Abstract
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Common causes include gram-negative and gram-positive bacteria as well as fungi. Neutrophils are among the first cells to arrive at an infection site where they function as important effector cells of the innate immune system and as regulators of the host immune response. The regulation of neutrophil migration is therefore important both for the infection-directed host response and for the development of organ dysfunctions in sepsis. Downregulation of CXCR4/CXCL12 stimulates neutrophil migration from the bone marrow. This is followed by transmigration/extravasation across the endothelial cell barrier at the infection site; this process is directed by adhesion molecules and various chemotactic gradients created by chemotactic cytokines, lipid mediators, bacterial peptides, and peptides from damaged cells. These mechanisms of neutrophil migration are modulated by sepsis, leading to reduced neutrophil migration and even reversed migration that contributes to distant organ failure. The sepsis-induced modulation seems to differ between neutrophil subsets. Furthermore, sepsis patients should be regarded as heterogeneous because neutrophil migration will possibly be further modulated by the infecting microorganisms, antimicrobial treatment, patient age/frailty/sex, other diseases (e.g., hematological malignancies and stem cell transplantation), and the metabolic status. The present review describes molecular mechanisms involved in the regulation of neutrophil migration; how these mechanisms are altered during sepsis; and how bacteria/fungi, antimicrobial treatment, and aging/frailty/comorbidity influence the regulation of neutrophil migration.
Keywords:
sepsis; neutrophils; neutrophil subsets; chemotaxis; aging; frailty; antibiotics; stem cell transplantation; metabolism 1. Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, i.e., the host response to the infection causes organ damage/failure [1,2]. The organ functions are evaluated by the Sequential/sepsis-related Organ Failure Assessment (SOFA) score that is based on scoring respiration, coagulation, liver function, circulation, consciousness, and renal function. Finally, septic shock is observed when the circulatory dysfunctions are severe enough to substantially increase mortality; these patients can be clinically identified by the fact that they require vasopressor therapy and by their increased serum lactate levels despite adequate volume resuscitation [1,2]. Septic shock patients have a hospital mortality exceeding 40%.
Neutrophils are important effector cells of the innate immune system and constitute an important part of the first line defense against infections [3,4,5,6]. However, neutrophils derived from sepsis patients are dysfunctional (e.g., with regard to chemotaxis/migration) [3,4,5], and this dysfunction is probably important in terms of the contribution of neutrophils to the development of the sepsis-associated organ failure that directly relates to mortality and morbidity [6]. Several observations suggest that organ dysfunction/failure is not due to hypoperfusion and hypoxia alone; additional mechanisms involve endothelial and microvascular dysfunctions, immune dysregulation, and (cellular) metabolic reprogramming [6]. Our aim was to review and discuss the molecular mechanisms involved in the regulation of neutrophil migration (Section 2, Section 3, Section 4, Section 5, Section 6 and Section 7); how these mechanisms operate during sepsis (Section 8, Section 9 and Section 10) how bacteria/fungi, antimicrobial treatment, and aging/frailty/comorbidity/sex influence neutrophil migration (Section 11, Section 12, Section 13, Section 14 and Section 15); and possible therapeutic strategies that have been tried or suggested to target neutrophil functions in sepsis patients (Section 16).
2. The Regulation of Neutrophil Migration during Bacterial Infections: Transendothelial Migration from the Bone Marrow and Later Extravasation at the Infection Site
Sepsis is characterized by increased neutrophil release from the bone marrow followed by transendothelial migration in small vessels and extravascular chemotaxis at the infection site (see Section 3) [7,8,9,10,11]. The final step in sepsis entails reversed migration contributing to sepsis-induced organ failure. Most studies referred to in this section and in the following Section 3, Section 4, Section 5, Section 6 and Section 7 are based on studies of circulating neutrophils, and it is stated in the text when neutrophils were derived from other compartments.
2.1. Migration of Neutrophils from the Bone Marrow to the Circulation
Neutrophils are produced in the bone marrow, and the retention of immature neutrophils in the marrow is ensured by their expression of the CXCR4 chemokine receptor and its ligand CXCL12 [5,12,13,14,15,16]. The hematopoietic growth factor granulocyte colony-stimulating factor (G-CSF) is a downregulator of CXCR4 expression and thereby a stimulator of neutrophil migration from the bone marrow [13].
2.2. Extravasation of Neutrophils at the Infection Site: Molecular Mechanisms Involved in the Regulation of Endothelial Adhesion, Transendothelial Migration, and Neutrophil Polarization
Neutrophil extravasation is a multistep process [17,18,19,20]. The first step is the capture of floating neutrophils by endothelial cell surfaces via adhesion molecules that are upregulated on endothelial cells in response to proinflammatory cytokines and/or microbial molecules. These initial weak and transient interactions are mediated by the selectin family of adhesion molecules, and they allow rolling of the neutrophils on the endothelial cells. Platelet P selectin contributes to the formation of platelet–leukocyte aggregates and functions as an adhesion mechanism by bridging neutrophils and endothelium [21].
The next step is the stimulation and activation of the neutrophils by chemokines that are presented on the endothelial luminal surface. This process allows firm adhesion, and the neutrophils then crawl on the surface to finally find a site that allows transmigration [22]. The neutrophils can cross the endothelial barrier either by the parallel route between endothelial cells or by the transcellular route. Several neutrophil integrins control the firm adhesion to the endothelium including αLβ2, αMβ2, and α4β1 integrins; their ligands are intercellular adhesion molecule 1 (ICAM-1), ICAM-2, and vascular adhesion molecule 1 (VCAM-1), respectively [22].
Neutrophil polarization is thereafter necessary for directional migration; the cells develop filamentous actin polymerization at the leading edge that faces the chemotactic gradients, whereas myosin filaments assemble at the opposite end where they form the uropod [22]. Differences in the lipid composition are probably essential for the cell membrane organization of the various proteins/adhesion molecules involved in the polarization, and therefore modulation of the lipid content/structure (e.g., cholesterol depletion) will inhibit polarization/migration [22,23,24,25]. The mechanisms behind these lipid effects seem to involve the cholesterol-induced modulation of intracellular PI3-kinase/RhoA signaling that leads to uropod formation [25]. Furthermore, cholesterol is a major constituent of the plasma membrane and has important effects on the physical properties of the lipid bilayer: it regulates the lateral mobility of membrane proteins and increases the strength of membrane–cytoskeleton interactions [26]. A general effect of cholesterol depletion is therefore cell stiffening with the strengthening of the membrane–cytoskeleton interactions and increased endothelial stiffening with strengthened cytoskeleton–membrane interactions is associated with enhanced endothelial contractility and increased neutrophil migration [27]. However, other membrane lipids including phosphoinositides, phosphoinositols, lipid phosphatases, and sphingomyelins/ceramides are also involved in regulation of neutrophil polarity/chemotaxis [28,29,30].
Endothelial heparan sulfate binds the CXCL1 and CXCL2 chemokines (see Section 3.5), and these chemokines thereby become involved in the process of neutrophil crawling/diapedesis [22,31]. The chemotactic leukotriene B4 (see Section 3.3) and the surrounding pericytes are also regulators in these early steps of neutrophil extravasation [22,32,33]. Thus, neutrophil transmigration/extravasation is regulated by an orchestrated action of endothelial/pericyte/neutrophil adhesion molecules and chemotactic agents, and one would also expect the binding of neutrophil integrins to various extracellular matrix proteins to further modulate these initial steps of neutrophil migration.
2.3. Caveola Are Important for Neutrophil Transmigration and the Formation of Chemotactic Gradients
The caveolar invaginations of the neutrophil plasma membrane are important in the regulation of neutrophil migration (see Section 11.4). Caveola and caveolin-1 signaling in endothelial cells have additional indirect effects on neutrophil chemotaxis. Caveolin-1 then interacts with endothelial nitric oxide (NO) synthase resulting in the modulation of toll-like receptor (TLR)-initiated Nuclear factor κ B (NFκB) activation and transcriptional regulation, including the regulation of endothelial chemokine expression [34]. Caveolin-1-initiated signaling seems to limit inflammation through this inhibition of TLR-induced cytokine/chemokine release. Furthermore, the level of endothelial caveolin-1 (responsible for induction of membrane caveola) is important in neutrophil extravasation. Chemotaxis preferentially recruits neutrophils to a subset of venules that express high levels of ICAM-1 and low levels of caveolin-1. The level of caveolin-1 expression will then determine the balance between parallel and transcellular neutrophil extravasation [35]. In addition, extravascular chemokines can traverse the endothelial barrier when establishing the chemotactic gradients (see above), and several neutrophil–chemotactic chemokines then seem to traverse the microvasculature through caveola [35]. Thus, caveola and caveolin-1 are important both for the regulation of neutrophil transmigration and for the initiation of chemokine-dependent chemotaxis.
3. Regulation of Neutrophil Migration during Bacterial Infections: The Chemoattractants
Chemotaxis is defined as guided cell movement in response to chemical signals released at a distant site; the cells then move along concentration gradients formed by receptor-binding chemoattractants [7]. The four main chemoattractant are N-formylated peptide, complement factors, lipids, and chemokines. In contrast, non-directional random migration is referred to as chemokinesis [8].
Chemoattractants are classified as either end targets or intermediary targets (Figure 1) [7,9,11]. The end targets are N-formylated peptides and complement chemoattractants, and they are dominant over the intermediary chemoattractants (i.e., lipid chemoattractants and chemokines) in case of opposing gradients. These two main classes show differences in downstream receptor signaling with end target attractants mainly signaling through phospholipase A2 and/or mitogen-activated protein kinase (MAP kinase) p38, whereas intermediary attractants signal through the phosphatidylinositol 3-kinase (PI3K) pathway [10,11]. Finally, lipid chemoattractants are essential for the initial neutrophil recruitment. The first step is followed by an amplification step of cytokine/chemokine release, and the overall process is often referred to as the lipid–cytokine–chemokine cascade [9]. It can be seen from the descriptions in Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6 that the different chemotactic ligands/receptors form an interacting chemotactic network.
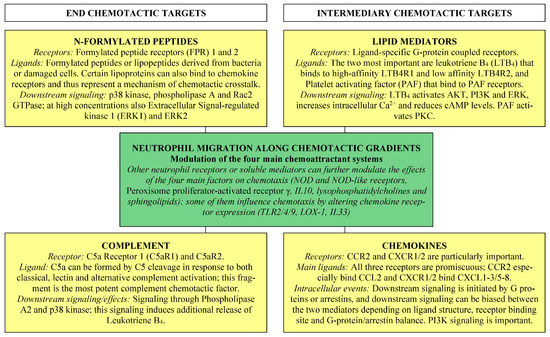
Figure 1.
An overview of the classification and important characteristics of the four (see the yellow boxes) main neutrophil chemoattractants (for a detailed discussion with references see Section 3).
3.1. Formylated Peptide Receptors and Their Binding of Bacteria-Derived and Endogenous N-Formylated Peptides and Lipopeptides
Neutrophils can respond to exogenous N-formylated peptides originating from various bacteria and to endogenous peptides derived from damaged cells (e.g., mitochondrial proteins); such peptides can be recognized by three different receptors and the formylated peptide receptors (FPR) 1 and FPR2 which are both expressed by neutrophils [7,36]. FPR1 and FPR2 bind structurally diverse ligands with either agonistic or antagonistic effects [37]. Agonistic ligation of FPR1 increases neutrophil chemotaxis [38]. These formylated peptides are regarded as end target chemoattractant, i.e., when the neutrophils experience opposing chemotactic signaling, they will preferentially migrate along the formylated peptide gradients [7,36]. Intracellular signaling downstream to the FPRs involves various pathways including the p38 kinase and the Rac2 GTPase, and at high concentrations, extracellular signal-regulated kinase 1 (ERK1) and ERK2 also [39,40].
FPR1 can also bind endogenous non-formylated ligands that include peptides derived from the neutrophil mediator cathepsin G and annexin A1/annexin A4 [41,42]. The same is true for FPR2 which is more promiscuous and can bind formylated peptides as well as serum amyloid A, β-amyloid, annexin A1, humanin, and possibly lipoxin A4 [41,42]. FPR2 can mediate both pro- and anti-inflammatory effects depending on the ligand, and various ligands seem to differ with regard to their effects on neutrophil migration [37,41]. Possible explanations for these ligand-dependent variations are the different dimerization states of the receptor, the altered receptor conformation, and/or differences in downstream signaling depending on the ligand [41].
Lipopeptides are short endogenous or microbial-derived peptides with a fatty acid linked at the N-terminal end [37]. These molecules have both activating and inhibitory effects on G-protein-coupled receptors, and they can modulate FPR-2-initiated intracellular signaling [37]. Due to their lipid domain, the molecules pass through the cell membrane and bind to the cytosolic parts of receptor complexes. Their protein part is usually identical or similar to one of the intracellular loops of the targeted receptor, although the receptor specificity is not always complete, e.g., a CXCR4 targeting lipoprotein was also found to target FPR2 [41,42,43]. This last observation suggests that lipopeptides represent a mechanism for crosstalk between various chemotactic mechanisms [43].
3.2. Complement Factors
The complement factor C5 is important for neutrophil chemotaxis, and the proteolytic fragment C5a is the most potent complement chemoattractant [7,44]. C5a is formed by C5 cleavage in response to both classical, lectin, and alternative complement activation [7], and its chemotactic effect is mainly mediated through the C5a Receptor 1 (C5aR1) and its downstream mediator p38 [10,11]. Neutrophil activation through C5aR1 can also lead to the release of the chemoattractant leukotriene B4, i.e., C5aR1 crosstalks with lipid chemoattractants [45]. Finally, the role of complement factor C3 in inflammation is complex, but it does not seem to have any major effect on neutrophil migration [44].
3.3. The Interacting Lipid Chemoattractants Leukotriene B4 and Platelet Activating Factor
Eicosanoids derived from arachidonic acid should be regarded as the main lipid chemoattractants [7]. Leukotriene B4 is the strongest lipid chemoattractant for neutrophils; it is mainly released by myeloid cells and binds to the G-protein-coupled receptors leukotriene B4 receptor 1 (LTB4R1) and 2 (LTB4R2) [46,47]. LTB4R1 is mainly expressed by neutrophils, and receptor ligation results in neutrophil polarization followed by migration [9]. The functional effects of LTB4R2 ligation are less well characterized [7].
Another lipid chemoattractant is platelet-activating factor (PAF) which is derived from phosphatidylcholine [7,48,49]. It binds to a specific G-protein-coupled receptor on the neutrophils and can thereby activate and polarize neutrophils through intracellular signaling involving protein kinase C (PKC) [39,50]. PAF can also induce the release of leukotriene B4 by neutrophils [48], and there is an additional crosstalk between these two main chemotactic mediators through leukotriene B4 modulation of intracellular signaling downstream to PAF receptors [51]. However, animal models suggest that PAF may also inhibit neutrophil chemotaxis in certain biological contexts [50].
3.4. Classification of Chemokines and Chemokine Receptors
The chemokines constitute a family of low molecular weight cytokines (8–12 kDa, 70–120 amino acid residues) with chemotactic activity and, based on their chemical structure, they are classified into two main subsets: CCL and CXCL chemokines [52,53]. These two subsets bind to CCR and CXCR chemokine receptors, respectively. Several of these receptors are promiscuous with regard to ligand binding, and several chemokines are promiscuous with regard to receptor binding. The CXCL chemokines can be further subclassified into chemokines with and without a glutamate–leucine–arginine (ELR) motif before the CXC motif. CXCL chemokines with the ELR motif are CXCL1-3 and CXCL5-8; these chemokines are most important for neutrophil chemotaxis and can bind to the CXCR1/CXCR2 receptors [52,53,54]. Finally, neutrophil recruitment can also be guided by CCR1 ligands (CCL3 and CCL4), whereas CCR2 (receptor for CCL2) is most important during chronic inflammation. Finally, collagen fragments can also have chemotactic activity by binding to the CXCR2 receptor [52,53].
CCR2, CXCR1, and CXCR2 chemokines show similarities in downstream intracellular signaling. The PI3K pathway is important together with the additional activation of phospholipase C and increased intracellular calcium levels [55].
3.5. The Diversity of the Chemokine Receptors CXCR1/CXCR2 and Their Signaling: Biased Intracellular Signaling Is Initiated Both at the Ligand, Receptor, and Signal Initiation Levels
The chemokine system is characterized by biased signaling. This means that either (i) the relative strength of the intracellular pathway activation by two receptors with similar cellular density differs even when binding the same ligand (i.e., different ligand affinity of the receptors) or (ii) the relative activation of various pathways downstream to a certain chemokine receptor depends on the binding ligand. CXCR1/CXCR2 show biased signaling, which can be due to various molecular mechanisms [52].
CXCR1 versus CXCR2 affinity of individual chemokines. All CXCR2 ligands bind to this receptor with a similar high affinity, whereas the CXCR1 affinity differs between ligands with CXCL8 showing the highest affinity [56]. Thus, the balance between CXCR1/CXCR2 signaling depends on the availability of various ligand in the microenvironment.
Chemokine receptors have two different ligand-binding sites. The chemokines interact with CXCR1 and CXCR2 through two different binding sites, i.e., the chemokine N-terminal loop can either bind to N-terminal receptor residues (site 1) or to extracellular loop residues (site 2). Furthermore, an initial Site-1 interaction causes conformational receptor changes that are essential for binding to Site 2; differences between two chemokines in regard to the affinity for the two binding sites may therefore lead to differences in downstream signaling [57,58].
Chemokine monomers and dimers differ in receptor affinity. Chemokines exist as monomers and dimers, and some of them even exist as high-order oligomers or heterodimers [52,57,58,59]. Chemokine monomers show biological activity at the nanomolar level, but at higher concentrations, this varies. For example, the CXCL8 dimers show an altered receptor affinity with lower CXCR1 affinity but similar high CXCR2 affinity compared with the monomer [58,59,60]. Thus, the local chemokine concentration and the equilibrium/ratio between monomeric and dimeric states will influence the balance between CXCR1 and CXCR2 ligation/signaling at least for certain CXCL chemokines [59,61,62].
Modulation of chemokine ligation by glycosaminoglycan binding. Chemokines can bind to extracellular glycosaminoglycans, and for certain chemokines (i.e., CXCL1/5/7/8) this binding is stronger for the dimers than for the monomers [52]. Chemokine binding to glycosaminoglycans on endothelial cells is also involved in the regulation of extravasation.
Downstream receptor signaling through G-proteins versus β-arrestin. The intracellular signaling of chemokine receptors is initiated immediately downstream to the receptor by either G-proteins or β-arrestin [52]. G-protein-mediated signaling is rapid in onset and is followed by gradual waning, whereas β-arrestin signaling has a slower onset with sustained duration [63]. β-arrestin is additionally involved in termination of the G-protein initiated signaling through recruitment of adaptor proteins and the enhancement of endocytosis of the chemokine/receptor complex [52,59].
Biased downstream signaling due to ligation of the same receptor by different chemokines. Several examples illustrate that ligation of the same receptor by different chemokines leads to different downstream effects. First, CXCR2 ligation by CXCL1 leads to an influx of extracellular calcium, but this is not observed after CXCL8 ligation [64]. Second, CXCL8 bound to CXCR1 but not to CXCR2 primes neutrophils for superoxide release, and this priming effect is also observed for CXCR2 after CXCL1 and CXCL5 binding [65,66]. Third, both G-protein and β-arrestin are important for the induction of the dynamic actin remodeling that is fundamental for neutrophil migration and for the integrin activation needed for transendothelial migration, but the balance between G-protein and β-arrestin signaling varies [52,59,67,68,69]. For example, CXCR2 ligation by CXCL6 is relatively G-protein biased whereas the CXCL8 seems to be relatively β-arrestin biased with regard to cytoskeletal effects compared with other CXCR2 ligands [70]. Finally, CXCL1 monomers are more active than dimers with regard to several functional effects (e.g., calcium mobilization, chemotaxis, and β-arrestin recruitment), whereas such differences are not observed between CXCL2 monomers and dimers [60]. Finally, monomers and dimers may also differ with regard to the initiation of CXCR1/2 internalization/recycling [60,71].
Receptor dimerization. Dimerization may influence receptor signaling. CXCR2 forms homodimers whereas CXCR1 does not. CXCR1/CXCR2 heterodimers also exist [72,73].
To summarize, binding of the same receptor (CXCR1 and/or CXCR2) by different ligands can influence the downstream regulatory signaling of neutrophil migration.
3.6. Other Receptors or Mediators Involved in the Regulation of Neutrophil Migration: Molecular Mechanisms That Function as Modulators of the Main Chemotactic Pathways
Several other receptors and soluble mediators are also involved in the regulation of neutrophil chemotaxis, including:
- Toll-like receptors 2 and 4 (TLR2/TLR4). These receptors have both exogenous (e.g., microbial molecules) and endogenous agonists [74,75]. Ligation of TLR2 and TLR4 alters the neutrophil expression of CXCR1 and CXCR2 [76]. However, this effect of TLR4 may be age-dependent because it differs between newborns and adults [77]. TLR4 also increases neutrophil expression of immunostimulatory HLA-DR and the immunosuppressive T cell checkpoint molecule PD-L1 (see later Section 9.4 and Section 10.1), but these two effects may differ between neutrophil subsets [78];
- TLR9. Ligation of this intracellular receptor in neutrophils by exogenous or endogenous ligands triggers CXCR2 downregulation [79], and TLR9 ligation in other immunocompetent cells seems to have additional indirect effects on neutrophil chemotaxis [80,81,82];
- NOD-like receptors. Several cytosolic nucleotide-binding oligomerization domains (NOD) and NOD-like receptors (NLRs) have been described. NOD2 and NLRP3 are expressed by neutrophils and ligation of both receptors facilitates migration [83]. These receptors can be activated by the peptidoglycans of most bacteria [84,85];
- LOX-1. The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is expressed by neutrophils after TLR2/4 ligation, it activates neutrophil NFκB [86,87] but decreases CXCR2 expression in animal models [88,89];
- Peroxisome proliferator-activated receptor γ (PPARγ). This nuclear receptor inhibits neutrophil chemotaxis in sepsis [90,91];
- IL10 receptor. The signaling inhibits neutrophil chemotaxis and cytokine release [91];
- IL33 receptor. IL33R ligation blocks TLR4-mediated CXCR2 internalization and enhances neutrophil migration in sepsis [92,93,94,95,96];
- Lysophosphatidylcholines. Lysophosphatidylcholines can modulate neutrophil migration/chemotaxis. These lipids mediate the direct effects on neutrophils by ligation of the G2A receptor, a member of the proton-sensing G-protein-coupled receptors [97]. The G2A ligation causes the recruitment of clathrin that is important for the signal transduction [97], modulates p38 signaling [98], and finally increases neutrophil expression of the αM/CD11b integrin and formylated peptide receptors [99]. In vivo animal studies have shown that these lipids can reduce neutrophil migration [100];
- Sphingolipids. Sphingolipids and sphingolipid metabolism are involved in the regulation of neutrophil migration [9,101,102,103,104,105,106]. First, lactosylceramide forms lipid raft microdomains coupled with the Src family kinase Lyn in neutrophils, and it can thereby initiate lipid raft-mediated regulation of neutrophil polarization and migration [10,104]. Second, sphingosine 1-phosphate (Sph-1-P) inhibits CXCL8- and formylated peptide-induced chemotactic migration of human neutrophils [103]. Third, glycosphingolipids seem to support slow neutrophil rolling on endothelial cells mediated by E-selectin and the transition to firm neutrophil adherence [105]. Finally, the neutral sphingomyelinase (N-SMase) is a plasma membrane enzyme that converts sphingomyelin to ceramide; it preferentially distributes towards the leading edge of neutrophils and is important for proper orientation in chemotactic gradients [30,104,106]. Thus, sphingolipids influence neutrophil migration through various molecular mechanisms.
