lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Handling and Sample Collection
2.2. Cell Lines Culture
2.3. 5′ and 3′ Rapid Amplification of cDNA Ends (RACE)
2.4. Cytoplasmic and Nuclear RNA Fractionation Assay
2.5. RNA-Fluorescence In Situ Hybridization (FISH) Assay
2.6. Overexpression Plasmid Construction and Small Interfering RNA Synthesis
2.7. Cell Transfection and CPB2 Toxin Infection
2.8. RNA Extraction and RT-qPCR
2.9. Cell Viability Assay
2.10. Proliferation Assay
2.11. Apoptosis Analysis
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Cytotoxicity Assay
2.14. Reactive Oxygen (ROS) Level Assessing
2.15. Superoxide Dismutase (SOD) Activity Testing
2.16. Dual Luciferase Reporter Assay
2.17. Western Blot
2.18. Statistical Analysis
3. Results
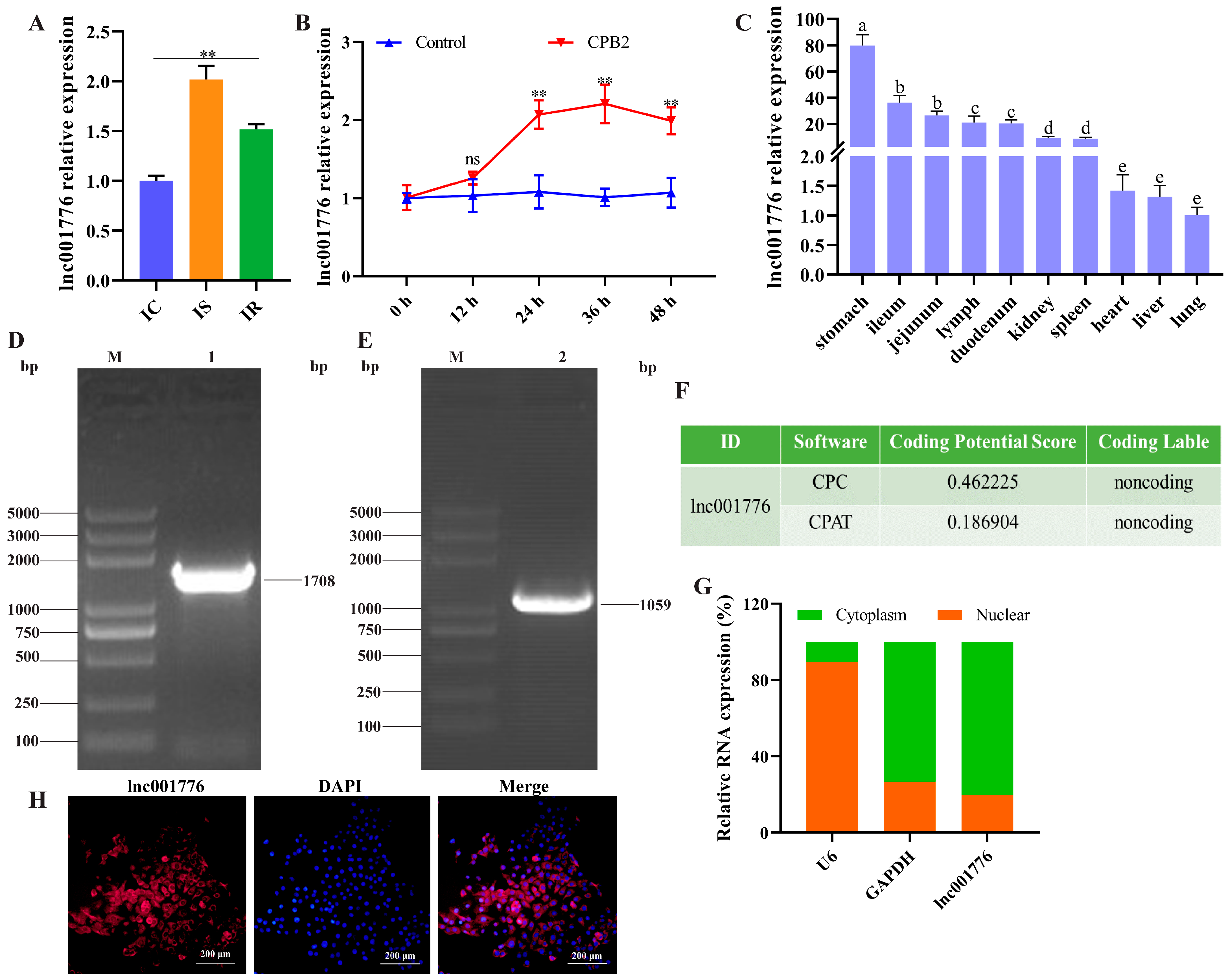
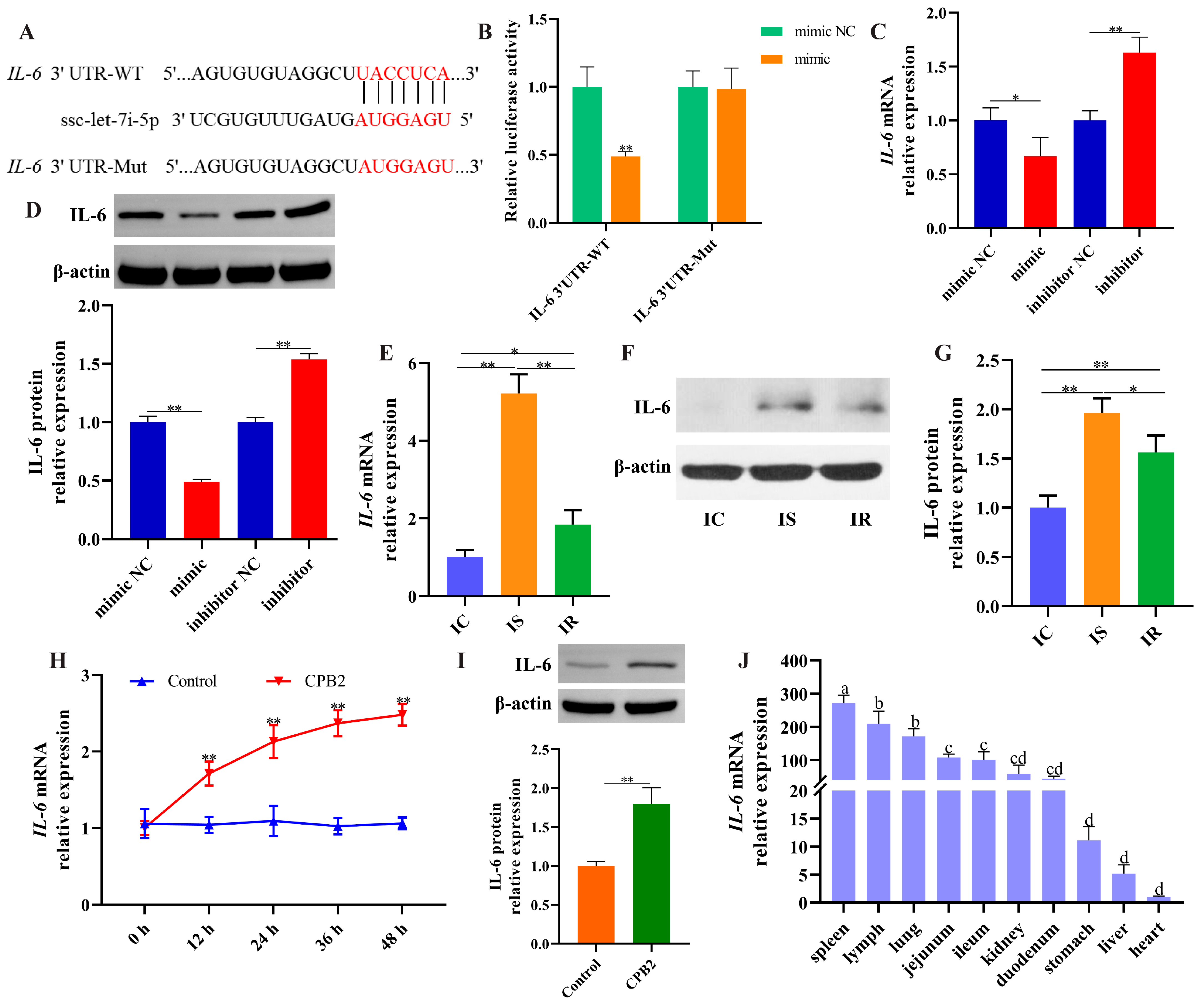
3.1. lnc001776 was Highly Expressed in the Ileum of Diarrhea Piglets and CPB2-Exposed IPEC-J2 Cells
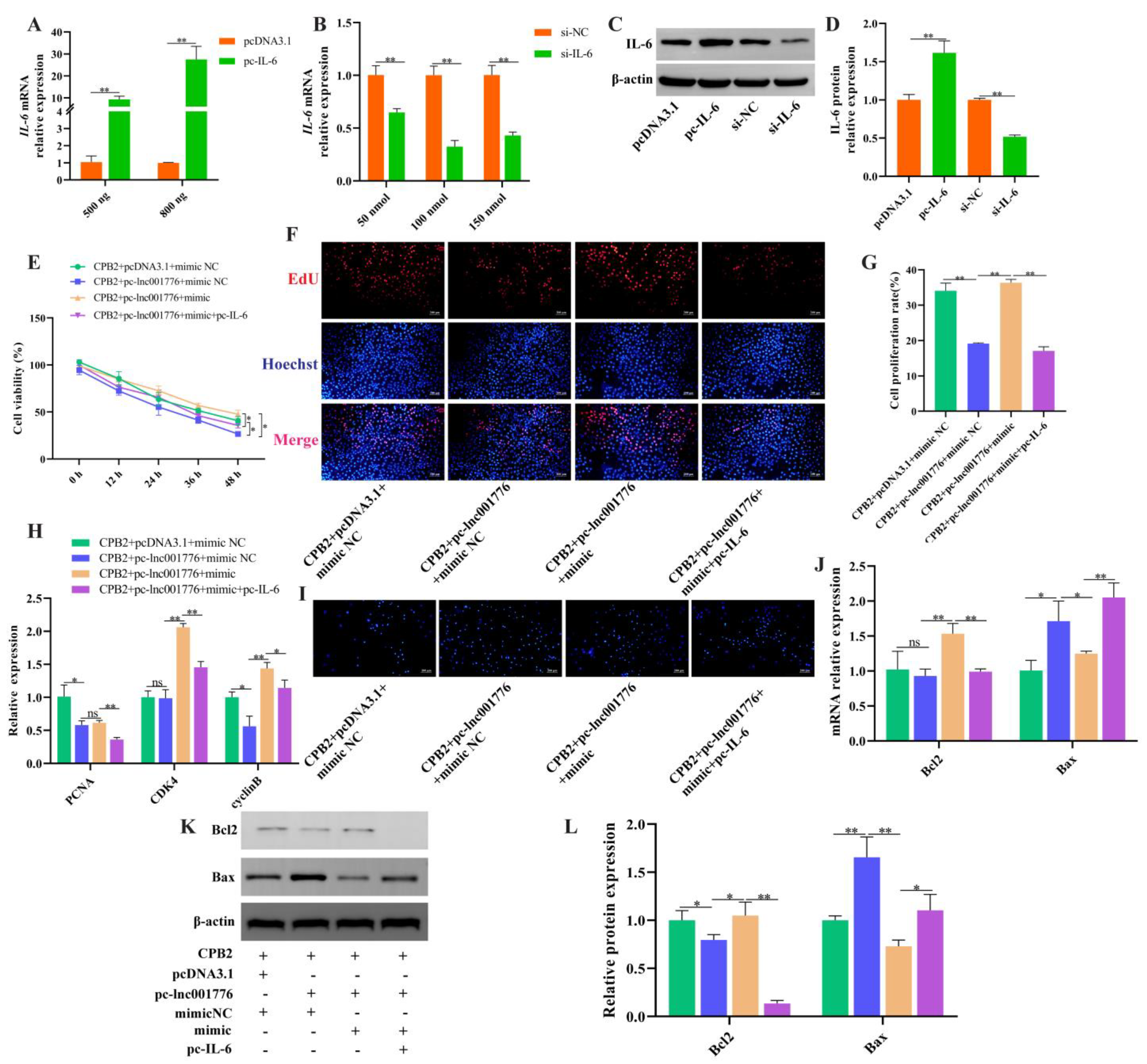
3.2. lnc001776 Deletion Suppressed CPB2 Toxin-Triggered Apoptosis in IPEC-J2 Cells
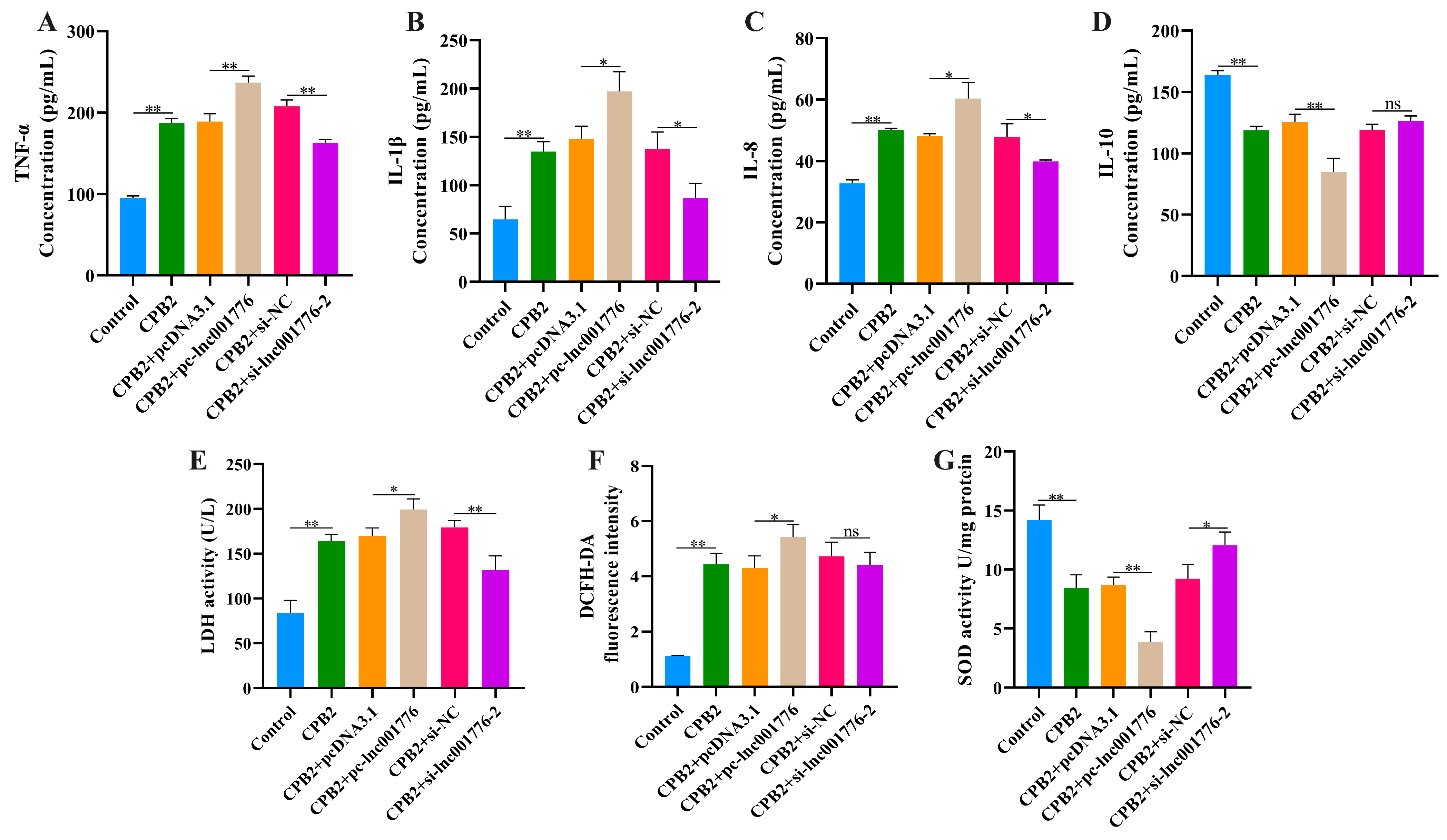
3.3. Overexpression of lnc001776 Exacerbated CPB2 Toxin-Treated IPEC-J2 Cell Inflammatory Injury
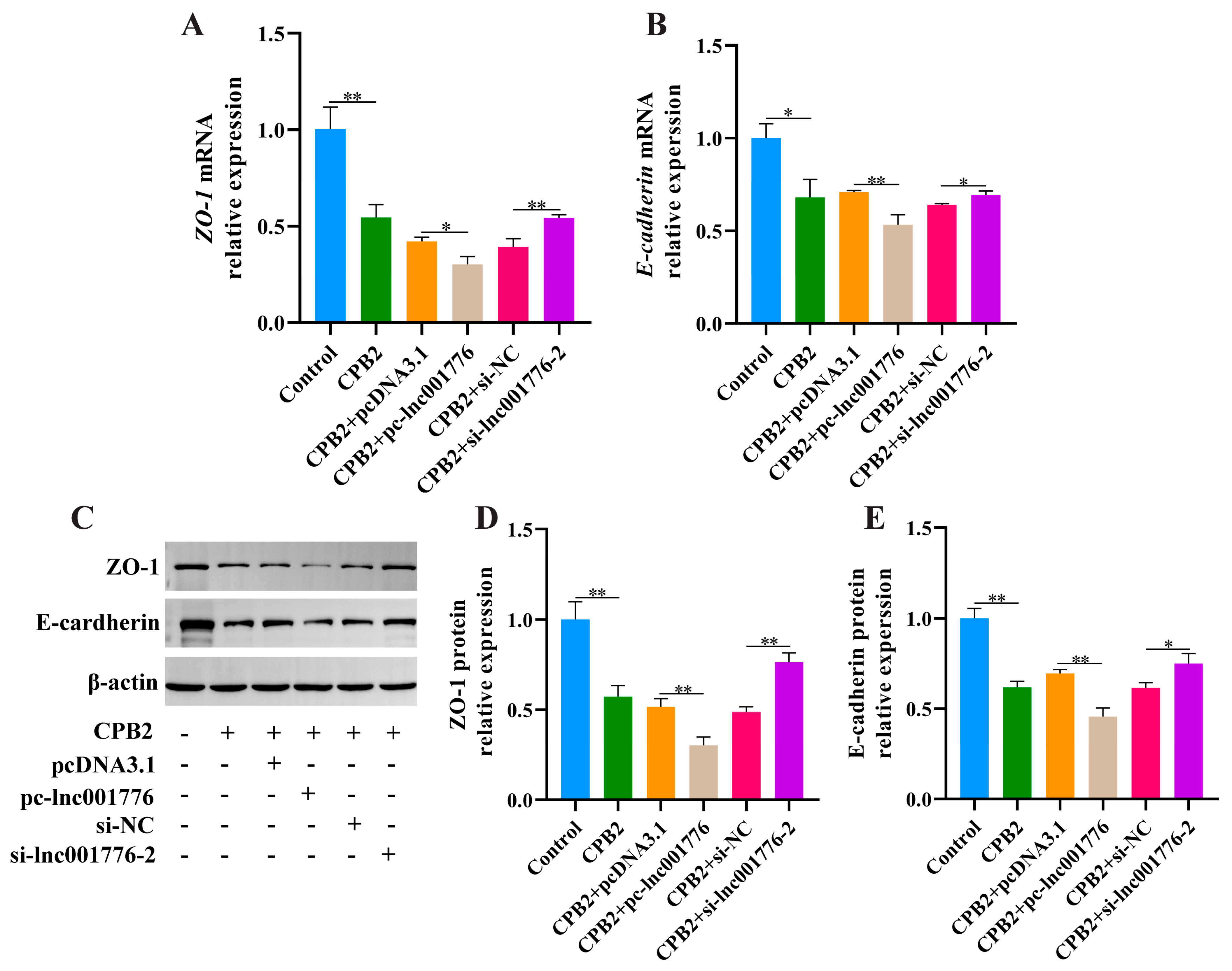
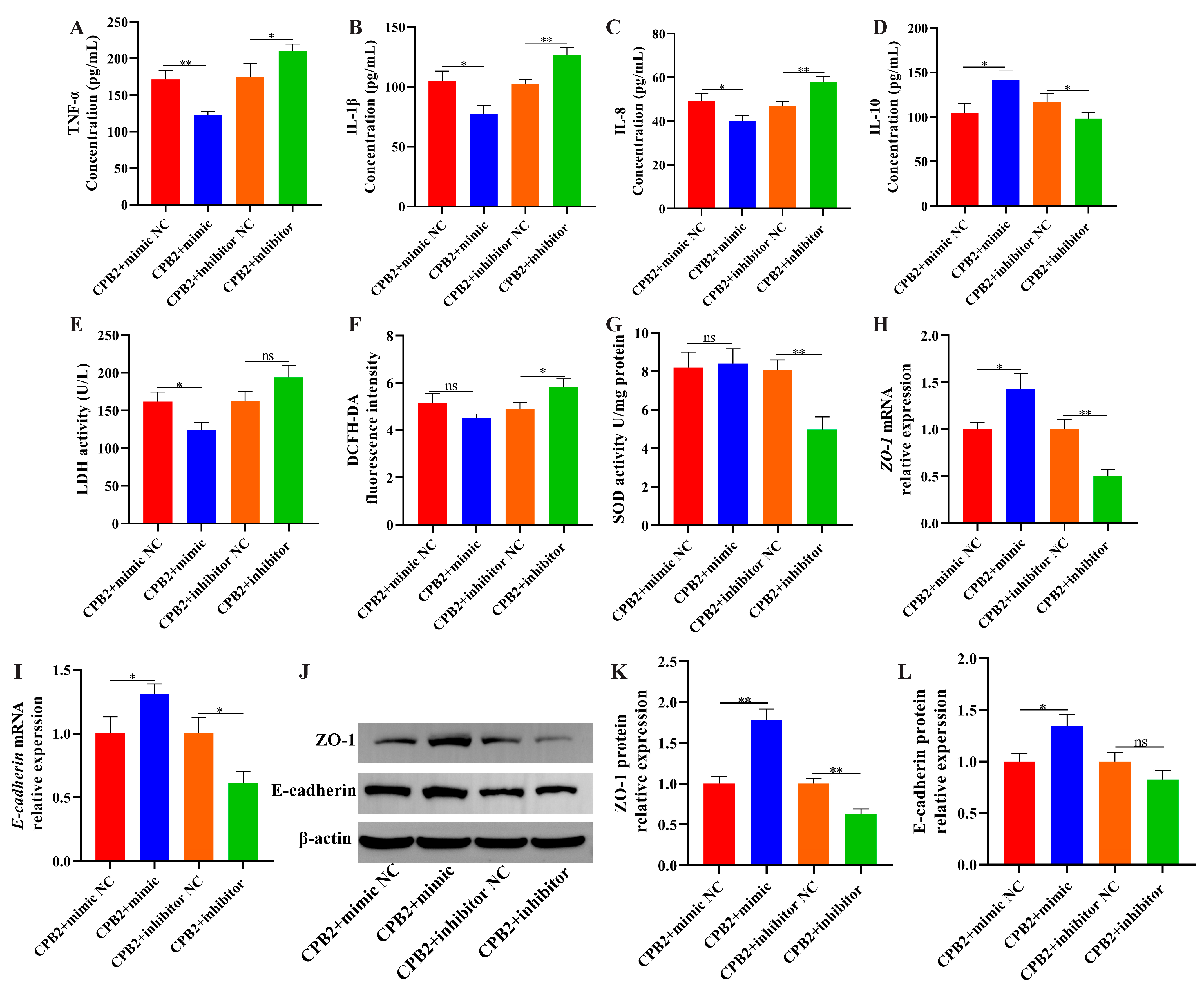
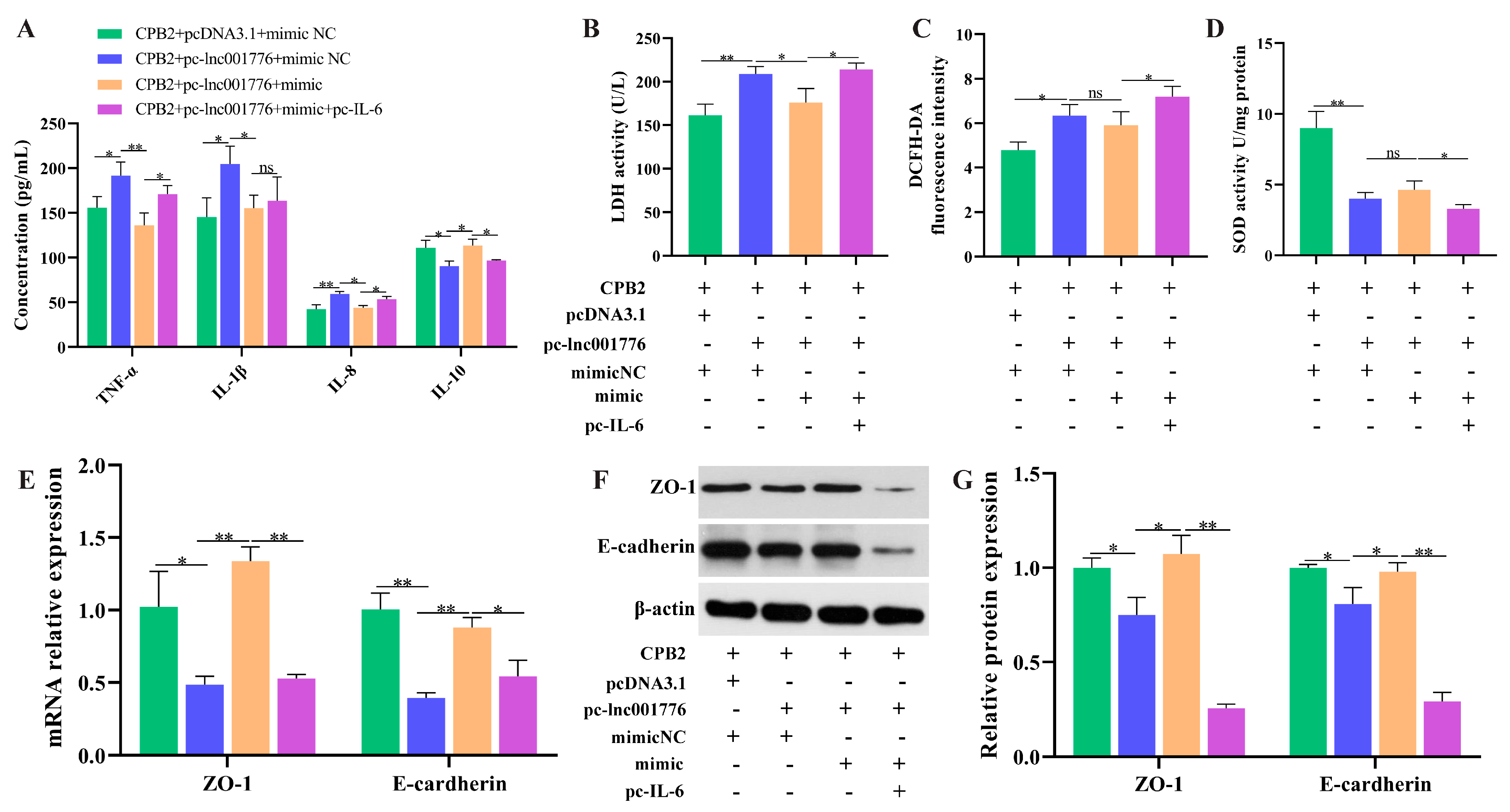
3.4. Suppression of lnc001776 Mitigated CPB2 Toxin-Triggered Barrier Disruption in IPEC-J2 Cells
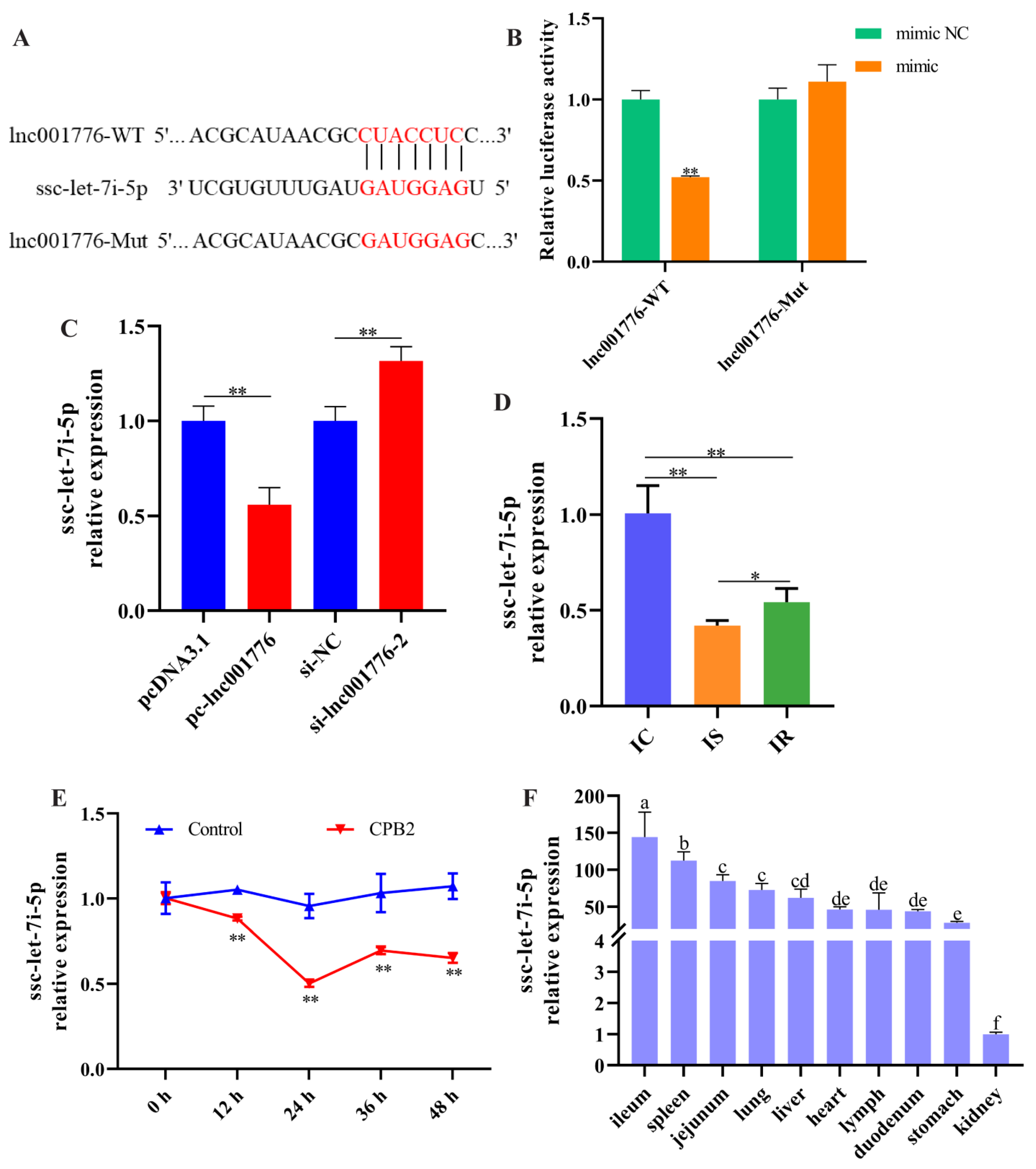
3.5. lnc001776 Acted as a Sponge for ssc-let-7i-5p
3.6. Overexpression of ssc-let-7i-5p Ameliorated CPB2 Toxin-Caused Damage in IPEC-J2 Cells
3.7. IL-6 Was a Target of ssc-let-7i-5p
3.8. Overexpression of lnc001776 Aggravated CPB2 Toxin-Triggered Damage in IPEC-J2 Cells by ssc-let-7i-5p/IL-6 Axis
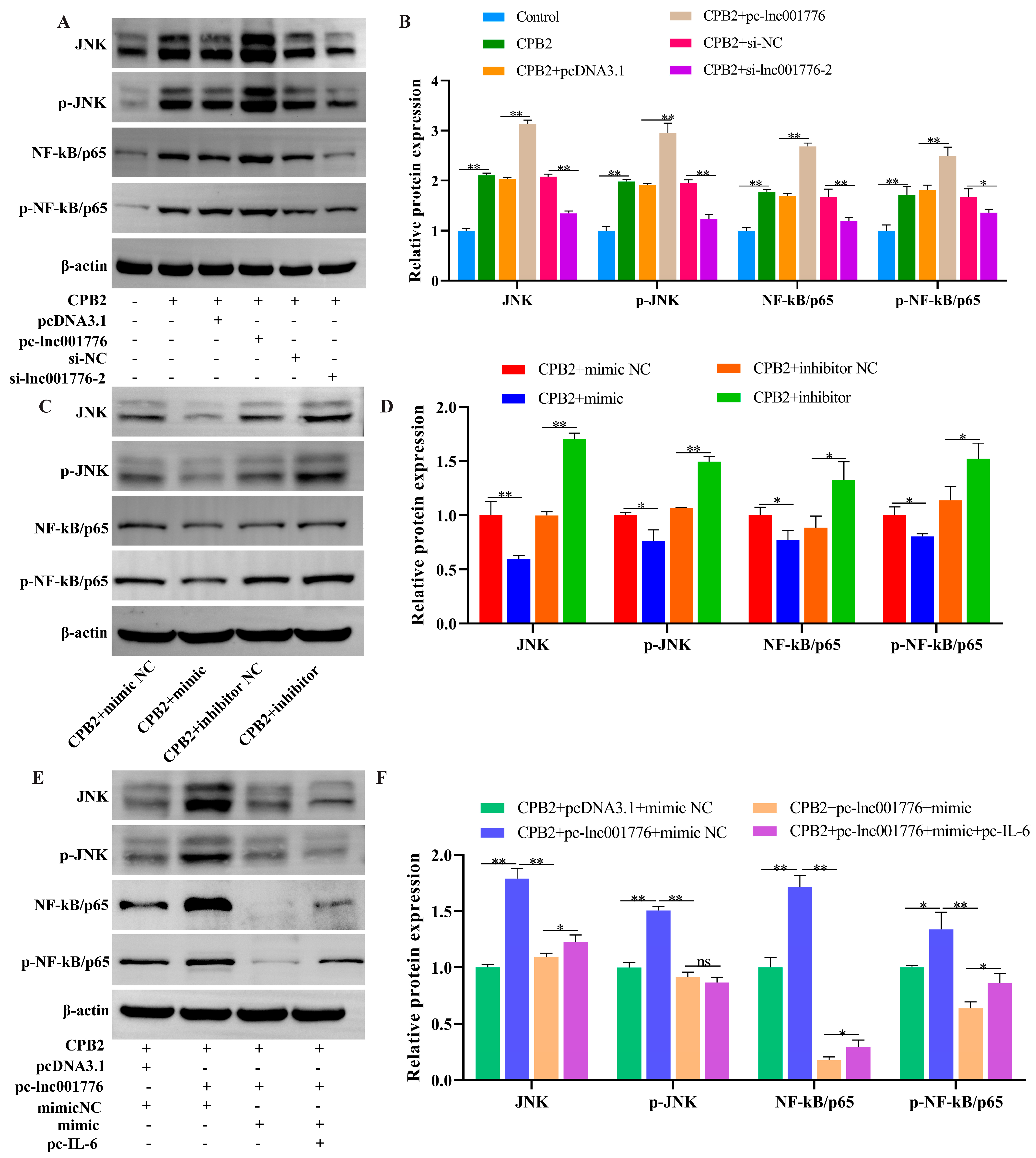
