Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics
Abstract
1. Introduction
2. LncRNAs as Mediators of the Autophagy Process
2.1. Initiation
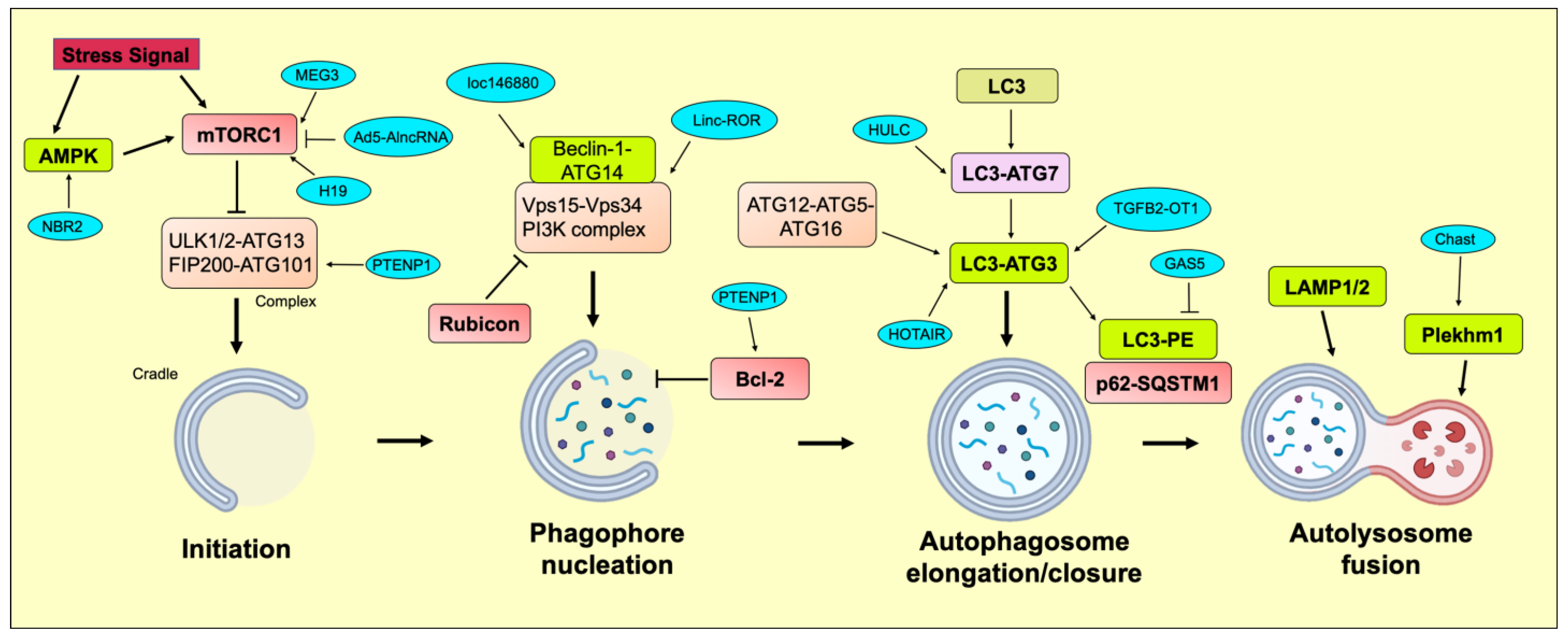
2.2. Phagophore Nucleation
2.3. Autophagosome Elongation/Closure
2.4. Autolysosome Fusion
3. LncRNAs Targeting Autophagy in Different Cancers
3.1. Bladder Cancer
3.2. Breast Cancer
| Cancer | LncRNAs | Clinical/In Vitro | Model | Expression | References |
|---|---|---|---|---|---|
| Acute myeloid leukemia | LINC00265 | Clinical | Peripheral venous blood | Up | [125] |
| In vitro | OCI/AML-2, and THP-1 | Up | |||
| DANCR | In vitro | Ara C-treated primary cell lines and HL60, U937, and KG1a | Up | [126] | |
| Breast Cancer | DANCR | Clinical | Breast cancer tissues | Up | [120] |
| In vitro | HCC1937, 1590, ZR-75-30, and MDA-MB-468 | Up | |||
| GAS5 | Clinical | Breast cancer tissues | Down | [121] | |
| In vitro | MCF-7 and MDA-MB-231 | Down | |||
| H19 | Clinical | Tamoxifen-resistant breast cancer tissues | Up | [119] | |
| In vitro | MCF7/TAMR | Up | |||
| AGAP2-AS1 | In vitro | Exosomes from SKBR-3-TR | Up | [124] | |
| Bladder Cancer | MEG3 | Clinical | Bladder cancer tissues | Down | [114] |
| UCA1 | Clinical | Bladder cancer tissues | Up | [115] | |
| In vitro | HT-1376, T24, J82, 5637, and EJ | Up | |||
| ADAMTS9-AS1 | In vitro | J82, EJ, 5637, and T24 | Up | [116] | |
| Cervical Cancer | ROR1-AS1 | Clinical | Cervical cancer tissues | Up | [127] |
| In vitro | SiHa, ME-180 and SW756 | Up | |||
| RP11-381N20.2 | Clinical | Cervical cancer tissues and chemotherapy insensitive cervical cancer tissues | Down | [128] | |
| Clear Cell Renal Cell Carcinoma | TUG1 | Clinical | Clear cell renal cell carcinoma tissues | Up | [129] |
| In vitro | 786-0 and A498 | Up | |||
| Colon Cancer | EGOT | Clinical | Colon cancer tissues and serum | Up | [130] |
| CASC2 | Clinical | Colon cancer tissues | Down | [131] | |
| In vitro | HT-29, SW948, RKO, and SW480 | Down | |||
| LINC00858 | Clinical | Colon cancer tissues | Up | [132] | |
| In vitro | CT26, SW480, HCT116, and SW620 | Up | |||
| KCNQ1OT1 | Clinical | Colon cancer tissues | Up | [133] | |
| Colorectal Cancer | NEAT1 | Clinical | Colon cancer tissues | Up | [134] |
| In vitro | HT29, HCT8, HCT116, SW480, and SW620 | Up | |||
| SLCO4A1-AS1 | Clinical | Colon cancer tissues | Up | [135] | |
| In vitro | SW620, SW480, HT29, DLD-1, and RKO | Up | |||
| SNHG14 | Clinical | Colon cancer tissues | Up | [136] | |
| In vitro | SW620 and SW480 | Up | |||
| UCA1 | Clinical | 5-FU resistant-CRC tissues | Up | [137] | |
| In vitro | SW480/5-FU and SW620/5-FU | Up | |||
| MALAT1 | Clinical | Colorectal cancer tissues | Up | [138] | |
| In vitro | HCT290, HCT116, SW480, and SW620 | Up | |||
| CPS1-IT1 | Clinical | Colorectal cancerous tissues | Down | [139] | |
| In vitro | LoVo, SW620, SW480, and LS174T | Down | |||
| H19 | Clinical | Colorectal cancer tissue | Up | [140] | |
| In vitro | 5-Fu resistant SW1116 and acquired 5-Fu resistant HCT8Fu | Up | |||
| CASC9 | In vitro | HT-29, SW480, and HCT-116 | Up | [141] | |
| SNHG8 | In vitro | HCT116, HCT8, HT29, and SW480 | Up | [109] | |
| TUG1 | Clinical | Colorectal cancer tissue | Up | [142] | |
| In vitro | HT-29, DLD-1, LS513, LoVo, and HCT15 | Up | |||
| Gastric Cancer | SNHG11 | Clinical | Gastric cancer tissues | Up | [143] |
| In vitro | AGS, BGC-823, HGC-27, MGC-803, SGC-7901, and MKN45 | Up | |||
| JPX | Clinical | Gastric cancer tissues | Up | [144] | |
| In vitro | NCI-N87 and MKN45 | Up | |||
| LINC01572 | Clinical | DDP-Resistant gastric cancer tissues | Up | [145] | |
| In vitro | BGC823/DDP and SGC7901/DDP | Up | |||
| CRNDE | In vitro | Oxaliplatin and 5-FU resistant MGC-803 | Down | [146] | |
| MALAT1 | Clinical | Gastric cancer tissues | Up | [147] | |
| MALAT1 | In vitro | SGC7901/VCR | Up | [148] | |
| HAGLROS | Clinical | Gastric cancer tissues | Up | [149] | |
| In vitro | SGC-7901, BGC-823, HGC-27, MGC-803, and AGS | Up | |||
| MALAT1 | In vitro | CDDP-resistant GC cell lines (AGS/CDDP and HGC-27/CDDP) | Up | [150] | |
| HULC | In vitro | SGC7901/CDDP and MGC-803/CDDP cells transfected with LV-METase | Down | [151] | |
| EIF3J-DT | In vitro | MGC-803/OXA and MGC-803/5Fu | Up | [152] | |
| DANCR | Clinical | Gastric cancer tissues | Up | [153] | |
| In vitro | SGC7901, MGC-803, and NCI-N87 | Up | |||
| CCAT1 | In vitro | AGS, and MKN-45 | Up | [154] | |
| LIT3527 | Clinical | Gastric cancer tissues | Up | [155] | |
| In vitro | AGS, MKN45, MKN74, SGC7901, and MGC-803 | Up | |||
| FEZF1-AS1 | Clinical | Gastric cancer tissues | Up | [108] | |
| In vitro | MKN-49P, MGC-803, BGC-823, SGC-7901, and NCI-N87 | Up | |||
| LINC00963 | Clinical | Gastric cancer tissues | Up | [156] | |
| In vitro | SGC-7901, MKN45, and MKN74 | Up | |||
| Glioma | MALAT1 | Clinical | Glioma tissues | Up | [105] |
| In vitro | U87, U118, U251, U373 and D247 | Up | |||
| CASC2 | Clinical | Glioma and peritumoral brain edema (PTBE) tissues | Down | [157] | |
| In vitro | Temozolomide resistance U257 and U87 | Down | |||
| MEG3 | Clinical | Glioma tissues | Down | [158] | |
| In vitro | U251 | Down | |||
| GAS5 | In vitro | U87, U251, U138 and LN18 | Down | [159] | |
| AC023115.3 | In vitro | Cisplatin-induced U87MG | Up | [90] | |
| Linc-RA1 | In vitro | Radioresistant glioma cell lines (M059K and U87) | Up | [160] | |
| H19 | In vitro | U87, and U251 | Up | [161] | |
| LINC00470 | In vitro | Exosomes from glioma patient’s serum | Up | [162] | |
| Lnc-NLC1-C | In vitro | U87MG | Up | [163] | |
| Head and Neck Squamous Cell Carcinoma | LINC00460 | Clinical | Head and neck squamous cell carcinoma tissues | Up | [164] |
| In vitro | PCI-13, FaDu, SCC-15, and UM-SCC-10A | Up | |||
| Hepatocellular Cancer | SNHG11 | Clinical | Hepatocellular cancer tissues | Up | [165] |
| In vitro | SK-HEP-1, Hep G2, HuH-7, and Li-7 | Up | |||
| HOTAIR | Clinical | Hepatocellular cancer tissues | Up | [98] | |
| In vitro | Huh7, HepG2, and BEL-7402 | Up | |||
| PVT1 | Clinical | Hepatocellular cancer tissues | Up | [166] | |
| In vitro | Bel-7402, Hep3B, and HepG2 | Up | |||
| CCAT1 | Clinical | Hepatocellular cancer tissues | Up | [167] | |
| In vitro | Huh7, HCCLM3, Hep3B, and HepG2 | Up | |||
| HNF1A-AS1 | Clinical | Hepatocellular cancer tissues | Up | [97] | |
| In vitro | HepG2, SMMC-7721, and Huh7 | Up | |||
| NBR2 | Clinical | Hepatocellular cancer tissues | Down | [168] | |
| In vitro | HepG2, PLC/PRF/5, Hep3B, Huh7, MHCC-97 L, MHCC-97H, SK-Hep1, and MHCC-LM3 | Down | |||
| NEAT1 | In vitro | HepG2, Huh7, Hep3B, and SMMC-7721 | Up | [169] | |
| H19 | In vitro | Hypoxia/reoxygenation (h/R)-induced HepG2 and HCCLM3 | Up | [170] | |
| DCST1-AS1 | Clinical | Hepatocellular cancer tissues | Up | [171] | |
| HAGLROS | Clinical | Hepatocellular cancer tissues | Up | [172] | |
| In vitro | SK-Hep1, MHCC97L, MHCC97H, Huh7, and HepG2.2.15 | Up | |||
| DANCR | Clinical | Hepatocellular cancer tissues | Up | [173] | |
| HULC | Clinical | Hepatocellular cancer tissues | Up | [174] | |
| ATB | Clinical | Hepatocellular cancer tissues | Up | [175] | |
| CRNDE | Clinical | Hepatocellular cancer tissues | Up | [103] | |
| In vitro | SMMC-7721, HepG2, Hep3B, Huh7, and PLC | Up | |||
| RP11-295G20.2 | Clinical | Hepatocellular cancer tissues | Up | [176] | |
| CCAT2 | Clinical | Hepatocellular cancer tissues | Up | [177] | |
| HnRNPU-AS1 | Clinical | Hepatocellular cancer tissues | Down | [178] | |
| Laryngeal Squamous Cell Carcinoma | H19 | Clinical | Laryngeal squamous cell carcinoma tissues | Up | [179] |
| In vitro | TU-177/R and AMC-HN-8/R | Up | |||
| Lung Cancer | MSTO2P | Clinical | Lung cancer tissues | Up | [180] |
| In vitro | H1299, H23, and A549 | Up | |||
| LCPAT1 | In vitro | CSE- or PM2.5-induced H1299 and H520 | Up | [181] | |
| PANDAR | Clinical | Lung cancer tissues | Down | [182] | |
| In vitro | L78, PC9, 95D, NCI-H460, and A549 | Down | |||
| MITA1 | In vitro | Gefitinib-resistant HCC827GR | Up | [110] | |
| Lymphoma | BCYRN1 | Clinical | Extranodal NK/T-cell lymphoma samples | Up | [183] |
| Multiple Myeloma | MALAT1 | Clinical | Bone marrow mononuclear cells | Up | [106] |
| In vitro | KM3 and U266 | Up | |||
| Nasopharyngeal Cancer | MEG3 | Clinical | Nasopharyngeal cancer tissues | Down | [184] |
| In vitro | C666-1, HK-1, 5-8F, and 6-10B | Down | |||
| Non-Small Cell Lung Cancer | NBAT1 | Clinical | Non-small cell lung cancer tissues | Down | [185] |
| BLACAT1 | In vitro | A549/DDP and H1299/DDP | Up | [186] | |
| GAS5 | Clinical | Non-small cell lung cancer tissues | Down | [187] | |
| PVT1 | Clinical | Non-small cell lung cancer and cisplatin-resistant tissues | Up | [188] | |
| In vitro | A549 and A549/DDP | Up | |||
| Osteosarcoma | CTA | Clinical | Osteosarcoma tissues | Down | [189] |
| In vitro | DOX-resistant MG-63 | Down | |||
| DICER1-AS1 | In vitro | MG-63, U2OS, HOS, 143B, and Saos-2 | Up | [107] | |
| SNHG15 | Clinical | Osteosarcoma tissues | Up | [190] | |
| In vitro | 143B, U2OS, HOS, MG63, and Saos-2 | Up | |||
| SNHG6 | Clinical | Osteosarcoma tissues | Up | [191] | |