4. Neutrophils Priming during Chemotaxis Modulates Neutrophil Migration
Neutrophil priming means that a primary agonist enhances the response to a second activator/event [107,108]. Priming may be transient or more durable, and the mechanisms mediating the priming effect involve various signaling pathways (e.g., p38, ERK1/2, and PI3K kinase), transcriptional regulators including NFκB, increased antiapoptotic signaling, granule mobilization, and the expression of surface molecules including the αMβ2 integrin and CD66 [109]. The ligand affinity of integrins as well as the cellular shape/deformability and polarization can be altered by priming [109]. Thus, neutrophil priming can modulate molecular mechanisms involved in neutrophil migration.
Neutrophils can be primed before (i.e., in the circulation), during, or following transmigration, and priming mechanisms that facilitate neutrophil migration involve endothelial cells, extravascular stromal cells, extracellular matrix proteins [110,111,112,113], platelets [110,111], and other immunocompetent cells [112]. Furthermore, the priming agents include the soluble mediators C3a and C5a complement components, interferon γ, CXCL8, and tumor necrosis factor (TNF) α together with pathogen-derived agents such as formylated peptides, peptidoglycan, and Staphylococcus aureus toxins [107,108,109,114]. Thus, neutrophils can be primed during chemotaxis, and this priming is at least partly caused by the chemotactic agents. However, there seems to be a possibility for depriming and recovery of primed neutrophils, but this process is less well characterized [109].
5. The Coagulation System: Regulation of Neutrophil Migration by Protease Receptors
Both activation of the coagulation system and thrombocytopenia occurs in a large number of sepsis patients [21,115,116,117]. Coagulation factors contribute to the regulation of inflammation through effects on protease-activated receptors 1-4 (PAR1-4) that can be activated by various proteases. Thrombin can activate PAR1/3/4, Granzyme A activates PAR1/2, cathepsin G activates PAR1/4, and matrix metalloprotease 1 (MMP1) activates PAR1 [118]. Several other proteases that are activated during coagulation and/or inflammation can also activate various PARs (e.g., plasmin, FVIIa, FXa, and C4a) [118]. The PARs are G-protein-coupled membrane receptors that are expressed by a wide range of cells involved in the regulation of inflammation and neutrophil migration, e.g., endothelial cells, platelets, and various leukocytes [119,120].
PARs are activated when thrombin binds to the receptor and cleaves the N-terminal domain to unmask a new N-terminus that acts as a ligand through its binding to the receptor body [120]. PAR2 agonists can then alter neutrophil expression of certain adhesion molecules (e.g., decreased L-selectin and increased α4β1 integrin), but at the same time these agonists also reduce the transendothelial migration of neutrophils [121]. This apparent discrepancy may be caused by indirect PAR effects, e.g., mediated by endothelial cells or platelets [121]. Furthermore, PAR2 agonists can also increase the neutrophil release of proinflammatory IL1β, CXCL8, and IL6 [122]. Finally, in vivo animal studies suggest that both PAR1 and PAR4 are involved in the regulation of neutrophil migration by increasing neutrophil rolling, migration, and chemotaxis [123,124].
6. Cytokine Release by Initially Recruited Neutrophils Increases the Further Recruitment of Neutrophils Together with a Wide Range of Immunocompetent Cells
Neutrophils can release a wide range of soluble mediators (Table 1) [125,126,127,128,129,130,131,132,133,134,135], including CXCL1/2/5/8 that bind to CXCR1 and/or CXCR2 on neutrophils; these cytokines are released through various mechanisms including exosomal release (see Section 9.9). The initial neutrophil recruitment in inflammation thereby leads to a further enhancement of the neutrophil infiltration. The other chemokines are chemoattractants for monocytes (CCL2-4), NK cells (CCL2-4 and CXCL10), mature (CCL19) and immature dendritic cells (CCL2/4/18/20), and a wide range of T cell subsets including naïve T cells (CCL18), CD8+ T cells (CXCL12), regulatory T cells (CCL17), Th1 cells (CXCL9-11), and Th17 cells (CCL2 and CCL20). Several neutrophil-secreted cytokines are additionally important for endothelial cell functions, angioregulation, or angiogenesis [125,126,127]. Thus, the neutrophils seem to amplify the transmigration and extravascular migration of additional neutrophils as well as a wide range of other immunocompetent cells through their cytokine release [136]. However, neutrophils also communicate and interact with their neighboring cells through several additional mechanisms, including (i) the release of extracellular vesicles/exosomes that contain various intracellular proteins and cytokines, (ii) binding to cell surface adhesion molecules and (iii) through their cytoneme, i.e., projections specialized for the exchange of mediators between cells [137].

Table 1.
Soluble mediators released by neutrophils [125,126,127,128,129,130,131,132,133,134,135]. For the TNF superfamily and angioregulatory/fibroid and other cytokines groups we have indicated important functions in parentheses. Mediators studied mainly in animal models are marked *, and mediators where controversial data exist are marked with (?). Abbreviations: IFN, interferon, and TGF, transforming growth factor.
7. Structural Lipids and Neutrophil Migration: The Membrane Structure, Lipid Interactions with Membrane Proteins, and the Function of Lipid Rafts
The plasma membrane is a dynamic structure assembled by lipids and proteins (see Section 2.3) [138]. The lipid composition varies between different regions of the membrane, e.g., the plasma membrane cytoplasmic leaflet usually contains more phosphatidylethanolamines and phosphatidylserines compared with the outer leaflet that is rich in sphingolipids [139]. Cholesterol deposition may also be asymmetrical, and sterols (i.e., a steroid subset including cholesterol) seem to increase membrane stiffness [140,141].
Phospholipids are important for the anchoring, orientation, and signaling of plasma membrane proteins [142], whereas gangliosides (i.e., glycosphingolipids linked to sugar chains) have effects on β1 integrins and cytoskeletal proteins that are involved in cell migration [142]. Furthermore, the modulation of fatty acid saturation is important to control the stiffness and elasticity of the cell membrane and for regulation of receptor-initiated signaling [143,144]. These lipid-associated modulations of cell signaling can be seen with relatively small changes in membrane lipid composition [145]. Finally, both the proportion of polyunsaturated fatty acids and the cholesterol level of the plasma membrane can be influenced by dietary interventions [146,147]. Although these studies were based on experimental in vitro models or animal models, the observations are possibly also relevant for neutrophil G-protein-coupled receptors.
Experimental models suggest that electrostatic interactions between the N-terminal parts of the CXCR1 receptor and the lipid parts of the cell membrane are important for the three-dimensional folding of the receptor chain [148,149,150,151]. These interactions increase the affinity of CXCR1 for CXCL8 [148]. Furthermore, after CXCR1 ligation by CXCL8, the receptor relocalizes to lipid rafts that facilitate its G-protein-mediated signaling [152]. The leukotriene B4 receptor BLT1 is also localized to lipid rafts in neutrophils, and cholesterol deprivation disrupts the lipid rafts and thereby reduces downstream BLT1 signaling [153,154]. In contrast, the FPR-1 receptor is localized outside the lipid rafts and its downstream signaling is therefore not altered by lipid raft disruption [153].
8. Modulation of Neutrophil Migration in Patients with Sepsis: Reverse Migration Due to Inflammation-Induced Modulation of Normal Chemotactic Mechanisms
Most neutrophil studies in sepsis patients (Section 8, Section 9 and Section 10) are based on circulating neutrophils; it is stated in the text when the cells were derived from other compartments.
The local clearance of neutrophils at inflammatory sites is caused by local necrosis or the induction of apoptosis with subsequent phagocytosis by macrophages, but neutrophils can additionally migrate back to the circulation [155]. Animal models have shown that up to 70% of recruited neutrophils can return to the circulation from inflammatory sites [156,157]. Reverse migrated neutrophils are characterized by a ICAM-1highCXCR1low phenotype, and this is different from the majority of circulating ICAM-1lowCXCR1high and tissue-resident ICAM-1highCXCR1high neutrophils [158,159]. The multistep process of reversed neutrophil migration is regarded as a programmed process characterized by defined molecular events [158,159,160,161,162,163,164,165,166,167,168,169,170]:
- Modulation of chemotactic responses. Neutrophils from sepsis patients show attenuated chemotaxis towards formylated peptides, leukotriene B4, and CXCL8 [170]. Furthermore, neutrophil C5aR expression reaches a peak early during sepsis and thereafter gradually declines [170];
- Altered microvascular permeability modulates chemokine gradients. The endothelial junctions are altered during inflammation, the microvascular permeability is thereby increased and the leakage of chemokines (e.g., CXCL1) from the circulation to the perivascular environment alters the chemokine gradients and contributes to reversed neutrophil migration [160,161,162]. Animal models suggest that CXCL8 and CXCR2 are also important for reversed neutrophil migration [164]; CXCL8 is a chemoattractant at lower concentrations but it can function as a chemorepellent at higher levels and thereby facilitate reversed migration [165];
- A possible role of altered receptor trafficking. Chemokine receptors can be inactivated by internalization and degradation, and end-target chemoattractants use this mechanism to desensitize neutrophils to intermediary chemoattractants [168]. Receptor levels can be quickly altered by the modulation of receptor internalization/degradation compared with mechanisms that require altered gene transcription. Animal models suggest that altered CXCR1/CXCR2 balance due to continued CXCR2 signaling together with reduced CXCR1 levels/signaling facilitates reverse neutrophil migration [153,158,159,168];
- CXCR4/CXCL12 signaling. Disruption of CXCR4/CXCL12 signaling would facilitate reversed neutrophil migration, but animal models suggest that CXCR4 is upregulated when reversed migrated neutrophils migrate to new peripheral organs [166]. These neutrophils show increased expression of the early apoptotic marker Annexin V, and the CXCR4 upregulation may facilitate their later migration back to the bone marrow [166]. Thus, at least some reverse migrated neutrophils possibly migrate via other peripheral organs to the bone marrow where they undergo apoptosis;
- Local release of neutrophil elastase alters endothelial cell junctions. Animal studies suggest that neutrophils can exhibit extravascular-to-luminal migration through endothelial cell junctions, and this process depends on the reduced expression and/or function of the endothelial junctional adhesion molecule-C (JAM-C) [167]. Neutrophil elastase cleaves JAM-C and thereby promotes neutrophil-reversed migration [167]. Furthermore, the local release of neutrophil elastase is promoted by leukotriene B4; this chemotactic signal thereby seems to promote reverse transendothelial migration of neutrophils during the late steps of inflammation [167]. Finally, neutrophil elastase seems to be presented to JAM-C by the neutrophil αMβ2 integrins (CD11b/CD18), but ICAM-1 (expressed at high levels by reversed migrating neutrophils) also seems to be important for the cleavage of JAM-C [167];
- Arachidonic acid metabolism. Leukotriene B4 is an early proinflammatory derivative of arachidonic acid that seems to facilitate the later resolution of inflammation through the release of neutrophil elastase (see above). Furthermore, neutrophils seem to shift to the production of the alternative anti-inflammatory arachidonic acid derivative Lipoxin A4 when they are localized in an inflammatory environment [168], and Lipoxin A4 enhances reversed migration [169];
- Proteases. The levels of proteases other than neutrophil elastase may also be involved in the regulation of reversed migration, e.g., Cathepsin C reduction reduces reversed neutrophil migration [166];
- Hypoxia-inducible factor 1 α (HIF-1α). The transcription factor HIF-1α is expressed by activated neutrophils; it is targeted for degradation by oxygen-dependent propyl oxygenase but this enzyme is suppressed in response to bacterial infection and HIF-1α will therefore accumulate [168]. Activated HIF-1α promotes neutrophil survival and reduces reversed migration [166,168], but it is not known whether or how HIF-1α is involved in the regulation of reverse neutrophil migration;
- Macrophages. Concomitant macrophage infiltration can also facilitate reversed neutrophil migration [155].
To conclude, the molecular mechanisms responsible for reversed neutrophil migration are complex and involve the modulation of several mechanisms that are important for the early steps of proinflammatory extravasation/tissue migration, i.e., arachidonic acid metabolism, CXCR1/2 signaling, neutrophil/endothelial adhesion, and microvessel permeability. Whether modulation of neutrophil retention signaling (e.g., CXCR4/CXCL12, HIF-1α, and semaphorins) also contributes needs to be further investigated [165,166,168,170].
9. Modulation of Neutrophil Migration and Communication in Patients with Sepsis; Several Cellular Mechanisms Are Altered by the Infection
9.1. Increased Bone Marrow Release of Neutrophils: Effects of Sepsis on G-CSF and CXCL12
Inflammation is often associated with increased levels of circulating neutrophils due to increased bone marrow release [171,172]. The growth factor G-CSF can be released by neutrophils together with a wide range of proinflammatory cytokines (Table 1); it is upregulated in sepsis as a part of the acute phase reaction and promotes the generation and release of mature and immature neutrophils [173,174]. Furthermore, under homeostatic conditions, CXCL12 is highly expressed in bone marrow stromal cells, and CXCL12/CXCR4 serves as a main mechanism for bone marrow retention of neutrophils [175]. Infectious stress causes rapid downregulation of CXCL12 mRNA and protein levels in the bone marrow, and neutrophil release is thereby facilitated [171,172].
9.2. Effects of Sepsis on Neutrophil Rigidity and F-Actin Accumulation: Increased Rigidity as a Possible Mechanism That Increases the Risk of Organ Failure
Proinflammatory mediators as well as bacterial products cause increased neutrophil rigidity and priming of the neutrophils at the same time [176,177]. The increased rigidity is associated with F-actin accumulation; it can be induced by proinflammatory TNFα and this TNFα effect seems to be mediated by the transcription factor PPARγ [90,178]. There is also reduced endothelial rolling of neutrophils during sepsis, and increased rigidity/priming combined with decreased rolling probably leads to neutrophil sequestering in the microcirculation as a part of the pathogenesis of organ dysfunction/failure [177,179].
9.3. Fatty Acids, Cell Membrane Structure, and Neutrophil Migration in Sepsis: Dietary Polyunsaturated Fatty Acids Decrease the Mortality in Experimental Sepsis
Recent studies investigated the effect of dietary polyunsaturated fatty acids in a murine model of Staphylococcus aureus sepsis [180,181,182,183]. Increased dietary polyunsaturated fatty acids then increased survival, decreased the bacterial load, and increased neutrophil infiltration at the inflammatory site. The cell membrane should be regarded as a dynamic regulator of neutrophil migration [144,145,146,184,185]. A possible explanation for this effect of dietary polyunsaturated fatty acids could therefore be modulation of neutrophil membranes with altered chemotactic signaling, increased neutrophil migration/infiltration, and thereby decreased mortality in sepsis. Alternatively, dietary modulation may alter the membrane stiffness and thus have a general effect on neutrophil migration that reduces the risk of multiorgan failure.
9.4. Effects of Sepsis on Neutrophil–Endothelial Interactions: The Importance of Adhesion Molecules, Integrins, and Heparinase
Studies in animal models have demonstrated that endothelial ICAM-1 expression is upregulated during sepsis, and there is also a transient increase in VCAM-1 [186,187,188,189,190]. Sepsis is also associated with increased degradation of the glycocalyx, and a TNFα-induced increase in endothelial expression of heparinase is important for this degradation [191]. A possible effect of this degradation is exposure of adhesion molecules on the endothelial cells leading to increased neutrophil adhesion.
Neutrophils upregulate β1 and β2 integrins in response to proinflammatory mediators; these adhesion molecules interact with endothelial ICAM-1/VCAM-1 and studies in animal models suggest that this upregulation enables firm adhesion to endothelial cells and thereby inhibition of the later extravascular neutrophil migration [95,96,192]. Animal models suggest that the combined upregulation of integrins in circulating neutrophils and endothelial adhesion molecule is seen especially for gram-positive bacteria, whereas for other bacteria the integrin upregulation is seen only for interstitial neutrophils [193]. Animal models also suggest that circulating neutrophils upregulate β1 integrins during sepsis, and this upregulation may influence neutrophil crawling and adhesion to endothelial cells [194,195].
Proteases can modulate endothelial cell regulation of inflammation through the ligation of protease-activated receptors (see Section 5), but further studies are needed to clarify whether these ligand/receptor interactions are involved in the pathogenesis of sepsis-associated organ failure.
9.5. Selectins and Selectin Receptors in Sepsis
Neutrophil L-selectin and its ligands (CD44, PSGL1 and ESEL-1) are important for neutrophil adhesion to endothelial cells [196,197], but the possible modulation of selectins/selectin ligands during sepsis has not been characterized in detail [197].
9.6. Soluble Adhesion Molecules as Modulators of Endothelial–Neutrophil Interactions in Sepsis?
Several adhesion molecules exist in biologically active soluble forms, and this is true both for selectins and ICAM-1 [198]. Membrane-bound forms of these adhesion molecules are expressed by endothelial cells and/or neutrophils and are important for extravasation and migration (see Section 2.2). The systemic levels of these soluble adhesion molecules can be altered in patients with bacterial infections/sepsis [198,199]. The serum levels of L-, P-, and E-selectins as well as ICAM-1 are then increased in immunocompetent patients. In contrast, patients with chemotherapy-induced neutropenia show only increased serum levels of L-selectin and ICAM-1 whereas P- and E-selectin levels are decreased during bacterial infections [198,199]. The possible influence of these infection-induced alterations on in vivo neutrophil migration has not been investigated, but the biologically active soluble forms will possibly compete with the corresponding membrane-expressed forms and thereby influence neutrophil migration [198].
Several other cell membrane molecules can also be released by neutrophils during sepsis [200]. First, CD14 is a part of the TLR4 receptor complex, and it is upregulated and released from the lipopolysaccharide-binding protein in sepsis. Second, the triggering receptor expressed on the myeloid cells 1 (TREM1) is also upregulated and released by neutrophils after exposure to bacteria. Third, azurocidin is released when neutrophils bind to selectins, and this protein can alter the endothelial cytoskeleton and thereby enhance neutrophil migration through the endothelial layer. However, additional studies are needed to clarify whether these soluble forms will influence neutrophil migration and/or can be used as a biomarker of sepsis in routine clinical practice.
The serum levels of soluble VCAM-1 and ICAM-1 correlate with organ dysfunction in sepsis [186,201,202,203], and these clinical observations support the hypothesis that soluble adhesion molecules are involved in the pathogenesis of sepsis.
9.7. Multifactorial Modulation of Neutrophil Chemotaxis in Sepsis
The regulation of neutrophil chemotaxis shows complex alteration in patients with sepsis [204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251], and Table 2 summarizes how various neutrophil receptors/mediators are involved in the sepsis-associated modulation of neutrophils.

Table 2.
The modulation of neutrophil receptors during sepsis; a summary of important effects on neutrophil migration.
General inhibition of neutrophil chemotaxis and associations with prognosis. Studies in animal models have described inadequate neutrophil migration towards infection foci during severe sepsis even though high chemokine levels are seen at the infection sites [74,204]. These abnormalities are probably caused by reduced responsiveness for all four main chemotactic mechanisms (see Figure 1). The migratory responses to formylated peptides, LTB4 and CXCL8 are reduced, and neutrophil C5aR expression reaches an early peak during sepsis before a gradual decline [154]. Furthermore, patients with the largest reduction of neutrophil migration towards formylated peptides or LTB4 have decreased survival [205]. Decreased neutrophil migration towards CXCL8 is seen especially in patients with severe sepsis; this chemotactic CXCL8 effect seems to be a part of a more complex dysregulated phenotype also including defects in NETosis and delayed apoptosis [206]. Finally, animal studies suggest that C5aR dysfunction alters neutrophil migration and thereby contributes to the development of organ failure [207,208,209].
The role of G-protein-coupled receptor (GPCR) kinases. Repeated exposure to agonists will lead to the internalization of many G-protein-coupled receptors, and this process seems to depend on G-protein-coupled receptor kinases (GPCR kinases) [210]. These kinases phosphorylate intracellular parts of activated receptors and facilitate the recruitment of arrestins that decouple the receptor from the G-protein and thereby lead to receptor internalization [211]:
- TLR2/4/9 ligation induces the upregulation of GPKR kinase 2 and thereby internalization of CXCR2 [74,79,93,212,213]. In contrast, IL33 prevents TLR-induced upregulation of GPCR kinase 2 and can thereby stimulate neutrophil chemotaxis [93,214];
- The TLR induction of CXCR2 internalization seems to depend on TNFα [235,236,237,238,239,240,241]. This TNFα effect is possibly mediated by an autocrine loop because TNFα can be released by neutrophils (Table 1), but TNFα release by neighboring immunocompetent cells (see Section 6) may also contribute to local effects and to the increased systemic TNFα levels in sepsis patients [215];
- Both TLR and TNF-dependent pathways upregulate NO synthase that in turn induces GPCR kinase 2 and finally reduces neutrophil expression of CXCR2 [216]. Taken together these observations suggest a signaling cascade involving TLR→TNFα→NO synthase→GPCR kinase 2→CXCR2 downregulation;
- Finally, PI3Kγ-initiated signaling can also cause GPCR kinase induction and thereby reduced CXCR2 expression [217,218].
Thus, there seems to be three different intracellular signaling events/pathways (i.e., TLR, TNFα, and PI3Kγ) that contribute to the internalization and thereby the reduction of CXCR2 cell surface expression in neutrophils in sepsis.
CXCR1 versus CXCR2 expression. As explained above, CXCR2 is internalized in circulating neutrophils during sepsis [219,220,221]. Decreased neutrophil CXCR2 expression in sepsis patients correlates with the Apache II score, a parameter for disease severity [219]. In contrast, neutrophil CXCR1 levels are not altered in sepsis patients [219].
The role of the CCR2 chemokine receptor. CCR2 is induced on the neutrophil surface during sepsis in a TLR2/4 dependent manner; this promiscuous chemokine receptor is probably not involved in neutrophil recruitment to the infectious site [223], but it seems to be involved in neutrophil recruitment to vital organs and the pathogenesis of organ failure [223,224]. Finally, non-surviving septic shock patients have higher neutrophil CCR2 expression than survivors [224].
