Effect of the Metabotropic Glutamate Receptor Type 5 Negative Allosteric Modulator Dipraglurant on Motor and Non-Motor Symptoms of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Drugs
2.3. Plasma and Cerebrospinal Fluid Sampling
2.4. Bioanalytical Method
2.5. Plasma Protein Binding
2.6. Haloperidol-Induced Catalepsy
2.7. Vogel Conflict-Drinking Test in Rats
2.8. Marble-Burying Test in Mice
2.9. Forced Swim Test
2.9.1. Mice
2.9.2. Rats
2.10. Rotarod Test
2.10.1. Mice
2.10.2. Rats
2.11. Locomotor Activity
2.11.1. Mice
2.11.2. Rats
2.12. Data Analysis
3. Results
3.1. Pharmacokinetic Profile in Rats
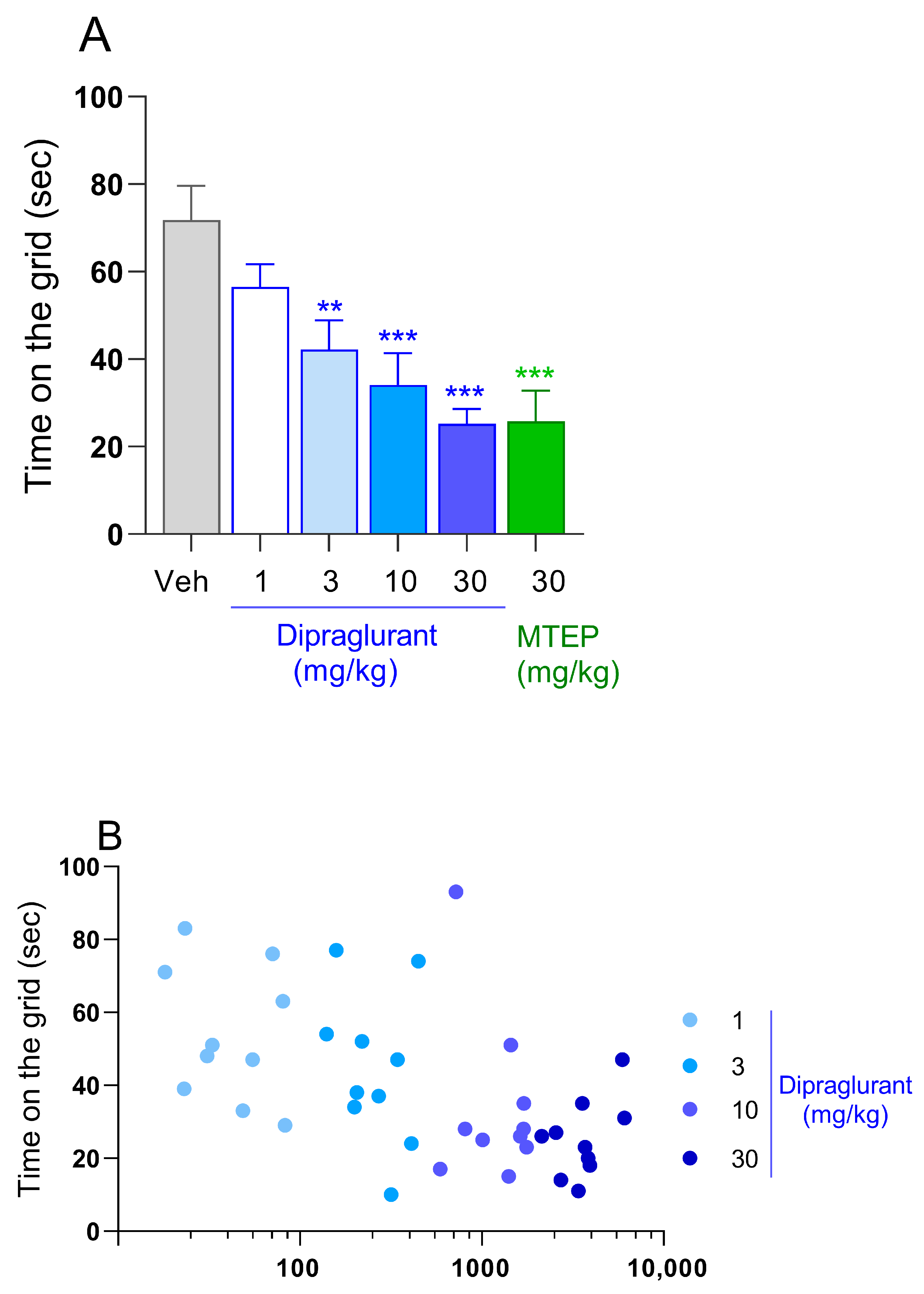
3.2. Dipraglurant Shows Antiparkinsonian Effect in Haloperidol-Induced Catalepsy Test
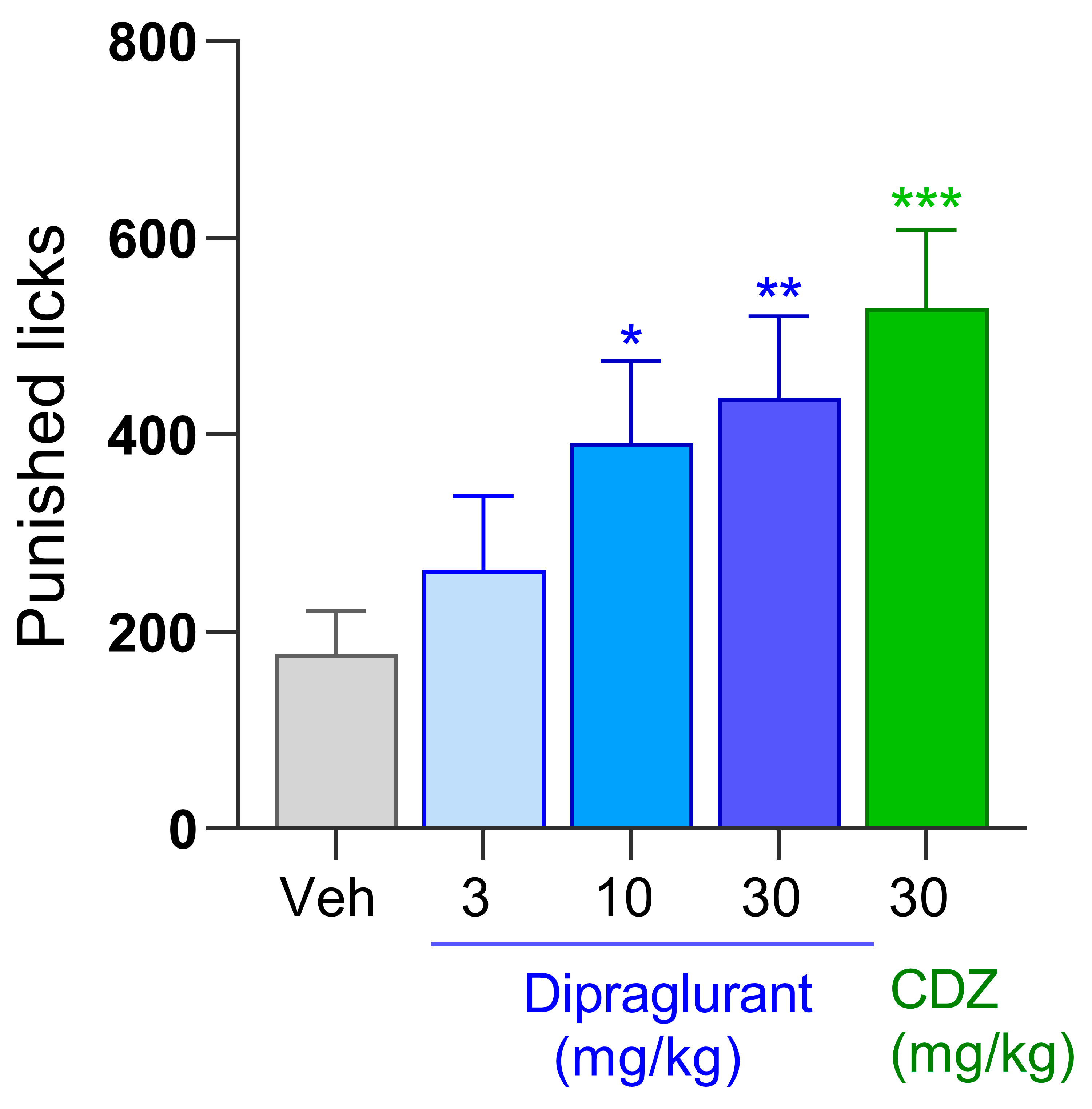
3.3. Anxiolytic Effect of Dipraglurant in the Vogel Test
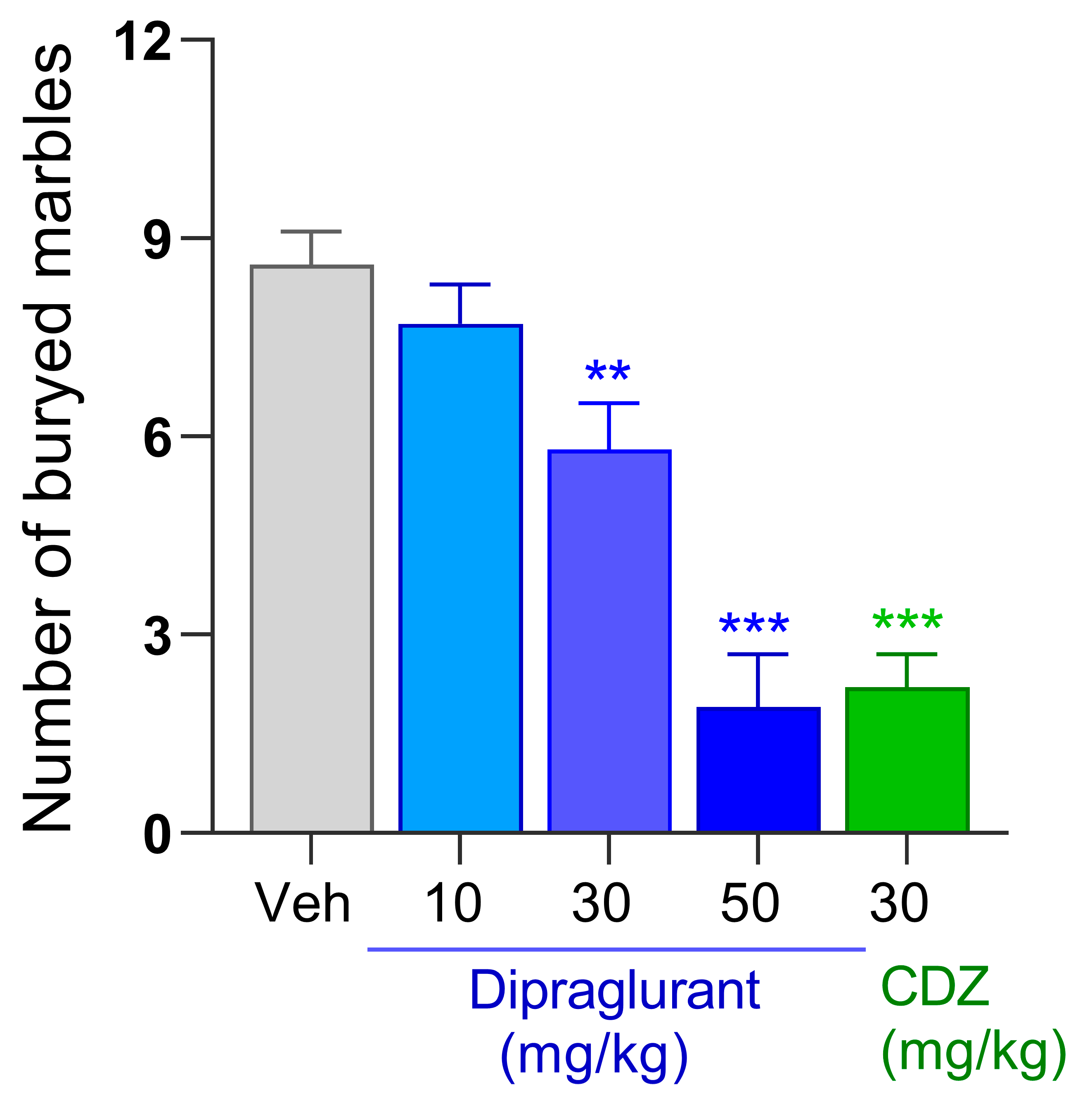
3.4. Anticompulsive Effect of Dipraglurant in the Marble Burying Test
3.5. Antidepressant Effect of Dipraglurant in the Forced Swim Test
3.6. Dipraglurant Shows No Motor Impairment, as Measured in the Rotarod Test
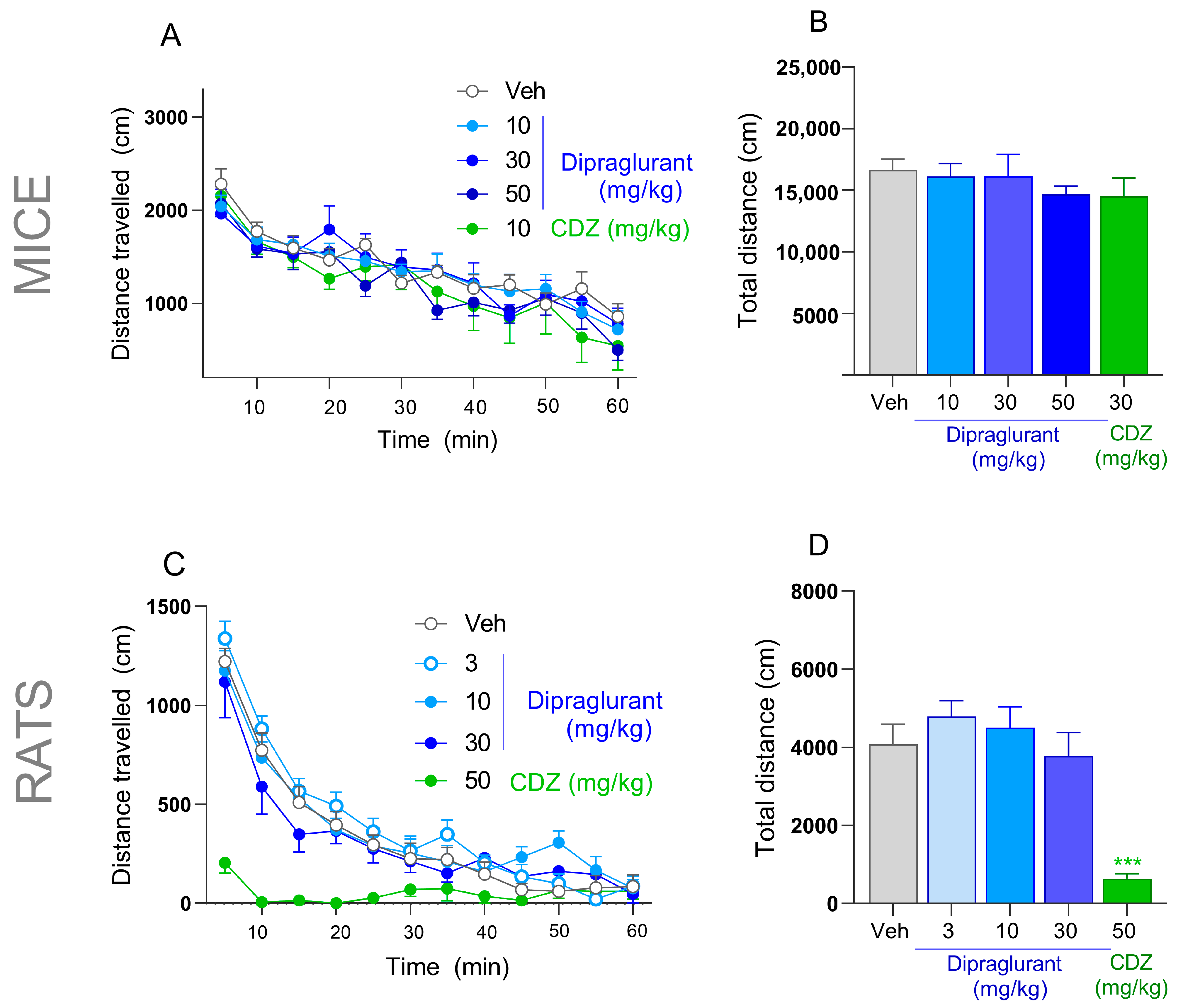
3.7. No Effect of Dipraglurant on Spontaneous Locomotor Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Jacob, E.L.; Gatto, N.M.; Thompson, A.; Bordelon, Y.; Ritz, B. Occurrence of depression and anxiety prior to Parkinson’s disease. Park. Relat. Disord. 2010, 16, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Pacheco, O.; Limotai, N.; Go, C.L.; Fernandez, H.H. Nonmotor manifestations in Parkinson disease. Neurol. 2012, 18, 1–16. [Google Scholar] [CrossRef]
- Richard, I.H. Anxiety disorders in Parkinson’s disease. Adv. Neurol. 2005, 96, 42–55. [Google Scholar]
- Quelhas, R.; Costa, M. Anxiety, depression, and quality of life in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.R.; Slaughter, K.A.; Nichols, D.; Holmes, S.E.; Martens, M.P. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 187–196. [Google Scholar] [CrossRef]
- Lemke, M.R. Depressive symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. 1), 21–25. [Google Scholar] [CrossRef]
- Nuti, A.; Ceravolo, R.; Piccinni, A.; Dell’Agnello, G.; Bellini, G.; Gambaccini, G.; Rossi, C.; Logi, C.; Dell’Osso, L.; Bonuccelli, U. Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur. J. Neurol. 2004, 11, 315–320. [Google Scholar] [CrossRef]
- Dissanayaka, N.N.W.; Sellbach, A.; Matheson, S.; O’Sullivan, J.D.; Silburn, P.A.; Byrne, G.J.; Marsh, R.; Mellick, G.D. Anxiety disorders in Parkinson’s disease: Prevalence and risk factors. Mov. Disord. 2010, 25, 838–845. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Rodríguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Leblois, A.; Boraud, T.; Meissner, W.; Bergman, H.; Hansel, D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J. Neurosci. 2006, 26, 3567–3583. [Google Scholar] [CrossRef]
