The Complement System in Kidney Transplantation
Abstract
1. Introduction
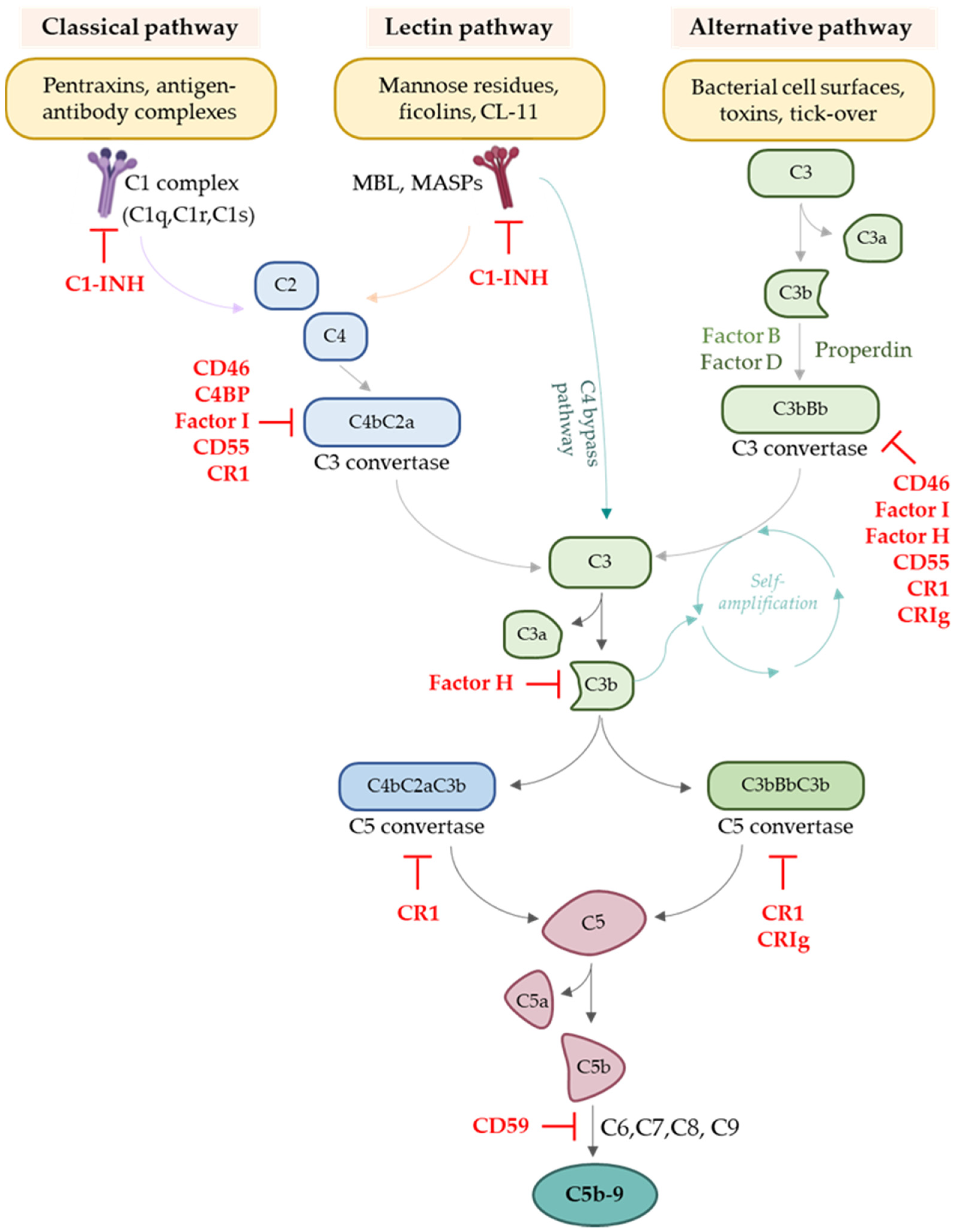
2. Overview of the Complement System
3. Involvement of the Complement System in Transplantation
3.1. Complement Activation in Donor Kidneys
3.2. Complement Activation in Transplant Candidates
3.3. Ischaemia/Reperfusion Injury (IRI) and Complement Activation
3.3.1. Ischaemia/Reperfusion Injury: The Contribution of the Different Pathways of Complement Activation
3.3.2. Ischaemia/Reperfusion Injury: The Contributions of Other Components of the Complement System, Regulators and Receptors
3.4. Complement Activation Modulates the Adaptive Immune Response against the Graft
3.4.1. The Role of Complement in Regulating T Cell Responses
3.4.2. The Role of Complement in Antibody-Mediated Rejection
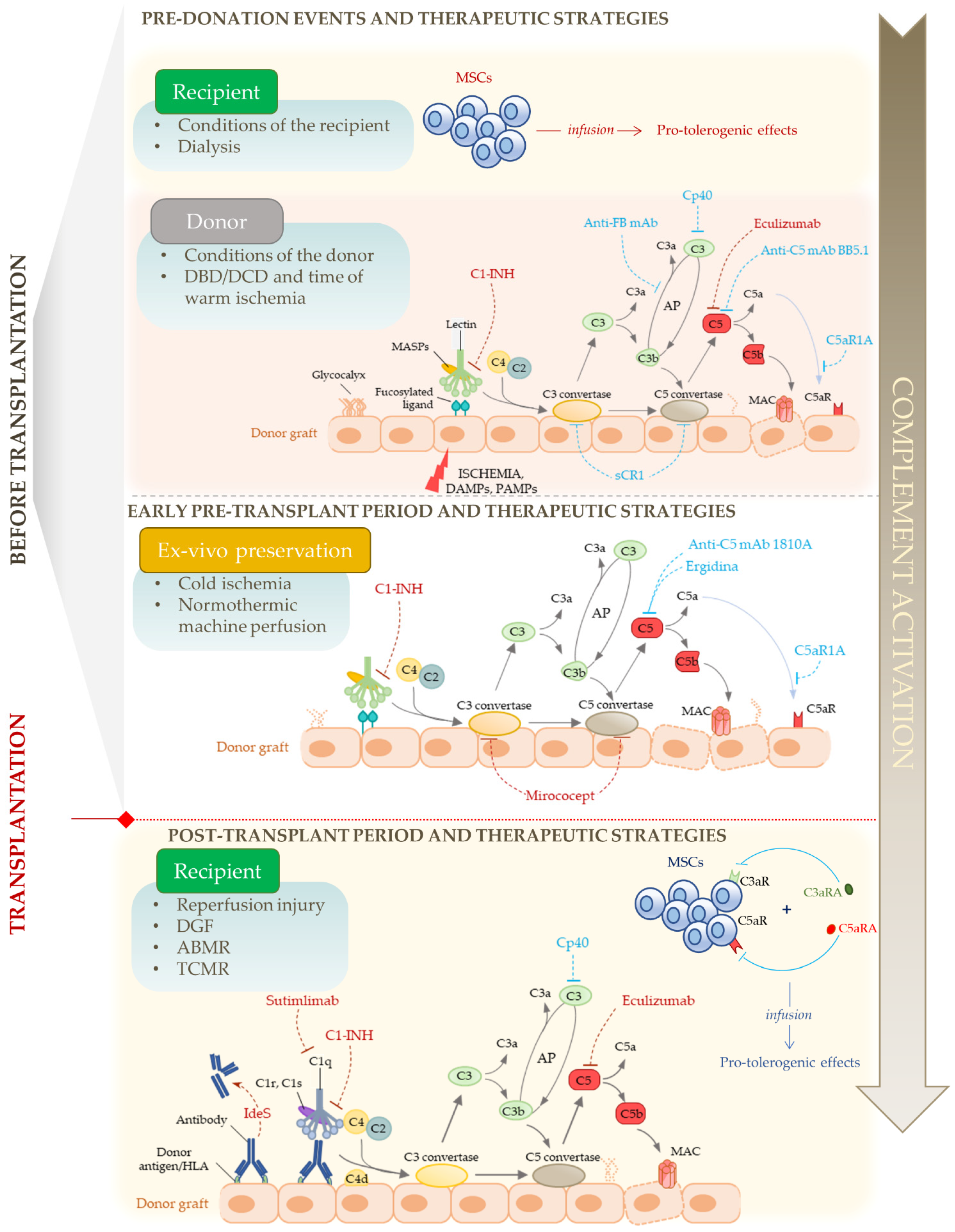
4. Complement as a Therapeutic Target in Kidney Transplantation
5. Blocking Complement to Help Pro-Tolerogenic Cell Therapies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farrar, C.A.; Kupiec-Weglinski, J.W.; Sacks, S.H. The Innate Immune System and Transplantation. Cold Spring Harb. Perspect. Med. 2013, 3, a015479. [Google Scholar] [CrossRef] [PubMed]
- Damman, J.; Daha, M.R.; van Son, W.J.; Leuvenink, H.G.; Ploeg, R.J.; Seelen, M.A. Crosstalk between Complement and Toll-like Receptor Activation in Relation to Donor Brain Death and Renal Ischemia-Reperfusion Injury: Complement and TLRs in Kidney Transplantation. Am. J. Transplant. 2011, 11, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Sacks, S.H.; Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 2012, 12, 431–442. [Google Scholar] [CrossRef]
- Cravedi, P.; Heeger, P.S. Complement as a multifaceted modulator of kidney transplant injury. J. Clin. Investig. 2014, 124, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hu, D.; Wang, M.; Zipfel, P.F.; Hu, Y. Complement in Hemolysis- and Thrombosis- Related Diseases. Front. Immunol. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Arbore, G.; Kemper, C.; Kolev, M. Intracellular complement − the complosome − in immune cell regulation. Mol. Immunol. 2017, 89, 2–9. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Merle, N.S.; Noé, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- De la O Becerra, K.I.; Oosterheert, W.; Bos, R.M.V.D.; Xenaki, K.T.; Lorent, J.H.; Ruyken, M.; Schouten, A.; Rooijakkers, S.H.M.; Henegouwen, P.M.P.V.B.E.; Gros, P. Multifaceted Activities of Seven Nanobodies against Complement C4b. J. Immunol. 2022, 208, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, Y.; Li, X.; Zeng, F.; Rao, Y.; He, Y.; Wang, Y.; Liu, M.; Li, D.; Xu, Z.; et al. Microglial debris is cleared by astrocytes via C4b-facilitated phagocytosis and degraded via RUBICON-dependent noncanonical autophagy in mice. Nat. Commun. 2022, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. New Engl. J. Med. 2001, 344, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Tew, J.G.; Kosco, M.H.; Burton, G.F.; Szakal, A.K. Follicular Dendritic Cells as Accessory Cells. Immunol. Rev. 1990, 117, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, N.; Haeffner-Cavaillon, N.; Haeffner, A.; Weiss, L.; Kazatchkine, M.D. Triggering of Complement Receptors CR1 (CD35) and CR3 (CD11b/CD18) Induces Nuclear Translocation of NF-Kappa B (P50/P65) in Human Monocytes and Enhances Viral Replication in HIV-Infected Monocytic Cells. J. Immunol. 1950, 155, 4861–4867. [Google Scholar] [CrossRef]
- Phan, T.; Grigorova, I.; Okada, T.; Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007, 8, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Abel, B.; Gallimore, A.; Carroll, M.; Bachmann, M.F. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 2002, 8, 373–378. [Google Scholar] [CrossRef]
- Soteros, B.M.; Sia, G.M. Complement and microglia dependent synapse elimination in brain development. Wiley Interdiscip. Rev. Syst. Biol. Med. 2021, 14, e1545. [Google Scholar] [CrossRef]
- Mevorach, D. Clearance of dying cells and systemic lupus erythematosus: The role of C1q and the complement system. Apoptosis 2010, 15, 1114–1123. [Google Scholar] [CrossRef]
- Defendi, F.; Thielens, N.M.; Clavarino, G.; Cesbron, J.-Y.; Dumestre-Pérard, C. The Immunopathology of Complement Proteins and Innate Immunity in Autoimmune Disease. Clin. Rev. Allergy Immunol. 2019, 58, 229–251. [Google Scholar] [CrossRef]
- Nauser, C.L.; Farrar, C.A.; Sacks, S.H. Complement Recognition Pathways in Renal Transplantation. J. Am. Soc. Nephrol. 2017, 28, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Grafals, M.; Thurman, J.M. The Role of Complement in Organ Transplantation. Front. Immunol. 2019, 10, 2380. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Menon, S.S.; Cortes, C.; Ferreira, V.P. Hijacking Factor H for Complement Immune Evasion. Front. Immunol. 2021, 12, 602277. [Google Scholar] [CrossRef] [PubMed]
- Lesher, A.M.; Nilsson, B.; Song, W.-C. Properdin in complement activation and tissue injury. Mol. Immunol. 2013, 56, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Blaum, B.; Hannan, J.; Herbert, A.P.; Kavanagh, D.; Uhrín, D.; Stehle, T. Structural basis for sialic acid–mediated self-recognition by complement factor H. Nat. Chem. Biol. 2014, 11, 77–82. [Google Scholar] [CrossRef]
- Harboe, M.; Ulvund, G.; Vien, L.; Fung, M.; Mollnes, T.E. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 2004, 138, 439–446. [Google Scholar] [CrossRef]
- Harboe, M.; Garred, P.; Karlstrøm, E.; Lindstad, J.K.; Stahl, G.L.; Mollnes, T.E. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol. Immunol. 2009, 47, 373–380. [Google Scholar] [CrossRef]
