High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Human iPSC-Derived Neurons and Human Primary Astrocytes
2.2. Two-Dimensional Cell Culture
2.3. Three-Dimensional Cell Culture
2.4. Calcium Transient Spontaneous Oscillation Waveform Recording in 2D and 3D
2.5. Compound Selection, Drug Dilution, and Addition
2.6. Data Analysis of Ca2+ Oscillation
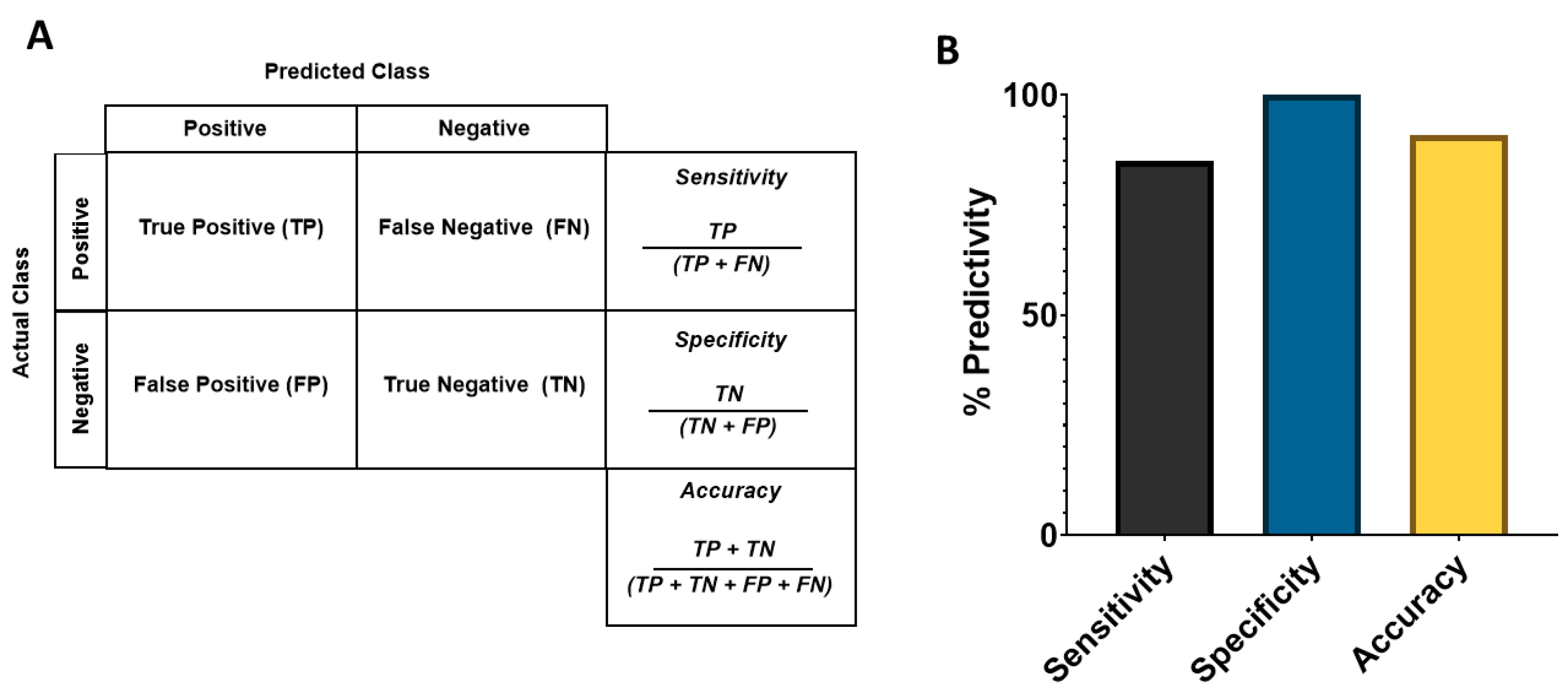
2.7. Sensitivity, Specificity, and Predictive Value of Potential Cardiac Risks in the Assay
2.8. Immunofluorescence
2.9. Characterization of Gene Expression (RNA-seq)
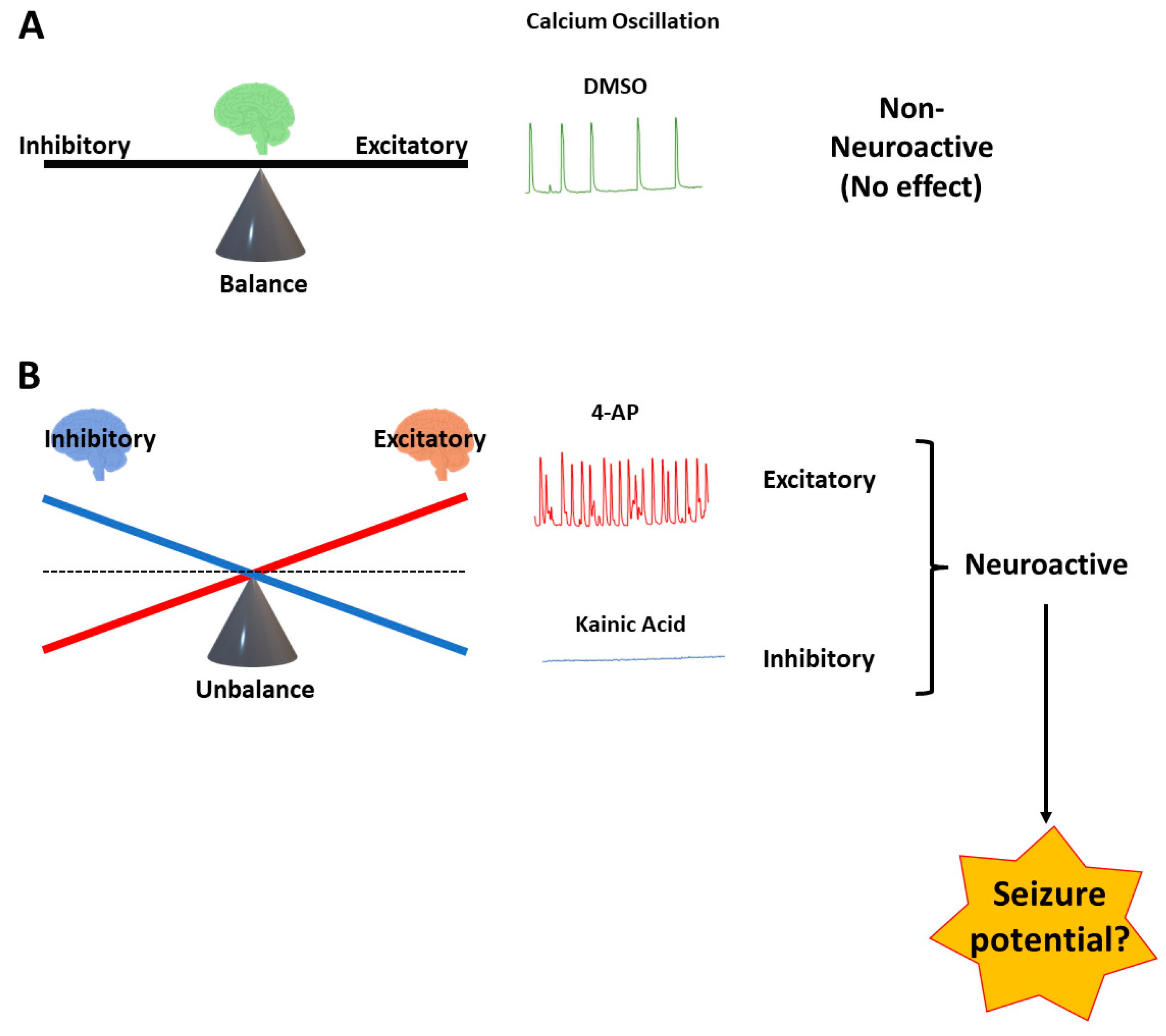
3. Results
3.1. Time Course of Spontaneous Ca2+ Oscillations in hiPSC-Derived Neurons in 2D and 3D Cultures
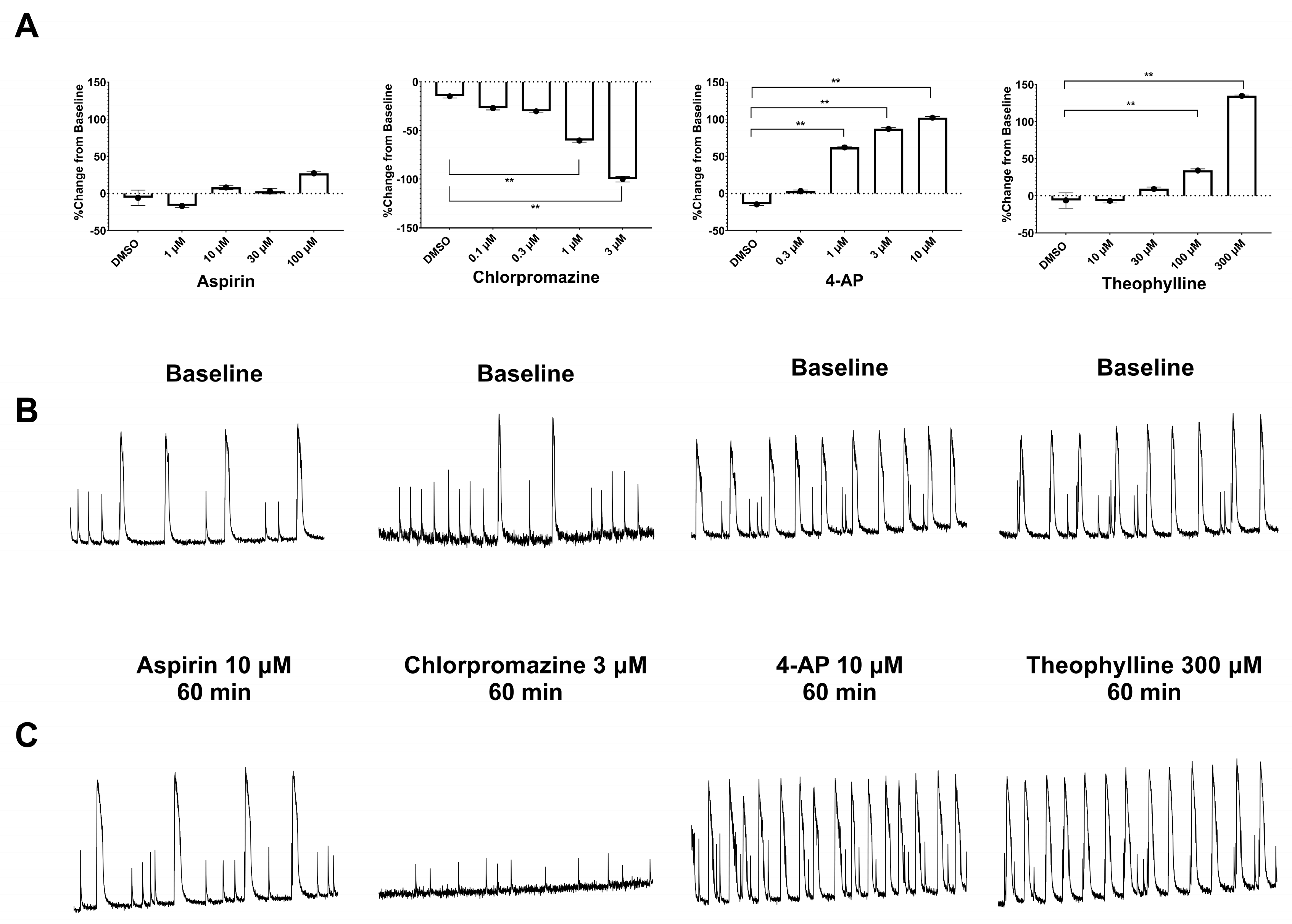
3.2. Measurement of Pharmacological Effects in hiPSC-Derived Neurons in 2D and 3D Cultures Using the Ca2+ Transient Assay
3.3. Analysis of Sensitivity, Specificity, and Balanced Accuracy
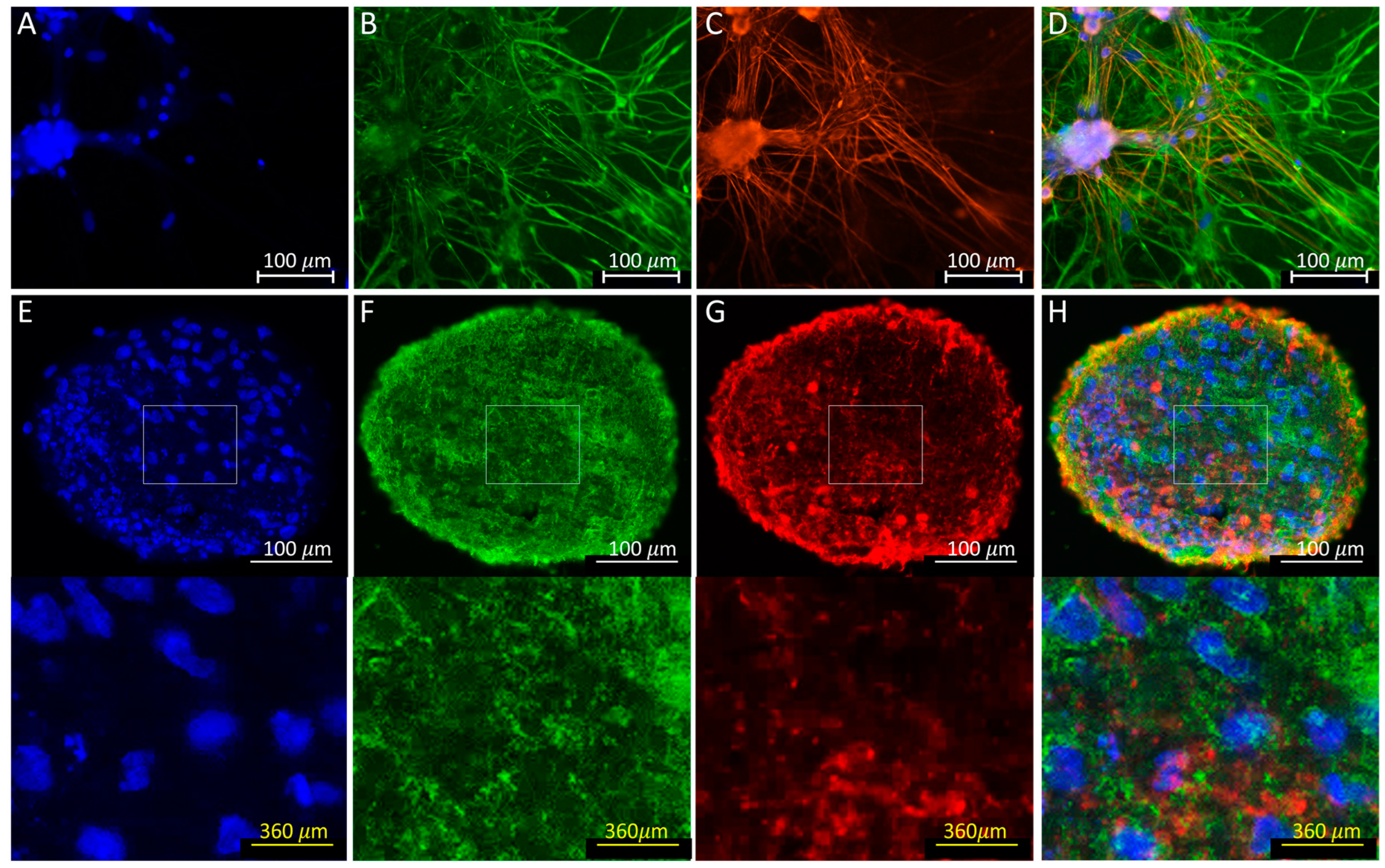
3.4. Immunofluorescence
3.5. Transcriptome Analysis of hiPSC-Derived Neurons Co-Cultured with Human Primary Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Authier, S.; Arezzo, J.; Delatte, M.S.; Kallman, M.-J.; Markgraf, C.; Paquette, D.; Pugsley, M.K.; Ratcliffe, S.; Redfern, W.S.; Stevens, J.; et al. Safety pharmacology investigations on the nervous system: An industry survey. J. Pharmacol. Toxicol. Methods 2016, 81, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Irwin, S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 1968, 13, 222–257. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Sablé, E.; Porsolt, R.D. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr. Protoc. Pharmacol. 2004, 27, 10. [Google Scholar] [CrossRef]
- Bielfelt, S.W.; Redman, H.C.; McClellan, R.O. Sire- and sex-related differences in rates of epileptiform seizures in a purebred beagle dog colony. Am. J. Vet. Res. 1971, 32, 2039–2048. [Google Scholar]
- Kelly, K.M. Spike-wave discharges: Absence or not, a common finding in common laboratory rats. Epilepsy Curr. 2004, 4, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Kreir, M.; Van Deuren, B.; Versweyveld, S.; De Bondt, A.; Van den Wyngaert, I.; Van der Linde, H.; Lu, H.R.; Teuns, G.; Gallacher, D.J. Do in vitro assays in rat primary neurons predict drug-induced seizure liability in humans? Toxicol. Appl. Pharmacol. 2018, 346, 45–57. [Google Scholar] [CrossRef]
- Tukker, A.M.; Westerink, R.H.S. Novel test strategies for in vitro seizure liability assessment. Expert. Opin. Drug. Metab. Toxicol. 2021, 17, 923–936. [Google Scholar] [CrossRef]