- Jenner, P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 2008, 9, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Verhagen Metman, L.; Blanchet, P.J.; van den Munckhof, P.; Del Dotto, P.; Natté, R.; Chase, T.N. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov. Disord. 1998, 13, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Chase, T.N.; Oh, J.D.; Konitsiotis, S. Antiparkinsonian and antidyskinetic activity of drugs targeting central glutamatergic mechanisms. J. Neurol. 2000, 247 (Suppl. 2), II36–II42. [Google Scholar] [CrossRef]
- Hadj Tahar, A.; Grégoire, L.; Darré, A.; Bélanger, N.; Meltzer, L.; Bédard, P.J. Effect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naive parkinsonian monkeys. Neurobiol. Dis. 2004, 15, 171–176. [Google Scholar] [CrossRef]
- Nutt, J.G.; Gunzler, S.A.; Kirchhoff, T.; Hogarth, P.; Weaver, J.L.; Krams, M.; Jamerson, B.; Menniti, F.S.; Landen, J.W. Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov. Disord. 2008, 23, 1860–1866. [Google Scholar] [CrossRef]
- Paik, J.; Keam, S.J. Amantadine Extended-Release (GOCOVRI™): A Review in Levodopa-Induced Dyskinesia in Parkinson’s Disease. CNS Drugs 2018, 32, 797–806. [Google Scholar] [CrossRef]
- Cenci, M.A.; Skovgård, K.; Odin, P. Non-dopaminergic approaches to the treatment of motor complications in Parkinson’s disease. Neuropharmacology 2022, 210, 109027. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Metman, L.V.; Chase, T.N. Renaissance of amantadine in the treatment of Parkinson’s disease. Adv. Neurol. 2003, 91, 251–257. [Google Scholar]
- Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992, 258, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.M.; Standaert, D.G.; Young, A.B.; Penney, J.B. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J. Neurosci. 1994, 14, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’Malley, K.; van den Pol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Tallaksen-Greene, S.J.; Kaatz, K.W.; Romano, C.; Albin, R.L. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998, 780, 210–217. [Google Scholar] [CrossRef]
- Rouse, S.T.; Marino, M.J.; Bradley, S.R.; Awad, H.; Wittmann, M.; Conn, P.J. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: Implications for treatment of Parkinson’s disease and related disorders. Pharmacol. Ther. 2000, 88, 427–435. [Google Scholar] [CrossRef]
- Ferraguti, F.; Shigemoto, R. Metabotropic glutamate receptors. Cell Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef]
- Pisani, A.; Gubellini, P.; Bonsi, P.; Conquet, F.; Picconi, B.; Centonze, D.; Bernardi, G.; Calabresi, P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 2001, 106, 579–587. [Google Scholar] [CrossRef]