- Morgan, B.P.; Walters, D.; Serna, M.; Bubeck, D. Terminal complexes of the complement system: New structural insights and their relevance to function. Immunol. Rev. 2016, 274, 141–151. [Google Scholar] [CrossRef]
- Qi, R.; Qin, W. Role of Complement System in Kidney Transplantation: Stepping From Animal Models to Clinical Application. Front. Immunol. 2022, 13, 811696. [Google Scholar] [CrossRef]
- Khera, R.; Das, N. Complement Receptor 1: Disease associations and therapeutic implications. Mol. Immunol. 2009, 46, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 2007, 219, 157–166. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Jensen, R.K. Structural Immunology of Complement Receptors 3 and 4. Front. Immunol. 2018, 9, 2716. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Hänsch, G.M.; Stegmaier, S.; Denefleh, B.; Hug, F.; Schoels, M. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: Activation-dependent up-regulation and regulatory function. Eur. J. Immunol. 2001, 31, 1173–1180. [Google Scholar] [CrossRef]
- Fayyazi, A.; Scheel, O.; Werfel, T.; Schweyer, S.; Oppermann, M.; Götze, O.; Radzun, H.J.; Zwirner, J. The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology 2000, 99, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Nester, C.M. All Things Complement. Clin. J. Am. Soc. Nephrol. 2016, 11, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Gastoldi, S.; Galbusera, M.; Ruggenenti, P.L.; Portalupi, V.; Rota, S.; Rubis, N.; Liguori, L.; Conti, S.; Tironi, M.; et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv. 2022, 6, 866–881. [Google Scholar] [CrossRef]
- Biglarnia, A.-R.; Huber-Lang, M.; Mohlin, C.; Ekdahl, K.N.; Nilsson, B. The multifaceted role of complement in kidney transplantation. Nat. Rev. Nephrol. 2018, 14, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Damman, J.; Bloks, V.W.; Daha, M.R.; Van Der Most, P.J.; Sanjabi, B.; Van Der Vlies, P.; Snieder, H.; Ploeg, R.J.; Krikke, C.; Leuvenink, H.G.D.; et al. Hypoxia and Complement-and-Coagulation Pathways in the Deceased Organ Donor as the Major Target for Intervention to Improve Renal Allograft Outcome. Transplantation 2015, 99, 1293–1300. [Google Scholar] [CrossRef]
- Damman, J.; Nijboer, W.N.; Schuurs, T.A.; Leuvenink, H.G.; Morariu, A.M.; Tullius, S.G.; Van Goor, H.; Ploeg, R.J.; Seelen, M.A. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol. Dial. Transplant. 2011, 26, 2345–2354. [Google Scholar] [CrossRef]
- Poppelaars, F.; Seelen, M.A. Complement-mediated inflammation and injury in brain dead organ donors. Mol. Immunol. 2017, 84, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Bartoszek, D.; Mazanowska, O.; Kościelska-Kasprzak, K.; Kamińska, D.; Lepiesza, A.; Chudoba, P.; Myszka, M.; Żabińska, M.; Klinger, M. Functional Activity of the Complement System in Deceased Donors in Relation to Kidney Allograft Outcome. Transplant. Proc. 2018, 50, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Damman, J.; Seelen, M.A.; Moers, C.; Daha, M.R.; Rahmel, A.; Leuvenink, H.G.; Paul, A.; Pirenne, J.; Ploeg, R.J. Systemic Complement Activation in Deceased Donors Is Associated With Acute Rejection After Renal Transplantation in the Recipient. Transplantation 2011, 92, 163–169. [Google Scholar] [CrossRef] [PubMed]
- van Werkhoven, M.B.; Damman, J.; van Dijk, M.C.R.F.; Daha, M.R.; de Jong, I.J.; Leliveld, A.; Krikke, C.; Leuvenink, H.G.; van Goor, H.; van Son, W.J.; et al. Complement Mediated Renal Inflammation Induced by Donor Brain Death: Role of Renal C5a-C5aR Interaction. Am. J. Transplant. 2013, 13, 875–882. [Google Scholar] [CrossRef] [PubMed]
- van Werkhoven, M.B.; Damman, J.; Daha, M.R.; Krikke, C.; van Goor, H.; van Son, W.J.; Hillebrands, J.-L.; van Dijk, M.C.R.F.; Seelen, M.A.J. Novel insights in localization and expression levels of C5aR and C5L2 under native and post-transplant conditions in the kidney. Mol. Immunol. 2012, 53, 237–245. [Google Scholar] [CrossRef]
- De Vries, D.K.; Van Der Pol, P.; Van Anken, G.E.; Van Gijlswijk, D.J.; Damman, J.; Lindeman, J.H.; Reinders, M.E.J.; Schaapherder, A.F.; van Kooten, C. Acute But Transient Release of Terminal Complement Complex After Reperfusion in Clinical Kidney Transplantation. Transplant. J. 2013, 95, 816–820. [Google Scholar] [CrossRef]
- Błogowski, W.; Dołęgowska, B.; Sałata, D.; Budkowska, M.; Domański, L.; Starzyńska, T. Clinical Analysis of Perioperative Complement Activity during Ischemia/Reperfusion Injury following Renal Transplantation. Clin. J. Am. Soc. Nephrol. 2012, 7, 1843–1851. [Google Scholar] [CrossRef]
- Damman, J.; Hoeger, S.; Boneschansker, L.; Theruvath, A.; Waldherr, R.; Leuvenink, H.G.; Ploeg, R.J.; Yard, B.A.; Seelen, M.A. Targeting complement activation in brain-dead donors improves renal function after transplantation. Transpl. Immunol. 2011, 24, 233–237. [Google Scholar] [CrossRef]
- Poppelaars, F.; Jager, N.M.; Kotimaa, J.; Leuvenink, H.G.; Daha, M.R.; van Kooten, C.; Seelen, M.A.; Damman, J. C1-Inhibitor Treatment Decreases Renal Injury in an Established Brain-Dead Rat Model. Transplantation 2018, 102, 79–87. [Google Scholar] [CrossRef]
- Danobeitia, J.S.; Zens, T.J.; Chlebeck, P.J.; Zitur, L.J.; Reyes, J.A.; Eerhart, M.J.; Coonen, J.; Capuano, S.; D’Alessandro, A.M.; Torrealba, J.R.; et al. Targeted donor complement blockade after brain death prevents delayed graft function in a nonhuman primate model of kidney transplantation. Am. J. Transplant. 2020, 20, 1513–1526. [Google Scholar] [CrossRef]
