 Ca2+ Channels Control Endothelin-1-Induced Mitogenesis and Melanogenesis in Primary Human Melanocytes. J. Investig. Dermatol. 2012, 132, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Oancea, E. UV light phototransduction depolarizes human melanocytes. Channels 2013, 7, 243–248. [Google Scholar] [CrossRef]
- Bentley, D.C.; Pulbutr, P.; Chan, S.; Smith, P.A. Etiology of the membrane potential of rat white fat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E161–E175. [Google Scholar] [CrossRef]
- Pangalos, M.; Bintig, W.; Schlingmann, B.; Feyerabend, F.; Witte, F.; Begandt, D.; Heisterkamp, A.; Ngezahayo, A. Action potentials in primary osteoblasts and in the MG-63 osteoblast-like cell line. J. Bioenerg. Biomembr. 2011, 43, 311–322. [Google Scholar] [CrossRef]
- Binggeli, R.; Weinstein, R.C. Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986, 123, 377–401. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Wright, S.H. Generation of resting membrane potential. Adv. Physiol. Educ. 2004, 28, 139–142. [Google Scholar] [CrossRef]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef]
- Renigunta, V.; Schlichthörl, G.; Daut, J. Much more than a leak: Structure and function of K2P-channels. Pflüg. Arch. Eur. J. Physiol. 2015, 467, 867–894. [Google Scholar] [CrossRef]
- Alexander, S.P.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Ion channels. Br. J. Pharmacol. 2021, 178 (Suppl. S1), S157–S245. [Google Scholar] [CrossRef]
- Janata, J. Historical review. Twenty years of ion-selective field-effect transistors. Analyst 1994, 119, 2275–2278. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. mAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Seal, R.L.; Braschi, B.; Gray, K.; Jones, T.E.M.; Tweedie, S.; Haim-Vilmovsky, L.; Bruford, E.A. Genenames.org: The HGNC resources in 2023. Nucleic Acids Res. 2023, 51, D1003–D1009. [Google Scholar] [CrossRef]
- Finn, R.N.; Cerda, J. Evolution and functional diversity of aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef]
- Cochet-Bissuel, M.; Lory, P.; Monteil, A. The sodium leak channel, NALCN, in health and disease. Front. Cell. Neurosci. 2014, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Scemes, E.; Spray, D.C.; Meda, P. Connexins, pannexins, innexins: Novel roles of “hemi-channels”. Pflug. Arch. Eur. J. Physiol. 2009, 457, 1207–1226. [Google Scholar] [CrossRef]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, G.T.; Burt, J.M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta BBA-Biomembr. 2005, 1711, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef]
- Adams, D.S.; Levin, M. Endogenous voltage gradients as mediators of cell-cell communication: Strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013, 352, 95–122. [Google Scholar] [CrossRef]
- Eom, D.S.; Bain, E.J.; Patterson, L.B.; Grout, M.E.; Parichy, D.M. Long-distance communication by specialized cellular projections during pigment pattern development and evolution. eLife 2015, 4, e12401. [Google Scholar] [CrossRef]
- Sherer, N.M.; Mothes, W. Cytonemes and tunneling nanotubules in cell–cell communication and viral pathogenesis. Trends Cell Biol. 2008, 18, 414–420. [Google Scholar] [CrossRef]
- Mese, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of connexin 43 in different forms of intercellular communication—Gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef]
- Lecanda, F.; Warlow, P.M.; Sheikh, S.; Furlan, F.; Steinberg, T.H.; Civitelli, R. Connexin43 Deficiency Causes Delayed Ossification, Craniofacial Abnormalities, and Osteoblast Dysfunction. J. Cell Sci. 2000, 151, 931–944. [Google Scholar] [CrossRef]
- Srinivas, M.; Verselis, V.K.; White, T.W. Human diseases associated with connexin mutations. Biochim. Biophys. Acta BBA-Biomembr. 2018, 1860, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Southan, C.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Transporters. Br. J. Pharmacol. 2021, 178 (Suppl. S1), S412–S513. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug. Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Liu, S.; Leach, S.D. Zebrafish models for cancer. Annu. Rev. Pathol. 2011, 6, 71–93. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Page-McCaw, P.S.; Chen, W. Zebrafish Genome Engineering Using the CRISPR-Cas9 System. Trends Genet. 2016, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Wolfe, S.A. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev. Cell 2011, 21, 48–64. [Google Scholar] [CrossRef]
- Kawakami, K. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007, 8 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Sprague, J.; Clements, D.; Conlin, T.; Edwards, P.; Frazer, K.; Schaper, K.; Segerdell, E.; Song, P.; Sprunger, B.; Westerfield, M. The Zebrafish Information Network (ZFIN): The zebrafish model organism database. Nucleic Acids Res. 2003, 31, 241–243. [Google Scholar] [CrossRef]
- Sprague, J.; Doerry, E.; Douglas, S.; Westerfield, M. The Zebrafish Information Network (ZFIN): A resource for genetic, genomic and developmental research. Nucleic Acids Res. 2001, 29, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef]
- Veldman, M.B.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Grandel, H.; Schulte-Merker, S. The development of the paired fins in the zebrafish (Danio rerio). Mech. Dev. 1998, 79, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Pfefferli, C.; Jazwinska, A. The art of fin regeneration in zebrafish. Regeneration 2015, 2, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Mabee, P.M.; Crotwell, P.L.; Bird, N.C.; Burke, A.C. Evolution of median fin modules in the axial skeleton of fishes. J. Exp. Zool. 2002, 294, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Mari-Beffa, M.; Murciano, C. Dermoskeleton morphogenesis in zebrafish fins. Dev. Dyn. 2010, 239, 2779–2794. [Google Scholar] [CrossRef]
- Yano, T.; Abe, G.; Yokoyama, H.; Kawakami, K.; Tamura, K. Mechanism of pectoral fin outgrowth in zebrafish development. Development 2012, 139, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Le Bleu, H.K.; Yette, G.A.; Henner, A.L.; Robbins, A.E.; Braunstein, J.A.; Stankunas, K. longfin causes cis-ectopic expression of the kcnh2a ether-a-go-go K+ channel to autonomously prolong fin outgrowth. Development 2021, 148, dev199384. [Google Scholar] [CrossRef]
