Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives
Abstract
1. Introduction
2. Human Definition of SIRS and MODS
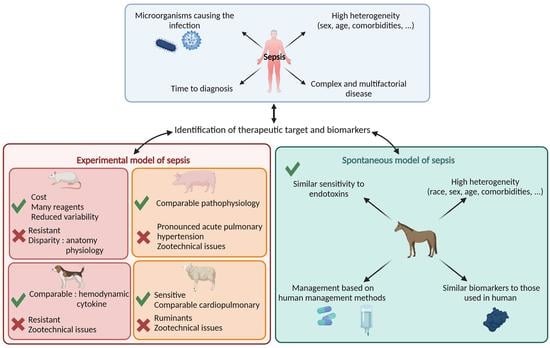
3. Preclinical Experimental Animal Models of Sepsis Benefits for Human Medicine
4. Spontaneous Equine Sepsis, a Pertinent Veterinary Disease for Modeling Human Sepsis
5. SIRS and MODS Definition for Adult Horse
5.1. Equine SIRS Definition
5.2. Equine MODS Definition
6. Main Biomarkers of Equine Sepsis: A Proof of Concept for Human Medicine
6.1. Main Biomarkers Currently Used to Diagnose Sepsis
6.1.1. Fibrinogen
6.1.2. D-Dimer
6.1.3. Serum Amyloid A
6.1.4. C-Reactive Protein
6.1.5. Procalcitonin
6.2. Clinical Perspective: Studying the Secretome to Identify New Biomarkers
7. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2015, 193, 259–272. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent Advances in Biosensors for Diagnosis and Detection of Sepsis: A Comprehensive Review. Biosens. Bioelectron. 2019, 124–125, 205–215. [Google Scholar] [CrossRef]
- Quenot, J.P.; Pavon, A.; Fournel, I.; Barbar, S.D.; Bruyère, R. Le choc septique de l’adulte en France: Vingt ans de données épidémiologiques. Réanimation 2015, 24, 303–309. [Google Scholar] [CrossRef]
- Monneret, G.; Gossez, M.; Aghaeepour, N.; Gaudilliere, B.; Venet, F. How Clinical Flow Cytometry Rebooted Sepsis Immunology. Cytom. A 2019, 95, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef]
- Murando, F.; Peloso, A.; Cobianchi, L. Experimental Abdominal Sepsis: Sticking to an Awkward but Still Useful Translational Model. Mediat. Inflamm 2019, 2019, 8971036. [Google Scholar] [CrossRef]
- Blangy-Letheule, A.; Persello, A.; Rozec, B.; Waard, M.D.; Lauzier, B. New Approaches to Identify Sepsis Biomarkers: The Importance of Model and Sample Source for Mass Spectrometry. Oxid. Med. Cell. Longev. 2020, 2020, 6681073. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- The Lancet Infectious Diseases. For Sepsis, the Drugs Don’t Work. Lancet Infect. Dis. 2012, 12, 89. [Google Scholar] [CrossRef]
- Guillon, A.; Preau, S.; Aboab, J.; Azabou, E.; Jung, B.; Silva, S.; Textoris, J.; Uhel, F.; Vodovar, D.; Zafrani, L.; et al. Preclinical Septic Shock Research: Why We Need an Animal ICU. Ann. Intensive Care 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Khan, M.; Liaw, P.; Fox-Robichaud, A. Experimental Sepsis Models; IntechOpen: London, UK, 2012; ISBN 978-953-51-0780-4. [Google Scholar]
- Nagy, S.; Tárnoky, K.; Tutsek, L.; Boros, M.; Karácsony, G. A Canine Model of Hyperdynamic Sepsis Induced by Intestinal Ischemia. Acta Physiol. Hung. 1990, 75, 303–320. [Google Scholar] [PubMed]
- Song, R.; Kim, J.; Yu, D.; Park, C.; Park, J. Kinetics of IL-6 and TNF-α Changes in a Canine Model of Sepsis Induced by Endotoxin. Vet. Immunol. Immunopathol. 2012, 146, 143–149. [Google Scholar] [CrossRef]
- Fink, M.P.; Heard, S.O. Laboratory Models of Sepsis and Septic Shock. J. Surg. Res. 1990, 49, 186–196. [Google Scholar] [CrossRef]
- Michie, H.R. The Value of Animal Models in the Development of New Drugs for the Treatment of the Sepsis Syndrome. J. Antimicrob. Chemother. 1998, 41, 47–49. [Google Scholar] [CrossRef]
