Altered Epigenetic Profiles in the Placenta of Preeclamptic and Intrauterine Growth Restriction Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Placental Tissues
2.2. DNA Extraction
2.3. Bisulfite Conversion
2.4. Data Processing and Global Epigenetic Analyses
2.5. USEQ Sliding Window Analysis
2.6. Great Analysis
2.7. Immunoblot
2.8. Statistical Analysis for Immunoblot
3. Results
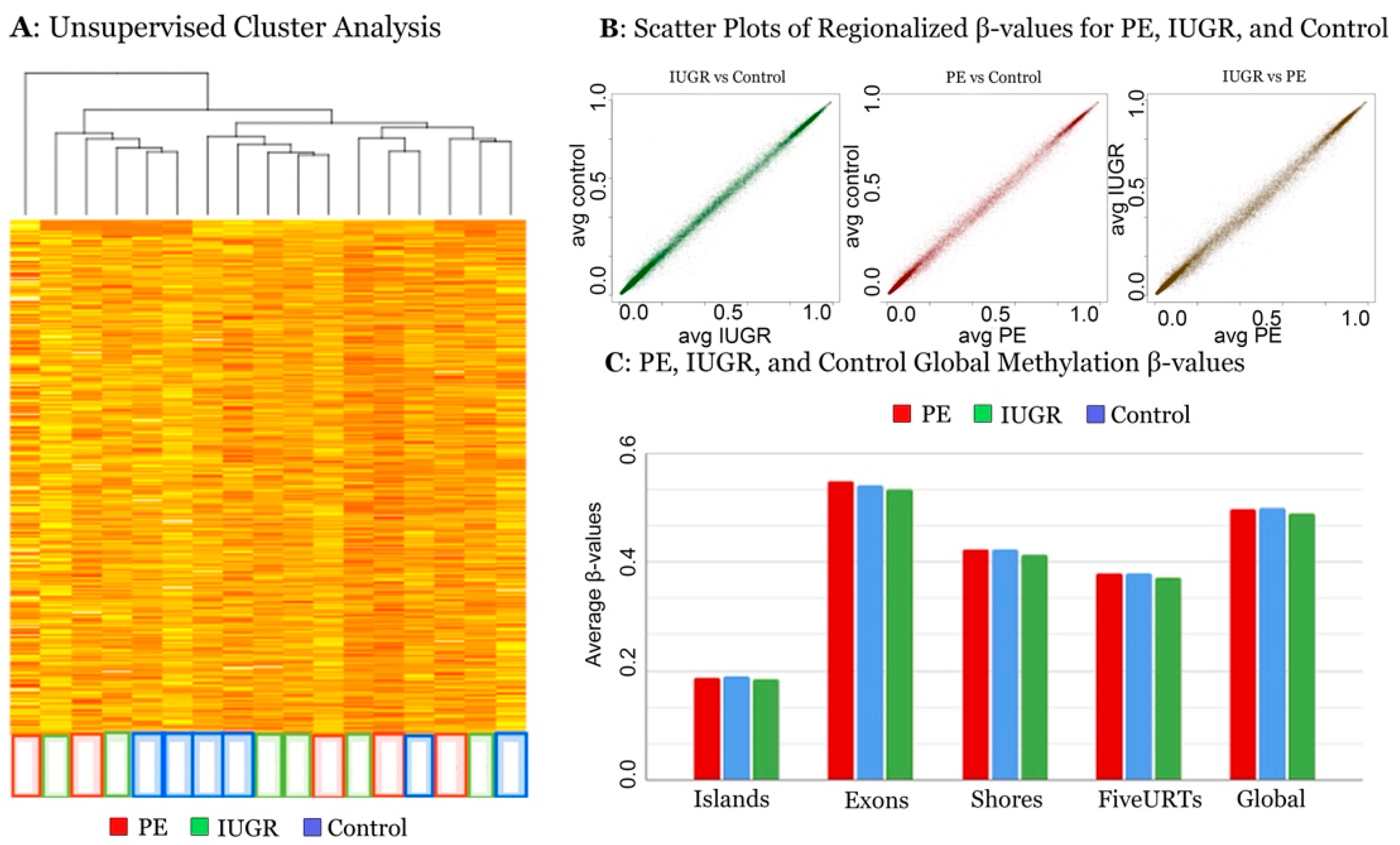
3.1. Global Methylation Analysis
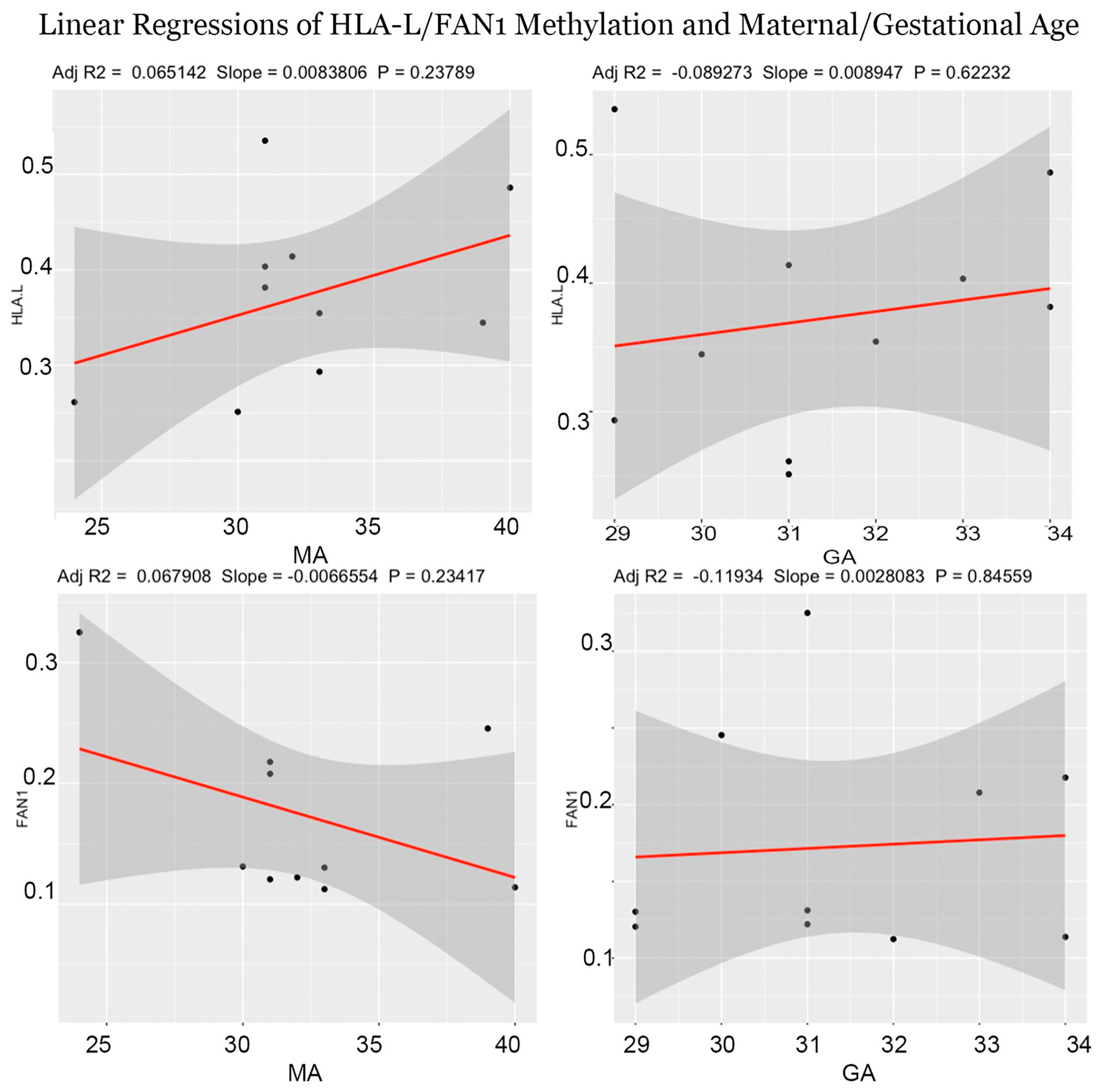
3.2. Regional Methylation Analysis
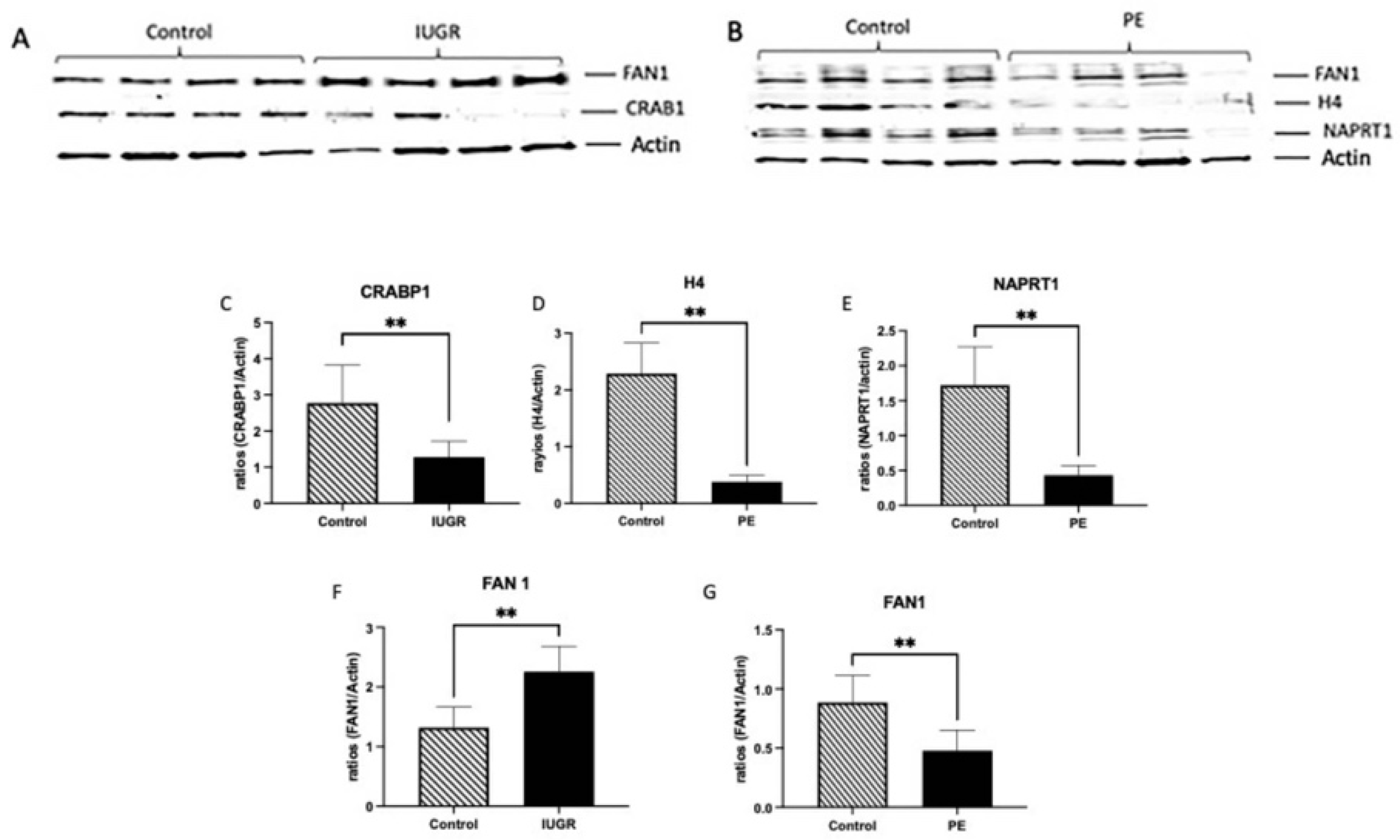
3.3. Immunoblot for CRABP1, FAN1, HISTH4L and NAPRT1
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsai, K.; Tullis, B.; Jensen, T.; Graff, T.; Reynolds, P.; Arroyo, J. Differential expression of mTOR related molecules in the placenta from gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR) and preeclampsia patients. Reprod. Biol. 2021, 21, 100503. [Google Scholar] [CrossRef] [PubMed]
- Bahr, B.L.; Price, M.D.; Merrill, D.; Mejia, C.; Call, L.; Bearss, D.; Arroyo, J. Different expression of placental pyruvate kinase in normal, preeclamptic and intrauterine growth restriction pregnancies. Placenta 2014, 35, 883–890. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.T.; Price, M.D.; Mejia, C.A.; Galan, H.L.; Arroyo, J.A. Increased trophoblast expression of NFAT5/TonEBP in pre-eclamptic placentas and hyperosmolar-treated BeWo cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 37–43. [Google Scholar] [CrossRef]
- Tsai, K.Y.F.; Tullis, B.; Mejia, J.; Reynolds, P.R.; Arroyo, J.A. Regulation of trophoblast cell invasion by Pyruvate Kinase isozyme M2 (PKM2). Placenta 2021, 103, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 6 (Suppl. 3), 332–336. [Google Scholar] [PubMed]
- Krebs, C.; Macara, L.M.; Leiser, R.; Bowman, A.W.; Greer, I.A.; Kingdom, J.C. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am. J. Obstet. Gynecol. 1996, 175, 1534–1542. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef]