- Alagarsamy, S.; Saugstad, J.; Warren, L.; Mansuy, I.M.; Gereau, R.W.; Conn, P.J. NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology 2005, 49 (Suppl. 1), 135–145. [Google Scholar] [CrossRef]
- Breysse, N.; Amalric, M.; Salin, P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J. Neurosci. 2003, 23, 8302–8309. [Google Scholar] [CrossRef]
- Ossowska, K.; Konieczny, J.; Wolfarth, S.; Pilc, A. MTEP, a new selective antagonist of the metabotropic glutamate receptor subtype 5 (mGluR5), produces antiparkinsonian-like effects in rats. Neuropharmacology 2005, 49, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Turle-Lorenzo, N.; Breysse, N.; Baunez, C.; Amalric, M. Functional interaction between mGlu 5 and NMDA receptors in a rat model of Parkinson’s disease. Psychopharmacology 2005, 179, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Spooren, W.; Lesage, A.; Lavreysen, H.; Gasparini, F.; Steckler, T. Metabotropic glutamate receptors: Their therapeutic potential in anxiety. Curr. Top. Behav. Neurosci. 2010, 2, 391–413. [Google Scholar] [CrossRef]
- Dekundy, A.; Pietraszek, M.; Schaefer, D.; Cenci, M.A.; Danysz, W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res. Bull. 2006, 69, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Mela, F.; Marti, M.; Dekundy, A.; Danysz, W.; Morari, M.; Cenci, M.A. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J. Neurochem. 2007, 101, 483–497. [Google Scholar] [CrossRef]
- Dekundy, A.; Gravius, A.; Hechenberger, M.; Pietraszek, M.; Nagel, J.; Tober, C.; van der Elst, M.; Mela, F.; Parsons, C.G.; Danysz, W. Pharmacological characterization of MRZ-8676, a novel negative allosteric modulator of subtype 5 metabotropic glutamate receptors (mGluR5): Focus on L: -DOPA-induced dyskinesia. J. Neural Transm. 2011, 118, 1703–1716. [Google Scholar] [CrossRef]
- Johnston, T.H.; Fox, S.H.; McIldowie, M.J.; Piggott, M.J.; Brotchie, J.M. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-(2-methyl-1,3-thiazol-4-yl)ethynylpyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2010, 333, 865–873. [Google Scholar] [CrossRef]
- Morin, N.; Grégoire, L.; Gomez-Mancilla, B.; Gasparini, F.; Di Paolo, T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology 2010, 58, 981–986. [Google Scholar] [CrossRef]
- Grégoire, L.; Morin, N.; Ouattara, B.; Gasparini, F.; Bilbe, G.; Johns, D.; Vranesic, I.; Sahasranaman, S.; Gomez-Mancilla, B.; Di Paolo, T. The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Park. Relat. Disord. 2011, 17, 270–276. [Google Scholar] [CrossRef]
