Oscillatory Deficits in the Sub-Chronic PCP Rat Model for Schizophrenia Are Reversed by mGlu5 Receptor-Positive Allosteric Modulators VU0409551 and VU0360172
Abstract
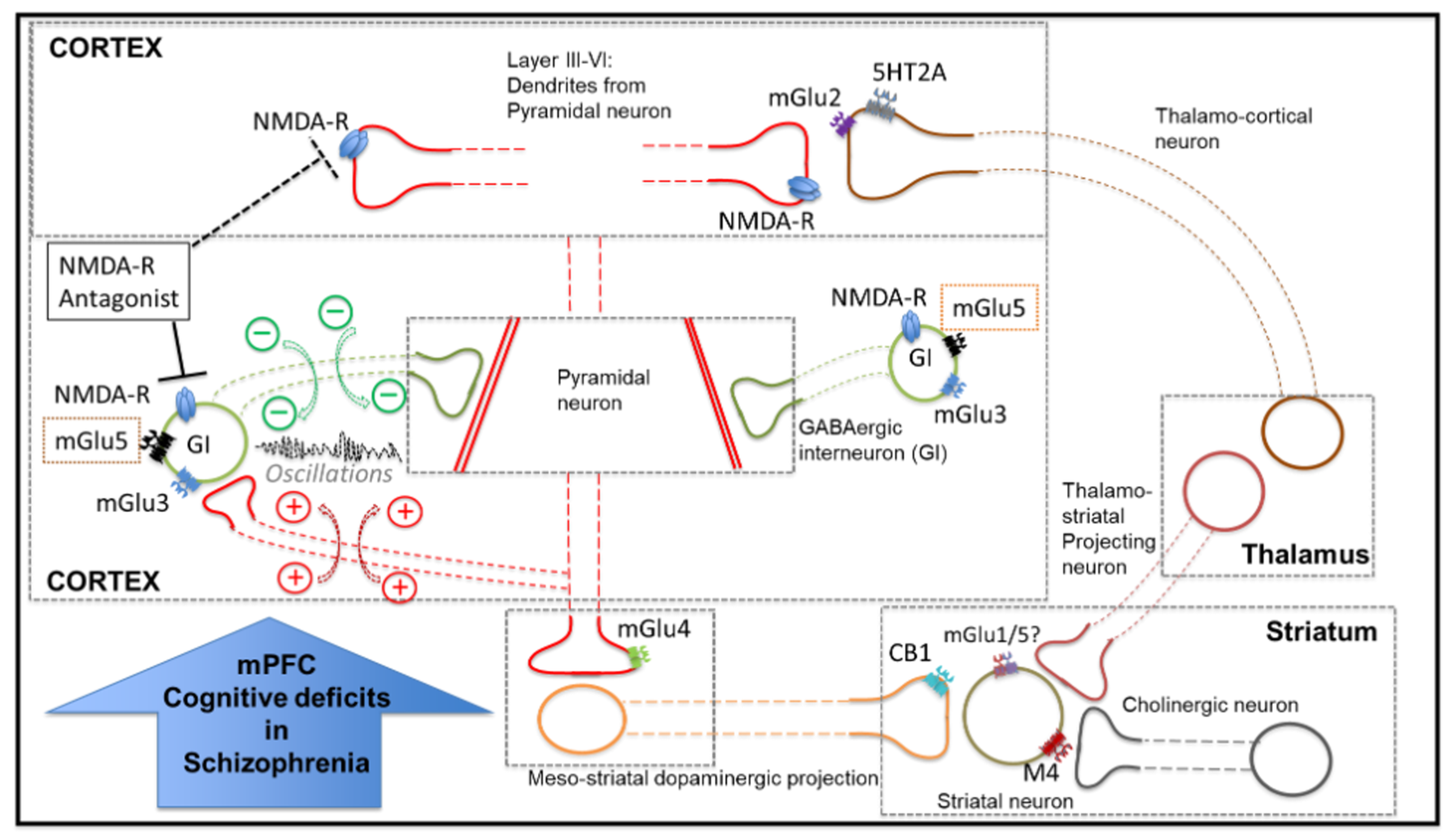
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Drug Treatments
2.4. Behaviour: Novel Object Recognition Paradigm
2.5. Slice Preparation and Incubation
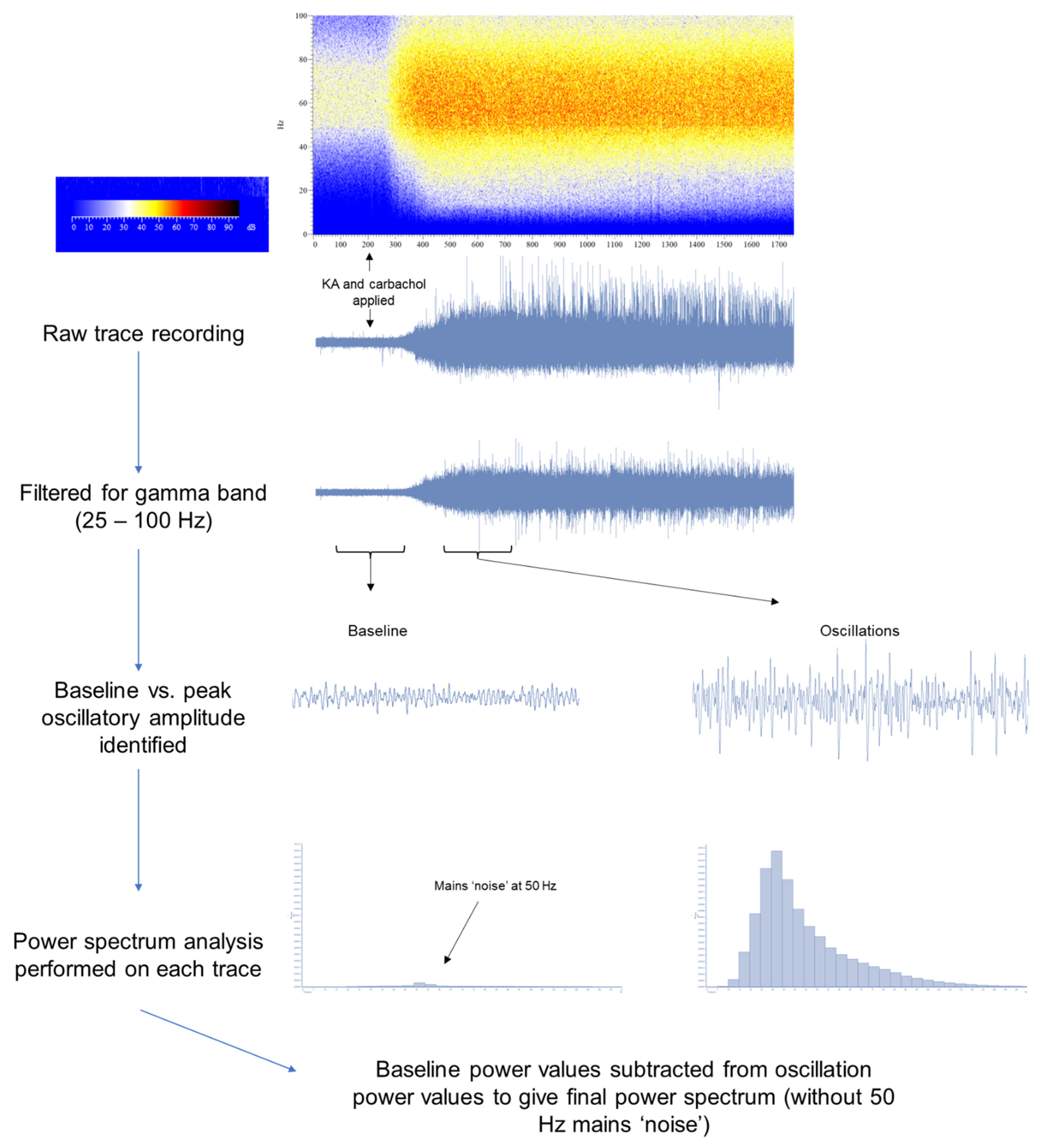
2.6. Induction of Oscillations Ex Vivo
2.7. Analysis
2.7.1. NOR Data
2.7.2. Electrophysiological Data
3. Results
3.1. Novel Object Recognition (NOR) Is Impaired in the scPCP Model
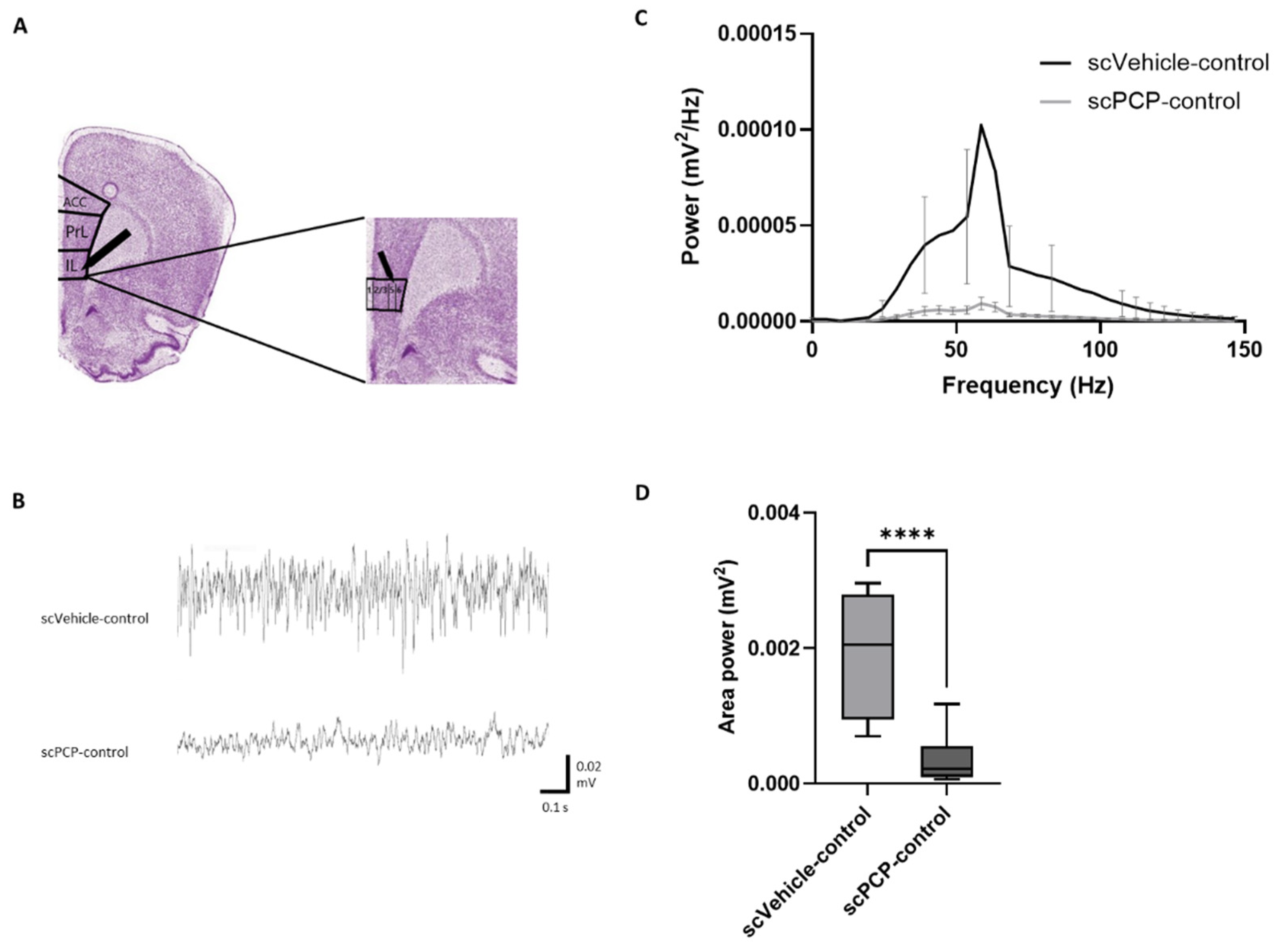
3.2. scPCP Treatment Is Associated with Diminished Gamma Oscillations
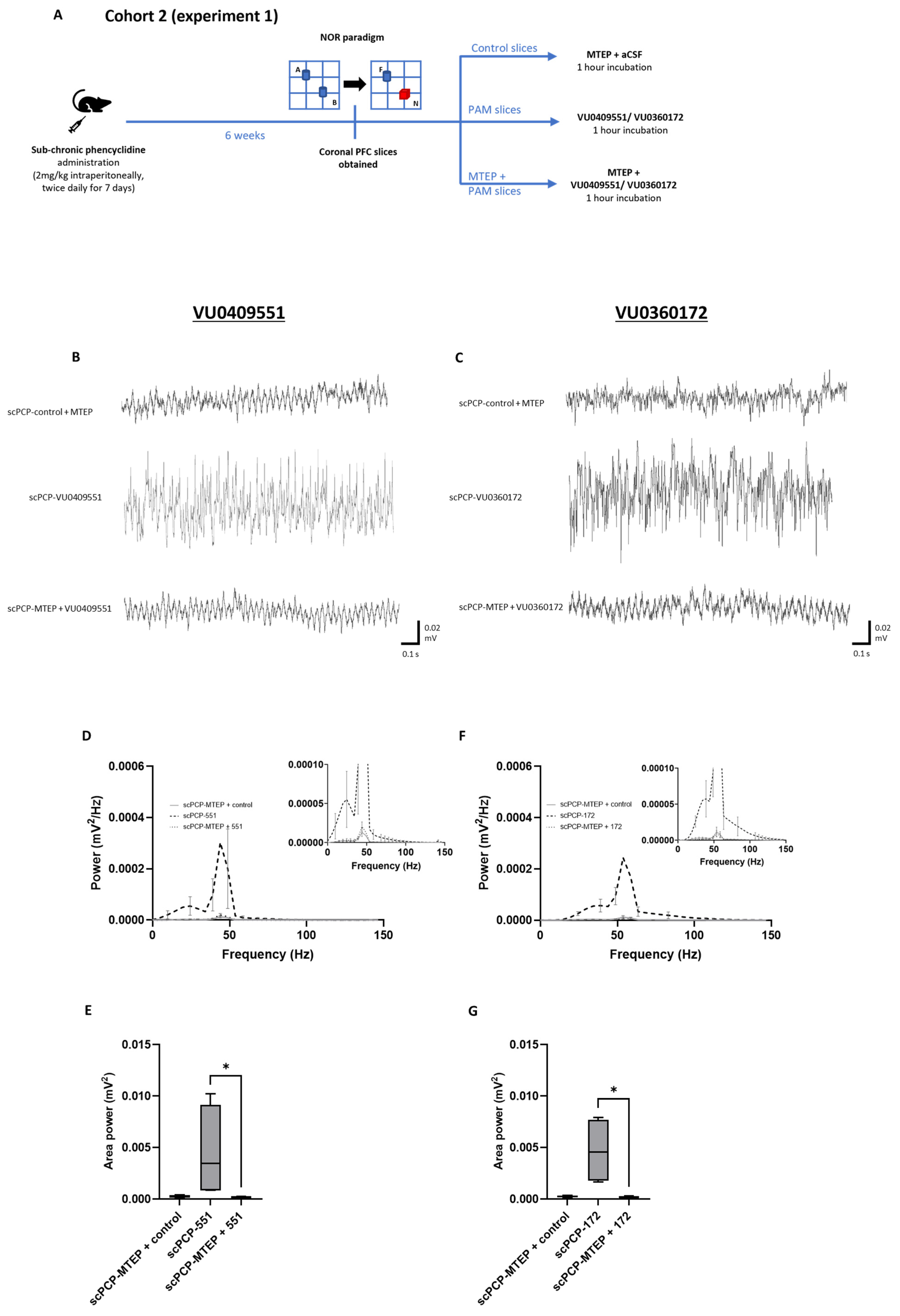
3.3. scPCP-Induced Impairments in Gamma Oscillations Are Restored by VU0409551 and VU0360172 in a Concentration-Dependent Manner
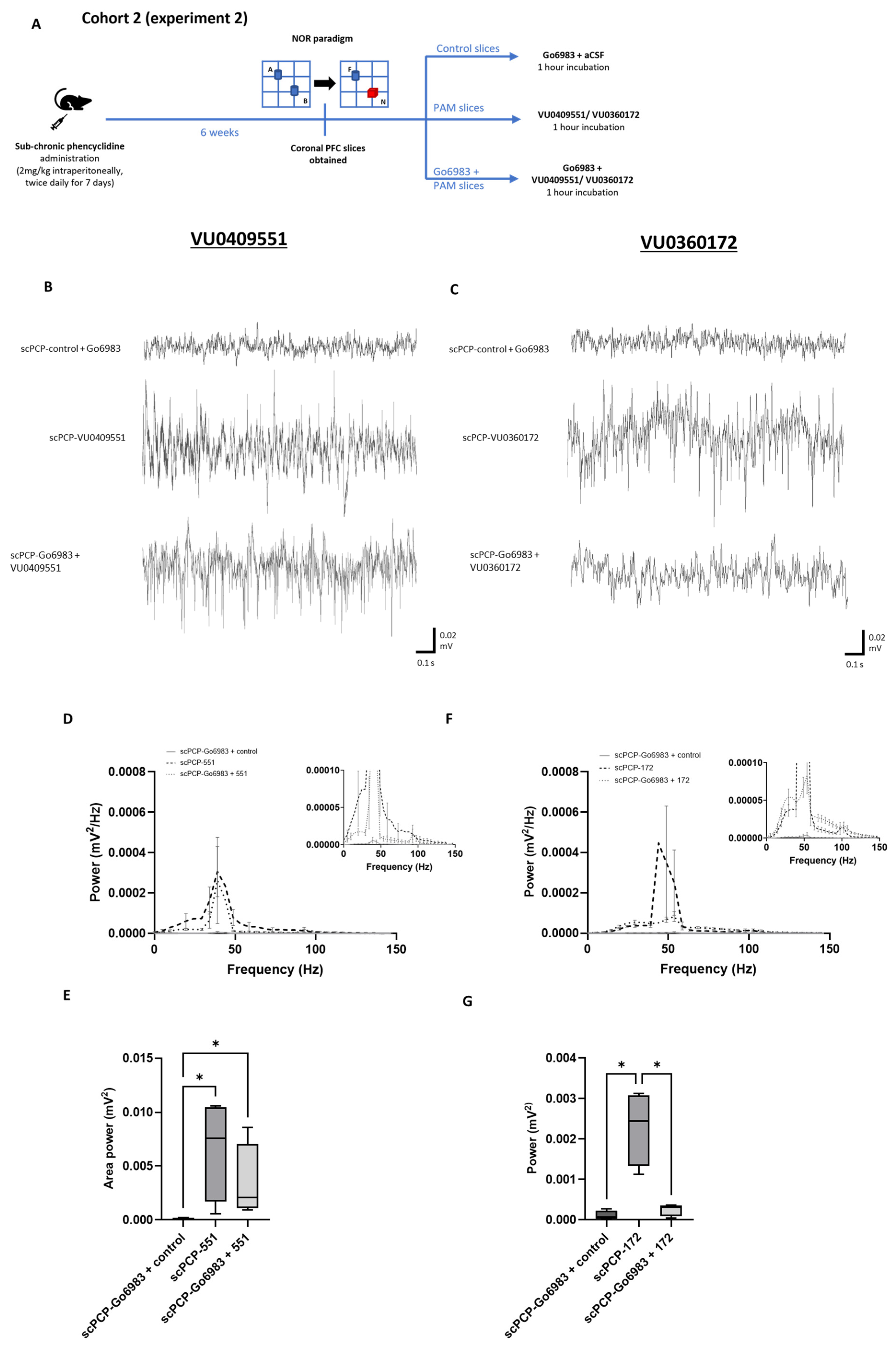
3.4. The Effect of mGlu5 Antagonism on the PAM-Mediated Enhancement of scPCP Gamma Oscillations
3.5. The Effect of PKC Inhibition on the PAM-Mediated Enhancement of scPCP Gamma Oscillations
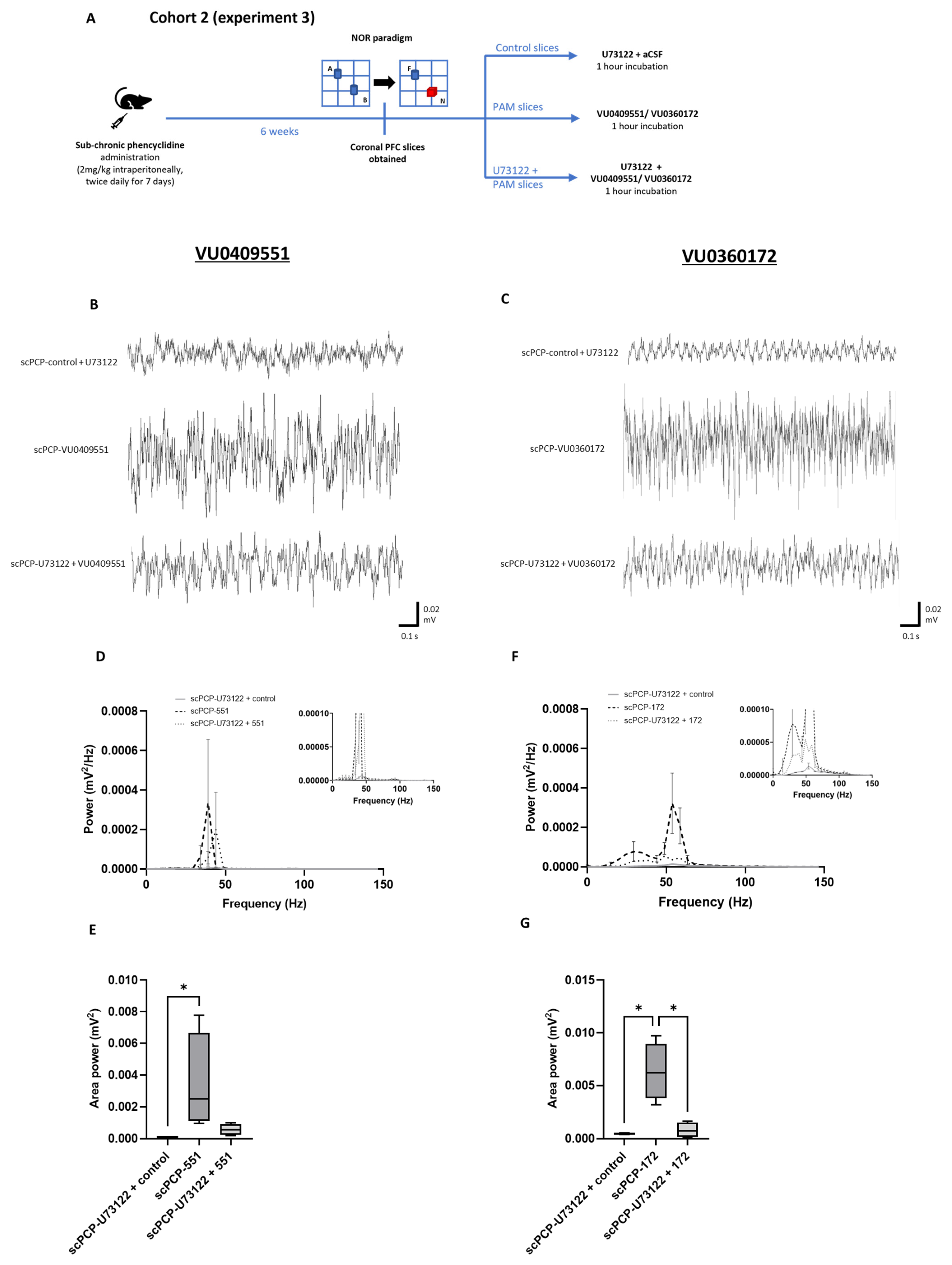
3.6. The Effect of PLC Inhibition on the PAM-Mediated Enhancement of scPCP Gamma Oscillations
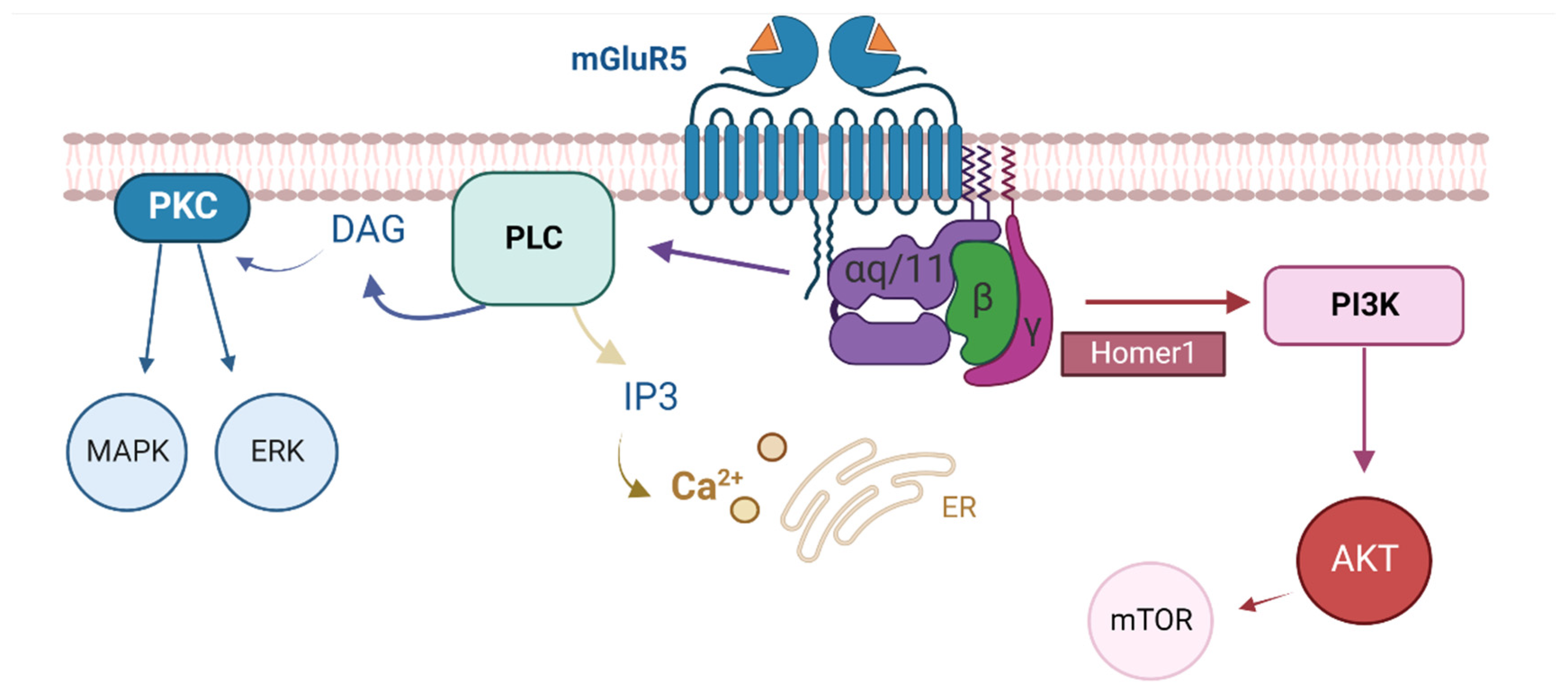
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Malaspina, D.; Walsh-Messinger, J.; Gaebel, W.; Smith, L.M.; Gorun, A.; Prudent, V.; Antonius, D.; Trémeau, F. Negative symptoms, past and present: A historical perspective and moving to DSM-5. Eur. Neuropsychopharmacol. 2014, 24, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Kar, S.K.; Shukla, R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 2018, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Koren, D.; Reichenberg, A.; Bowie, C.R. Negative Symptoms and Cognitive Deficits: What Is the Nature of Their Relationship? Schizophr. Bull. 2005, 32, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Savilla, K.; Kettler, L.; Galletly, C. Relationships between Cognitive Deficits, Symptoms and Quality of Life in Schizophrenia. Aust. N. Z. J. Psychiatry 2008, 42, 496–504. [Google Scholar] [CrossRef]
- Tsapakis, E.M.; Dimopoulou, T.; Tarazi, F.I. Clinical management of negative symptoms of schizophrenia: An update. Pharmacol. Ther. 2015, 153, 135–147. [Google Scholar] [CrossRef]
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar] [CrossRef]
- Neill, J.C.; Harte, M.K.; Haddad, P.M.; Lydall, E.S.; Dwyer, D.M. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: A translational link to humans. Eur. Neuropsychopharmacol. 2014, 24, 822–835. [Google Scholar] [CrossRef]
- Jonas, K.; Lian, W.; Callahan, J.; Ruggero, C.J.; Clouston, S.; Reichenberg, A.; Carlson, G.A.; Bromet, E.J.; Kotov, R. The Course of General Cognitive Ability in Individuals with Psychotic Disorders. JAMA Psychiatry 2022, 79, 659–666. [Google Scholar] [CrossRef]
- Li, P.; Snyder, G.L.; Vanover, K.E. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef]
