Advances in Innate Immunity to Overcome Immune Rejection during Xenotransplantation
Abstract
1. Introduction
2. The Complement and Natural Antibodies System
3. The Innate Cellular Immune Response in Xenotransplantation
3.1. Macrophages-Mediated Xenograft Rejection
3.2. Natural Killer Cells-Mediated Xenograft Rejection
3.3. Neutrophils-Mediated Xenograft Rejection
4. Toll-Like Receptors
5. Xenotransplantation in Clinical Grade
6. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascalho, M.; Platt, J.L. Xenotransplantation and other means of organ replacement. Nat. Rev. Immunol. 2001, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Vandeputte, M.; Waer, M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J. Immunol. 1997, 158, 5658–5667. [Google Scholar] [PubMed]
- Schuurman, H.J.; Cheng, J.; Lam, T. Pathology of xenograft rejection: A commentary. Xenotransplantation 2003, 10, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Frank, M.M. Therapeutic potential of complement modulation. Nat. Rev. Drug Discov. 2010, 9, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahmad, A. Role of MAIT cells in the immunopathogenesis of inflammatory diseases: New players in old game. Int. Rev. Immunol. 2018, 37, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Fearon, D.T.; Locksley, R.M. The instructive role of innate immunity in the acquired immune response. Science 1996, 272, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Akira, S. Identification and functions of pattern-recognition receptors. J. Allergy Clin. Immunol. 2010, 125, 985–992. [Google Scholar] [CrossRef]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Le Bas-Bernardet, S.; Tillou, X.; Branchereau, J.; Dilek, N.; Poirier, N.; Châtelais, M.; Charreau, B.; Minault, D.; Hervouet, J.; Renaudin, K.; et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am. J. Transplant. 2015, 15, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fang, C.; Fu, W.; Jiang, B.; Li, G.; Qin, L.; Rosenbluth, J.; Gong, G.; Xie, C.B.; Yoo, P.; et al. Endothelial Cell-Derived Interleukin-18 Released During Ischemia Reperfusion Injury Selectively Expands T Peripheral Helper Cells to Promote Alloantibody Production. Circulation 2020, 141, 464–478. [Google Scholar] [CrossRef]
- Dempsey, P.W.; Allison, M.E.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Solvik, U.O.; Haraldsen, G.; Fiane, A.E.; Boretti, E.; Lambris, J.D.; Fung, M.; Thorsby, E.; Mollnes, T.E. Human serum-induced expression of E-selectin on porcine aortic endothelial cells in vitro is totally complement mediated. Transplantation 2001, 72, 1967–1973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyagawa, S.; Hirose, H.; Shirakura, R.; Naka, Y.; Nakata, S.; Kawashima, Y.; Seya, T.; Matsumoto, M.; Uenaka, A.; Kitamura, H. The mechanism of discordant xenograft rejection. Transplantation 1988, 46, 825–830. [Google Scholar] [CrossRef]
- Miyagawa, S.; Maeda, A.; Toyama, C.; Kogata, S.; Okamatsu, C.; Yamamoto, R.; Masahata, K.; Kamiyama, M.; Eguchi, H.; Watanabe, M.; et al. Aspects of the Complement System in New Era of Xenotransplantation. Front. Immunol. 2022, 13, 860165. [Google Scholar] [CrossRef]
- Ramírez, P.; Montoya, M.J.; Ríos, A.; García Palenciano, C.; Majado, M.; Chávez, R.; Muñoz, A.; Fernández, O.M.; Sánchez, A.; Segura, B.; et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant. Proc. 2005, 37, 4103–4106. [Google Scholar] [CrossRef]
- McGregor, C.G.; Ricci, D.; Miyagi, N.; Stalboerger, P.G.; Du, Z.; Oehler, E.A.; Tazelaar, H.D.; Byrne, G.W. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation 2012, 93, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Tazelaar, H.D.; Byrne, G.W.; McGregor, C.G. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation 2011, 91, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Houser, S.L.; Kuwaki, K.; Knosalla, C.; Dor, F.J.; Gollackner, B.; Cheng, J.; Shimizu, A.; Schuurman, H.J.; Cooper, D.K. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 2004, 11, 416–425. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.G.; Teotia, S.S.; Byrne, G.W.; Michaels, M.G.; Risdahl, J.M.; Schirmer, J.M.; Tazelaar, H.D.; Walker, R.C.; Logan, J.S. Cardiac xenotransplantation: Progress toward the clinic. Transplantation 2004, 78, 1569–1575. [Google Scholar] [CrossRef]
- Lin, C.C.; Ezzelarab, M.; Shapiro, R.; Ekser, B.; Long, C.; Hara, H.; Echeverri, G.; Torres, C.; Watanabe, H.; Ayares, D.; et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am. J. Transplant. 2010, 10, 1556–1568. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Hoyt, R.F.; Thomas, M.L., 3rd; Ayares, D.; Horvath, K.A. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 1106–1113; discussion 1113–1114. [Google Scholar] [CrossRef]
