Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders
Abstract
1. Introduction
2. Other Sources of Free Radicals Contribute to OS
2.1. Environmental Factors: Radiation/UV Rays and Pollution
2.2. Lifestyle Related Factors
2.3. Genetic Factors
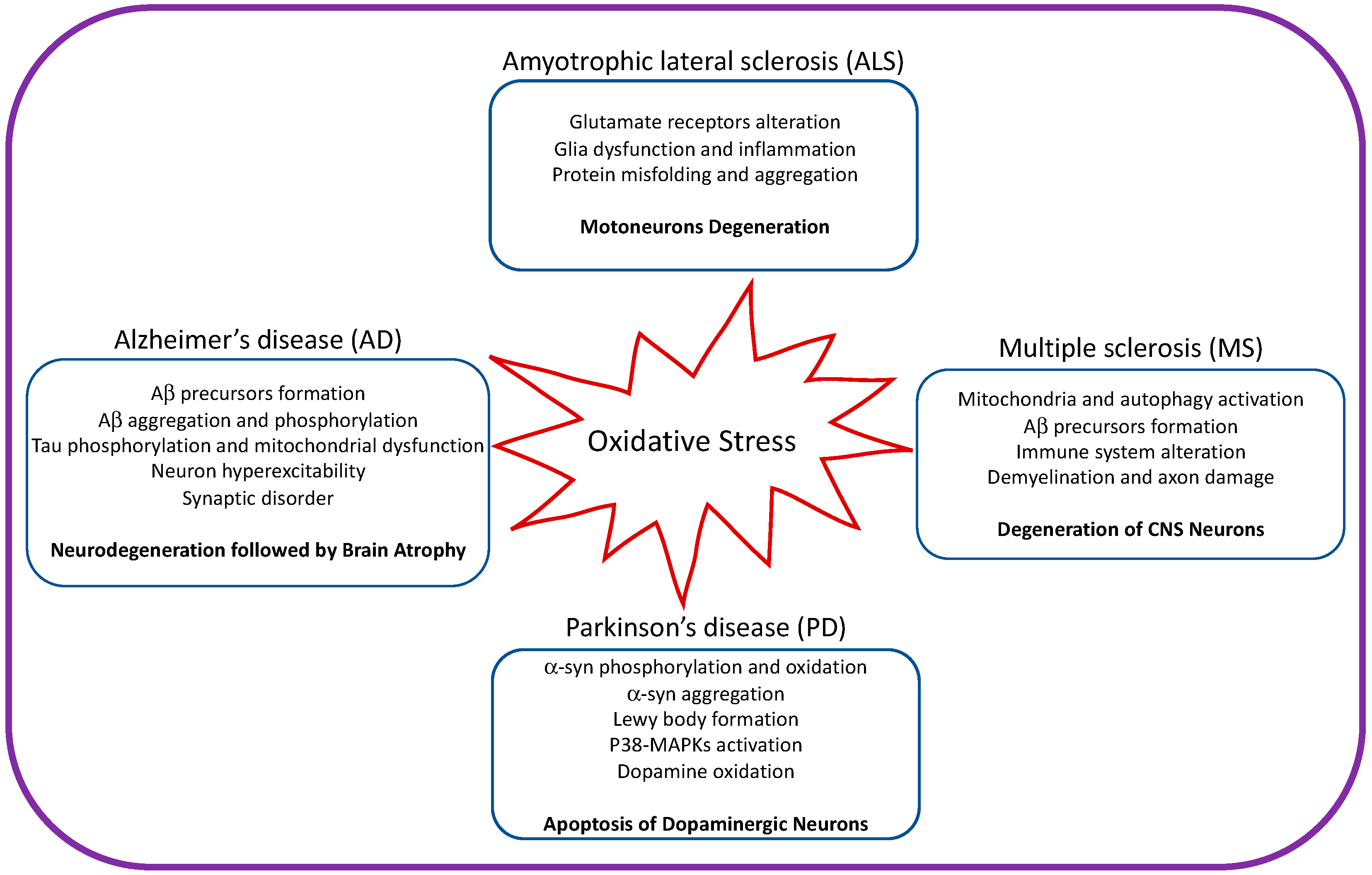
3. Mechanistic Evolution of Neurodegenerative Diseases Caused by OS
4. Major Degenerative Disorders Caused by OS
4.1. OS in Amyotrophic Lateral Sclerosis (ALS)
4.2. OS in Alzheimer’s Disease (AD)
4.3. OS in Parkinson’s Disease (PD)
4.4. OS in Multiple Sclerosis (MS)
5. Significance of Non-Coding RNAs in OS
6. Methods to Measure OS in Neurodegenerative Diseases
6.1. Peripheral Blood OS
6.2. Magnetic Resonance Spectroscopy (MRS)
6.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
6.4. Positron Emission Tomography (PET)
7. Approaches to Slow Aging by Fighting OS
8. Conclusions and Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E., Jr. Mitochondrial Role in Cell Aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The Role of Mitochondria in Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities. Antioxid. Redox Signal. 2019, 30, 1450–1499. [Google Scholar] [CrossRef]
- Zuo, L.; Koozechian, M.S.; Chen, L.L. Characterization of Reactive Nitrogen Species in Allergic Asthma. Ann. Allergy Asthma Immunol. 2014, 112, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and Physiological Role of Reactive Oxygen Species—The Good, the Bad and the Ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Taso, O.V.; Philippou, A.; Moustogiannis, A.; Zevolis, E.; Koutsilieris, M. Lipid Peroxidation Products and Their Role in Neurodegenerative Diseases. Ann. Res. Hosp. 2019, 3, 2. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Lovell, M.A. Biomarkers of Lipid Peroxidation in Alzheimer Disease (AD): An Update. Arch. Toxicol. 2015, 89, 1035–1044. [Google Scholar] [CrossRef]
- Ciamporcero, E.; Daga, M.; Pizzimenti, S.; Roetto, A.; Dianzani, C.; Compagnone, A.; Palmieri, A.; Ullio, C.; Cangemi, L.; Pili, R.; et al. Crosstalk between Nrf2 and YAP Contributes to Maintaining the Antioxidant Potential and Chemoresistance in Bladder Cancer. Free Radic. Biol. Med. 2018, 115, 447–457. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-Nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an Abundant Form of Oxidative DNA Damage, Causes G—T and A—C Substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Antunes dos Santos, A.; Ferrer, B.; Marques Gonçalves, F.; Tsatsakis, A.M.; Renieri, E.A.; Skalny, A.V.; Farina, M.; Rocha, J.B.T.; Aschner, M. Oxidative Stress in Methylmercury-Induced Cell Toxicity. Toxics 2018, 6, 47. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-Mapping Type 2 Diabetes Loci to Single-Variant Resolution Using High-Density Imputation and Islet-Specific Epigenome Maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Jian Li, J.; Gius, D. Metabolic Oxidation/Reduction Reactions and Cellular Responses to Ionizing Radiation: A Unifying Concept in Stress Response Biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Spitz, D.R.; Hauer-Jensen, M. Ionizing Radiation-Induced Responses: Where Free Radical Chemistry Meets Redox Biology and Medicine. Antioxid. Redox Signal. 2014, 20, 1407–1409. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Chen, Y.; Thompson, D.C.; Vasiliou, V. Ultraviolet Radiation: Cellular Antioxidant Response and the Role of Ocular Aldehyde Dehydrogenase Enzymes. Eye Contact Lens 2011, 37, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ściskalska, M.; Zalewska, M.; Grzelak, A.; Milnerowicz, H. The Influence of the Occupational Exposure to Heavy Metals and Tobacco Smoke on the Selected Oxidative Stress Markers in Smelters. Biol. Trace Elem. Res. 2014, 159, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. The Haber-Weiss Cycle—70 Years Later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s Disease. Arch. Neurosci. 