- Daane, J.M.; Blum, N.; Lanni, J.; Boldt, H.; Iovine, M.K.; Higdon, C.W.; Johnson, S.L.; Lovejoy, N.R.; Harris, M.P. Modulation of bioelectric cues in the evolution of flying fishes. Curr. Biol. 2021, 31, 5052–5061.e8. [Google Scholar] [CrossRef] [PubMed]
- Perathoner, S.; Daane, J.M.; Henrion, U.; Seebohm, G.; Higdon, C.W.; Johnson, S.L.; Nüsslein-Volhard, C.; Harris, M.P. Bioelectric Signaling Regulates Size in Zebrafish Fins. PLoS Genet. 2014, 10, e1004080. [Google Scholar] [CrossRef] [PubMed]
- Lanni, J.S.; Peal, D.; Ekstrom, L.; Chen, H.; Stanclift, C.; Bowen, M.E.; Mercado, A.; Gamba, G.; Kahle, K.T.; Harris, M.P. Integrated K+ channel and K+Cl− cotransporter functions are required for the coordination of size and proportion during development. Dev. Biol. 2019, 456, 164–178. [Google Scholar] [CrossRef]
- Iovine, M.K.; Higgins, E.P.; Hindes, A.; Coblitz, B.; Johnson, S.L. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev. Biol. 2005, 278, 208–219. [Google Scholar] [CrossRef]
- Eskova, A.; Chauvigné, F.; Maischein, H.-M.; Ammelburg, M.; Cerdà, J.; Nüsslein-Volhard, C.; Irion, U. Gain-of-function mutations in Aqp3a influence zebrafish pigment pattern formation through the tissue environment. Development 2017, 144, 2059–2069. [Google Scholar] [CrossRef]
- Iovine, M.K.; Johnson, S.L. Genetic analysis of isometric growth control mechanisms in the zebrafish caudal Fin. Genetics 2000, 155, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Silic, M.R.; Wu, Q.; Kim, B.H.; Golling, G.; Chen, K.H.; Freitas, R.; Chubykin, A.A.; Mittal, S.K.; Zhang, G. Potassium Channel-Associated Bioelectricity of the Dermomyotome Determines Fin Patterning in Zebrafish. Genetics 2020, 215, 1067–1084. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Brandt, D.Y.C.; Hu, B.; Sheng, J.; Wang, M.; Luo, H.; Li, Y.; Guo, S.; Sheng, B.; et al. The genetic architecture of phenotypic diversity in the Betta fish (Betta splendens). Sci. Adv. 2022, 8, eabm4955. [Google Scholar] [CrossRef]
- Schartl, M.; Kneitz, S.; Ormanns, J.; Schmidt, C.; Anderson, J.L.; Amores, A.; Catchen, J.; Wilson, C.; Geiger, D.; Du, K.; et al. The Developmental and Genetic Architecture of the Sexually Selected Male Ornament of Swordtails. Curr. Biol. 2021, 31, 911–922.e4. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Omori, Y.; Fukuta, K.; Wada, H.; Watanabe, M.; Chen, Z.; Iwasaki, M.; Mishina, T.; Matsuzaki, S.S.; Yoshihara, D.; et al. The Genetic Basis of Morphological Diversity in Domesticated Goldfish. Curr. Biol. 2020, 30, 2260–2274.e6. [Google Scholar] [CrossRef]
- Singh, A.P.; Nüsslein-Volhard, C. Zebrafish Stripes as a Model for Vertebrate Colour Pattern Formation. Curr. Biol. 2015, 25, R81–R92. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.B.; Parichy, D.M. Zebrafish Pigment Pattern Formation: Insights into the Development and Evolution of Adult Form. Annu. Rev. Genet. 2019, 53, 505–530. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.A.; Barsh, G.S. How the zebrafish got its stripes. eLife 2016, 5, e14239. [Google Scholar] [CrossRef]
- Dooley, C.M.; Schwarz, H.; Mueller, K.P.; Mongera, A.; Konantz, M.; Neuhauss, S.C.; Nusslein-Volhard, C.; Geisler, R. Slc45a2 and V-ATPase are regulators of melanosomal pH homeostasis in zebrafish, providing a mechanism for human pigment evolution and disease. Pigment. Cell Melanoma Res. 2013, 26, 205–217. [Google Scholar] [CrossRef]
- Lamason, R.L.; Mohideen, M.A.; Mest, J.R.; Wong, A.C.; Norton, H.L.; Aros, M.C.; Jurynec, M.J.; Mao, X.; Humphreville, V.R.; Humbert, J.E.; et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005, 310, 1782–1786. [Google Scholar] [CrossRef]
- Le, L.; Escobar, I.E.; Ho, T.; Lefkovith, A.J.; Latteri, E.; Haltaufderhyde, K.D.; Dennis, M.K.; Plowright, L.; Sviderskaya, E.V.; Bennett, D.C.; et al. SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation. Mol. Biol. Cell 2020, 31, 2687–2702. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, G.; Beltre, R.; Sessa, A.; Burger, A.; Zhou, Y.; Mosimann, C.; White, R.M. A defect in the mitochondrial protein Mpv17 underlies the transparent casper zebrafish. Dev. Biol. 2017, 430, 11–17. [Google Scholar] [CrossRef]
- Antonenkov, V.D.; Isomursu, A.; Mennerich, D.; Vapola, M.H.; Weiher, H.; Kietzmann, T.; Hiltunen, J.K. The Human Mitochondrial DNA Depletion Syndrome Gene MPV17 Encodes a Non-selective Channel That Modulates Membrane Potential. J. Biol. Chem. 2015, 290, 13840–13861. [Google Scholar] [CrossRef]
- Irion, U.; Frohnhöfer, H.G.; Krauss, J.; Çolak Champollion, T.; Maischein, H.-M.; Geiger-Rudolph, S.; Weiler, C.; Nüsslein-Volhard, C. Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. eLife 2014, 3, e05125. [Google Scholar] [CrossRef]
- Watanabe, M.; Sawada, R.; Aramaki, T.; Skerrett, I.M.; Kondo, S. The Physiological Characterization of Connexin41.8 and Connexin39.4, Which Are Involved in the Striped Pattern Formation of Zebrafish. J. Biol. Chem. 2016, 291, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, M.; Watanabe, M.; Ishii, M.; Chen, T.; Johnson, S.L.; Kurachi, Y.; Okada, N.; Kondo, S. Pigment Pattern in jaguar/obelix Zebrafish Is Caused by a Kir7.1 Mutation: Implications for the Regulation of Melanosome Movement. PLoS Genet. 2006, 2, e197. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Frohnhofer, H.G.; Dooley, C.M.; Eskova, A.; Nusslein-Volhard, C.; Irion, U. Evolution of the potassium channel gene Kcnj13 underlies colour pattern diversification in Danio fish. Nat. Commun. 2020, 11, 6230. [Google Scholar] [CrossRef]
- Inaba, M.; Yamanaka, H.; Kondo, S. Pigment Pattern Formation by Contact-Dependent Depolarization. Science 2012, 335, 677. [Google Scholar] [CrossRef]
- Williams, K. Interactions of polyamines with ion channels. Biochem. J. 1997, 325 Pt 2, 289–297. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lee, S.J. Polyamines and potassium channels: A 25-year romance. J. Biol. Chem. 2018, 293, 18779–18788. [Google Scholar] [CrossRef]