- Dwyer, K.M.; Robson, S.C.; Nandurkar, H.H.; Campbell, D.J.; Gock, H.; Murray-Segal, L.J.; Fisicaro, N.; Mysore, T.B.; Kaczmarek, E.; Cowan, P.J.; et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J. Clin. Investig. 2004, 113, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Mathews, D.V.; Breeden, C.P.; Higginbotham, L.B.; Ladowski, J.; Martens, G.; Stephenson, A.; Farris, A.B.; Strobert, E.A.; Jenkins, J.; et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am. J. Transplant. 2019, 19, 2174–2185. [Google Scholar] [CrossRef]
- Fiorante, P.; Banz, Y.; Mohacsi, P.J.; Kappeler, A.; Wuillemin, W.A.; Macchiarini, P.; Roos, A.; Daha, M.R.; Schaffner, T.; Haeberli, A.; et al. Low molecular weight dextran sulfate prevents complement activation and delays hyperacute rejection in pig-to-human xenotransplantation models. Xenotransplantation 2001, 8, 24–35. [Google Scholar] [CrossRef]
- Laumonier, T.; Mohacsi, P.J.; Matozan, K.M.; Banz, Y.; Haeberli, A.; Korchagina, E.Y.; Bovin, N.V.; Vanhove, B.; Rieben, R. Endothelial cell protection by dextran sulfate: A novel strategy to prevent acute vascular rejection in xenotransplantation. Am. J. Transplant. 2004, 4, 181–187. [Google Scholar] [CrossRef]
- Laumonier, T.; Walpen, A.J.; Maurus, C.F.; Mohacsi, P.J.; Matozan, K.M.; Korchagina, E.Y.; Bovin, N.V.; Vanhove, B.; Seebach, J.D.; Rieben, R. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation 2003, 76, 838–843. [Google Scholar] [CrossRef][Green Version]
- Sandrin, M.S.; Vaughan, H.A.; Dabkowski, P.L.; McKenzie, I.F. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proc. Natl. Acad. Sci. USA 1993, 90, 11391–11395. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between Human Natural Anti-Alpha-Galactosyl Immunoglobulin-G and Bacteria of the Human Flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Lambrigts, D.; Sachs, D.H.; Cooper, D.K.C. Discordant organ xenotransplantation in primates—World experience and current status. Transplantation 1998, 66, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Trucco, M. Use of genetically-engineered pig donors in islet transplantation. World J. Transplant. 2015, 5, 243–250. [Google Scholar] [CrossRef]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef]
- Yamada, K.; Yazawa, K.; Shimizu, A.; Iwanaga, T.; Hisashi, Y.; Nuhn, M.; O’Malley, P.; Nobori, S.; Vagefi, P.A.; Patience, C.; et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005, 11, 32–34. [Google Scholar] [CrossRef]
- Kuwaki, K.; Tseng, Y.L.; Dor, F.J.; Shimizu, A.; Houser, S.L.; Sanderson, T.M.; Lancos, C.J.; Prabharasuth, D.D.; Cheng, J.; Moran, K.; et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat. Med. 2005, 11, 29–31. [Google Scholar] [CrossRef]
- Shimizu, A.; Hisashi, Y.; Kuwaki, K.; Tseng, Y.L.; Dor, F.J.; Houser, S.L.; Robson, S.C.; Schuurman, H.J.; Cooper, D.K.; Sachs, D.H.; et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am. J. Pathol. 2008, 172, 1471–1481. [Google Scholar] [CrossRef]
- Burdorf, L.; Stoddard, T.; Zhang, T.; Rybak, E.; Riner, A.; Avon, C.; Laaris, A.; Cheng, X.; Sievert, E.; Braileanu, G.; et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am. J. Transplant. 2014, 14, 1084–1095. [Google Scholar] [CrossRef]
- Taylor, S.G.; McKenzie, I.F.; Sandrin, M.S. Characterization of the rat alpha(1,3)galactosyltransferase: Evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1,3)Gal by two separate glycosylation pathways. Glycobiology 2003, 13, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Milland, J.; Christiansen, D.; Lazarus, B.D.; Taylor, S.G.; Xing, P.X.; Sandrin, M.S. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J. Immunol. 2006, 176, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.C.; Stussi, G.; Huggel, K.; Rieben, R.; Seebach, J.D. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation 2007, 83, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, T.; Du, J.; Xia, Q.; Wang, L.; Chen, S.; Zhu, L.; Pan, D.; Wang, Y.; Chen, G. Both Natural and Induced Anti-Sda Antibodies Play Important Roles in GTKO Pig-to-Rhesus Monkey Xenotransplantation. Front. Immunol. 2022, 13, 849711. [Google Scholar] [CrossRef]
- Lutz, A.J.; Li, P.; Estrada, J.L.; Sidner, R.A.; Chihara, R.K.; Downey, S.M.; Burlak, C.; Wang, Z.Y.; Reyes, L.M.; Ivary, B.; et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Estrada, J.L.; Burlak, C.; Montgomery, J.; Butler, J.R.; Santos, R.M.; Wang, Z.Y.; Paris, L.L.; Blankenship, R.L.; Downey, S.M.; et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation 2015, 22, 20–31. [Google Scholar] [CrossRef]