3.9. Elevated lnc001776 Activated JNK/NF-kB Pathway Through ssc-let-7i-5p/IL-6 Axis
4. Discussion
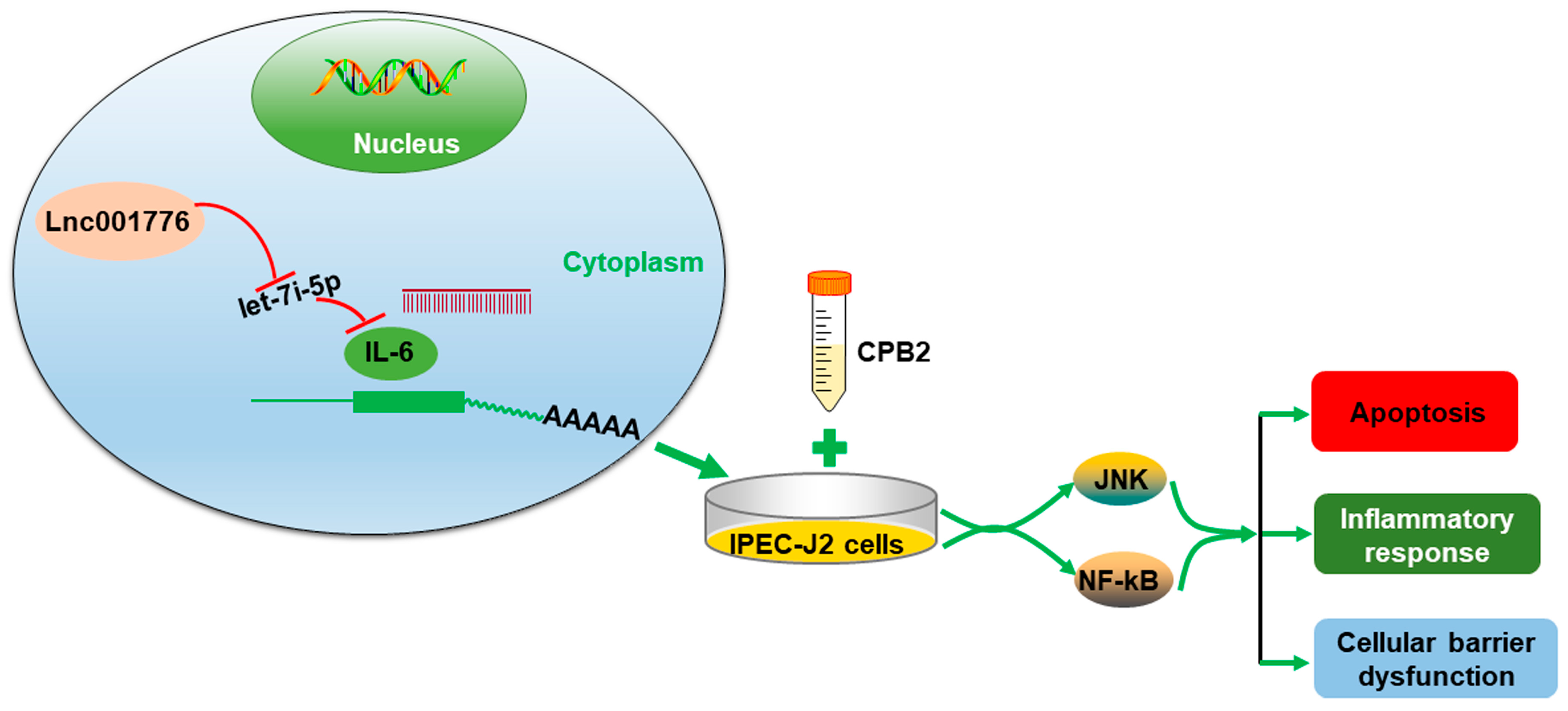
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uthe, J.J.; Royaee, A.; Lunney, J.K.; Stabel, T.J.; Zhao, S.H.; Tuggle, C.K.; Bearson, S.M. Porcine differential gene expression in response to Salmonella enterica serovars Choleraesuis and Typhimurium. Mol. Immunol. 2007, 44, 2900–2914. [Google Scholar] [CrossRef]
- Wu, Z.; Qin, W.; Wu, S.; Zhu, G.; Bao, W.; Wu, S. Identification of microRNAs regulating Escherichia coli F18 infection in Meishan weaned piglets. Biol. Direct 2016, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cai, L.; Huang, X.; Sun, W.; Li, S.; Wang, P.; Yang, Q.; Jiang, T.; Gun, S. Histological and Comparative Transcriptome Analyses Provide Insights into Small Intestine Health in Diarrheal Piglets after Infection with Clostridium Perfringens Type C. Animals 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, P.; Ke, W.; Wang, J.; Wang, X.; Xiao, S.; Fang, L. Porcine deltacoronavirus (PDCoV) infection antagonizes interferon-λ1 production. Vet. Microbiol. 2020, 247, 108785. [Google Scholar] [CrossRef]
- Zhou, X.; Niu, J.W.; Zhang, J.F.; Liao, M.; Zhai, S.L. Commentary: Identification of pulmonary infections with porcine Rotavirus A in pigs with respiratory disease. Front. Vet. Sci. 2023, 10, 1102602. [Google Scholar] [CrossRef]
- Camargo, A.; Guerrero-Araya, E.; Castañeda, S.; Vega, L.; Cardenas-Alvarez, M.X.; Rodríguez, C.; Paredes-Sabja, D.; Ramírez, J.D.; Muñoz, M. Intra-species diversity of Clostridium perfringens: A diverse genetic repertoire reveals its pathogenic potential. Front. Microbiol. 2022, 13, 952081. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Adams, V.; Bannam, T.L.; Miyamoto, K.; Garcia, J.P.; Uzal, F.A.; Rood, J.I.; McClane, B.A. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 2013, 77, 208–233. [Google Scholar] [CrossRef]