- Ceciliani, F.; Restelli, L.; Lecchi, C. Proteomics in Farm Animals Models of Human Diseases. PROTEOMICS—Clin. Appl. 2014, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Groenen, M.A.M.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.-J.; et al. Analyses of Pig Genomes Provide Insight into Porcine Demography and Evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The Porcine Innate Immune System: An Update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The Pig as an Animal Model for Human Pathologies: A Proteomics Perspective. PROTEOMICS—Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, E. Animal Models for Translational Proteomics. PROTEOMICS—Clin. Appl. 2014, 8, 637–639. [Google Scholar] [CrossRef]
- Cohen, R.I.; Hassell, A.-M.; Marzouk, K.; Marini, C.; Liu, S.F.; Scharf, S.M. Renal Effects of Nitric Oxide in Endotoxemia. Am. J. Respir. Crit. Care Med. 2001, 164, 1890–1895. [Google Scholar] [CrossRef]
- Ji, M.-H.; Yang, J.-J.; Wu, J.; Li, R.-Q.; Li, G.-M.; Fan, Y.-X.; Li, W.-Y. Experimental Sepsis in Pigs—Effects of Vasopressin on Renal, Hepatic, and Intestinal Dysfunction. Upsala J. Med. Sci. 2012, 117, 257–263. [Google Scholar] [CrossRef]
- Maybauer, D.M.; Maybauer, M.O.; Szabó, C.; Cox, R.A.; Westphal, M.; Kiss, L.; Horvath, E.M.; Traber, L.D.; Hawkins, H.K.; Salzman, A.L.; et al. The peroxynitrite catalyst WW-85 improves pulmonary function in ovine septic shock. Shock 2011, 35, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Traber, D.L.; Redl, H.; Schlag, G.; Herndon, D.N.; Kimura, R.; Prien, T.; Traber, L.D. Cardiopulmonary Responses to Continuous Administration of Endotoxin. Am. J. Physiol. Heart Circ. Physiol. 1988, 254, H833–H839. [Google Scholar] [CrossRef]
- Taylor, S. A Review of Equine Sepsis. Equine Vet. Educ. 2015, 27, 99–109. [Google Scholar] [CrossRef]
- McGovern, K.F.; Lascola, K.M.; Smith, S.A.; Clark-Price, S.C.; Wilkins, P.A.; Schaeffer, D.J.; Foreman, J.H. The Effects of Hyperglycemia and Endotoxemia on Coagulation Parameters in Healthy Adult Horses. J. Vet. Intern. Med. 2013, 27, 347–353. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Jacobs, C.C.; Cook, V.L.; Gandy, J.C.; Hauptman, J.G.; Sordillo, L.M. Duration of in Vivo Endotoxin Tolerance in Horses. Vet. Immunol. Immunopathol. 2016, 173, 10–16. [Google Scholar] [CrossRef]
- Scavone, D.; Sgorbini, M.; Borges, A.S.; Oliveira-Filho, J.P.; Vitale, V.; Paltrinieri, S. Serial Measurements of Paraoxonase-1 (PON-1) Activity in Horses with Experimentally Induced Endotoxemia. BMC Vet. Res. 2020, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Stasi, A.; Intini, A.; Gigante, M.; Di Palma, A.M.; Divella, C.; Netti, G.S.; Prattichizzo, C.; Pontrelli, P.; Crovace, A.; et al. Endothelial Dysfunction and Renal Fibrosis in Endotoxemia-Induced Oliguric Kidney Injury: Possible Role of LPS-Binding Protein. Crit. Care 2014, 18, 520. [Google Scholar] [CrossRef]
- Anderson, J.R.; Smagul, A.; Simpson, D.; Clegg, P.D.; Rubio-Martinez, L.M.; Peffers, M.J. The Synovial Fluid Proteome Differentiates between Septic and Nonseptic Articular Pathologies. J Proteom. 2019, 202, 103370. [Google Scholar] [CrossRef] [PubMed]