- Lim, J.H.; Kang, Y.J.; Bak, H.J.; Kim, M.S.; Lee, H.J.; Kwak, D.W.; Han, Y.J.; Kim, M.Y.; Boo, H.; Kim, S.Y.; et al. Epigenome-wide DNA methylation profiling of preeclamptic placenta according to severe features. Clin. Epigenet. 2020, 12, 128. [Google Scholar] [CrossRef]
- Nelissen, E.C.; van Montfoort, A.P.; Dumoulin, J.C.; Evers, J.L. Epigenetics and the placenta. Hum. Reprod. Update 2011, 17, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Novielli, C.; Mando, C.; Tabano, S.; Anelli, G.M.; Fontana, L.; Antonazzo, P.; Miozzo, M.; Cetin, I. Mitochondrial DNA content and methylation in fetal cord blood of pregnancies with placental insufficiency. Placenta 2017, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Mayne, B.T.; Buckberry, S.; Breen, J.; Rodriguez Lopez, C.M.; Roberts, C.T. Recent progress towards understanding the role of DNA methylation in human placental development. Reproduction 2016, 152, R23–R30. [Google Scholar] [CrossRef]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in the human placenta and fetal growth (review). Mol. Med. Rep. 2012, 5, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.P.; Price, E.M. The human placental methylome. Cold Spring Harb. Perspect. Med. 2015, 5, a023044. [Google Scholar] [CrossRef]
- Banister, C.E.; Koestler, D.C.; Maccani, M.A.; Padbury, J.F.; Houseman, E.A.; Marsit, C.J. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics 2011, 6, 920–927. [Google Scholar] [CrossRef]
- Chu, T.; Bunce, K.; Shaw, P.; Shridhar, V.; Althouse, A.; Hubel, C.; Peters, D. Comprehensive analysis of preeclampsia-associated DNA methylation in the placenta. PLoS ONE 2014, 9, e107318. [Google Scholar] [CrossRef]
- Anderson, C.M.; Ralph, J.L.; Wright, M.L.; Linggi, B.; Ohm, J.E. DNA methylation as a biomarker for preeclampsia. Biol. Res. Nurs. 2014, 16, 409–420. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Fortin, J.P.; Triche, T.J., Jr.; Hansen, K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef]