- Berg, D.; Godau, J.; Trenkwalder, C.; Eggert, K.; Csoti, I.; Storch, A.; Huber, H.; Morelli-Canelo, M.; Stamelou, M.; Ries, V.; et al. AFQ056 treatment of levodopa-induced dyskinesias: Results of 2 randomized controlled trials. Mov. Disord. 2011, 26, 1243–1250. [Google Scholar] [CrossRef]
- Tison, F.; Keywood, C.; Wakefield, M.; Durif, F.; Corvol, J.-C.; Eggert, K.; Lew, M.; Isaacson, S.; Bezard, E.; Poli, S.-M.; et al. A Phase 2A Trial of the Novel mGluR5-Negative Allosteric Modulator Dipraglurant for Levodopa-Induced Dyskinesia in Parkinson’s Disease. Mov. Disord. 2016, 31, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Busse, C.S.; Brodkin, J.; Tattersall, D.; Anderson, J.J.; Warren, N.; Tehrani, L.; Bristow, L.J.; Varney, M.A.; Cosford, N.D.P. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-(2-methyl-1,3-thiazol-4-yl)ethynylpyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 2004, 29, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Pałucha, A.; Brański, P.; Szewczyk, B.; Wierońska, J.M.; Kłak, K.; Pilc, A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol. Biochem. Behav. 2005, 81, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Belozertseva, I.V.; Kos, T.; Popik, P.; Danysz, W.; Bespalov, A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 2007, 17, 172–179. [Google Scholar] [CrossRef]
- Thomas, A.M.; Bui, N.; Perkins, J.R.; Yuva-Paylor, L.A.; Paylor, R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology 2012, 219, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Goudet, C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 464. [Google Scholar] [CrossRef]
- Rocher, J.-P.; Bonnet, B.; Boléa, C.; Lütjens, R.; Le Poul, E.; Poli, S.; Epping-Jordan, M.; Bessis, A.-S.; Ludwig, B.; Mutel, V. mGluR5 negative allosteric modulators overview: A medicinal chemistry approach towards a series of novel therapeutic agents. Curr. Top. Med. Chem. 2011, 11, 680–695. [Google Scholar] [CrossRef]
- El Mouedden, M.; Haseldonckx, M.; Mackie, C.; Meert, T.; Mercken, M. Method for the determination of the levels of beta-amyloid peptide in the CSF sampled from freely moving rats. J. Pharmacol. Toxicol. Methods 2005, 52, 229–233. [Google Scholar] [CrossRef]
- Jenner, P. Preventing and controlling dyskinesia in Parkinson’s disease--a view of current knowledge and future opportunities. Mov. Disord. 2008, 23 (Suppl. 3), S585–S598. [Google Scholar] [CrossRef]
- Ku, S.; Glass, G.A. Age of Parkinson’s disease onset as a predictor for the development of dyskinesia. Mov. Disord. 2010, 25, 1177–1182. [Google Scholar] [CrossRef]
- Coric, V.; Taskiran, S.; Pittenger, C.; Wasylink, S.; Mathalon, D.H.; Valentine, G.; Saksa, J.; Wu, Y.-T.; Gueorguieva, R.; Sanacora, G.; et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. Biol. Psychiatry 2005, 58, 424–428. [Google Scholar] [CrossRef]