| In vitro | SOSP-9607 and MG63 | Up | |||
| Ovarian Cancer | HOXA11-AS | In vitro | SKOV3, OVCAR3, and A2780 | Up | [192] |
| TUG1 | Clinical | Ovarian cancer tissues | Up | [193] | |
| In vitro | A2780, A2780/R, and SKOV3 | Up | |||
| XIST | In vitro | SKOV3, A2780, and HO-8910 | Up | [194] | |
| Pancreatic Cancer | LINC01207 | Clinical | Pancreatic cancer tissues | Up | [195] |
| PVT1 | In vitro | Gemcitabine-resistant PANC-1 and SW1990 | Up | [196] | |
| MALAT1 | Clinical | Pancreatic ductal adenocarcinoma tissues | Up | [197] | |
| In vitro | CFPAC, Bxpc-3, and PANC-1 | Up | |||
| SNHG14 | In vitro | SW1990 | Up | [198] | |
| ANRIL | Clinical | Pancreatic cancer tissues | Up | [199] | |
| In vitro | PANC-1, ASPC-1, HPAC, and BxPC-3 | Up | |||
| Papillary Thyroid Cancer | BANCR | Clinical | Papillary thyroid cancer tissues | Up | [200] |
| In vitro | IHH-4 | Up | |||
| Prostate Cancer | SNHG1 | Clinical | Prostate cancer tissues | Up | [201] |
| In vitro | LNCaP, PC-3, and DU-145 | Up | |||
| PRRT3-AS1 | In vitro | PC3, DU145, LNCaP, IA8, and IF11 | Up | [104] | |
| Retinoblastoma | MALAT1 | In vitro | Y79, Weri-Rb1, SO-Rb50, and HXO-RB44 | Up | [202] |
3.3. Cervical Cancer
3.4. Colorectal Cancer
| Cancer | LncRNA | Target miRNA/Gene | Effect after Overexpression/Knockdown | References |
|---|---|---|---|---|
| Acute Myeloid Leukemia | LINC00265 b | miR-485-5p | ↓LC3-II/LC3-I ratio, ↓Beclin-1, ↑p62, ↓IRF2, ↑Apoptosis | [125] |
| UCA1 a | miR-96-5p | ↑ATG7, ↑Beclin-1, ↑Proliferation | [212] | |
| DANCR a | miR-874-3p | ↑ATG16L1, ↑Cytarabine resistance, ↑LC3-II, ↓SQSTM1/p62 | [126] | |
| Breast Cancer | DANCR b | miR-758-3p | ↑ATG5, ↑Caspase 3, ↑Caspase 9, ↑Bax, ↓Bcl-2, ↑LC3B, ↓Beclin-1, ↓PAX6 | [120] |
| GAS5 a | - | ↑LC3B, ↑Beclin-1, ↑ULK1, ↑ULK2, ↑Chemosensitivity | [121] | |
| H19 b | - | ↓Tamoxifen resistance, ↓Beclin-1, ↓LC3-II, ↑DNMT3B | [119] | |
| OTUD6B-AS1 a | miR-26a-5p | ↑LC3B-II, ↑γ-H2AX, ↓p-ATR, ↓p-ATM, ↓p-RAD51 | [123] | |
| AGAP2-AS1 b | ELAVL1 | ↓ATG10, ↓ATG5, ↓LC3-II, ↑p62, ↓Trastuzumab resistance | [124] | |
| Bladder Cancer | MEG3 b | - | ↑LC3-II, ↓Apoptosis, ↓G0/G1 phase populations | [114] |
| UCA1 b | miR-582-5p | ↓ATG7, ↑p62, ↑LC3-I/LC3-II ratio, ↑E- cadherin, ↓Zeb1, ↓Zeb2, ↓Twist, ↓Snail, ↓MRP1, ↓LRP, ↓GST, ↑TOPO-II | [115] | |
| ADAMTS9-AS1 b | AMDAMT9 | ↑Beclin-1, ↑LC3-II/LC3-I ratio, ↑Caspase 9, ↑Bax, ↓vimentin, ↓N-cadherin, ↓Snail, ↑E-cadherin, ↓p62, ↓Bcl-2, ↓PIK3CB, ↓p-AKT, ↓p-mTOR | [116] | |
| Cervical Cancer | ROR1-AS1 b | miR-670-3p | ↓Beclin 1, ↑LC3-I, ↓LC3-II, ↓Proliferation, ↑Apoptosis | [127] |
| RP11-381N20.2 a | - | ↓Paclitaxel-induced autophagy, ↓ATG7, ↑ chemosensitivity | [128] | |
| Clear Cell Renal Cell Carcinoma | TUG1 b | miR-31-5p | ↑LC3-II/LC3-I ratio, ↓p62, ↓PCNA, ↑cle-Caspase 3, ↓FLOT1 | [129] |
| Colon Cancer | EGOT a | - | ↓cle-Caspase 3, ↓Bax, ↑Bcl-2, ↓Beclin-1, ↑p62, ↓LC3-II/LC3-I, ↑Proliferation, ↑Invasion | [130] |
| CASC2 a | miR-214 | ↓TRIM16, ↑Beclin-1, ↑LC3-II, ↑Bax, ↓Bcl-2, ↑cle-Caspase 3, ↓Proliferation | [131] | |
| LINC00858 b | - | ↑Beclin-1, ↑LC3Ⅱ/I, ↑Bax, ↓Bcl-2, ↑cle-Caspase 3, ↑p27 | [132] | |
| KCNQ1OT1 b | miR-34a | ↓Atg4B, ↑cle-PARP, ↓LC3Ⅱ, ↑Chemosensitivity, ↓Proliferation | [133] | |
| Colorectal Cancer | NEAT1 b | miR-34a | ↓ATG9A, ↓ATG4B, ↓Beclin-1, ↓LC3II/I ratio, ↑cle-Caspase 3, ↓ULK1, ↓HMGB1, ↑Chemosensitivity | [134] |
| SLCO4A1-AS1 a | miR-508-3p | ↑Proliferation, ↓Apoptosis ↑LC3B-II, ↑PARD3 | [135] | |
| SNHG14 b | miR-186 | ↓Proliferation, ↓Migration, ↓Invasion, ↓ATG14, ↓LC3B, ↓Cisplatin resistance | [136] | |
| UCA1 b | miR-23b-3p | ↓LC3-II/LC3-I ratio, ↓Beclin-1, ↑p62, ↑Bax, ↑Caspase 3, ↓5-FU resistance, ↓ZNF281 | [137] | |
| MALAT1 b | miR-101 | ↓Proliferation, ↑cle-Caspase 3, ↓LC3-II/LC3-I ratio, ↑p62 | [138] | |
| CPS1-IT1 a | - | ↓LC3-II, ↓HIF-1α, ↓Beclin-1, ↓N-cadherin, ↓Vimentin, ↑E-cadherin, ↑ZO-1 | [139] | |
| H19 a | miR-194-5p | ↑Proliferation, ↑LC3-II, ↓p62, ↑SIRT1, ↑Chemoresistance | [140] | |
| SNHG6 b | miR-26a-5p | ↓Proliferation, ↑cle-Caspase 3, ↑cle- PARP, ↓p-ULK1, ↓ATG13, ↓ULK1, ↓Chemoresistance | [211] | |
| CASC9 b | - | ↓Proliferation, ↓Migration, ↑LC3B-II, ↓p62, ↓Vimentin, ↑E-cadherin, ↑p-AMPKα/AMPKα, ↓p-AKT, ↓p-mTOR | [141] | |
| SNHG8 a | miR-588 | ↑Proliferation, ↑LC3-II, ↑ATG7, | [109] | |
| TUG1 a | miR-195-5p | ↑Proliferation ↑LC3II, ↑Beclin-1, ↓p53, ↓Bax, ↑Bcl-2, ↓Caspase 3, ↑HDGF, ↑DDX5, ↑β-catenin | [142] | |
| Gastric Cancer | SNHG11 b | miR-483-3p/miR-1276 | ↓LC3-II/LC3-I ratio, ↑p62, ↓LAMP1, ↓Twist, ↓Nanog, ↓LRG5, ↓CD133, ↓EpCAM, ↓Sox2, ↓Bcl-2, ↑Bax, ↓MMP-2, ↓MMP-7, ↑E-cadherin, ↓N-cadherin, ↓CUL4A, ↑GSK-3β, ↓β-catenin, ↑cle- PARP, ↑cle-Caspase 3, ↑cle-Caspase 6 | [143] |
| JPX b | miR-197 | ↓Proliferation, ↓Migration, ↓Invasion | [144] | |
| LINC01572 b | miR-497-5p | ↓Autophagy, ↓Proliferation, ↓Migration, ↓Invasion, ↓Cisplatin resistance | [145] | |
| CRNDE a | - | ↑Apoptosis, ↓LC3-II, ↑cle- PARP, ↑cle-Caspase 3, ↓Chemoresistance | [146] | |
| MALAT1 a | miR-204 | ↑Proliferation, ↑LC3B, ↑Ki67, ↑TRMP3 | [147] | |
| MALAT1 b | miR-23b-3p | ↓LC3-II/LC3-I ratio, ↑p62, ↓ATG12, ↓Chemoresistance | [148] | |
| MALAT1 a | miR-30b | ↑Proliferation, ↑LC3-II, ↓p62, ↑ATG5, ↑Cisplatin resistance | [150] | |
| HULC a | - | ↑LC3-II/LC3-I, ↑Beclin-1, ↓p62, ↑FoxM1, ↑MDR1, ↑Cisplatin resistance | [151] | |
| HAGLROS b | miR-100-5p | ↑LC3-II/LC3-I, ↓p62, ↓p-mTOR, ↓mTOR, ↓p-4E-BP1, ↓Proliferation, ↓Migration, ↓Invasion | [149] | |
| EIF3J-DT b | miR-188-3p | ↓Proliferation, ↑cle-PARP, ↑cle-Caspase 3, ↓LC3-II, ↓ATG14, ↓Chemoresistance | [152] | |
| DANCR b | miR-194 | ↑LC3-II/LC3-I ratio, ↑Beclin-1, ↑Apoptosis | [153] | |
| CCAT1 a | miR-140-3p | ↑Proliferation, ↑Migration, ↑Invasion, ↑LC3A/B, ↑Beclin-1, ↑ATG5, ↑ATG12 | [154] | |
| LIT3527 b | - | ↓Proliferation, ↓Migration, ↑LC3-II ↑Apoptosis, ↓p-AKT, ↓p-mTOR, ↓p-ERK, ↓4EBP1, ↓Metastasis | [155] | |
| FEZF1-AS1 b | - | ↓LC3-II, ↓ATG5, ↑Bax, ↓Bcl-2, ↑cle-Caspase 3, ↓MDR1, ↓MPR1, ↓S-phase cell populations, ↓Chemoresistance | [108] | |
| LINC00963 b | miR-4458 | ↓LC3-II, ↑p62, ↓Proliferation, ↓Migration | [156] | |
| Glioblastoma | LINC00470 a | miR-101 | ↑ELFN2, ↓Dicer, ↓LC3-II, ↓ATG7, ↓ATG3, ↓Beclin-1 | [213] |
| Glioma | MALAT1 b | miR-101-3p | ↓LC3-II, ↑p62, ↓Proliferation, ↓STMN1, ↓RAB5A, ↓ATG4D | [105] |
| CASC2 a | miR-193a-5p | ↓LC3-II, ↓Beclin-1, ↑p62, ↑mTOR, ↓Migration, ↓Invasion | [157] | |
| GAS5 a | - | ↓Proliferation, ↓LC3-II, ↑p62, ↑p-mTOR ↑Chemosenstivity | [159] | |
| AC023115.3 b | miR-26a | ↑LC3-II, ↓p62, ↓cle-Caspase 3, ↓cle- PARP, ↓Mcl1, ↓Chemoresistance | [90] | |
| Linc-RA1 a | - | ↓% DNA damage, ↓% Irradiation-induced death, ↑H2Bub1, ↓LC3B-II/I ratio, ↑p62, ↓γ-H2AX, ↑Radioresistance | [160] | |
| H19 a | - | ↑Proliferation, ↑Migration, ↓Autophagy, ↓p-mTOR, ↑p-ULK1 | [161] | |
| LINC00470 a | miR-580-3p | ↓LC3-II/LC3-I, ↓Beclin-1, ↑p62, ↑Proliferation, ↓G1phase cell population, ↑p-PI3K, ↑p-mTOR, ↑p-AKT | [162] | |
| Lnc-NLC1-C b | - | ↓Proliferation, ↓Migration, ↓Invasion, ↑ROS generation, ↓LC3II/I, ↓p62, ↑ATG9, ↑Rab1, ↓PRDX-3 | [163] | |
| DRAIC a | - | ↓LC3-II, ↑p62, ↓Migration, ↓Invasion, ↓p-ULK1 (S757), ↓p-S6K, ↑p-AMPK, ↑p-RPTOR, ↑p-FoxO3a | [214] | |
| Head and Neck Squamous Cell Carcinoma | LINC00460 b | miR-206 | ↓STC2, ↓AKT, ↓ERK, ↓p-ERK, ↓p-AKT, ↑G0/G1-phase cell arrest, ↑Bax, ↑cle-PARP, ↑cle-Caspase 3, ↑LC3-II/I ratio, ↑Beclin-1 | [164] |
| Hepatocellular Cancer | SNHG11 b | mir-184 | ↓AGO2, ↓Beclin-1, ↓LC3-II/I ratio, ↑cle-Caspase 3, ↓Migration, ↓Invasion | [165] |
| HOTAIR a | - | ↑LC3-II, ↑ATG3, ↑ATG7, ↑Proliferation | [98] | |
| H19 b | - | ↓Proliferation, ↓G0/G1-phase cell population, ↑cle-Caspase 3, ↑cle-Caspase 9, ↓Bcl-2, ↑Cyt c, ↓LC3-II/1 ratio, ↓Beclin-1, ↑p62, ↑p-PI3K, ↑p-AKT, ↑p-mTOR | [170] | |
| PVT1 a | miR-365 | ↑Proliferation, ↑Ki67, ↑LC3-II, ↑ATG3 | [166] | |
| CCAT1 a | miR-181a-5p | ↑Proliferation, ↑LC3-II, ↓p62, ↑ATG7 | [167] | |
| MEG3 a | - | ↓Proliferation, ↓Migration, ↓LC3-II/LC3-I, ↓Beclin-1, ↓ILF3, ↑p-PI3K, ↑p-AKT, ↑p-mTOR | [215] | |
| HNF1A-AS1 a | miR-30b-5p | ↑Proliferation, ↑LC3BII/I, ↓p62, ↑ATG5, ↑Bcl-2 | [97] | |
| MCM3AP-AS1 b | miR-455 | ↓Migration, ↓Vessel formation | [216] | |
| NBR2 a | - | ↓Proliferation, ↓Migration, ↓Invasion, ↓LC3 II/I ratio, ↓Beclin-1, ↑p62, ↓p-ERK, ↓p-JNK | [168] | |
| NEAT1 a | miR-204 | ↓Sorafenib-induced growth inhibition, ↑LC3-II/I ratio, ↑p-AKT, ↑p-mTOR, ↑ATG3 | [169] | |
| DCST1-AS1 b | - | ↓Proliferation, ↓Migration, ↑Autophagy, ↑Apoptosis | [171] | |
| HAGLROS b | miR-5095 | ↓LC3 II/I ratio, ↓Beclin-1, ↑p62, ↑Bax, ↑cle-Caspase 3, ↑cle-Caspase 9, ↓Bcl-2, ↓p-PI3K, ↓p-AKT, ↓p-mTOR, ↑PTEN | [172] | |
| DANCR b | miR-222-3p | ↓Proliferation, ↓Autophagy | [173] | |
| HULC a | miR-15a | ↑Proliferation, ↑LC3 II/I ratio, ↑Sirt1, ↓PTEN, ↑JAK, ↑PKM2, ↑CDK2, ↑p-PI3K, ↑p-AKT, ↑p-mTOR, ↑Jun, ↑Survivin | [174] | |
| ATB a | - | ↑Proliferation, ↑LC3 II/I ratio, ↓p-YAP ↑ATG5 | [175] | |
| CRNDE a | miR-543 | ↑ATG4B, ↑LC3-II/I ratio, ↓p62 | [103] | |
| RP11-295G20.2 a | PTEN | ↓LC3B, ↓PTEN, ↑p-AKT, ↓FOXO3a | [176] | |
| CCAT2 b | miR-4496/ELAVL1 | ↓Migration, ↓Invasion, ↓LC3 II/I ratio, ↓Beclin-1, ↑p62 | [177] | |