- Avoli, M.; Jefferys, J.G.R. Models of drug-induced epileptiform synchronization in vitro. J. Neurosci. Methods 2016, 260, 26–32. [Google Scholar] [CrossRef]
- DaSilva, J.K.; Breidenbach, L.; Deats, T.; Li, D.; Treinen, K.; Dinklo, T.; Kervyn, S.; Teuns, G.; Traebert, M.; Hempel, K. Nonclinical species sensitivity to convulsions: An IQ DruSafe consortium working group initiative. J. Pharm. Toxicol. Methods 2020, 103, 106683. [Google Scholar] [CrossRef]
- Zhai, J.; Zhou, Y.-Y.; Lagrutta, A. Sensitivity, specificity and limitation of in vitro hippocampal slice and neuron-based assays for assessment of drug-induced seizure liability. Toxicol. Appl. Pharmacol. 2021, 430, 115725. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.A.; Accardi, M.V.; Zhong, Y.; Paquette, D.; Authier, S. Drug-Induced Seizures: Considerations for Underlying Molecular Mechanisms. Int. J. Toxicol. 2021, 40, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Luithardt, H.H.; Metea, M.R.; Strock, C.J. In Vitro Screening for Seizure Liability Using Microelectrode Array Technology. Toxicol. Sci. 2018, 163, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Odawara, A.; Saitoh, Y.; Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Long-term electrophysiological activity and pharmacological response of a human induced pluripotent stem cell-derived neuron and astrocyte co-culture. Biochem. Biophys. Res. Commun. 2014, 443, 1176–1181. [Google Scholar] [CrossRef]
- Grainger, A.I.; King, M.C.; Nagel, D.A.; Parri, H.R.; Coleman, M.D.; Hill, E.J. In vitro Models for Seizure-Liability Testing Using Induced Pluripotent Stem Cells. Front. Neurosci. 2018, 12, 590. [Google Scholar] [CrossRef]
- Woodruff, G.; Phillips, N.; Carromeu, C.; Guicherit, O.; White, A.; Johnson, M.; Zanella, F.; Anson, B.; Lovenberg, T.; Bonaventure, P.; et al. Screening for modulators of neural network activity in 3D human iPSC-derived cortical spheroids. PLoS ONE 2020, 15, e0240991. [Google Scholar] [CrossRef]
- Hogberg, H.T.; Smirnova, L. The Future of 3D Brain Cultures in Developmental Neurotoxicity Testing. Front. Toxicol. 2022, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Cohen, J.D.; Yukawa, T.; Estrella, H.; Leonard, C.; Nunes, J.; Choi, C.; Lewis, L.; Baker, K.S.; Kuga, K.; et al. Assessment of a 3D neural spheroid model to detect pharmaceutical-induced neurotoxicity. ALTEX—Altern. Anim. Exp. 2022, 39, 560–582. [Google Scholar] [CrossRef]