- Kong, M.; Ba, M.; Ren, C.; Yu, L.; Dong, S.; Yu, G.; Liang, H. An updated meta-analysis of amantadine for treating dyskinesia in Parkinson’s disease. Oncotarget 2017, 8, 57316–57326. [Google Scholar] [CrossRef] [PubMed]
- Leta, V.; Jenner, P.; Chaudhuri, K.R.; Antonini, A. Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update. Expert Opin. Drug Saf. 2019, 18, 1203–1218. [Google Scholar] [CrossRef]
- Breysse, N.; Baunez, C.; Spooren, W.; Gasparini, F.; Amalric, M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J. Neurosci. 2002, 22, 5669–5678. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.J.; Bubser, M. Anticataleptic effects of the N-methyl-D-aspartate antagonist MK-801 in rats. Pharmacol. Biochem. Behav. 1989, 32, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.A.; Blackman, A.; Awere, S.; Leander, J.D. NMDA receptor antagonists inhibit catalepsy induced by either dopamine D1 or D2 receptor antagonists. Eur. J. Pharmacol. 1993, 237, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Gossel, M.; Zajaczkowski, W.; Dill, D.; Quack, G. Are NMDA antagonistic properties relevant for antiparkinsonian-like activity in rats?--case of amantadine and memantine. J. Neural Transm. Park. Dis. Dement. Sect. 1994, 7, 155–166. [Google Scholar] [CrossRef]
- Rylander, D.; Recchia, A.; Mela, F.; Dekundy, A.; Danysz, W.; Cenci, M.A. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: Effects on motor behavior and striatal nuclear signaling. J. Pharmacol. Exp. Ther. 2009, 330, 227–235. [Google Scholar] [CrossRef]
- Iravani, M.M.; Syed, E.; Jackson, M.J.; Johnston, L.C.; Smith, L.A.; Jenner, P. A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur. J. Neurosci. 2005, 21, 841–854. [Google Scholar] [CrossRef]
- Battaglia, G.; Busceti, C.L.; Molinaro, G.; Biagioni, F.; Storto, M.; Fornai, F.; Nicoletti, F.; Bruno, V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J. Neurosci. 2004, 24, 828–835. [Google Scholar] [CrossRef]
- Aguirre, J.A.; Kehr, J.; Yoshitake, T.; Liu, F.-L.; Rivera, A.; Fernandez-Espinola, S.; Andbjer, B.; Leo, G.; Medhurst, A.D.; Agnati, L.F.; et al. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005, 1033, 216–220. [Google Scholar] [CrossRef]
- Armentero, M.-T.; Fancellu, R.; Nappi, G.; Bramanti, P.; Blandini, F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2006, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vernon, A.C.; Zbarsky, V.; Datla, K.P.; Croucher, M.J.; Dexter, D.T. Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J. Neurochem. 2007, 103, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.J.; Liu, J.; Wang, S.; Ali, U.; Gui, Z.H.; Wang, Y. Chronic, systemic treatment with a metabotropic glutamate receptor 5 antagonist in 6-hydroxydopamine partially lesioned rats reverses abnormal firing of dopaminergic neurons. Brain Res. 2009, 1286, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Black, Y.D.; Xiao, D.; Pellegrino, D.; Kachroo, A.; Brownell, A.-L.; Schwarzschild, M.A. Protective effect of metabotropic glutamate mGluR5 receptor elimination in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 161–165. [Google Scholar] [CrossRef]