- Nix, D.A.; Courdy, S.J.; Boucher, K.M. Empirical methods for controlling false positives and estimating confidence in ChIP-Seq peaks. BMC Bioinform. 2008, 9, 523. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Luo, H.; Fan, Z.; Luo, J.; He, S.; Yue, H.; Zhang, P.; Chen, R. BioCircos.js: An interactive Circos JavaScript library for biological data visualization on web applications. Bioinformatics 2016, 32, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.Y.; Bristor, D.; Hiller, M.; Clarke, S.L.; Schaar, B.T.; Lowe, C.B.; Wenger, A.M.; Bejerano, G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010, 28, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Enikeev, A.D.; Komelkov, A.V.; Axelrod, M.E.; Galetsky, S.A.; Kuzmichev, S.A.; Tchevkina, E.M. CRABP1 and CRABP2 Protein Levels Correlate with Each Other but Do Not Correlate with Sensitivity of Breast Cancer Cells to Retinoic Acid. Biochemistry 2021, 86, 217–229. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, Y.W.; Nhieu, J.; Zhang, X.; Wei, L.N. Sonic Hedgehog-Gli1 Signaling and Cellular Retinoic Acid Binding Protein 1 Gene Regulation in Motor Neuron Differentiation and Diseases. Int. J. Mol. Sci. 2020, 21, 4125. [Google Scholar] [CrossRef]
- Huebner, H.; Hartner, A.; Rascher, W.; Strick, R.R.; Kehl, S.; Heindl, F.; Wachter, D.L.; Beckmann Md, M.W.; Fahlbusch, F.B.; Ruebner, M. Expression and Regulation of Retinoic Acid Receptor Responders in the Human Placenta. Reprod. Sci. 2018, 25, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Moirangthem, R.; Kaur, R. Genome protection: Histone H4 and beyond. Curr. Genet. 2020, 66, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Pereira, S.; Silva, S.S.; Azevedo, L.; Castro, L.; Amorim, A.; Silva, R.M. NAMPT and NAPRT1: Novel polymorphisms and distribution of variants between normal tissues and tumor samples. Sci. Rep. 2014, 4, 6311. [Google Scholar] [CrossRef]