The role of Toll-like receptors. Neutrophil expression of TLR2 and TLR4 seems to be downregulated in patients with septic shock, and gene expression studies show an additional downregulation of several inflammatory response gene clusters, including TLR-dependent genes [225,226]. However, despite this TLR downregulation, these receptors mediate important inhibitory effects on CXCR3 expression (see above).
Platelet-activating factor (PAF). The PAF receptor activates MAP kinases and induces the production of proinflammatory cytokines including CXCL8 [227,228]. However, despite the increased cytokine releases the final effect of receptor ligation is reduced neutrophil migration, and receptor blocking increases survival in animal sepsis models [227,228].
Hydrogensulfide-synthesis and its influence on endothelial adhesion. The activity of the hydrogen sulfide-synthesizing enzyme cystathionine g-lyase is increased in sepsis; its increased activity seems to be associated with increased neutrophil adhesion to endothelium, increased migration/chemotaxis, and maintained/increased CXCR2 expression in murine sepsis [229,230].
The possible importance of LOX-1, IL17, and Peroxisome proliferator-activated receptor γ (PPARγ). Both LOX-1 and the acute phase protein α-1 acid protein contribute to the failure of neutrophil migration [89,231,232]; this is possibly mediated by the LOX-1 receptor recognition of several inflammatory products including CRP, apoptotic cells, various bacterial products, and activated platelets [89,233]. Finally, IL17 seems to facilitate the recruitment of neutrophils to the infection site [234], whereas the results from the studies of PPAR effects on neutrophil migration in sepsis are conflicting [204].
9.8. Reversed Neutrophil Migration in Patients with Sepsis: Is It a Sign of Immunological Imbalance and a Mechanism in the Pathogenesis of Sepsis-Associated Organ Dysfunction/Failure?
It is well-accepted that immunological dysregulation is essential in the development and progression of sepsis [204]. Neutrophils then have effector mechanisms that are essential for the removal of the infecting agent, including degranulation, phagocytosis, and the formation of extracellular traps, but they are also important for local immunoregulation at the infectious site and in the pathogenesis of distant organ dysfunctions [204].
Neutrophils are among the first immunocompetent cells that are recruited to an infectious site; they become important through several mechanisms that amplify inflammation during the progression of sepsis. Reversely migrating neutrophils still seem to have a proinflammatory phenotype characterized by their increased capacity of superoxide release, high ICAM-1 membrane expression, likely increased levels of inducible nitric oxide synthase, increased capacity of extracellular trap formation, and increased lifespan due to antiapoptotic signaling [252,253,254,255,256]. Experimental studies have also shown an association between the level of reverse-migrated neutrophils in the circulation and organ failure [253,257,258]. Taken together these observations are consistent with the hypothesis that reversed migration contributes to the systemic dissemination of the inflammatory response and to organ failure distant to the infection site. Experimental animal models of sepsis also suggest that LTB4 promotes reverse neutrophil migration in sepsis and thereby contributes to the pathogenesis of organ failure [257,258]. Thus, even though available data on reversed neutrophil migration in human sepsis are limited, experimental evidence suggests that reversed migration is involved in the pathogenesis of sepsis-associated organ failure.
9.9. Exosomes in Sepsis: A Possible Mechanism for Neutrophil Communication with Neighboring Cells and for Their Influence on Distant Organs
Exosomes are extracellular 40–150 nm endosome-derived vesicles; they carry proteins and nucleotides/noncoding RNA as well as metabolites/lipids that can be transferred between cells [259]. Animal models have shown that neutrophil-derived exosomes are increased in the blood during sepsis, and these exosomes carry high levels of proinflammatory mediators, including cytokines and DAMP molecules. The exosomes probably represent an important form of communication between neutrophils and neighboring as well as distant cells/organs, and they possibly have a role in the development of distant organ dysfunctions. Studies in hepatocytes have shown that CXCR1 and CXCR2 regulate exosome release [260], but it is not known whether similar effects are seen in neutrophils during chemotaxis. The effect of microparticles on neutrophil migration depends on the cell surface molecules of the particles. Some microparticles seem to enhance neutrophil chemotaxis to CXCL8, and this is mediated by L-selectin and P-selectin glycoprotein ligand-1 present on the surface of these particles [261]. In contrast, particles may also mediate anti-inflammatory effects and inhibit neutrophil chemotaxis; this effect is associated with the surface expression of annexin-1 [262].
10. Several Molecular Markers of Neutrophil Heterogeneity Are Involved in the Regulation of Neutrophil Migration: The Possible Importance of Various Subsets in Sepsis
The modulation of neutrophil migration occurs in a complex context of a more general sepsis-induced immunosuppression [263,264]. The complexity is further increased by the identification of several neutrophil subsets that seem to differ with regard to regulation of migration [265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306].
10.1. Neutrophil Heterogeneity
The circulating neutrophils were previously regarded as fully differentiated phagocytic defender cells, but they are now regarded as a heterogeneous population including different effector and immunoregulatory subsets [270,284]. Several of the molecular markers used to identify various neutrophil subsets are important regulators of fundamental neutrophil functions, including neutrophil migration (Table 3). The following neutrophil subsets were identified:

Table 3.
Individual molecular markers used to characterize neutrophil subsets and their involvement in neutrophil migration.
- Mature or classic neutrophils have the phenotype CD16+ (Fc receptor), CD177+ (cell surface glycoprotein/CD32 binding), CTL-2+ (von Willebrand’s factor receptor), CD11b/CD18+, and CD11a/CD18+ (two integrins) [281]. An alternative strategy to identify neutrophil subsets is based on differences in cell density [284,285,286], and the low-density cells can then be further subclassified based on their expression of CD11b, CD16, and CD86 [284];
- Animal studies suggest that aged neutrophils have a different phenotype [287], but the survival time seems to differ between neutrophil subsets and cells with the HLA-DR+CD49+CD80+ phenotype have an extended survival of up to 72 h [288];
- CD177 is a cell surface glycoprotein; most humans have both CD177negative and CD177positive neutrophils [289]. One study investigated 535 healthy individuals: 65% of them had >60% CD177+ circulating neutrophils, 25% had intermediate levels (20–60% CD177+ neutrophils), and a small minority of 14 patients had only CD177- neutrophils. Proteinase 3 is a neutrophil intracellular protease; the fraction of circulating protease 3 positive neutrophils in healthy individuals varies between 0 and 100% [282] and there is a cellular covariation between CD177 and protease 3 [283];
- The minority of mature CD66b+CD10+ neutrophils differ in their regulation of T cell activation compared with CD66b+CD10- neutrophils [281,290], a subset of neutrophils seem to have a specialized B cell helper function [291], and neutrophil subsets differ even in their capacity to support cancer cell proliferation [284].
Thus, several neutrophil subsets were identified, and animal studies suggest that the balance between neutrophil subsets can be important for the capacity to resist certain bacterial infections [281,292]. Furthermore, studies in human inflammatory diseases suggest that the neutrophil heterogeneity is even more extensive, including CD63+ or PD-L1+ subsets identified in patients with cystic fibrosis [293,294], CD64+ neutrophils as a marker of sepsis (see below) [270], subsets expressing the receptor activator of nuclear factor κB ligand (RANKL) identified in chronic obstructive pulmonary disease [295], CD49d+ neutrophils detected in the nasal fluid during viral infections [296], and HLA-DR+ subsets in patients with Leishmaniosis [297]. It is difficult to judge whether these subsets represent true differentiation or whether they are markers of activation, and it is not known whether these subsets can be detected in patients with sepsis.
10.2. Neutrophil Expression of the Fcγ Receptor CD64 in Patients with Sepsis: Increased Levels That Associate with Prognosis
The CD64 FcγR1 is expressed only at low levels on resting neutrophils; this expression is increased during sepsis, and the level of circulating CD64+ neutrophils is regarded as a possible diagnostic or prognostic biomarker in sepsis [272,273]. CD64 expression is increased early during sepsis, and the levels are generally higher for patients with septic shock. Furthermore, the CD64 level correlates with severity scores such as APACHE II and total SOFA scores and predicts survival/mortality for intensive-care sepsis patients. A recent study of sepsis patients showed that immature CD64+ neutrophils could be further subclassified; two new neutrophil subsets were then identified based on the expression of CD123 (the IL3 receptor) and PD-L1 [298]. Thus, CD64+ neutrophils are heterogeneous, but it is not known whether the diagnostic/prognostic impact of circulating CD64+ neutrophil levels is caused by all or only a subset of the CD64+ neutrophils.
10.3. Neutrophils from Patients with Sepsis Show Increased CD177 (a Cell-Surface Glycoprotein) and Decreased CD10 Expression, and the Levels of the Two Markers Show Inverse Correlation
A study of neutrophils derived from 45 patients with septic shock identified 364 upregulated and 328 downregulated genes. CD177 mRNA had the highest fold change alteration when patients were compared with healthy individuals, and this difference was also confirmed at the protein level [299]. However, the sepsis patients were also characterized by a constant CD177 negative neutrophil subset, CD177 was coexpressed with protease 3, and circulating neutrophils in addition showed decreased CD10 levels with a significant inverse correlation between CD177 and CD10 protein levels. The CD10 metalloprotease is a negative regulator of chemotaxis [279], and the increased CD177/decreased CD10 combination will thus facilitate the extravasation/migration of this subset (Table 4).
10.4. The Fraction of Olfactomedin 4 (OLFM4) Positive Neutrophils Is Increased in Patients with Sepsis, and High Levels Are Associated with Adverse Prognosis Even in Adjusted Analyses
OLFM4 is a neutrophil-specific granule protein secreted through exosomes [278]. The percentage of OLFM4-expressing neutrophils in healthy individuals varies between 8 and 57% (Table 4), and the levels of positive cells increase in sepsis. However, the levels can also be increased especially in other bacterial infections, but also in viral and parasitic infections, sterile inflammatory disorders, and in cancer patients [274,278,300].
A recent study described the increased mortality of sepsis patients with high levels of circulating OLFM4+ neutrophils and the same was true for non-septic patients with infections and Systemic Inflammatory Response Syndrome (SIRS). In total, 56% of sepsis patients with elevated OLFM4+ neutrophils died compared to 18% of patients with OLFM4+ neutrophils below 37.6% [301]. These associations between the OLFM4+ neutrophil subset and mortality were significant when adjusting for age, sex, absolute neutrophil count, comorbidities, and the total SOFA score. Furthermore, similar observations were made in a prospective pediatric study including patients with septic shock; patients with at least two organ failures at day 7 of septic shock or dying before day 28 had a higher percentage of OLFM4+ neutrophils compared to patients with less severe disease [302]. These associations were also significant after adjusted analyses. Thus, high levels in the circulation of the OLFM4+ neutrophil subset are associated with severe/multiple organ failures, and this cell subset may therefore have a role in the pathogenesis of organ failure [302,303,304].
OLFM4 can be released by neutrophils, and the serum level of OLFM4 is increased in sepsis patients, especially patients with severe disease [303]. Finally, OLFM4 inhibits cathepsin C which activates cathepsin G [305], and cathepsin G is a regulator of neutrophil chemotaxis [195,276,277,278,306]. Thus, the modulation of neutrophil migration may contribute to the adverse prognostic impact of the ILFM4+ neutrophil subset and/or soluble OLFM4.
10.5. Myeloid-Derived Suppressor Cells in Sepsis; Further Neutrophil Development to an Immunosuppressive Neutrophil Subset Associated with Adverse Prognosis in Sepsis Patients
The two main myeloid-derived suppressor cell (MDSC) subsets are monocytic and granulocyte (G)-MDSC; the latter has the phenotype CD33+CD11b+CD14+HLA-DRlow [307]. Thus, G-MDSCs represent a subset or a further neutrophil differentiation/development. Several neutrophil-secreted cytokines can function as expanders of MDSCs, and the TLR-target NFκB can function as an MDSC activator [307].
There is an increased expression of the PD-1 ligand by MDSCs in sepsis patients, and ligation of the PD-1 checkpoint on T cells leads to T cell apoptosis that can contribute to the observed lymphopenia during sepsis [307]. Furthermore, high G-MDSC levels are associated with adverse prognosis in sepsis patients, whereas a similar association is not observed for monocytic MDSCs [306,308,309,310]. Furthermore, typical mature G-MDSCs as well as a CD14low mature G-MDSC subset show increased levels especially in gram-positive sepsis [311]. Finally, low CD10 (CD10dim) and/or CD16 (CD16dim) expression by G-MDSC were associated with an adverse prognosis in sepsis [312]. Thus, G-MDSCs subsets are included among neutrophils and increased MDSCs seem to contribute to the immunocompromised state and an adverse prognosis in sepsis.
11. Important Bacterial Products That Interfere with Neutrophil Functions
Sepsis can be caused by a wide range of bacteria; gram-positive infections have increased over time and are now almost as common as gram-negative infections [313,314]. This increase is probably due to the increased use of invasive procedures. Various Staphylococcus species are the most common among gram-positive infections (30–35% of cases), although Enterococcus infections (10%) are also common. A wide range of gram-negative bacteria can cause sepsis (55–60%), whereas fungal infections constitute a smaller subset (15%) with Candida species being the most common. The most common sites of infection are respiratory (40% of cases), genitourinal (15–20%), and abdominal (5–10%), but 6–8% of patients have an unknown site [84,85,314,315,316]. In this section, we will describe how common sepsis-causing bacteria interact with neutrophils and thereby modulate their migration [84,85,214,243,269,317]. Most neutrophil studies referred to in this section and the following Section 12, Section 13, Section 14, Section 15 and Section 16 are based on circulating neutrophils; it is stated in the text when cells were derived from other compartments.
11.1. Modulation of Neutrophil Migration by Staphylococcus aureus
The effects of Staphylococcus aureus on neutrophils, including neutrophil migration, have been extensively studied, and several bacterial molecules can modulate neutrophil migration [107,318]:
- The chemotaxis inhibitory protein of Staphylococcus aureus (CHIP) can bind and thereby block the extracellular parts of the chemotactic C5a and formylated peptide receptors but without initiation of intracellular signaling [319,320,321,322,323,324];
- Agonistic ligation of the neutrophil formyl peptide receptor-like-1 stimulates neutrophil migration [38]. Staphylococcus aureus releases additional proteins that bind to and block this receptor and thereby inhibit neutrophil chemotaxis [317,324,325]. Some of these antagonists also block Fcγ receptors [107,318];
- The cysteine protease Staphopain A cleaves the N-terminal part of the CXCR2 receptor and thereby inhibits its binding of chemokine ligands [326];
- Selectins are important for the initial steps of neutrophil extravasation, and neutrophils express the P-selectin glycoprotein ligand 1 [22,107,318]. Staphylococcus aureus secretes two proteins that bind to this selectin ligand and thereby inhibit neutrophil rolling on endothelial cells [327,328]. One of these proteins can also bind to the N-terminal end of other G-protein-coupled receptors and thereby inhibit their binding with other ligands; this mechanism inhibits effects of complement factors and CXCL chemokines on neutrophil migration [329];
- TLR2 can recognize several staphylococcal lipoproteins that have a TLR2 antagonizing effect [330,331];
- Staphylococcal superantigen-like proteins 1 and 5 are broad protease inhibitors; they inhibit the neutrophil proteases MMP8 and MMP9 and thereby the proteolytic cleavage and potentiation of CXCL8 [332].
These observations clearly illustrate that bacterial molecules can modulate/inhibit neutrophil migration through various molecular mechanisms. These mechanisms may also contribute to the increased levels of certain MDSC subsets especially in gram-positive infections [311].
11.2. Streptococcal Modulation of Neutrophil Migration
Several cell wall components derived from Streptococcus pneumoniae, including lipoteichoic acid and other lipoproteins, are recognized by TLR2, and downregulation of chemokine receptors would therefore be expected [242]. Furthermore, TLR4 can bind the exotoxin pneumolysin, and pneumococcal DNA can bind to TLR9 [242]. One would expect ligation of these TLRs to cause downregulation of chemokine receptors and thereby inhibition of neutrophil migration (see Section 9.7).
11.3. Gram-Negative Bacteria; TLR4 Binding and Binding of Microorganisms to Lipid Rafts
Lipopolysaccharide is a ligand for TLR4 [243,333], and gram-negative bacteria would therefore be expected to inhibit neutrophil migration through TLR4-mediated downregulation of chemokine receptors (see Section 9.7).
Lipid rafts are membrane domains rich in sphingolipids/cholesterol, and they contain glycosylphosphatidylinositol-anchored proteins as well as other signaling molecules also anchored via lipids (see Section 7) [334]. The lipid rafts are important for chemotactic signaling in neutrophils, but certain components can also function as receptors for pathogen-associated molecular patterns [334,335]. This is true for lactocylceramides that are able to bind directly to pathogen components including molecules derived from mycobacteria and candida species, such as Pneumocystis jeroveci beta-glycan and Escherichia coli proteins [335]. Other glycosphingolipids are also able to bind microbiological molecules including Escherichia coli, Pseudomonas aeruginosa, and shigella enterotoxins. Thus, the glycosphingolipids in lipid rafts are able to function as pathogen-associated molecular pattern receptors, and at least some of these (e.g., soluble beta-glucan from Candida albicans) can induce neutrophil migration [334,335].
Caveola are plasma membrane invaginations with a diameter of 60–80 nm; they are a subset of lipid rafts enriched in cholesterol and sphingolipids (see Section 2.3 and Section 7) [34]. Caveolin is a major constituent of the caveola; it exists in the isoforms caveolin-1-3 and is involved in cholesterol transport [336,337]. However, several G-protein-coupled receptors are sequestered within the caveola through interactions with caveolin-1, a protein that is expressed by many different cells including neutrophils [338]. Caveolins can also bind various microorganisms [34]. Furthermore, caveolin-1-deficient mice show increased mortality during infections with Salmonella and Pseudomonas aeruginosa, but at the same time, these mice have increased neutrophil infiltration at the infection site [34]. Thus, caveola are involved both in microbial binding as well as in G-protein-coupled receptor signaling, and experimental models suggest that caveolin is important for neutrophil migration [338]. Future studies have to clarify whether caveola-mediated effects on neutrophil migration are caused by binding to neutrophil caveola or whether indirect effects mediated by binding to endothelial caveola, for example, also contribute.
11.4. Candida Species and Neutrophil Migration
Candida species and especially Candida albicans show several molecular interactions that can modulate neutrophil migration and the proinflammatory neutrophil phenotype. First, various chemotactic formyl peptide receptors bind fungal molecules [339]. Second, the αMβ2 integrin has two distinct ligand-binding sites that cause different cellular responses; one of these sites is highly promiscuous and can bind both endogenous and microbial ligands [340]. Dual ligation of αMβ2 by the extracellular matrix protein fibronectin and candida β-glucan will then enhance neutrophil chemotaxis, and at the same time, there is a shift in fibronectin binding from α5β1 to α3β1 integrins. Thus, these effects of candida on neutrophils are caused by other molecular mechanisms aside from the bacterial effects.
Recent studies have described that the β-1,6-long glucosyl side-chain-branched beta-glucan derived from Candida albicans causes the dose-dependent induction of neutrophil migration by binding to lactosylceramide followed by activation of the Src family kinase/PI-3K/G-protein signaling pathway [341]. Furthermore, resolvins are a class of anti-inflammatory lipids derived from omega-3 polyunsaturated fatty acids, and Candida albicans can synthesize resolvin E1 that reduces neutrophil chemotaxis in response to CXCL8 but enhances the phagocytosis of Candida [342]. Finally, secretory aspartyl proteinases (Saps) of Candida albicans increase in vitro chemotaxis of neutrophils. Sap2 and Sap6 then induce neutrophil migration and the release of chemotactic chemokines [343,344,345,346].
12. Effects of Antimicrobial Drugs on Neutrophil Migration
Several studies have described the effects of antimicrobial agents on neutrophil migration, including agents used in the treatment of sepsis:
- The penicillins carbenicillin and piperacillin together with the carbapenem thienpenem and three cephalosporins (cefotetan, ceftazidime, and moxalactam) had no effect on the migration of neutrophils against Staphylococcus aureus. In contrast, the third-generation cephalosporin cefoperazone inhibited neutrophil migration [347];
- Fusidic acid, rifampicin, and doxycycline can also decrease neutrophil chemotaxis, whereas no inhibition of neutrophil chemotaxis could be detected for penicillins, cephalosporins, nalidixic acid, sulfamethoxazole, or trimethoprim [348];
- Another study showed that tetracycline, minocycline, and erythromycin could inhibit neutrophil chemotaxis [349];
- In total, 14 cephalosporins, 11 penicillins, and 1 monobactam were evaluated for their in vitro modulation of murine neutrophil migration [350]. The beta-lactam antibiotics could be classified into distinct groups based on their effects on formylated peptide-directed migration: (i) cephalosporin C and cephaloridine had no effect; (ii) decreased migration was observed for cloxacillin, cefotaxime, ceftazadime, cefuroxime, cephalothin, cephapirin, cephadine, cefoperazone, cefoxitin, ceftriaxone, cefadroxil, cefazolin, penicillin G, methicillin, 6-amino-penicillanic acid, nafcillin, piperacillin, ticarcillin, ampicillin, oxacillin, and aztreonam; and (iii) increased migration was observed for cefsulodin [350];
- Amoxicillin can increase the neutrophil expression of certain adhesion molecules [351]. However, another study described increased neutrophil migration through endothelial cell monolayers by co-amoxiclav, and this was probably due to effects on both cell types [352];
- The triazoles itraconazole and fluconazole differ in their effect on chemotaxis; only intraconazole inhibits neutrophil chemotaxis [353,354];
- With regard to formylated peptide-initiated chemotaxis, amphotericin B mediated inhibition whereas ketoconazole caused enhancement and 5-flucytosine, fluconazole, and ciloflucin had no effects [355];
- Fluconazole (TLR9), voriconazole (TLR2/4/9), liposomal amphotericin B (TLR4), and caspofungin (TLR2/4/9, dectin-1) increased the expression of various pattern-recognizing neutrophil receptors [356].
We emphasize that there are relatively few studies, hence the effects have mainly been observed in experimental models, the molecular mechanisms have not been characterized, and the possible importance of these effects in sepsis has not been investigated.
13. Neutrophil Migration in Certain Groups of Patients with an Increased Risk of Infection
13.1. Elderly Patients
Neutrophils from elderly individuals were investigated in several studies (Table 4) [357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377]. Functional studies have confirmed that both endothelial adhesion and chemotaxis are altered in the elderly [357,358,359,368,369,370,371]. Elderly individuals show preserved chemokinesis (i.e., random migration) whereas chemotaxis is reduced, and this chemotactic defect can be detected from the sixth decade of life [359]. A possible overall effect is therefore less accurate neutrophil migration towards inflammatory sites [359,369,371]. Increased basal PI3K activity seems to be at least partly responsible for the reduced chemotaxis, and inhibition of PI3Kγ/PI3Kδ was suggested as a possible strategy to restore neutrophil migration [359].