- Coyle, J.T. Glutamate and Schizophrenia: Beyond the Dopamine Hypothesis. Cell. Mol. Neurobiol. 2006, 26, 363–382. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D.C. From Revolution to Evolution: The Glutamate Hypothesis of Schizophrenia and its Implication for Treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef]
- Filice, F.; Vörckel, K.J.; Sungur, A.; Wöhr, M.; Schwaller, B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol. Brain 2016, 9, 10. [Google Scholar] [CrossRef]
- Glausier, J.R.; Lewis, D.A. GABA and schizophrenia: Where we stand and where we need to go. Schizophr. Res. 2017, 181, 2–3. [Google Scholar] [CrossRef]
- Staff, N.P.; Spruston, N. Intracellular correlate of EPSP-spike potentiation in CA1 pyramidal neurons is controlled by GABAergic modulation. Hippocampus 2003, 13, 801–805. [Google Scholar] [CrossRef]
- Marder, C.P.; Buonomano, D.V. Timing and Balance of Inhibition Enhance the Effect of Long-Term Potentiation on Cell Firing. J. Neurosci. 2004, 24, 8873–8884. [Google Scholar] [CrossRef]
- Sakurai, T.; Gamo, N.J.; Hikida, T.; Kim, S.-H.; Murai, T.; Tomoda, T.; Sawa, A. Converging models of schizophrenia—Network alterations of prefrontal cortex underlying cognitive impairments. Prog. Neurobiol. 2015, 134, 178–201. [Google Scholar] [CrossRef]
- Hashimoto, T.; Volk, D.W.; Eggan, S.M.; Mirnics, K.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Gene Expression Deficits in a Subclass of GABA Neurons in the Prefrontal Cortex of Subjects with Schizophrenia. J. Neurosci. 2003, 23, 6315–6326. [Google Scholar] [CrossRef]
- Enomoto, T.; Tse, M.T.; Floresco, S.B. Reducing Prefrontal Gamma-Aminobutyric Acid Activity Induces Cognitive, Behavioral, and Dopaminergic Abnormalities That Resemble Schizophrenia. Biol. Psychiatry 2011, 69, 432–441. [Google Scholar] [CrossRef]
- Auger, M.L.; Floresco, S.B. Prefrontal Cortical GABA Modulation of Spatial Reference and Working Memory. Int. J. Neuropsychopharmacol. 2014, 18, pyu013. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Haenschel, C.; Nikolic, D.; Singer, W. The Role of Oscillations and Synchrony in Cortical Networks and Their Putative Relevance for the Pathophysiology of Schizophrenia. Schizophr. Bull. 2008, 34, 927–943. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.M.; McCarley, R.W.; Brown, R.E. Chronic Ketamine Reduces the Peak Frequency of Gamma Oscillations in Mouse Prefrontal Cortex Ex vivo. Front. Psychiatry 2013, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, F.; Massimini, M.; Peterson, M.J.; Riedner, B.A.; Lazar, M.; Murphy, M.J.; Huber, R.; Rosanova, M.; Alexander, A.L.; Kalin, N.H.; et al. Reduced Evoked Gamma Oscillations in the Frontal Cortex in Schizophrenia Patients: A TMS/EEG Study. Am. J. Psychiatry 2008, 165, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.B.; Lasagna, C.A.; Martínez-Cancino, R.; Pamidighantam, P.; Deldin, P.J.; Tso, I.F. Neural Oscillatory Abnormalities During Gaze Processing in Schizophrenia: Evidence of Reduced Theta Phase Consistency and Inter-areal Theta-Gamma Coupling. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 370–379. [Google Scholar] [CrossRef]