| HnRNPU-AS1 a | miR-556-3p/miR-580-3p | ↓Proliferation, ↓Migration, ↑Autophagy, | [178] | |
| Hypoxic Tumor | LincRNA-p21 b | - | ↓Proliferation, ↑G2/M arrest of cell populations, ↓Migration, ↓HIF-1α, ↓LC3 II, ↑p62 | [217] |
| Laryngeal Squamous Cell Carcinoma | H19 b | miR-107 | ↓LC3 II/I ratio, ↓Beclin-1, ↑p62, ↓LAMP2, ↓Chemoresistance | [179] |
| Lung Cancer | MSTO2P b | - | ↓Proliferation, ↓Agt5, ↓LC-3II, ↓EZH2 | [180] |
| LCPAT1 b | RCC2 | ↓Proliferation, ↓Migration, ↓Invasion, Autophagy halted after CSE/ PM2.5 exposure | [181] | |
| LINC00857 b | YBX1 | ↓Proliferation, ↑LC3 II/I ratio, ↑cle-PARP, ↓YBX1, ↓p-MET, ↑p-AMPKa | [218] | |
| PANDAR a | - | ↓Proliferation, ↑Autophagy, ↑Beclin-1 | [182] | |
| MITA1 a | - | ↓Apoptosis, ↑LC3 II/I ratio, ↑Beclin-1, ↓p62, ↑Gefitinib resistance | [110] | |
| Lymphoma | BCYRN1 a | - | ↑Proliferation, ↑Bcl-2, ↑Cyclin D1, ↓p53, ↓Bax, ↓p21, ↑Autophagy, ↑Beclin-1, ↑LC3-II, ↓p-mTOR, ↓p-AKT | [183] |
| Multiple Myeloma | MALAT1 b | HMGB1 | ↓Proliferation, ↑Apoptosis, ↓Beclin-1, ↓LC3B, ↓HMBG1 | [106] |
| Nasopharyngeal Cancer | MEG3 a | miR-21 | ↑LC3 II/I ratio, ↑Beclin-1, ↓p62, ↑Bax, ↑cle-Caspase 3, ↓Bcl-2, ↑PTEN | [184] |
| Neuroblastoma | SNHG7 b | miR-329-3p | ↓Proliferation, ↓LC3B-I/LC3B-II, ↓Beclin-1, ↑p62, ↓Chemoresistance, | [219] |
| Non-Small Cell Lung Cancer | UCA1 b | miR-185-5p | ↓Proliferation, ↓Ki67, ↑Caspase 3, ↓LC3 II/I ratio, ↓Beclin-1, ↑p62, ↓WISP2, ↓β-catenin, ↓TCF4 | [220] |
| NBAT1 b | PSMD10 | ↑LC3-II, ↓p62, ↑ATG7, ↑PSMD10, ↑Proliferation, ↑Chemoresistance | [185] | |
| BLACAT1 a | miR-17 | ↑LC3 II/I ratio, ↑Beclin-1, ↑MRP1, ↑Chemoresistance, ↑Proliferation ↑ATG7 | [186] | |
| GAS5 a | - | ↓Proliferation, ↑LC3-II ↓Chemoresistance | [187] | |
| PVT1 b | miR-216b | ↓LC3B II/I, ↑p62, ↓Beclin-1, ↑Apoptosis, ↑Cisplatin sensitivity, | [188] | |
| Osteosarcoma | CTA a | miR-210 | ↓LC3-II, ↓BNIP3/BNIP3L, ↑cle-Caspase 3, ↑Doxorubicin sensitivity, ↑Apoptosis | [189] |
| DICER1-AS1 b | miR-30b | ↓ATG5, ↓LC3-II, ↓Beclin-1, ↓Proliferation, ↓Migration, ↓Invasion | [107] | |
| SNHG15 b | miR-141 | ↓LC3-II/LC3-I, ↓ATG5, ↑p62, ↓Proliferation, ↓Migration, ↓Invasion | [190] | |
| SNHG6 b | miR-26a-5p | ↓ULK1, ↑ATF3, ↑cle-Caspase 3, ↓Proliferation, ↓Migration, ↓Invasion | [191] | |
| Ovarian Cancer | HOXA11-AS b | - | ↑LC3II/I ratio, ↑Beclin-1, ↓p62, ↓Migration, ↓Invasion, ↑Cisplatin sensitivity | [192] |
| TUG1 b | miR-29b-3p | ↓Beclin-1, ↓LC3B II/I, ↑cle-Caspase 3, ↑cle-Caspase 7, ↓Proliferation, ↑Paclitaxel sensitivity | [193] | |
| XIST b | miR-506-3p | ↓LC3 II/I ratio, ↑p62, ↑Bax, ↓Bcl-2, ↓FOXP1, ↑Carboplatin sensitivity | [194] | |
| Pancreatic Cancer | LINC01207 b | miR-143-5p | ↓AGR2, ↓Cell growth, ↑Apoptosis, ↑LC3II, ↑Beclin-1, ↓p62, ↓Bcl-2/Bax | [195] |
| PVT1 b | miR-619-5p | ↓ATG14, ↓Pygo2, ↓Cyclin-D1, ↓c-Myc, ↓LC3-II, ↑p62, ↓Axin2, ↓Gemcitabine resistance | [196] | |
| MALAT1 b | HuR | ↓LC3B II/I, ↑p62, ↓LAMP-2, ↓MMP-3, ↓MUC4 | [197] | |
| SNHG14 b | miR-101 | ↓RAB5A, ↓ATG4D, ↓Gemcitabine resistance, ↓Migration, ↓Invasion | [198] | |
| ANRIL b | miR-181a | ↓LC3 II, ↑Beclin-1, ↓HMGB1, ↓Proliferation, ↓Snail, ↓Vimentin, ↑E- cadherin, ↓N-cadherin | [199] | |
| Papillary Thyroid Cancer | RP11-476D10.1 b | miR-138-5p | ↓LRRK2, ↑Beclin1, ↑LC3B, ↑Bax ↓Bcl-2 | [221] |
| BANCR b | - | ↓LC3-II/LC3-I, ↑Apoptosis, ↑Cell population in the G1 phase | [200] | |
| Prostate Cancer | HULC b | - | ↑p-Beclin-1, ↑Bax, ↑Caspase 3, ↓PCNA, ↓Cyclin D1, ↑LC3B-II, ↑Irradiation sensitivity | [222] |
| SNHG1 b | EZH2 | ↑LC3-II, ↑Beclin-1, ↓p62, ↓p-PI3K, ↓p-AKT, ↓p-mTOR, ↓p-p70S6K, ↓Wnt1, ↓β-catenin, ↓c-Myc, ↓Cyclin D1, ↓EZH2 | [201] | |
| PRRT3-AS1 b | PPARγ | ↑LC3A, ↑LC3B, ↑Beclin-1, ↓p-S6K1, ↓NF-κB1, ↓COX2, ↓p-4EPB1, ↓PCNA, ↓Ki67, ↑PPARγ, ↑Bax, ↑cle-Caspase 3, ↓Bcl-2, ↓Migration, ↓Invasion | [104] | |
| Retinoblastoma | MALAT1 b | miR-124 | ↓LC3-II, ↓Beclin-1, ↑p62 | [202] |
| Uveal melanoma | ZNNT1 a | - | ↑ATG12, ↓SQSTM1, ↓Tumor cell growth, ↓Migration, ↓Invasion | [223] |
3.5. Gastric Cancer
3.6. Glioma
3.7. Hepatocellular Cancer
3.8. Hematological Malignancies
3.9. Lung Cancer
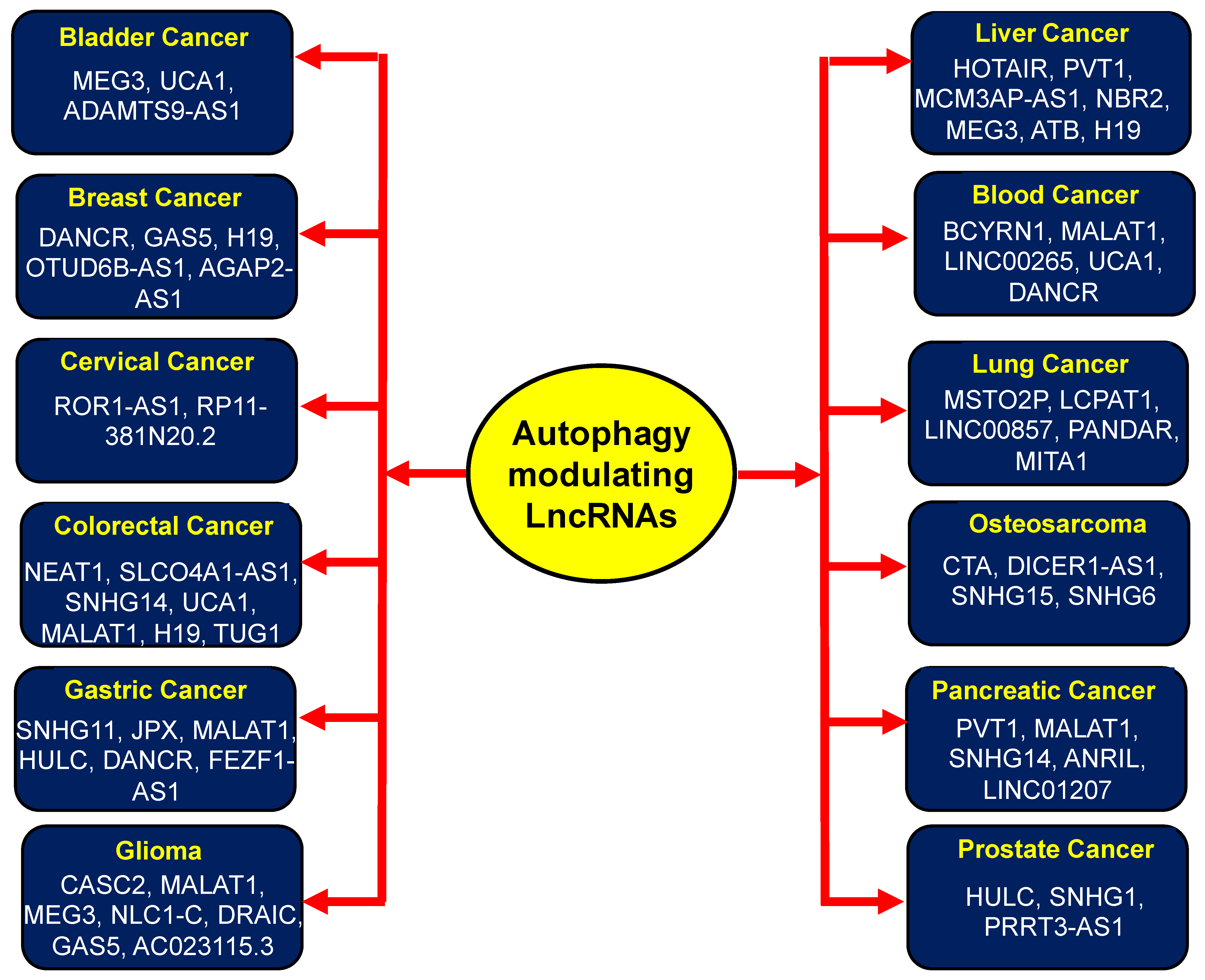
3.10. Osteosarcoma
3.11. Ovarian Cancer
3.12. Pancreatic Cancer
3.13. Prostate Cancer
3.14. Other Cancers
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jiang, G.M.; Tan, Y.; Wang, H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 2019, 18, 17. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Murrow, L.; Debnath, J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 105–137. [Google Scholar] [CrossRef]
- Gewirtz, D.A. The four faces of autophagy: Implications for cancer therapy. Cancer Res. 2014, 74, 647–651. [Google Scholar] [CrossRef]
- Chen, H.Y.; White, E. Role of autophagy in cancer prevention. Cancer Prev. Res. 2011, 4, 973–983. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Klionsky, D.J. Autophagy revisited: A conversation with Christian de Duve. Autophagy 2008, 4, 740–743. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Butsch, T.J.; Ghosh, B.; Bohnert, K.A. Organelle-Specific Autophagy in Cellular Aging and Rejuvenation. Adv. Geriatr. Med. Res. 2021, 3, e210010. [Google Scholar] [CrossRef]
- Li, C.J.; Liao, W.T.; Wu, M.Y.; Chu, P.Y. New Insights into the Role of Autophagy in Tumor Immune Microenvironment. Int. J. Mol. Sci. 2017, 18, 1566. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Um, J.Y.; Chinnathambi, A.; Govindasamy, C.; Sethi, G.; Ahn, K.S. Leelamine Modulates STAT5 Pathway Causing Both Autophagy and Apoptosis in Chronic Myelogenous Leukemia Cells. Biology 2022, 11, 366. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2022, 80, 205–217. [Google Scholar] [CrossRef]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 2009, 335, 1–32. [Google Scholar] [CrossRef]
- Galluzzi, L.; Green, D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2022, 24, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Um, J.Y.; Chinnathambi, A.; Govindasamy, C.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Withanolide modulates the potential crosstalk between apoptosis and autophagy in different colorectal cancer cell lines. Eur. J. Pharmacol. 2022, 928, 175113. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Marinkovic, M.; Sprung, M.; Buljubasic, M.; Novak, I. Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 8023821. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Mukhopadhyay, S.; Sinha, N.; Das, D.N.; Panda, P.K.; Patra, S.K.; Maiti, T.K.; Mandal, M.; Dent, P.; Wang, X.Y.; et al. Autophagy: Cancer’s friend or foe? Adv. Cancer Res. 2013, 118, 61–95. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Vega-Rubin-de-Celis, S. The Role of Beclin 1-Dependent Autophagy in Cancer. Biology 2019, 9, 4. [Google Scholar] [CrossRef]
- Schmukler, E.; Kloog, Y.; Pinkas-Kramarski, R. Ras and autophagy in cancer development and therapy. Oncotarget 2014, 5, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, Y.; Peker, N.; Akcay, A.; Gozuacik, D. Autophagy and Cancer Dormancy. Front. Oncol. 2021, 11, 627023. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.B.; Schiemann, W.P. Autophagy in breast cancer metastatic dormancy: Tumor suppressing or tumor promoting functions? J. Cancer Metastasis Treat. 2019, 5, 43. [Google Scholar] [CrossRef]
- Akkoc, Y.; Gozuacik, D. Autophagy and Hepatic Tumor Microenvironment Associated Dormancy. J. Gastrointest. Cancer 2021, 52, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, L.; Ishola, T. Dormancy in Breast Cancer, the Role of Autophagy, lncRNAs, miRNAs and Exosomes. Int. J. Mol. Sci. 2022, 23, 5271. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L. Cell-intrinsic survival signals. The role of autophagy in metastatic dissemination and tumor cell dormancy. Semin. Cancer Biol. 2020, 60, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. AMPK-mTOR Signaling and Cellular Adaptations in Hypoxia. Int. J. Mol. Sci. 2021, 22, 9765. [Google Scholar] [CrossRef]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-Tumor Activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rabiee, N.; Kumar, A.P.; Sethi, G.; Zarrabi, A.; Wang, Y. Long noncoding RNAs (lncRNAs) in pancreatic cancer progression. Drug Discov. Today 2022, 27, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Pandya, G.; Kirtonia, A.; Sethi, G.; Pandey, A.K.; Garg, M. The implication of long non-coding RNAs in the diagnosis, pathogenesis and drug resistance of pancreatic ductal adenocarcinoma and their possible therapeutic potential. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188423. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20. [Google Scholar] [CrossRef]
- Chen, X.; Tang, F.R.; Arfuso, F.; Cai, W.Q.; Ma, Z.; Yang, J.; Sethi, G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomolecules 2019, 10, 66. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Yu, W.; Zhang, Y.; Ao, X.; Wang, J. Long non-coding RNAs: Biogenesis, functions, and clinical significance in gastric cancer. Mol. Ther. Oncolytics 2021, 23, 458–476. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Chen, L.L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef]