- Frohnhofer, H.G.; Geiger-Rudolph, S.; Pattky, M.; Meixner, M.; Huhn, C.; Maischein, H.M.; Geisler, R.; Gehring, I.; Maderspacher, F.; Nusslein-Volhard, C.; et al. Spermidine, but not spermine, is essential for pigment pattern formation in zebrafish. Biol. Open 2016, 5, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Watanabe, D.; Kondo, S. Polyamine sensitivity of gap junctions is required for skin pattern formation in zebrafish. Sci. Rep. 2012, 2, 473. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.; Krauss, J.; Frohnhofer, H.G.; Irion, U.; Nusslein-Volhard, C. Tight Junction Protein 1a regulates pigment cell organisation during zebrafish colour patterning. eLife 2015, 4, e06545. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins at a glance. J. Cell Sci. 2011, 124, 2107–2112. [Google Scholar] [CrossRef]
- Mollinedo-Gajate, I.; Song, C.; Knopfel, T. Genetically Encoded Voltage Indicators. Adv. Exp. Med. Biol. 2021, 1293, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.; Titov, D.V. Genetically encoded tools for measuring and manipulating metabolism. Nat. Chem. Biol. 2022, 18, 451–460. [Google Scholar] [CrossRef]
- Poth, K.M.; Texakalidis, P.; Boulis, N.M. Chemogenetics: Beyond Lesions and Electrodes. Neurosurgery 2021, 89, 185–195. [Google Scholar] [CrossRef]
- Ozawa, A.; Arakawa, H. Chemogenetics drives paradigm change in the investigation of behavioral circuits and neural mechanisms underlying drug action. Behav. Brain Res. 2021, 406, 113234. [Google Scholar] [CrossRef]
- Keifer, O.; Kambara, K.; Lau, A.; Makinson, S.; Bertrand, D. Chemogenetics a robust approach to pharmacology and gene therapy. Biochem. Pharmacol. 2020, 175, 113889. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Liang, W.; Li, Y.; Zou, Z.; Xie, Y.; Liao, Y.; Yu, L.; Lin, Q.; Huang, M.; et al. The Roles of Optogenetics and Technology in Neurobiology: A Review. Front. Aging Neurosci. 2022, 14, 867863. [Google Scholar] [CrossRef]
- Antinucci, P.; Dumitrescu, A.; Deleuze, C.; Morley, H.J.; Leung, K.; Hagley, T.; Kubo, F.; Baier, H.; Bianco, I.H.; Wyart, C. A calibrated optogenetic toolbox of stable zebrafish opsin lines. eLife 2020, 9, e54937. [Google Scholar] [CrossRef] [PubMed]
- Josselyn, S.A. The past, present and future of light-gated ion channels and optogenetics. eLife 2018, 7, e42367. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cui, J. Patch-Clamp and Perfusion Techniques to Study Ion Channels Expressed in Xenopus Oocytes. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot099051. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Z.; Schnitzer, M.J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 2016, 19, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Ikegaya, Y.; Matsumoto, N. In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives. Sensors 2021, 21, 1448. [Google Scholar] [CrossRef]
- Muto, A.; Kawakami, K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun. Integr. Biol. 2011, 4, 566–568. [Google Scholar] [CrossRef]
- Kim, C.K.; Miri, A.; Leung, L.C.; Berndt, A.; Mourrain, P.; Tank, D.W.; Burdine, R.D. Prolonged, brain-wide expression of nuclear-localized GCaMP3 for functional circuit mapping. Front. Neural Circuits 2014, 8, 138. [Google Scholar] [CrossRef]
- DeMarco, E.; Xu, N.; Baier, H.; Robles, E. Neuron types in the zebrafish optic tectum labeled by an id2b transgene. J. Comp. Neurol. 2020, 528, 1173–1188. [Google Scholar] [CrossRef]
- Beerman, R.W.; Matty, M.A.; Au, G.G.; Looger, L.L.; Choudhury, K.R.; Keller, P.J.; Tobin, D.M. Direct In Vivo Manipulation and Imaging of Calcium Transients in Neutrophils Identify a Critical Role for Leading-Edge Calcium Flux. Cell Rep. 2015, 13, 2107–2117. [Google Scholar] [CrossRef]
- Shelef, M.A.; Tauzin, S.; Huttenlocher, A. Neutrophil migration: Moving from zebrafish models to human autoimmunity. Immunol. Rev. 2013, 256, 269–281. [Google Scholar] [CrossRef]
- Mizuno, H.; Sassa, T.; Higashijima, S.; Okamoto, H.; Miyawaki, A. Transgenic zebrafish for ratiometric imaging of cytosolic and mitochondrial Ca2+ response in teleost embryo. Cell Calcium 2013, 54, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, L.; Bruchas, M.R.; Solnica-Krezel, L. Imaging early embryonic calcium activity with GCaMP6s transgenic zebrafish. Dev. Biol. 2017, 430, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, R.; Emfinger, C.H.; Walcher, A.; Giolai, M.; Krautgasser, C.; Remedi, M.S.; Nichols, C.G.; Meyer, D. In vivo monitoring of intracellular Ca2+ dynamics in the pancreatic beta-cells of zebrafish embryos. Islets 2018, 10, 221–238. [Google Scholar] [CrossRef]
- Li, F.; Long, Y.; Xie, J.; Ren, J.; Zhou, T.; Song, G.; Li, Q.; Cui, Z. Generation of GCaMP6s-Expressing Zebrafish to Monitor Spatiotemporal Dynamics of Calcium Signaling Elicited by Heat Stress. Int. J. Mol. Sci. 2021, 22, 5551. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Morales, M.; Garteizgogeascoa, I.; Perazzolo, C.; So, J.; Shin, D.; Singh, S.P. In vivo imaging of calcium dynamics in zebrafish hepatocytes. Hepatology 2022, 77, 789–801. [Google Scholar] [CrossRef]
- Kettunen, P. Calcium Imaging in the Zebrafish. Adv. Exp. Med. Biol. 2020, 1131, 901–942. [Google Scholar] [CrossRef]
- Lukasz, D.; Kindt, K.S. In Vivo Calcium Imaging of Lateral-line Hair Cells in Larval Zebrafish. J. Vis. Exp. 2018, e58794. [Google Scholar] [CrossRef]
- Debnath, A.; Williams, P.D.E.; Bamber, B.A. Reduced Ca(2+) transient amplitudes may signify increased or decreased depolarization depending on the neuromodulatory signaling pathway. Front. Neurosci. 2022, 16, 931328. [Google Scholar] [CrossRef]
- Zhu, M.H.; Jang, J.; Milosevic, M.M.; Antic, S.D. Population imaging discrepancies between a genetically-encoded calcium indicator (GECI) versus a genetically-encoded voltage indicator (GEVI). Sci. Rep. 2021, 11, 5295. [Google Scholar] [CrossRef]
- Gest, A.M.M.; Yaeger-Weiss, S.K.; Lazzari-Dean, J.R.; Miller, E.W. VoltageFluor dyes and fluorescence lifetime imaging for optical measurement of membrane potential. In Methods in Enzymology; Minor, D.L., Colecraft, H.M., Eds.; Ion Channels: Channel Production and Optical Methods; Academic Press: Cambridge, MA, USA, 2021; Volume 653, pp. 267–293. [Google Scholar]
- Yang, H.H.; St-Pierre, F. Genetically Encoded Voltage Indicators: Opportunities and Challenges. J. Neurosci. 2016, 36, 9977–9989. [Google Scholar] [CrossRef] [PubMed]
- Platisa, J.; Pieribone, V.A. Genetically encoded fluorescent voltage indicators: Are we there yet? Curr. Opin. Neurobiol. 2018, 50, 146–153. [Google Scholar] [CrossRef]