- Borde, L.; Amory, H.; Grulke, S.; Leroux, A.A.; Houben, R.M.; Detilleux, J.; Sandersen, C.C. Prognostic Value of Echocardiographic and Doppler Parameters in Horses Admitted for Colic Complicated by Systemic Inflammatory Response Syndrome. J. Vet. Emerg. Crit. Care 2014, 24, 302–310. [Google Scholar] [CrossRef]
- McConachie, E.; Giguère, S.; Barton, M.H. Scoring System for Multiple Organ Dysfunction in Adult Horses with Acute Surgical Gastrointestinal Disease. J. Vet. Intern. Med. 2016, 30, 1276–1283. [Google Scholar] [CrossRef]
- Satué, K.; Gardon, J.C.; Muñoz, A. Clinical and Laboratorial Description of the Differential Diagnoses of Hemostatic Disorders in the Horse. Iran. J. Vet. Res. 2020, 21, 1–8. [Google Scholar]
- Senior, J.M.; Proudman, C.J.; Leuwer, M.; Carter, S.D. Plasma Endotoxin in Horses Presented to an Equine Referral Hospital: Correlation to Selected Clinical Parameters and Outcomes. Equine Vet. J. 2011, 43, 585–591. [Google Scholar] [CrossRef]
- Osterbur, K.; Mann, F.A.; Kuroki, K.; DeClue, A. Multiple Organ Dysfunction Syndrome in Humans and Animals. J. Vet. Intern. Med. 2014, 28, 1141–1151. [Google Scholar] [CrossRef]
- Roy, M.-F.; Kwong, G.P.S.; Lambert, J.; Massie, S.; Lockhart, S. Prognostic Value and Development of a Scoring System in Horses With Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2017, 31, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.N.; Vandenplas, M.L. Is It the Systemic Inflammatory Response Syndrome or Endotoxemia in Horses with Colic? Vet. Clin. N. Am. Equine Pract. 2014, 30, 337–351. [Google Scholar] [CrossRef]
- Schwarz, B.C.; van den Hoven, R.; Schwendenwein, I. Diagnostic Value of the Neutrophil Myeloperoxidase Index in Horses with Systemic Inflammation. Vet. J. 2012, 191, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.N.; Talavera, J.; Fernández Del Palacio, M.J. Usefulness of Doppler Ultrasonography to Assess Digital Vascular Dynamics in Horses with Systemic Inflammatory Response Syndrome or Laminitis. J. Am. Vet. Med. Assoc. 2013, 243, 1756–1761. [Google Scholar] [CrossRef]
- Epstein, K.L.; Brainard, B.M.; Giguere, S.; Vrono, Z.; Moore, J.N. Serial Viscoelastic and Traditional Coagulation Testing in Horses with Gastrointestinal Disease. J. Vet. Emerg. Crit. Care 2013, 23, 504–516. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Staempfli, H.R.; Willan, A. Prognostic Variables for Survival of Neonatal Foals Under Intensive Care. J. Vet. Intern. Med. 1992, 6, 89–95. [Google Scholar] [CrossRef]
- Cohen, N.D.; Parson, E.M.; Seahorn, T.L.; Carter, G.K. Prevalence and Factors Associated with Development of Laminitis in Horses with Duodenitis/Proximal Jejunitis: 33 Cases (1985–1991). J. Am. Vet. Med. Assoc. 1994, 204, 250–254. [Google Scholar]
- Werners, A.H.; Bull, S.; Fink-Gremmels, J. Endotoxaemia: A Review with Implications for the Horse. Equine Vet. J. 2005, 37, 371–383. [Google Scholar] [CrossRef]