- Nicoletti, F.; Orlando, R.; Di Menna, L.; Cannella, M.; Notartomaso, S.; Mascio, G.; Iacovelli, L.; Matrisciano, F.; Fazio, F.; Caraci, F.; et al. Targeting mGlu Receptors for Optimization of Antipsychotic Activity and Disease-Modifying Effect in Schizophrenia. Front. Psychiatry 2019, 10, 49. [Google Scholar] [CrossRef]
- Dogra, S.; Conn, P.J. Metabotropic Glutamate Receptors As Emerging Targets for the Treatment of Schizophrenia. Mol. Pharmacol. 2022, 101, 275–285. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Su, L.-D.; Wang, N.; Han, J.; Shen, Y. Group 1 Metabotropic Glutamate Receptors in Neurological and Psychiatric Diseases: Mechanisms and Prospective. Neuroscientist 2021, 28, 453–468. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Macdonald, M.L.; Borgmann-Winter, K.E.; Banerjee, A.; Sleiman, P.; Tom, A.; Khan, A.; Lee, K.-C.; Roussos, P.; Siegel, S.J.; et al. mGluR5 hypofunction is integral to glutamatergic dysregulation in schizophrenia. Mol. Psychiatry 2020, 25, 750–760. [Google Scholar] [CrossRef]
- Sengmany, K.; Singh, J.; Stewart, G.D.; Conn, P.J.; Christopoulos, A.; Gregory, K.J. Biased allosteric agonism and modulation of metabotropic glutamate receptor 5: Implications for optimizing preclinical neuroscience drug discovery. Neuropharmacology 2017, 115, 60–72. [Google Scholar] [CrossRef]
- Huang, H.; Degnan, A.P.; Balakrishnan, A.; Easton, A.; Gulianello, M.; Huang, Y.; Matchett, M.; Mattson, G.; Miller, R.; Santone, K.S.; et al. Oxazolidinone-based allosteric modulators of mGluR5: Defining molecular switches to create a pharmacological tool box. Bioorganic Med. Chem. Lett. 2016, 26, 4165–4169. [Google Scholar] [CrossRef]
- Liu, F.; Grauer, S.; Kelley, C.; Navarra, R.; Graf, R.; Zhang, G.; Atkinson, P.J.; Popiolek, M.; Wantuch, C.; Khawaja, X.; et al. ADX47273 [S-(4-Fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: A Novel Metabotropic Glutamate Receptor 5-Selective Positive Allosteric Modulator with Preclinical Antipsychotic-Like and Procognitive Activities. J. Pharmacol. Exp. Ther. 2008, 327, 827–839. [Google Scholar] [CrossRef]
- Schlumberger, C.; Pietraszek, M.; Gravius, A.; Danysz, W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol. Biochem. Behav. 2010, 95, 23–30. [Google Scholar] [CrossRef]
- Uslaner, J.M.; Parmentier-Batteur, S.; Flick, R.B.; Surles, N.O.; Lam, J.S.; McNaughton, C.H.; Jacobson, M.A.; Hutson, P.H. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 2009, 57, 531–538. [Google Scholar] [CrossRef]
- Yang, F.; Snyder, L.B.; Balakrishnan, A.; Brown, J.M.; Sivarao, D.V.; Easton, A.; Fernandes, A.; Gulianello, M.; Hanumegowda, U.M.; Huang, H.; et al. Discovery and Preclinical Evaluation of BMS-955829, a Potent Positive Allosteric Modulator of mGluR5. ACS Med. Chem. Lett. 2016, 7, 289–293. [Google Scholar] [CrossRef]