2015, 2, e60038. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, Incidence, and Outcome of Non-Alcoholic Fatty Liver Disease in Asia, 1999–2019: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Späte, U.; Kränkel, N.; Christian, S.P.; Linke, A.; Schuler, G.; Hambrecht, R. Nuclear Factor-Kappa B Activation in Skeletal Muscle of Patients with Chronic Heart Failure: Correlation with the Expression of Inducible Nitric Oxide Synthase. Eur. J. Cardiovasc. Prev. Rehabil. 2003, 10, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Role of Nuclear Factor kappaB and Mitogen-Activated Protein Kinase Signaling in Exercise-Induced Antioxidant Enzyme Adaptation. Appl. Physiol. Nutr. Metab. 2007, 32, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox Homeostasis in Sport: Do Athletes Really Need Antioxidant Support? Res. Sport. Med. 2019, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Taegtmeyer, H. Too Much or Not Enough of a Good Thing—The Janus Faces of Autophagy in Cardiac Fuel and Protein Homeostasis. J. Mol. Cell. Cardiol. 2015, 84, 223–226. [Google Scholar] [CrossRef]
- Wu, N.N.; Tian, H.; Chen, P.; Wang, D.; Ren, J.; Zhang, Y. Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells 2019, 8, 1436. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and Antioxidant Balance in the Airways and Airway Diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Salminen, L.E.; Paul, R.H. Oxidative Stress and Genetic Markers of Suboptimal Antioxidant Defense in the Aging Brain: A Theoretical Review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, e8416763. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Górny, M.; Bilska-Wilkosz, A.; Iciek, M.; Hereta, M.; Kamińska, K.; Kamińska, A.; Chwatko, G.; Rogóż, Z.; Lorenc-Koci, E. Alterations in the Antioxidant Enzyme Activities in the Neurodevelopmental Rat Model of Schizophrenia Induced by Glutathione Deficiency during Early Postnatal Life. Antioxidants 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Chiras, D.; Kitsos, G.; Petersen, M.B.; Skalidakis, I.; Kroupis, C. Oxidative Stress in Dry Age-Related Macular Degeneration and Exfoliation Syndrome. Crit. Rev. Clin. Lab. Sci. 2015, 52, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Wize, K.; Prendecki, M.; Lianeri, M.; Kozubski, W.; Dorszewska, J. Genetic Variants and Oxidative Stress in Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 208–223. [Google Scholar] [CrossRef]
- Mailloux, R.J. Teaching the Fundamentals of Electron Transfer Reactions in Mitochondria and the Production and Detection of Reactive Oxygen Species. Redox Biol. 2015, 4, 381–398. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Salacinski, H.J.; Yang, W.; Davidson, B.R.; Seifalian, A.M. The Contemporary Role of Antioxidant Therapy in Attenuating Liver Ischemia-Reperfusion Injury: A Review. Liver Transplant. 2005, 11, 1031–1047. [Google Scholar] [CrossRef]
- Panov, A.V.; Dikalov, S.I. Cardiolipin, Perhydroxyl Radicals, and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxid. Med. Cell. Longev. 2020, 2020, 1323028. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c Oxidase Dysfunction in Oxidative Stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar]
- Jellinger, K.A. Basic Mechanisms of Neurodegeneration: A Critical Update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Neergheen, V.S.; Bahorun, T.; Jen, L.-S. Free Radicals, Antioxidants and Diabetes: Embryopathy, Retinopathy, Neuropathy, Nephropathy and Cardiovascular Complications. Neuroembryol. Aging 2006, 4, 117–137. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ingre, C.; Roos, P.M.; Piehl, F.; Kamel, F.; Fang, F. Risk Factors for Amyotrophic Lateral Sclerosis. Clin. Epidemiol. 2015, 7, 181–193. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Carrì, M.T.; Valle, C.; Bozzo, F.; Cozzolino, M. Oxidative Stress and Mitochondrial Damage: Importance in Non-SOD1 ALS. Front. Cell. Neurosci. 2015, 9, 41. [Google Scholar] [CrossRef]