- Adams, A.B.; Kim, S.C.; Martens, G.R.; Ladowski, J.M.; Estrada, J.L.; Reyes, L.M.; Breeden, C.; Stephenson, A.; Eckhoff, D.E.; Tector, M.; et al. Xenoantigen Deletion and Chemical Immunosuppression Can Prolong Renal Xenograft Survival. Ann. Surg. 2018, 268, 564–573. [Google Scholar] [CrossRef]
- Martens, G.R.; Reyes, L.M.; Li, P.; Butler, J.R.; Ladowski, J.M.; Estrada, J.L.; Sidner, R.A.; Eckhoff, D.E.; Tector, M.; Tector, A.J. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation 2017, 101, e86–e92. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, H.; Patel, D.; Jagdale, A.; Ayares, D.; Anderson, D.; Eckhoff, D.E.; Cooper, D.K.C.; Hara, H. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci. Rep. 2020, 10, 9771. [Google Scholar] [CrossRef]
- Estrada, J.L.; Martens, G.; Li, P.; Adams, A.; Newell, K.A.; Ford, M.L.; Butler, J.R.; Sidner, R.; Tector, M.; Tector, J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef]
- Ma, D.; Hirose, T.; Lassiter, G.; Sasaki, H.; Rosales, I.; Coe, T.M.; Rickert, C.G.; Matheson, R.; Colvin, R.B.; Qin, W.; et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am. J. Transplant. 2022, 22, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Machaidze, Z.; Wamala, I.; Fraser, J.W.; Navarro-Alvarez, N.; Kim, K.; Schuetz, C.; Shi, S.; Zhu, A.; Hertl, M.; et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation 2014, 21, 454–464. [Google Scholar] [CrossRef]
- Shah, J.A.; Patel, M.S.; Elias, N.; Navarro-Alvarez, N.; Rosales, I.; Wilkinson, R.A.; Louras, N.J.; Hertl, M.; Fishman, J.A.; Colvin, R.B.; et al. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am. J. Transplant. 2017, 17, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.; McCurry, K.R.; Martin, M.J.; McClellan, S.M.; Platt, J.L.; Logan, J.S. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation 1997, 63, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lee, E.M.; Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Hwang, J.I.; Alam, Z.; Ahn, C.; Lee, B.C. Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs. Transgenic Res. 2017, 26, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Candinas, D.; Belliveau, S.; Koyamada, N.; Miyatake, T.; Hechenleitner, P.; Mark, W.; Bach, F.H.; Hancock, W.W. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation 1996, 62, 1920–1927. [Google Scholar] [CrossRef]
- Itescu, S.; Kwiatkowski, P.; Artrip, J.H.; Wang, S.F.; Ankersmit, J.; Minanov, O.P.; Michler, R.E. Role of natural killer cells, macrophages, and accessory molecule interactions in the rejection of pig-to-primate xenografts beyond the hyperacute period. Hum. Immunol. 1998, 59, 275–286. [Google Scholar] [CrossRef]
- Fox, A.; Mountford, J.; Braakhuis, A.; Harrison, L.C. Innate and adaptive immune responses to nonvascular xenografts: Evidence that macrophages are direct effectors of xenograft rejection. J. Immunol. 2001, 166, 2133–2140. [Google Scholar] [CrossRef]
- Yi, S.N.; Hawthorne, W.J.; Lehnert, A.M.; Ha, H.; Wong, J.K.W.; van Rooijen, N.; Davey, K.; Patel, A.T.; Walters, S.N.; Chandra, A.; et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J. Immunol. 2003, 170, 2750–2758. [Google Scholar] [CrossRef]
- Chandra, A.P.; Ouyang, L.; Yi, S.; Wong, J.K.; Ha, H.; Walters, S.N.; Patel, A.T.; Stokes, R.; Jardine, M.; Hawthorne, W.J.; et al. Chemokine and toll-like receptor signaling in macrophage mediated islet xenograft rejection. Xenotransplantation 2007, 14, 48–59. [Google Scholar] [CrossRef]
- Wu, G.S.; Korsgren, O.; Zhang, J.G.; Song, Z.S.; van Rooijen, N.; Tibell, A. Pig islet xenograft rejection is markedly delayed in macrophage-depleted mice: A study in streptozotocin diabetic animals. Xenotransplantation 2000, 7, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Toso, C.; Morel, P.; Bosco, D.; Bucher, P.; Oberholzer, J.; Mathe, Z.; Mai, G.; Wekerle, T.; Berney, T.; et al. Macrophage depletion prolongs discordant but not concordant islet xenograft survival. Transplantation 2005, 79, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Basker, M.; Alwayn, I.P.J.; Buhler, L.; Harper, D.; Abraham, S.; Gray, H.K.; DeAngelis, H.; Awwad, M.; Down, J.; Rieben, R.; et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the pragocytic reticuloendothelial system in baboons. Transplantation 2001, 72, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Buhler, L.; Alwayn, I.P.J.; Basker, M.; Oravec, G.; Thall, A.; White-Scharf, M.E.; Sachs, D.H.; Awwad, M.; Cooper, D.K.C. CD40-CD154 pathway blockade requires host macrophages to induce humoral unresponsieveness to pig hematopoietic cells in baboons. Transplantation 2001, 72, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Gresham, H.D.; Lindberg, F.P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Tena, A.A.; Sachs, D.H.; Mallard, C.; Yang, Y.G.; Tasaki, M.; Farkash, E.; Rosales, I.A.; Colvin, R.B.; Leonard, D.A.; Hawley, R.J. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation 2017, 101, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.Y.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007, 8, 1313–1323. [Google Scholar] [CrossRef]