- Waters, M.; Savoie, A.; Garmory, H.S.; Bueschel, D.; Popoff, M.R.; Songer, J.G.; Titball, R.W.; McClane, B.A.; Sarker, M.R. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J. Clin. Microbiol. 2003, 41, 3584–3591. [Google Scholar] [CrossRef]
- Gibert, M.; Jolivet-Reynaud, C.; Popoff, M.R. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 1997, 203, 65–73. [Google Scholar] [CrossRef]
- Zeng, J.; Song, F.; Yang, Y.; Ma, C.; Deng, G.; Li, Y.; Wang, Y.; Liu, X. The Generation and Characterization of Recombinant Protein and Antibodies of Clostridium perfringens Beta2 Toxin. J. Immunol. Res. 2016, 2016, 5708468. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, Q.; Huang, X.; Yan, Z.; Zhang, S.; Luo, R.; Wang, P.; Wang, W.; Xie, K.; Jiang, T.; et al. Effects of Clostridium perfringens beta2 toxin on apoptosis, inflammation, and barrier function of intestinal porcine epithelial cells. Microb. Pathog. 2020, 147, 104379. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Yang, Q.; Huang, X.; Yan, Z.; Gao, X.; Wang, W.; Xie, K.; Wang, P.; Gun, S. Clostridium perfringens beta2 toxin induced in vitro oxidative damage and its toxic assessment in porcine small intestinal epithelial cell lines. Gene 2020, 759, 144999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, Z.; Lu, S.; Fu, W.; Liu, Z.; Jiang, X.; Tai, S. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin. Chim. Acta 2018, 485, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Fico, A.; Fiorenzano, A.; Pascale, E.; Patriarca, E.J.; Minchiotti, G. Long non-coding RNA in stem cell pluripotency and lineage commitment: Functions and evolutionary conservation. Cell. Mol. Life Sci. 2019, 76, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Q.; Tian, Z.; Lu, X.; Sun, Y.; Chen, Z.; Zhang, H.; Mao, Y.; Yang, Z. MIR221HG Is a Novel Long Noncoding RNA that Inhibits Bovine Adipocyte Differentiation. Genes 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liao, K.; Niu, F.; Yang, L.; Dallon, B.W.; Callen, S.; Tian, C.; Shu, J.; Cui, J.; Sun, Z.; et al. Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol. Ther.-Nucleic Acids 2018, 13, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, Z.; Liu, H.; Li, W.; Guo, X.; Zhang, Z.; Liu, Y.; Jia, L.; Li, Y.; Ren, Y.; et al. lincRNA-Cox2 regulates NLRP3 inflammasome and autophagy mediated neuroinflammation. Cell Death Differ. 2019, 26, 130–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Hou, L.; Cao, Z.; Zhu, G.; Vongsangnak, W.; Xu, Q.; Chen, G. Identification of Differentially Expressed Non-coding RNA Networks with Potential Immunoregulatory Roles During Salmonella Enteritidis Infection in Ducks. Front. Vet. Sci. 2021, 8, 692501. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lv, X.; Zhang, W.; Hu, T.; Cao, X.; Ren, Z.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. Insights into long non-coding RNA and mRNA expression in the jejunum of lambs challenged with Escherichia coli F17. Front. Vet. Sci. 2022, 9, 819917. [Google Scholar] [CrossRef]
- Zhao, Y.; Srivastava, D. A developmental view of microRNA function. Trends Biochem. Sci. 2007, 32, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Sun, X.; Xue, H.; Xiong, Y.; Yu, R.; Gao, X.; Qian, M.; Wang, S.; Wang, H.; Xu, J.; Chen, Z.; et al. GALE Promotes the Proliferation and Migration of Glioblastoma Cells and Is Regulated by miR-let-7i-5p. Cancer Manag. Res. 2019, 11, 10539–10554. [Google Scholar] [CrossRef]