- Segui, N.; Mina, L.B.; Lazaro, C.; Sanz-Pamplona, R.; Pons, T.; Navarro, M.; Bellido, F.; Lopez-Doriga, A.; Valdes-Mas, R.; Pineda, M.; et al. Germline Mutations in FAN1 Cause Hereditary Colorectal Cancer by Impairing DNA Repair. Gastroenterology 2015, 149, 563–566. [Google Scholar] [CrossRef]
- Zhou, W.; Otto, E.A.; Cluckey, A.; Airik, R.; Hurd, T.W.; Chaki, M.; Diaz, K.; Lach, F.P.; Bennett, G.R.; Gee, H.Y.; et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012, 44, 910–915. [Google Scholar] [CrossRef]
- Wang, R.; Persky, N.S.; Yoo, B.; Ouerfelli, O.; Smogorzewska, A.; Elledge, S.J.; Pavletich, N.P. DNA repair. Mechanism of DNA interstrand cross-link processing by repair nuclease FAN1. Science 2014, 346, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Almomani, S.N.; Alsaleh, A.A.; Weeks, R.J.; Chatterjee, A.; Day, R.C.; Honda, I.; Homma, H.; Fukuzawa, R.; Slatter, T.L.; Hung, N.A.; et al. Identification and validation of DNA methylation changes in pre-eclampsia. Placenta 2021, 110, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Li, Q.; Li, W.; Thamotharan, S.; Tosevska, A.; Morselli, M.; Sung, K.; Janzen, C.; Zhou, X.; Pellegrini, M.; et al. Cell-free DNA Methylation and Transcriptomic Signature Prediction of Pregnancies with Adverse Outcomes. Epigenetics 2021, 16, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Chu, A.; Liao, W.W.; Rubbi, L.; Janzen, C.; Hsu, F.M.; Thamotharan, S.; Ganguly, A.; Lam, L.; Montoya, D.; et al. Prenatal Growth Patterns and Birthweight Are Associated With Differential DNA Methylation and Gene Expression of Cardiometabolic Risk Genes in Human Placentas: A Discovery-Based Approach. Reprod. Sci. 2018, 25, 523–539. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.B.; Herzog, E.M.; Duvekot, J.J.; van der Spek, P.J.; Steegers, E.A.P.; Stoop, M.P.; Willemsen, S.P.; Steegers-Theunissen, R.P.M. Differences in DNA methylation of insulin-like growth factor 2 and cadherin 13 in patients with preeclampsia. Pregnancy Hypertens. 2020, 19, 150–158. [Google Scholar] [CrossRef]