- Ambrosi, G.; Armentero, M.-T.; Levandis, G.; Bramanti, P.; Nappi, G.; Blandini, F. Effects of early and delayed treatment with an mGluR5 antagonist on motor impairment, nigrostriatal damage and neuroinflammation in a rodent model of Parkinson’s disease. Brain Res. Bull. 2010, 82, 29–38. [Google Scholar] [CrossRef]
- Caraci, F.; Battaglia, G.; Sortino, M.A.; Spampinato, S.; Molinaro, G.; Copani, A.; Nicoletti, F.; Bruno, V. Metabotropic glutamate receptors in neurodegeneration/neuroprotection: Still a hot topic? Neurochem. Int. 2012, 61, 559–565. [Google Scholar] [CrossRef]
- Youssef, E.A.; Berry-Kravis, E.; Czech, C.; Hagerman, R.J.; Hessl, D.; Wong, C.Y.; Rabbia, M.; Deptula, D.; John, A.; Kinch, R.; et al. Effect of the mGluR5-NAM Basimglurant on Behavior in Adolescents and Adults with Fragile X Syndrome in a Randomized, Double-Blind, Placebo-Controlled Trial: FragXis Phase 2 Results. Neuropsychopharmacology 2018, 43, 503–512. [Google Scholar] [CrossRef]
- Hagerman, R.; Jacquemont, S.; Berry-Kravis, E.; Des Portes, V.; Stanfield, A.; Koumaras, B.; Rosenkranz, G.; Murgia, A.; Wolf, C.; Apostol, G.; et al. Mavoglurant in Fragile X Syndrome: Results of two open-label, extension trials in adults and adolescents. Sci. Rep. 2018, 8, 16970. [Google Scholar] [CrossRef]
- Ballard, T.M.; Woolley, M.L.; Prinssen, E.; Huwyler, J.; Porter, R.; Spooren, W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: A comparison. Psychopharmacology 2005, 179, 218–229. [Google Scholar] [CrossRef]
- Varty, G.B.; Grilli, M.; Forlani, A.; Fredduzzi, S.; Grzelak, M.E.; Guthrie, D.H.; Hodgson, R.A.; Lu, S.X.; Nicolussi, E.; Pond, A.J.; et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: A comparison of efficacy and side-effect profiles. Psychopharmacology 2005, 179, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, K.; Gołembiowska, K.; Sowa, M.; Nowak, G.; Chojnacka-Wójcik, E.; Pilc, A. Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent. Neuropharmacology 2007, 53, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, L.; Jaeschke, G.; Michalon, A.; Vieira, E.; Honer, M.; Spooren, W.; Porter, R.; Hartung, T.; Kolczewski, S.; Büttelmann, B.; et al. CTEP: A novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J. Pharmacol. Exp. Ther. 2011, 339, 474–486. [Google Scholar] [CrossRef]