- Sakata, S.; Okamura, Y. Phosphatase activity of the voltage-sensing phosphatase, VSP, shows graded dependence on the extent of activation of the voltage sensor. J. Physiol. 2014, 592, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Jinno, Y.; Tomita, A.; Niino, Y.; Yamada, Y.; Mikoshiba, K.; Miyawaki, A.; Okamura, Y. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J. Physiol. 2013, 591, 4427–4437. [Google Scholar] [CrossRef]
- St-Pierre, F.; Marshall, J.D.; Yang, Y.; Gong, Y.; Schnitzer, M.J.; Lin, M.Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 2014, 17, 884–889. [Google Scholar] [CrossRef]
- Chamberland, S.; Yang, H.H.; Pan, M.M.; Evans, S.W.; Guan, S.; Chavarha, M.; Yang, Y.; Salesse, C.; Wu, H.; Wu, J.C.; et al. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. eLife 2017, 6, e25690. [Google Scholar] [CrossRef]
- Villette, V.; Chavarha, M.; Dimov, I.K.; Bradley, J.; Pradhan, L.; Mathieu, B.; Evans, S.W.; Chamberland, S.; Shi, D.; Yang, R.; et al. Ultrafast Two-Photon Imaging of a High-Gain Voltage Indicator in Awake Behaving Mice. Cell 2019, 179, 1590–1608.e23. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; St-Pierre, F.; Sun, X.; Ding, X.; Lin, M.Z.; Clandinin, T.R. Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo. Cell 2016, 166, 245–257. [Google Scholar] [CrossRef]
- Silic, M.R.; Zhang, G. Visualization of Cellular Electrical Activity in Zebrafish Early Embryos and Tumors. J. Vis. Exp. 2018, e57330. [Google Scholar] [CrossRef]
- Silic, M.R.; Dong, Z.; Chen, Y.; Kimbrough, A.; Zhang, G. Zebrafish Embryos Display Characteristic Bioelectric Signals during Early Development. Cells 2022, 11, 3586. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, K.; Shiraishi, A.; Fukuda, N.; Tsuda, S. In vivo wide-field voltage imaging in zebrafish with voltage-sensitive dye and genetically encoded voltage indicator. Dev. Growth Differ. 2021, 63, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, H.; Okumura, K.; Hiyoshi, K.; Maruyama, K.; Kakinuma, H.; Amo, R.; Okamoto, H.; Yamasu, K.; Tsuda, S. Optical interrogation of neuronal circuitry in zebrafish using genetically encoded voltage indicators. Sci. Rep. 2018, 8, 6048. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fairn, G.D.; Ceccarelli, D.F.; Sicheri, F.; Wilde, A. Cleavage Furrow Organization Requires PIP2-Mediated Recruitment of Anillin. Curr. Biol. 2012, 22, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Field, S.J.; Madson, N.; Kerr, M.L.; Galbraith, K.A.A.; Kennedy, C.E.; Tahiliani, M.; Wilkins, A.; Cantley, L.C. PtdIns(4,5)P2 Functions at the Cleavage Furrow during Cytokinesis. Curr. Biol. 2005, 15, 1407–1412. [Google Scholar] [CrossRef]
- Raccuglia, D.; Huang, S.; Ender, A.; Heim, M.M.; Laber, D.; Suárez-Grimalt, R.; Liotta, A.; Sigrist, S.J.; Geiger, J.R.P.; Owald, D. Network-Specific Synchronization of Electrical Slow-Wave Oscillations Regulates Sleep Drive in Drosophila. Curr. Biol. 2019, 29, 3611–3621.e3. [Google Scholar] [CrossRef] [PubMed]
- Raccuglia, D.; McCurdy, L.Y.; Demir, M.; Gorur-Shandilya, S.; Kunst, M.; Emonet, T.; Nitabach, M.N. Presynaptic GABA Receptors Mediate Temporal Contrast Enhancement in Drosophila Olfactory Sensory Neurons and Modulate Odor-Driven Behavioral Kinetics. eNeuro 2016, 3, ENEURO.0080-0016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, X.; Villette, V.; Gou, Y.; Colbert, K.L.; Lai, S.; Guan, S.; Land, M.A.; Lee, J.; Assefa, T.; et al. Sustained deep-tissue voltage recording using a fast indicator evolved for two-photon microscopy. Cell 2022, 185, 3408–3425.e29. [Google Scholar] [CrossRef]
- Shimaoka, D.; Steinmetz, N.A.; Harris, K.D.; Carandini, M. The impact of bilateral ongoing activity on evoked responses in mouse cortex. eLife 2019, 8, e43533. [Google Scholar] [CrossRef]
- Song, C.; Piscopo, D.M.; Niell, C.M.; Knopfel, T. Cortical signatures of wakeful somatosensory processing. Sci. Rep. 2018, 8, 11977. [Google Scholar] [CrossRef]
- Platisa, J.; Zeng, H.; Madisen, L.; Cohen, L.B.; Pieribone, V.A.; Storace, D.A. Voltage imaging in the olfactory bulb using transgenic mouse lines expressing the genetically encoded voltage indicator ArcLight. Sci. Rep. 2022, 12, 1875. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Haziza, S.; Huang, C.; Chrapkiewicz, R.; Luo, J.; Cardin, J.A.; Schnitzer, M.J.; Pieribone, V.A. Dual-polarity voltage imaging of the concurrent dynamics of multiple neuron types. Science 2022, 378, eabm8797. [Google Scholar] [CrossRef] [PubMed]
- Treger, J.S.; Priest, M.F.; Bezanilla, F. Single-molecule fluorimetry and gating currents inspire an improved optical voltage indicator. eLife 2015, 4, e10482. [Google Scholar] [CrossRef]
- Zhang, Y.; Shannonhouse, J.; Gomez, R.; Son, H.; Ishida, H.; Evans, S.; Chavarha, M.; Shi, D.; Zhang, G.; Lin, M.Z.; et al. Imaging sensory transmission and neuronal plasticity in primary sensory neurons with genetically-encoded voltage indicator, ASAP4.4-Kv. bioRxiv 2021. [Google Scholar] [CrossRef]
- Evans, S.W.; Shi, D.; Chavarha, M.; Plitt, M.H.; Taxidis, J.; Madruga, B.; van Keulen, S.C.; Pang, M.M.; Su, S.; Hwang, F.-J.; et al. A positively Tuned Voltage Indicator Reveals Electrical Correlates of Calcium Activity in the Brain. bioRxiv 2022. [Google Scholar] [CrossRef]
- Platisa, J.; Vasan, G.; Yang, A.; Pieribone, V.A. Directed Evolution of Key Residues in Fluorescent Protein Inverses the Polarity of Voltage Sensitivity in the Genetically Encoded Indicator ArcLight. ACS Chem. Neurosci. 2017, 8, 513–523. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Farhi, S.L.; Zhao, Y.; Brinks, D.; Zou, P.; Ruangkittisakul, A.; Platisa, J.; Pieribone, V.A.; Ballanyi, K.; Cohen, A.E.; et al. A Bright and Fast Red Fluorescent Protein Voltage Indicator That Reports Neuronal Activity in Organotypic Brain Slices. J. Neurosci. 2016, 36, 2458–2472. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.R.; Cohen, L.B.; Pieribone, V.A. Single Action Potentials and Subthreshold Electrical Events Imaged in Neurons with a Fluorescent Protein Voltage Probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Jin, L.; Platisa, J.; Cohen, L.B.; Baker, B.J.; Pieribone, V.A. Fluorescent protein voltage probes derived from ArcLight that respond to membrane voltage changes with fast kinetics. PLoS ONE 2013, 8, e81295. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Geiller, T.; Jung, A.; Nakajima, R.; Song, Y.K.; Baker, B.J. Improving a genetically encoded voltage indicator by modifying the cytoplasmic charge composition. Sci. Rep. 2017, 7, 8286. [Google Scholar] [CrossRef]