- Ide, K.; Wang, H.; Tahara, H.; Liu, J.; Wang, X.; Asahara, T.; Sykes, M.; Yang, Y.G.; Ohdan, H. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 5062–5066. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Ide, K.; Wang, Y.; Van Rooijen, N.; Ohdan, H.; Yang, Y.G. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPα capable of binding to human CD47. Cell Transplant. 2011, 20, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Tena, A.; Kurtz, J.; Leonard, D.A.; Dobrinsky, J.R.; Terlouw, S.L.; Mtango, N.; Verstegen, J.; Germana, S.; Mallard, C.; Arn, J.S.; et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am. J. Transplant. 2014, 14, 2713–2722. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Wang, H.; Sun, L.; Fu, Y.; Yang, Y.G. Elimination of donor CD47 protects against vascularized allograft rejection in mice. Xenotransplantation 2019, 26, e12459. [Google Scholar] [CrossRef]
- Watanabe, H.; Sahara, H.; Nomura, S.; Tanabe, T.; Ekanayake-Alper, D.K.; Boyd, L.K.; Louras, N.J.; Asfour, A.; Danton, M.A.; Ho, S.H.; et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation 2018, 25, e12391. [Google Scholar] [CrossRef]
- Yan, J.J.; Koo, T.Y.; Lee, H.S.; Lee, W.B.; Kang, B.; Lee, J.G.; Jang, J.Y.; Fang, T.; Ryu, J.H.; Ahn, C.; et al. Role of Human CD200 Overexpression in Pig-to-Human Xenogeneic Immune Response Compared With Human CD47 Overexpression. Transplantation 2018, 102, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Jiaravuthisan, P.; Maeda, A.; Takakura, C.; Wang, H.T.; Sakai, R.; Shabri, A.M.; Lo, P.C.; Matsuura, R.; Kodama, T.; Eguchi, H.; et al. A membrane-type surfactant protein D (SP-D) suppresses macrophage-mediated cytotoxicity in swine endothelial cells. Transpl. Immunol. 2018, 47, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 2002, 12, 609–615. [Google Scholar] [CrossRef]
- Maeda, A.; Kawamura, T.; Nakahata, K.; Ueno, T.; Usui, N.; Eguchi, H.; Miyagawa, S. Regulation of macrophage-mediated xenocytotoxicity by overexpression of alpha-2,6-sialyltransferase in swine endothelial cells. Transplant. Proc. 2014, 46, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Goodman, J.; Sasaki, H.; Lowell, J.; Mohanakumar, T. Activation of natural killer cells and macrophages by porcine endothelial cells augments specific T-cell xenoresponse. Am. J. Transplant. 2002, 2, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hauzenberger, E.; Klominek, J.; Holgersson, J. Anti-Gal IgG potentiates natural killer cell migration across porcine endothelium via endothelial cell activation and increased natural killer cell motility triggered by CD16 cross-linking. Eur. J. Immunol. 2004, 34, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.X.; Weber, M.; Lechler, R.; Dorling, A. NK-cell-dependent acute xenograft rejection in the mouse heart-to-rat model. Xenotransplantation 2006, 13, 408–414. [Google Scholar] [CrossRef]
- Khalfoun, B.; Barrat, D.; Watier, H.; Machet, M.C.; Arbeille-Brassart, B.; Riess, J.G.; Salmon, H.; Gruel, Y.; Bardos, P.; Lebranchu, Y. Development of an ex vivo model of pig kidney perfused with human lymphocytes. Analysis of xenogeneic cellular reactions. Surgery 2000, 128, 447–457. [Google Scholar] [CrossRef]
- Andre, P.; Biassoni, R.; Colonna, M.; Cosman, D.; Lanier, L.L.; Long, E.O.; Lopez-Botet, M.; Moretta, A.; Moretta, L.; Parham, P.; et al. New nomenclature for MHC receptors. Nat. Immunol. 2001, 2, 661. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, W.H.; Uehara, S.; Chase, C.M.; Colvin, R.B.; Russell, P.S.; Madsen, J.C. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation 2006, 81, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.R.; Vidal, A.C.; Malyguine, A.M. Natural killer cell-endothelial cell interactions in xenotransplantation. Immunol. Res. 2000, 22, 165–176. [Google Scholar] [CrossRef]
- Yin, D.P.; Zeng, H.S.; Ma, L.L.; Shen, J.U.; Xu, H.; Byrne, G.W.; Chong, A.S. Cutting edge: NK cells mediate IgG1-Dependent hyperacute rejection of xenografts. J. Immunol. 2004, 172, 7235–7238. [Google Scholar] [CrossRef] [PubMed]
- Rieben, R.; Seebach, J.D. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005, 26, 2–5. [Google Scholar] [CrossRef]