- Naganuma, T.; Hirose, T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013, 10, 456–461. [Google Scholar] [CrossRef]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-Transcriptional Gene Regulation by Long Non-Coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Vallino, L.; Ferraresi, A.; Secomandi, E.; Salwa, A.; Chinthakindi, M.; Galetto, A.; Dhanasekaran, D.N.; Isidoro, C. Epigenetic control of autophagy in women’s tumors: Role of non-coding RNAs. J. Cancer Metastasis Treat. 2021, 7, 4. [Google Scholar] [CrossRef]
- Sanchez Calle, A.; Kawamura, Y.; Yamamoto, Y.; Takeshita, F.; Ochiya, T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018, 109, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. [Google Scholar] [CrossRef]
- Kansara, S.; Pandey, V.; Lobie, P.E.; Sethi, G.; Garg, M.; Pandey, A.K. Mechanistic Involvement of Long Non-Coding RNAs in Oncotherapeutics Resistance in Triple-Negative Breast Cancer. Cells 2020, 9, 1511. [Google Scholar] [CrossRef]
- Uzhytchak, M.; Smolkova, B.; Lunova, M.; Jirsa, M.; Frtus, A.; Kubinova, S.; Dejneka, A.; Lunov, O. Iron Oxide Nanoparticle-Induced Autophagic Flux Is Regulated by Interplay between p53-mTOR Axis and Bcl-2 Signaling in Hepatic Cells. Cells 2020, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef]
- Entezari, M.; Sadrkhanloo, M.; Rashidi, M.; Asnaf, S.E.; Taheriazam, A.; Hashemi, M.; Ashrafizadeh, M.; Zarrabi, A.; Rabiee, N.; Hushmandi, K.; et al. Non-coding RNAs and macrophage interaction in tumor progression. Crit. Rev. Oncol. Hematol. 2022, 173, 103680. [Google Scholar] [CrossRef]
- Tang, J.Y.; Chuang, Y.T.; Shiau, J.P.; Yang, K.H.; Chang, F.R.; Hou, M.F.; Farooqi, A.A.; Chang, H.W. Long Noncoding RNAs and Circular RNAs Regulate AKT and Its Effectors to Control Cell Functions of Cancer Cells. Cells 2022, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Islam Khan, M.Z.; Tam, S.Y.; Law, H.K.W. Autophagy-Modulating Long Non-coding RNAs (LncRNAs) and Their Molecular Events in Cancer. Front. Genet. 2018, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, M.; Zhang, J.; Guo, B.; Singh, S.; Lin, X.; Xiong, H.; Ju, S.; Wang, L.; Zhou, Y.; et al. The role of lncRNA H19 in tumorigenesis and drug resistance of human Cancers. Front. Genet. 2022, 13, 1005522. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Tee, A.R. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- Suzuki, K.; Kirisako, T.; Kamada, Y.; Mizushima, N.; Noda, T.; Ohsumi, Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001, 20, 5971–5981. [Google Scholar] [CrossRef]
- Papinski, D.; Kraft, C. Atg1 kinase organizes autophagosome formation by phosphorylating Atg9. Autophagy 2014, 10, 1338–1340. [Google Scholar] [CrossRef]
- Davies, C.W.; Stjepanovic, G.; Hurley, J.H. How the Atg1 complex assembles to initiate autophagy. Autophagy 2015, 11, 185–186. [Google Scholar] [CrossRef]
- Zhuo, C.; Jiang, R.; Lin, X.; Shao, M. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget 2017, 8, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xue, D.; Song, F.; Liu, X.; Li, W.; Wang, Y. DUSP5 (dual-specificity protein phosphatase 5) suppresses BCG-induced autophagy via ERK 1/2 signaling pathway. Mol. Immunol. 2020, 126, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Z.D.; Han, L.; Zhang, J.; Lee, S.W.; Wang, W.; Lee, H.; Zhuang, L.; Chen, J.; Lin, H.K.; et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016, 18, 431–442. [Google Scholar] [CrossRef]
- Liu, Y.M.; Ma, J.H.; Zeng, Q.L.; Lv, J.; Xie, X.H.; Pan, Y.J.; Yu, Z.J. MiR-19a Affects Hepatocyte Autophagy via Regulating lncRNA NBR2 and AMPK/PPARalpha in D-GalN/Lipopolysaccharide-Stimulated Hepatocytes. J. Cell. Biochem. 2018, 119, 358–365. [Google Scholar] [CrossRef]
- Tang, S.; Tan, G.; Jiang, X.; Han, P.; Zhai, B.; Dong, X.; Qiao, H.; Jiang, H.; Sun, X. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget 2016, 7, 73257–73269. [Google Scholar] [CrossRef]
- Isakson, P.; Bjoras, M.; Boe, S.O.; Simonsen, A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 2010, 116, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, L.; Kang, R.; Yang, M.; Liu, L.; Zhao, Y.; Yu, Y.; Xie, M.; Yin, X.; Livesey, K.M.; et al. Autophagy regulates myeloid cell differentiation by p62/SQSTM1-mediated degradation of PML-RARalpha oncoprotein. Autophagy 2011, 7, 401–411. [Google Scholar] [CrossRef]
- Xiu, Y.L.; Sun, K.X.; Chen, X.; Chen, S.; Zhao, Y.; Guo, Q.G.; Zong, Z.H. Upregulation of the lncRNA Meg3 induces autophagy to inhibit tumorigenesis and progression of epithelial ovarian carcinoma by regulating activity of ATG3. Oncotarget 2017, 8, 31714–31725. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yao, W.; Gumireddy, K.; Li, A.; Wang, J.; Xiao, W.; Chen, K.; Xiao, H.; Li, H.; Tang, K.; et al. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol. Cancer Ther. 2014, 13, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tseng, Y.W.; Wu, J.C.; Chen, G.Y.; Lin, K.C.; Hwang, S.M.; Hu, Y.C. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 2015, 44, 71–81. [Google Scholar] [CrossRef]
- He, C.; Levine, B. The Beclin 1 interactome. Curr. Opin. Cell Biol. 2010, 22, 140–149. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009, 10, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Akioka, M.; Kondo-Kakuta, C.; Yamamoto, H.; Ohsumi, Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J. Cell Sci. 2013, 126, 2534–2544. [Google Scholar] [CrossRef]
- Lindqvist, L.M.; Vaux, D.L. BCL2 and related prosurvival proteins require BAK1 and BAX to affect autophagy. Autophagy 2014, 10, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Q.J.; Yue, Z. Atg14L and Rubicon: Yin and yang of Beclin 1-mediated autophagy control. Autophagy 2009, 5, 890–891. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhu, H.; Qu, X.; Zhao, L.; Tan, Y.; Jiang, Y.; Liao, M.; Wu, X. Inhibition of long non-coding RNA ROR reverses resistance to Tamoxifen by inducing autophagy in breast cancer. Tumour Biol. 2017, 39, 1010428317705790. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, Y.; Wei, H.Y.; Lv, K.Z.; Fu, P.F. Large intergenic non-coding RNA-ROR reverses gemcitabine-induced autophagy and apoptosis in breast cancer cells. Oncotarget 2016, 7, 59604–59617. [Google Scholar] [CrossRef]
- Deng, X.; Feng, N.; Zheng, M.; Ye, X.; Lin, H.; Yu, X.; Gan, Z.; Fang, Z.; Zhang, H.; Gao, M.; et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 112–125. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Z.; Zhang, L.; Lv, P.; Yang, T.; Gao, J.; Pan, N.; Wu, Q.; Lou, J.; Han, C.; et al. Long non-coding RNA AC023115.3 suppresses chemoresistance of glioblastoma by reducing autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1393–1404. [Google Scholar] [CrossRef]
- Fujioka, Y.; Noda, N.N.; Nakatogawa, H.; Ohsumi, Y.; Inagaki, F. Dimeric coiled-coil structure of Saccharomyces cerevisiae Atg16 and its functional significance in autophagy. J. Biol. Chem. 2010, 285, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Noda, N.N.; Fujii, K.; Yoshimoto, K.; Ohsumi, Y.; Inagaki, F. In vitro reconstitution of plant Atg8 and Atg12 conjugation systems essential for autophagy. J. Biol. Chem. 2008, 283, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lu, W.; Ge, D.; Meng, N.; Li, Y.; Su, L.; Zhang, S.; Zhang, Y.; Zhao, B.; Miao, J. A new microRNA signal pathway regulated by long noncoding RNA TGFB2-OT1 in autophagy and inflammation of vascular endothelial cells. Autophagy 2015, 11, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ahn, C.; Chun, C.H.; Jin, E.J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014, 32, 1628–1635. [Google Scholar] [CrossRef]
- Kang, Y.; Song, J.; Kim, D.; Ahn, C.; Park, S.; Chun, C.H.; Jin, E.J. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J. Orthop. Res. 2016, 34, 412–418. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, X.; Zhang, A.; Li, C.; Bai, J.; Dong, J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem. Biophys. Res. Commun. 2016, 473, 1268–1275. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Li, H.; Liu, J. The long noncoding RNA HOTAIR activates autophagy by upregulating ATG3 and ATG7 in hepatocellular carcinoma. Mol. Biosyst. 2016, 12, 2605–2612. [Google Scholar] [CrossRef]
- Huynh, K.K.; Eskelinen, E.L.; Scott, C.C.; Malevanets, A.; Saftig, P.; Grinstein, S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007, 26, 313–324. [Google Scholar] [CrossRef]
- Saftig, P.; Beertsen, W.; Eskelinen, E.L. LAMP-2: A control step for phagosome and autophagosome maturation. Autophagy 2008, 4, 510–512. [Google Scholar] [CrossRef]