- Sirenko, O.; Hesley, J.; Rusyn, I.; Cromwell, E.F. High-content high-throughput assays for characterizing the viability and morphology of human iPSC-derived neuronal cultures. Assay Drug Dev Technol. 2014, 12, 536–547. [Google Scholar] [CrossRef]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca(2+) waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359–1392. [Google Scholar] [CrossRef]
- Fu, M.; Sun, Z.-h.; Zong, M.; He, X.-p.; Zuo, H.-c.; Xie, Z.-p. Deoxyschisandrin modulates synchronized Ca2+ oscillations and spontaneous synaptic transmission of cultured hippocampal neurons. Acta Pharmacol. Sin. 2008, 29, 891–898. [Google Scholar] [CrossRef]
- Pacico, N.; Mingorance-Le Meur, A. New in vitro phenotypic assay for epilepsy: Fluorescent measurement of synchronized neuronal calcium oscillations. PLoS ONE 2014, 9, e84755. [Google Scholar] [CrossRef] [PubMed]
- Hemstapat, K.; Smith, M.T.; Monteith, G.R. Measurement of intracellular Ca2+ in cultured rat embryonic hippocampal neurons using a fluorescence microplate reader: Potential application to biomolecular screening. J. Pharm. Toxicol. Methods 2004, 49, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Smetters, D.; Majewska, A.; Yuste, R. Detecting action potentials in neuronal populations with calcium imaging. Methods 1999, 18, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kumlien, E.; Lundberg, P.O. Seizure risk associated with neuroactive drugs: Data from the WHO adverse drug reactions database. Seizure 2010, 19, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; DeLaura, S.; Jones, E.M. The CIRM iPSC repository. Stem Cell. Res. 2020, 44, 101671. [Google Scholar] [CrossRef]
- Peña, F.; Tapia, R. Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: Role of glutamate- and GABA-mediated neurotransmission and of ion channels. Neuroscience 2000, 101, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-K.; Kim, Y.-I.; Seo, M.-S.; Sung, S.-E.; Choi, J.-H.; Lee, S.; Jung, Y.-S.; Cho, J.Y.; Hwang, D.Y.; Park, S.-J.; et al. A comparative study of the phenotype with kainic acid-induced seizure in DBA/2 mice from three different sources. Lab. Anim. Res. 2020, 36, 39. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, T.; Hyysalo, A.; Kapucu, F.E.; Aarnos, L.; Vinogradov, A.; Eglen, S.J.; Ylä-Outinen, L.; Narkilahti, S. Functional characterization of human pluripotent stem cell-derived cortical networks differentiated on laminin-521 substrate: Comparison to rat cortical cultures. Sci. Rep. 2019, 9, 17125. [Google Scholar] [CrossRef]
- Talbot, J.; Chear, S.; Phipps, A.; Pébay, A.; Hewitt, A.W.; Vickers, J.C.; King, A.E.; Cook, A.L. Image-Based Quantitation of Kainic Acid-Induced Excitotoxicity as a Model of Neurodegeneration in Human iPSC-Derived Neurons. Methods Mol. Biol. 2022, 2549, 187–207. [Google Scholar] [CrossRef]