Table 4.
Important aging-associated direct and indirect effects on neutrophil migration.
Table 4.
Important aging-associated direct and indirect effects on neutrophil migration.
| Modulation of cell surface molecules, release of soluble mediators |
| CD11a and CD11b integrin expression are not altered in elderly, but CD16 expression is significantly reduced [357,358]. Plasma levels of neutrophil elastase are increased, suggesting a preactivated basal state of neutrophils [359] |
| Altered effects of receptor ligation or altered downstream signaling |
| Fc receptors and formylated peptide receptors show altered downstream signaling through MAP kinases, the Janus kinase (JAK)/Signal transducer and activation of transcription (STAT) and PI3K-Akt pathways [360,361]. These effects are due to altered membrane structure with altered receptor recruitment (including TLRs) to lipid rafts [362,363,364]. The altered signaling possibly influences cytokine/chemokine expression/release [366]. Neutrophils show increased basal PI3K signaling [359] but decreased TLR signaling [365]. |
| Functional effects |
| Neutrophils from elderly do not have general defects in endothelial adherence or transendothelial migration [367,368]. Neutrophil chemotaxis in the elderly is impaired due to increased PI3K signaling [359,369]. This impairment is worsened in patients with pneumonia and the worsening is associated with disease severity [359]. Chemotaxis is decreased by surgery; patients recover partly within six weeks and completely within six months [370]. Chemokinesis (random movements) is increased in the elderly whereas chemotaxis is impaired; the final effect is possibly inaccurate neutrophil migration in the direction of infections [359,369,371]. Murine models of aging have shown high reversed transendothelial migration by neutrophils in inflamed tissues; this is due to the desensitization of CXCR1 by CXCL1 releasing cells localized at endothelial cell junctions [372]. These neutrophils then re-enter the circulation and disseminate into the lungs where they cause organ failure [372]. |
| In vivo observations |
| Aged individuals show increased neutrophil levels in airways/bronchoalveolar lavage fluid [373,374,375]. Elderly patients with pneumococcal pneumonia show increased pulmonary infiltration of neutrophils [376]. Some animal models show reduced neutrophil numbers at infection sites in aged mice (Staphylococcus aureus and Pseudomonas aeruginosa), even though local chemokine levels are high and circulating neutrophils express high CXCR2 levels [359,371,376]. However, other models of inflammation have given different results [377]. |
Animal studies have shown dysfunctional neutrophil migration to infection sites together with increased reversed migration in aging, and this relocalization contributes to the pathogenesis of organ failure [373,374,375,376,377]. Several molecular mechanisms are involved in this dysfunction, including: (i) reduced integrin expression that influences the endothelial adhesion and transendothelial migration [357,358]; (ii) altered signaling downstream to several receptors, including receptors for chemotactic mediators and signaling initiated by receptors in lipid rafts [359,360,361,362,363,364]; and (iii) altered basal expression/activation of several intracellular pathways [359,365,366,367,368,369,370,371,372,373,374,375,376,377].
13.2. Sepsis, Frailty, and Neutrophils
Frailty should be regarded as a manifestation of unhealthy aging and is defined as an aging-related syndrome of physiological decline with marked vulnerability to adverse outcomes [378]. Frail older individuals often present with an increased burden of symptoms (including weakness/fatigue), medical complexity, and reduced tolerance to medical and surgical interventions. Frailty can be measured based on several factors including comorbidity, use of medication, nutritional status, blood tests, or physical/emotional/cognitive function, and multiple tools for screening and assessment of frailty have been developed (e.g., The Clinical Frailty Scale, CFS) [379,380].
Frail elderly people are vulnerable to adverse health outcomes, and frailty has a prognostic accuracy higher than both SIRS and Quick SOFA (qSOFA) criteria among elderly patients admitted to hospital with suspected infection, implying that frailty as a prognostic factor can be used for risk-stratification [381]. Furthermore, preexisting clinical frailty is associated with worse outcomes, including increased mortality for elderly sepsis patients [382], and frailty together with age and disease severity are identified as predictors of long-term mortality in very old intensive care patients with sepsis [383]. Finally, a longer duration of mechanical ventilation in frail sepsis patients who underwent protocol-based weaning was also observed [384].
Frailty can be associated with systemic signs of inflammation, and aging characterized by additional frailty is associated with abnormalities in neutrophil levels and neutrophil migration [385,386]. Some studies describe that frail elderly people, compared to age- and sex-matched controls, show a stable and low-grade inflammation according to C-reactive protein levels (referred to as inflammageing), and this is associated with an expansion of circulating myeloid cells [387,388]. A positive correlation between the frailty score and neutrophil count was described for frail elderly women, whereas a negative correlation was seen for the frailty score and lymphocyte counts [387]. Other studies have also described associations between white blood cell counts and frailty, particularly neutrophil and monocyte counts [389]. Hence, both total neutrophil counts and neutrophil to lymphocyte ratio are increased in frail elderly people [390].
A previous study described that neutrophils from frail elderly individuals showed increased expression of integrin αM/CD11b and HLA-DR. The frequency of CD11b+ neutrophils correlated with plasma TNFα levels whereas the frequency of HLA-DR+ neutrophils correlated with circulating mitochondrial DNA [391]. Additional experimental studies suggested that these two soluble markers were responsible for the induction of this neutrophil phenotype. Furthermore, the aging-associated reduction in the accuracy of migration is worsened by frailty, and this inaccuracy seems to correlate both with physical and cognitive markers of frailty [392]. Additional regression analysis suggested that the frailty index together with age and Charlson’s comorbidity index were able to predict neutrophil chemotaxis. Finally, the reduced neutrophil chemotaxis showed no association with systemic signs of inflammation (inflammageing), but it could be reversed by selective PI3K inhibitors [392].
Studies of the effects of physical activity/lifestyle on neutrophil migration and levels of circulating neutrophils have given conflicting results [393,394]. Taken together these studies suggest that lifestyle differences do not have any major impact on neutrophil migration and cannot explain the neutrophil dysfunctions in elderly/frail individual.
13.3. Neutrophil Migration in Patients with Myelodysplastic Syndrome
Myelodysplastic syndrome (MDS) is a hematological malignancy characterized by the abnormal differentiation of myeloid cells and usually one or more cytopenias in peripheral blood; the patients have dysplastic neutrophils and an increased risk of severe bacterial infections [395,396]. Various defects in neutrophil migration were described in MDS, especially for patients with advanced disease. First, MDS patients show decreased in vitro neutrophil chemotaxis in response to the formylated peptides CXCL1 and CXCL8 [397]. This reduction is associated with altered signaling through the ERK1/2 and PI-3K-Akt pathways [398]. Second, MDS neutrophils show decreased expression of the αMβ2 integrin that is important for transendothelial migration [399,400]. Third, MDS neutrophils show increased levels of miR-34a and miR-155 that reduce neutrophil migration towards formylated peptides and CXCL8 [401]. Finally, reduced neutrophil migration was also described for the chronic myelomonocytic leukemia variant [402]. Thus, reduced neutrophil migration seems to contribute to the increased risk of severe bacterial infections in these patients.
13.4. Neutrophil Migration after Stem Cell Transplantation
Autologous or allogeneic hematopoietic stem cell transplantation is used in the treatment of several hematological malignancies [403]. The increased risk of severe bacterial infections is associated with the duration and severity of the early posttransplant neutropenia [404]. However, reduced neutrophil chemotaxis [405,406] together with reduced CD16 expression [406] can be observed even after neutrophil reconstitution, whereas β2 integrin expression is normal [406]. Other neutrophil effector functions can also be altered in these patients [407,408,409], although the phagocytic capacity seems to be normal [405,408,409]. Finally, neutrophil functions are further modulated during sepsis in these patients [410]. To conclude, reduced neutrophil migration is observed after stem cell transplantation, but it should be emphasized that the available studies are few and the question of patient heterogeneity was not addressed.
14. Is There an Effect of Gender with Regard to the Severity and Mortality of Sepsis?
Previous studies suggest that sex hormones are important metabolic regulators in patients with sepsis [411], and several studies have even described gender differences with regard to the severity and/or mortality of sepsis patients [412,413,414,415]. Increased male mortality was described for patients with surgical septic shock [416], and male gender was also described as an independent risk factor for developing post-traumatic sepsis [417] as well as other major infections after surgery [418]. The molecular and cellular mechanisms behind this gender difference were not characterized in detail, but sex hormones are also involved in immunoregulation [419,420] and one hypothesis has therefore been that females develop stronger innate and adaptive immune responses than males. This hypothesis is supported by observations suggesting that estrogens, progesterone, testosterone, and other androgens are involved in the regulation of neutrophil activation and migration [419,420,421,422,423,424]. The targeting of sex hormone metabolism has even been suggested as a possible therapeutic strategy in sepsis [413,415].
15. The Metabolic Heterogeneity of Sepsis Patients: A Subset of Patients Has a Systemic Metabolic Profile Similar to SIRS Patients
The systemic metabolic profiles at the time of hospital admission for patients with bacterial infections and either SIRS or sepsis according to the 2016 criteria [1,2] have recently been characterized [425,426]. These two patient groups differed with regard to the amino acid, lysophosphatidylcholine, and sphingolipid metabolism, but a minor subset of exceptional sepsis patients had a metabolomic profile similar to SIRS patients. Sphingolipids (see Section 2.2, Section 3.6, Section 11.3 and Section 11.4), lysophosphatidylcholines (see Section 3.6), and amino acids [427,428,429,430] are important regulators of neutrophil migration, and the metabolic heterogeneity of sepsis patients may therefore reflect heterogeneity with regard to the regulation of neutrophil migration.
16. Therapeutic Targeting of Neutrophils in Sepsis: Tried and Suggested Strategies
Several strategies for the inhibition of molecular mechanisms involved in the regulation of neutrophil migration were tried or considered as possible therapeutic approaches in sepsis. Targeting of neutrophil migration as a possible strategy is supported by the observation that reduced chemotaxis is associated with advanced disease and reduced survival in sepsis patients [205,206,431]. Indeed, decreased neutrophil CXCR2 expression in sepsis correlates with the Apache II severity score [219] and there is an association between high levels of circulating OLFM4+ neutrophils and mortality [301]. The strategies listed below were considered and/or tried in humans.
Targeting of lipid mediators. Targeting of lipids is suggested both by the importance of neutrophil membrane stiffness/lipid profile, neutrophil polarization, lipid rafts, and caveola as well as chemotactic lipid mediators in the regulation of neutrophil migration, and lipids are also important for the endothelial cell stiffness that influence neutrophil transmigration (Section 2.3 and Section 7; [25,26,27,46,47,88,89,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,148,149,150,151,152,153]). Statins were tried in the treatment of sepsis, especially in patients with septic pulmonary infections [369,432,433]. Simvastatin can improve/increase neutrophil chemotaxis in healthy elderly individuals; this effect is also observed in elderly with less severe sepsis but not in patients with more severe disease. Simvastatin may also improve the prognosis in septic elderly patients with pulmonary infection [433], but additional clinical studies are needed to verify this observation. Finally, the modulation of arachidonic acid metabolism by ibuprofen was also tried in a small study of sepsis patients, but neutrophil migration was not examined for these patients [434].
Hematopoietic growth factor therapy. In vivo G-CSF therapy (see Section 2.1 [13,171,172]) does not alter the expression of CD16, CD64, or L-selectin by circulating and bronchoalveolar lavage fluid neutrophils derived from patients with pneumonia [435]. G-CSF therapy of healthy stem cell donors decreases neutrophil chemotaxis and this effect may also contribute to the reduced chemotaxis in sepsis patients even though the G-CSF responsiveness of neutrophils seems to be decreased in sepsis patients compared with healthy individuals [405,436]. Both GM-CSF and the GM-CSF/IL3 fusion protein also decrease neutrophil chemotaxis [437,438]. G-CSF and GM-CSF therapy was tried in sepsis, but a recent met analysis concluded that none of these growth factors should be recommended for the routine treatment of patients with sepsis [439].
Activated protein C treatment. Neutrophils express receptors for this coagulation mediator and recombinant human-activated protein C was tried in the treatment of sepsis (see also Section 5); treatment with this agent decreases chemotaxis for neutrophils derived from the circulation and bronchoalveolar lavage fluid in a human endotoxin-induced pulmonary inflammation model [440].
Polymyxin B hemoperfusion. This procedure can reduce endotoxin levels but did not provide a benefit for a group of highly selected sepsis patients; the procedure reduced neutrophil CD11b expression but did not alter chemotaxis [441].
Inhibition of intracellular signaling. PI3K contributes to organ failure in sepsis, and inhibition of this pathway may be a possible strategy for targeting neutrophils and inhibiting neutrophil migration (Section 13.1) [217,218,317,442]. Furthermore, observations in an animal model of endotoxin-induced inflammation have shown that p38 inhibition suppresses pulmonary neutrophil infiltration [442]. These observations suggest that pathway inhibitors should be further investigated as a possible therapeutic strategy in sepsis.
Checkpoint inhibitors. MDSCs can be increased in sepsis, and these cells show increased expression of the PD-1 ligand (see Section 10.5) [307]. The use of T cell checkpoint inhibitors to restore T cell dysfunctions was investigated in two early clinical studies [443,444]; this strategy may have indirect effects on neutrophil migration and/or alter the effects of local neutrophil-induced recruitment of various T cell subsets to inflammatory sites (Section 6) [125,126,127].
Targeting of single proinflammatory mediators. The targeting of TNFα [215], IL1, IFNγ [125,126,127,136,137], proteases (Section 5 [115,116,117,118,119,120,121,122,123]), and female sex hormones [413,415,445] has been tried and/or suggested by animal studies, but the results so far have not been convincing, even though IL1 inhibition and immunostimulation seemed to improve survival for subsets of sepsis patients in two of these studies [446,447,448,449,450].
None of these strategies should be regarded as an established treatment for the routine handling of patients with sepsis, but several of them show that such strategies have the capacity to alter neutrophil functionand several of them even show the capacity for neutrophil migration. Furthermore, some of these studies suggest that certain strategies are effective only for subsets of patients. In our opinion, future clinical studies of such therapeutic approaches should therefore have a focus on the biological and clinical heterogeneity of the patients.
17. Conclusions
Neutrophil migration is regulated by complex molecular mechanisms involving both endothelial cell adhesion/transmigration and extravascular chemotactic migration. These mechanisms are modulated in sepsis; common observations include reduced neutrophil migration toward infection sites and even reversed migration that possibly contributes to organ failures. However, sepsis patients should be regarded as heterogeneous with regard to neutrophil migration because the migration is possibly also influenced by the infecting agent, antimicrobial treatment, patient age/frailty, and other diseases predisposing to bacterial infections. Future clinical studies should clarify whether the treatment of sepsis patients needs to be individualized based on this heterogeneity.
Author Contributions
Conceptualization, Ø.B. (Øystein Bruserud), Ø.B. (Øyvind Bruserud); Methodology, Ø.B. (Øystein Bruserud); original draft preparation, Ø.B. (Øystein Bruserud), Ø.B. (Øyvind Bruserud), H.R.; writing—review and editing; Ø.B. (Øystein Bruserud), K.A.M.; Ø.B. (Øyvind Bruserud), H.R., Ø.W.; visualization, Ø.B. (Øystein Bruserud). All authors have read and agreed to the published version of the manuscript.
Funding
The research of KAM is supported by the Norwegian Research Council under the frame NordForsk project number 90456; ERA PerMed project 2018-151 and PerMIT; and by the Swedish Research Council (project number 2018-02475).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G.; et al. 2001 CM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Janicova, A.; Relja, B. Neutrophil Phenotypes and Functions in Trauma and Trauma-Related Sepsis. Shock 2021, 56, 16–29. [Google Scholar] [CrossRef]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [CrossRef]
- Petri, B.; Sanz, M.J. Neutrophil chemotaxis. Cell Tissue Res. 2018, 371, 425–436. [Google Scholar] [CrossRef]
- Corriden, R.; Insel, P.A. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal 2012, 8, 587–598. [Google Scholar] [CrossRef]
- Sadik, C.D.; Luster, A.D. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 2012, 91, 207–215. [Google Scholar] [CrossRef]
- Heit, B.; Robbins, S.M.; Downey, C.M.; Guan, Z.; Colarusso, P.; Miller, B.J.; Jirik, F.R.; Kubes, P. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 2008, 9, 743–752. [Google Scholar] [CrossRef]
- Heit, B.; Tavener, S.; Raharjo, E.; Kubes, P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 2002, 159, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Henson, P.M.; Worthen, G.S. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 2004, 104, 565–571. [Google Scholar]
- Kim, H.K.; De La Luz Sierra, M.; Williams, C.K.; Gulino, A.V.; Tosato, G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood 2006, 108, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Malech, H.L. WHIM syndrome: Congenital immune deficiency disease. Curr. Opin. Hematol. 2009, 16, 20–26. [Google Scholar] [CrossRef]
- Wang, J.; Tannous, B.A.; Poznansky, M.C.; Chen, H. CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a therapeutic agent. Pharmacol. Res. 2020, 159, 105010. [Google Scholar] [CrossRef]
- Theyab, A.; Algahtani, M.; Alsharif, K.F.; Hawsawi, Y.M.; Alghamdi, A.; Alghamdi, A.; Akinwale, J. New insight into the mechanism of granulocyte colony-stimulating factor (G-CSF) that induces the mobilization of neutrophils. Hematology 2021, 26, 628–636. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Muller, W.A. Localized signals that regulate transendothelial migration. Curr. Opin. Immunol. 2016, 38, 24–29. [Google Scholar] [CrossRef]
- Nourshargh, S.; Hordijk, P.L.; Sixt, M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010, 11, 366–378. [Google Scholar] [CrossRef]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by ‘invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.D. Neutrophil transendothelial migration: Updates and new perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Pierini, L.M.; Eddy, R.J.; Fuortes, M.; Seveau, S.; Casulo, C.; Maxfield, F.R. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 2003, 278, 10831–10841. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; de la Fuente, H.; Mittelbrunn, M.; Sánchez-Madrid, F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol. Rev. 2007, 218, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Bodin, S.; Welch, M.D. Plasma membrane organization is essential for balancing competing pseudopod- and uropod-promoting signals during neutrophil polarization and migration. Mol. Biol. Cell 2005, 16, 5773–5783. [Google Scholar] [CrossRef]