- Peek, S.F.; Semrad, S.; McGuirk, S.M.; Riseberg, A.; Slack, J.A.; Marques, F.; Coombs, D.; Lien, L.; Keuler, N.; Darien, B.J. Prognostic Value of Clinicopathologic Variables Obtained at Admission and Effect of Antiendotoxin Plasma on Survival in Septic and Critically Ill Foals. J. Vet. Intern. Med. 2006, 20, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Okabayashi, K.; Wada, H.; Ohta, S.; Shiku, H.; Nobori, T.; Maruyama, K. Hemostatic Markers and the Sepsis-Related Organ Failure Assessment Score in Patients with Disseminated Intravascular Coagulation in an Intensive Care Unit. Am. J. Hematol. 2004, 76, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Manocha, S.; Russell, J.A.; Sutherland, A.M.; Wattanathum, A.; Walley, K.R. Fibrinogen-Beta Gene Haplotype Is Associated with Mortality in Sepsis. J. Infect. 2007, 54, 572–577. [Google Scholar] [CrossRef]
- Garcia-Obregon, S.; Azkargorta, M.; Seijas, I.; Pilar-Orive, J.; Borrego, F.; Elortza, F.; Boyano, M.D.; Astigarraga, I. Identification of a Panel of Serum Protein Markers in Early Stage of Sepsis and Its Validation in a Cohort of Patients. J. Microbiol. Immunol. Infect. 2018, 51, 465–472. [Google Scholar] [CrossRef]
- Kibe, S.; Adams, K.; Barlow, G. Diagnostic and Prognostic Biomarkers of Sepsis in Critical Care. J. Antimicrob. Chemother. 2011, 66, ii33–ii40. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.V.; Kold, S.E. Fibrinogen Response to Surgical Tissue Trauma in the Horse. Equine Vet. J. UK 1988, 20, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Crisman, M.V.; Scarratt, W.K.; Zimmerman, K.L. Blood Proteins and Inflammation in the Horse. Vet. Clin. N. Am Equine Pr. 2008, 24, 285–297. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and Sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Prasse, K.W.; Topper, M.J.; Moore, J.N.; Welles, E.G. Analysis of Hemostasis in Horses with Colic. J. Am. Vet. Med. Assoc. 1993, 203, 685–693. [Google Scholar]
- Topper, M.J.; Prasse, K.W. Analysis of Coagulation Proteins as Acute-Phase Reactants in Horses with Colic. Am. J. Vet. Res. 1998, 59, 542–545. [Google Scholar]
- Collatos, C.; Barton, M.H.; Prasse, K.W.; Moore, J.N. Intravascular and Peritoneal Coagulation and Fibrinolysis in Horses with Acute Gastrointestinal Tract Diseases. J. Am. Vet. Med. Assoc. 1995, 207, 465–470. [Google Scholar] [PubMed]
- Borges, A.S.; Divers, T.J.; Stokol, T.; Mohammed, O.H. Serum Iron and Plasma Fibrinogen Concentrations as Indicators of Systemic Inflammatory Diseases in Horses. J. Vet. Intern. Med. 2007, 21, 489–494. [Google Scholar] [CrossRef]
- Jacobsen, S. Review of Equine Acute-Phase Proteins. Infect. Dis. 2007, 53, 230–235. [Google Scholar]
- Jacobsen, S.; Andersen, P.H. The Acute Phase Protein Serum Amyloid A (SAA) as a Marker of Inflammation in Horses. Equine Vet. Educ. 2010, 19, 38–46. [Google Scholar] [CrossRef]
- Andrews, D.A.; Reagan, W.J.; DeNicola, D.B. Plasma Fibrinogen in Recognizing Equine Inflammatory Disease. Compend. Contin. Educ. Pract. Vet. USA 1994, 16, 1354–1366. [Google Scholar]
- Prinsen, B.H.C.M.T.; Rabelink, T.J.; Beutler, J.J.; Kaysen, G.A.; De Boer, J.; Boer, W.H.; Hagen, E.C.; Berger, R.; De Sain-Van Der Velden, M.G.M. Increased Albumin and Fibrinogen Synthesis Rate in Patients with Chronic Renal Failure. Kidney Int. 2003, 64, 1495–1504. [Google Scholar] [CrossRef]