- Caceres, A.; Banker, G.; Steward, O.; Binder, L.; Payne, M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984, 315, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Fields, K.L.; Hakomori, S.I.; Mirsky, R.; Pruss, R.M.; Winter, J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979, 174, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Dravid, S.M.; Murray, T.F. Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [Mg(2+)]: Involvement of AMPA/kainate and metabotropic glutamate receptors. Brain Res. 2004, 1006, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, O.; Parham, F.; Dea, S.; Sodhi, N.; Biesmans, S.; Mora-Castilla, S.; Ryan, K.; Behl, M.; Chandy, G.; Crittenden, C.; et al. Functional and Mechanistic Neurotoxicity Profiling Using Human iPSC-Derived Neural 3D Cultures. Toxicol. Sci. Off. J. Soc. Toxicol. 2019, 167, 58–76. [Google Scholar] [CrossRef]
- Wang, X.; Gruenstein, E.I. Mechanism of synchronized Ca2+ oscillations in cortical neurons. Brain Res. 1997, 767, 239–249. [Google Scholar] [CrossRef]
- Lerche, H.; Shah, M.; Beck, H.; Noebels, J.; Johnston, D.; Vincent, A. Ion channels in genetic and acquired forms of epilepsy. J. Physiol. 2013, 591, 753–764. [Google Scholar] [CrossRef]
- Boutin, M.E.; Strong, C.E.; Van Hese, B.; Hu, X.; Itkin, Z.; Chen, Y.C.; LaCroix, A.; Gordon, R.; Guicherit, O.; Carromeu, C.; et al. A multiparametric calcium signal screening platform using iPSC-derived cortical neural spheroids. SLAS Discov. Adv. Life Sci. R. D. 2022, 27, 209–218. [Google Scholar] [CrossRef]
- Koo, J.; Choe, H.K.; Kim, H.-D.; Chun, S.K.; Son, G.H.; Kim, K. Effect of Mefloquine, a Gap Junction Blocker, on Circadian Period2 Gene Oscillation in the Mouse Suprachiasmatic Nucleus Ex Vivo. Endocrinol. Metab. 2015, 30, 361–370. [Google Scholar] [CrossRef]
- Strickland, M.R.; Alvarez-Breckenridge, C.; Gainor, J.F.; Brastianos, P.K. Tumor Immune Microenvironment of Brain Metastases: Toward Unlocking Antitumor Immunity. Cancer Discov. 2022, 12, 1199–1216. [Google Scholar] [CrossRef]
- Fucile, S.; Miledi, R.; Eusebi, F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 2943–2947. [Google Scholar] [CrossRef]
- Bishnoi, M.; Chopra, K.; Kulkarni, S.K. Theophylline, adenosine receptor antagonist prevents behavioral, biochemical and neurochemical changes associated with an animal model of tardive dyskinesia. Pharmacol. Rep. PR 2007, 59, 181–191. [Google Scholar] [PubMed]