- Kibat, C.; Krishnan, S.; Ramaswamy, M.; Baker, B.J.; Jesuthasan, S. Imaging voltage in zebrafish as a route to characterizing a vertebrate functional connectome: Promises and pitfalls of genetically encoded indicators. J. Neurogenet. 2016, 30, 80–88. [Google Scholar] [CrossRef]
- Sepehri Rad, M.; Cohen, L.B.; Braubach, O.; Baker, B.J. Monitoring voltage fluctuations of intracellular membranes. Sci. Rep. 2018, 8, 6911. [Google Scholar] [CrossRef]
- Akemann, W.; Mutoh, H.; Perron, A.; Rossier, J.; Knopfel, T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat. Methods 2010, 7, 643–649. [Google Scholar] [CrossRef]
- Lam, A.J.; St-Pierre, F.; Gong, Y.; Marshall, J.D.; Cranfill, P.J.; Baird, M.A.; McKeown, M.R.; Wiedenmann, J.; Davidson, M.W.; Schnitzer, M.J.; et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 2012, 9, 1005–1012. [Google Scholar] [CrossRef]
- Akemann, W.; Mutoh, H.; Perron, A.; Park, Y.K.; Iwamoto, Y.; Knopfel, T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 2012, 108, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Mishina, Y.; Mutoh, H.; Song, C.; Knopfel, T. Exploration of genetically encoded voltage indicators based on a chimeric voltage sensing domain. Front. Mol. Neurosci. 2014, 7, 78. [Google Scholar] [CrossRef]
- Gautam, S.G.; Perron, A.; Mutoh, H.; Knopfel, T. Exploration of fluorescent protein voltage probes based on circularly permuted fluorescent proteins. Front. Neuroeng. 2009, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Perron, A.; Mutoh, H.; Launey, T.; Knopfel, T. Red-shifted voltage-sensitive fluorescent proteins. Chem. Biol. 2009, 16, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- van Opbergen, C.J.M.; Koopman, C.D.; Kok, B.J.M.; Knopfel, T.; Renninger, S.L.; Orger, M.B.; Vos, M.A.; van Veen, T.A.B.; Bakkers, J.; de Boer, T.P. Optogenetic sensors in the zebrafish heart: A novel in vivo electrophysiological tool to study cardiac arrhythmogenesis. Theranostics 2018, 8, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Koopman, C.D.; De Angelis, J.; Iyer, S.P.; Verkerk, A.O.; Da Silva, J.; Berecki, G.; Jeanes, A.; Baillie, G.J.; Paterson, S.; Uribe, V.; et al. The zebrafish grime mutant uncovers an evolutionarily conserved role for Tmem161b in the control of cardiac rhythm. Proc. Natl. Acad. Sci. USA 2021, 118, e2018220118. [Google Scholar] [CrossRef]
- Tsutsui, H.; Karasawa, S.; Okamura, Y.; Miyawaki, A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat. Methods 2008, 5, 683–685. [Google Scholar] [CrossRef]
- Sung, U.; Sepehri-Rad, M.; Piao, H.H.; Jin, L.; Hughes, T.; Cohen, L.B.; Baker, B.J. Developing Fast Fluorescent Protein Voltage Sensors by Optimizing FRET Interactions. PLoS ONE 2015, 10, e0141585. [Google Scholar] [CrossRef]
- Kralj, J.M.; Douglass, A.D.; Hochbaum, D.R.; Maclaurin, D.; Cohen, A.E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods 2012, 9, 90–95. [Google Scholar] [CrossRef]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Adam, Y.; Kim, J.J.; Lou, S.; Zhao, Y.; Xie, M.E.; Brinks, D.; Wu, H.; Mostajo-Radji, M.A.; Kheifets, S.; Parot, V.; et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 2019, 569, 413–417. [Google Scholar] [CrossRef]
- Zou, P.; Zhao, Y.; Douglass, A.D.; Hochbaum, D.R.; Brinks, D.; Werley, C.A.; Harrison, D.J.; Campbell, R.E.; Cohen, A.E. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun. 2014, 5, 4625. [Google Scholar] [CrossRef]
- Hou, J.H.; Kralj, J.M.; Douglass, A.D.; Engert, F.; Cohen, A.E. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol. 2014, 5, 344. [Google Scholar] [CrossRef]
- Dempsey, G.T.; Chaudhary, K.W.; Atwater, N.; Nguyen, C.; Brown, B.S.; McNeish, J.D.; Cohen, A.E.; Kralj, J.M. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods 2016, 81, 240–250. [Google Scholar] [CrossRef]
- Piatkevich, K.D.; Jung, E.E.; Straub, C.; Linghu, C.; Park, D.; Suk, H.-J.; Hochbaum, D.R.; Goodwin, D.; Pnevmatikakis, E.; Pak, N.; et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 2018, 14, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bohm, U.L.; Kimura, Y.; Kawashima, T.; Ahrens, M.B.; Higashijima, S.I.; Engert, F.; Cohen, A.E. Voltage imaging identifies spinal circuits that modulate locomotor adaptation in zebrafish. Neuron 2022, 110, 1211–1222.e4. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Gong, Y. A high-speed, bright, red fluorescent voltage sensor to detect neural activity. Sci. Rep. 2019, 9, 15878. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef]
- Kannan, M.; Vasan, G.; Huang, C.; Haziza, S.; Li, J.Z.; Inan, H.; Schnitzer, M.J.; Pieribone, V.A. Fast, in vivo voltage imaging using a red fluorescent indicator. Nat. Methods 2018, 15, 1108–1116. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Kawashima, T.; Singh, A.; Novak, O.; Liu, H.; Shuai, Y.; Huang, Y.C.; Campagnola, L.; Seeman, S.C.; Yu, J.; et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 2019, 365, 699–704. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Zheng, J.; Singh, A.; Huang, Y.C.; Reep, D.; Tsegaye, G.; Tsang, A.; Arthur, B.J.; Rehorova, M.; Olson, C.V.L.; et al. Sensitivity optimization of a rhodopsin-based fluorescent voltage indicator. Neuron 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.S.; Valenti, R.; Zheng, J.; Wong, A.; Team, G.P.; Podgorski, K.; Koyama, M.; Kim, D.S.; Schreiter, E.R. A general approach to engineer positive-going eFRET voltage indicators. Nat. Commun. 2020, 11, 3444. [Google Scholar] [CrossRef] [PubMed]
- Chanda, B.; Blunck, R.; Faria, L.C.; Schweizer, F.E.; Mody, I.; Bezanilla, F. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat. Neurosci. 2005, 8, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Grenier, V.; Daws, B.R.; Liu, P.; Miller, E.W. Spying on Neuronal Membrane Potential with Genetically Targetable Voltage Indicators. J. Am. Chem. Soc. 2019, 141, 1349–1358. [Google Scholar] [CrossRef]