- Fiusa, M.M.L.; Carvalho-Filho, M.A.; Annichino-Bizzacchi, J.M.; de Paula, E.V. Causes and Consequences of Coagulation Activation in Sepsis: An Evolutionary Medicine Perspective. BMC Med. 2015, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Rodelo, J.R.; de la Rosa, G.; Valencia, M.L.; Ospina, S.; Arango, C.M.; Gómez, C.I.; García, A.; Nuñez, E.; Jaimes, F.A. D-Dimer Is a Significant Prognostic Factor in Patients with Suspected Infection and Sepsis. Am. J. Emerg. Med. 2012, 30, 1991–1999. [Google Scholar] [CrossRef]
- Sandholm, M.; Vidovic, A.; Puotunen-Reinert, A.; Sankari, S.; Nyholm, K.; Rita, H. D-Dimer Improves the Prognostic Value of Combined Clinical and Laboratory Data in Equine Gastrointestinal Colic. Acta Vet. Scand. 1995, 36, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Monreal, L.; Anglés, A.; Espada, Y.; Monasterio, J.; Monreal, M. Hypercoagulation and Hypofibrinolysis in Horses with Colic and DIC. Equine Vet. J. 2000, 32, 19–25. [Google Scholar] [CrossRef]
- Stokol, T.; Erb, H.N.; De Wilde, L.; Tornquist, S.J.; Brooks, M. Evaluation of Latex Agglutination Kits for Detection of Fibrin(Ogen) Degradation Products and D-Dimer in Healthy Horses and Horses with Severe Colic. Vet. Clin. Pathol. 2005, 34, 375–382. [Google Scholar] [CrossRef]
- Delgado, M.A.; Monreal, L.; Armengou, L.; Ríos, J.; Segura, D. Peritoneal D-Dimer Concentration for Assessing Peritoneal Fibrinolytic Activity in Horses with Colic. J. Vet. Intern. Med. 2009, 23, 882–889. [Google Scholar] [CrossRef]
- Cesarini, C.; Monreal, L.; Armengou, L.; Delgado, M.á.; Ríos, J.; Jose-Cunilleras, E. Association of Admission Plasma D-Dimer Concentration with Diagnosis and Outcome in Horses with Colic. J. Vet. Intern. Med. 2010, 24, 1490–1497. [Google Scholar] [CrossRef]
- Ye, R.D.; Sun, L. Emerging Functions of Serum Amyloid A in Inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Tan, L. Serum Amyloid a Promotes Visfatin Expression in Macrophages. BioMed Res. Int. 2016, 2016, e4819327. [Google Scholar] [CrossRef]
- Vandenplas, M.L.; Moore, J.N.; Barton, M.H.; Roussel, A.J.; Cohen, N.D. Concentrations of Serum Amyloid A and Lipopolysaccharide-Binding Protein in Horses with Colic. Am. J. Vet. Res. 2005, 66, 1509–1516. [Google Scholar] [CrossRef]
- Jacobsen, S.; Nielsen, J.V.; Kjelgaard-Hansen, M.; Toelboell, T.; Fjeldborg, J.; Halling-Thomsen, M.; Martinussen, T.; Thoefner, M.B. Acute Phase Response to Surgery of Varying Intensity in Horses: A Preliminary Study. Vet. Surg. 2009, 38, 762–769. [Google Scholar] [CrossRef]
- Jacobsen, S.; Jensen, J.C.; Frei, S.; Jensen, A.L.; Thoefner, M.B. Use of Serum Amyloid A and Other Acute Phase Reactants to Monitor the Inflammatory Response after Castration in Horses: A Field Study. Equine Vet. J. 2005, 37, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.J.; Leise, B.S.; Burgess, B.A.; Morley, P.S.; Cloninger, M.; Hassel, D.M. Concentrations of Serum Amyloid A and Plasma Fibrinogen in Horses Undergoing Emergency Abdominal Surgery. J. Vet. Emerg. Crit. Care 2016, 26, 344–351. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.D.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum Amyloid A in Equine Health and Disease. Equine Vet. J. 2019, 51, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sinovich, M.; Villarino, N.F.; Singer, E.; Robinson, C.S.; Rubio-Martínez, L.M. Can Blood Serum Amyloid A Concentrations in Horses Differentiate Synovial Sepsis from Extrasynovial Inflammation and Determine Response to Treatment? Vet. Rec. 2020, 187, 235. [Google Scholar] [CrossRef]