- Yang, H.D.; Kim, H.S.; Kim, S.Y.; Na, M.J.; Yang, G.; Eun, J.W.; Wang, H.J.; Cheong, J.Y.; Park, W.S.; Nam, S.W. HDAC6 Suppresses Let-7i-5p to Elicit TSP1/CD47-Mediated Anti-Tumorigenesis and Phagocytosis of Hepatocellular Carcinoma. Hepatology 2019, 70, 1262–1279. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Wang, W.; Gao, Y.; Li, W.Y.; Tan, X.; Wang, Y.K.; Wang, W.Z. The Peripheral Circulating Exosomal microRNAs Related to Central Inflammation in Chronic Heart Failure. J. Cardiovasc. Transl. Res. 2022, 15, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Harrington, E.O.; Braza, J.; Shil, A.; Chichger, H. Extracellular vesicles released from p18 overexpressing pulmonary endothelial cells are barrier protective—Potential implications for acute respiratory distress syndrome. Pulm. Circ. 2020, 10, 2045894020951759. [Google Scholar] [CrossRef]
- Huang, X.Y.; Sun, W.Y.; Yan, Z.Q.; Shi, H.R.; Yang, Q.L.; Wang, P.F.; Li, S.G.; Liu, L.X.; Zhao, S.G.; Gun, S.B. Novel Insights reveal Anti-microbial Gene Regulation of Piglet Intestine Immune in response to Clostridium perfringens Infection. Sci. Rep. 2019, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, X.; Sun, W.; Yang, Q.; Shi, H.; Jiang, T.; Li, S.; Wang, P.; Gun, S. Analyses of long non-coding RNA and mRNA profiling in the spleen of diarrheic piglets caused by Clostridium perfringens type C. PeerJ 2018, 6, e5997. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, W.; Yan, Z.; Shi, H.; Yang, Q.; Wang, P.; Li, S.; Liu, L.; Zhao, S.; Gun, S. Integrative Analyses of Long Non-coding RNA and mRNA Involved in Piglet Ileum Immune Response to Clostridium perfringens Type C Infection. Front. Cell. Infect. Microbiol. 2019, 9, 130. [Google Scholar] [CrossRef]
- Zou, M.; Zhai, Y.; Mei, X.; Wei, X. Lactate dehydrogenase and the severity of adenoviral pneumonia in children: A meta-analysis. Front. Pediatr. 2023, 10, 1059728. [Google Scholar] [CrossRef]