- Kiss, T.; László, Z.; Szabadics, J. Mechanism of 4-aminopyridine block of the transient outward K-current in identified Helix neuron. Brain Res. 2002, 927, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.S.; Sato, W.; Ataka, K.; Yagisawa, K.; Omori, Y.; Kanbayashi, T.; Shimizu, T. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2014, 10, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A. Pilocarpine-induced seizures revisited: What does the model mimic? Epilepsy Curr. 2009, 9, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Pakdaman, H.; Harandi, A.A.; Gharagozli, K.; Alaeddini, F.; Esfandani, A.; Mirbehbahani, S.H.; Doroudi, T.; Kolivand, P.; Bahrami, P.; Kazemi, H. Epilepsy lifetime prevalence in Iran: A large population- based national survey. Sci. Rep. 2021, 11, 9437. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Suntharampillai, N.; Iwanowski, P. Antibiotic-induced epileptic seizures: Mechanisms of action and clinical considerations. Seizure 2020, 81, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Akaike, N.; Shirasaki, T.; Yakushiji, T. Quinolones and fenbufen interact with GABAA receptor in dissociated hippocampal cells of rat. J. Neurophysiol. 1991, 66, 497–504. [Google Scholar] [CrossRef]
- Hill, S.; Sikand, H.; Lee, J. A case report of seizure induced by bupropion nasal insufflation. Prim. Care Companion J. Clin. Psychiatry 2007, 9, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Wang, C.C.; Wang, S.J. Bupropion attenuates kainic acid-induced seizures and neuronal cell death in rat hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 207–214. [Google Scholar] [CrossRef]
- Starr, P.; Klein-Schwartz, W.; Spiller, H.; Kern, P.; Ekleberry, S.E.; Kunkel, S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am. J. Emerg. Med. 2009, 27, 911–915. [Google Scholar] [CrossRef]


| Compounds | Cas Number | Source | Free Cmax (µM) | Tested Concentrations (µM) |
|---|---|---|---|---|
| DMSO | - | Sigma | na | - |
| Aspirin | 50-78-2 | Sigma | 0.04–0.16 | 3–100 |
| Amoxicillin | 26787-78-0 | Sigma | 0.003–32 | 0.1–3 |
| Acetaminophen | 103-90-2 | Sigma | 0.46 | 1–30 |
| Acetylcholine | 66-23-9 | Sigma | 1–10 | 10–300 |
| Serotonin | 153-98-0 | Tocris Biosciences | 1–2 | 3–100 |
| Bicuculline | 485-49-4 | Sigma | na | 0.3–10 |
| Glutamate | 56-86-0 | Sigma | 1–2 | 1–30 |
| Enoxacin | 74011-58-8 | Sigma | 1–4 | 0.3–10 |
| Cyclothiazide | 2259-96-3 | Sigma | 30 | 3–100 |
| Theophylline | 58-55-9 | Sigma | 8–15 | 10–300 |
| Maprotilene | 10262-69-8 | Sigma | 0.06 | 0.3–10 |
| Bupropion | 31677-93-7 | Sigma | 0.07 | 0.3–10 |
| Clozapine | 5786-21-0 | Sequoia research products Ltd. | 0.3 | 0.03–1 |
| 4-Aminopyridine | 504-24-5 | Sigma | 0.025–0.075 | 0.3–10 |
| Amoxapine | 14028-44-5 | Sigma | 0.04–0.6 | 0.1–3 |