- Brown, J.; Iacovelli, L.; Di Cicco, G.; Grayson, B.; Rimmer, L.; Fletcher, J.; Neill, J.C.; Wall, M.J.; Ngomba, R.T.; Harte, M. The comparative effects of mGlu5 receptor positive allosteric modulators VU0409551 and VU0360172 on cognitive deficits and signalling in the sub-chronic PCP rat model for schizophrenia. Neuropharmacology 2022, 208, 108982. [Google Scholar] [CrossRef]
- Rodriguez, A.L.; Grier, M.D.; Jones, C.K.; Herman, E.J.; Kane, A.S.; Smith, R.L.; Williams, R.; Zhou, Y.; Marlo, J.E.; Days, E.L.; et al. Discovery of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5 Reveals Chemical and Functional Diversity and In Vivo Activity in Rat Behavioral Models of Anxiolytic and Antipsychotic Activity. Mol. Pharmacol. 2010, 78, 1105–1123. [Google Scholar] [CrossRef]
- Rook, J.M.; Xiang, Z.; Lv, X.; Ghoshal, A.; Dickerson, J.W.; Bridges, T.M.; Johnson, K.A.; Foster, D.; Gregory, K.J.; Vinson, P.N.; et al. Biased mGlu 5 -Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu 5 Modulation of NMDAR Currents. Neuron 2015, 86, 1029–1040. [Google Scholar] [CrossRef]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar] [CrossRef]
- Hermans, E.; Challiss, R.A.J. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: Prototypic family C G-protein-coupled receptors. Biochem. J. 2001, 359, 465–484. [Google Scholar] [CrossRef]
- Conn, P.J.; Lindsley, C.W.; Jones, C.K. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009, 30, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Paquet, M.; Ferreira, L.T.; Cregan, T.; Swan, P.; Cregan, S.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptor-Mediated Cell Signaling Pathways Are Altered in a Mouse Model of Huntington’s Disease. J. Neurosci. 2010, 30, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.O.; Rittenhouse, S.E.; Tsichlis, P.N. AKT/PKB and Other D3 Phosphoinositide-Regulated Kinases: Kinase Activation by Phosphoinositide-Dependent Phosphorylation. Annu. Rev. Biochem. 1999, 68, 965–1014. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Klann, E. Activation of the Phosphoinositide 3-Kinase-Akt-Mammalian Target of Rapamycin Signaling Pathway Is Required for Metabotropic Glutamate Receptor-Dependent Long-Term Depression. J. Neurosci. 2004, 24, 6352–6361. [Google Scholar] [CrossRef]
- Noetzel, M.J.; Gregory, K.J.; Vinson, P.N.; Manka, J.T.; Stauffer, S.R.; Lindsley, C.W.; Niswender, C.M.; Xiang, Z.; Conn, P.J. A Novel Metabotropic Glutamate Receptor 5 Positive Allosteric Modulator Acts at a Unique Site and Confers Stimulus Bias to mGlu5 Signaling. Mol. Pharmacol. 2013, 83, 835–847. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodriguez, A.L.; Conn, P.J. Allosteric Potentiators of Metabotropic Glutamate Receptor Subtype 5 Have Differential Effects on Different Signaling Pathways in Cortical Astrocytes. J. Pharmacol. Exp. Ther. 2005, 315, 1212–1219. [Google Scholar] [CrossRef]