- Jager, N.M.; van Zanden, J.E.; Subías, M.; Leuvenink, H.G.D.; Daha, M.R.; de Córdoba, S.R.; Poppelaars, F.; Seelen, M.A. Blocking Complement Factor B Activation Reduces Renal Injury and Inflammation in a Rat Brain Death Model. Front. Immunol. 2019, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Suzuki, Y.; Ito, Y. Complement regulation and kidney diseases: Recent knowledge of the double-edged roles of complement activation in nephrology. Clin. Exp. Nephrol. 2017, 22, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Jiao, Y.; Zou, G.; Gao, H.; Zhuo, L.; Li, W. Activation of Complement Pathways in Kidney Tissue May Mediate Tubulointerstitial Injury in Diabetic Nephropathy. Front. Med. 2022, 9, 845679. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellström, B.; Nilsson, B. Cardiovascular disease in haemodialysis: Role of the intravascular innate immune system. Nat. Rev. Nephrol. 2017, 13, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L. Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Heylen, L.; Pirenne, J.; Naesens, M.; Sprangers, B.; Jochmans, I. “Time is tissue”—A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am. J. Transplant. 2021, 21, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef]
- Sim, E.; Sim, R.B. Enzymic assay of C3b receptor on intact cells and solubilized cells. Biochem. J. 1983, 210, 567–576. [Google Scholar] [CrossRef]
- Jager, N.M.; Venema, L.H.; Arykbaeva, A.S.; Meter-Arkema, A.H.; Ottens, P.J.; van Kooten, C.; Mollnes, T.E.; Alwayn, I.P.J.; Leuvenink, H.G.D.; Pischke, S.E.; et al. Complement Is Activated During Normothermic Machine Perfusion of Porcine and Human Discarded Kidneys. Front. Immunol. 2022, 13, 831371. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, B.; Chen, G.; Zhang, X.; Li, M.; Sun, H.; Liu, W.; Vladau, C.; Liu, R.; Jevnikar, A.M.; et al. Preventing Renal IschemiaReperfusion Injury Using Small Interfering RNA by Targeting Complement 3 Gene. Am. J. Transplant. 2006, 6, 2099–2108. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Sun, H.; Feng, B.; Li, M.; Chen, G.; Vladau, C.; Chen, D.; Suzuki, M.; Min, L.; et al. Protection of Renal Ischemia Injury using Combination Gene Silencing of Complement 3 and Caspase 3 Genes. Transplantation 2006, 82, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Farrar, C.A.; Zhou, W.; Lin, T.; Sacks, S.H. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 2006, 20, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Sieve, I.; Münster-Kühnel, A.K.; Hilfiker-Kleiner, D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vasc. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bongoni, A.K.; Lu, B.; McRae, J.L.; Salvaris, E.J.; Toonen, E.; Vikstrom, I.; Morelli, A.B.; Pearse, M.J.; Cowan, P.J. Complement-mediated Damage to the Glycocalyx Plays a Role in Renal Ischemia-reperfusion Injury in Mice. Transplant. Direct 2019, 5, e341. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Ljubanovic, D.; Edelstein, C.L.; Gilkeson, G.S.; Holers, V.M. Lack of a Functional Alternative Complement Pathway Ameliorates Ischemic Acute Renal Failure in Mice. J. Immunol. 2003, 170, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Royer, P.A.; Ljubanović, D.; Dursun, B.; Lenderink, A.M.; Edelstein, C.L.; Holers, V.M. Treatment with an Inhibitory Monoclonal Antibody to Mouse Factor B Protects Mice from Induction of Apoptosis and Renal Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2006, 17, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Azzollini, N.; Todeschini, M.; Fiori, S.; Cavinato, R.A.; Cassis, P.; Solini, S.; Pezzuto, F.; Mister, M.; Thurman, J.M.; et al. Complement Alternative Pathway Deficiency in Recipients Protects Kidney Allograft From Ischemia/Reperfusion Injury and Alloreactive T Cell Response. Am. J. Transplant. 2017, 17, 2312–2325. [Google Scholar] [CrossRef]
- Goetz, L.; Laskowski, J.; Renner, B.; Pickering, M.C.; Kulik, L.; Klawitter, J.; Stites, E.; Christians, U.; Van Der Vlag, J.; Ravichandran, K.; et al. Complement factor H protects mice from ischemic acute kidney injury but is not critical for controlling complement activation by glomerular IgM. Eur. J. Immunol. 2018, 48, 791–802. [Google Scholar] [CrossRef]
- Wu, Y.; Zwaini, Z.D.; Brunskill, N.J.; Zhang, X.; Wang, H.; Chana, R.; Stover, C.M.; Yang, B. Properdin Deficiency Impairs Phagocytosis and Enhances Injury at Kidney Repair Phase Post Ischemia–Reperfusion. Front. Immunol. 2021, 12, 697760. [Google Scholar] [CrossRef]
- Zhou, W.; Farrar, C.A.; Abe, K.; Pratt, J.R.; Marsh, J.E.; Wang, Y.; Stahl, G.L.; Sacks, S.H. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J. Clin. Investig. 2000, 105, 1363–1371. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef] [PubMed]
- Genster, N.; Takahashi, M.; Sekine, H.; Endo, Y.; Garred, P.; Fujita, T. Lessons learned from mice deficient in lectin complement pathway molecules. Mol. Immunol. 2014, 61, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Moller-Kristensen, M.; Wang, W.; Ruseva, M.; Thiel, S.; Nielsen, S.; Takahashi, K.; Shi, L.; Ezekowitz, A.; Jensenius, J.C.; Gadjeva, M. Mannan-Binding Lectin Recognizes Structures on Ischaemic Reperfused Mouse Kidneys and is Implicated in Tissue Injury. Scand. J. Immunol. 2005, 61, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.P.; Roos, A.; Mallat, M.J.; Fujita, T.; de Fijter, J.W.; Daha, M.R. Association Between Mannose-Binding Lectin Levels and Graft Survival in Kidney Transplantation. Am. J. Transplant. 2005, 5, 1361–1366. [Google Scholar] [CrossRef]
- Asgari, E.; Farrar, C.A.; Lynch, N.; Ali, Y.M.; Roscher, S.; Stover, C.; Zhou, W.; Schwaeble, W.J.; Sacks, S.H. Mannan-binding lectin-associated serine protease 2 is critical for the development of renal ischemia reperfusion injury and mediates tissue injury in the absence of complement C4. FASEB J. 2014, 28, 3996–4003. [Google Scholar] [CrossRef]