| Pilocarpine | 54-71-7 | Sigma | 0.03–0.55 | 0.3–10 |
| Chlorpromazine | 69-09-0 | Sigma | 0.05–0.15 | 0.1–3 |
| Pazopanib | 444731-52-6 | Sigma | 0.6–1.2 | 0.3–10 |
| Phenytoin | 57-41-0 | Sigma | 4–15 | 3–100 |
| Dizocilpine (MK-801) | 77086-21-6 | Sequoia research products Ltd. | na | 0.1–3 |
| Mefloquine | 51773-92-3 | Sigma | 0.095 | 1–30 |
| quinacrine | 69-05-6 | Sigma | 0.05 | 0.3–10 |
| Carbamazepine | 298-46-4 | Sigma | 10 | 3–100 |
| GABA | 56-12-2 | Sigma | ~1 | 0.1–3 |
| Kainic Acid | 487-79-6 | Sigma | na | 0.3–10 |
| Compounds | Class of Drugs | Free Cmax (µM) | Ca²⁺ Transient Peak Number (2D) | Ca²⁺ Transient Peak Number (3D) |
|---|---|---|---|---|
| Aspirin | Negative control | 0.04–0.16 | ± (100 µM) | ± (100 µM) |
| Amoxicillin | Negative control | 0.003 | ± (3 µM) | ± (3 µM) |
| Acetaminophen | Negative control | 0.46 | ± (10 µM) | ± (10 µM) |
| Bicuculline | GABAA receptor antagonist | na | ± (10 µM) | ± (10 µM) |
| Enoxacin | Antibiotic | 1 | ± (10 µM) | ± (10 µM) |
| GABA | GABA receptor agonist | ~1 | ↓ (1 µM) | ± (3 µM) |
| Cyclothiazide | AMPA PAM | 30 | ↑ (10 µM) | ↑ (10 µM) |
| Kainic Acid | AMPA/NMDA/Glutamate receptor agonist | na | ↓ (3 µM) | ↑ (3 µM) |
| Dizocilpine (MK-801) | NMDA antagonist | na | ↓ (0.1 µM) | ↓ (0.1 µM) |
| Glutamate | Glutamate receptor agonist | 1–2 | ↓ (1 µM) | ± (30 µM) |
| Theophylline | Adenosine receptor antagonist | 8–15 | ↑ (30 µM) | ↑ (30 µM) |
| Mefloquine | Adenosine receptor A2a/Connexin43 antagonist | 0.12 | ↓ (1 µM) | ± (30 µM) |
| Maprotiline | Norepinephrine reuptake inhibitor | 0.06 | ↓ (3 µM) | ± (10 µM) |
| Bupropion | Norepinephrine–dopamine reuptake inhibitor | 0.07 | ± (10 µM) | ± (10 µM) |
| Clozapine | D2/5-HT2A antagonist | 0.3 | ± (1 µM) | ± (1 µM) |
| Chlorpromazine | Dopamine/5HTR receptor antagonist | 0.05–0.15 | ↓ (0.3 µM) | ± (3 µM) |
| Amoxapine | Reuptake of norepinephrine and serotonin inhibitor | 0.04–0.6 | ↓ (3 µM) | ± (3 µM) |
| Serotonin | 5HTR agonist | 1–2 | ↓ (3 µM) | ↓ (3 µM) |
| Acetylcholine | Acetylcholine receptor agonist | 1–10 | ↓ (30 µM) | ↓ (30 µM) |
| Pilocarpine | Muscarinic acetylcholine receptor agonist | 0.03–0.55 | ↑ (3 µM) | ± (10 µM) |
| 4-Aminopyridine | KCNA1 antagonist | 0.025–0.075 | ↑ (1 µM) | ↑ (1 µM) |
| Phenytoin | Sodium channel SCN1A antagonist | 4–15 | ↓ (3 µM) | ± (100 µM) |
| Carbamazepine | Sodium channel antagonist | 10 | ↓ (30 µM) | ± (100 µM) |
| Quinacrine | Phospholipase A2 inhibitor | 0.05 | ↓ (10 µM) | ± (10 µM) |
| Pazopanib | Tyrosine kinase inhibitor | 0.6–1.2 | ± (10 µM) | ± (10 µM) |