- Balu, D.T.; Li, Y.; Takagi, S.; Presti, K.T.; Ramikie, T.S.; Rook, J.M.; Jones, C.K.; Lindsley, C.W.; Conn, P.J.; Bolshakov, V.Y.; et al. An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacology 2016, 41, 2052–2061. [Google Scholar] [CrossRef]
- Jentsch, J.D.; Wise, A.; Katz, Z.; Roth, R.H. α-noradrenergic receptor modulation of the phencyclidine- and Δ9-tetrahydrocannabinol-induced increases in dopamine utilization in rat prefrontal cortex. Synapse 1998, 28, 21–26. [Google Scholar] [CrossRef]
- Abdul-Monim, Z.; Neill, J.C.; Reynolds, G.P. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J. Psychopharmacol. 2007, 21, 198–205. [Google Scholar] [CrossRef]
- Leger, M.; Alvaro, G.; Large, C.; Harte, M.; Neill, J.C. Efficacy of AUT6, a novel and selective Kv3 channel modulator, to alleviate cognitive and neurobiological dysfunction in the sub-chronic PCP rat model of schizophrenia symptomatology. J. Psychopharmacol. Suppl. 2015, 29, A66. [Google Scholar]
- Grayson, B.; Idris, N.; Neill, J. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav. Brain Res. 2007, 184, 31–38. [Google Scholar] [CrossRef]
- Neill, J.C.; Grayson, B.; Kiss, B.; Gyertyán, I.; Ferguson, P.; Adham, N. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur. Neuropsychopharmacol. 2016, 26, 3–14. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Grayson, B.; Leger, M.; Piercy, C.; Adamson, L.; Harte, M.; Neill, J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015, 285, 176–193. [Google Scholar] [CrossRef]
- Neill, J.C.; Barnes, S.; Cook, S.; Grayson, B.; Idris, N.F.; McLean, S.L.; Snigdha, S.; Rajagopal, L.; Harte, M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol. Ther. 2010, 128, 419–432. [Google Scholar] [CrossRef]
- Sutcliffe, J.; Marshall, K.; Neill, J. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav. Brain Res. 2007, 177, 117–125. [Google Scholar] [CrossRef]
- Wessinger, W.D. Sexual dimorphic effects of chronic phencyclidine in rats. Eur. J. Pharmacol. 1995, 277, 107–112. [Google Scholar] [CrossRef]
- Walf, A.A.; Rhodes, M.E.; Frye, C.A. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006, 86, 35–46. [Google Scholar] [CrossRef]
- Walker, Q.; Nelson, C.J.; Smith, D.; Kuhn, C.M. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol. Biochem. Behav. 2002, 73, 743–752. [Google Scholar] [CrossRef]
- Becegato, M.; Meurer, Y.S.; Paiva-Santos, M.A.; Lima, A.C.; Marinho, G.F.; Bioni, V.S.; Soares, M.B.; Leão, A.H.; Suchecki, D.; Silva, R.H. Impaired discriminative avoidance and increased plasma corticosterone levels induced by vaginal lavage procedure in rats. Physiol. Behav. 2021, 232, 113343. [Google Scholar] [CrossRef]
- Dere, E.; Huston, J.P.; Silva, M.A.D.S. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007, 31, 673–704. [Google Scholar] [CrossRef]