- Baumann, B.C.; Forte, P.; Hawley, R.J.; Rieben, R.; Schneider, M.K.; Seebach, J.D. Lack of galactose-alpha-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J. Immunol. 2004, 172, 6460–6467. [Google Scholar] [CrossRef]
- Schneider, M.K.; Strasser, M.; Gilli, U.O.; Kocher, M.; Moser, R.; Seebach, J.D. Rolling adhesion of human NK cells to porcine endothelial cells mainly relies on CD49d-CD106 interactions. Transplantation 2002, 73, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, B.G.; Garcia-Borges, C.; Crew, M.D.; Seebach, J.D. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J. Immunol. 2006, 177, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kim, N.; Kim, E.O.; Choi, J.R.; Bluestone, J.A.; Lee, K.M. Suppression of human anti-porcine natural killer cell xenogeneic responses by combinations of monoclonal antibodies specific to CD2 and NKG2D and extracellular signal-regulated kinase kinase inhibitor. Immunology 2010, 130, 545–555. [Google Scholar] [CrossRef]
- Crew, M.D. Play it in E or G: Utilization of HLA-E and -G in xenotransplantation. Xenotransplantation 2007, 14, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Kawamura, T.; Ueno, T.; Usui, N.; Eguchi, H.; Miyagawa, S. The suppression of inflammatory macrophage-mediated cytotoxicity and proinflammatory cytokine production by transgenic expression of HLA-E. Transpl. Immunol. 2013, 29, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Laird, C.T.; Burdorf, L.; French, B.M.; Kubicki, N.; Cheng, X.; Braileanu, G.; Sun, W.; O’Neill, N.A.; Cimeno, A.; Parsell, D.; et al. Transgenic expression of human leukocyte antigen-E attenuates GalKO.hCD46 porcine lung xenograft injury. Xenotransplantation 2017, 24, e12294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, Y.; Lin, Y.; Bullens, D.M.; Rutgeerts, O.; Goebels, J.; Segers, C.; Boon, L.; Kasran, A.; De Vos, R.; et al. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood 2007, 110, 3926–3935. [Google Scholar] [CrossRef]
- Bottino, R.; Knoll, M.F.; Graeme-Wilson, J.; Klein, E.C.; Ayares, D.; Trucco, M.; Cooper, D.K. Safe use of anti-CD154 monoclonal antibody in pig islet xenotransplantation in monkeys. Xenotransplantation 2017, 24, e12283. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Kikuchi, H.; Suzuki, T.; Yamaki, J.; Homma, M.K.; Oshima, Y.; Homma, Y. Prenylated quinolinecarboxylic acid derivative suppresses immune response through inhibition of PAK2. Biochem. Pharmacol. 2016, 105, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.; Maeda, A.; Kodama, T.; Takakura, C.; Yoneyama, T.; Sakai, R.; Noguchi, Y.; Matsuura, R.; Eguchi, H.; Matsunami, K.; et al. The novel immunosuppressant prenylated quinolinecarboxylic acid-18 (PQA-18) suppresses macrophage differentiation and cytotoxicity in xenotransplantation. Immunobiology 2019, 224, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Chuang, Y.H.; Tsai, Y.F.; Yu, H.P. Role of neutrophil extracellular traps following injury. Shock 2014, 41, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, G.M.; Platt, J.L.; Bach, F.H.; Dalmasso, A.P. Neutrophil adhesion to xenogeneic endothelium via iC3b. J. Immunol. 1991, 146, 730–734. [Google Scholar] [PubMed]
- Bastian, F.; Stelzmüller, M.E.; Kratochwill, K.; Kasimir, M.T.; Simon, P.; Weigel, G. IgG deposition and activation of the classical complement pathway involvement in the activation of human granulocytes by decellularized porcine heart valve tissue. Biomaterials 2008, 29, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Ferreira, A.; Mitroulis, I.; Ricklin, D.; Bornstein, S.R.; Waskow, C.; Lambris, J.D.; Chavakis, T. Complement inhibition in a xenogeneic model of interactions between human whole blood and porcine endothelium. Horm. Metab. Res. 2015, 47, 36–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Mohanna, F.; Saleh, S.; Parhar, R.S.; Khabar, K.; Collison, K. Human neutrophil gene expression profiling following xenogeneic encounter with porcine aortic endothelial cells: The occult role of neutrophils in xenograft rejection revealed. J. Leukoc. Biol. 2005, 78, 51–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- al-Mohanna, F.; Collison, K.; Parhar, R.; Kwaasi, A.; Meyer, B.; Saleh, S.; Allen, S.; al-Sedairy, S.; Stern, D.; Yacoub, M. Activation of naive xenogeneic but not allogeneic endothelial cells by human naive neutrophils: A potential occult barrier to xenotransplantation. Am. J. Pathol. 1997, 151, 111–120. [Google Scholar] [PubMed]