| Peak Number—2D | Peak Number—3D | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Dose (µM) | Dose 1 | Dose 2 | Dose 3 | Dose 4 | Dose 1 | Dose 2 | Dose 3 | Dose 4 | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 4-AP | 0.37, 1.11, 3.33, 10 | 9.0 | 22.2 | 70.3 ** | 29.6 | 85.8 ** | 76.0 | 110.0 ** | 69.0 | 16.7 | 9.1 | 62.4 * | 69.9 | 24.1 * | 20.7 | 89.0 ** | 27.1 |
| Acetylcholine | 11.11, 33.33, 100, 300 | 2.4 | 26.9 | −31.5 | 51.8 | −47.2 * | 38.9 | −63.5 ** | 19.9 | −33.8 | 23.9 | −51.6 * | 20.6 | −34.9 | 38.3 | −68.3 ** | 19.1 |
| Amoxapine | 0.11, 0.33, 1, 3 | −5.2 | 8.5 | 25.3 | 51.8 | 12.5 | 27.9 | −35.3 * | 14.8 | −10.1 | 16.0 | −2.2 | 17.8 | −10.8 | 18.4 | −7.5 | 22.9 |
| Amoxicillin | 0.11, 0.33, 1, 3 | −10.5 | 29.7 | 8.3 | 38.2 | 27.2 * | 7.9 | 16.2 | 42.7 | −8.0 | 8.8 | 1.4 | 17.2 | 9.3 | 35.0 | 18.3 | 28.6 |
| Aspirin | 3.7, 11.11, 33.33, 100 | −10.2 | 14.5 | 17.6 | 21.1 | 10.1 | 32.9 | 27.9 * | 35.6 | −0.4 | 11.4 | 0.0 | 16.9 | 10.0 | 32.5 | −8.2 | 17.6 |
| Bicuculine | 0.37, 1.11, 3.33, 10 | −29.4 | 33.4 | −3.5 | 30.0 | 9.9 | 60.8 | 5.8 | 53.6 | −14.9 | 23.0 | −13.0 | 23.0 | 5.8 | 35.2 | −5.8 | 31.1 |
| Bupropion | 0.37, 1.11, 3.33, 10 | 12.7 | 16.6 | 6.6 | 34.9 | −12.0 | 17.9 | 6.0 | 41.8 | 1.3 | 28.1 | −8.4 | 9.1 | −2.2 | 13.6 | 12.5 | 46.0 |
| Carbamazepine | 3.7, 11.11, 33.33, 100 | −15.3 | 41.7 | −17.0 | 21.0 | −76.8 ** | 27.3 | −100.0 ** | 0.0 | 11.4 | 12.9 | 3.9 | 24.7 | −19.3 | 19.6 | −21.5 | 40.8 |
| Chlorpromazine | 0.11, 0.33, 1, 3 | −27.0 | 19.3 | −29.5 | 6.7 | −59.8 ** | 12.9 | −100.0 ** | 0.0 | 6.5 | 32.0 | −2.6 | 23.2 | 11.5 | 32.3 | −4.8 | 15.4 |
| Clozapine | 0.04, 0.11, 0.33, 1 | 15.3 | 50.6 | 24.2 | 24.9 * | −15.0 | 32.3 | −8.3 | 43.3 | 11.1 | 17.2 | −10.0 | 14.9 | −2.8 | 17.2 | −13.9 | 16.4 |
| Cyclothiazide | 3.7, 11.11, 33.33, 100 | 2.7 | 30.9 | 178.3 ** | 116.3 | −25.2 | 30.7 | −82.0 * | 33.4 | 0.0 | 27.6 | 21.5 | 33.0 | 63.6 * | 50.0 | 102.9 ** | 50.5 |
| Enoxacin | 0.37, 1.11, 3.33, 10 | 1.3 | 26.3 | 2.3 | 32.6 | 5.5 | 22.6 | 2.0 | 17.3 | −16.9 | 25.0 | 5.5 | 14.7 | 13.2 | 21.3 | 2.2 | 21.2 |
| GABA | 0.11, 0.33, 1, 3 | −1.6 | 27.3 | −4.4 | 34.6 | −12.4 | 51.3 | −36.5 * | 34.7 | 5.0 | 23.4 | −1.8 | 19.9 | −0.6 | 28.3 | 2.2 | 24.8 |
| Glutamate | 1.11, 3.33, 10, 30 | −9.3 | 60.3 | −8.9 * | 68.5 | −60.3 * | 49.9 | −100.0 * | 0.0 | −7.0 | 20.1 | 12.3 | 33.6 | 20.0 | 34.4 | 8.4 | 17.3 |
| Kainic Acid | 0.37, 1.11, 3.33, 10 | 21.0 | 42.1 | 26.8 * | 13.7 | −53.3 | 43.8 | −100.0 ** | 0.0 | 1.3 | 36.9 | 16.4 | 15.3 | 18.7 * | 19.7 | 102.8 ** | 62.2 |
| Maprotiline | 0.37, 1.11, 3.33, 10 | −0.7 | 18.1 | 0.5 | 39.7 | −100.0 ** | 0.0 | −100.0 ** | 0.0 | −16.5 | 18.4 | −8.2 | 15.9 | −7.9 | 15.5 | 1.9 | 21.9 |