- Ayee, M.A.; Levitan, I. Paradoxical impact of cholesterol on lipid packing and cell stiffness. Front. Biosci. 2016, 21, 1245–1259. [Google Scholar]
- Stroka, K.M.; Levitan, I.; Aranda-Espinoza, H. OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1. J. Biomech. 2012, 45, 1828–1834. [Google Scholar] [CrossRef]
- Gambardella, L.; Vermeren, S. Molecular players in neutrophil chemotaxis--focus on PI3K and small GTPases. J. Leukoc Biol. 2013, 94, 603–612. [Google Scholar] [CrossRef]
- Mócsai, A.; Walzog, B.; Lowell, C.A. Intracellular signalling during neutrophil recruitment. Cardiovasc Res. 2015, 107, 373–385. [Google Scholar] [CrossRef]
- Sitrin, R.G.; Sassanella, T.M.; Petty, H.R. An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils. Am. J. Respir. Cell Mol. Biol. 2011, 44, 205–212. [Google Scholar] [CrossRef]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.G.; Zhang, K.; Conly, J.; Kubes, P. Neutrophil crawling in capillaries; a novel immune response to Staphylococcus aureus. PLoS Pathog. 2014, 10, e1004379. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.C.; Melis, N.; Chen, D.; Wang, W.; Gallardo, D.; Weigert, R.; Parent, C.A. The LTB4-BLT1 axis regulates actomyosin and β2-integrin dynamics during neutrophil extravasation. J. Cell Biol. 2020, 219, e201910215. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guo, W.; Han, J.; Li, X.A. Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci. 2013, 93, 1–6. [Google Scholar] [CrossRef]
- Marmon, S.; Hinchey, J.; Oh, P.; Cammer, M.; de Almeida, C.J.; Gunther, L.; Raine, C.S.; Lisanti, M.P. Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am. J. Pathol. 2009, 174, 684–692. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Dahlgren, C.; Gabl, M.; Holdfeldt, A.; Winther, M.; Forsman, H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem. Pharmacol. 2016, 114, 22–39. [Google Scholar] [CrossRef]
- McDonald, B.; Kubes, P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. 2011, 89, 1079–1088. [Google Scholar] [CrossRef]
- Edwards, L.J.; Constantinescu, C.S. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm. Allergy Drug Targets 2009, 8, 182–190. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Malik, A.B.; Tang, H.; Yang, T.; Sun, B.; Wang, G.; Minshall, R.D.; Li, Y.; Zhao, Y.; et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 2012, 13, 457–464. [Google Scholar] [CrossRef]
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015, 185, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Hwang, T.L. The potential impacts of formyl peptide receptor 1 in inflammatory diseases. Front. Biosci. 2016, 8, 436–449. [Google Scholar]
- Gabl, M.; Holdfeldt, A.; Winther, M.; Oprea, T.; Bylund, J.; Dahlgren, C.; Forsman, H. A pepducin designed to modulate P2Y2R function interacts with FPR2 in human neutrophils and transfers ATP to an NADPH-oxidase-activating ligand through a receptor cross-talk mechanism. Biochim. Biophys. Acta 2016, 1863, 1228–1237. [Google Scholar] [CrossRef]
- Muldur, S.; Vadysirisack, D.D.; Ragunathan, S.; Tang, Y.; Ricardo, A.; Sayegh, C.E.; Irimia, D. Human Neutrophils Respond to Complement Activation and Inhibition in Microfluidic Devices. Front. Immunol. 2021, 12, 777932. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, D.J.; Yan, J.; Ross, G.; Hansen, R.D.; Baran, J.T.; Subbarao, K.; Wang, L.; Haribabu, B. C5a-mediated leukotriene B4-amplified neutrophil chemotaxis is essential in tumor immunotherapy facilitated by anti-tumor monoclonal antibody and beta-glucan. J. Immunol. 2005, 174, 7050–7056. [Google Scholar] [CrossRef]
- Kato, K.; Yokomizo, T.; Izumi, T.; Shimizu, T. Cell-specific transcriptional regulation of human leukotriene B(4) receptor gene. J. Exp. Med. 2000, 192, 413–420. [Google Scholar] [CrossRef]
- Yokomizo, T.; Kato, K.; Terawaki, K.; Izumi, T.; Shimizu, T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000, 192, 421–432. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.; Hosseini, E. Intravascular leukocyte migration through platelet thrombi: Directing leukocytes to sites of vascular injury. Thromb. Haemost. 2015, 113, 1224–1235. [Google Scholar]
- Harayama, T.; Shindou, H.; Ogasawara, R.; Suwabe, A.; Shimizu, T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J. Biol. Chem. 2008, 283, 11097–11106. [Google Scholar] [CrossRef]
- Moreno, S.E.; Alves-Filho, J.C.; Rios-Santos, F.; Silva, J.S.; Ferreira, S.H.; Cunha, F.Q.; Teixeira, M.M. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J. Immunol. 2006, 177, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, E.; Stankova, J.; Rola-Pleszczynski, M. Involvement of leukotriene B4 receptor 1 signaling in platelet-activating factor-mediated neutrophil degranulation and chemotaxis. Prostaglandins Other Lipid Mediat. 2005, 75, 25–34. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Schnoor, M.; Richardson, R.M.; Rajagopal, S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal 2019, 54, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M. Chemokines in pathology and medicine. J. Int. Med. 2001, 250, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Kubes, P. Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur. J. Immunol. 2012, 42, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Wolf, M.; Qin, S.; Mackay, C.R.; Baggiolini, M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc. Natl. Acad. Sci. USA 1996, 93, 6682–6686. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; Handel, T.M. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: The role of structural dynamics in function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef]
- Wu, L.; Ruffing, N.; Shi, X.; Newman, W.; Soler, D.; Mackay, C.R.; Qin, S. Discrete steps in binding and signaling of interleukin-8 with its receptor. J. Biol. Chem. 1996, 271, 31202–31209. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Prado, G.N.; Fernando, H.; Clark-Lewis, I.; Navarro, J. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry 2006, 45, 7882–7888. [Google Scholar] [CrossRef]
- Nasser, M.W.; Raghuwanshi, S.K.; Grant, D.J.; Jala, V.R.; Rajarathnam, K.; Richardson, R.M. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J. Immunol. 2009, 183, 3425–3432. [Google Scholar] [CrossRef]
- Fernando, H.; Chin, C.; Rösgen, J.; Rajarathnam, K. Dimer dissociation is essential for interleukin-8 (IL-8) binding to CXCR1 receptor. J. Biol. Chem. 2004, 279, 36175–36178. [Google Scholar] [CrossRef]
- Joseph, P.R.; Rajarathnam, K. Solution NMR characterization of WT CXCL8 monomer and dimer binding to CXCR1 N-terminal domain. Protein Sci. 2015, 24, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Damaj, B.B.; McColl, S.R.; Neote, K.; Hébert, C.A.; Naccache, P.H. Diverging signal transduction pathways activated by interleukin 8 (IL-8) and related chemokines in human neutrophils. IL-8 and Gro-alpha differentially stimulate calcium influx through IL-8 receptors A and B. J. Biol. Chem. 1996, 271, 20540–20544. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Ali, H.; Pridgen, B.C.; Haribabu, B.; Snyderman, R. Multiple signaling pathways of human interleukin-8 receptor A. Independent regulation by phosphorylation. J. Biol. Chem. 1998, 273, 10690–10695. [Google Scholar] [CrossRef]
- Ben-Baruch, A.; Grimm, M.; Bengali, K.; Evans, G.A.; Chertov, O.; Wang, J.M.; Howard, O.M.; Mukaida, N.; Matsushima, K.; Oppenheim, J.J. The differential ability of IL-8 and neutrophil-activating peptide-2 to induce attenuation of chemotaxis is mediated by their divergent capabilities to phosphorylate CXCR2 (IL-8 receptor B). J. Immunol. 1997, 158, 5927–5933. [Google Scholar] [CrossRef]
- Alon, R.; van Buul, J.D. Leukocyte Breaching of Endothelial Barriers: The Actin Link. Trends Immunol. 2017, 38, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Schnoor, M.; Alcaide, P.; Voisin, M.B.; van Buul, J.D. Crossing the Vascular Wall: Common and Unique Mechanisms Exploited by Different Leukocyte Subsets during Extravasation. Mediat. Inflamm. 2015, 2015, 946509. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Bassoni, D.L.; Campbell, J.J.; Gerard, N.P.; Gerard, C.; Wehrman, T.S. Biased agonism as a mechanism for differential signaling by chemokine receptors. J. Biol. Chem. 2013, 288, 35039–35048. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Trettel, F.; Di Bartolomeo, S.; Lauro, C.; Catalano, M.; Ciotti, M.T.; Limatola, C. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 2003, 278, 40980–40988. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Wilkinson, G.; Milligan, G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J. Biol. Chem. 2005, 280, 28663–28674. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Freitas, A.; Souto, F.O.; Spiller, F.; Paula-Neto, H.; Silva, J.S.; Gazzinelli, R.T.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4018–4023. [Google Scholar] [CrossRef]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef]
- Sabroe, I.; Jones, E.C.; Whyte, M.K.; Dower, S.K. Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 2005, 115, 90–98. [Google Scholar] [CrossRef]
- Raymond, S.L.; Hawkins, R.B.; Murphy, T.J.; Rincon, J.C.; Stortz, J.A.; López, M.C.; Ungaro, R.; Ellett, F.; Baker, H.V.; Wynn, J.L.; et al. Impact of toll-like receptor 4 stimulation on human neonatal neutrophil spontaneous migration, transcriptomics, and cytokine production. J. Mol. Med. 2018, 96, 673–684. [Google Scholar] [CrossRef]
- Qi, X.; Yu, Y.; Sun, R.; Huang, J.; Liu, L.; Yang, Y.; Rui, T.; Sun, B. Identification and characterization of neutrophil heterogeneity in sepsis. Crit. Care 2021, 25, 50. [Google Scholar] [CrossRef]
- Trevelin, S.C.; Alves-Filho, J.C.; Sônego, F.; Turato, W.; Nascimento, D.C.; Souto, F.O.; Cunha, T.M.; Gazzinelli, R.T.; Cunha, F.Q. Toll-like receptor 9 activation in neutrophils impairs chemotaxis and reduces sepsis outcome. Crit. Care Med. 2012, 40, 2631–2637. [Google Scholar] [CrossRef]
- Mallavia, B.; Liu, F.; Lefrançais, E.; Cleary, S.J.; Kwaan, N.; Tian, J.J.; Magnen, M.; Sayah, D.M.; Soong, A.; Chen, J.; et al. Mitochondrial DNA Stimulates TLR9-Dependent Neutrophil Extracellular Trap Formation in Primary Graft Dysfunction. Am. J. Respir. Cell Mol. Biol. 2020, 62, 364–372. [Google Scholar] [CrossRef]
- Bao, W.; Xia, H.; Liang, Y.; Ye, Y.; Lu, Y.; Xu, X.; Duan, A.; He, J.; Chen, Z.; Wu, Y.; et al. Toll-like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci. Rep. 2016, 6, 22579. [Google Scholar] [CrossRef]
- Sacramento, L.; Trevelin, S.C.; Nascimento, M.S.; Lima-Jùnior, D.S.; Costa, D.L.; Almeida, R.P.; Cunha, F.Q.; Silva, J.S.; Carregaro, V. Toll-like receptor 9 signaling in dendritic cells regulates neutrophil recruitment to inflammatory foci following Leishmania infantum infection. Infect Immun. 2015, 83, 4604–4616. [Google Scholar] [CrossRef] [PubMed]
- Ekman, A.K.; Cardell, L.O. The expression and function of Nod-like receptors in neutrophils. Immunology 2010, 130, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.G.; Sorbara, M.T.; Girardin, S.E.; Philpott, D.J. What is new with Nods? Curr. Opin. Immunol. 2011, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Philpott, D.J. Peptidoglycan: A critical activator of the mammalian immune system during infection and homeostasis. Immunol. Rev. 2011, 243, 40–60. [Google Scholar] [CrossRef]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar] [CrossRef]
- Everhart, M.B.; Han, W.; Sherrill, T.P.; Arutiunov, M.; Polosukhin, V.V.; Burke, J.R.; Sadikot, R.T.; Christman, J.W.; Yull, F.E.; Blackwell, T.S. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 2006, 176, 4995–5005. [Google Scholar] [CrossRef]
- Shimaoka, T.; Kume, N.; Minami, M.; Hayashida, K.; Sawamura, T.; Kita, T.; Yonehara, S. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J. Immunol. 2001, 166, 5108–5114. [Google Scholar] [CrossRef]
- Wu, Z.; Sawamura, T.; Kurdowska, A.K.; Ji, H.L.; Idell, S.; Fu, J. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun. 2011, 79, 2865–2870. [Google Scholar] [CrossRef]
- Reddy, R.C.; Narala, V.R.; Keshamouni, V.G.; Milam, J.E.; Newstead, M.W.; Standiford, T.J. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-γ. Blood 2008, 112, 4250–4258. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Sisti, F.; Sônego, F.; Wang, S.; Filgueiras, L.R.; Brandt, S.; Serezani, A.P.; Du, H.; Cunha, F.Q.; Alves-Filho, J.C.; et al. PPAR-γ/IL-10 axis inhibits MyD88 expression and ameliorates murine polymicrobial sepsis. J. Immunol. 2014, 192, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sônego, F.; Alves-Filho, J.C.; Cunha, F.Q. Targeting neutrophils in sepsis. Expert Rev. Clin. Immunol. 2014, 10, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Sônego, F.; Souto, F.O.; Freitas, A.; Verri, W.A., Jr.; Auxiliadora-Martins, M.; Basile-Filho, A.; McKenzie, A.N.; Xu, D.; Cunha, F.Q.; et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 2010, 16, 708–712. [Google Scholar] [CrossRef]
- Lerman, Y.V.; Kim, M. Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 19–28. [Google Scholar] [CrossRef]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef]
- Khan, S.Y.; McLaughlin, N.J.; Kelher, M.R.; Eckels, P.; Gamboni-Robertson, F.; Banerjee, A.; Silliman, C.C. Lysophosphatidylcholines activate G2A inducing G(αi)₋₁-/G(αq/)₁₁-Ca²(+) flux, G(βγ)-Hck activation and clathrin/β-arrestin-1/GRK6 recruitment in PMNs. Biochem. J. 2010, 432, 35–45. [Google Scholar] [CrossRef]
- Hong, C.W.; Kim, T.K.; Ham, H.Y.; Nam, J.S.; Kim, Y.H.; Zheng, H.; Pang, B.; Min, T.K.; Jung, J.S.; Lee, S.N.; et al. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J. Immunol. 2010, 184, 4401–4413. [Google Scholar] [CrossRef]
- Silliman, C.C.; Elzi, D.J.; Ambruso, D.R.; Musters, R.J.; Hamiel, C.; Harbeck, R.J.; Paterson, A.J.; Bjornsen, A.J.; Wyman, T.H.; Kelher, M.; et al. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J. Leukoc. Biol. 2003, 73, 511–524. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Byun, D.J.; Jin, S.; Seo, B.S.; Shin, M.H.; Leem, A.Y.; Choung, J.J.; Park, M.S.; Hyun, Y.M. Lysophosphatidylcholine Alleviates Acute Lung Injury by Regulating Neutrophil Motility and Neutrophil Extracellular Trap Formation. Front. Cell Dev. Biol. 2022, 10, 941914. [Google Scholar] [CrossRef]
- Snider, A.J.; Orr Gandy, K.A.; Obeid, L.M. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010, 92, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Prinetti, A.; Sonnino, S.; Mauri, L.; Kobayashi, T.; Ishii, K.; Kaga, N.; Murayama, K.; Kurihara, H.; Nakayama, H.; et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 2008, 25, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Kimura, S.; Hakomori, S.; Igarashi, Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett. 1997, 420, 196–200. [Google Scholar] [CrossRef]
- Sitrin, R.G.; Sassanella, T.M.; Landers, J.J.; Petty, H.R. Migrating human neutrophils exhibit dynamic spatiotemporal variation in membrane lipid organization. Am. J. Respir. Cell Mol. Biol. 2010, 43, 498–506. [Google Scholar] [CrossRef]
- Mondal, N.; Stolfa, G.; Antonopoulos, A.; Zhu, Y.; Wang, S.S.; Buffone, A., Jr.; Atilla-Gokcumen, G.E.; Haslam, S.M.; Dell, A.; Neelamegham, S. Glycosphingolipids on Human Myeloid Cells Stabilize E-Selectin-Dependent Rolling in the Multistep Leukocyte Adhesion Cascade. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Masuda, H.; Kaga, N.; Nakayama, H.; Matsumoto, R.; Iwahara, C.; Yoshizaki, F.; Tamaki, Y.; Kobayashi, T.; Hayakawa, T.; et al. Properties and functions of lactosylceramide from mouse neutrophils. Glycobiology 2015, 25, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.E.; Borgogna, T.R.; Patel, D.M.; Sward, E.W.; Voyich, J.M. Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front. Cell Infect Microbiol. 2017, 7, 286. [Google Scholar] [CrossRef]
- Vogt, K.L.; Summers, C.; Condliffe, A.M. The clinical consequences of neutrophil priming. Curr. Opin. Hematol. 2019, 26, 22–27. [Google Scholar] [CrossRef]
- Vogt, K.L.; Summers, C.; Chilvers, E.R.; Condliffe, A.M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12967. [Google Scholar] [CrossRef]
- Montrucchio, G.; Bosco, O.; Del Sorbo, L.; Fascio Pecetto, P.; Lupia, E.; Goffi, A.; Omedè, P.; Emanuelli, G.; Camussi, G. Mechanisms of the priming effect of low doses of lipopoly-saccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb. Haemost. 2003, 90, 872–881. [Google Scholar] [CrossRef]
- Li, J.; Kim, K.; Barazia, A.; Tseng, A.; Cho, J. Platelet-neutrophil interactions under thromboinflammatory conditions. Cell Mol. Life Sci. 2015, 72, 2627–2643. [Google Scholar] [CrossRef] [PubMed]
- Kliger, E.; Kristal, B.; Shapiro, G.; Chezar, J.; Sela, S. Primed polymorphonuclear leukocytes from hemodialysis patients enhance monocyte transendothelial migration. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H974–H987. [Google Scholar] [CrossRef] [PubMed]
- Koehl, B.; Nivoit, P.; El Nemer, W.; Lenoir, O.; Hermand, P.; Pereira, C.; Brousse, V.; Guyonnet, L.; Ghinatti, G.; Benkerrou, M.; et al. The endothelin B receptor plays a crucial role in the adhesion of neutrophils to the endothelium in sickle cell disease. Haematologica 2017, 102, 1161–1172. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Ravi, S.; Budhathoki, R.; Arjyal, L.; Hamal, S.; Bista, A.; Khadka, S.; Uprety, D. Current understanding and future implications of sepsis-induced thrombocytopenia. Eur. J. Haematol. 2021, 106, 301–305. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95. [Google Scholar]
- Iba, T.; Connors, J.M.; Nagaoka, I.; Levy, J.H. Recent advances in the research and management of sepsis-associated DIC. Int. J. Hematol. 2021, 113, 24–33. [Google Scholar] [CrossRef]
- Lucena, F.; McDougall, J.J. Protease Activated Receptors and Arthritis. Int. J. Mol. Sci. 2021, 22, 9352. [Google Scholar] [CrossRef]
- Pawlinski, R.; Pedersen, B.; Schabbauer, G.; Tencati, M.; Holscher, T.; Boisvert, W.; Andrade-Gordon, P.; Frank, R.D.; Mackman, N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood 2004, 103, 1342–1347. [Google Scholar] [CrossRef]
- Coughlin, S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. USA 1999, 96, 11023–11027. [Google Scholar] [CrossRef]
- Shpacovitch, V.M.; Seeliger, S.; Huber-Lang, M.; Balkow, S.; Feld, M.; Hollenberg, M.D.; Sarma, V.J.; Ward, P.A.; Strey, A.; Gerke, V.; et al. Agonists of proteinase-activated receptor-2 affect transendothelial migration and apoptosis of human neutrophils. Exp. Dermatol. 2007, 16, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Shpacovitch, V.M.; Varga, G.; Strey, A.; Gunzer, M.; Mooren, F.; Buddenkotte, J.; Vergnolle, N.; Sommerhoff, C.P.; Grabbe, S.; Gerke, V.; et al. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. J. Leukoc. Biol. 2004, 76, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Gomides, L.F.; Lima, O.C.; Matos, N.A.; Freitas, K.M.; Francischi, J.N.; Tavares, J.C.; Klein, A. Blockade of proteinase-activated receptor 4 inhibits neutrophil recruitment in experimental inflammation in mice. Inflamm. Res. 2014, 63, 935–941. [Google Scholar] [PubMed]
- Liu, Y.G.; Teng, Y.S.; Shan, Z.G.; Cheng, P.; Hao, C.J.; Lv, Y.P.; Mao, F.Y.; Yang, S.M.; Chen, W.; Zhao, Y.L.; et al. Arrestin domain containing 3 promotes Helicobacter pylori-associated gastritis by regulating protease-activated receptor 1. JCI Insight 2020, 5, e135849. [Google Scholar] [CrossRef]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the cytokines produced by human neutrophils in tumors. Semin. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Martucci, C.; Franchi, S.; Giannini, E.; Tian, H.; Melchiorri, P.; Negri, L.; Sacerdote, P. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br. J. Pharmacol. 2006, 147, 225–234. [Google Scholar] [CrossRef]
- Hult, E.M.; Gurczynski, S.J.; O’Dwyer, D.N.; Zemans, R.L.; Rasky, A.; Wang, Y.; Murray, S.; Crawford, H.C.; Moore, B.B. Myeloid- and Epithelial-Derived HBEGF Promotes Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 641–653. [Google Scholar] [CrossRef]
- Gruss, H.-J.; Herrmann, F. CD30 ligand, a member of the TNF ligand superfamily, with growth and activation control CD30+ lymphoid and lymphoma cells. Leuk. Lymphoma. 1996, 20, 397–409. [Google Scholar] [CrossRef]
- Kuhn, N.F.; Purdon, T.J.; van Leeuwen, D.G.; Lopez, A.V.; Curran, K.J.; Daniyan, A.F.; Brentjens, R.J. CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response. Cancer Cell 2019, 35, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Krammer, P.H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp. Cell Res. 2000, 256, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Wang, P.; Yoshimura, K.; Choi, I.H.; Hilliard, A.; Chen, Y.H.; Wang, C.R.; Schulick, R.; Flies, A.S.; Flies, D.B.; et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J. Clin. Investig. 2006, 116, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Kim, H.Y.; Min, J.Y.; Kim, H.M.; Jeong, H.J. An osteoclastogenesis system, the RANKL/RANK signalling pathway, contributes to aggravated allergic inflammation. Br. J. Pharmacol. 2019, 176, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Tersteegen, A.; Sorg, U.R.; Virgen-Slane, R.; Helle, M.; Petzsch, P.; Dunay, I.R.; Köhrer, K.; Degrandi, D.; Ware, C.F.; Pfeffer, K. Lymphotoxin β Receptor: A Crucial Role in Innate and Adaptive Immune Responses against Toxoplasma gondii. Infect Immun. 2021, 89, e00026-21. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Jain, R.; Cassatella, M.A.; Lood, C. Editorial: Neutrophil Communication. Front. Immunol. 2020, 11, 871. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- de Kroon, A.I.; Rijken, P.J.; De Smet, C.H. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog. Lipid Res. 2013, 52, 374–394. [Google Scholar] [CrossRef]