- Hultén, C.; Demmers, S. Serum Amyloid A (SAA) as an Aid in the Management of Infectious Disease in the Foal: Comparison with Total Leucocyte Count, Neutrophil Count and Fibrinogen. Equine Vet. J. 2002, 34, 693–698. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Zeller, J.; Potempa, L.A.; Pietersz, G.A.; Eisenhardt, S.U.; Peter, K. C-Reactive Protein and Its Structural Isoforms: An Evolutionary Conserved Marker and Central Player in Inflammatory Diseases and Beyond. Subcell. Biochem. 2020, 94, 499–520. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-Phase Proteins: As Diagnostic Tool. J. Pharm. Bioallied Sci 2011, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Camacho, C.; Losa, J. Biomarkers for Sepsis. Biomed Res. Int. 2014, 2014, 547818. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, M.J.; Franco-Martínez, L.; Martínez-Subiela, S.; Cerón, J.J. Biomarkers of Sepsis in Pigs, Horses and Cattle: From Acute Phase Proteins to Procalcitonin. Anim. Health Res. Rev. 2022, 23, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Fujinaga, T.; Naiki, M.; Mizuno, S.; Otomo, K. Isolation, Characterization, and Quantitative Analysis of C-Reactive Protein from Horses. Am. J. Vet. Res. 1990, 51, 1215–1220. [Google Scholar]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in Sepsis and Systemic Inflammation: A Harmful Biomarker and a Therapeutic Target. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High Serum Procalcitonin Concentrations in Patients with Sepsis and Infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Afsar, I.; Sener, A.G. Is Procalcitonin a Diagnostic and/or Prognostic Marker in Sepsis? Infect. Dis. Clin. Pract. 2015, 23, 3–6. [Google Scholar] [CrossRef]
- Rieger, M.; Kochleus, C.; Teschner, D.; Rascher, D.; Barton, A.K.; Geerlof, A.; Kremmer, E.; Schmid, M.; Hartmann, A.; Gehlen, H. A New ELISA for the Quantification of Equine Procalcitonin in Plasma as Potential Inflammation Biomarker in Horses. Anal. Bioanal. Chem. 2014, 406, 5507–5512. [Google Scholar] [CrossRef]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Jose-Cunilleras, E.; Corazza, M.; Tognetti, R.; Guidi, G.; Intorre, L.; Sgorbini, M. Plasma Procalcitonin Concentration in Healthy Horses and Horses Affected by Systemic Inflammatory Response Syndrome. J. Vet. Intern. Med. 2015, 29, 1689–1691. [Google Scholar] [CrossRef]
- Teschner, D.; Rieger, M.; Koopmann, C.; Gehlen, H. Procalcitonin in horses with an acute colic. Pferdeheilkunde 2015, 31, 371–377. [Google Scholar] [CrossRef]
- Kilcoyne, I.; Nieto, J.E.; Dechant, J.E. Diagnostic Value of Plasma and Peritoneal Fluid Procalcitonin Concentrations in Horses with Strangulating Intestinal Lesions. J. Am. Vet. Med. Assoc. 2020, 256, 927–933. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Nocera, I.; Bonelli, F.; Vitale, V.; Meucci, V.; Conte, G.; Jose-Cunilleras, E.; Gracia-Calvo, L.A.; Sgorbini, M. Evaluation of Plasmatic Procalcitonin in Healthy, and in Systemic Inflammatory Response Syndrome (SIRS) Negative or Positive Colic Horses. Animals 2021, 11, 2015. [Google Scholar] [CrossRef]