- Agar, J.; Durham, H. Relevance of Oxidative Injury in the Pathogenesis of Motor Neuron Diseases. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2003, 4, 232–242. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Kraft, A.D.; Resch, J.M.; Johnson, D.A.; Johnson, J.A. Activation of the Nrf2–ARE Pathway in Muscle and Spinal Cord during ALS-like Pathology in Mice Expressing Mutant SOD1. Exp. Neurol. 2007, 207, 107–117. [Google Scholar] [CrossRef]
- Velde, C.V.; McDonald, K.K.; Boukhedimi, Y.; McAlonis-Downes, M.; Lobsiger, C.S.; Hadj, S.B.; Zandona, A.; Julien, J.-P.; Shah, S.B.; Cleveland, D.W. Misfolded SOD1 Associated with Motor Neuron Mitochondria Alters Mitochondrial Shape and Distribution Prior to Clinical Onset. PLoS ONE 2011, 6, e22031. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.N.; Kumar, A.; Chandra, R.; Puri, S.K.; Singh, R.L.; Kalita, J.; Misra, U.K. Oxidant–Antioxidant Imbalance in the Erythrocytes of Sporadic Amyotrophic Lateral Sclerosis Patients Correlates with the Progression of Disease. Neurochem. Int. 2008, 52, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Keon, M.; Musrie, B.; Dinger, M.; Brennan, S.E.; Santos, J.; Saksena, N.K. Destination Amyotrophic Lateral Sclerosis. Front. Neurol. 2021, 12, 596006. [Google Scholar] [CrossRef]
- Anderson, C.J.; Bredvik, K.; Burstein, S.R.; Davis, C.; Meadows, S.M.; Dash, J.; Case, L.; Milner, T.A.; Kawamata, H.; Zuberi, A.; et al. ALS/FTD Mutant CHCHD10 Mice Reveal a Tissue-Specific Toxic Gain-of-Function and Mitochondrial Stress Response. Acta Neuropathol. 2019, 138, 103–121. [Google Scholar] [CrossRef]
- Bannwarth, S.; Ait-El-Mkadem, S.; Chaussenot, A.; Genin, E.C.; Lacas-Gervais, S.; Fragaki, K.; Berg-Alonso, L.; Kageyama, Y.; Serre, V.; Moore, D.G.; et al. A Mitochondrial Origin for Frontotemporal Dementia and Amyotrophic Lateral Sclerosis through CHCHD10 Involvement. Brain 2014, 137, 2329–2345. [Google Scholar] [CrossRef]
- Genin, E.C.; Bannwarth, S.; Ropert, B.; Lespinasse, F.; Mauri-Crouzet, A.; Augé, G.; Fragaki, K.; Cochaud, C.; Donnarumma, E.; Lacas-Gervais, S.; et al. CHCHD10 and SLP2 Control the Stability of the PHB Complex: A Key Factor for Motor Neuron Viability. Brain 2022, 145, 3415–3430. [Google Scholar] [CrossRef] [PubMed]
- Fontana, I.C.; Zimmer, A.R.; Rocha, A.S.; Gosmann, G.; Souza, D.O.; Lourenco, M.V.; Ferreira, S.T.; Zimmer, E.R. Amyloid-β Oligomers in Cellular Models of Alzheimer’s Disease. J. Neurochem. 2020, 155, 348–369. [Google Scholar] [CrossRef]
- Fernandez-Perez, E.J.; Peters, C.; Aguayo, L.G. Membrane Damage Induced by Amyloid Beta and a Potential Link with Neuroinflammation. Curr. Pharm. Des. 2016, 22, 1295–1304. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Lira-De León, K.I.; Campos-Peña, V.; De Anda-Hernández, M.A.; Mena-López, R. Tau Oligomers and Aggregation in Alzheimer’s Disease. J. Neurochem. 2010, 112, 1353–1367. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Li, P.; Wei, N.; Zhao, Z.; Liang, H.; Ji, X.; Chen, W.; Xue, M.; Wei, J. The Ambiguous Relationship of Oxidative Stress, Tau Hyperphosphorylation, and Autophagy Dysfunction in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, e352723. [Google Scholar] [CrossRef] [PubMed]
- Rojas Quijano, F.A.; Morrow, D.; Wise, B.M.; Brancia, F.L.; Goux, W.J. Prediction of Nucleating Sequences from Amyloidogenic Propensities of Tau-Related Peptides. Biochemistry 2006, 45, 4638–4652. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Tau Protein and the Neurofibrillary Pathology of Alzheimer’s Disease. Trends Neurosci. 1993, 16, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.L.; Espinoza, M.; Kress, Y.; Davies, P. Conformational Change as One of the Earliest Alterations of Tau in Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 719–727. [Google Scholar] [CrossRef]