- Inagaki, S.; Tsutsui, H.; Suzuki, K.; Agetsuma, M.; Arai, Y.; Jinno, Y.; Bai, G.; Daniels, M.J.; Okamura, Y.; Matsuda, T.; et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Rep. 2017, 7, 42398. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Griffin, N.M.; Thakur, D.; Joshi, P.; Nguyen-Le, A.; McCotter, S.; Jain, A.; Saeidi, M.; Kulkarni, P.; Eisdorfer, J.T.; et al. An Autonomous Molecular Bioluminescent Reporter (AMBER) for Voltage Imaging in Freely Moving Animals. Adv. Biol. 2021, 5, e2100842. [Google Scholar] [CrossRef]
- Sternson, S.M.; Roth, B.L. Chemogenetic Tools to Interrogate Brain Functions. Annu. Rev. Neurosci. 2014, 37, 387–407. [Google Scholar] [CrossRef]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Sternson, S.M. Chemogenetic Tools for Causal Cellular and Neuronal Biology. Physiol. Rev. 2018, 98, 391–418. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Li, H.; Spudich, J.L. Microbial Rhodopsins: Diversity, Mechanisms, and Optogenetic Applications. Annu. Rev. Biochem. 2017, 86, 845–872. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Vierock, J.; Yizhar, O.; Fenno, L.E.; Tsunoda, S.; Kianianmomeni, A.; Prigge, M.; Berndt, A.; Cushman, J.; Polle, J.; et al. The microbial opsin family of optogenetic tools. Cell 2011, 147, 1446–1457. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.; Zhang, F.; Hegemann, P.; Diesseroth, K. Microbial opsins: A family of single-component tools for optical control of neural activity. Cold Spring Harb. Protoc. 2011, 2011, top102. [Google Scholar] [CrossRef]
- Arrenberg, A.B.; Del Bene, F.; Baier, H. Optical control of zebrafish behavior with halorhodopsin. Proc. Natl. Acad. Sci. USA 2009, 106, 17968–17973. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, E.E.; Haupt, S.; Ausborn, J.; Ampatzis, K.; El Manira, A. Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J. Neurosci. 2014, 34, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Monesson-Olson, B.D.; Browning-Kamins, J.; Aziz-Bose, R.; Kreines, F.; Trapani, J.G. Optical stimulation of zebrafish hair cells expressing channelrhodopsin-2. PLoS ONE 2014, 9, e96641. [Google Scholar] [CrossRef]
- Harris, J.M.; Wang, A.Y.; Boulanger-Weill, J.; Santoriello, C.; Foianini, S.; Lichtman, J.W.; Zon, L.I.; Arlotta, P. Long-Range Optogenetic Control of Axon Guidance Overcomes Developmental Boundaries and Defects. Dev. Cell. 2020, 53, 577–588.e7. [Google Scholar] [CrossRef]
- Jeong, Y.-M.; Choi, T.-I.; Hwang, K.-S.; Lee, J.-S.; Gerlai, R.; Kim, C.-H. Optogenetic Manipulation of Olfactory Responses in Transgenic Zebrafish: A Neurobiological and Behavioral Study. Int. J. Mol. Sci. 2021, 22, 7191. [Google Scholar] [CrossRef]
- Arrenberg, A.B.; Stainier, D.Y.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Kopton, R.A.; Baillie, J.S.; Rafferty, S.A.; Moss, R.; Zgierski-Johnston, C.M.; Prykhozhij, S.V.; Stoyek, M.R.; Smith, F.M.; Kohl, P.; Quinn, T.A.; et al. Cardiac Electrophysiological Effects of Light-Activated Chloride Channels. Front. Physiol. 2018, 9, 1806. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, T.; Kondo, S. Method for disarranging the pigment pattern of zebrafish by optogenetics. Dev. Biol. 2020, 460, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Varady, A.; Distel, M. Non-neuromodulatory Optogenetic Tools in Zebrafish. Front. Cell. Dev. Biol. 2020, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.; Izquierdo, E.; Viswanathan, R.; Hartmann, J.; Pallares Cartes, C.; De Renzis, S. Principles and applications of optogenetics in developmental biology. Development 2019, 146, dev175067. [Google Scholar] [CrossRef] [PubMed]
- Vardy, E.; Robinson, J.E.; Li, C.; Olsen, R.H.J.; DiBerto, J.F.; Giguere, P.M.; Sassano, F.M.; Huang, X.-P.; Zhu, H.; Urban, D.J.; et al. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron 2015, 86, 936–946. [Google Scholar] [CrossRef]
- Whissell, P.D.; Tohyama, S.; Martin, L.J. The Use of DREADDs to Deconstruct Behavior. Front. Genet. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.S.; Tescarollo, F.C.; Sun, H. DREADDs in Epilepsy Research: Network-Based Review. Front. Mol. Neurosci. 2022, 15, 863003. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Bonaventura, J.; Lesniak, W.; Mathews, W.B.; Sysa-Shah, P.; Rodriguez, L.A.; Ellis, R.J.; Richie, C.T.; Harvey, B.K.; Dannals, R.F.; et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357, 503–507. [Google Scholar] [CrossRef]
- Bonaventura, J.; Eldridge, M.A.G.; Hu, F.; Gomez, J.L.; Sanchez-Soto, M.; Abramyan, A.M.; Lam, S.; Boehm, M.A.; Ruiz, C.; Farrell, M.R.; et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat. Commun. 2019, 10, 4627. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Miyakawa, N.; Takuwa, H.; Hori, Y.; Oyama, K.; Ji, B.; Takahashi, M.; Huang, X.P.; Slocum, S.T.; DiBerto, J.F.; et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020, 23, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gumpper, R.H.; Huang, X.P.; Liu, Y.; Krumm, B.E.; Cao, C.; Fay, J.F.; Roth, B.L. Molecular basis for selective activation of DREADD-based chemogenetics. Nature 2022, 612, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Goutaudier, R.; Coizet, V.; Carcenac, C.; Carnicella, S. DREADDs: The Power of the Lock, the Weakness of the Key. Favoring the Pursuit of Specific Conditions Rather than Specific Ligands. eNeuro 2019, 6, ENEURO.0171-19. [Google Scholar] [CrossRef]
- Urban, D.J.; Roth, B.L. DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Meister, J.; Bone, D.B.J.; Pydi, S.P.; Rossi, M.; Wess, J. Use of DREADD Technology to Identify Novel Targets for Antidiabetic Drugs. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Jain, S.; McMillin, S.M.; Cui, Y.; Gautam, D.; Sakamoto, W.; Lu, H.; Jou, W.; McGuinness, O.P.; Gavrilova, O.; et al. A novel experimental strategy to assess the metabolic effects of selective activation of a G(q)-coupled receptor in hepatocytes in vivo. Endocrinology 2013, 154, 3539–3551. [Google Scholar] [CrossRef]
- Chen, S.; Chiu, C.N.; McArthur, K.L.; Fetcho, J.R.; Prober, D.A. TRP channel mediated neuronal activation and ablation in freely behaving zebrafish. Nat. Methods 2016, 13, 147–150. [Google Scholar] [CrossRef]
- Silic, M.R.; Zhang, G. Tissue-specific modification of cellular bioelectrical activities using the chemogenetic tool, DREADD, in zebrafish. bioRxiv 2021. [Google Scholar] [CrossRef]