- McEwan, D.G.; Popovic, D.; Gubas, A.; Terawaki, S.; Suzuki, H.; Stadel, D.; Coxon, F.P.; Miranda de Stegmann, D.; Bhogaraju, S.; Maddi, K.; et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 2015, 57, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Viereck, J.; Kumarswamy, R.; Foinquinos, A.; Xiao, K.; Avramopoulos, P.; Kunz, M.; Dittrich, M.; Maetzig, T.; Zimmer, K.; Remke, J.; et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl. Med. 2016, 8, 326ra322. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, L.; Dai, X.; Li, T.; Yan, X.; Zhang, Y.; Xiao, H.; Shen, X.; Huang, G.; Xiang, W.; et al. LncRNA CRNDE Promotes ATG4B-Mediated Autophagy and Alleviates the Sensitivity of Sorafenib in Hepatocellular Carcinoma Cells. Front. Cell Dev. Biol. 2021, 9, 687524. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Wang, W. Long non-coding RNA PRRT3-AS1 silencing inhibits prostate cancer cell proliferation and promotes apoptosis and autophagy. Exp. Physiol. 2020, 105, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Luo, W.; Wang, J.; Peng, T.; Sun, G.; Shi, J.; Li, Z.; Zhang, B. Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma. Biochem. Biophys. Res. Commun. 2017, 492, 480–486. [Google Scholar] [CrossRef]
- Gao, D.; Lv, A.E.; Li, H.P.; Han, D.H.; Zhang, Y.P. LncRNA MALAT-1 Elevates HMGB1 to Promote Autophagy Resulting in Inhibition of Tumor Cell Apoptosis in Multiple Myeloma. J. Cell. Biochem. 2017, 118, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Hou, Z.; Zheng, L.; Wang, X.; Wu, L.; Zhang, C. LncRNA DICER1-AS1 promotes the proliferation, invasion and autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed. Pharmacother. 2018, 104, 110–118. [Google Scholar] [CrossRef]
- Gui, Z.; Zhao, Z.; Sun, Q.; Shao, G.; Huang, J.; Zhao, W.; Kuang, Y. LncRNA FEZF1-AS1 Promotes Multi-Drug Resistance of Gastric Cancer Cells via Upregulating ATG5. Front. Cell Dev. Biol. 2021, 9, 749129. [Google Scholar] [CrossRef]
- He, C.; Fu, Y.; Chen, Y.; Li, X. Long non-coding RNA SNHG8 promotes autophagy as a ceRNA to upregulate ATG7 by sponging microRNA-588 in colorectal cancer. Oncol. Lett. 2021, 22, 577. [Google Scholar] [CrossRef]
- Hu, J.; Dong, S.W.; Pei, Y.; Wang, J.; Zhang, J.; Wei, X.P. LncRNA MITA1 promotes gefitinib resistance by inducing autophagy in lung cancer cells. Biochem. Biophys. Res. Commun. 2021, 551, 21–26. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Mirzaei, S.; Barati, M.; Hejazi, E.S.; Kakavand, A.; Entezari, M.; Salimimoghadam, S.; Kalbasi, A.; Rashidi, M.; Taheriazam, A.; et al. Curcumin in the treatment of urological cancers: Therapeutic targets, challenges and prospects. Life Sci. 2022, 309, 120984. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, T.; Cheng, X.; Liu, F.; Wu, Y.; Ma, L.; Li, W. LINC00958 Inhibits Autophagy of Bladder Cancer Cells via Sponge Adsorption of miR-625-5p to Promote Tumor Angiogenesis and Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 2435114. [Google Scholar] [CrossRef]
- Ying, L.; Huang, Y.; Chen, H.; Wang, Y.; Xia, L.; Chen, Y.; Liu, Y.; Qiu, F. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol. Biosyst. 2013, 9, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, W.; Ning, J.; Yu, W.; Rao, T.; Cheng, F. Long noncoding RNA UCA1 targets miR-582-5p and contributes to the progression and drug resistance of bladder cancer cells through ATG7-mediated autophagy inhibition. OncoTargets Ther. 2019, 12, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Z.; Dong, L.; Zhou, F. lncRNA ADAMTS9-AS1 promotes bladder cancer cell invasion, migration, and inhibits apoptosis and autophagy through PI3K/AKT/mTOR signaling pathway. Int. J. Biochem. Cell Biol. 2021, 140, 106069. [Google Scholar] [CrossRef]
- Maruthanila, V.L.; Elancheran, R.; Kunnumakkara, A.B.; Kabilan, S.; Kotoky, J. Recent development of targeted approaches for the treatment of breast cancer. Breast Cancer 2017, 24, 191–219. [Google Scholar] [CrossRef]
- Thakur, K.K.; Kumar, A.; Banik, K.; Verma, E.; Khatoon, E.; Harsha, C.; Sethi, G.; Gupta, S.C.; Kunnumakkara, A.B. Long noncoding RNAs in triple-negative breast cancer: A new frontier in the regulation of tumorigenesis. J. Cell. Physiol. 2021, 236, 7938–7965. [Google Scholar] [CrossRef]
- Wang, J.; Xie, S.; Yang, J.; Xiong, H.; Jia, Y.; Zhou, Y.; Chen, Y.; Ying, X.; Chen, C.; Ye, C.; et al. The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J. Hematol. Oncol. 2019, 12, 81. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, B.F.; Ding, J.; Shi, L.; Ren, H.M.; Liu, K.; Huang, C.C.; Ma, F.X.; Wu, X.Y. LncRNA DANCR-miR-758-3p-PAX6 Molecular Network Regulates Apoptosis and Autophagy of Breast Cancer Cells. Cancer Manag. Res. 2020, 12, 4073–4084. [Google Scholar] [CrossRef]
- Li, G.; Qian, L.; Tang, X.; Chen, Y.; Zhao, Z.; Zhang, C. Long noncoding RNA growth arrestspecific 5 (GAS5) acts as a tumor suppressor by promoting autophagy in breast cancer. Mol. Med. Rep. 2020, 22, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. The Long Noncoding RNA HOTAIR in Breast Cancer: Does Autophagy Play a Role? Int. J. Mol. Sci. 2017, 18, 2317. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Li, R.G.; Huang, Y.Q.; Lu, J.P.; Zhang, W.J.; Wang, Z.Y. LncRNA OTUD6B-AS1 promotes paclitaxel resistance in triple negative breast cancer by regulation of miR-26a-5p/MTDH pathway-mediated autophagy and genomic instability. Aging 2021, 13, 24171–24191. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Qu, H.; Zhang, F.; Peng, S.; Dou, D.; Yang, Y.; Ding, Y.; Xie, M.; Dong, H.; Liao, Y.; et al. Exosomal long noncoding RNA AGAP2-AS1 regulates trastuzumab resistance via inducing autophagy in breast cancer. Am. J. Cancer Res. 2021, 11, 1962–1981. [Google Scholar] [PubMed]
- Zhang, F.; Li, Q.; Zhu, K.; Zhu, J.; Li, J.; Yuan, Y.; Zhang, P.; Zhou, L.; Liu, L. LncRNA LINC00265/miR-485-5p/IRF2-mediated autophagy suppresses apoptosis in acute myeloid leukemia cells. Am. J. Transl. Res. 2020, 12, 2451–2462. [Google Scholar] [PubMed]
- Zhang, H.; Liu, L.; Chen, L.; Liu, H.; Ren, S.; Tao, Y. Long noncoding RNA DANCR confers cytarabine resistance in acute myeloid leukemia by activating autophagy via the miR-874-3P/ATG16L1 axis. Mol. Oncol. 2021, 15, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, Z.; Wang, Y.; Xu, F.; Cheng, Z. Long noncoding RNA ROR1-AS1 enhances STC2-mediated cell growth and autophagy in cervical cancer through miR-670-3p. J. Recept. Signal Transduct. 2021, 41, 582–592. [Google Scholar] [CrossRef]
- Zou, S.H.; Du, X.; Lin, H.; Wang, P.C.; Li, M. Paclitaxel inhibits the progression of cervical cancer by inhibiting autophagy via lncRNARP11-381N20.2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3010–3017. [Google Scholar] [CrossRef]
- Lv, D.; Xiang, Y.; Yang, Q.; Yao, J.; Dong, Q. Long Non-Coding RNA TUG1 Promotes Cell Proliferation and Inhibits Cell Apoptosis, Autophagy in Clear Cell Renal Cell Carcinoma via MiR-31-5p/FLOT1 Axis. OncoTargets Ther. 2020, 13, 5857–5868. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Cao, W.B.; Wang, H.Y.; Niu, L.; Zhang, G.Z. Study on Clinical Significance of LncRNA EGOT Expression in Colon Cancer and Its Effect on Autophagy of Colon Cancer Cells. Cancer Manag. Res. 2020, 12, 13501–13512. [Google Scholar] [CrossRef]
- Ju, B.; Liu, Z.; Nai, C.; Zhu, X. Long non-coding RNA CASC2 induces apoptosis and autophagy in human colon cancer cells via modulation of TRIM16 expression. Am. J. Transl. Res. 2020, 12, 2695–2702. [Google Scholar] [PubMed]
- Wu, J.; Meng, X.; Gao, R.; Jia, Y.; Chai, J.; Zhou, Y.; Wang, J.; Xue, X.; Dang, T. Long non-coding RNA LINC00858 inhibits colon cancer cell apoptosis, autophagy, and senescence by activating WNK2 promoter methylation. Exp. Cell Res. 2020, 396, 112214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Li, D.; Yang, L.; Jin, J.; Zhang, B. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. OncoTargets Ther. 2019, 12, 2649–2660. [Google Scholar] [CrossRef]
- Liu, F.; Ai, F.Y.; Zhang, D.C.; Tian, L.; Yang, Z.Y.; Liu, S.J. LncRNA NEAT1 knockdown attenuates autophagy to elevate 5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer Med. 2020, 9, 1079–1091. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J. LncRNA SLCO4A1-AS1 promotes colorectal cancer cell proliferation by enhancing autophagy via miR-508-3p/PARD3 axis. Aging 2019, 11, 4876–4889. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, S.; Wang, X.; Mao, E.; Huang, L. SNHG14 stimulates cell autophagy to facilitate cisplatin resistance of colorectal cancer by regulating miR-186/ATG14 axis. Biomed. Pharmacother. 2020, 121, 109580. [Google Scholar] [CrossRef]
- Xian, Z.; Hu, B.; Wang, T.; Zeng, J.; Cai, J.; Zou, Q.; Zhu, P. lncRNA UCA1 Contributes to 5-Fluorouracil Resistance of Colorectal Cancer Cells through miR-23b-3p/ZNF281 Axis. OncoTargets Ther. 2020, 13, 7571–7583. [Google Scholar] [CrossRef]
- Si, Y.; Yang, Z.; Ge, Q.; Yu, L.; Yao, M.; Sun, X.; Ren, Z.; Ding, C. Long non-coding RNA Malat1 activated autophagy, hence promoting cell proliferation and inhibiting apoptosis by sponging miR-101 in colorectal cancer. Cell. Mol. Biol. Lett. 2019, 24, 50. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, W.; Song, J.; Wang, S.; Gu, X. LncRNA CPS1-IT1 suppresses EMT and metastasis of colorectal cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1alpha. Biochimie 2018, 144, 21–27. [Google Scholar] [CrossRef]