- Mathiasen, J.R.; DiCamillo, A. Novel Object Recognition in the Rat: A Facile Assay for Cognitive Function. Curr. Protoc. Pharmacol. 2010, 49, 5.59.1–5.59.15. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2008, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hjerling-Leffler, J.; Zagha, E.; Fishell, G.; Rudy, B. The Largest Group of Superficial Neocortical GABAergic Interneurons Expresses Ionotropic Serotonin Receptors. J. Neurosci. 2010, 30, 16796–16808. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Roby, K.D.; Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010, 518, 389–404. [Google Scholar] [CrossRef]
- Bartos, M.; Vida, I.; Jonas, P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007, 8, 45–56. [Google Scholar] [CrossRef]
- Cunningham, M.O.; Hunt, J.; Middleton, S.; LeBeau, F.E.N.; Gillies, M.G.; Davies, C.H.; Maycox, P.R.; Whittington, M.A.; Racca, C. Region-Specific Reduction in Entorhinal Gamma Oscillations and Parvalbumin-Immunoreactive Neurons in Animal Models of Psychiatric Illness. J. Neurosci. 2006, 26, 2767–2776. [Google Scholar] [CrossRef]
- Hájos, N.; Pálhalmi, J.; Mann, E.O.; Németh, B.; Paulsen, O.; Freund, T.F. Spike Timing of Distinct Types of GABAergic Interneuron during Hippocampal Gamma Oscillations In Vitro. J. Neurosci. 2004, 24, 9127–9137. [Google Scholar] [CrossRef]
- Klausberger, T.; Marton, L.F.; O’Neill, J.; Huck, J.H.J.; Dalezios, Y.; Fuentealba, P.; Suen, W.Y.; Papp, E.; Kaneko, T.; Watanabe, M.; et al. Complementary Roles of Cholecystokinin- and Parvalbumin-Expressing GABAergic Neurons in Hippocampal Network Oscillations. J. Neurosci. 2005, 25, 9782–9793. [Google Scholar] [CrossRef]
- Soltesz, I.; Deschenes, M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J. Neurophysiol. 1993, 70, 97–116. [Google Scholar] [CrossRef]
- Glykos, V.; Whittington, M.A.; LeBeau, F.E.N. Subregional differences in the generation of fast network oscillations in the rat medial prefrontal cortex (mPFC) in vitro. J. Physiol. 2015, 593, 3597–3615. [Google Scholar] [CrossRef]
- Woo, T.-U.; Crowell, A. Targeting synapses and myelin in the prevention of schizophrenia. Schizophr. Res. 2005, 73, 193–207. [Google Scholar] [CrossRef]
- Woo, T.-U.; Pucak, M.; Kye, C.; Matus, C.; Lewis, D. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience 1997, 80, 1149–1158. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Bourgeois, J.-P.; Goldman-Rakic, P.S.; Rakic, P. Synaptogenesis in the Prefrontal Cortex of Rhesus Monkeys. Cereb. Cortex 1994, 4, 78–96. [Google Scholar] [CrossRef]
- Anderson, S.; Classey, J.; Condé, F.; Lund, J.; Lewis, D. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience 1995, 67, 7–22. [Google Scholar] [CrossRef]
- Erickson, S.L.; Lewis, D.A. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J. Comp. Neurol. 2002, 448, 186–202. [Google Scholar] [CrossRef]
- Poulsen, C.; Picton, T.W.; Paus, T. Age-Related Changes in Transient and Oscillatory Brain Responses to Auditory Stimulation in Healthy Adults 19–45 Years Old. Cereb. Cortex 2006, 17, 1454–1467. [Google Scholar] [CrossRef]