- Grabowski, T.J.; Cho, H.S.; Vonsattel, J.P.G.; Rebeck, G.W.; Greenberg, S.M. Novel Amyloid Precursor Protein Mutation in an Iowa Family with Dementia and Severe Cerebral Amyloid Angiopathy. Ann. Neurol. 2001, 49, 697–705. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for Neurodegenerative Diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories from 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Chen, K.; Quiroz, Y.T.; Jakimovich, L.J.; Gomez, M.G.; Langois, C.M.; Langbaum, J.B.; Ayutyanont, N.; Roontiva, A.; Thiyyagura, P. Florbetapir PET Analysis of Amyloid-β Deposition in the Presenilin 1 E280A Autosomal Dominant Alzheimer’s Disease Kindred: A Cross-Sectional Study. Lancet Neurol. 2012, 11, 1057–1065. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Herholz, K. Amyloid Imaging for Dementia in Clinical Practice. BMC Med. 2015, 13, 163. [Google Scholar] [CrossRef]
- Gunasingh Masilamoni, J.; Philip Jesudason, E.; Dhandayuthapani, S.; Ashok, B.S.; Vignesh, S.; Jebaraj, W.C.E.; Paul, S.F.; Jayakumar, R. The Neuroprotective Role of Melatonin against Amyloid β Peptide Injected Mice. Free Radic. Res. 2008, 42, 661–673. [Google Scholar] [CrossRef]
- Smith, D.G.; Cappai, R.; Barnham, K.J. The Redox Chemistry of the Alzheimer’s Disease Amyloid Beta Peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional Changes of the Basal Ganglia Circuitry in Parkinson’s Disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Weiler, M.; Filippi, M. Propagation of Pathology through Brain Networks in Neurodegenerative Diseases: From Molecules to Clinical Phenotypes. CNS Neurosci. Ther. 2015, 21, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Rensburg, Z.; Abrahams, S.; Bardien, S.; Kenyon, C. Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson’s Disease and Intervention with Turmeric. Mol. Neurobiol. 2021, 58, 5920–5936. Available online: https://link.springer.com/article/10.1007/s12035-021-02516-5 (accessed on 3 January 2023). [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative Stress in Parkinson’s Disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Fahn, S.; Cohen, G. The Oxidant Stress Hypothesis in Parkinson’s Disease: Evidence Supporting It. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef]
- Delcambre, S.; Nonnenmacher, Y.; Hiller, K. Dopamine Metabolism and Reactive Oxygen Species Production. In Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease; Buhlman, L.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–47. ISBN 978-3-319-42139-1. [Google Scholar]
- Nunomura, A.; Moreira, P.I.; Castellani, R.J.; Lee, H.; Zhu, X.; Smith, M.A.; Perry, G. Oxidative Damage to RNA in Aging and Neurodegenerative Disorders. Neurotox. Res. 2012, 22, 231–248. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent Advances: Molecular Mechanism of RNA Oxidation and Its Role in Various Diseases. Front. Mol. Biosci. 2020, 7, 184. [Google Scholar] [CrossRef]
- Jellinger, K.A. Recent Advances in Our Understanding of Neurodegeneration. J. Neural Transm. 2009, 116, 1111–1162. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxid. Med. Cell. Longev. 2020, 2020, e7191080. [Google Scholar] [CrossRef] [PubMed]
- Compston, A. Genetic Epidemiology of Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 1997, 62, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Galiniak, S.; Żyracka, E.; Grzesik, M.; Naparło, K.; Sowa, P.; Bartosz, G.; Sadowska-Bartosz, I. Oxidative Modification of Blood Serum Proteins in Multiple Sclerosis after Interferon Beta and Melatonin Treatment. Oxid. Med. Cell. Longev. 2017, 2017, e7905148. [Google Scholar] [CrossRef] [PubMed]
- Hernangómez, M.; Carrillo-Salinas, F.J.; Mecha, M.; Correa, F.; Mestre, L.; Loría, F.; Feliú, A.; Docagne, F.; Guaza, C. Brain Innate Immunity in the Regulation of Neuroinflammation: Therapeutic Strategies by Modulating CD200-CD200R Interaction Involve the Cannabinoid System. Curr. Pharm. Des. 2014, 20, 4707–4722. [Google Scholar] [CrossRef]