- Wang, M.; Han, D.; Yuan, Z.; Hu, H.; Zhao, Z.; Yang, R.; Jin, Y.; Zou, C.; Chen, Y.; Wang, G.; et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Islam Khan, M.Z.; Law, H.K.W. Cancer Susceptibility Candidate 9 (CASC9) Promotes Colorectal Cancer Carcinogenesis via mTOR-Dependent Autophagy and Epithelial-Mesenchymal Transition Pathways. Front. Mol. Biosci. 2021, 8, 627022. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Li, Q.; Cheng, X.; Wu, T.; Gao, P.; Gu, Y. Insulin-like growth factor 2 mRNA-binding protein 2-stabilized long non-coding RNA Taurine up-regulated gene 1 (TUG1) promotes cisplatin-resistance of colorectal cancer via modulating autophagy. Bioengineered 2022, 13, 2450–2469. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ma, J.; Wei, J.; Meng, W.; Wang, Y.; Shi, M. lncRNA SNHG11 Promotes Gastric Cancer Progression by Activating the Wnt/beta-Catenin Pathway and Oncogenic Autophagy. Mol. Ther. 2021, 29, 1258–1278. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, Z. Long noncoding RNA JPX promotes gastric cancer progression by regulating CXCR6 and autophagy via inhibiting miR197. Mol. Med. Rep. 2021, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Jia, N.; Li, W.; Zhang, X.Y. LINC01572 Regulates Cisplatin Resistance in Gastric Cancer Cells by Mediating miR-497-5p. OncoTargets Ther. 2020, 13, 10877–10887. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Q.; Chen, P.; Liu, C.; Wang, H.; Zhao, L. The lncRNA CRNDE is regulated by E2F6 and sensitizes gastric cancer cells to chemotherapy by inhibiting autophagy. J. Cancer 2022, 13, 3061–3072. [Google Scholar] [CrossRef]
- Shao, G.; Zhao, Z.; Zhao, W.; Hu, G.; Zhang, L.; Li, W.; Xing, C.; Zhang, X. Long non-coding RNA MALAT1 activates autophagy and promotes cell proliferation by downregulating microRNA-204 expression in gastric cancer. Oncol. Lett. 2020, 19, 805–812. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, Y.; You, S.; Li, K.; Tong, X.; Chen, S.; Chen, E.; Lin, X.; Chen, Y. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol. Cancer 2017, 16, 174. [Google Scholar] [CrossRef]
- Chen, J.F.; Wu, P.; Xia, R.; Yang, J.; Huo, X.Y.; Gu, D.Y.; Tang, C.J.; De, W.; Yang, F. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol. Cancer 2018, 17, 6. [Google Scholar] [CrossRef]
- Xi, Z.; Si, J.; Nan, J. LncRNA MALAT1 potentiates autophagyassociated cisplatin resistance by regulating the microRNA30b/autophagyrelated gene 5 axis in gastric cancer. Int. J. Oncol. 2019, 54, 239–248. [Google Scholar] [CrossRef]
- Xin, L.; Zhou, Q.; Yuan, Y.W.; Zhou, L.Q.; Liu, L.; Li, S.H.; Liu, C. METase/lncRNA HULC/FoxM1 reduced cisplatin resistance in gastric cancer by suppressing autophagy. J. Cancer Res. Clin. Oncol. 2019, 145, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, S.; Wu, Q.; Wu, J.; Zhou, R.; Wang, C.; Wu, Z.; Rong, X.; Huang, N.; Sun, L.; et al. Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy 2021, 17, 4083–4101. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, G.; Huang, C.; Zhao, X. KLF5 activates lncRNA DANCR and inhibits cancer cell autophagy accelerating gastric cancer progression. NPJ Genom. Med. 2021, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Peng, Z.X.; Ji, W.D.; Yu, J.D.; Qian, C.; Liu, J.D.; Fang, G.E. LncRNA CCAT1 Upregulates ATG5 to Enhance Autophagy and Promote Gastric Cancer Development by Absorbing miR-140-3p. Dig. Dis. Sci. 2022, 67, 3725–3741. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Deng, Y.; Jin, J.; Wu, Y.; Shen, L. Long Noncoding RNA LIT3527 Knockdown induces Apoptosis and Autophagy through inhibiting mTOR pathway in Gastric Cancer Cells. J. Cancer 2021, 12, 4901–4911. [Google Scholar] [CrossRef]
- Hou, M.; Li, C.; Dong, S. LINC00963/miR-4458 regulates the effect of oxaliplatin in gastric cancer by mediating autophagic flux through targeting of ATG16L1. Sci. Rep. 2021, 11, 20951. [Google Scholar] [CrossRef]
- Jiang, C.; Shen, F.; Du, J.; Fang, X.; Li, X.; Su, J.; Wang, X.; Huang, X.; Liu, Z. Upregulation of CASC2 sensitized glioma to temozolomide cytotoxicity through autophagy inhibition by sponging miR-193a-5p and regulating mTOR expression. Biomed. Pharmacother. 2018, 97, 844–850. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Feng, X.; Li, X.; Pan, L.; Liu, J.; Wang, F.; Yuan, Z.; Yang, L.; Yu, J.; et al. Long non-coding RNA MEG3 regulates proliferation, apoptosis, and autophagy and is associated with prognosis in glioma. J. Neurooncol. 2018, 140, 281–288. [Google Scholar] [CrossRef]
- Huo, J.F.; Chen, X.B. Long noncoding RNA growth arrest-specific 5 facilitates glioma cell sensitivity to cisplatin by suppressing excessive autophagy in an mTOR-dependent manner. J. Cell. Biochem. 2019, 120, 6127–6136. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Zheng, R.; Zhang, J.; Huang, C.; Zheng, R.; Huang, Z.; Qiu, W.; Liu, M.; Yang, K.; et al. Linc-RA1 inhibits autophagy and promotes radioresistance by preventing H2Bub1/USP44 combination in glioma cells. Cell Death Dis. 2020, 11, 758. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, X.; Han, H.; Zhang, H.; Li, X.; Jiang, C.; Feng, M. Long noncoding RNA H19 contributes to the proliferation and autophagy of glioma cells through mTOR/ULK1 pathway. Neuroreport 2021, 32, 352–358. [Google Scholar] [CrossRef]
- Ma, W.; Zhou, Y.; Liu, M.; Qin, Q.; Cui, Y. Long non-coding RNA LINC00470 in serum derived exosome: A critical regulator for proliferation and autophagy in glioma cells. Cancer Cell Int. 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Q.; Zeng, X.; Li, M.; Liao, J. lnc-NLC1-C inhibits migration, invasion and autophagy of glioma cells by targeting miR-383 and regulating PRDX-3 expression. Oncol. Lett. 2021, 22, 640. [Google Scholar] [CrossRef]
- Xue, K.; Li, J.; Nan, S.; Zhao, X.; Xu, C. Downregulation of LINC00460 decreases STC2 and promotes autophagy of head and neck squamous cell carcinoma by up-regulating microRNA-206. Life Sci. 2019, 231, 116459. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, F.; Lei, Z.; Luo, H. LncRNA SNHG11 Promotes Proliferation, Migration, Apoptosis, and Autophagy by Regulating hsa-miR-184/AGO2 in HCC. OncoTargets Ther. 2020, 13, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peng, X.; Jin, H.; Liu, J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma. Gene 2019, 697, 94–102. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Y.; Peng, X.; Jin, H.; Liu, J. LncRNA CCAT1 promotes autophagy via regulating ATG7 by sponging miR-181 in hepatocellular carcinoma. J. Cell. Biochem. 2019, 120, 17975–17983. [Google Scholar] [CrossRef]
- Sheng, J.Q.; Wang, M.R.; Fang, D.; Liu, L.; Huang, W.J.; Tian, D.A.; He, X.X.; Li, P.Y. LncRNA NBR2 inhibits tumorigenesis by regulating autophagy in hepatocellular carcinoma. Biomed. Pharmacother. 2021, 133, 111023. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Li, H.; Liu, J. LncRNA NEAT1 promotes autophagy via regulating miR-204/ATG3 and enhanced cell resistance to sorafenib in hepatocellular carcinoma. J. Cell. Physiol. 2020, 235, 3402–3413. [Google Scholar] [CrossRef]
- Cui, C.; Li, Z.; Wu, D. The long non-coding RNA H19 induces hypoxia/reoxygenation injury by up-regulating autophagy in the hepatoma carcinoma cells. Biol. Res. 2019, 52, 32. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.S.; Huang, Q.; Chen, H.L.; Zhang, Z.; Tan, Q.F. LncRNA DCST1-AS1 accelerates the proliferation, metastasis and autophagy of hepatocellular carcinoma cell by AKT/mTOR signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6091–6104. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hu, J.; Pu, J.; Tang, Q.; Li, W.; Ma, R.; Xu, Z.; Tan, C.; Yao, T.; Wu, X.; et al. Long noncoding RNA HAGLROS promotes cell proliferation, inhibits apoptosis and enhances autophagy via regulating miR-5095/ATG12 axis in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 73, 72–80. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.L.; Gong, Y.; Ma, W.J.; Li, B.; Jiang, Y.Z. LncRNA DANCR promotes ATG7 expression to accelerate hepatocellular carcinoma cell proliferation and autophagy by sponging miR-222-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8778–8787. [Google Scholar] [CrossRef]
- Xin, X.; Wu, M.; Meng, Q.; Wang, C.; Lu, Y.; Yang, Y.; Li, X.; Zheng, Q.; Pu, H.; Gui, X.; et al. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol. Cancer 2018, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Yan, G.X.; Dong, D.S.; Xin, H.; Liu, Z.Y. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 5310–5322. [Google Scholar] [CrossRef]
- Liang, L.; Huan, L.; Wang, J.; Wu, Y.; Huang, S.; He, X. LncRNA RP11-295G20.2 regulates hepatocellular carcinoma cell growth and autophagy by targeting PTEN to lysosomal degradation. Cell Discov. 2021, 7, 118. [Google Scholar] [CrossRef]
- Shi, J.; Guo, C.; Ma, J. CCAT2 enhances autophagy-related invasion and metastasis via regulating miR-4496 and ELAVL1 in hepatocellular carcinoma. J. Cell. Mol. Med. 2021, 25, 8985–8996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Guan, H.; Zhang, D. HnRNPU-AS1 inhibits the proliferation, migration and invasion of HCC cells and induces autophagy through miR-556-3p/ miR-580-3p/SOCS6 axis. Cancer Biomark. 2022, 34, 443–457. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Z.; Zhao, J.; Zhai, X.; Li, J.; Zhang, Y.; Zong, L.; Peng, H.; Qi, J.; Kong, X.; et al. H19/miR-107/HMGB1 axis sensitizes laryngeal squamous cell carcinoma to cisplatin by suppressing autophagy in vitro and in vivo. Cell Biol. Int. 2021, 45, 674–685. [Google Scholar] [CrossRef]