- Magnus, C.J.; Lee, P.H.; Bonaventura, J.; Zemla, R.; Gomez, J.L.; Ramirez, M.H.; Hu, X.; Galvan, A.; Basu, J.; Michaelides, M.; et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 2019, 364, eaav5282. [Google Scholar] [CrossRef]
- Magnus, C.J.; Lee, P.H.; Atasoy, D.; Su, H.H.; Looger, L.L.; Sternson, S.M. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 2011, 333, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Beckwith-Cohen, B.; Holzhausen, L.C.; Nawy, S.; Kramer, R.H. Controlling Horizontal Cell-Mediated Lateral Inhibition in Transgenic Zebrafish Retina with Chemogenetic Tools. eNeuro 2020, 7, ENEURO.0022-20. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Kikuta, H.; Urasaki, A.; Asakawa, K.; Kawakami, K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol. Biol. 2009, 561, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, S.; Flossmann, T.; Gao, S.; Witte, O.W.; Nagel, G.; Holthoff, K.; Kirmse, K. Optimized photo-stimulation of halorhodopsin for long-term neuronal inhibition. BMC Biol. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Ganjawala, T.H.; Lu, Q.; Fenner, M.D.; Abrams, G.W.; Pan, Z.-H. Improved CoChR Variants Restore Visual Acuity and Contrast Sensitivity in a Mouse Model of Blindness under Ambient Light Conditions. Mol. Ther. 2019, 27, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Ochs, A.R.; Karathanos, T.V.; Trayanova, N.A.; Boyle, P.M. Optogenetic Stimulation Using Anion Channelrhodopsin (GtACR1) Facilitates Termination of Reentrant Arrhythmias with Low Light Energy Requirements: A Computational Study. Front. Physiol. 2021, 12, 718622. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Cheng, R.K.; Ho, J.; Krishnan, S.; Mohammad, F.; Claridge-Chang, A.; Jesuthasan, S. Optical inhibition of larval zebrafish behaviour with anion channelrhodopsins. BMC Biol. 2017, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, A.Y.; Roshchin, M.V.; Smirnova, G.R.; Dolgikh, D.A.; Balaban, P.M.; Ostrovsky, M.A. Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light. Neurosci. Lett. 2017, 640, 76–80. [Google Scholar] [CrossRef]
- Mahn, M.; Gibor, L.; Patil, P.; Cohen-Kashi Malina, K.; Oring, S.; Printz, Y.; Levy, R.; Lampl, I.; Yizhar, O. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. Nat. Commun. 2018, 9, 4125. [Google Scholar] [CrossRef]
- Alberio, L.; Locarno, A.; Saponaro, A.; Romano, E.; Bercier, V.; Albadri, S.; Simeoni, F.; Moleri, S.; Pelucchi, S.; Porro, A.; et al. A light-gated potassium channel for sustained neuronal inhibition. Nat. Methods 2018, 15, 969–976. [Google Scholar] [CrossRef]
- Hight, A.E.; Kozin, E.D.; Darrow, K.; Lehmann, A.; Boyden, E.; Brown, M.C.; Lee, D.J. Superior temporal resolution of Chronos versus channelrhodopsin-2 in an optogenetic model of the auditory brainstem implant. Hear. Res. 2015, 322, 235–241. [Google Scholar] [CrossRef]
- Krol, A.; Lopez-Huerta, V.G.; Corey, T.E.C.; Deisseroth, K.; Ting, J.T.; Feng, G. Two eARCHT3.0 Lines for Optogenetic Silencing of Dopaminergic and Serotonergic Neurons. Front. Neural Circuits 2019, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.A.; Middleton, S.J.; Clark, A.J.; Daniel, T.; Khovanov, N.; McMahon, S.B.; Bennett, D.L. Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain 2017, 140, 2570–2585. [Google Scholar] [CrossRef]
- Hobson, C.M.; Guo, M.; Vishwasrao, H.D.; Wu, Y.; Shroff, H.; Chew, T.L. Practical considerations for quantitative light sheet fluorescence microscopy. Nat. Methods 2022, 19, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Park, O.K.; Kwak, J.; Jung, Y.J.; Kim, Y.H.; Hong, H.S.; Hwang, B.J.; Kwon, S.H.; Kee, Y. 3D Light-Sheet Fluorescence Microscopy of Cranial Neurons and Vasculature during Zebrafish Embryogenesis. Mol. Cells 2015, 38, 975–981. [Google Scholar] [CrossRef]
- Levin, M.; Martyniuk, C.J. The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems 2018, 164, 76–93. [Google Scholar] [CrossRef]
- Tseng, A.; Levin, M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 2013, 6, e22595. [Google Scholar] [CrossRef]
- Silver, B.B.; Nelson, C.M. The Bioelectric Code: Reprogramming Cancer and Aging From the Interface of Mechanical and Chemical Microenvironments. Front. Cell. Dev. Biol. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wen, Y.; Serre, N.B.C.; Laursen, C.C.H.; Dietz, A.G.; Taylor, B.R.; Drobizhev, M.; Molina, R.S.; Aggarwal, A.; Rancic, V.; et al. A sensitive and specific genetically-encoded potassium ion biosensor for in vivo applications across the tree of life. PLoS Biol. 2022, 20, e3001772. [Google Scholar] [CrossRef]
- Lodovichi, C.; Ratto, G.M.; Trevelyan, A.J.; Arosio, D. Genetically encoded sensors for Chloride concentration. J. Neurosci. Methods 2022, 368, 109455. [Google Scholar] [CrossRef]
- Moresco, E.M.; Li, X.; Beutler, B. Going forward with genetics: Recent technological advances and forward genetics in mice. Am. J. Pathol. 2013, 182, 1462–1473. [Google Scholar] [CrossRef]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Kutscher, L.M.; Shaham, S. Forward and reverse mutagenesis in C. elegans. WormBook 2014, 17, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Almen, M.S.; Nordstrom, K.J.; Fredriksson, R.; Schioth, H.B. Mapping the human membrane proteome: A majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.; Williams, A.H.; Caplan, J.S.; Marder, E. Correlations in ion channel expression emerge from homeostatic tuning rules. Proc. Natl. Acad. Sci. USA 2013, 110, E2645–E2654. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The technology and biology of single-cell RNA sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Silic, M.R.; Black, M.M.; Zhang, G. Phylogenetic and developmental analyses indicate complex functions of calcium-activated potassium channels in zebrafish embryonic development. Dev. Dyn. 2021, 250, 1477–1493. [Google Scholar] [CrossRef] [PubMed]
- Silic, M.R.; Murata, S.H.; Park, S.J.; Zhang, G.J. Evolution of inwardly rectifying potassium channels and their gene expression in zebrafish embryos. Dev. Dynam 2022, 251, 687–713. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Maddison, L.A.; Chen, W. Multiplex conditional mutagenesis in zebrafish using the CRISPR/Cas system. Methods Cell Biol. 2016, 135, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Rolf, A.; Mustata, R.C.; Merenda, A.; Kim, J.; Perera, S.; Grego, T.; Andrews, K.; Tremble, K.; Silva, J.C.; Fink, J.; et al. One-step generation of conditional and reversible gene knockouts. Nat. Methods 2017, 14, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Zoller, D.; Hammer, J.; Stucke, J.; Spiess, S.; Kesavan, G.; Kroehne, V.; Eguiguren, J.S.; Ezhkova, D.; Petzold, A.; et al. Cre-Controlled CRISPR mutagenesis provides fast and easy conditional gene inactivation in zebrafish. Nat. Commun. 2021, 12, 1125. [Google Scholar] [CrossRef]