- Jin, H.; Cho, Y. Structural and functional relationships of FAN1. DNA Repair 2017, 56, 135–143. [Google Scholar] [CrossRef]
- Airik, R.; Schueler, M.; Airik, M.; Cho, J.; Porath, J.D.; Mukherjee, E.; Sims-Lucas, S.; Hildebrandt, F. A FANCD2/FANCI-Associated Nuclease 1-Knockout Model Develops Karyomegalic Interstitial Nephritis. J. Am. Soc. Nephrol. 2016, 27, 3552–3559. [Google Scholar] [CrossRef]
- Deshmukh, A.L.; Porro, A.; Mohiuddin, M.; Lanni, S.; Panigrahi, G.B.; Caron, M.C.; Masson, J.Y.; Sartori, A.A.; Pearson, C.E. FAN1, a DNA Repair Nuclease, as a Modifier of Repeat Expansion Disorders. J. Huntingt. Dis. 2021, 10, 95–122. [Google Scholar] [CrossRef]
- Thongthip, S.; Bellani, M.; Gregg, S.Q.; Sridhar, S.; Conti, B.A.; Chen, Y.; Seidman, M.M.; Smogorzewska, A. Fan1 deficiency results in DNA interstrand cross-link repair defects, enhanced tissue karyomegaly, and organ dysfunction. Genes Dev. 2016, 30, 645–659. [Google Scholar] [CrossRef]
- Furness, D.L.; Dekker, G.A.; Roberts, C.T. DNA damage and health in pregnancy. J. Reprod. Immunol. 2011, 89, 153–162. [Google Scholar] [PubMed]
- Tsai, K.Y.F.; Tullis, B.; Breithaupt, K.L.; Fowers, R.; Jones, N.; Grajeda, S.; Reynolds, P.R.; Arroyo, J.A. A Role for RAGE in DNA Double Strand Breaks (DSBs) Detected in Pathological Placentas and Trophoblast Cells. Cells 2021, 10, 857. [Google Scholar] [CrossRef]
- Sueoka, T.; Koyama, K.; Hayashi, G.; Okamoto, A. Chemistry-Driven Epigenetic Investigation of Histone and DNA Modifications. Chem. Rec. 2018, 18, 1727–1744. [Google Scholar] [CrossRef] [PubMed]
- Megee, P.C.; Morgan, B.A.; Smith, M.M. Histone H4 and the maintenance of genome integrity. Genes Dev. 1995, 9, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.L.; Portoso, M.; Mata, J.; Bahler, J.; Allshire, R.C.; Kouzarides, T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 2004, 119, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dutt, S.; Xu, R.; Graves, K.; Juszczynski, P.; Manis, J.P.; Shipp, M.A. BBAP monoubiquitylates histone H4 at lysine 91 and selectively modulates the DNA damage response. Mol. Cell. 2009, 36, 110–120. [Google Scholar] [CrossRef]