- Parrillo, J.E.; Burch, C.; Shelhamer, J.H.; Parker, M.M.; Natanson, C.; Schuette, W. A Circulating Myocardial Depressant Substance in Humans with Septic Shock. Septic Shock Patients with a Reduced Ejection Fraction Have a Circulating Factor That Depresses in Vitro Myocardial Cell Performance. J. Clin. Investig. 1985, 76, 1539–1553. [Google Scholar] [CrossRef]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.H.; Bron, S.; van Dijl, J.M. Signal Peptide-Dependent Protein Transport in Bacillus Subtilis: A Genome-Based Survey of the Secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef] [PubMed]
- Chenau, J.; Michelland, S.; Seve, M. Le sécrétome: Définitions et intérêt biomédical. La Rev. Méd. Interne 2008, 29, 606–608. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- van Hees, H.W.; Schellekens, W.-J.M.; Linkels, M.; Leenders, F.; Zoll, J.; Donders, R.; Dekhuijzen, P.R.; van der Hoeven, J.G.; Heunks, L.M. Plasma from Septic Shock Patients Induces Loss of Muscle Protein. Crit. Care 2011, 15, R233. [Google Scholar] [CrossRef] [PubMed]
- Blangy-Letheule, A.; Persello, A.; Michelland, S.; Cunin, V.; Souab, F.; Aillerie, V.; Dhot, J.; Erraud, A.; Montnach, J.; Seve, M.; et al. The Secretome Deregulations in a Rat Model of Endotoxemic Shock. Oxid. Med. Cell. Longev. 2021, 2021, 6650464. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Salluh, J.I.F. Use of Biomarkers in Sepsis: Many Questions, Few Answers. Rev. Bras. Ter. Intensiv. 2013, 25, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, P.A. What’s in a Word? The Need for SIRS and Sepsis Definitions in Equine Medicine and Surgery. Equine Vet. J. 2018, 50, 7–9. [Google Scholar] [CrossRef] [PubMed]
| Calculation of the SOFA Score | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Respiration PaO2/FiO2 (kPa) | ≥53.3 | <53.3 | <40 | <26.7 with ventilation support | <13.3 with ventilation support |
| Coagulation Platelets (103/mm3) | >150 | <150 | <100 | <50 | <20 |
| Hepatic Bilirubin (µmol/L) | <20 | 20–32 | 33–101 | 102–204 | ≥204 |
| Cardiovascular | |||||
| Hypotension (mmHg) | MAP > 70 | MAP < 70 | |||
| Dopamine (µg/kg/min) | <5 | 5.1–15 | >15 | ||
| Central Nervous System Glasgow score | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Kidney | |||||
| Creatinine (µmol/L) | <110 | 110–170 | 171–299 | 300–400 | >440 |
| Urine (mL/day) | - | - | - | <500 | <500 |
| Models | Advantages | Disadvantages |
|---|---|---|
| Rodent | - Less expensive - Wide range of reagents - Wide range of transgenic model - Reduced variability - Short reproduction time | - Resistance to LPS - Zootechnical issues - Anatomy and physiology disparity with human |
| Canine | - Hemodynamic similarity - Cytokine similarity | - Resistance to LPS - Zootechnical issues - Long reproduction time |
| Sheep | - Diversity of races - Extremely sensitive to LPS - Cardiopulmonary similarity | - Ruminants - Zootechnical issues - Long reproduction time |