- Liu, F.; Kambakam, S.; Almeida, M.P.; Ming, Z.; Welker, J.M.; Wierson, W.A.; Schultz-Rogers, L.E.; Ekker, S.C.; Clark, K.J.; Essner, J.J.; et al. Cre/lox regulated conditional rescue and inactivation with zebrafish UFlip alleles generated by CRISPR-Cas9 targeted integration. eLife 2022, 11, e71478. [Google Scholar] [CrossRef]
- Wang, Y.; Hsu, A.Y.; Walton, E.M.; Park, S.J.; Syahirah, R.; Wang, T.; Zhou, W.; Ding, C.; Lemke, A.P.; Zhang, G.; et al. A robust and flexible CRISPR/Cas9-based system for neutrophil-specific gene inactivation in zebrafish. J. Cell Sci. 2021, 134, jcs258574. [Google Scholar] [CrossRef]
- Lee, R.T.; Knapik, E.W.; Thiery, J.P.; Carney, T.J. An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 2013, 140, 2923–2932. [Google Scholar] [CrossRef]
- Lee, R.T.; Thiery, J.P.; Carney, T.J. Dermal fin rays and scales derive from mesoderm, not neural crest. Curr. Biol. 2013, 23, R336–R337. [Google Scholar] [CrossRef]
- Levin, M.; Pietak, A.M.; Bischof, J. Planarian regeneration as a model of anatomical homeostasis: Recent progress in biophysical and computational approaches. Semin. Cell Dev. Biol. 2019, 87, 125–144. [Google Scholar] [CrossRef]
- Durant, F.; Bischof, J.; Fields, C.; Morokuma, J.; LaPalme, J.; Hoi, A.; Levin, M. The Role of Early Bioelectric Signals in the Regeneration of Planarian Anterior/Posterior Polarity. Biophys. J. 2019, 116, 948–961. [Google Scholar] [CrossRef]
- Rogers, K.W.; Schier, A.F. Morphogen gradients: From generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011, 27, 377–407. [Google Scholar] [CrossRef]
- Tabata, T.; Takei, Y. Morphogens, their identification and regulation. Development 2004, 131, 703–712. [Google Scholar] [CrossRef]
- Pai, V.P.; Lemire, J.M.; Pare, J.F.; Lin, G.; Chen, Y.; Levin, M. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via Notch signaling and regulation of proliferation. J. Neurosci. 2015, 35, 4366–4385. [Google Scholar] [CrossRef] [PubMed]
- Belus, M.T.; Rogers, M.A.; Elzubeir, A.; Josey, M.; Rose, S.; Andreeva, V.; Yelick, P.C.; Bates, E.A. Kir2.1 is important for efficient BMP signaling in mammalian face development. Dev. Biol. 2018, 444 (Suppl. S1), S297–S307. [Google Scholar] [CrossRef] [PubMed]
- Dahal, G.R.; Rawson, J.; Gassaway, B.; Kwok, B.; Tong, Y.; Ptáček, L.J.; Bates, E. An inwardly rectifying K+ channel is required for patterning. Development 2012, 139, 3653–3664. [Google Scholar] [CrossRef] [PubMed]
- Dahal, G.R.; Pradhan, S.J.; Bates, E.A. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development 2017, 144, 2771–2783. [Google Scholar] [CrossRef]
- Muccioli, S.; Brillo, V.; Chieregato, L.; Leanza, L.; Checchetto, V.; Costa, R. From Channels to Canonical Wnt Signaling: A Pathological Perspective. Int. J. Mol. Sci. 2021, 22, 4613. [Google Scholar] [CrossRef] [PubMed]
- Delling, M.; DeCaen, P.G.; Doerner, J.F.; Febvay, S.; Clapham, D.E. Primary cilia are specialized calcium signalling organelles. Nature 2013, 504, 311–314. [Google Scholar] [CrossRef] [PubMed]
- DeCaen, P.G.; Delling, M.; Vien, T.N.; Clapham, D.E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 2013, 504, 315–318. [Google Scholar] [CrossRef]
- Emmons-Bell, M.; Hariharan, I.K. Membrane potential regulates Hedgehog signalling in the Drosophila wing imaginal disc. EMBO Rep. 2021, 22, e51861. [Google Scholar] [CrossRef]
- Petrov, K.; Wierbowski, B.M.; Liu, J.; Salic, A. Distinct Cation Gradients Power Cholesterol Transport at Different Key Points in the Hedgehog Signaling Pathway. Dev. Cell 2020, 55, 314–327.e7. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.R.; Neahring, L.; Zhang, Y.; Roberts, K.J.; Beachy, P.A. Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl. Acad. Sci. USA 2017, 114, E11141–E11150. [Google Scholar] [CrossRef] [PubMed]
| Somatic Cells | Embryonic Origin | Millivolts (mV) | References |
|---|---|---|---|
| Skeletal myocyte | Paraxial mesoderm | From −91 to −65 | [42] |
| Heart myocyte | Lateral plate mesoderm | From −95 to −40 | [43] |
| Gut smooth muscle myocyte | Lateral plate mesoderm | From −70 to −35 | [44] |
| Gliocyte | Neuroectoderm or neural crest | About −80 | [45] |
| Neuron | Neuroectoderm | From −85 to −65 | [46,47] |
| Adrenal cortex | Intermediate mesoderm | From −71 to −66 | [48] |
| Adrenal medulla | Neural crest | From −32 to −20 | [48] |
| Lymphocyte | Mesoderm | From −70 to −50 | [49] |
| Thyroid follicular cell | Foregut endoderm | From −70 to −60 | [50] |
| Chondrocyte | Mesoderm and neural crest | From −64 to −48 | [51] |
| Fibroblast | All three embryological germ layers | From −25 to −16 | [52,53] |
| Liver hepatocyte | Ventral foregut endoderm | From −50 to −20 | [54] |
| Pancreas β-cell | Foregut endoderm | From −80 to −60 | [35] |
| Epithelial cell | All three embryological germ layers | From −70 to −20 | [55] |
| Melanocyte | Neural crest | From −50 to −40 | [56,57,58] |
| White adipocyte | Mesoderm and neuroectoderm | From −69 to −17 | [59] |
| Osteocytes | Mesoderm and neural crest | About −60 | [60] |
| Cancer and tumor cells | All three embryological germ layers | From −50 to −5 | [10,56,61,62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silic, M.R.; Zhang, G. Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model. Cells 2023, 12, 1148. https://doi.org/10.3390/cells12081148
Silic MR, Zhang G. Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model. Cells. 2023; 12(8):1148. https://doi.org/10.3390/cells12081148
Chicago/Turabian StyleSilic, Martin R., and GuangJun Zhang. 2023. "Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model" Cells 12, no. 8: 1148. https://doi.org/10.3390/cells12081148
APA StyleSilic, M. R., & Zhang, G. (2023). Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model. Cells, 12(8), 1148. https://doi.org/10.3390/cells12081148