- Rojas, D.C.; Maharajh, K.; Teale, P.D.; Kleman, M.R.; Benkers, T.L.; Carlson, J.P.; Reite, M.L. Development of the 40 Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin. Neurophysiol. 2006, 117, 110–117. [Google Scholar] [CrossRef]
- Di Menna, L.; Joffe, M.E.; Iacovelli, L.; Orlando, R.; Lindsley, C.W.; Mairesse, J.; Gressèns, P.; Cannella, M.; Caraci, F.; Copani, A.; et al. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 2018, 128, 301–313. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Sarasso, S.; Guller, Y.; Riedner, B.A.; Peterson, M.J.; Bellesi, M.; Massimini, M.; Postle, B.R.; Tononi, G. Reduced Natural Oscillatory Frequency of Frontal Thalamocortical Circuits in Schizophrenia. Arch. Gen. Psychiatry 2012, 69, 766–774. [Google Scholar] [CrossRef]
- Hudson, M.R.; Rind, G.; O’Brien, T.J.; Jones, N.C. Reversal of evoked gamma oscillation deficits is predictive of antipsychotic activity with a unique profile for clozapine. Transl. Psychiatry 2016, 6, e784. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, I.I.; Gould, T.D. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Basar-Eroglu, C.; Brand, A.; Hildebrandt, H.; Kedzior, K.K.; Mathes, B.; Schmiedt, C. Working memory related gamma oscillations in schizophrenia patients. Int. J. Psychophysiol. 2007, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gruetzner, C.; Wibral, M.; Sun, L.; Rivolta, D.; Singer, W.; Maurer, K.; Uhlhaas, P. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front. Hum. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Bikbaev, A.; Neyman, S.; Ngomba, R.T.; Conn, J.; Nicoletti, F.; Manahan-Vaughan, D. MGluR5 Mediates the Interaction between Late-LTP, Network Activity, and Learning. PLoS ONE 2008, 3, e2155. [Google Scholar] [CrossRef]
- Aguilar, D.D.; Strecker, R.E.; Basheer, R.; McNally, J.M. Alterations in sleep, sleep spindle, and EEG power in mGluR5 knockout mice. J. Neurophysiol. 2020, 123, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.A.; Pinto-Duarte, A.; Kappe, A.; Zembrzycki, A.; Metzler, A.; Mukamel, E.; Lucero, J.; Wang, X.; Sejnowski, T.J.; Markou, A.; et al. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol. Psychiatry 2015, 20, 1161–1172. [Google Scholar] [CrossRef]
- Bikbaev, A.; Manahan-Vaughan, D. Metabotropic glutamate receptor, mGlu5, regulates hippocampal synaptic plasticity and is required for tetanisation-triggered changes in theta and gamma oscillations. Neuropharmacology 2017, 115, 20–29. [Google Scholar] [CrossRef]
- Celli, R.; Wall, M.J.; Santolini, I.; Vergassola, M.; Di Menna, L.; Mascio, G.; Cannella, M.; van Luijtelaar, G.; Pittaluga, A.; Ciruela, F.; et al. Pharmacological activation of mGlu5 receptors with the positive allosteric modulator VU0360172, modulates thalamic GABAergic transmission. Neuropharmacology 2020, 178, 108240. [Google Scholar] [CrossRef]
- Kenakin, T.; Christopoulos, A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013, 12, 205–216. [Google Scholar] [CrossRef]