- Schwaeble, W.J.; Lynch, N.J.; Clark, J.E.; Marber, M.; Samani, N.J.; Ali, Y.M.; Dudler, T.; Parent, B.; Lhotta, K.; Wallis, R.; et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. USA 2011, 108, 7523–7528. [Google Scholar] [CrossRef]
- Henriksen, M.L.; Brandt, J.; Iyer, S.S.; Thielens, N.M.; Hansen, S. Characterization of the interaction between collectin 11 (CL-11, CL-K1) and nucleic acids. Mol. Immunol. 2013, 56, 757–767. [Google Scholar] [CrossRef]
- Farrar, C.A.; Tran, D.; Li, K.; Wu, W.; Peng, Q.; Schwaeble, W.; Zhou, W.; Sacks, S.H. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J. Clin. Investig. 2016, 126, 1911–1925. [Google Scholar] [CrossRef]
- Howard, M.C.; Nauser, C.L.; Farrar, C.A.; Wallis, R.; Sacks, S.H. l -Fucose prevention of renal ischaemia/reperfusion injury in Mice. FASEB J. 2019, 34, 822–834. [Google Scholar] [CrossRef]
- Howard, M.C.; Nauser, C.L.; Vizitiu, D.A.; Sacks, S.H. Fucose as a new therapeutic target in renal transplantation. Pediatr. Nephrol. 2020, 36, 1065–1073. [Google Scholar] [CrossRef]
- Farrar, C.A.; Easgari, E.; Schwaeble, W.J.; Sacks, S.H. Which pathways trigger the role of complement in ischaemia/reperfusion injury? Front. Immunol. 2012, 3, 341. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Li, K.; Sacks, S.H.; Sheerin, N.S. The membrane attack complex, C5b-9, up regulates collagen gene expression in renal tubular epithelial cells. Clin. Exp. Immunol. 2004, 136, 60–66. [Google Scholar] [CrossRef]
- Yu, Z.X.; Qi, S.; Lasaro, M.A.; Bouchard, K.; Dow, C.; Moore, K.; Wu, Z.; Barama, A.; Xu, J.; Johnson, K.; et al. Targeting Complement Pathways During Cold Ischemia and Reperfusion Prevents Delayed Graft Function. Am. J. Transplant. 2016, 16, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Durigutto, P.; Sblattero, D.; Biffi, S.; De Maso, L.; Garrovo, C.; Baj, G.; Colombo, F.; Fischetti, F.; Di Naro, A.F.; Tedesco, F.; et al. Targeted Delivery of Neutralizing Anti-C5 Antibody to Renal Endothelium Prevents Complement-Dependent Tissue Damage. Front. Immunol. 2017, 8, 01093. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Song, W.-C. Membrane complement regulatory proteins: Insight from animal studies and relevance to human diseases. Int. Immunopharmacol. 2001, 1, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Yamada, K.; Miwa, T.; Liu, J.; Nangaku, M.; Song, W.-C. Critical Protection from Renal Ischemia Reperfusion Injury by CD55 and CD59. J. Immunol. 2004, 172, 3869–3875. [Google Scholar] [CrossRef]
- Bongoni, A.K.; Lu, B.; Salvaris, E.J.; Roberts, V.; Fang, D.; McRae, J.L.; Fisicaro, N.; Dwyer, K.M.; Cowan, P.J. Overexpression of Human CD55 and CD59 or Treatment with Human CD55 Protects against Renal Ischemia-Reperfusion Injury in Mice. J. Immunol. 2017, 198, 4837–4845. [Google Scholar] [CrossRef]
- Thurman, J.M.; Lenderink, A.M.; Royer, P.A.; Coleman, K.E.; Zhou, J.; Lambris, J.D.; Nemenoff, R.A.; Quigg, R.J.; Holers, V.M. C3a Is Required for the Production of CXC Chemokines by Tubular Epithelial Cells after Renal Ishemia/Reperfusion. J. Immunol. 2007, 178, 1819–1828. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.-Q.; He, Q.-H.; Li, Y.; Tang, M.; Zheng, Q.-Y.; Xu, G.-L.; Zhang, K.-Q. C5a/C5aR pathway accelerates renal ischemia-reperfusion injury by downregulating PGRN expression. Int. Immunopharmacol. 2017, 53, 17–23. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Smyth, L.A.; Xing, G.; Wang, N.; Meader, L.; Lu, B.; Sacks, S.H.; Zhou, W. C3a and C5a Promote Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2012, 23, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.V.; Shiels, I.A.; Strachan, A.J.; Abbenante, G.; Fairlie, D.P.; Taylor, S.M. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int. 2003, 63, 134–142. [Google Scholar] [CrossRef] [PubMed]
- de Vries, B.; Kóhl, J.; Leclercq, W.K.G.; Wolfs, T.G.A.M.; van Bijnen, A.A.J.H.M.; Heeringa, P.; Buurman, W.A. Complement Factor C5a Mediates Renal Ischemia-Reperfusion Injury Independent from Neutrophils. J. Immunol. 2003, 170, 3883–3889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, X.; Feng, B.; Sun, H.; Suzuki, M.; Ichim, T.; Kubo, N.; Wong, A.; Min, L.R.; Budohn, M.E.; et al. Gene Silencing of Complement C5a Receptor Using siRNA for Preventing Ischemia/Reperfusion Injury. Am. J. Pathol. 2008, 173, 973–980. [Google Scholar] [CrossRef]
- Lewis, A.G.; Köhl, G.; Ma, Q.; Devarajan, P.; Köhl, J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin. Exp. Immunol. 2008, 153, 117–126. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Patel, H.; Sacks, S.H.; Zhou, W. Dendritic Cell Synthesis of C3 Is Required for Full T Cell Activation and Development of a Th1 Phenotype. J. Immunol. 2006, 176, 3330–3341. [Google Scholar] [CrossRef]
- Pratt, J.R.; Basheer, S.A.; Sacks, S.H. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat. Med. 2002, 8, 582–587. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Anderson, K.; Farrar, C.A.; Lu, B.; Smith, R.A.G.; Sacks, S.H.; Zhou, W. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a–C3aR interaction. Blood 2008, 111, 2452–2461. [Google Scholar] [CrossRef]
- Heeger, P.S.; Lalli, P.N.; Lin, F.; Valujskikh, A.; Liu, J.; Muqim, N.; Xu, Y.; Medof, M.E. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005, 201, 1523–1530. [Google Scholar] [CrossRef]
- Sheen, J.-H.; Strainic, M.G.; Liu, J.; Zhang, W.; Yi, Z.; Medof, M.E.; Heeger, P.S. TLR-Induced Murine Dendritic Cell (DC) Activation Requires DC-Intrinsic Complement. J. Immunol. 2017, 199, 278–291. [Google Scholar] [CrossRef]