- Li, X.; Need, A.B.; Baez, M.; Witkin, J.M. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J. Pharmacol. Exp. Ther. 2006, 319, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Pomierny-Chamioło, L.; Poleszak, E.; Pilc, A.; Nowak, G. NMDA but not AMPA glutamatergic receptors are involved in the antidepressant-like activity of MTEP during the forced swim test in mice. Pharmacol. Rep. 2010, 62, 1186–1190. [Google Scholar] [CrossRef]
- Wieronska, J.M.; Szewczyk, B.; Branski, P.; Palucha, A.; Pilc, A. Antidepressant-like effect of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist in the olfactory bulbectomized rats. Amino Acids 2002, 23, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Brodkin, J.; Busse, C.; Sukoff, S.J.; Varney, M.A. Anxiolytic-like activity of the mGluR5 antagonist MPEP: A comparison with diazepam and buspirone. Pharmacol. Biochem. Behav. 2002, 73, 359–366. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 2011, 6, e21175. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Bhattacharyya, S.; Christopher, R.; Khanna, S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology 2005, 30, 1735–1740. [Google Scholar] [CrossRef]
- Grant, P.; Lougee, L.; Hirschtritt, M.; Swedo, S.E. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2007, 17, 761–767. [Google Scholar] [CrossRef]
- Aboujaoude, E.; Barry, J.J.; Gamel, N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. J. Clin. Psychopharmacol. 2009, 29, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Krystal, J.H.; Coric, V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx 2006, 3, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Berg, D.; Rascol, O.; Eggert, K.; Ceballos-Baumann, A.; Corvol, J.-C.; Storch, A.; Zhang, L.; Azulay, J.-P.; Broussolle, E.; et al. A Placebo-Controlled Trial of AQW051 in Patients with Moderate to Severe Levodopa-Induced Dyskinesia. Mov. Disord. 2016, 31, 1049–1054. [Google Scholar] [CrossRef] [PubMed]

| Domain | Model | Doses (mg/kg) | Plasma Conc. (ng/mL) | Calculated CSF Conc. (ng/mL) | Calculated CSF Conc. (nM) | CSF/IC50 | ED50 (mg/kg) |
|---|---|---|---|---|---|---|---|
| Learned anxiety | Vogel conflict drinking test (rat) | 3 | 501 ± 97 | 9.5 | 25.6 | 0.54 | 7.4 ± 0.9 |
| 10 * | 1120 ± 148 | 21.3 | 57.5 | 1.22 | |||
| 30 ** | 2043 ± 420 | 38.8 | 104.8 | 2.23 | |||
| Innate anxiety (OCD–like) | Marble burying (mouse) | 10 | 26 ± 5 | 2.4 | 6.5 | 0.14 | 43 ± 6 |
| 30 * | 212 ± 28 | 19.3 | 52.1 | 1.11 | |||
| 50 *** | 865 ± 156 | 78.7 | 212.5 | 4.52 | |||
| Depression | Forced swim test (mouse) | 30 ** | 946 ± 264 | 86.1 | 232.5 | 4.95 | ~30 |
| 50 *** | 2266 ± 627 | 206.2 | 556.7 | 11.84 | |||
| Forced swim test (rat) | 3 **, 10 *, 30 ** | N.D. | N.D. | ||||