- Sengmany, K.; Gregory, K.J. Metabotropic glutamate receptor subtype 5: Molecular pharmacology, allosteric modulation and stimulus bias. Br. J. Pharmacol. 2016, 173, 3001–3017. [Google Scholar] [CrossRef]
- Emery, A.C.; DiRaddo, J.O.; Miller, E.; Hathaway, H.A.; Pshenichkin, S.; Takoudjou, G.R.; Grajkowska, E.; Yasuda, R.P.; Wolfe, B.B.; Wroblewski, J.T. Ligand Bias at Metabotropic Glutamate 1a Receptors: Molecular Determinants That Distinguish β-Arrestin-Mediated from G Protein-Mediated Signaling. Mol. Pharmacol. 2012, 82, 291–301. [Google Scholar] [CrossRef]
- Hathaway, H.A.; Pshenichkin, S.; Grajkowska, E.; Gelb, T.; Emery, A.C.; Wolfe, B.B.; Wroblewski, J.T. Pharmacological characterization of mGlu1 receptors in cerebellar granule cells reveals biased agonism. Neuropharmacology 2015, 93, 199–208. [Google Scholar] [CrossRef]
- Teleuca, A.E.; Alemà, G.S.; Casolini, P.; Barberis, I.; Ciabattoni, F.; Orlando, R.; Di Menna, L.; Iacovelli, L.; Scioli, M.R.; Nicoletti, F.; et al. Changes in mGlu5 Receptor Signaling Are Associated with Associative Learning and Memory Extinction in Mice. Life 2022, 12, 463. [Google Scholar] [CrossRef]
- Tabata, T.; Kano, M. Calcium Dependence of Native Metabotropic Glutamate Receptor Signaling in Central Neurons. Mol. Neurobiol. 2004, 29, 261–270. [Google Scholar] [CrossRef]
- Selemon, L.D.; Goldman-Rakic, P.S. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol. Psychiatry 1999, 45, 17–25. [Google Scholar] [CrossRef]
- Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A.; Jarskog, L.F. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr. Res. 2006, 81, 47–63. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Moghaddam, B. Bringing order to the glutamate chaos in schizophrenia. Neuron 2003, 40, 881–884. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, J.; Grayson, B.; Neill, J.C.; Harte, M.; Wall, M.J.; Ngomba, R.T. Oscillatory Deficits in the Sub-Chronic PCP Rat Model for Schizophrenia Are Reversed by mGlu5 Receptor-Positive Allosteric Modulators VU0409551 and VU0360172. Cells 2023, 12, 919. https://doi.org/10.3390/cells12060919
Brown J, Grayson B, Neill JC, Harte M, Wall MJ, Ngomba RT. Oscillatory Deficits in the Sub-Chronic PCP Rat Model for Schizophrenia Are Reversed by mGlu5 Receptor-Positive Allosteric Modulators VU0409551 and VU0360172. Cells. 2023; 12(6):919. https://doi.org/10.3390/cells12060919
Chicago/Turabian StyleBrown, Jessica, Ben Grayson, Joanna C. Neill, Michael Harte, Mark J. Wall, and Richard T. Ngomba. 2023. "Oscillatory Deficits in the Sub-Chronic PCP Rat Model for Schizophrenia Are Reversed by mGlu5 Receptor-Positive Allosteric Modulators VU0409551 and VU0360172" Cells 12, no. 6: 919. https://doi.org/10.3390/cells12060919
APA StyleBrown, J., Grayson, B., Neill, J. C., Harte, M., Wall, M. J., & Ngomba, R. T. (2023). Oscillatory Deficits in the Sub-Chronic PCP Rat Model for Schizophrenia Are Reversed by mGlu5 Receptor-Positive Allosteric Modulators VU0409551 and VU0360172. Cells, 12(6), 919. https://doi.org/10.3390/cells12060919