- Jackson, D.E.; Ward, C.M.; Wang, R.; Newman, P.J. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J. Biol. Chem. 1997, 272, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Maeda, A.; Sakai, R.; Lo, P.C.; Takakura, C.; Jiaravuthisan, P.; Mod Shabri, A.; Matsuura, R.; Kodama, T.; Hiwatashi, S.; et al. Human CD31 on porcine cells suppress xenogeneic neutrophil-mediated cytotoxicity via the inhibition of NETosis. Xenotransplantation 2018, 25, e12396. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.K.; Ghielmetti, M.; Rhyner, D.M.; Antsiferova, M.A.; Seebach, J.D. Human leukocyte transmigration across Galalpha(1,3)Gal-negative porcine endothelium is regulated by human CD18 and CD99. Transplantation 2009, 87, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Parhar, R.S.; Al-Hejailan, R.S.; Bakheet, R.H.; Khaleel, H.S.; Khalak, H.G.; Halees, A.S.; Zaidi, M.Z.; Meyer, B.F.; Yung, G.P.; et al. Identification of the tetraspanin CD82 as a new barrier to xenotransplantation. J. Immunol. 2013, 191, 2796–2805. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 2006, 84, 712–725. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Toldo, S.; Quader, M.; Salloum, F.N.; Mezzaroma, E.; Abbate, A. Targeting the Innate Immune Response to Improve Cardiac Graft Recovery after Heart Transplantation: Implications for the Donation after Cardiac Death. Int. J. Mol. Sci. 2016, 17, 958. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-kappaB signaling: Balancing life and death—A new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Dessing, M.C.; Schouten, M.; Draing, C.; Levi, M.; von Aulock, S.; van der Poll, T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 2008, 197, 245–252. [Google Scholar] [CrossRef]
- van Zoelen, M.A.; Yang, H.; Florquin, S.; Meijers, J.C.; Akira, S.; Arnold, B.; Nawroth, P.P.; Bierhaus, A.; Tracey, K.J.; van der Poll, T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 2009, 31, 280–284. [Google Scholar] [CrossRef]
- Rusai, K.; Sollinger, D.; Baumann, M.; Wagner, B.; Strobl, M.; Schmaderer, C.; Roos, M.; Kirschning, C.; Heemann, U.; Lutz, J. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr. Nephrol. 2010, 25, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Ro, H.; Lee, E.W.; Hong, J.H.; Han, K.H.; Yeom, H.J.; Kim, H.J.; Kim, M.G.; Jung, H.S.; Oh, K.H.; Park, K.S.; et al. Roles of islet Toll-like receptors in pig to mouse islet xenotransplantation. Cell Transplant. 2013, 22, 1709–1722. [Google Scholar] [CrossRef]
- Benda, B.; Korsgren, O. Interleukin-6 in islet xenograft rejection. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2001, 14, 63–71. [Google Scholar] [CrossRef]
- Tsung, A.; Klune, J.R.; Zhang, X.; Jeyabalan, G.; Cao, Z.; Peng, X.; Stolz, D.B.; Geller, D.A.; Rosengart, M.R.; Billiar, T.R. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007, 204, 2913–2923. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Yang, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Wang, H.; Ochani, M.; Li, J.; Lu, B.; Chavan, S.; Rosas-Ballina, M.; Al-Abed, Y.; et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 2010, 107, 11942–11947. [Google Scholar] [CrossRef]
- Lv, Q.; Li, C.; Mo, Y.; He, L. The role of HMGB1 in heart transplantation. Immunol. Lett. 2018, 194, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef]
- Krüger, B.; Krick, S.; Dhillon, N.; Lerner, S.M.; Ames, S.; Bromberg, J.S.; Lin, M.; Walsh, L.; Vella, J.; Fischereder, M.; et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 3390–3395. [Google Scholar] [CrossRef]
- Li, J.H.; Zhao, B.; Zhu, X.H.; Wang, L.; Zou, H.J.; Chen, S.; Guo, H.; Ruan, Y.L.; Zheng, F.; Xiang, Y.; et al. Blockade of Extracellular HMGB1 Suppresses Xenoreactive B Cell Responses and Delays Acute Vascular Xenogeneic Rejection. Am. J. Transplant. 2015, 15, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef]
- Porrett, P.M.; Orandi, B.J.; Kumar, V.; Houp, J.; Anderson, D.; Cozette Killian, A.; Hauptfeld-Dolejsek, V.; Martin, D.E.; Macedon, S.; Budd, N.; et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 2022, 22, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Rao, A.S.; Murase, N.; Fung, J.; Demetris, A.J. Will xenotransplantation ever be feasible? J. Am. Coll. Surg. 1998, 186, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Bühler, L.; Awwad, M.; Basker, M.; Gojo, S.; Watts, A.; Treter, S.; Nash, K.; Oravec, G.; Chang, Q.; Thall, A.; et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation 2000, 69, 2296–2304. [Google Scholar] [CrossRef]