- Laude, A.J.; Prior, I.A. Plasma membrane microdomains: Organization, function and trafficking. Mol. Membr. Biol. 2004, 21, 193–205. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Buck, M. Computational Modeling Reveals that Signaling Lipids Modulate the Orientation of K-Ras4A at the Membrane Reflecting Protein Topology. Structure 2017, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, S.M.; Stadnyk, A.W.; Lin, T.J. Toll-like receptors in the host defense against Pseudomonas aeruginosa respiratory infection and cystic fibrosis. J. Leukoc. Biol. 2012, 92, 977–985. [Google Scholar] [CrossRef]
- Schumann, J.; Leichtle, A.; Thiery, J.; Fuhrmann, H. Fatty acid and peptide profiles in plasma membrane and membrane rafts of PUFA supplemented RAW264.7 macrophages. PLoS ONE 2011, 6, e24066. [Google Scholar] [CrossRef] [PubMed]
- Schoeniger, A.; Fuhrmann, H.; Schumann, J. LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition. PeerJ 2016, 4, e1663. [Google Scholar] [CrossRef]
- Randall, A.S.; Liu, C.H.; Chu, B.; Zhang, Q.; Dongre, S.A.; Juusola, M.; Franze, K.; Wakelam, M.J.; Hardie, R.C. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J. Neurosci. 2015, 35, 2731–2746. [Google Scholar] [CrossRef]
- Briot, A.; Civelek, M.; Seki, A.; Hoi, K.; Mack, J.J.; Lee, S.D.; Kim, J.; Hong, C.; Yu, J.; Fishbein, G.A.; et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J. Exp. Med. 2015, 212, 2147–2163. [Google Scholar] [CrossRef]
- Kharche, S.; Joshi, M.; Sengupta, D.; Chattopadhyay, A. Membrane-induced organization and dynamics of the N-terminal domain of chemokine receptor CXCR1:insights from atomistic simulations. Chem. Phys. Lipids 2018, 210, 142–148. [Google Scholar] [CrossRef]
- Haldar, S.; Raghuraman, H.; Namani, T.; Rajarathnam, K.; Chattopadhyay, A. Membrane interaction of the N-terminal domain of chemokine receptor CXCR1. Biochim. Biophys. Acta. 2010, 1798, 1056–1061. [Google Scholar] [CrossRef]
- Park, S.H.; Das, B.B.; Casagrande, F.; Tian, Y.; Nothnagel, H.J.; Chu, M.; Kiefer, H.; Maier, K.; De Angelis, A.A.; Marassi, F.M.; et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012, 491, 779–783. [Google Scholar] [CrossRef]
- Park, S.H.; Casagrande, F.; Cho, L.; Albrecht, L.; Opella, S.J. Interactions of interleukin-8 with the human chemokine receptor CXCR1 in phospholipid bilayers by NMR spectroscopy. J. Mol. Biol. 2011, 414, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Zhang, N.; Xu, X.; Oppenheim, J.J.; Jin, T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol. Cell Biol. 2005, 25, 5752–5762. [Google Scholar] [PubMed]
- Sitrin, R.G.; Emery, S.L.; Sassanella, T.M.; Blackwood, R.A.; Petty, H.R. Selective localization of recognition complexes for leukotriene B4 and formyl-Met-Leu-Phe within lipid raft microdomains of human polymorphonuclear neutrophils. J. Immunol. 2006, 177, 8177–8184. [Google Scholar] [CrossRef] [PubMed]
- Serezani, C.H.; Aronoff, D.M.; Sitrin, R.G.; Peters-Golden, M. FcgammaRI ligation leads to a complex with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions. Blood 2009, 114, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Fan, J. Neutrophil in Reverse Migration: Role in Sepsis. Front. Immunol. 2021, 12, 656039. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Johnson, R.J.; Mooney, A.; Hugo, C.; Gordon, K.; Savill, J. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am. J. Pathol. 1997, 150, 223–234. [Google Scholar]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef]
- Huang, A.J.; Manning, J.E.; Bandak, T.M.; Ratau, M.C.; Hanser, K.R.; Silverstein, S.C. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 1993, 120, 1371–1380. [Google Scholar] [CrossRef]
- Owen-Woods, C.; Joulia, R.; Barkaway, A.; Rolas, L.; Ma, B.; Nottebaum, A.F.; Arkill, K.P.; Stein, M.; Girbl, T.; Golding, M.; et al. Local microvascular leakage promotes trafficking of activated neutrophils to remote organs. J. Clin. Investig. 2020, 130, 2301–2318. [Google Scholar] [CrossRef] [PubMed]
- Marki, A.; Ley, K. Leaking chemokines confuse neutrophils. J. Clin. Investig. 2020, 130, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Ross, E.A.; McGettrick, H.M.; Osborne, C.E.; Haworth, O.; Schmutz, C.; Stone, P.C.; Salmon, M.; Matharu, N.M.; Vohra, R.K.; et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 2006, 79, 303–311. [Google Scholar] [CrossRef]
- Powell, D.; Tauzin, S.; Hind, L.E.; Deng, Q.; Beebe, D.J.; Huttenlocher, A. Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep. 2017, 19, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Tharp, W.G.; Yadav, R.; Irimia, D.; Upadhyaya, A.; Samadani, A.; Hurtado, O.; Liu, S.Y.; Munisamy, S.; Brainard, D.M.; Mahon, M.J.; et al. Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo. J. Leukoc. Biol. 2006, 79, 539–554. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Bodkin, J.V.; Beyrau, M.; Woodfin, A.; Ody, C.; Rourke, C.; Chavakis, T.; Brohi, K.; Imhof, B.A.; Nourshargh, S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 2015, 42, 1075–1086. [Google Scholar] [CrossRef]
- Rocha-Gregg, B.; Huttenlocher, A. Signal integration in forward and reverse neutrophil migration: Fundamentals and emerging mechanisms. Curr. Opin. Cell Biol. 2021, 72, 124–130. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Sun, B.W. Recent advances in neutrophil chemotaxis abnormalities during sepsis. Chin. J. Traumatol. 2022, 25, 317–324. [Google Scholar] [CrossRef]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Kelly-Scumpia, K.M.; Thayer, T.C.; Winfield, R.D.; Scumpia, P.O.; Cuenca, A.G.; Harrington, P.B.; O’Malley, K.A.; Warner, E.; Gabrilovich, S.; et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J. Immunol. 2011, 187, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Foss, B.; Petersen, H. Hematopoietic growth factors in patients receiving intensive chemotherapy for malignant disorders: Studies of granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interleukin-3 (IL-3) and Flt-3 ligand (Flt3L). Eur. Cytokine. Netw. 2001, 12, 231–238. [Google Scholar] [PubMed]
- Mehta, H.M.; Corey, S.J. G-CSF, the guardian of granulopoiesis. Semin. Immunol. 2021, 54, 101515. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.J.; Link, D.C. Regulation of neutrophil homeostasis. Curr. Opin. Hematol. 2007, 14, 3–8. [Google Scholar] [CrossRef]
- Drost, E.M.; Kassabian, G.; Meiselman, H.J.; Gelmont, D.; Fisher, T.C. Increased rigidity and priming of polymorphonuclear leukocytes in sepsis. Am. J. Respir. Crit. Care Med. 1999, 159, 1696–1702. [Google Scholar] [CrossRef]
- Skoutelis, A.T.; Kaleridis, V.; Athanassiou, G.M.; Kokkinis, K.I.; Missirlis, Y.F.; Bassaris, H.P. Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Crit. Care Med. 2000, 28, 2355–2359. [Google Scholar] [CrossRef]
- Saito, H.; Lai, J.; Rogers, R.; Doerschuk, C.M. Mechanical properties of rat bone marrow and circulating neutrophils and their responses to inflammatory mediators. Blood 2002, 99, 2207–2213. [Google Scholar] [CrossRef]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Svahn, S.L.; Gutiérrez, S.; Ulleryd, M.A.; Nookaew, I.; Osla, V.; Beckman, F.; Nilsson, S.; Karlsson, A.; Jansson, J.O.; Johansson, M.E. Dietary Polyunsaturated Fatty Acids Promote Neutrophil Accumulation in the Spleen by Altering Chemotaxis and Delaying Cell Death. Infect Immun. 2019, 87, e00270-19. [Google Scholar] [CrossRef]
- Svahn, S.L.; Ulleryd, M.A.; Grahnemo, L.; Ståhlman, M.; Borén, J.; Nilsson, S.; Jansson, J.O.; Johansson, M.E. Dietary Omega-3 Fatty Acids Increase Survival and Decrease Bacterial Load in Mice Subjected to Staphylococcus aureus-Induced Sepsis. Infect. Immun. 2016, 84, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Svahn, S.L.; Grahnemo, L.; Pálsdóttir, V.; Nookaew, I.; Wendt, K.; Gabrielsson, B.; Schéle, E.; Benrick, A.; Andersson, N.; Nilsson, S.; et al. Dietary polyunsaturated fatty acids increase survival and decrease bacterial load during septic Staphylococcus aureus infection and improve neutrophil function in mice. Infect. Immun. 2015, 83, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Mahata, B.; Banerjee, A.; Kundu, M.; Bandyopadhyay, U.; Biswas, K. TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells. Sci. Rep. 2015, 5, 9048. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Mahata, B.; Banerjee, A.; Chakraborty, S.; Debnath, S.; Ray, S.S.; Ghosh, Z.; Biswas, K. Ganglioside GM2 mediates migration of tumor cells by interacting with integrin and modulating the downstream signaling pathway. Biochim. Biophys. Acta 2016, 1863 Pt A, 1472–1489. [Google Scholar] [CrossRef]
- Laudes, I.J.; Guo, R.F.; Riedemann, N.C.; Speyer, C.; Craig, R.; Sarma, J.V.; Ward, P.A. Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am. J. Pathol. 2004, 164, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Zeuke, S.; Ulmer, A.J.; Kusumoto, S.; Katus, H.A.; Heine, H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc. Res. 2002, 56, 126–134. [Google Scholar] [CrossRef]
- Sawa, Y.; Ueki, T.; Hata, M.; Iwasawa, K.; Tsuruga, E.; Kojima, H.; Ishikawa, H.; Yoshida, S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J. Histochem. Cytochem. 2008, 56, 97–109. [Google Scholar] [CrossRef]
- Wu, R.Q.; Xu, Y.X.; Song, X.H.; Chen, L.J.; Meng, X.J. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J. Gastroenterol. 2001, 7, 128–130. [Google Scholar] [CrossRef]
- Ogawa, H.; Rafiee, P.; Heidemann, J.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Kalyanaraman, B.; Pritchard, K.A., Jr.; Binion, D.G. Mechanisms of endotoxin tolerance in human intestinal microvascular endothelial cells. J. Immunol. 2003, 170, 5956–5964. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Driscoll, K.E.; Roth, R.A. Inhibition of pulmonary neutrophil trafficking during endotoxemia is dependent on the stimulus for migration. Am. J. Respir. Cell Mol. Biol. 1999, 20, 769–776. [Google Scholar] [CrossRef]
- Burns, A.R.; Doerschuk, C.M. Quantitation of L-selectin and CD18 expression on rabbit neutrophils during CD18-independent and CD18-dependent emigration in the lung. J. Immunol. 1994, 153, 3177–3188. [Google Scholar] [CrossRef]
- Guo, R.F.; Riedemann, N.C.; Laudes, I.J.; Sarma, V.J.; Kunkel, R.G.; Dilley, K.A.; Paulauskis, J.D.; Ward, P.A. Altered neutrophil trafficking during sepsis. J. Immunol. 2002, 169, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ibbotson, G.C.; Doig, C.; Kaur, J.; Gill, V.; Ostrovsky, L.; Fairhead, T.; Kubes, P. Functional alpha4-integrin: A newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat. Med. 2001, 7, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, B. Pleiotropic regulations of neutrophil receptors response to sepsis. Inflamm. Res. 2017, 66, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Hidalgo, A.; Chang, J.; Peired, A.; Frenette, P.S. CD44 is a physiological E-selectin ligand on neutrophils. J. Exp. Med. 2005, 201, 1183–1189. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Halstensen, A.; Peen, E.; Solberg, C.O. Serum levels of adhesion molecules and cytokines in patients with acute leukaemia. Leuk. Lymphoma 1996, 23, 423–430. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Akselen, P.E.; Bergheim, J.; Nesthus, I. Serum concentrations of E-selectin, P-selectin, ICAM-1 and interleukin 6 in acute leukaemia patients with chemotherapy-induced leucopenia and bacterial infections. Br. J. Haematol. 1995, 91, 394–402. [Google Scholar] [CrossRef]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef]
- Gearing, A.J.; Newman, W. Circulating adhesion molecules in disease. Immunol. Today 1993, 14, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Ward, P.A. Role of oxidants in lung injury during sepsis. Antioxid. Redox. Signal 2007, 9, 1991–2002. [Google Scholar] [CrossRef]
- van Den Engel, N.K.; Heidenthal, E.; Vinke, A.; Kolb, H.; Martin, S. Circulating forms of intercellular adhesion molecule (ICAM)-1 in mice lacking membranous ICAM-1. Blood 2000, 95, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sônego, F.; Castanheira, F.V.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.; Nascimento, D.C.; Colón, D.F.; de Fátima Borges, V.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Murta, B.M.; Zaparoli, M.; Ferreira, R.B.; Silva-Vergara, M.L.; Oliveira, C.H.; Murta, E.F.; Ferreira, S.H.; Cunha, F.Q. Failure of neutrophil chemotactic function in septic patients. Crit. Care Med. 2002, 30, 1056–1061. [Google Scholar] [CrossRef]
- Patel, J.M.; Sapey, E.; Parekh, D.; Scott, A.; Dosanjh, D.; Gao, F.; Thickett, D.R. Sepsis Induces a Dysregulated Neutrophil Phenotype That Is Associated with Increased Mortality. Mediat. Inflamm. 2018, 2018, 4065362. [Google Scholar] [CrossRef]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.; Naud, J.F.; Campisi, G.; Giannias, B.; Liu, S.; DiCarlo, A.; Ferri, L.E.; Pascual, J.L.; Tchervenkov, J.; Christou, N.V. Alteration of chemoattractant receptor expression regulates human neutrophil chemotaxis in vivo. Ann. Surg. 2002, 235, 550–559. [Google Scholar] [CrossRef]
- Guo, R.F.; Riedemann, N.C.; Bernacki, K.D.; Sarma, V.J.; Laudes, I.J.; Reuben, J.; Younkin, E.; Neff, T.A.; Paulauskis, J.D.; Zetoune, F.S.; et al. Neutrophil C5a receptor and the outcome in a rat model of sepsis. FASEB J. 2003, 17, 1889–1891. [Google Scholar] [CrossRef]
- Moore, C.A.; Milano, S.K.; Benovic, J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007, 69, 451–482. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Paula-Neto, H.A.; Alves-Filho, J.C.; Souto, F.O.; Spiller, F.; Amêndola, R.S.; Freitas, A.; Cunha, F.Q.; Barja-Fidalgo, C. Inhibition of guanylyl cyclase restores neutrophil migration and maintains bactericidal activity increasing survival in sepsis. Shock 2011, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, S.D.; Kook, M.; Lee, H.Y.; Ghim, J.; Choi, Y.; Zabel, B.A.; Ryu, S.H.; Bae, Y.S. Phospholipase D2 drives mortality in sepsis by inhibiting neutrophil extracellular trap formation and down-regulating CXCR2. J. Exp. Med. 2015, 212, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Tran, V.G.; Kim, W.; Kim, J.; Cho, H.R.; Kwon, B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J. Immunol. 2012, 189, 287–295. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Cunha, F.Q.; Assreuy, J.; Moss, D.W.; Rees, D.; Leal, L.M.; Moncada, S.; Carrier, M.; O’Donnell, C.A.; Liew, F.Y. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: Role of TNF-alpha and IL-1-beta. Immunology 1994, 81, 211–215. [Google Scholar]
- Martin, E.L.; Souza, D.G.; Fagundes, C.T.; Amaral, F.A.; Assenzio, B.; Puntorieri, V.; Del Sorbo, L.; Fanelli, V.; Bosco, M.; Delsedime, L.; et al. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am. J. Respir. Crit. Care Med. 2010, 182, 762–773. [Google Scholar] [CrossRef]
- Sakai, K.; Suzuki, H.; Oda, H.; Akaike, T.; Azuma, Y.; Murakami, T.; Sugi, K.; Ito, T.; Ichinose, H.; Koyasu, S.; et al. Phosphoinositide 3-kinase in nitric oxide synthesis in macrophage: Critical dimerization of inducible nitric-oxide synthase. J. Biol. Chem. 2006, 281, 17736–17742. [Google Scholar] [CrossRef]
- Chishti, A.D.; Shenton, B.K.; Kirby, J.A.; Baudouin, S.V. Neutrophil chemotaxis and receptor expression in clinical septic shock. Int. Care Med. 2004, 30, 605–611. [Google Scholar] [CrossRef]
- Parvataneni, S.; Gonipeta, B.; Packiriswamy, N.; Lee, T.; Durairaj, H.; Parameswaran, N. Role of myeloid-specific G-protein coupled receptor kinase-2 in sepsis. Int. J. Clin. Exp. Med. 2011, 4, 320–330. [Google Scholar]
- Rios-Santos, F.; Alves-Filho, J.C.; Souto, F.O.; Spiller, F.; Freitas, A.; Lotufo, C.M.; Soares, M.B.; Dos Santos, R.R.; Teixeira, M.M.; Cunha, F.Q. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am. J. Respir. Crit. Care Med. 2007, 175, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Fredly, H.; Kittang, A.O.; Bruserud, Ø. The possible diagnostic and prognostic use of systemic chemokine profiles in clinical medicine—The experience in acute myeloid leukemia from disease development and diagnosis via conventional chemotherapy to allogeneic stem cell transplantation. Toxins 2013, 5, 336–362. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.L.; Gao, H.; Rancilio, N.J.; Neff, T.A.; Huffnagle, G.B.; Sarma, J.V.; Ward, P.A. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am. J. Pathol. 2004, 165, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Souto, F.O.; Alves-Filho, J.C.; Turato, W.M.; Auxiliadora-Martins, M.; Basile-Filho, A.; Cunha, F.Q. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am. J. Respir. Crit. Care Med. 2011, 183, 234–242. [Google Scholar] [CrossRef]
- Salomao, R.; Brunialti, M.K.; Gomes, N.E.; Mendes, M.E.; Diaz, R.S.; Komninakis, S.; Machado, F.R.; da Silva, I.D.; Rigato, O. Toll-like receptor pathway signaling is differently regulated in neutrophils and peripheral mononuclear cells of patients with sepsis, severe sepsis, and septic shock. Crit. Care Med. 2009, 37, 132–139. [Google Scholar] [CrossRef]
- Tang, B.M.; McLean, A.S.; Dawes, I.W.; Huang, S.J.; Lin, R.C. The use of gene-expression profiling to identify candidate genes in human sepsis. Am. J. Respir. Crit. Care Med. 2007, 176, 676–684. [Google Scholar] [CrossRef]
- McCullers, J.A.; Rehg, J.E. Lethal synergism between influenza virus and Streptococcus pneumoniae: Characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Halbgebauer, R.; Schmidt, C.Q.; Karsten, C.M.; Ignatius, A.; Huber-Lang, M. Janus face of complement-driven neutrophil activation during sepsis. Semin. Immunol. 2018, 37, 12–20. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Dis. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Spiller, F.; Orrico, M.I.; Nascimento, D.C.; Czaikoski, P.G.; Souto, F.O.; Alves-Filho, J.C.; Freitas, A.; Carlos, D.; Montenegro, M.F.; Neto, A.F.; et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 2010, 182, 360–368. [Google Scholar] [CrossRef]
- Mestriner, F.L.; Spiller, F.; Laure, H.J.; Souto, F.O.; Tavares-Murta, B.M.; Rosa, J.C.; Basile-Filho, A.; Ferreira, S.H.; Greene, L.J.; Cunha, F.Q. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 19595–19600. [Google Scholar] [CrossRef] [PubMed]
- Torres-Dueñas, D.; Celes, M.R.; Freitas, A.; Alves-Filho, J.C.; Spiller, F.; Dal-Secco, D.; Dalto, V.F.; Rossi, M.A.; Ferreira, S.H.; Cunha, F.Q. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br. J. Pharmacol. 2007, 152, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Cao, K.; Jiang, J.P.; Guan, W.X.; Du, J.F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Alves-Filho, J.C.; Victoni, T.; Secher, T.; Lemos, H.P.; Sônego, F.; Cunha, F.Q.; Ryffel, B. IL-17 receptor signaling is required to control polymicrobial sepsis. J. Immunol. 2009, 182, 7846–7854. [Google Scholar] [CrossRef]
- Cummings, C.J.; Martin, T.R.; Frevert, C.W.; Quan, J.M.; Wong, V.A.; Mongovin, S.M.; Hagen, T.R.; Steinberg, K.P.; Goodman, R.B. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J. Immunol. 1999, 162, 2341–2346. [Google Scholar] [CrossRef]
- Arraes, S.M.; Freitas, M.S.; da Silva, S.V.; De Paula Neto, H.A.; Alves-Filho, J.C.; Auxiliadora Martins, M.; Basile-Filho, A.; Tavares-Murta, B.M.; Barja-Fidalgo, C.; Cunha, F.Q. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood 2006, 108, 2906–2913. [Google Scholar] [CrossRef]
- Korbecki, J.; Kupnicka, P.; Chlubek, M.; Gorący, J.; Gutowska, I.; Baranowska-Bosiacka, I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. Int. J. Mol. Sci. 2022, 23, 2168. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; de Freitas, A.; Russo, M.; Cunha, F.Q. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 2006, 34, 461–470. [Google Scholar] [CrossRef]
- Dal Secco, D.; Moreira, A.P.; Freitas, A.; Silva, J.S.; Rossi, M.A.; Ferreira, S.H.; Cunha, F.Q. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: Role of soluble guanylate cyclase. Nitric Oxide 2006, 15, 77–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Brandish, P.E.; Di Valentin, M.; Schelvis, J.P.; Babcock, G.T.; Marletta, M.A. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry 2000, 39, 10848–10854. [Google Scholar] [CrossRef]
- Mishra, H.K.; Johnson, T.J.; Seelig, D.M.; Walcheck, B. Targeting ADAM17 in leukocytes increases neutrophil recruitment and reduces bacterial spread during polymicrobial sepsis. J. Leukoc. Biol. 2016, 100, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Koppe, U.; Suttorp, N.; Opitz, B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell Microbiol. 2012, 14, 460–466. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Zhang, B.; Loomba, R.; Seki, E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G30–G41. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, M.A. The neutrophil: One of the cellular targets of interleukin-10. Int. J. Clin. Lab. Res. 1998, 28, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, L.; Gasperini, S.; Lapinet, J.A.; Calzetti, F.; Pinardi, C.; Liu, Y.; Zurawski, S.; de Waal Malefyt, R.; Moore, K.W.; Cassatella, M.A. Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J. Immunol. 2001, 167, 2312–2322. [Google Scholar] [CrossRef]
- Takanabe-Mori, R.; Ono, K.; Wada, H.; Takaya, T.; Ura, S.; Yamakage, H.; Satoh-Asahara, N.; Shimatsu, A.; Takahashi, Y.; Fujita, M.; et al. Lectin-like oxidized low-density lipoprotein receptor-1 plays an important role in vascular inflammation in current smokers. J. Atheroscler. Thromb. 2013, 20, 585–590. [Google Scholar] [CrossRef]