| Parkinson’s disease | Haloperidol-induced catalepsy (rat) | 1 | 54 ± 10 | 1.0 | 2.7 | 0.06 | 2.83 ± 0.3 |
| 3 | 328 ± 25 | 6.2 | 16.7 | 0.35 | |||
| 10 ** | 1723 ± 218 | 32.7 | 88.3 | 1.88 | |||
| 30 *** | 3617 ± 383 | 68.7 | 185.5 | 3.95 | |||
| Species | Compound | Administration Route | Dose (mg/kg) | Immobility Duration (s) Mean ± S.E.M. |
|---|---|---|---|---|
| Mouse | Dipraglurant | p.o. | 0 | 188.2 ± 9.5 |
| 30 | 134.4 ± 16.7 ** | |||
| 50 | 113.5 ± 10.4 *** | |||
| Desipramine | i.p. | 10 | 93.9 ± 9.7 *** | |
| Rat | Dipraglurant | p.o. | 0 | 214.5 ± 6.1 |
| 3 | 177.5 ± 5.3 ** | |||
| 10 | 184.7 ± 9.2 * | |||
| 30 | 167.5 ± 11.8 ** | |||
| Imipramine | i.p. | 64 | 101.8 ± 7.8 *** |
| Species | Compound | Administration Route | Dose (mg/kg) | Time on the Rotarod (s) Mean ± S.E.M. |
|---|---|---|---|---|
| Mouse | Dipraglurant | p.o. | 0 | 144.1 ± 8.6 |
| 10 | 142.9 ± 7.7 | |||
| 30 | 158.3 ± 8.4 | |||
| 50 | 137.0 ± 10.6 | |||
| Chlordiazepoxide | i.p. | 30 | 8.3 ± 2.5 *** | |
| Rat | Dipraglurant | p.o. | 0 | 103.2 ± 17.5 |
| 3 | 137.1 ± 21.9 | |||
| 10 | 124.2 ± 17.7 | |||
| 30 | 102.1 ± 19.6 | |||
| Diazepam | i.p. | 8 | 18.7 ± 7.0 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epping-Jordan, M.P.; Girard, F.; Bessis, A.-S.; Mutel, V.; Boléa, C.; Derouet, F.; Bessif, A.; Mingard, B.; Barbier, S.; Paradis, J.S.; et al. Effect of the Metabotropic Glutamate Receptor Type 5 Negative Allosteric Modulator Dipraglurant on Motor and Non-Motor Symptoms of Parkinson’s Disease. Cells 2023, 12, 1004. https://doi.org/10.3390/cells12071004
Epping-Jordan MP, Girard F, Bessis A-S, Mutel V, Boléa C, Derouet F, Bessif A, Mingard B, Barbier S, Paradis JS, et al. Effect of the Metabotropic Glutamate Receptor Type 5 Negative Allosteric Modulator Dipraglurant on Motor and Non-Motor Symptoms of Parkinson’s Disease. Cells. 2023; 12(7):1004. https://doi.org/10.3390/cells12071004
Chicago/Turabian StyleEpping-Jordan, Mark P., Françoise Girard, Anne-Sophie Bessis, Vincent Mutel, Christelle Boléa, Francis Derouet, Abdelhak Bessif, Brice Mingard, Stéphanie Barbier, Justine S. Paradis, and et al. 2023. "Effect of the Metabotropic Glutamate Receptor Type 5 Negative Allosteric Modulator Dipraglurant on Motor and Non-Motor Symptoms of Parkinson’s Disease" Cells 12, no. 7: 1004. https://doi.org/10.3390/cells12071004
APA StyleEpping-Jordan, M. P., Girard, F., Bessis, A.-S., Mutel, V., Boléa, C., Derouet, F., Bessif, A., Mingard, B., Barbier, S., Paradis, J. S., Rocher, J.-P., Lütjens, R., Kalinichev, M., & Poli, S. (2023). Effect of the Metabotropic Glutamate Receptor Type 5 Negative Allosteric Modulator Dipraglurant on Motor and Non-Motor Symptoms of Parkinson’s Disease. Cells, 12(7), 1004. https://doi.org/10.3390/cells12071004