- Lalli, P.N.; Strainic, M.; Yang, M.; Lin, F.; Medof, M.E.; Heeger, P.S. Locally produced C5a binds to T cell–expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 2008, 112, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Leventhal, J.; Lakhani, P.; Ward, S.C.; Donovan, M.J.; Heeger, P.S. Immune Cell-Derived C3a and C5a Costimulate Human T Cell Alloimmunity. Am. J. Transplant. 2013, 13, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, Q.; Xing, G.; Li, K.; Wang, N.; Farrar, C.A.; Meader, L.; Sacks, S.H.; Zhou, W. Deficiency of C5aR Prolongs Renal Allograft Survival. J. Am. Soc. Nephrol. 2010, 21, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Gueler, F.; Rong, S.; Gwinner, W.; Mengel, M.; Diaeresiscker, V.B.; Diaeresisn, S.S.; Greten, T.F.; Hawlisch, H.; Polakowski, T.; Schnatbaum, K.; et al. Complement 5a Receptor Inhibition Improves Renal Allograft Survival. J. Am. Soc. Nephrol. 2008, 19, 2302–2312. [Google Scholar] [CrossRef]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic Regulation of T Lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Kolev, M.; Le Friec, G.; Leung, M.; Bertram, P.G.; Fara, A.F.; Subias, M.; Pickering, M.C.; Drouet, C.; Meri, S.; et al. Intracellular Complement Activation Sustains T Cell Homeostasis and Mediates Effector Differentiation. Immunity 2013, 39, 1143–1157. [Google Scholar] [CrossRef]
- Kolev, M.; Dimeloe, S.; Le Friec, G.; Navarini, A.; Arbore, G.; Povoleri, G.A.; Fischer, M.; Belle, R.; Loeliger, J.; Develioglu, L.; et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 2015, 42, 1033–1047. [Google Scholar] [CrossRef]
- Arbore, G.; West, E.E.; Rahman, J.; Le Friec, G.; Niyonzima, N.; Pirooznia, M.; Tunc, I.; Pavlidis, P.; Powell, N.; Li, Y.; et al. Complement receptor CD46 co-stimulates optimal human CD8+ T cell effector function via fatty acid metabolism. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Arbore, G.; West, E.E.; Spolski, R.; Robertson, A.A.B.; Klos, A.; Rheinheimer, C.; Dutow, P.; Woodruff, T.M.; Yu, Z.X.; O’Neill, L.A.; et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4 + T cells. Science 2016, 352, aad1210. [Google Scholar] [CrossRef]
- Chun, N.; Heeger, P.S. Blurring the Lines Between Innate and Adaptive Immunity. Transplantation 2016, 100, 1789–1790. [Google Scholar] [CrossRef]
- Kemper, C.; Chan, A.C.; Green, J.M.; Brett, K.A.; Murphy, K.M.; Atkinson, J.P. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 2003, 421, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.-G.; Levings, M.K. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr. Opin. Immunol. 2000, 12, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Ni Choileain, S.; Weyand, N.J.; Neumann, C.; Thomas, J.; So, M.; Astier, A.L. The Dynamic Processing of CD46 Intracellular Domains Provides a Molecular Rheostat for T Cell Activation. PLoS ONE 2011, 6, e16287. [Google Scholar] [CrossRef]
- Castellano, G.; Woltman, A.M.; Schlagwein, N.; Xu, W.; Schena, F.P.; Daha, M.R.; van Kooten, C. Immune modulation of human dendritic cells by complement. Eur. J. Immunol. 2007, 37, 2803–2811. [Google Scholar] [CrossRef]
- Lu, J.; Teh, B.K.; Wang, L.; Wang, Y.; Tan, Y.S.; Lai, M.C.; Reid, K.B.M. The Classical and Regulatory Functions of C1q in Immunity and Autoimmunity. Cell. Mol. Immunol. 2008, 5, 9–21. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Dijkstra, D.J.; van Kooten, C.; Gelderman, K.A.; Trouw, L.A. Circulating C1q levels in health and disease, more than just a biomarker. Mol. Immunol. 2021, 140, 206–216. [Google Scholar] [CrossRef]
- Zimmer, A.; Bouley, J.; Le Mignon, M.; Pliquet, E.; Horiot, S.; Turfkruyer, M.; Baron-Bodo, V.; Horak, F.; Nony, E.; Louise, A.; et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1020–1030. [Google Scholar] [CrossRef]
- Mascarell, L.; Airouche, S.; Berjont, N.; Gary, C.; Gueguen, C.; Fourcade, G.; Bellier, B.; Togbe, D.; Ryffel, B.; Klatzmann, D.; et al. The regulatory dendritic cell marker C1q is a potent inhibitor of allergic inflammation. Mucosal Immunol. 2017, 10, 695–704. [Google Scholar] [CrossRef]
- Zang, X.; Allison, J.P. To be or not to be B7. J. Clin. Investig. 2006, 116, 2590–2593. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, B.-H.; Dong, Y.; Yamamura, A.; Fu, W. CRIg, a tissue-resident macrophage specific immune checkpoint molecule, promotes immunological tolerance in NOD mice, via a dual role in effector and regulatory T cells. Elife 2017, 6, e29540. [Google Scholar] [CrossRef] [PubMed]
- Einecke, G.; Sis, B.; Reeve, J.; Mengel, M.; Campbell, P.M.; Hidalgo, L.G.; Kaplan, B.; Halloran, P.F. Antibody-Mediated Microcirculation Injury Is the Major Cause of Late Kidney Transplant Failure. Am. J. Transplant. 2009, 9, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Chang, J.; Famulski, K.; Hidalgo, L.G.; Salazar, I.D.; Lopez, M.M.; Matas, A.; Picton, M.; de Freitas, D.; Bromberg, J.; et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2015, 26, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Ugurlar, D.; Howes, S.C.; de Kreuk, B.-J.; Koning, R.I.; de Jong, R.N.; Beurskens, F.J.; Schuurman, J.; Koster, A.J.; Sharp, T.H.; Parren, P.W.H.I.; et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 2018, 359, 794–797. [Google Scholar] [CrossRef]
- De Clippel, D.; Baeten, M.; Torfs, A.; Emonds, M.-P.; Feys, H.B.; Compernolle, V.; Vandekerckhove, P. Screening for HLA antibodies in plateletpheresis donors with a history of transfusion or pregnancy. Transfusion 2014, 54, 3036–3042. [Google Scholar] [CrossRef]
- Lederer, S.R.; Schneeberger, H.; Albert, E.; Johnson, J.P.; Gruber, R.; Land, W.; Burkhardt, K.; Hillebrand, G.; Feucht, H.E. Early renal graft dysfunction: The Role of Preformed Antibodies to DR-Typed Lymphoblastoid Cell Lines: 1. Transplantation 1996, 61, 313–319. [Google Scholar] [CrossRef]