- Van Wilpe, S.; Koornstra, R.; Den Brok, M.; De Groot, J.W.; Blank, C.; De Vries, J.; Gerritsen, W.; Mehra, N. Lactate dehydrogenase: A marker of diminished antitumor immunity. Oncoimmunology 2020, 9, 1731942. [Google Scholar] [CrossRef]
- Park, S.G.; Kim, J.H.; Xia, Y.; Sung, J.H. Generation of reactive oxygen species in d stem cells: Friend or foe? Expert Opin. Ther. Targets 2011, 15, 1297–1306. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Merlin, J.P.J.; Dellaire, G.; Xu, Z.; Rupasinghe, H.P.V. Genistein and Procyanidin B2 Reduce Carcinogen-Induced Reactive Oxygen Species and DNA Damage through the Activation of Nrf2/ARE Cell Signaling in Bronchial Epithelial Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 3676. [Google Scholar] [CrossRef]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Xikeranmu, Z.; Abdunasir, M.; Ma, J.; Tusong, K.; Liu, X. Characterization of two copper/zinc superoxide dismutases (Cu/Zn-SODs) from the desert beetle Microdera punctipennis and their activities in protecting E. coli cells against cold. Cryobiology 2019, 87, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fan, H.; Jin, J.; Gao, S.; Huang, R.; Wu, S.; Bao, W. Insight into mechanisms of pig lncRNA FUT3-AS1 regulating E. coli F18-bacterial diarrhea. PLoS Pathog. 2022, 18, e1010584. [Google Scholar] [CrossRef]
- Gao, X.; Sun, X.; Yao, X.; Wang, Y.; Li, Y.; Jiang, X.; Han, Y.; Zhong, L.; Wang, L.; Song, H.; et al. Downregulation of the Long Noncoding RNA IALNCR Targeting MAPK8/JNK1 Promotes Apoptosis and Antagonizes Bovine Viral Diarrhea Virus Replication in Host Cells. J. Virol. 2022, 96, e01113-22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, J.; Ye, J.; Xia, L.; Lu, N. Emerging role of lncRNAs in the normal and diseased intestinal barrier. Inflamm. Res. 2018, 67, 757–764. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Xie, X.; Chen, C.Y.; Huang, D.; Shen, L.; Zhang, H.; Chen, Z.W.; Zeng, G. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc. Natl. Acad. Sci. USA 2015, 112, E3883–E3892. [Google Scholar] [CrossRef]
- Li, F.; Sun, J.; Huang, S.; Su, G.; Pi, G. LncRNA GAS5 Overexpression Reverses LPS-Induced Inflammatory Injury and Apoptosis Through Up-Regulating KLF2 Expression in ATDC5 Chondrocytes. Cell. Physiol. Biochem. 2018, 45, 1241–1251. [Google Scholar] [CrossRef]
- Chen, S.W.; Wang, P.Y.; Liu, Y.C.; Sun, L.; Zhu, J.; Zuo, S.; Ma, J.; Li, T.Y.; Zhang, J.L.; Chen, G.W.; et al. Effect of Long Noncoding RNA H19 Overexpression on Intestinal Barrier Function and Its Potential Role in the Pathogenesis of Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 2582–2592. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Zhan, H.; Dai, J.; Chang, Y.; Wu, F.; Liu, T.; Liu, Z.; Gao, C.; Li, L.; et al. A long noncoding RNA distributed in both nucleus and cytoplasm operates in the PYCARD-regulated apoptosis by coordinating the epigenetic and translational regulation. PLoS Genet. 2019, 15, e1008144. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Wen, L.; Lin, A. Membrane-lipid associated lncRNA: A new regulator in cancer signaling. Cancer Lett. 2018, 419, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, C.; Cao, Y.; Yin, H.; Shen, G.; Lu, W.; Ding, W. LncRNA DANCR improves the dysfunction of the intestinal barrier and alleviates epithelial injury by targeting the miR-1306-5p/PLK1 axis in sepsis. Cell Biol. Int. 2021, 45, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, T.; Chen, Z.; Huang, J.; Qin, Z.; Li, L. Overexpression of lncRNA TUG1 Alleviates NLRP3 Inflammasome-Mediated Cardiomyocyte Pyroptosis Through Targeting the miR-186-5p/XIAP Axis in Coronary Microembolization-Induced Myocardial Damage. Front. Immunol. 