- Nair, A.; Frederick, T.J.; Miller, S.D. Astrocytes in Multiple Sclerosis: A Product of Their Environment. Cell. Mol. Life Sci. 2008, 65, 2702–2720. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Yenari, M.A. Inflammatory Responses in Brain Ischemia. Curr. Med. Chem. 2015, 22, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Degan, D.; Ornello, R.; Tiseo, C.; Carolei, A.; Sacco, S.; Pistoia, F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018, 24, 1485–1501. [Google Scholar] [CrossRef]
- Kasschau, M.; Sherman, K.; Haider, L.; Frontario, A.; Shaw, M.; Datta, A.; Bikson, M.; Charvet, L. A Protocol for the Use of Remotely-Supervised Transcranial Direct Current Stimulation (TDCS) in Multiple Sclerosis (MS). J. Vis. Exp. 2015, 106, e53542. [Google Scholar]
- Gitik, M.; Liraz-Zaltsman, S.; Oldenborg, P.-A.; Reichert, F.; Rotshenker, S. Myelin Down-Regulates Myelin Phagocytosis by Microglia and Macrophages through Interactions between CD47 on Myelin and SIRPα (Signal Regulatory Protein-α) on Phagocytes. J. Neuroinflamm. 2011, 8, 24. [Google Scholar] [CrossRef]
- Perry, V.H. Contribution of Systemic Inflammation to Chronic Neurodegeneration. Acta Neuropathol. 2010, 120, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.M.; Miller, S.D. Cytokine Control of Inflammation and Repair in the Pathology of Multiple Sclerosis. Yale J. Biol. Med. 2012, 85, 447. [Google Scholar] [PubMed]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS Immune Privilege: Hiding in Plain Sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Holman, D.W.; Klein, R.S.; Ransohoff, R.M. The Blood–Brain Barrier, Chemokines and Multiple Sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Song, H.; Küpfer, P.A.; Leumann, C.J.; Sonntag, W.E. An Assay for RNA Oxidation Induced Abasic Sites Using the Aldehyde Reactive Probe. Free Radic. Res. 2011, 45, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; Verma, A.; Zhang, J.; Snyder, S.H. Poly (ADP-Ribose) Polymerase, Nitric Oxide and Cell Death. Trends Pharmacol. Sci. 1999, 20, 171–181. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G. RNA and Oxidative Stress in Alzheimer’s Disease: Focus on MicroRNAs. Oxid. Med. Cell. Longev. 2020, 2020, e2638130. [Google Scholar] [CrossRef]
- Moreira, P.I.; Nunomura, A.; Nakamura, M.; Takeda, A.; Shenk, J.C.; Aliev, G.; Smith, M.A.; Perry, G. Nucleic Acid Oxidation in Alzheimer Disease. Free Radic. Biol. Med. 2008, 44, 1493–1505. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef]
- Johnson, R.; Noble, W.; Tartaglia, G.G.; Buckley, N.J. Neurodegeneration as an RNA Disorder. Prog. Neurobiol. 2012, 99, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; De Strooper, B. Noncoding RNAs in Neurodegeneration. Nat. Rev. Neurosci. 2017, 18, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.F.; Ogawa, K.; Beck, G.; Ikenaka, K.; Takeuchi, E.; Yasumizu, Y.; Jinno, J.; Kimura, Y.; Baba, K.; Nagai, Y.; et al. PiRNA/PIWI Protein Complex as a Potential Biomarker in Sporadic Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Chen, J.; Tao, Z.; Ren, J. Non-Coding RNAs: New Players in Mitophagy and Neurodegeneration. Neurochem. Int. 2022, 152, 105253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Liu, Y.; Liu, J.; Gong, X.; Li, G.; Tang, M. Crosstalk between Regulatory Non-Coding RNAs and Oxidative Stress in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 975248. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [PubMed]
- Cervinkova, B.; Krcmova, L.K.; Sestakova, V.; Solichova, D.; Solich, P. A Fully Validated Bioanalytical Method Using an UHPLC–MS/MS System for Quantification of DNA and RNA Oxidative Stress Biomarkers. Anal Bioanal. Chem. 2017, 409, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Duszenko, M.; Gospodaryov, D.V.; Garaschuk, O. Oxidative Stress and Energy Metabolism in the Brain: Midlife as a Turning Point. Antioxidants 2021, 10, 1715. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L. The Significance of 8-OxoGsn in Aging-Related Diseases. Aging Dis. 2020, 11, 1329–1338. [Google Scholar] [CrossRef]