| Mefloquine | 1.11, 3.33, 10, 30 | −30.8 | 21.7 | 16.2 | 60.2 | −100.0 ** | 0.0 | −100.0 ** | 0.0 | 2.8 | 21.5 | −11.1 | 9.1 | 7.2 | 19.8 | −3.7 | 26.5 |
| MK−801 | 0.11, 0.33, 1, 3 | −82.8 ** | 10.9 | −90.4 ** | 9.3 | −84.6 ** | 17.7 | −96.3 ** | 5.7 | −49.1 * | 16.7 | −67.3 ** | 24.4 | −97.2 ** | 6.8 | −84.8 ** | 9.4 |
| Paracetamol (acetaminophen) | 1.11, 3.33, 10, 30 | −11.7 | 20.4 | −25.0 | 32.0 | −18.2 | 56.4 | −94.5 ** | 13.5 | 2.2 | 37.2 | −3.0 | 22.0 | 12.5 | 24.6 | 47.2 * | 42.4 |
| Pazopanib | 0.37, 1.11, 3.33, 10 | −49.0 | 31.8 | 4.4 | 27.5 | 11.3 | 60.3 | 10.0 | 54.1 | 6.4 | 38.1 | −4.2 | 10.2 | 0.8 | 26.5 | −2.0 | 36.4 |
| Phenytoin | 3.7, 11.11, 33.33, 100 | −33.5 | 9.0 | −15.2 | 14.9 | −51.2 ** | 8.2 | −27.5 | 14.4 | 1.4 | 18.4 | −11.8 | 9.4 | −14.6 | 11.9 | −9.9 | 11.3 |
| Pilocarpine | 0.37, 1.11, 3.33, 10 | 11.5 | 18.4 | 8.3 | 36.2 | 34.3 * | 41.0 | −19.2 | 15.6 | 16.7 | 18.2 | 23.9 | 43.8 | 12.9 | 22.8 | 23.1 | 26.1 |
| Quinacrine | 0.37, 1.11, 3.33, 10 | −2.7 | 27.8 | −2.8 | 6.9 | −11.0 | 55.8 | −92.2 ** | 12.3 | 3.2 | 28.2 | 0.4 | 37.4 | 14.1 | 12.7 | 12.5 | 14.7 |
| Serotonin | 3.7, 11.11, 33.33, 100 | −23.4 | 31.7 | −1.7 | 74.7 | −43.8 | 39.3 | −45.8 ** | 22.5 | −70.7 ** | 32.7 | −69.1 ** | 29.8 | −82.1 ** | 16.6 | −80.3 ** | 29.4 |
| Theophylline | 11.11, 33.33, 100, 300 | −5.7 | 22.7 | 24.7 | 43.3 | 39.3 * | 23.5 | 118.4 ** | 105.4 | 2.0 | 14.7 | 53.5 * | 60.4 | 78.1 * | 80.0 | 114.2 * | 98.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.-R.; Seo, M.; Kreir, M.; Tanaka, T.; Yamoto, R.; Altrocchi, C.; van Ammel, K.; Tekle, F.; Pham, L.; Yao, X.; et al. High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures. Cells 2023, 12, 958. https://doi.org/10.3390/cells12060958
Lu H-R, Seo M, Kreir M, Tanaka T, Yamoto R, Altrocchi C, van Ammel K, Tekle F, Pham L, Yao X, et al. High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures. Cells. 2023; 12(6):958. https://doi.org/10.3390/cells12060958
Chicago/Turabian StyleLu, Hua-Rong, Manabu Seo, Mohamed Kreir, Tetsuya Tanaka, Rie Yamoto, Cristina Altrocchi, Karel van Ammel, Fetene Tekle, Ly Pham, Xiang Yao, and et al. 2023. "High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures" Cells 12, no. 6: 958. https://doi.org/10.3390/cells12060958
APA StyleLu, H.-R., Seo, M., Kreir, M., Tanaka, T., Yamoto, R., Altrocchi, C., van Ammel, K., Tekle, F., Pham, L., Yao, X., Teisman, A., & Gallacher, D. J. (2023). High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures. Cells, 12(6), 958. https://doi.org/10.3390/cells12060958