- Bentall, A.; Cornell, L.D.; Gloor, J.M.; Park, W.D.; Gandhi, M.J.; Winters, J.L.; Chedid, M.F.; Dean, P.G.; Stegall, M.D. Five-Year Outcomes in Living Donor Kidney Transplants With a Positive Crossmatch. Am. J. Transplant. 2012, 13, 76–85. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Tedesco, F.; Pausa, M.; Nardon, E.; Introna, M.; Mantovani, A.; Dobrina, A. The Cytolytically Inactive Terminal Complement Complex Activates Endothelial Cells to Express Adhesion Molecules and Tissue Factor Procoagulant Activity. J. Exp. Med. 1997, 185, 1619–1628. [Google Scholar] [CrossRef]
- Bettoni, S.; Galbusera, M.; Gastoldi, S.; Donadelli, R.; Tentori, C.; Spartà, G.; Bresin, E.; Mele, C.; Alberti, M.; Tortajada, A.; et al. Interaction between Multimeric von Willebrand Factor and Complement: A Fresh Look to the Pathophysiology of Microvascular Thrombosis. J. Immunol. 2017, 199, 1021–1040. [Google Scholar] [CrossRef]
- Jane-wit, D.; Manes, T.D.; Yi, T.; Qin, L.; Clark, P.; Kirkiles-Smith, N.C.; Abrahimi, P.; Devalliere, J.; Moeckel, G.; Kulkarni, S.; et al. Alloantibody and Complement Promote T Cell-Mediated Cardiac Allograft Vasculopathy through Non-Canonical NF-ΚB Signaling in Endothelial Cells. Circulation 2013, 128, 002972. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, C.; Fu, Y.-X.; Holers, V.M.; Molina, H. Expression of Complement Receptors 1 and 2 on Follicular Dendritic Cells Is Necessary for the Generation of a Strong Antigen-Specific IgG Response. J. Immunol. 1998, 160, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.W.; Allison, M.E.D.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of Complement as a Molecular Adjuvant: Bridging Innate and Acquired Immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.F.; Lukacs-Kornek, V.; Kuligowski, M.P.; Pitcher, L.A.; Degn, S.E.; Turley, S.J.; Carroll, M.C. Complement-Dependent Transport of Antigen into B Cell Follicles. J. Immunol. 2010, 185, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Fitch, Z.W.; Schroder, P.M.; Choi, A.Y.; Manook, M.; Yoon, J.; Song, M.; Yi, J.S.; Khandelwal, S.; Arepally, G.M.; et al. C3 complement inhibition prevents antibody-mediated rejection and prolongs renal allograft survival in sensitized non-human primates. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Bekassy, Z.; Grunenwald, A.; Roumenina, L.T. A role for complement blockade in kidney transplantation. Cell. Mol. Immunol. 2022, 19, 755–757. [Google Scholar] [CrossRef]
- Tatapudi, V.S.; Montgomery, R.A. Therapeutic Modulation of the Complement System in Kidney Transplantation: Clinical Indications and Emerging Drug Leads. Front. Immunol. 2019, 10, 2306. [Google Scholar] [CrossRef]
- Kamel, M.H.; Jaberi, A.; Gordon, C.E.; Beck, L.H.; Francis, J. The Complement System in the Modern Era of Kidney Transplantation: Mechanisms of Injury and Targeted Therapies. Semin. Nephrol. 2022, 42, 14–28. [Google Scholar] [CrossRef]
- Frémeaux-Bacchi, V.; Legendre, C.M. The emerging role of complement inhibitors in transplantation. Kidney Int. 2015, 88, 967–973. [Google Scholar] [CrossRef]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- Legendre, C.M.; Licht, C.; Muus, P.; Greenbaum, L.A.; Babu, S.; Bedrosian, C.; Bingham, C.; Cohen, D.J.; Delmas, Y.; Douglas, K.; et al. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. N. Engl. J. Med. 2013, 368, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Galbusera, M.; Gastoldi, S.; Macor, P.; Banterla, F.; Bresin, E.; Tripodo, C.; Bettoni, S.; Donadelli, R.; Valoti, E.; et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 2014, 124, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Schwotzer, N.; Golshayan, D.; Frémeaux-Bacchi, V. The Rational Use of Complement Inhibitors in Kidney Diseases. Kidney Int. Rep. 2022, 7, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Weitz, M.; Amon, O.; Bassler, D.; Koenigsrainer, A.; Nadalin, S. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatr. Nephrol. 2011, 26, 1325–1329. [Google Scholar] [CrossRef]
- Siedlecki, A.M.; Isbel, N.; Walle, J.V.; Eggleston, J.J.; Cohen, D.J.; Licht, C.; Frémeaux-Bacchi, V.; Ariceta, G.; Ardissino, G.; Fakhouri, F.; et al. Eculizumab Use for Kidney Transplantation in Patients With a Diagnosis of Atypical Hemolytic Uremic Syndrome. Kidney Int. Rep. 2018, 4, 434–446. [Google Scholar] [CrossRef]
- Lonze, B.E.; Singer, A.L.; Montgomery, R.A. Eculizumab and Renal Transplantation in a Patient with CAPS. N. Engl. J. Med. 2010, 362, 1744–1745. [Google Scholar] [CrossRef]
- Lonze, B.E.; Zachary, A.A.; Magro, C.M.; Desai, N.M.; Orandi, B.J.; Dagher, N.N.; Singer, A.L.; Carter-Monroe, N.; Nazarian, S.M.; Segev, D.L.; et al. Eculizumab Prevents Recurrent Antiphospholipid Antibody Syndrome and Enables Successful Renal Transplantation. Am. J. Transp. 2014, 14, 459–465. [Google Scholar] [CrossRef]
- Garg, N.; Zhang, Y.; Nicholson-Weller, A.; Khankin, E.V.; Borsa, N.G.; Meyer, N.C.; McDermott, S.; E Stillman, I.; Rennke, H.G.; Smith, R.J.; et al. C3 glomerulonephritis secondary to mutations in factors H and I: Rapid recurrence in deceased donor kidney transplant effectively treated with eculizumab. Nephrol. Dial. Transplant. 2018, 33, 2260–2265. [Google Scholar] [CrossRef]
- Assistance Publique. Hôpitaux de Paris Treatment of Subclinical Antibody-Mediated Acute Rejection in Kidney Transplant Recipients with the Complement Inhibitor Eculizumab. 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02113891 (accessed on 26 October 2022).
- Heeger, P.M.D. Eculizumab for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplantation. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01919346 (accessed on 24 November 2022).