2021, 12, 637598. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, X.; Liu, F. Silencing TTTY15 mitigates hypoxia-induced mitochondrial energy metabolism dysfunction and cardiomyocytes apoptosis via TTTY15/let-7i-5p and TLR3/NF-κB pathways. Cell. Signal. 2020, 76, 109779. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.J.; Harp, K.O.; Bashi, A.; Hood, J.L.; Botchway, F.; Wilson, M.D.; Thompson, W.E.; Stiles, J.K.; Driss, A. MiR-451a and let-7i-5p loaded extracellular vesicles attenuate heme-induced inflammation in hiPSC-derived endothelial cells. Front. Immunol. 2022, 13, 1082414. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Killeen, M.J.; Linder, M.; Pontoniere, P.; Crea, R. NF-κB signaling and chronic inflammatory diseases: Exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today 2014, 19, 373–378. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Yan, Z.; Yang, Q.; Huang, X.; Wang, P.; Gao, X.; Li, J.; Gun, S. lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis. Cells 2023, 12, 1036. https://doi.org/10.3390/cells12071036
Xie K, Yan Z, Yang Q, Huang X, Wang P, Gao X, Li J, Gun S. lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis. Cells. 2023; 12(7):1036. https://doi.org/10.3390/cells12071036
Chicago/Turabian StyleXie, Kaihui, Zunqiang Yan, Qiaoli Yang, Xiaoyu Huang, Pengfei Wang, Xiaoli Gao, Jie Li, and Shuangbao Gun. 2023. "lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis" Cells 12, no. 7: 1036. https://doi.org/10.3390/cells12071036
APA StyleXie, K., Yan, Z., Yang, Q., Huang, X., Wang, P., Gao, X., Li, J., & Gun, S. (2023). lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis. Cells, 12(7), 1036. https://doi.org/10.3390/cells12071036