- Wang, L.J.; Sun, G.Z.; Chen, Y.F. LncRNA MSTO2P promotes proliferation and autophagy of lung cancer cells by up-regulating EZH2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3375–3382. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Feng, N.; Wang, R.; Zhang, W.; Deng, X.; Wang, Y.; Yu, X.; Ye, X.; Li, L.; et al. LncRNA LCPAT1 Mediates Smoking/ Particulate Matter 2.5-Induced Cell Autophagy and Epithelial-Mesenchymal Transition in Lung Cancer Cells via RCC2. Cell. Physiol. Biochem. 2018, 47, 1244–1258. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xia, S.; Yang, L.; Wu, D.; Zhou, Y.; Lu, J. Long noncoding RNA PANDAR inhibits the development of lung cancer by regulating autophagy and apoptosis pathways. J. Cancer 2020, 11, 4783–4790. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Wang, H.N.; Fu, R.Y.; Liu, X.D.; Piao, Y.S.; Wei, L.Q.; Wang, J.W.; Zhang, L. LncRNA BCYRN1-induced autophagy enhances asparaginase resistance in extranodal NK/T-cell lymphoma. Theranostics 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.; Lv, B. Long non-coding RNA MEG3 promotes autophagy and apoptosis of nasopharyngeal carcinoma cells via PTEN up-regulation by binding to microRNA-21. J. Cell. Mol. Med. 2021, 25, 61–72. [Google Scholar] [CrossRef]
- Zheng, T.; Li, D.; He, Z.; Feng, S.; Zhao, S. Long noncoding RNA NBAT1 inhibits autophagy via suppression of ATG7 in non-small cell lung cancer. Am. J. Cancer Res. 2018, 8, 1801–1811. [Google Scholar]
- Huang, F.X.; Chen, H.J.; Zheng, F.X.; Gao, Z.Y.; Sun, P.F.; Peng, Q.; Liu, Y.; Deng, X.; Huang, Y.H.; Zhao, C.; et al. LncRNA BLACAT1 is involved in chemoresistance of nonsmall cell lung cancer cells by regulating autophagy. Int. J. Oncol. 2019, 54, 339–347. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.Q.; Shao, X.M.; Wei, L. GAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2271–2277. [Google Scholar]
- Chen, L.; Han, X.; Hu, Z.; Chen, L. The PVT1/miR-216b/Beclin-1 regulates cisplatin sensitivity of NSCLC cells via modulating autophagy and apoptosis. Cancer Chemother. Pharmacol. 2019, 83, 921–931. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Wu, S. Long non-coding RNA CTA sensitizes osteosarcoma cells to doxorubicin through inhibition of autophagy. Oncotarget 2017, 8, 31465–31477. [Google Scholar] [CrossRef]
- Liu, K.; Hou, Y.; Liu, Y.; Zheng, J. LncRNA SNHG15 contributes to proliferation, invasion and autophagy in osteosarcoma cells by sponging miR-141. J. Biomed. Sci. 2017, 24, 46. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, G.; Xu, J.; Zhang, C. Silencing of SNHG6 induced cell autophagy by targeting miR-26a-5p/ULK1 signaling pathway in human osteosarcoma. Cancer Cell Int. 2019, 19, 82. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Wu, Q.; Wang, H.; Xia, H.; Sun, Y. Long non-coding RNA HOXA11-AS knockout inhibits proliferation and overcomes drug resistance in ovarian cancer. Bioengineered 2022, 13, 13893–13905. [Google Scholar] [CrossRef]
- Gu, L.; Li, Q.; Liu, H.; Lu, X.; Zhu, M. Long Noncoding RNA TUG1 Promotes Autophagy—Associated Paclitaxel Resistance by Sponging miR-29b-3p in Ovarian Cancer Cells. OncoTargets Ther. 2020, 13, 2007–2019. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Li, Y.; Ye, F.; Zhou, X. LncRNA XIST promotes carboplatin resistance of ovarian cancer through activating autophagy via targeting miR-506-3p/FOXP1 axis. J. Gynecol. Oncol. 2022, 33, e81. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.O.; Zhou, W.Y.; Chang, X.Y.; Zhang, M.M.; Zhang, Y.; Yang, X.H. Long non-coding RNA LINC01207 silencing suppresses AGR2 expression to facilitate autophagy and apoptosis of pancreatic cancer cells by sponging miR-143-5p. Mol. Cell. Endocrinol. 2019, 493, 110424. [Google Scholar] [CrossRef]
- Zhou, C.; Yi, C.; Yi, Y.; Qin, W.; Yan, Y.; Dong, X.; Zhang, X.; Huang, Y.; Zhang, R.; Wei, J.; et al. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/beta-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol. Cancer 2020, 19, 118. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Gao, Y.; Wang, Y.W.; Zhang, G.Q.; Pan, S.H.; Ji, L.; Kong, R.; Wang, G.; Jia, Y.H.; et al. Long Noncoding RNA MALAT1 Promotes Aggressive Pancreatic Cancer Proliferation and Metastasis via the Stimulation of Autophagy. Mol. Cancer Ther. 2016, 15, 2232–2243. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, P.; Wang, C.; Xin, B. SNHG14 enhances gemcitabine resistance by sponging miR-101 to stimulate cell autophagy in pancreatic cancer. Biochem. Biophys. Res. Commun. 2019, 510, 508–514. [Google Scholar] [CrossRef]
- Wang, L.; Bi, R.; Li, L.; Zhou, K.; Yin, H. lncRNA ANRIL aggravates the chemoresistance of pancreatic cancer cells to gemcitabine by targeting inhibition of miR-181a and targeting HMGB1-induced autophagy. Aging 2021, 13, 19272–19281. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Q.; Zhao, Y.; Chen, J.; Wang, S.; Hu, J.; Sun, Y. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol. Lett. 2014, 8, 1947–1952. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Xu, H.; Xu, L.; Chen, D.; Wang, J.; Huang, S.; Wen, Y.; Fang, L. Long Non-Coding RNA SNHG1 Regulates the Wnt/beta-Catenin and PI3K/AKT/mTOR Signaling Pathways via EZH2 to Affect the Proliferation, Apoptosis, and Autophagy of Prostate Cancer Cell. Front. Oncol. 2020, 10, 552907. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, Y.; Fang, F.; Liu, K. MALAT1 modulates the autophagy of retinoblastoma cell through miR-124-mediated stx17 regulation. J. Cell. Biochem. 2018, 119, 3853–3863. [Google Scholar] [CrossRef]
- Ningegowda, R.; Shivananju, N.S.; Rajendran, P.; Basappa; Rangappa, K.S.; Chinnathambi, A.; Li, F.; Achar, R.R.; Shanmugam, M.K.; Bist, P.; et al. A novel 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFalpha by abrogating NF-kappaB activation cascade. Apoptosis 2017, 22, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Mohan, C.D.; Eng, H.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Rangappa, K.S.; Sethi, G.; Kumar, A.P.; Ahn, K.S. 2,3,5,6-Tetramethylpyrazine Targets Epithelial-Mesenchymal Transition by Abrogating Manganese Superoxide Dismutase Expression and TGFbeta-Driven Signaling Cascades in Colon Cancer Cells. Biomolecules 2022, 12, 891. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kunnumakkara, A.B.; Popper, B.; Majeed, M.; Aggarwal, B.B.; Shakibaei, M. Calebin A Potentiates the Effect of 5-FU and TNF-beta (Lymphotoxin alpha) against Human Colorectal Cancer Cells: Potential Role of NF-kappaB. Int. J. Mol. Sci. 2020, 21, 2393. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That Calebin A, a Component of Curcuma Longa Suppresses NF-B Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-beta (Lymphotoxin). Nutrients 2019, 11, 2904. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the Epithelial-to-Mesenchymal Transition of Human Colorectal Cancer by Human TNF-beta (Lymphotoxin) and its Reversal by Resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef]
- Kourani, K.; Jain, P.; Kumar, A.; Jangid, A.K.; Swaminathan, G.; Durgempudi, V.R.; Jose, J.; Reddy, R.; Pooja, D.; Kulhari, H.; et al. Inulin coated Mn3O4 nanocuboids coupled with RNA interference reverse intestinal tumorigenesis in Apc knockout murine colon cancer models. Nanomedicine 2022, 40, 102504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singam, A.; Swaminathan, G.; Killi, N.; Tangudu, N.K.; Jose, J.; Gundloori VN, R.; Dinesh Kumar, L. Combinatorial therapy using RNAi and curcumin nano-architectures regresses tumors in breast and colon cancer models. Nanoscale 2022, 14, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Z.; He, J.; Lai, Q.; Yao, X.; Li, Q.; Liu, Y.; Lai, H.; Gu, C.; Yan, Q.; et al. LncRNA SNHG6 promotes chemoresistance through ULK1-induced autophagy by sponging miR-26a-5p in colorectal cancer cells. Cancer Cell Int. 2019, 19, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chen, X.F.; Wang, M.; Zhang, P.P.; Zhang, F.; Zhang, J.J. Long non-coding RNA UCA1 promotes autophagy by targeting miR-96-5p in acute myeloid leukaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fu, H.; Liu, X.; Lei, Q.; Zhang, Y.; She, X.; Liu, Q.; Liu, Q.; Sun, Y.; Li, G.; et al. LINC00470 Coordinates the Epigenetic Regulation of ELFN2 to Distract GBM Cell Autophagy. Mol. Ther. 2018, 26, 2267–2281. [Google Scholar] [CrossRef]
- Saha, S.; Zhang, Y.; Wilson, B.; Abounader, R.; Dutta, A. The tumor-suppressive long noncoding RNA DRAIC inhibits protein translation and induces autophagy by activating AMPK. J. Cell Sci. 2021, 134, jcs259306. [Google Scholar] [CrossRef]
- Pu, Z.; Wu, L.; Guo, Y.; Li, G.; Xiang, M.; Liu, L.; Zhan, H.; Zhou, X.; Tan, H. LncRNA MEG3 contributes to adenosine-induced cytotoxicity in hepatoma HepG2 cells by downregulated ILF3 and autophagy inhibition via regulation PI3K-AKT-mTOR and beclin-1 signaling pathway. J. Cell. Biochem. 2019, 120, 18172–18185. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, C.; Zhang, G. LncRNA MCM3AP-AS1 Regulates Epidermal Growth Factor Receptor and Autophagy to Promote Hepatocellular Carcinoma Metastasis by Interacting with miR-455. DNA Cell Biol. 2019, 38, 857–864. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Sun, T.; Yang, W. LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathway. Exp. Cell Res. 2017, 358, 188–198. [Google Scholar] [CrossRef]