- Yeung, K.R.; Chiu, C.L.; Pidsley, R.; Makris, A.; Hennessy, A.; Lind, J.M. DNA methylation profiles in preeclampsia and healthy control placentas. Am. J. Physiol. Heart. Circ. Physiol. 2016, 310, H1295–H1303. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhao, Y. Inflammation in Preeclampsia: Genetic Biomarkers, Mechanisms, and Therapeutic Strategies. Front. Immunol. 2022, 13, 883404. [Google Scholar] [CrossRef]
- Chu, T.; Shaw, P.; McClain, L.; Simhan, H.; Peters, D. High-resolution epigenomic liquid biopsy for noninvasive phenotyping in pregnancy. Prenat. Diagn. 2021, 41, 61–69. [Google Scholar] [CrossRef]
- Navajas, R.; Ramos-Fernandez, A.; Herraiz, I.; Galindo, A.; Bartha, J.L.; Corrales, F.; Paradela, A. Quantitative proteomic analysis of serum-purified exosomes identifies putative pre-eclampsia-associated biomarkers. Clin. Proteom. 2022, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Meyberg, R.; Boos, R.; Babajan, A.; Ertan, A.K.; Schmidt, W. Intrauterine growth retardation--perinatal mortality and postnatal morbidity in a perinatal center. Z. Geburtshilfe Neonatol. 2000, 204, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]


| Control | IUGR | PE | |
|---|---|---|---|
| Maternal Age | 34 ± 2.8 | 30 ± 1.3 | 36 ± 1.7 |
| Gestational Age (wks) | 38 ± 0.01 | 31 ± 0.5 | 32 ± 1.02 |
| Fetal Weight (g) | 3498 ± 45 | 972 ± 40.1 | 1813 ± 301 |
| BMI (gestational) | 22 ± 0.9 | 28 ± 4.64 | 38 ± 3.6 |
| Blood pressure (Average S/D) | 122/83 | 127/79 | 161/107 |
| Umbilical Artery Resistance (Average Doppler) | NA | 1.7 ± 0.14 | NA |
| Proteinuria | Trae | Trace | +3 to +4 |
| Female/Male ratios | 1.0 | 0.8 | 1.0 |
| PE | |||||||
| Chromosome | Num. Array Cpgs | Start Loc. | Stop Loc. | Associated Genes | Region | Log2 Ratio | Phred-FDR |