- Johns Hopkins University Eculizumab to Enable Renal Transplantation in Patients with History of Antiphospholipid Antibody Syndrome or Catastrophic Antiphospholipid Antibody Syndrome. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT01029587 (accessed on 26 October 2022).
- Stegall, M. A Single Center, Open-Label Study to Determine the Safety and Efficacy of a Dosing Regimen of Eculizumab Added to Conventional Treatment in the Prevention of Antibody-Mediated Rejection (AMR) in ABO Blood Group Incompatible Living Donor Kidney Transplantation (ABOi LDKTx). 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT01095887 (accessed on 26 October 2022).
- Kulkarni, S. Eculizumab Therapy for Chronic Complement-Mediated Injury in Kidney Transplantation: A Randomized, Open-Label, Pilot Intervention Trial. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01327573 (accessed on 26 October 2022).
- Stegall, M. A Single Center, Open-Label Study to Determine the Safety and Efficacy of a Dosing Regimen of Eculizumab Added to Conventional Treatment in the Prevention of Antibody-Mediated Rejection (AMR) in Positive Crossmatch Living Donor Kidney Transplantation. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT00670774 (accessed on 26 October 2022).
- Bentall, A.; Tyan, D.B.; Sequeira, F.; Everly, M.J.; Gandhi, M.J.; Cornell, L.D.; Li, H.; Henderson, N.A.; Raghavaiah, S.; Winters, J.L.; et al. Antibody-mediated rejection despite inhibition of terminal complement. Transpl. Int. 2014, 27, 1235–1243. [Google Scholar] [CrossRef]
- Stegall, M. A Single Center, Open-Label Study to Determine the Safety and Efficacy of a Dosing Regimen of Eculizumab Added to Conventional Treatment in the Prevention of Acute Humoral Rejection (AHR) in Positive Crossmatch Deceased Donor Kidney Transplantation. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01106027 (accessed on 26 October 2022).
- MD, A.K.C. Efficacy and Safety of Eculizumab for Treatment of Antibody-Mediated Rejection Following Renal Transplantation. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01895127 (accessed on 26 October 2022).
- Alexion Pharmaceuticals A Randomized, Open-Label, Multicenter Trial to Determine Safety and Efficacy of Eculizumab in the Prevention of Antibody Mediated Rejection (AMR) in Living Donor Kidney Transplant Recipients Requiring Desensitization Therapy. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01399593 (accessed on 26 October 2022).
- Marks, W.H.; Mamode, N.; Montgomery, R.A.; Stegall, M.D.; Ratner, L.E.; Cornell, L.D.; Rowshani, A.T.; Colvin, R.B.; Dain, B.; Boice, J.A.; et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 2876–2888. [Google Scholar] [CrossRef]
- Alexion Pharmaceuticals An Open-Label, Single-Arm, Multicenter Trial to Determine Safety and Efficacy of Eculizumab in the Prevention of Antibody Mediated Rejection (AMR) in Sensitized Recipients of a Kidney Transplant From a Deceased Donor. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01567085 (accessed on 26 October 2022).
- Alexion Pharmaceuticals A Randomized, Parallel-Group, Double-Blind, Placebo-Controlled, Multi-Center Study of Eculizumab for the Prevention of Delayed Graft Function After Kidney Transplantation in Adult Subjects at Increased Risk of Delayed Graft Function. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02145182 (accessed on 24 November 2022).
- Kaabak, M. Study of Eculizumab for Prevention and Treatment Reperfusion Injury in Kidney Transplantation. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01756508 (accessed on 26 October 2022).
- Schroppel, B.M.D. Pilot Study of the Clinical Activity of Eculizumab for Prevention of Delayed Graft Function in Patients Undergoing Deceased Donor Kidney Transplantation. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01403389 (accessed on 26 October 2022).
- Schröppel, B.; Akalin, E.; Baweja, M.; Bloom, R.D.; Florman, S.; Goldstein, M.; Haydel, B.; Hricik, D.E.; Kulkarni, S.; Levine, M.; et al. Peritransplant eculizumab does not prevent delayed graft function in deceased donor kidney transplant recipients: Results of two randomized controlled pilot trials. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.E.; Mejia, P.; Lu, F. Biological activities of C1 inhibitor. Mol. Immunol. 2008, 45, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Vo, A.; Choi, J.; Ammerman, N.; Lim, K.; Sethi, S.; Kim, I.; Kumar, S.; Najjar, R.; Peng, A.; et al. Three-Year Outcomes of a Randomized, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2019, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- MD, S.J. A Phase I/II, Double-Blind, Placebo-Controlled Study: Assessing Safety and Efficacy of Preoperative Renal Allograft Infusions of C1 Inhibitor (Berinert®) (Human) (C1INH) vs. Placebo Administration in Recipients of a Renal Allograft From Deceased High Risk Donors and Its Impact on Delayed Graft Function (DGF) and Ischemia/Reperfusion Injury (IRI). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04696146 (accessed on 26 October 2022).
- University of Wisconsin, Madison A Phase I/II, Single Center, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Feasibility of Using Human Recombinant C1 Inhibitor(RUCONEST®) as a Therapeutic Strategy to Reduce the Incidence of Delayed Graft Function in Recipients of Kidneys From Donation After Cardio-Circulatory Death. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03791476 (accessed on 26 October 2022).
- University of Wisconsin, Madison A Phase I, Single Center, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Tolerability of C1 Inhibitor (CINRYZE) as a Donor Pre-Treatment Strategy in Brain Dead Donors Who Meet a Kidney Donor Risk Index (KDRI) Above 60%. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02435732 (accessed on 26 October 2022).
- Berger, M.; Lefaucheur, C.; Jordan, S.C. Update on C1 Esterase Inhibitor in Human Solid Organ Transplantation. Transplantation 2019, 103, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Pharming Technologies, B.V. Recombinant Human C1 Inhibitor for the Treatment of Early Antibody-Mediated Rejection in Renal Transplantation. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01035593 (accessed on 26 October 2022).
- Vo, A.A.; Zeevi, A.; Choi, J.; Cisneros, K.; Toyoda, M.; Kahwaji, J.; Peng, A.; Villicana, R.; Puliyanda, D.; Reinsmoen, N.; et al. A Phase I/II Placebo-Controlled Trial of C1-Inhibitor for Prevention of Antibody-Mediated Rejection in HLA Sensitized Patients. Transplantation 2015, 99, 299–308. [Google Scholar] [CrossRef]
- Shire, A. Randomized Double-Blind Placebo-Controlled Study to Evaluate the Efficacy and Safety of Cinryze® (C1 Esterase Inhibitor [Human]) for the Treatment of Acute Antibody-Mediated Rejection in Kidney Transplant Patients. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02547220 (accessed on 26 October 2022).
- Shire, A. Randomized Double-Blind Placebo-Controlled Pilot Study to Evaluate the Safety and Effect of CINRYZE® (C1 Esterase Inhibitor [Human]) for the Treatment of Acute Antibody-Mediated Rejection in Recipients of Donor-Sensitized Kidney Transplants. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT01147302 (accessed on 26 October 2022).
- Montgomery, R.A.; Orandi, B.J.; Racusen, L.; Jackson, A.M.; Garonzik-Wang, J.M.; Shah, T.; Woodle, E.S.; Sommerer, C.; Fitts, D.; Rockich, K.; et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody Mediated Rejection Following Kidney Transplantation: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Am. J. Transplant. 2016, 16, 3468–3478. [Google Scholar] [CrossRef]
- C1-Inhibitor (INH) for Refractory Antibody Mediated Renal Allograft Rejection—Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02936479 (accessed on 28 October 2022).