- Dunn, S.; Vohra, R.S.; Murphy, J.E.; Homer-Vanniasinkam, S.; Walker, J.H.; Ponnambalam, S. The lectin-like oxidized low-density-lipoprotein receptor: A pro-inflammatory factor in vascular disease. Biochem. J. 2008, 409, 349–355. [Google Scholar] [CrossRef]
- Reddy, R.C.; Standiford, T.J. Effects of sepsis on neutrophil chemotaxis. Curr. Opin. Hematol. 2010, 17, 18–24. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Vieira, S.M.; Dal-Secco, D.; Freitas, A.; Souto, F.O.; Mestriner, F.L.; Alves-Filho, J.C.; Grespan, R.; Kawai, T.; Ferreira, S.H.; et al. Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J. Immunol. 2008, 180, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.B.; Beyrau, M.; Colom, B.; Caille, D.; Diapouli, F.M.; Nash, G.B.; Chavakis, T.; Albelda, S.M.; Rainger, G.E.; et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 2011, 12, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ode, Y.; Aziz, M.; Wang, P. CIRP increases ICAM-1+ phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J. Leukoc. Biol. 2018, 103, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Hesse, A.K.; Dörger, M.; Kupatt, C.; Krombach, F. Proinflammatory role of inducible nitric oxide synthase in acute hyperoxic lung injury. Respir. Res. 2004, 5, 11. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef]
- Li, B.; Han, X.; Ye, X.; Ni, J.; Wu, J.; Dai, J.; Wu, Z.; Chen, C.; Wan, R.; Wang, X.; et al. Substance P-regulated leukotriene B4 production promotes acute pancreatitis-associated lung injury through neutrophil reverse migration. Int. Immunopharmacol. 2018, 57, 147–156. [Google Scholar] [CrossRef]
- Jin, H.; Aziz, M.; Ode, Y.; Wang, P. CIRP Induces Neutrophil Reverse Transendothelial Migration in Sepsis. Shock 2019, 51, 548–556. [Google Scholar] [CrossRef]
- Murao, A.; Brenner, M.; Aziz, M.; Wang, P. Exosomes in Sepsis. Front. Immunol. 2020, 11, 2140. [Google Scholar] [CrossRef]
- Nojima, H.; Konishi, T.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Chemokine Receptors, CXCR1 and CXCR2, Differentially Regulate Exosome Release in Hepatocytes. PLoS ONE 2016, 11, e0161443. [Google Scholar] [CrossRef]
- Nolan, S.; Dixon, R.; Norman, K.; Hellewell, P.; Ridger, V. Nitric oxide regulates neutrophil migration through microparticle formation. Am. J. Pathol. 2008, 172, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Norling, L.V.; Renshaw, D.; Cooper, D.; Leung, K.Y.; Perretti, M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112, 2512–2519. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Demaret, J.; Gossez, M.; Monneret, G. Myeloid cells in sepsis-acquired immunodeficiency. Ann. N. Y. Acad. Sci. 2021, 1499, 3–17. [Google Scholar] [CrossRef]
- Heit, B.; Colarusso, P.; Kubes, P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J. Cell Sci. 2005, 118, 5205–5220. [Google Scholar] [CrossRef] [PubMed]
- Østerud, B. The role of platelets in decrypting monocyte tissue factor. Dis. Mon. 2003, 49, 7–13. [Google Scholar] [CrossRef]
- Kim, D.; Haynes, C.L. On-chip evaluation of neutrophil activation and neutrophil-endothelial cell interaction during neutrophil chemotaxis. Anal. Chem. 2013, 85, 10787–10796. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, L.; Yan, D.; Chen, H.; Xu, G.; Jian, W.; Cui, P.; Chao, W. Extracellular MicroRNAs Induce Potent Innate Immune Responses via TLR7/MyD88-Dependent Mechanisms. J. Immunol. 2017, 199, 2106–2117. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Zhang, Y.; Boesen, C.C.; Radaev, S.; Brooks, A.G.; Fridman, W.H.; Sautes-Fridman, C.; Sun, P.D. Crystal structure of the extracellular domain of a human Fc gamma RIII. Immunity 2000, 13, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, R.; Azim, A.; Agarwal, V. Neutrophil CD64 a Diagnostic and Prognostic Marker of Sepsis in Adult Critically Ill Patients: A Brief Review. Indian J. Crit. Care Med. 2020, 24, 1242–1250. [Google Scholar] [PubMed]
- Hoffmann, J.J. Neutrophil CD64 as a sepsis biomarker. Biochem. Med. 2011, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rodgers, G.P. Olfactomedin 4 Is a Biomarker for the Severity of Infectious Diseases. Open Forum. Infect Dis. 2022, 9, ofac061. [Google Scholar] [CrossRef]
- Lomas, D.A.; Stone, S.R.; Llewellyn-Jones, C.; Keogan, M.T.; Wang, Z.M.; Rubin, H.; Carrell, R.W.; Stockley, R.A. The control of neutrophil chemotaxis by inhibitors of cathepsin G and chymotrypsin. J. Biol. Chem. 1995, 270, 23437–23443. [Google Scholar] [CrossRef]
- Tralau, T.; Meyer-Hoffert, U.; Schröder, J.M.; Wiedow, O. Human leukocyte elastase and cathepsin G are specific inhibitors of C5a-dependent neutrophil enzyme release and chemotaxis. Exp. Dermatol. 2004, 13, 316–325. [Google Scholar] [CrossRef]
- Chertov, O.; Ueda, H.; Xu, L.L.; Tani, K.; Murphy, W.J.; Wang, J.M.; Howard, O.M.; Sayers, T.J.; Oppenheim, J.J. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 1997, 186, 739–747. [Google Scholar] [CrossRef]
- Shipp, M.A.; Stefano, G.B.; Switzer, S.N.; Griffin, J.D.; Reinherz, E.L. CD10 (CALLA)/neutral endopeptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood 1991, 78, 1834–1841. [Google Scholar] [CrossRef]
- Zhang, L.X.; Ye, J.; Chen, Y.B.; Peng, H.L.; Chen, X.; Liu, L.; Jiang, A.G.; Huang, J.X. The effect of CD33 expression on inflammatory response in chronic obstructive pulmonary disease. Immunol. Investig. 2013, 42, 701–710. [Google Scholar] [CrossRef]
- Garley, M.; Jabloňská, E.; Suraźyński, A.; Grubczak, K.; Ratajczak-Wrona, W.; Iwaniuk, A.; Dabrovska, D.; Palka, J.A.; Moniuszko, M. Cytokine Network & NETs. Folia Biol. 2017, 63, 182–189. [Google Scholar]
- Witko-Sarsat, V.; Lesavre, P.; Lopez, S.; Bessou, G.; Hieblot, C.; Prum, B.; Noël, L.H.; Guillevin, L.; Ravaud, P.; Sermet-Gaudelus, I.; et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J. Am. Soc. Nephrol. 1999, 10, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Abdgawad, M.; Gunnarsson, L.; Segelmark, M.; Tapper, H.; Hellmark, T. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J. Leukoc. Biol. 2007, 81, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Garley, M.; Jabłońska, E. Heterogeneity Among Neutrophils. Arch. Immunol. Ther. Exp. 2018, 66, 21–30. [Google Scholar] [CrossRef]
- Jiang, J.; Guo, W.; Liang, X. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum. Immunol. 2014, 75, 1128–1137. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Chakravarti, A.; Rusu, D.; Flamand, N.; Borgeat, P.; Poubelle, P.E. Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Lab. Investig. 2009, 89, 1084–1099. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, R.; Ohnesorg, T.; Cho, V.; Lam, W.; Abhayaratna, W.P.; Gatenby, P.A.; Perera, C.; Zhang, Y.; Whittle, B.; et al. Heterogeneity of Human Neutrophil CD177 Expression Results from CD177P1 Pseudogene Conversion. PLoS Genet. 2016, 12, e1006067. [Google Scholar] [CrossRef]
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10+ and immature CD10- neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 2017, 129, 1343–1356. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Tirouvanziam, R.; Gernez, Y.; Conrad, C.K.; Moss, R.B.; Schrijver, I.; Dunn, C.E.; Davies, Z.A.; Herzenberg, L.A.; Herzenberg, L.A. Profound functional and signaling changes inviable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 2008, 105, 4335–4339. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Laval, J.; Forrest, O.A.; Preininger, M.; Brown, M.R.; Arafat, D.; Gibson, G.; Tangpricha, V.; Tirouvanziam, R. Mature cystic fibrosis airway neutrophils suppress T cell function: Evidence for a role of arginase 1 but not programmed death-ligand 1. J. Immunol. 2015, 194, 5520–5528. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Y.; Xu, W.; Lin, T.; Zeng, H. Expression of RANKL by peripheral neutrophils and its association with bone mineral density in COPD. Respirology 2017, 22, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.S.; Sigua, J.A.; Simpson, P.M.; Yan, K.; Hussain, S.A.; Santoro, J.L.; Buell, E.J.; Hunter, D.A.; Rohlfing, M.; Patadia, D.; et al. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J. Allergy Clin. Immunol. 2018, 142, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Sharma, S.; Conceição, J.; Carneiro, P.; Novais, F.; Scott, P.; Sundar, S.; Bacellar, O.; Carvalho, E.M.; Wilson, M.E. Phenotypic and functional characteristics of HLA-DR<sup>+</sup> neutrophils in Brazilians with cutaneous leishmaniasis. J. Leukoc. Biol. 2017, 101, 739–749. [Google Scholar]
- Meghraoui-Kheddar, A.; Chousterman, B.G.; Guillou, N.; Barone, S.M.; Granjeaud, S.; Vallet, H.; Corneau, A.; Guessous, K.; de Roquetaillade, C.; Boissonnas, A.; et al. Two New Neutrophil Subsets Define a Discriminating Sepsis Signature. Am. J. Respir. Crit. Care Med. 2022, 205, 46–59. [Google Scholar] [CrossRef]
- Demaret, J.; Venet, F.; Plassais, J.; Cazalis, M.A.; Vallin, H.; Friggeri, A.; Lepape, A.; Rimmelé, T.; Textoris, J.; Monneret, G. Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol. Lett. 2016, 178, 122–130. [Google Scholar] [CrossRef]
- Welin, A.; Amirbeagi, F.; Christenson, K.; Björkman, L.; Björnsdottir, H.; Forsman, H.; Dahlgren, C.; Karlsson, A.; Bylund, J. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS ONE 2013, 8, e69575. [Google Scholar] [CrossRef]
- Kangelaris, K.N.; Clemens, R.; Fang, X.; Jauregui, A.; Liu, T.; Vessel, K.; Deiss, T.; Sinha, P.; Leligdowicz, A.; Liu, K.D.; et al. A neutrophil subset defined by intracellular olfactomedin 4 is associated with mortality in sepsis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2021, 320, L892–L902. [Google Scholar] [CrossRef] [PubMed]
- Alder, M.N.; Opoka, A.M.; Lahni, P.; Hildeman, D.A.; Wong, H.R. Olfactomedin-4 Is a Candidate Marker for a Pathogenic Neutrophil Subset in Septic Shock. Crit. Care Med. 2017, 45, e426–e432. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am. J. Respir. Crit. Care Med. 2015, 191, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Ramakers, B.P.; Kamp, V.M.; Loi, A.L.; Lam, S.W.; Hietbrink, F.; Leenen, L.P.; Tool, A.T.; Pickkers, P.; Koenderman, L. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J. Leukoc. Biol. 2010, 88, 211–220. [Google Scholar] [CrossRef]
- Korkmaz, B.; Lesner, A.; Letast, S.; Mahdi, Y.K.; Jourdan, M.L.; Dallet-Choisy, S.; Marchand-Adam, S.; Kellenberger, C.; Viaud-Massuard, M.C.; Jenne, D.E.; et al. Neutrophil proteinase 3 and dipeptidyl peptidase I (cathepsin C) as pharmacological targets in granulomatosis with polyangiitis (Wegener granulomatosis). Semin. Immunopathol. 2013, 35, 411–421. [Google Scholar] [CrossRef]
- Ferrer-Lopez, P.; Renesto, P.; Schattner, M.; Bassot, S.; Laurent, P.; Chignard, M. Activation of human platelets by C5a-stimulated neutrophils: A role for cathepsin G. Am. J. Physiol. 1990, 258, C1100–C1107. [Google Scholar] [CrossRef]
- Malavika, M.; Sanju, S.; Poorna, M.R.; Vishnu Priya, V.; Sidharthan, N.; Varma, P.; Mony, U. Role of myeloid derived suppressor cells in sepsis. Int. Immunopharmacol. 2022, 104, 108452. [Google Scholar] [CrossRef] [PubMed]
- 308 Sayyadioskoie, S.R.; Schwacha, M.G. Myeloid-Derived Suppressor Cells (MDSCs) and the Immunoinflammatory Response to Injury (Mini Review). Shock 2021, 56, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yang, Y.; Okiji, T.; Azuma, M.; Nagai, S. Polymorphonuclear Myeloid-Derived Cells That Contribute to the Immune Paralysis Are Generated in the Early Phase of Sepsis via PD-1/PD-L1 Pathway. Infect Immun. 2021, 89, e00771-20. [Google Scholar] [CrossRef]
- Ruan, W.S.; Feng, M.X.; Xu, J.; Xu, Y.G.; Song, C.Y.; Lin, L.Y.; Li, L.; Lu, Y.Q. Early Activation of Myeloid-Derived Suppressor Cells Participate in Sepsis-Induced Immune Suppression via PD-L1/PD-1 Axis. Front. Immunol. 2020, 11, 1299. [Google Scholar] [CrossRef]
- Janols, H.; Bergenfelz, C.; Allaoui, R.; Larsson, A.M.; Rydén, L.; Björnsson, S.; Janciauskiene, S.; Wullt, M.; Bredberg, A.; Leandersson, K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 2014, 96, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Guérin, E.; Orabona, M.; Raquil, M.A.; Giraudeau, B.; Bellier, R.; Gibot, S.; Béné, M.C.; Lacombe, F.; Droin, N.; Solary, E.; et al. Circulatingimmature granulocytes with T-cell killing functions predict sepsis deterioration. Crit. Care Med. 2014, 42, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.B.; Harthug, S.; Kalager, T.; Digranes, A.; Solberg, C.O. Bloodstream infections at a Norwegian university hospital, 1974-1979 and 1988-1989: Changing etiology, clinical features, and outcome. Clin. Infect Dis. 1994, 19, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Linde-Zwirble, W.T.; Peck-Palmer, O.M.; Barnato, A.E.; Weissfeld, L.A.; Angus, D.C. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA 2010, 303, 2495–2503. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Li, X. Peptidoglycan-based immunomodulation. Appl. Microbiol. Biotechnol. 2022, 106, 981–993. [Google Scholar] [CrossRef]
- de Jong, N.W.M.; van Kessel, K.P.M.; van Strijp, J.A.G. Immune Evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Postma de Haas, C.J.; Veldkamp, K.E.; Peschel, A.; Weerkamp, F.; Van Wamel, W.J.; Heezius, E.C.; Poppelier, M.J.; Van Kessel, K.P.; van Strijp, J.A. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004, 199, 687–695. [Google Scholar] [CrossRef]
- Postma, B.; Poppelier, M.J.; van Galen, J.C.; Prossnitz, E.R.; van Strijp, J.A.; de Haas, C.J.; van Kessel, K.P. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 2004, 172, 6994–7001. [Google Scholar] [CrossRef]
- Haas, P.J.; de Haas, C.J.; Kleibeuker, W.; Poppelier, M.J.; van Kessel, K.P.; Kruijtzer, J.A.; Liskamp, R.M.; van Strijp, J.A. N-terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J. Immunol. 2004, 173, 5704–5711. [Google Scholar] [CrossRef] [PubMed]
- Postma, B.; Kleibeuker, W.; Poppelier, M.J.; Boonstra, M.; Van Kessel, K.P.; Van Strijp, J.A.; de Haas, C.J. Residues 10-18 within the C5a receptor N terminus compose a binding domain for chemotaxis inhibitory protein of Staphylococcus aureus. J. Biol. Chem. 2005, 280, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Haas, P.; de Haas, C.J.; Poppelier, M.J.; van Kessel, K.P.; van Strijp, J.A.; Dijkstra, K.; Scheek, R.M.; Fan, H.; Kruijtzer, J.A.; Liskamp, R.M.; et al. The structure of the C5a receptor-blocking domain of chemotaxis inhibitory protein of Staphylococcus aureus is related to a group of immune evasive molecules. J. Mol. Biol. 2005, 353, 859–872. [Google Scholar] [CrossRef]
- Prat, C.; Bestebroer, J.; de Haas, C.J.; van Strijp, J.A.; van Kessel, K.P. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 2006, 177, 8017–8026. [Google Scholar] [CrossRef]
- Prat, C.; Haas, P.J.; Bestebroer, J.; de Haas, C.J.; van Strijp, J.A.; van Kessel, K.P. A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J. Immunol. 2009, 183, 6569–6578. [Google Scholar] [CrossRef]
- Laarman, A.J.; Mijnheer, G.; Mootz, J.M.; van Rooijen, W.J.; Ruyken, M.; Malone, C.L.; Heezius, E.C.; Ward, R.; Milligan, G.; van Strijp, J.A.; et al. Staphylococcus aureus Staphopain A inhibits CXCR2-dependent neutrophil activation and chemotaxis. EMBO J. 2012, 31, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Seo, K.S.; Cartwright, R.A.; Connelley, T.; Chuang-Smith, O.N.; Merriman, J.A.; Guinane, C.M.; Park, J.Y.; Bohach, G.A.; Schlievert, P.M.; et al. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 2011, 7, e1002271. [Google Scholar] [CrossRef] [PubMed]
- Bestebroer, J.; Poppelier, M.J.; Ulfman, L.H.; Lenting, P.J.; Denis, C.V.; van Kessel, K.P.; van Strijp, J.A.; de Haas, C.J. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 2007, 109, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Bestebroer, J.; van Kessel, K.P.; Azouagh, H.; Walenkamp, A.M.; Boer, I.G.; Romijn, R.A.; van Strijp, J.A.; de Haas, C.J. Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 2009, 113, 328–337. [Google Scholar] [CrossRef]
- Bardoel, B.W.; Vos, R.; Bouman, T.; Aerts, P.C.; Bestebroer, J.; Huizinga, E.G.; Brondijk, T.H.; van Strijp, J.A.; de Haas, C.J. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J. Mol. Med. 2012, 90, 1109–1120. [Google Scholar] [CrossRef]
- Yokoyama, R.; Itoh, S.; Kamoshida, G.; Takii, T.; Fujii, S.; Tsuji, T.; Onozaki, K. Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect Immun. 2012, 80, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Koymans, K.J.; Bisschop, A.; Vughs, M.M.; van Kessel, K.P.; de Haas, C.J.; van Strijp, J.A. Staphylococcal Superantigen-Like Protein 1 and 5 (SSL1 & SSL5) Limit Neutrophil Chemotaxis and Migration through MMP-Inhibition. Int. J. Mol. Sci. 2016, 17, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sunagar, R.; Gosselin, E. Bacterial Protein Toll-Like-Receptor Agonists: A Novel Perspective on Vaccine Adjuvants. Front. Immunol. 2019, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Nakayama, H.; Hanafusa, K. Lactosylceramide-enriched microdomains mediate human neutrophil immunological functions via carbohydrate-carbohydrate interaction. Glycoconj. J. 2022, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Nagafuku, M.; Suzuki, A.; Iwabuchi, K.; Inokuchi, J.I. The regulatory roles of glycosphingolipid-enriched lipid rafts in immune systems. FEBS Lett. 2018, 592, 3921–3942. [Google Scholar] [CrossRef]
- Yuan, K.; Huang, C.; Fox, J.; Gaid, M.; Weaver, A.; Li, G.; Singh, B.B.; Gao, H.; Wu, M. Elevated inflammatory response in caveolin-1-deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor kappaB (NF-kappaB). J. Biol. Chem. 2011, 286, 21814–21825. [Google Scholar] [CrossRef]
- Medina, F.A.; de Almeida, C.J.; Dew, E.; Li, J.; Bonuccelli, G.; Williams, T.M.; Cohen, A.W.; Pestell, R.G.; Frank, P.G.; Tanowitz, H.B.; et al. Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006, 74, 6665–6674. [Google Scholar] [CrossRef]
- Hu, G.; Ye, R.D.; Dinauer, M.C.; Malik, A.B.; Minshall, R.D. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am. J. Physiol. Lung. Cell Mol. Physiol. 2008, 294, L178–L186. [Google Scholar] [CrossRef]
- Edens, H.A.; Parkos, C.A.; Liang, T.W.; Jesaitis, A.J.; Cutler, J.E.; Miettinen, H.M. Non-serum-dependent chemotactic factors produced by Candida albicans stimulate chemotaxis by binding to the formyl peptide receptor on neutrophils and to an unknown receptor on macrophages. Infect Immun. 1999, 67, 1063–1071. [Google Scholar] [CrossRef]
- O’Brien, X.M.; Reichner, J.S. Neutrophil Integrins and Matrix Ligands and NET Release. Front. Immunol. 2016, 7, 363. [Google Scholar] [CrossRef]
- Sato, T.; Iwabuchi, K.; Nagaoka, I.; Adachi, Y.; Ohno, N.; Tamura, H.; Seyama, K.; Fukuchi, Y.; Nakayama, H.; Yoshizaki, F.; et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J. Leukoc. Biol. 2006, 80, 204–211. [Google Scholar] [CrossRef]
- Haas-Stapleton, E.J.; Lu, Y.; Hong, S.; Arita, M.; Favoreto, S.; Nigam, S.; Serhan, C.N.; Agabian, N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS ONE 2007, 2, e1316. [Google Scholar] [CrossRef]
- Bryan, A.M.; Del Poeta, M. Secretory aspartyl proteinases induce neutrophil chemotaxis in vivo. Virulence 2016, 7, 737–739. [Google Scholar] [CrossRef]
- Gabrielli, E.; Sabbatini, S.; Roselletti, E.; Kasper, L.; Perito, S.; Hube, B.; Cassone, A.; Vecchiarelli, A.; Pericolini, E. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence 2016, 7, 819–825. [Google Scholar] [CrossRef]
- Ran, Y.; Iwabuchi, K.; Yamazaki, M.; Tsuboi, R.; Ogawa, H. Secreted aspartic proteinase from Candida albicans acts as a chemoattractant for peripheral neutrophils. J. Dermatol. Sci. 2013, 72, 191–193. [Google Scholar] [CrossRef]
- Bruno, V.M.; Shetty, A.C.; Yano, J.; Fidel, P.L., Jr.; Noverr, M.C.; Peters, B.M. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. mBio 2015, 6, e00182-15. [Google Scholar] [CrossRef]
- Fietta, A.; Sacchi, F.; Bersani, C.; Grassi, F.; Mangiarotti, P.; Grassi, G.G. Effect of beta-lactam antibiotics on migration and bactericidal activity of human phagocytes. Antimicrob. Agents. Chemother. 1983, 23, 930–931. [Google Scholar] [CrossRef]
- Forsgren, A.; Banck, G.; Beckman, H.; Bellahsène, A. Antibiotic-host defence interactions in vitro and in vivo. Scand. J. Infect Dis. Suppl. 1980, 24, 195–203. [Google Scholar]
- Webster, G.F.; Leyden, J.J.; McGinley, K.J.; McArthur, W.P. Suppression of polymorphonuclear leukocyte chemotactic factor production in Propionibacterium acnes by subminimal inhibitory concentrations of tetracycline, ampicillin, minocycline, and erythromycin. Antimicrob. Agents Chemother. 1982, 21, 770–772. [Google Scholar] [CrossRef]
- Kenny, M.T.; Balistreri, F.J.; Torney, H.L. beta-Lactam antibiotic modulation of murine neutrophil cytokinesis. Immunopharmacol. Immunotoxicol. 1992, 14, 797–811. [Google Scholar] [CrossRef]
- Hbabi, L.; Roques, C.; Michel, G.; Perruchet, A.M.; Benoist, H. In vitro stimulation of polymorphonuclear cell adhesion by ribomunyl and antibiotic + ribomunyl combinations: Effects on CD18, CD35 and CD16 expression. Int. J. Immunopharmacol. 1993, 15, 163–173. [Google Scholar] [CrossRef]
- Hofbauer, R.; Moser, D.; Gmeiner, B.; Kaye, A.D.; Kapiotis, S.; Wagner, O.; Frass, M. Amoxycillin/clavulanic acid combinations increase transmigration of leucocytes through endothelial cell monolayers: Endothelial cells play a key role. J. Antimicrob. Chemother. 1999, 44, 465–469. [Google Scholar] [CrossRef]