- Chen, G.; Qian, H.; Starzl, T.; Sun, H.; Garcia, B.; Wang, X.; Wise, Y.; Liu, Y.; Xiang, Y.; Copeman, L.; et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med. 2005, 11, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Wijkstrom, M.; Iwase, H.; Paris, W.; Hara, H.; Ezzelarab, M.; Cooper, D.K. Renal xenotransplantation: Experimental progress and clinical prospects. Kidney Int. 2017, 91, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, X.; Mannon, R.B.; Kirk, A.D. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J. Clin. Investig. 2006, 116, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Koyama, I.; Kawai, T.; Andrews, D.; Boskovic, S.; Nadazdin, O.; Wee, S.L.; Sogawa, H.; Wu, D.L.; Smith, R.N.; Colvin, R.B.; et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation 2004, 77, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.; Magill, M. The Inhibition of CD40/CD154 Costimulatory Signaling in the Prevention of Renal Transplant Rejection in Nonhuman Primates: A Systematic Review and Meta Analysis. Front. Immunol. 2022, 13, 861471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Wakwe, W.; Higginbotham, L.B.; Mathews, D.V.; Breeden, C.P.; Stephenson, A.C.; Jenkins, J.; Strobert, E.; Price, K.; Price, L.; et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am. J. Transplant. 2017, 17, 1182–1192. [Google Scholar] [CrossRef]
- Shock, A.; Burkly, L.; Wakefield, I.; Peters, C.; Garber, E.; Ferrant, J.; Taylor, F.R.; Su, L.; Hsu, Y.M.; Hutto, D.; et al. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: An in vivo study. Arthritis Res. Ther. 2015, 17, 234. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, H.J.; Kang, S.J.; Park, C.G. Novel Immunomodulatory Approaches for Porcine Islet Xenotransplantation. Curr. Diabetes Rep. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Kobayashi, T. Current status of pig kidney xenotransplantation. Int. J. Surg. 2015, 23, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.M.; Reichart, B.; Byrne, G.W.; McGregor, C.G.A. Current status of pig heart xenotransplantation. Int. J. Surg. 2015, 23, 234–239. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Hara, H.; Iwase, H.; Yamamoto, T.; Jagdale, A.; Kumar, V.; Mannon, R.B.; Hanaway, M.J.; Anderson, D.J.; Eckhoff, D.E. Clinical Pig Kidney Xenotransplantation: How Close Are We? J. Am. Soc. Nephrol. 2020, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Dagher, N.N.; Simpkins, C.E.; Locke, J.E.; Singer, A.L.; Segev, D.L.; Zachary, A.A.; Montgomery, R.A. Eculizumab, bortezomib and kidney paired donation facilitate transplantation of a highly sensitized patient without vascular access. Am. J. Transplant. 2010, 10, 2154–2160. [Google Scholar] [CrossRef]
- Walker, S.; Appari, M.; Forbes, S. Considerations and challenges of islet transplantation and future therapies on the horizon. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E109–E117. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Cui, Y.; Patel, D.; Jagdale, A.; Iwase, H.; Ayares, D.; Cooper, D.K.C.; Hara, H. Effect of intravenous immunoglobulin (IVIg) on primate complement-dependent cytotoxicity of genetically engineered pig cells: Relevance to clinical xenotransplantation. Sci. Rep. 2020, 10, 11747. [Google Scholar] [CrossRef]
- Magee, J.C.; Collins, B.H.; Harland, R.C.; Lindman, B.J.; Bollinger, R.R.; Frank, M.M.; Platt, J.L. Immunoglobulin prevents complement-mediated hyperacute rejection in swine-to-primate xenotransplantation. J. Clin. Investig. 1995, 96, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Striz, I. Cytokines of the IL-1 family: Recognized targets in chronic inflammation underrated in organ transplantations. Clin. Sci. 2017, 131, 2241–2256. [Google Scholar] [CrossRef]
- Min, B.H.; Shin, J.S.; Kim, J.M.; Kang, S.J.; Kim, H.J.; Yoon, I.H.; Park, S.K.; Choi, J.W.; Lee, M.S.; Park, C.G. Delayed revascularization of islets after transplantation by IL-6 blockade in pig to non-human primate islet xenotransplantation model. Xenotransplantation 2018, 25, e12374. [Google Scholar] [CrossRef]
- Zhang, G.; Iwase, H.; Li, Q.; Yamamoto, T.; Jagdale, A.; Ezzelarab, M.B.; Ayares, D.; Cooper, D.K.C.; Hara, H.; Wang, G. The Role of Interleukin-6 (IL-6) in the Systemic Inflammatory Response in Xenograft Recipients and in Pig Kidney Xenograft Failure. Front. Immunol. 2021, 12, 788949. [Google Scholar] [CrossRef]
- Burke, G.W.; Ciancio, G.; Sollinger, H.W. Advances in pancreas transplantation. Transplantation 2004, 77, S62–S67. [Google Scholar] [CrossRef]
- Starzl, T.E.; Murase, N.; Abu-Elmagd, K.; Gray, E.A.; Shapiro, R.; Eghtesad, B.; Corry, R.J.; Jordan, M.L.; Fontes, P.; Gayowski, T.; et al. Tolerogenic immunosuppression for organ transplantation. Lancet 2003, 361, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef]
- Wagner, M.; Earley, A.K.; Webster, A.C.; Schmid, C.H.; Balk, E.M.; Uhlig, K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2015, 12, Cd007746. [Google Scholar] [CrossRef] [PubMed]