- Yan, L.L.; Simms, C.L.; McLoughlin, F.; Vierstra, R.D.; Zaher, H.S. Oxidation and Alkylation Stresses Activate Ribosome-Quality Control. Nat. Commun. 2019, 10, 5611. [Google Scholar] [CrossRef]
- Je, G.; Kim, Y.-S. Mitochondrial ROS-Mediated Post-Transcriptional Regulation of α-Synuclein through MiR-7 and MiR-153. Neurosci. Lett. 2017, 661, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Tang, J.; Su, Q.; Chou, W.-C.; Zheng, F.; Guo, Z.; Yu, G.; Shao, W.; Li, H.; Wu, S. Paraquat-Induced Oxidative Stress Regulates N6-Methyladenosine (M6A) Modification of Circular RNAs. Environ. Pollut. 2021, 290, 117816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, L.; Liu, N.; Luo, X.; He, Z. Mir-141-3p Regulates Apoptosis and Mitochondrial Membrane Potential via Targeting Sirtuin1 in a 1-Methyl-4-Phenylpyridinium In Vitro Model of Parkinson’s Disease. BioMed Res. Int. 2020, 2020, e7239895. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Yan, Z.; Zhu, H.; Zhao, H. MiR-410 Exerts Neuroprotective Effects in a Cellular Model of Parkinson’s Disease Induced by 6-Hydroxydopamine via Inhibiting the PTEN/AKT/MTOR Signaling Pathway. Exp. Mol. Pathol. 2019, 109, 16–24. [Google Scholar] [CrossRef]
- Li, X.; Si, W.; Li, Z.; Tian, Y.; Liu, X.; Ye, S.; Huang, Z.; Ji, Y.; Zhao, C.; Hao, X.; et al. MiR-335 Promotes Ferroptosis by Targeting Ferritin Heavy Chain 1 in In Vivo and In Vitro Models of Parkinson’s Disease. Int. J. Mol. Med. 2021, 47, 61. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Zhou, Z.; Zhou, Q.; Sun, S.; Jin, Z.; Xi, Z.; Wei, J. Downregulation of LncRNA BACE1-AS Improves Dopamine-Dependent Oxidative Stress in Rats with Parkinson’s Disease by Upregulating MicroRNA-34b-5p and Downregulating BACE1. Cell Cycle 2020, 19, 1158–1171. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zhou, C.; Zhu, R.; Xiao, X.; Zhou, B.; Wan, D. LncRNA MiR-17-92a-1 Cluster Host Gene (MIR17HG) Promotes Neuronal Damage and Microglial Activation by Targeting the MicroRNA-153-3p/Alpha-Synuclein Axis in Parkinson’s Disease. Bioengineered 2022, 13, 4493–4516. [Google Scholar] [CrossRef]
- Xie, N.; Qi, J.; Li, S.; Deng, J.; Chen, Y.; Lian, Y. Upregulated LncRNA Small Nucleolar RNA Host Gene 1 Promotes 1-Methyl-4-Phenylpyridinium Ion-Induced Cytotoxicity and Reactive Oxygen Species Production through MiR-15b-5p/GSK3β Axis in Human Dopaminergic SH-SY5Y Cells. J. Cell. Biochem. 2019, 120, 5790–5801. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Oxidative Stress-Related Biomarkers in Essential Hypertension and Ischemia-Reperfusion Myocardial Damage. Dis. Mark. 2013, 35, 773–790. [Google Scholar] [CrossRef]
- Cherubini, A.; Ruggiero, C.; Polidori, M.C.; Mecocci, P. Potential Markers of Oxidative Stress in Stroke. Free Radic. Biol. Med. 2005, 39, 841–852. [Google Scholar] [CrossRef]
- Boskovic, M.; Vovk, T.; Kores Plesnicar, B.; Grabnar, I. Oxidative Stress in Schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-Glutathione Peroxidase, Catalase, and Cu/Zn-SOD for Cell Survival against Oxidative Stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lane, H.-Y. Plasma Glutathione Levels Decreased with Cognitive Decline among People with Mild Cognitive Impairment (MCI): A Two-Year Prospective Study. Antioxidants 2021, 10, 1839. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Polidori, A.; Arhami, M.; Gillen, D.L.; Kleinman, M.T.; Vaziri, N.D.; Longhurst, J.; Zaldivar, F. Circulating Biomarkers of Inflammation, Antioxidant Activity, and Platelet Activation Are Associated with Primary Combustion Aerosols in Subjects with Coronary Artery Disease. Environ. Health Perspect. 2008, 116, 898–906. [Google Scholar] [CrossRef]