- Viklicky, O.; Slatinska, J.; Novotny, M.; Hruba, P. Developments in immunosuppression. Curr. Opin. Organ Transplant. 2020, 26, 91–96. [Google Scholar] [CrossRef]
- Eskandary, F.; Jilma, B.; Mühlbacher, J.; Wahrmann, M.; Regele, H.; Kozakowski, N.; Firbas, C.; Panicker, S.; Parry, G.C.; Gilbert, J.C.; et al. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am. J. Transplant. 2018, 18, 916–926. [Google Scholar] [CrossRef]
- Anwar, I.J.; DeLaura, I.; Ladowski, J.; Gao, Q.; Knechtle, S.J.; Kwun, J. Complement-targeted therapies in kidney transplantation—Insights from preclinical studies. Front. Immunol. 2022, 13, 984090. [Google Scholar] [CrossRef]
- Amyndas Pharmaceuticals, S.A. Safety, Tolerability, Pharmacokinetics (PK) and Pharmacodynamics (PD) of a Single Ascending Dose (SAD) and a Multiple Dose (MD) of the Complement Inhibitor AMY-101. A Prospective, Single-Center, Open-Label, First-In-Human (FIH) Clinical Study in Healthy Male Volunteers. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03316521 (accessed on 26 October 2022).
- Jordan, S.C.; Lorant, T.; Choi, J.; Kjellman, C.; Winstedt, L.; Bengtsson, M.; Zhang, X.; Eich, T.; Toyoda, M.; Eriksson, B.-M.; et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 442–453. [Google Scholar] [CrossRef]
- Hansa Biopharma, A.B. A Phase II Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Efficacy of Intravenous IdeS After Administration of Ascending Doses in Chronic Kidney Disease Patients. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02224820 (accessed on 26 October 2022).
- Hansa Biopharma, A.B. A Phase II Study to Evaluate the Safety, Tolerability, Efficacy and Pharmacokinetics of Intravenous Ascending Doses of IdeS in Kidney Transplantation. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02475551 (accessed on 26 October 2022).
- MD, S.J. A Phase I/II Trial to Evaluate the Safety and Tolerability of Ides® (IgG Endopeptidase) to Eliminate Donor Specific HLA Antibodies (DSAs) and Prevent Antibody-Mediated Rejection Post-Transplant in Highly-HLA Sensitized Patients. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02426684 (accessed on 26 October 2022).
- Pratt, J.R.; Jones, M.E.; Dong, J.; Zhou, W.; Chowdhury, P.; Smith, R.A.; Sacks, S.H. Nontransgenic Hyperexpression of a Complement Regulator in Donor Kidney Modulates Transplant Ischemia/Reperfusion Damage, Acute Rejection, and Chronic Nephropathy. Am. J. Pathol. 2003, 163, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Smith, R.A.; Sacks, S.H.; Zhou, W. Therapeutic Strategy with a Membrane-Localizing Complement Regulator to Increase the Number of Usable Donor Organs after Prolonged Cold Storage. J. Am. Soc. Nephrol. 2006, 17, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Kassimatis, T.; Qasem, A.; Douiri, A.; Ryan, E.G.; Rebollo-Mesa, I.; Nichols, L.L.; Greenlaw, R.; Olsburgh, J.; Smith, R.A.; Sacks, S.H.; et al. A double-blind randomised controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): Study protocol for a randomised controlled trial. Trials 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kassimatis, T.; Greenlaw, R.; Hunter, J.P.; Douiri, A.; Flach, C.; Rebollo-Mesa, I.; Nichols, L.L.; Qasem, A.; Danzi, G.; Olsburgh, J.; et al. Ex vivo delivery of Mirococept: A dose-finding study in pig kidney after showing a low dose is insufficient to reduce delayed graft function in human kidney. Am. J. Transplant. 2020, 21, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Casiraghi, F.; Introna, M.; Gotti, E.; Todeschini, M.; Cavinato, R.A.; Capelli, C.; Rambaldi, A.; Cassis, P.; Rizzo, P.; et al. Autologous Mesenchymal Stromal Cells and Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2011, 6, 412–422. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Gotti, E.; Introna, M.; Todeschini, M.; Cavinato, R.A.; Capelli, C.; Rambaldi, A.; Cassis, P.; Rizzo, P.; et al. Mesenchymal stromal cells and kidney transplantation: Pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl. Int. 2013, 26, 867–878. [Google Scholar] [CrossRef]
- Casiraghi, F.; Perico, N.; Cortinovis, F.C.M.; Remuzzi, G. Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat. Rev. Nephrol. 2016, 12, 241–253. [Google Scholar] [CrossRef]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction Therapy With Autologous Mesenchymal Stem Cells in Living-Related Kidney Transplants. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef]
- Peng, Y.; Ke, M.; Xu, L.; Liu, L.; Chen, X.; Xia, W.; Li, X.; Chen, Z.; Ma, J.; Liao, D.; et al. Donor-Derived Mesenchymal Stem Cells Combined With Low-Dose Tacrolimus Prevent Acute Rejection After Renal Transplantation. Transplantation 2013, 95, 161–168. [Google Scholar] [CrossRef]
- Reinders, M.; Van Kooten, C.; Rabelink, T.; De Fijter, J.W. Mesenchymal Stromal Cell Therapy for Solid Organ Transplantation. Transplantation 2018, 102, 35–43. [Google Scholar] [CrossRef]
- Casiraghi, F.; Azzollini, N.; Todeschini, M.; Cavinato, R.A.; Cassis, P.; Solini, S.; Rota, C.; Morigi, M.; Introna, M.; Maranta, R.; et al. Localization of Mesenchymal Stromal Cells Dictates Their Immune or Proinflammatory Effects in Kidney Transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2012, 12, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Bussolati, B.; Bruno, S.; Morando, L.; Mauriello-Romanazzi, G.; Sanavio, F.; Stamenkovic, I.; Biancone, L.; Camussi, G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007, 72, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Harvey, J.; Finan, A.; Weber, K.; Agarwal, U.; Penn, M.S. Myocardial CXCR4 Expression Is Required for Mesenchymal Stem Cell Mediated Repair Following Acute Myocardial Infarction. Circulation 2012, 126, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; DiScipio, R.G.; Zhao, M.; Khaldoyanidi, S.K. C3a and C5a Are Chemotactic Factors for Human Mesenchymal Stem Cells, Which Cause Prolonged ERK1/2 Phosphorylation. J. Immunol. 2009, 182, 3827–3836. [Google Scholar] [CrossRef]
- Hengartner, N.-E.; Fiedler, J.; Schrezenmeier, H.; Huber-Lang, M.; Brenner, R.E. Crucial Role of IL1beta and C3a in the In Vitro-Response of Multipotent Mesenchymal Stromal Cells to Inflammatory Mediators of Polytrauma. PLoS ONE 2015, 10, e0116772. [Google Scholar] [CrossRef]
- Casiraghi, F.; Todeschini, M.; Azzollini, N.; Cravedi, P.; Cassis, P.; Solini, S.; Fiori, S.; Rota, C.; Karachi, A.; Carrara, C.; et al. Effect of Timing and Complement Receptor Antagonism on Intragraft Recruitment and Protolerogenic Effects of Mesenchymal Stromal Cells in Murine Kidney Transplantation. Transplantation 2019, 103, 1121–1130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarsiero, D.; Aiello, S. The Complement System in Kidney Transplantation. Cells 2023, 12, 791. https://doi.org/10.3390/cells12050791
Santarsiero D, Aiello S. The Complement System in Kidney Transplantation. Cells. 2023; 12(5):791. https://doi.org/10.3390/cells12050791
Chicago/Turabian StyleSantarsiero, Donata, and Sistiana Aiello. 2023. "The Complement System in Kidney Transplantation" Cells 12, no. 5: 791. https://doi.org/10.3390/cells12050791
APA StyleSantarsiero, D., & Aiello, S. (2023). The Complement System in Kidney Transplantation. Cells, 12(5), 791. https://doi.org/10.3390/cells12050791