- Su, W.; Wang, L.; Zhao, H.; Hu, S.; Zhou, Y.; Guo, C.; Wu, B.; Li, L.; Yang, Z.; Beer, D.G.; et al. LINC00857 Interacting with YBX1 to Regulate Apoptosis and Autophagy via MET and Phosphor-AMPKa Signaling. Mol. Ther. Nucleic Acids 2020, 22, 1164–1175. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wang, X.; Zhang, C.Y. LncRNA SNHG7 enhances chemoresistance in neuroblastoma through cisplatin-induced autophagy by regulating miR-329-3p/MYO10 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.R.; Jin, Z. Effects of Interference with UCA1 and Inhibition of miR-185-5p on Activation, Autophagy and Survival of beta-Catenin Pathway in Non-small Cell Lung Cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 2019, 50, 157–163. [Google Scholar]
- Zhao, Y.; Zhao, L.; Li, J.; Zhong, L. Silencing of long noncoding RNA RP11-476D10.1 enhances apoptosis and autophagy while inhibiting proliferation of papillary thyroid carcinoma cells via microRNA-138-5p-dependent inhibition of LRRK2. J. Cell. Physiol. 2019, 234, 20980–20991. [Google Scholar] [CrossRef]
- Chen, C.; Wang, K.; Wang, Q.; Wang, X. LncRNA HULC mediates radioresistance via autophagy in prostate cancer cells. Braz. J. Med. Biol. Res. 2018, 51, e7080. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, J.; Yang, Z.; Ge, S.; Zhang, H.; Zhong, Q.; Fan, X. ZNNT1 long noncoding RNA induces autophagy to inhibit tumorigenesis of uveal melanoma by regulating key autophagy gene expression. Autophagy 2020, 16, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef]
- Bishayee, A.; Haskell, Y.; Do, C.; Siveen, K.S.; Mohandas, N.; Sethi, G.; Stoner, G.D. Potential Benefits of Edible Berries in the Management of Aerodigestive and Gastrointestinal Tract Cancers: Preclinical and Clinical Evidence. Crit. Rev. Food Sci. Nutr. 2016, 56, 1753–1775. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanalakshmi, G.; Gamit, N.; Patil, M.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Kumar, A.P.; Warrier, S. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers 2018, 11, 25. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Y.J.; Jiang, S.Y.; Xu, Z.; Cao, B.Y.; Sethi, G.; Zeng, Y.Y.; Kong, Y.; Mao, X.L. Suppression of the USP10/CCND1 axis induces glioblastoma cell apoptosis. Acta Pharmacol. Sin. 2021, 42, 1338–1346. [Google Scholar] [CrossRef]
- Escamilla-Ramirez, A.; Castillo-Rodriguez, R.A.; Zavala-Vega, S.; Jimenez-Farfan, D.; Anaya-Rubio, I.; Briseno, E.; Palencia, G.; Guevara, P.; Cruz-Salgado, A.; Sotelo, J.; et al. Autophagy as a Potential Therapy for Malignant Glioma. Pharmaceuticals 2020, 13, 156. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, Z.; Dai, S.; Qian, L.; Sun, L.; Gong, Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 23. [Google Scholar] [CrossRef]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Padmavathi, G.; Kotoky, J.; Golla, R.; Kunnumakkara, A.B. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Qian, H.; Wang, S.; Fulte, S.; Ding, W.X. Autophagy and liver cancer. Clin. Mol. Hepatol. 2020, 26, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, C.; Li, Y.; Ma, J. The long noncoding RNA TINCR promotes self-renewal of human liver cancer stem cells through autophagy activation. Cell Death Dis. 2022, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Pandya, G.; Sethi, G.; Pandey, A.K.; Das, B.C.; Garg, M. A comprehensive review of genetic alterations and molecular targeted therapies for the implementation of personalized medicine in acute myeloid leukemia. J. Mol. Med. 2020, 98, 1069–1091. [Google Scholar] [CrossRef]
- Cai, W.; Xiong Chen, Z.; Rane, G.; Satendra Singh, S.; Choo, Z.; Wang, C.; Yuan, Y.; Zea Tan, T.; Arfuso, F.; Yap, C.T.; et al. Wanted DEAD/H or Alive: Helicases Winding Up in Cancers. J. Natl. Cancer Inst. 2017, 109, djw278. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A. Hematological malignancies and molecular targeting therapy. Eur. J. Pharmacol. 2019, 862, 172641. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Padmavathi, G.; Vikkurthi, R.; Harsha, C.; Roy, N.K.; Singh, A.K.; Monisha, J.; Wang, H.; Kumar, A.P.; et al. TIPE2 Induced the Proliferation, Survival, and Migration of Lung Cancer Cells through Modulation of Akt/mTOR/NF-kappaB Signaling Cascade. Biomolecules 2019, 9, 836. [Google Scholar] [CrossRef]
- Bordoloi, D.; Banik, K.; Vikkurthi, R.; Thakur, K.K.; Padmavathi, G.; Sailo, B.L.; Girisa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Inflection of Akt/mTOR/STAT-3 cascade in TNF-alpha induced protein 8 mediated human lung carcinogenesis. Life Sci. 2020, 262, 118475. [Google Scholar] [CrossRef]
- Bordoloi, D.; Harsha, C.; Padmavathi, G.; Banik, K.; Sailo, B.L.; Roy, N.K.; Girisa, S.; Thakur, K.K.; Khwairakpam, A.D.; Chinnathambi, A.; et al. Loss of TIPE3 reduced the proliferation, survival and migration of lung cancer cells through inactivation of Akt/mTOR, NF-kappaB, and STAT-3 signaling cascades. Life Sci. 2022, 293, 120332. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Padmavathi, G.; Banik, K.; Devi, K.A.; Harsha, C.; Girisa, S.; Buhrmann, C.; Shakibaei, M.; Kunnumakkara, A.B. Human tumor necrosis factor alpha-induced protein eight-like 1 exhibited potent anti-tumor effect through modulation of proliferation, survival, migration and invasion of lung cancer cells. Mol. Cell. Biochem. 2021, 476, 3303–3318. [Google Scholar] [CrossRef]
- Su, M.; Xiao, Y.; Tang, J.; Wu, J.; Ma, J.; Tian, B.; Zhou, Y.; Wang, H.; Yang, D.; Liao, Q.J.; et al. Role of lncRNA and EZH2 Interaction/Regulatory Network in Lung Cancer. J. Cancer 2018, 9, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Z.; Wang, R. Long non-coding RNAs in lung cancer: Regulation patterns, biologic function and diagnosis implications (Review). Int. J. Oncol. 2019, 55, 585–596. [Google Scholar] [CrossRef]
- Ginn, L.; Shi, L.; Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Noncoding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.J.; Taran, R.; Malipatil, N.B. Pediatric Osteosarcoma: An Updated Review. Indian J. Med. Paediatr. Oncol. 2017, 38, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Yang, Y.; Ma, Y.; Wang, F.; Xue, A.; Zhu, J.; Yang, H.; Chen, Q.; Chen, M.; Ye, L.; et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed. Pharmacother. 2020, 121, 109627. [Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Long non-coding RNAs in osteosarcoma. Oncotarget 2017, 8, 20462–20475. [Google Scholar] [CrossRef]
- Han, J.; Shen, X. Long noncoding RNAs in osteosarcoma via various signaling pathways. J. Clin. Lab. Anal. 2020, 34, e23317. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Khatoon, E.; Parama, D.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Targeting PD-1/PD-L1 axis as new horizon for ovarian cancer therapy. Life Sci. 2022, 306, 120827. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Tong, Z.; Gelovani, J.G.; Guha, S.; et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int. J. Cancer 2012, 131, E292–E303. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Ang, H.L.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Delfi, M.; Khan, H.; Ashrafizadeh, M.; et al. Pre-Clinical and Clinical Applications of Small Interfering RNAs (siRNA) and Co-Delivery Systems for Pancreatic Cancer Therapy. Cells 2021, 10, 3348. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Fathi, M.; Zhai, T.; Taheri, M.; Dong, P. LncRNAs: Novel Biomarkers for Pancreatic Cancer. Biomolecules 2021, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2017, 8, 17700–17711. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Ko, J.H.; Um, J.Y.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J. Cell. Physiol. 2021, 236, 5253–5264. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef]
- Dimaras, H.; Corson, T.W. Retinoblastoma, the visible CNS tumor: A review. J. Neurosci. Res. 2019, 97, 29–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Hegde, M.; Sethi, G.; Kunnumakkara, A.B. Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics. Cells 2023, 12, 810. https://doi.org/10.3390/cells12050810
Kumar A, Girisa S, Alqahtani MS, Abbas M, Hegde M, Sethi G, Kunnumakkara AB. Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics. Cells. 2023; 12(5):810. https://doi.org/10.3390/cells12050810
Chicago/Turabian StyleKumar, Aviral, Sosmitha Girisa, Mohammed S. Alqahtani, Mohamed Abbas, Mangala Hegde, Gautam Sethi, and Ajaikumar B. Kunnumakkara. 2023. "Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics" Cells 12, no. 5: 810. https://doi.org/10.3390/cells12050810
APA StyleKumar, A., Girisa, S., Alqahtani, M. S., Abbas, M., Hegde, M., Sethi, G., & Kunnumakkara, A. B. (2023). Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics. Cells, 12(5), 810. https://doi.org/10.3390/cells12050810