- Sussulini, A.; Hauser-Davis, R.A. Metallomics Applied to the Study of Neurodegenerative and Mental Diseases. In Metallomics: The Science of Biometals; Springer: Berlin/Heidelberg, Germany, 2018; pp. 21–37. [Google Scholar]
- Crotty, G.F.; Ascherio, A.; Schwarzschild, M.A. Targeting Urate to Reduce Oxidative Stress in Parkinson Disease. Exp. Neurol. 2017, 298, 210–224. [Google Scholar] [CrossRef]
- Das, T.K.; Javadzadeh, A.; Dey, A.; Sabesan, P.; Theberge, J.; Radua, J.; Palaniyappan, L. Antioxidant Defense in Schizophrenia and Bipolar Disorder: A Meta-Analysis of MRS Studies of Anterior Cingulate Glutathione. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 94–102. [Google Scholar] [CrossRef]
- Shukla, D.; Mandal, P.K.; Tripathi, M.; Vishwakarma, G.; Mishra, R.; Sandal, K. Quantitation of In Vivo Brain Glutathione Conformers in Cingulate Cortex among Age-Matched Control, MCI, and AD Patients Using MEGA-PRESS. Hum. Brain Mapp. 2020, 41, 194–217. [Google Scholar] [CrossRef]
- Mandal, P.K.; Saharan, S.; Tripathi, M.; Murari, G. Brain Glutathione Levels–a Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease. Biol. Psychiatry 2015, 78, 702–710. [Google Scholar] [CrossRef]
- Shih, Y.-Y.; Büchert, M.; Chung, H.-W.; Hennig, J.; von Elverfeldt, D. Vitamin C Estimation with Standard 1H Spectroscopy Using a Clinical 3T MR System: Detectability and Reliability within the Human Brain. J. Magn. Reson. Imaging 2008, 28, 351–358. [Google Scholar] [CrossRef]
- Terpstra, M.; Gruetter, R. 1H NMR Detection of Vitamin C in Human Brain In Vivo. Magn. Reson. Med. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Sepkhanova, I.; Drescher, M.; Meeuwenoord, N.J.; Limpens, R.W.; Koning, R.I.; Filippov, D.V.; Huber, M. Monitoring Alzheimer Amyloid Peptide Aggregation by EPR. Appl. Magn. Reson. 2009, 36, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, G.; Komsiyska, D.; Karamalakova, Y.; Petkov, Y.; Ivanov, V.; Manolova, T.; Gadjeva, V. Real Time Oxidative Stress Markers of Patients with Post-Stroke Depression: EPR Study. Bulg. Chem. Commun. 2018, 50, 64–68. [Google Scholar]
- Ikawa, M.; Okazawa, H.; Kudo, T.; Kuriyama, M.; Fujibayashi, Y.; Yoneda, M. Evaluation of Striatal Oxidative Stress in Patients with Parkinson’s Disease Using [62Cu]ATSM PET. Nucl. Med. Biol. 2011, 38, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Okazawa, H.; Tsujikawa, T.; Matsunaga, A.; Yamamura, O.; Mori, T.; Hamano, T.; Kiyono, Y.; Nakamoto, Y.; Yoneda, M. Increased Oxidative Stress Is Related to Disease Severity in the ALS Motor Cortex: A PET Study. Neurology 2015, 84, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Saleh, M.G.; Edden, R.A. Edited 1H Magnetic Resonance Spectroscopy In Vivo: Methods and Metabolites. Magn. Reson. Med. 2017, 77, 1377–1389. [Google Scholar] [CrossRef]
- Matsuzawa, D.; Hashimoto, K. Magnetic Resonance Spectroscopy Study of the Antioxidant Defense System in Schizophrenia. Antioxid. Redox Signal. 2011, 15, 2057–2065. [Google Scholar] [CrossRef]
- Yahya, A. Metabolite Detection by Proton Magnetic Resonance Spectroscopy Using PRESS. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 183–198. [Google Scholar] [CrossRef]
- Johnson, C.C.; Guy, A.W. Nonionizing Electromagnetic Wave Effects in Biological Materials and Systems. Proc. IEEE 1972, 60, 692–718. [Google Scholar] [CrossRef]