- Vuddhakul, V.; Mai, G.T.; McCormack, J.G.; Seow, W.K.; Thong, Y.H. Suppression of neutrophil and lymphoproliferative responses in vitro by itraconazole but not fluconazole. Int. J. Immunopharmacol. 1990, 12, 639–645. [Google Scholar] [CrossRef]
- Gürer, U.S.; Cevikbaş, A.; Johansson, C.; Derici, K.; Yardimci, T. Effect of fluconazole on human polymorphonuclear leucocyte functions ex vivo against Candida albicans. Chemotherapy 1999, 45, 277–283. [Google Scholar] [CrossRef]
- Roilides, E.; Walsh, T.J.; Rubin, M.; Venzon, D.; Pizzo, P.A. Effects of antifungal agents on the function of human neutrophils in vitro. Antimicrob. Agents Chemother. 1990, 34, 196–201. [Google Scholar] [CrossRef]
- Simitsopoulou, M.; Roilides, E.; Walsh, T.J. Immunomodulatory properties of antifungal agents on phagocytic cells. Immunol. Investig. 2011, 40, 809–824. [Google Scholar] [CrossRef]
- Butcher, S.K.; Chahal, H.; Nayak, L.; Sinclair, A.; Henriquez, N.V.; Sapey, E.; O’Mahony, D.; Lord, J.M. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 2001, 70, 881–886. [Google Scholar] [CrossRef]
- De Martinis, M.; Modesti, M.; Ginaldi, L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol. Cell Biol. 2004, 82, 415–420. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Fortin, C.F.; McDonald, P.P.; Lesur, O.; Fülöp, T., Jr. Aging and neutrophils: There is still much to do. Rejuvenation Res. 2008, 11, 873–882. [Google Scholar] [CrossRef]
- Larbi, A.; Douziech, N.; Fortin, C.; Linteau, A.; Dupuis, G.; Fulop, T., Jr. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun. Ageing 2005, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Larbi, A.; Lesur, O.; Douziech, N.; Fulop, T., Jr. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J. Leukoc. Biol. 2006, 79, 1061–1072. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Douziech, N.; Fortin, C.; Guérard, K.P.; Lesur, O.; Khalil, A.; Dupuis, G. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004, 3, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Panda, A.; Joshi, S.R.; Qian, F.; Allore, H.G.; Montgomery, R.R. Dysregulation of human Toll-like receptor function in aging. Ageing Res. Rev. 2011, 10, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Zhang, Y.; Shaw, A.C.; Lee, P.J. The role of Toll-like receptors in age-associated lung diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.; Simone, O.; Piazzolla, G.; Stella, I.; Antonaci, S. Age-related impairment of GM-CSF-induced signalling in neutrophils: Role of SHP-1 and SOCS proteins. Ageing Res. Rev. 2007, 6, 81–93. [Google Scholar] [CrossRef]
- Nogueira-Neto, J.; Cardoso, A.S.; Monteiro, H.P.; Fonseca, F.L.; Ramos, L.R.; Junqueira, V.B.; Simon, K.A. Basal neutrophil function in human aging: Implications in endothelial cell adhesion. Cell Biol. Int. 2016, 40, 796–802. [Google Scholar] [CrossRef]
- Bou Ghanem, E.N.; Clark, S.; Du, X.; Wu, D.; Camilli, A.; Leong, J.M.; Meydani, S.N. The α-tocopherol form of vitamin E reverses age-associated susceptibility to streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J. Immunol. 2015, 194, 1090–1099. [Google Scholar] [CrossRef]
- Sapey, E.; Patel, J.M.; Greenwood, H.L.; Walton, G.M.; Hazeldine, J.; Sadhra, C.; Parekh, D.; Dancer, R.C.A.; Nightingale, P.; Lord, J.M.; et al. Pulmonary Infections in the Elderly Lead to Impaired Neutrophil Targeting, Which Is Improved by Simvastatin. Am. J. Respir. Crit. Care Med. 2017, 196, 1325–1336. [Google Scholar] [CrossRef]
- Baëhl, S.; Garneau, H.; Le Page, A.; Lorrain, D.; Viens, I.; Svotelis, A.; Lord, J.M.; Phillips, A.C.; Cabana, F.; Larbi, A.; et al. Altered neutrophil functions in elderly patients during a 6-month follow-up period after a hip fracture. Exp. Gerontol. 2015, 65, 58–68. [Google Scholar] [CrossRef]
- Chen, M.M.; Palmer, J.L.; Plackett, T.P.; Deburghgraeve, C.R.; Kovacs, E.J. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp. Gerontol. 2014, 54, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Barkaway, A.; Rolas, L.; Joulia, R.; Bodkin, J.; Lenn, T.; Owen-Woods, C.; Reglero-Real, N.; Stein, M.; Vázquez-Martínez, L.; Girbl, T.; et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021, 54, 1494–1510. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, P.; Ragnoli, B.; Radaeli, A.; Moscato, G.; Malerba, M. Age-related increase of airway neutrophils in older healthy nonsmoking subjects. Rejuvenation Res. 2011, 14, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Ershler, W.; Rosenthal, N.S.; Lu, X.G.; Peterson, K. Immune dysregulation in the aging human lung. Am. J. Respir. Crit. Care Med. 1996, 153, 1072–1079. [Google Scholar] [CrossRef]
- Meyer, K.C.; Rosenthal, N.S.; Soergel, P.; Peterson, K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech. Ageing Dev. 1998, 104, 169–181. [Google Scholar] [CrossRef]
- Menter, T.; Giefing-Kroell, C.; Grubeck-Loebenstein, B.; Tzankov, A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiology 2014, 81, 160–167. [Google Scholar] [CrossRef]
- Tseng, C.W.; Liu, G.Y. Expanding roles of neutrophils in aging hosts. Curr. Opin. Immunol. 2014, 29, 43–48. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Int. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Fernando, S.M.; Guo, K.H.; Lukasik, M.; Rochwerg, B.; Cook, D.J.; Kyeremanteng, K.; Perry, J.J. Frailty and associated prognosis among older emergency department patients with suspected infection: A prospective, observational cohort study. CJEM 2020, 22, 687–691. [Google Scholar]
- Lee, H.Y.; Lee, J.; Jung, Y.S.; Kwon, W.Y.; Oh, D.K.; Park, M.H.; Lim, C.M.; Lee, S.M.; Korean Sepsis Alliance (KSA) Investigators. Preexisting Clinical Frailty Is Associated With Worse Clinical Outcomes in Patients With Sepsis. Crit. Care Med. 2022, 50, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.E.M.; Boumendil, A.; Flaatten, H.; Guidet, B.; Ibarz, M.; Jung, C.; Moreno, R.; Morandi, A.; Andersen, F.H.; Zafeiridis, T.; et al. Frailty is associated with long-term outcome in patients with sepsis who are over 80 years old: Results from an observational study in 241 European ICUs. Age Ageing 2021, 50, 1719–1727. [Google Scholar]
- Matsuda, W.; Uemura, T.; Yamamoto, M.; Uemura, Y.; Kimura, A. Impact of frailty on protocol-based weaning from mechanical ventilation in patients with sepsis: A retrospective cohort study. Acute Med. Surg. 2020, 7, e608. [Google Scholar]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Picca, A.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Bossola, M.; Cesari, M.; Onder, G.; Landi, F.; et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Exp. Gerontol. 2019, 122, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Garrido, J.; Navarro-Martínez, R.; Buigues-González, C.; Martínez-Martínez, M.; Ruiz-Ros, V.; Cauli, O. The value of neutrophil and lymphocyte count in frail older women. Exp. Gerontol. 2014, 54, 35–41. [Google Scholar]
- Samson, L.D.; Boots, A.M.H.; Verschuren, W.M.M.; Picavet, H.S.J.; Engelfriet, P.; Buisman, A.M. Frailty is associated with elevated CRP trajectories and higher numbers of neutrophils and monocytes. Exp. Gerontol. 2019, 125, 110674. [Google Scholar]
- Leng, S.X.; Xue, Q.L.; Tian, J.; Huang, Y.; Yeh, S.H.; Fried, L.P. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: Results from the Women’s Health and Aging Studies, I. Exp. Gerontol. 2009, 44, 511–516. [Google Scholar] [CrossRef]
- Leng, S.X.; Xue, Q.L.; Tian, J.; Walston, J.D.; Fried, L.P. Inflammation and frailty in older women. J. Am. Geriatr. Soc. 2007, 55, 864–871. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Loukov, D.; Naidoo, A.; Puchta, A.; Johnstone, J.; Millar, J.; Lelic, A.; Novakowski, K.E.; Dorrington, M.G.; Loeb, M.; et al. Circulating TNF and mitochondrial DNA are major determinants of neutrophil phenotype in the advanced-age, frail elderly. Mol. Immunol. 2015, 65, 148–156. [Google Scholar] [PubMed]
- Wilson, D.; Drew, W.; Jasper, A.; Crisford, H.; Nightingale, P.; Newby, P.; Jackson, T.; Lord, J.M.; Sapey, E. Frailty Is Associated With Neutrophil Dysfunction Which Is Correctable With Phosphoinositol-3-Kinase Inhibitors. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Suzuki, K.; Machida, K.; Saiki, C.; Murayama, R.; Sugita, M. Relationships between lifestyle factors and neutrophil functions in the elderly. J. Clin. Lab. Anal. 2002, 16, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Hasserjian, R.P. Myelodysplastic Syndrome Updated. Pathobiology 2019, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Vo, A.K.; Rekvam, H. Hematopoiesis, Inflammation and Aging-The Biological Background and Clinical Impact of Anemia and Increased C-Reactive Protein Levels on Elderly Individuals. J. Clin. Med. 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Moeller, M.; Bornemann, L.; Bessen, C.; Sobczak, C.; Schmitz, S.; Witjes, L.; Kruithoff, K.; Kohn, C.; Just, O.; et al. Surveillance of Myelodysplastic Syndrome via Migration Analyses of Blood Neutrophils: A Potential Prognostic Tool. J. Immunol. 2018, 201, 3546–3557. [Google Scholar] [CrossRef]
- Fuhler, G.M.; Knol, G.J.; Drayer, A.L.; Vellenga, E. Impaired interleuk in-8- and GROalpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. J. Leukoc. Biol. 2005, 77, 257–266. [Google Scholar] [CrossRef]
- Mazzone, A.; Ricevuti, G.; Pasotti, D.; Fossati, G.; Mazzucchelli, I.; Cavigliano, P.; Notario, A. The CD11/CD18 granulocyte adhesion molecules in myelodysplastic syndromes. Br. J. Haematol. 1993, 83, 245–252. [Google Scholar] [CrossRef]
- Ricevuti, G.; Mazzone, A.; Pasotti, D.; Fossati, G.; Mazzucchelli, I.; Notario, A. The role of integrins in granulocyte dysfunction in myelodysplastic syndrome. Leuk. Res. 1993, 17, 609–619. [Google Scholar] [CrossRef]
- Cao, M.; Shikama, Y.; Kimura, H.; Noji, H.; Ikeda, K.; Ono, T.; Ogawa, K.; Takeishi, Y.; Kimura, J. Mechanisms of Impaired Neutrophil Migration by MicroRNAs in Myelodysplastic Syndromes. J. Immunol. 2017, 198, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, L.; Schuster, M.; Schmitz, S.; Sobczak, C.; Bessen, C.; Merz, S.F.; Jöckel, K.H.; Haverkamp, T.; Gunzer, M.; Göthert, J.R. Defective migration and dysmorphology of neutrophil granulocytes in atypical chronic myeloid leukemia treated with ruxolitinib. BMC Cancer 2020, 20, 650. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; Mohty, M.; Montoto, S.; et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transpl. 2020, 55, 1604–1613. [Google Scholar] [CrossRef]
- Hansen, B.A.; Wendelbo, Ø.; Bruserud, Ø.; Hemsing, A.L.; Mosevoll, K.A.; Reikvam, H. Febrile Neutropenia in Acute Leukemia. Epidemiology, Etiology, Pathophysiology and Treatment. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020009. [Google Scholar] [PubMed]
- Thunström Salzer, A.; Niemiec, M.J.; Hosseinzadeh, A.; Stylianou, M.; Åström, F.; Röhm, M.; Ahlm, C.; Wahlin, A.; Ermert, D.; Urban, C.F. Assessment of Neutrophil Chemotaxis Upon G-CSF Treatment of Healthy Stem Cell Donors and in Allogeneic Transplant Recipients. Front. Immunol. 2018, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, F.; Minonzio, F.; Carbonelli, V.; Ongari, A.M.; Mocellin, M.C.; Soligo, D.; Annaloro, C.; Della Volpe, A.; Lambertenghi Dliliers, G. Abnormal neutrophil chemotaxis in bone marrow transplant patients correlates with impaired 31D8 monoclonal antibody binding. Haematologica 1995, 80, 123–129. [Google Scholar] [PubMed]
- Baron, S.; Binenbaum, Y.; Berger Achituv, S.; Tene, Y.; Elhasid, R. Neutrophil Elastase Activity as a Surrogate Marker for Neutrophil Extracellular Trap Formation following Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2019, 25, 2350–2356. [Google Scholar] [CrossRef]
- Miyagawa, B.; Klingemann, H.G. Phagocytosis and burst activity of granulocytes and monocytes after stem cell transplantation. J. Lab. Clin. Med. 1997, 129, 634–637. [Google Scholar] [PubMed]
- Rommeley, M.; Spies-Weisshart, B.; Schilling, K.; Hochhaus, A.; Sayer, H.G.; Scholl, S. Reconstitution and functional analyses of neutrophils and distinct subsets of monocytes after allogeneic stem cell transplantation. J. Cancer Res. Clin. Oncol. 2011, 137, 1293–1300. [Google Scholar] [CrossRef]
- Döring, M.; Cabanillas Stanchi, K.M.; Erbacher, A.; Haufe, S.; Schwarze, C.P.; Handgretinger, R.; Hofbeck, M.; Kerst, G. Phagocytic activity of monocytes, their subpopulations and granulocytes during post-transplant adverse events after hematopoietic stem cell transplantation. Immunobiology 2015, 220, 605–613. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Frink, M.; Choudhry, M.A.; Bland, K.I.; Chaudry, I.H. Metabolic modulators following trauma sepsis: Sex hormones. Crit. Care Med. 2007, 35 (Suppl. S9), S621–S629. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, J.; Yang, F.; Chen, J.J.; Li, Z.F.; Yi, C.L.; Gao, W.; Bai, X.J. The influence of sex on outcomes in trauma patients: A meta-analysis. Am. J. Surg. 2015, 210, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence 2014, 5, 12–19. [Google Scholar] [CrossRef]
- Sakr, Y.; Elia, C.; Mascia, L.; Barberis, B.; Cardellino, S.; Livigni, S.; Fiore, G.; Filippini, C.; Ranieri, V.M. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit. Care 2013, 17, R50. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; D’Haese, J.G.; Angele, M.K.; Chaudry, I.H. Potential therapeutic targets for sepsis in women. Exp. Opin. Ther. Targets 2015, 19, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Kahlke, V.; Staubach, K.H.; Zabel, P.; Stüber, F. Gender differences in human sepsis. Arch. Surg. 1998, 133, 1200–1205. [Google Scholar] [CrossRef]
- Kisat, M.; Villegas, C.V.; Onguti, S.; Zafar, S.N.; Latif, A.; Efron, D.T.; Haut, E.R.; Schneider, E.B.; Lipsett, P.A.; Zafar, H.; et al. Predictors of sepsis in moderately severely injured patients: An analysis of the National Trauma Data Bank. Surg. Infect. 2013, 14, 62–68. [Google Scholar] [CrossRef]
- Offner, P.J.; Moore, E.E.; Biffl, W.L. Male gender is a risk factor for major infections after surgery. Arch. Surg. 1999, 134, 935–938. [Google Scholar] [CrossRef]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef]
- Kwak, S.H.; Mitra, S.; Bdeir, K.; Strassheim, D.; Park, J.S.; Kim, J.Y.; Idell, S.; Cines, D.; Abraham, E. The kringle domain of urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation through interaction with αVβ3 integrins. J. Leukoc. Biol. 2005, 78, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Cody, V.; Tang, H.Y.; Lin, H.Y. Small molecule hormone or hormone-like ligands of integrin αVβ3: Implications for cancer cell behavior. Horm. Cancer 2013, 4, 335–342. [Google Scholar] [PubMed]
- Ho, Y.; Li, Z.L.; Shih, Y.J.; Chen, Y.R.; Wang, K.; Whang-Peng, J.; Lin, H.Y.; Davis, P.J. Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 2906. [Google Scholar] [CrossRef] [PubMed]
- Guidet, B.; Maury, E. Sex and severe sepsis. Crit. Care 2013, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Mosevoll, K.A.; Hansen, B.A.; Gundersen, I.M.; Reikvam, H.; Bruserud, Ø.; Bruserud, Ø.; Wendelbo, Ø. Patients with Bacterial Sepsis Are Heterogeneous with Regard to Their Systemic Lipidomic Profiles. Metabolites 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Mosevoll, K.A.; Hansen, B.A.; Gundersen, I.M.; Reikvam, H.; Bruserud, Ø.; Bruserud, Ø.; Wendelbo, Ø. Systemic Metabolomic Profiles in Adult Patients with Bacterial Sepsis: Characterization of Patient Heterogeneity at the Time of Diagnosis. Biomolecules 2023, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Baixauli, F.; Palacin, M. Editorial: Amino Acid Transport and Metabolism During Homeostasis and Inflammation. Front. Immunol. 2022, 12, 833258. [Google Scholar]
- Halaby, M.J.; McGaha, T.L. Amino Acid Transport and Metabolism in Myeloid Function. Front. Immunol. 2021, 12, 695238. [Google Scholar] [CrossRef]
- Tomé, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Eur. J. Clin. Nutr. 2021, 75, 1319–1327. [Google Scholar]
- Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef]
- Srisawat, N.; Kulvichit, W.; Tungsanga, S.; Peerapornratana, S.; Vorasitchai, S.; Tangkanakul, C.; Lumlertgul, N.; Komaenthammasophon, C.; Praditpornsilpa, K.; Tungsanga, K.; et al. The role of neutrophil chemotaxis activity as an immunologic biomarker to predict mortality in critically-ill patients with severe sepsis. J. Crit. Care 2020, 56, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.; Patel, J.; Mahida, R.; Wang, Q.; Parekh, D.; Dancer, R.C.; Khiroya, H.; Sapey, E.; Thickett, D.R. Simvastatin to modify neutrophil function in older patients with septic pneumonia (SNOOPI): Study protocol for a randomised placebo-controlled trial. Trials 2014, 15, 332. [Google Scholar] [PubMed]
- Sapey, E.; Patel, J.M.; Greenwood, H.; Walton, G.M.; Grudzinska, F.; Parekh, D.; Mahida, R.Y.; Dancer, R.C.A.; Lugg, S.T.; Howells, P.A.; et al. Simvastatin Improves Neutrophil Function and Clinical Outcomes in Pneumonia. A Pilot Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1282–1293. [Google Scholar] [CrossRef]
- Bernard, G.R.; Reines, H.D.; Halushka, P.V.; Higgins, S.B.; Metz, C.A.; Swindell, B.B.; Wright, P.E.; Watts, F.L.; Vrbanac, J.J. Prostacyclin and thromboxane A2 formation is increased in human sepsis syndrome. Effects of cyclooxygenase inhibition. Am. Rev. Respir. Dis. 1991, 144, 1095–1101. [Google Scholar] [CrossRef]
- Hustinx, W.N.; Van Kessel, C.P.; Heezius, E.; Burgers, S.; Lammers, J.W.; Hoepelman, I.M. Effects of granulocyte colony-stimulating factor (G-CSF) treatment on granulocyte function and receptor expression in patients with ventilator-dependent pneumonia. Clin. Exp. Immunol. 1998, 112, 334–340. [Google Scholar] [CrossRef]
- Grudzinska, F.S.; Brodlie, M.; Scholefield, B.R.; Jackson, T.; Scott, A.; Thickett, D.R.; Sapey, E. Neutrophils in community-acquired pneumonia: Parallels in dysfunction at the extremes of age. Thorax 2020, 75, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gurney, H.; Anderson, H.; Radford, J.; Potter, M.R.; Swindell, R.; Steward, W.; Kamthan, A.; Chang, J.; Weiner, J.; Thatcher, N.; et al. Infection risk in patients with small cell lung cancer receiving intensive chemotherapy and recombinant human ranulocyte-macrophage colony-stimulating factor. Eur. J. Cancer 1992, 28, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.S.; McIlheran, S.M.; Banks, S.M.; Vadhan-Raj, S. The effects of treatmentwith PIXY321 (GM-CSF/IL-3 fusion protein) on human polymorphonuclear leukocyte function. Exp. Hematol. 1993, 21, 1467–1472. [Google Scholar] [PubMed]
- Bo, L.; Wang, F.; Zhu, J.; Li, J.; Deng, X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit. Care 2011, 15, R58. [Google Scholar] [CrossRef]
- Nick, J.A.; Coldren, C.D.; Geraci, M.W.; Poch, K.R.; Fouty, B.W.; O’Brien, J.; Gruber, M.; Zarini, S.; Murphy, R.C.; Kuhn, K.; et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 2004, 104, 3878–3885. [Google Scholar]
- Srisawat, N.; Tungsanga, S.; Lumlertgul, N.; Komaenthammasophon, C.; Peerapornratana, S.; Thamrongsat, N.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit. Care 2018, 22, 279. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Young, S.K.; Arndt, P.G.; Lieber, J.G.; Suratt, B.T.; Poch, K.R.; Avdi, N.J.; Malcolm, K.C.; Taube, C.; Henson, P.M.; et al. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase. J. Immunol. 2002, 169, 5260–5269. [Google Scholar] [PubMed]
- Hotchkiss, R.S.; Colston, E.; Yende, S.; Angus, D.C.; Moldawer, L.L.; Crouser, E.D.; Martin, G.S.; Coopersmith, C.M.; Brakenridge, S.; Mayr, F.B.; et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit. Care Med. 2019, 47, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Colston, E.; Yende, S.; Crouser, E.D.; Martin, G.S.; Albertson, T.; Bartz, R.R.; Brakenridge, S.C.; Delano, M.J.; Park, P.K.; et al. Immune checkpoint inhibition in sepsis: A Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Int. Care Med. 2019, 45, 1360–1371. [Google Scholar] [CrossRef]
- Xerri, A.; Gallardo, F.; Kober, F.; Mathieu, C.; Fourny, N.; Tran, T.T.; Mege, J.L.; Singer, M.; Lalevée, N.; Bernard, M.; et al. Female hormones prevent sepsis-induced cardiac dysfunction: An experimental randomized study. Sci. Rep. 2022, 12, 4939. [Google Scholar] [CrossRef]
- Opal, S.M.; Fisher, C.J., Jr.; Dhainaut, J.F.; Vincent, J.L.; Brase, R.; Lowry, S.F.; Sadoff, J.C.; Slotman, G.J.; Levy, H.; Balk, R.A.; et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 1997, 25, 1115–1124. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar]
- Leventogiannis, K.; Kyriazopoulou, E.; Antonakos, N.; Kotsaki, A.; Tsangaris, I.; Markopoulou, D.; Grondman, I.; Rovina, N.; Theodorou, V.; Antoniadou, E.; et al. Toward personalized immunotherapy in sepsis: The PROVIDE randomized clinical trial. Cell Rep. Med. 2022, 3, 100817. [Google Scholar] [CrossRef]
- Nukarinen, E.; Tervahartiala, T.; Valkonen, M.; Hynninen, M.; Kolho, E.; Pettilä, V.; Sorsa, T.; Backman, J.; Hästbacka, J. Targeting matrix metalloproteinases with intravenous doxycycline in severe sepsis--A randomised placebo-controlled pilot trial. Pharmacol. Res. 2015, 99, 44–51. [Google Scholar] [CrossRef]
- Clark, M.A.; Plank, L.D.; Connolly, A.B.; Streat, S.J.; Hill, A.A.; Gupta, R.; Monk, D.N.; Shenkin, A.; Hill, G.L. Effect of a chimeric antibody to tumor necrosis factor-alpha on cytokine and physiologic responses in patients with severe sepsis—A randomized, clinical trial. Crit. Care Med. 1998, 26, 1650–1659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).