- Scandling, J.D.; Busque, S.; Lowsky, R.; Shizuru, J.; Shori, A.; Engleman, E.; Jensen, K.; Strober, S. Macrochimerism and clinical transplant tolerance. Hum. Immunol. 2018, 79, 266–271. [Google Scholar] [CrossRef]
- Lee, L.A.; Gritsch, H.A.; Sergio, J.J.; Arn, J.S.; Glaser, R.M.; Sablinski, T.; Sachs, D.H.; Sykes, M. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc. Natl. Acad. Sci. USA 1994, 91, 10864–10867. [Google Scholar] [CrossRef]
- Zhao, Y.; Rodriguez-Barbosa, J.I.; Swenson, K.; Zhao, G.; Arn, J.S.; Sykes, M. Highly disparate xenogeneic skin graft tolerance induction by fetal pig thymus in thymectomized mice: Conditioning requirements and the role of coimplantation of fetal pig liver. Transplantation 2001, 72, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Avalos, A.M.; Mao, S.Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C.; et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e747. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yamaguchi, T.; Hamanaka, S.; Kato-Itoh, M.; Yamazaki, Y.; Ibata, M.; Sato, H.; Lee, Y.S.; Usui, J.; Knisely, A.S.; et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 2010, 142, 787–799. [Google Scholar] [CrossRef]
- Goto, T.; Hara, H.; Sanbo, M.; Masaki, H.; Sato, H.; Yamaguchi, T.; Hochi, S.; Kobayashi, T.; Nakauchi, H.; Hirabayashi, M. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat. Commun. 2019, 10, 451. [Google Scholar] [CrossRef]
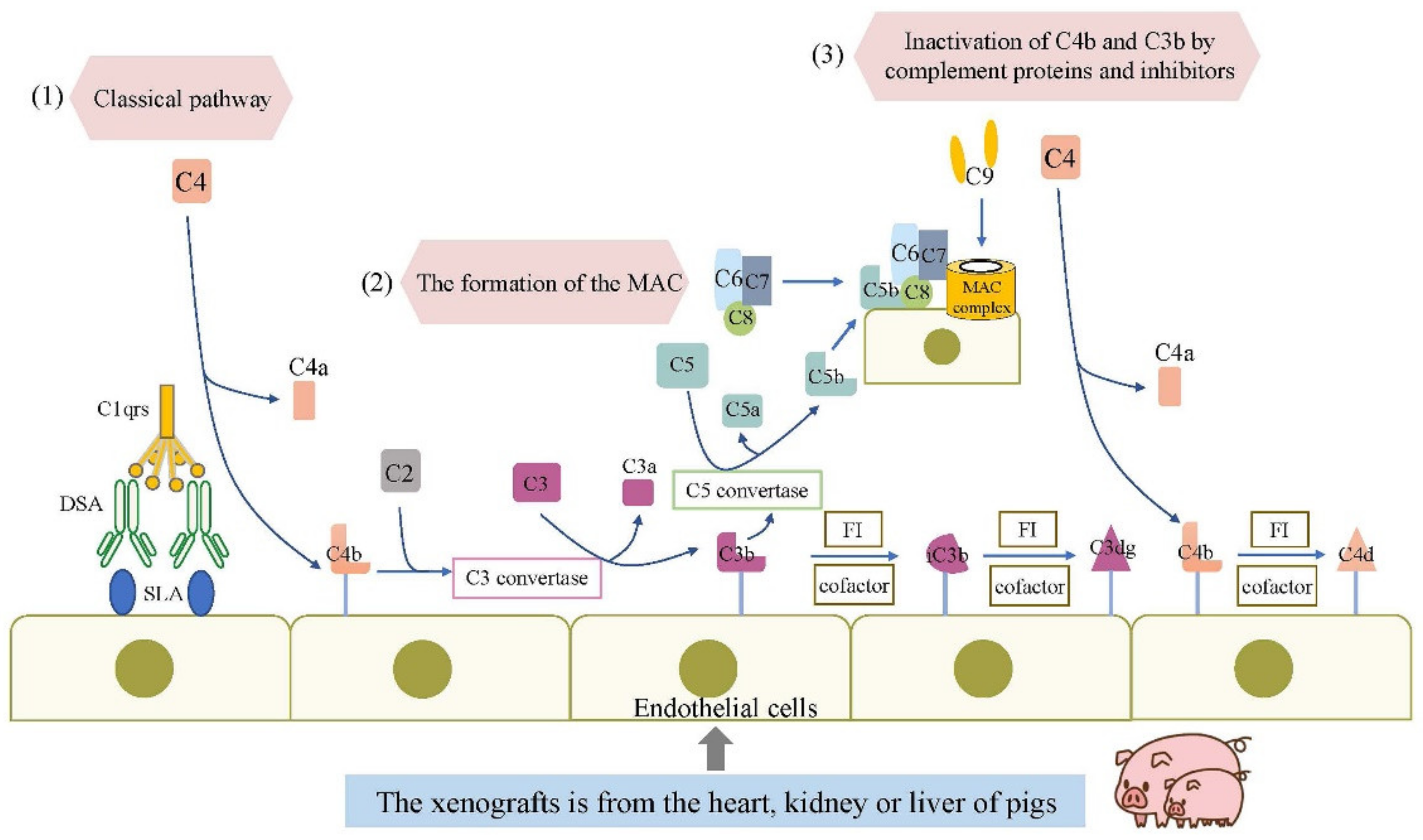
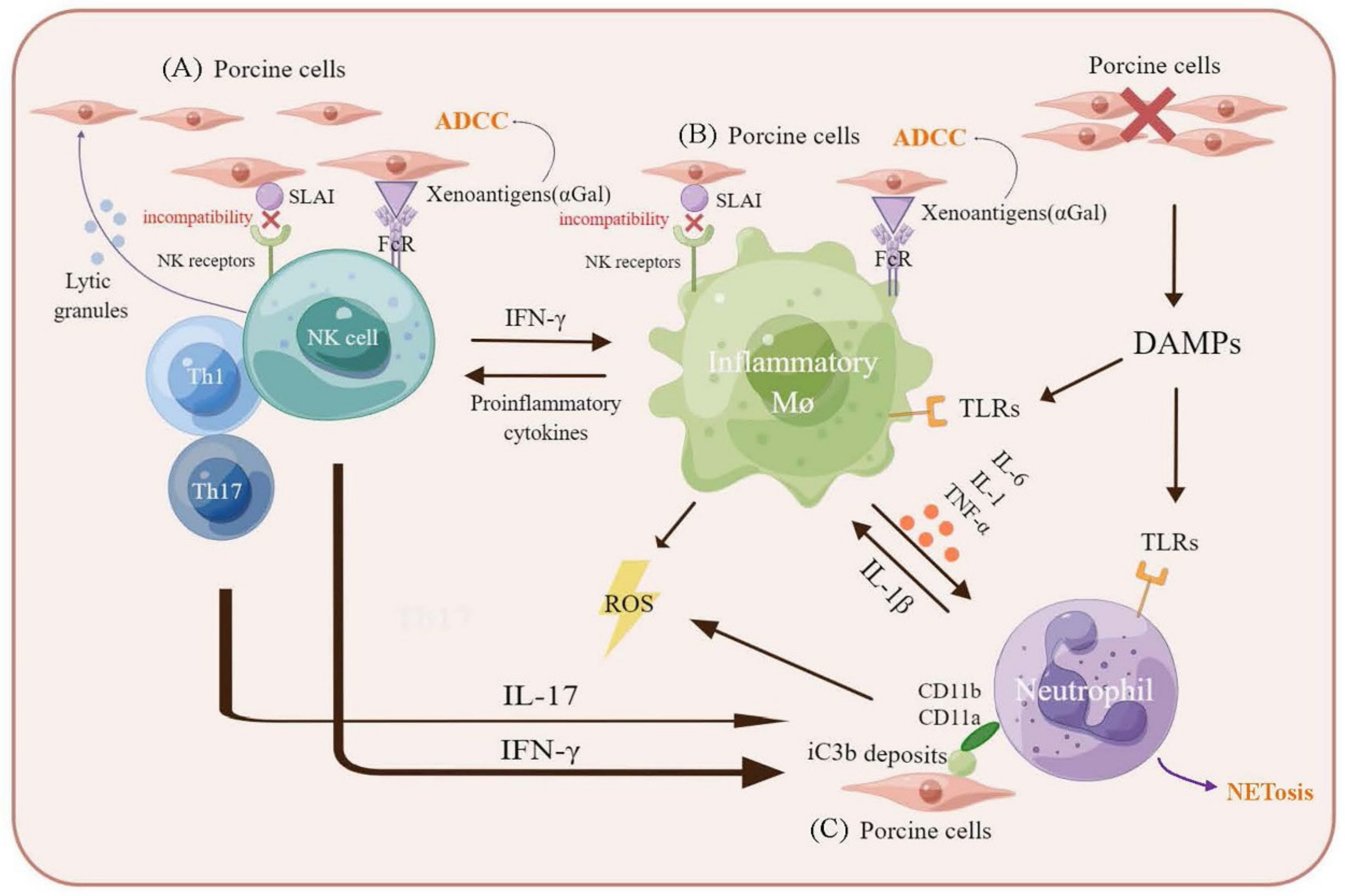

| Immune Response | Component | Rejection Mechanism | Target Gene Editing | Rejection Period |
|---|---|---|---|---|
| Innate | Complement alternative pathway | Inhibits complement activation | factor H | HAR |
| Complement regulatory proteins | Complement regulation | hCD55; hCD46; hCD59 expression | HAR; AVR | |
| Natural antibody | Reduces natural anti- αGal and non-Gal antibodies responses | GGTA1-KO; CMAH-KO; β4GALNT2-KO | HAR; AVR | |
| Platelet/Thrombin | Coagulation/thrombosis reduction | Human Thrombomodulin; human CD39 expression | AVR | |
| Macrophage | Macrophages regulation | hCD47 expression (a marker for “self”); human signal regulatory protein alpha expression; human heme oxygenase 1 expression; human A20 expression; human CD39 expression. | AVR | |
| NK cell | Natural NK cells regulation | human HLA-G, HLA-E expression; human beta2 microglobulin expression | AVR | |
| Neutrophil | Neutrophils regulation | Integrins | AVR |
| Sources | Immunomodulating Agents | Component Mechanism | Pig-to-NHP Xenotransplantation | Reference |
|---|---|---|---|---|
| Under investigation | Anti-CD154 Ab | Blockade of CD40−CD154 | Kidney, heart, islets | [145,146,147] |
| Cobra venom factor | Inhibition of complement system | Islets | [34] | |
| Anti-CD40 Ab | Blockade of CD40−CD154 costimulatory signal | Kidney, heart, islets | [145,147,148] | |
| FDA non-approved, commercially available | Eculizumab | Blockade of the C5b-9 MAC | Pancreas | [149] |
| Etanercept | Anti-TNF-alpha inhibitor | Islets | [150] | |
| Intravenous immunoglobulin | Modulating antigen presenting cell activity and complement activation | Hearts | [151,152] | |
| IL-1 receptor antagonist | IL-1 inhibitor | Heart | [153] | |
| IL-6 receptor antagonist | IL-6 inhibitor | Islet, Kidney, Heart | [154,155] | |
| FDA approved for transplantation | Tacrolimus | Inhibition of the enzyme calcineurin by binding of FKBP-12 | Pancreas | [156,157] |
| Mycophenolic acids | Inhibition of the lymphocyte cycle by blocking inosine monophosphate dehydrogenase | Kidney, heart, islets | [158,159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.-Y.; Xu, X.-L.; Du, X.-G.; Wei, J.-H.; Yu, J.-N.; Deng, S.-L.; Qin, C. Advances in Innate Immunity to Overcome Immune Rejection during Xenotransplantation. Cells 2022, 11, 3865. https://doi.org/10.3390/cells11233865
Lu T-Y, Xu X-L, Du X-G, Wei J-H, Yu J-N, Deng S-L, Qin C. Advances in Innate Immunity to Overcome Immune Rejection during Xenotransplantation. Cells. 2022; 11(23):3865. https://doi.org/10.3390/cells11233865
Chicago/Turabian StyleLu, Tian-Yu, Xue-Ling Xu, Xu-Guang Du, Jin-Hua Wei, Jia-Nan Yu, Shou-Long Deng, and Chuan Qin. 2022. "Advances in Innate Immunity to Overcome Immune Rejection during Xenotransplantation" Cells 11, no. 23: 3865. https://doi.org/10.3390/cells11233865
APA StyleLu, T.-Y., Xu, X.-L., Du, X.-G., Wei, J.-H., Yu, J.-N., Deng, S.-L., & Qin, C. (2022). Advances in Innate Immunity to Overcome Immune Rejection during Xenotransplantation. Cells, 11(23), 3865. https://doi.org/10.3390/cells11233865