- Samuni, A.; Carmichael, A.J.; Russo, A.; Mitchell, J.B.; Riesz, P. On the Spin Trapping and ESR Detection of Oxygen-Derived Radicals Generated inside Cells. Proc. Natl. Acad. Sci. USA 1986, 83, 7593–7597. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection and Characterisation of Radicals in Biological Materials Using EPR Methodology. Biochim. Et Biophys. Acta Gen. Subj. 2014, 1840, 708–721. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a Standardized ROS Production Profile in Humans by Electron Paramagnetic Resonance. Oxid. Med. Cell. Longev. 2012, 2012, e973927. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.-R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The Links between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer’s Disease. Front. Cell. Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and Probiotics Attenuate the Development of Alzheimer’s Disease in Transgenic Mice: Role of Microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial Effects of Exercise on Gut Microbiota Functionality and Barrier Integrity, and Gut-Liver Crosstalk in an In Vivo Model of Early Obesity and Non-Alcoholic Fatty Liver Disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Yahaya, M.F.; Tooyama, I.; Damanhuri, H.A. A Mechanistic Evaluation of Antioxidant Nutraceuticals on Their Potential against Age-Associated Neurodegenerative Diseases. Antioxidants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Khan, M.S.; Muhammad, T.; Ikram, M.; Kim, M.O. Dietary Supplementation of the Antioxidant Curcumin Halts Systemic LPS-Induced Neuroinflammation-Associated Neurodegeneration and Memory/Synaptic Impairment via the JNK/NF-κB/Akt Signaling Pathway in Adult Rats. Oxid. Med. Cell. Longev. 2019, 2019, e7860650. [Google Scholar] [CrossRef]
- Dong, H.; Xu, L.; Wu, L.; Wang, X.; Duan, W.; Li, H.; Li, C. Curcumin Abolishes Mutant TDP-43 Induced Excitability in a Motoneuron-like Cellular Model of ALS. Neuroscience 2014, 272, 141–153. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Liang, Q.; Sun, Q.; Chen, C.; Zhang, Y.; Ding, Y.; Zhou, P. Metal Chelator EGCG Attenuates Fe(III)-Induced Conformational Transition of α-Synuclein and Protects AS-PC12 Cells against Fe(III)-Induced Death. J. Neurochem. 2017, 143, 136–146. [Google Scholar] [CrossRef]
- Wan Nasri, W.N.; Makpol, S.; Mazlan, M.; Tooyama, I.; Wan Ngah, W.Z.; Damanhuri, H.A. Tocotrienol Rich Fraction Supplementation Modulate Brain Hippocampal Gene Expression in APPswe/PS1dE9 Alzheimer’s Disease Mouse Model. J. Alzheimer’s Dis. 2019, 70, S239–S254. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tu, Y.; Jia, X.; Fang, K.; Liu, L.; Wan, L.; Xiang, C.; Wang, Y.; Sun, X.; Liu, T.; et al. Resveratrol Protects Against Pulmonary Arterial Hypertension in Rats via Activation of Silent Information Regulator 1. Cell. Physiol. Biochem. 2017, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Vitamin E and Oxidative Stress. Free Radic. Biol. Med. 1991, 11, 215–232. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Blumberg, J.B. Role of Vitamin E and Oxidative Stress in Exercise. Nutrition 2001, 17, 809–814. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Sample | Method | Disease Detected | References |
|---|---|---|---|---|
| LPO, GSH, GPx, SOD and vitamins C and E. | Peripheral blood | Biochemical analysis (Blood screening) | AD, PD | [133,134,135,136,137,138,139,140] |
| GSH and Vitamin C. using 1H MRS | Brain | 1H Magnetic Resonance Spectroscopy (MRS) | AD, MCI | [141,142,143,144] |
| Intracellular over-reductive state using redox sensitive probes | Brain | Electron Paramagnetic Resonance (EPR) | AD, Depressive disorders in post-stroke patients | [145,146] |
| Intracellular over-reductive state using Radiotracer. | Brain | Positron Emission Tomography(PET) | PD, ALS | [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, R.F.; Nagano, S. Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells 2023, 12, 753. https://doi.org/10.3390/cells12050753
Abdelhamid RF, Nagano S. Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells. 2023; 12(5):753. https://doi.org/10.3390/cells12050753
Chicago/Turabian StyleAbdelhamid, Rehab F., and Seiichi Nagano. 2023. "Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders" Cells 12, no. 5: 753. https://doi.org/10.3390/cells12050753
APA StyleAbdelhamid, R. F., & Nagano, S. (2023). Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells, 12(5), 753. https://doi.org/10.3390/cells12050753