| chr15 | 14 | 31194984 | 31196600 | FAN1 | Promoter | 1.605 | 44.51 |
| chr6 | 15 | 27840957 | 27841866 | HIST1H4L | Promoter | 0.841 | 44.51 |
| chr6 | 43 | 30226874 | 30228432 | HLA-L | Promoter | 0.562 | 44.51 |
| chr2 | 5 | 64834106 | 64834431 | None | NA | 0.646 | 44.25 |
| chr8 | 15 | 144659831 | 144661520 | NAPRT-1 | Promoter | 1.285 | 44.06 |
| chr6 | 11 | 27106564 | 27107757 | H4C9 | Promoter | .476 | 40.26 |
| IUGR | |||||||
| Chromosome | Num. Array Cpgs | Start Loc. | Stop Loc. | Associated Genes | Region | Log2 Ratio | Phred-FDR |
| chr15 | 14 | 31194984 | 31196600 | FAN1 | Promoter | 1.644 | 43.05 |
| chr15 | 17 | 78632109 | 78633663 | CRABP1 | Promoter | 0.971 | 43.05 |
| chr6 | 43 | 30226874 | 30228432 | HLA-L | Promoter | 0.538 | 43.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, C.; Clarke, D.; Holmstrom, J.; Stirland, I.; Reynolds, P.R.; Jenkins, T.G.; Arroyo, J.A. Altered Epigenetic Profiles in the Placenta of Preeclamptic and Intrauterine Growth Restriction Patients. Cells 2023, 12, 1130. https://doi.org/10.3390/cells12081130
Norton C, Clarke D, Holmstrom J, Stirland I, Reynolds PR, Jenkins TG, Arroyo JA. Altered Epigenetic Profiles in the Placenta of Preeclamptic and Intrauterine Growth Restriction Patients. Cells. 2023; 12(8):1130. https://doi.org/10.3390/cells12081130
Chicago/Turabian StyleNorton, Carter, Derek Clarke, Joshua Holmstrom, Isaac Stirland, Paul R. Reynolds, Tim G. Jenkins, and Juan A. Arroyo. 2023. "Altered Epigenetic Profiles in the Placenta of Preeclamptic and Intrauterine Growth Restriction Patients" Cells 12, no. 8: 1130. https://doi.org/10.3390/cells12081130
APA StyleNorton, C., Clarke, D., Holmstrom, J., Stirland, I., Reynolds, P. R., Jenkins, T. G., & Arroyo, J. A. (2023). Altered Epigenetic Profiles in the Placenta of Preeclamptic and Intrauterine Growth Restriction Patients. Cells, 12(8), 1130. https://doi.org/10.3390/cells12081130