| Swine | - Omnivorous as humans - Anatomy and physiology similar to humans - Diversity of races - Reproduce the thermogenesis response to stress and the systemic energetic failure associated with septic shock | - Zootechnical issues - Long reproduction time - Pronounced acute pulmonary hypertension |
| Schwarz et al., 2012 | Aguirre et al., 2013 | Epstein et al., 2013 | Borde et al., 2014 | |
|---|---|---|---|---|
| Heart rate (Bpm) | >60 | >50 | ||
| Respiratory rate (Mpm) | >30 | >30 or PaCO2 < 32 mmHg | >30 | |
| Temperature (°C) | >38.5 | <37.2 or >38.5 | >38.6 | <36.7 or >38.6 |
| Cell counts of leukocytes (× 109 cells/L) | <5 or >10 | <4 or >12.5 or >10% immature GNN | <4.5 or >12.5 or >10% GNN immatures | <5 or >14.5 |
| Additional criteria | / | Lactate > 2 mmol/L or MAP < 90 mmHg | ||
| Clinical Sign | Threshold Values |
|---|---|
| Temperature (°C) | <37 or >38.5 |
| Heart rate (Bpm) | >52 |
| Respiratory rate (Mpm) | >20 or PaCO2 < 32 mmHg |
| Cell count of leukocytes (×109 cells/L) | <5 or >12.5 |
| Biomarkers | Advantages | Disadvantages |
|---|---|---|
| Fibrinogen | - Markers of inflammation - Inexpensive - Easy measurement | - Lack of specificity |
| Serum amyloid A | - Major acute phase proteins in horses - Distinguish between septic and non-septic inflammatory states - Rapid and pronounced increase in response to inflammatory disease | - Lack of specificity |
| Procalcitonin | - Increase markedly - Early marker of microbial infection | - Lack of specificity |
| Biomarkers | Human | Horses |
|---|---|---|
| Fibrinogen | -  or or  in sepsis [50,51] in sepsis [50,51]-  associated with mortality [50] associated with mortality [50] | -  in sepsis in sepsis-  24 h after induction of inflammation 24 h after induction of inflammation |
| D-dimer | -  in sepsis in sepsis | -  in sepsis in sepsis |
| C-reactive Protein | -  in sepsis in sepsis | -  in sepsis in sepsis |
| Serum amyloid A | -  in sepsis [52] in sepsis [52] | -  in sepsis in sepsis-  12 h after surgery 12 h after surgery |
| Procalcitonin | -  in septic patients in septic patients- Levels begin to rise 4 h after the onset of systemic infection and peak between 8 and 24 h [53] | -  in sepsis in sepsis- PCT remained higher in colic horses compared with healthy horses up to 96 h after admission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blangy-Letheule, A.; Vergnaud, A.; Dupas, T.; Rozec, B.; Lauzier, B.; Leroux, A.A. Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells 2023, 12, 1052. https://doi.org/10.3390/cells12071052
Blangy-Letheule A, Vergnaud A, Dupas T, Rozec B, Lauzier B, Leroux AA. Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells. 2023; 12(7):1052. https://doi.org/10.3390/cells12071052
Chicago/Turabian StyleBlangy-Letheule, Angélique, Amandine Vergnaud, Thomas Dupas, Bertrand Rozec, Benjamin Lauzier, and Aurélia A. Leroux. 2023. "Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives" Cells 12, no. 7: 1052. https://doi.org/10.3390/cells12071052
APA StyleBlangy-Letheule, A., Vergnaud, A., Dupas, T., Rozec, B., Lauzier, B., & Leroux, A. A. (2023). Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells, 12(7), 1052. https://doi.org/10.3390/cells12071052