A Novel Technique of Amniotic Membrane Preparation Mimicking Limbal Epithelial Crypts Enhances the Number of Progenitor Cells upon Expansion
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Amniotic Membrane (HAM)
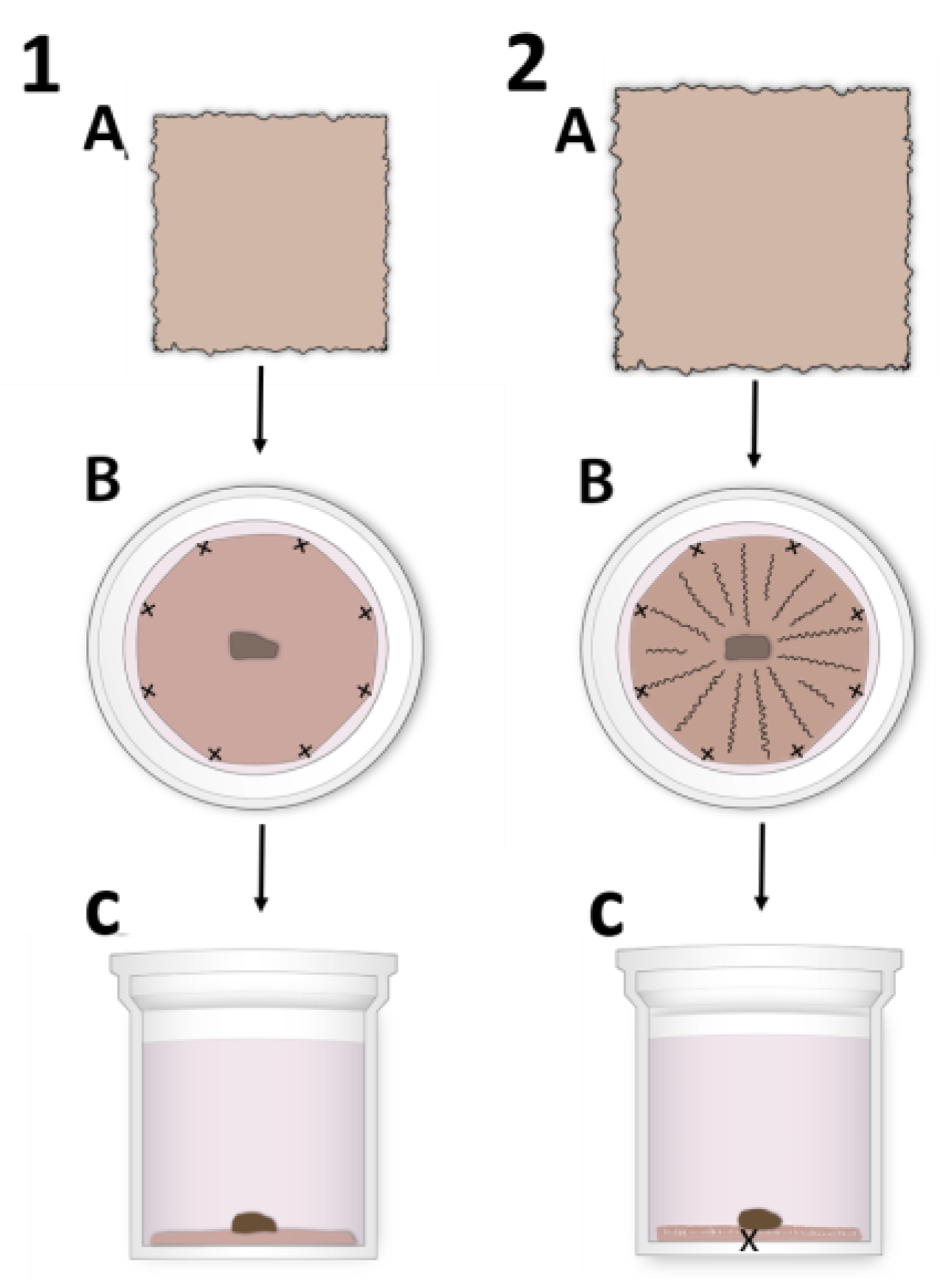
2.2. HAM Preparation for hLESC Expansion and Cultivation
2.3. Limbal Biopsies and Human LESC Harvesting
2.4. Immunohistochemistry (IHC) and Immunofluorescence Microscopy
2.5. Statistical Analysis
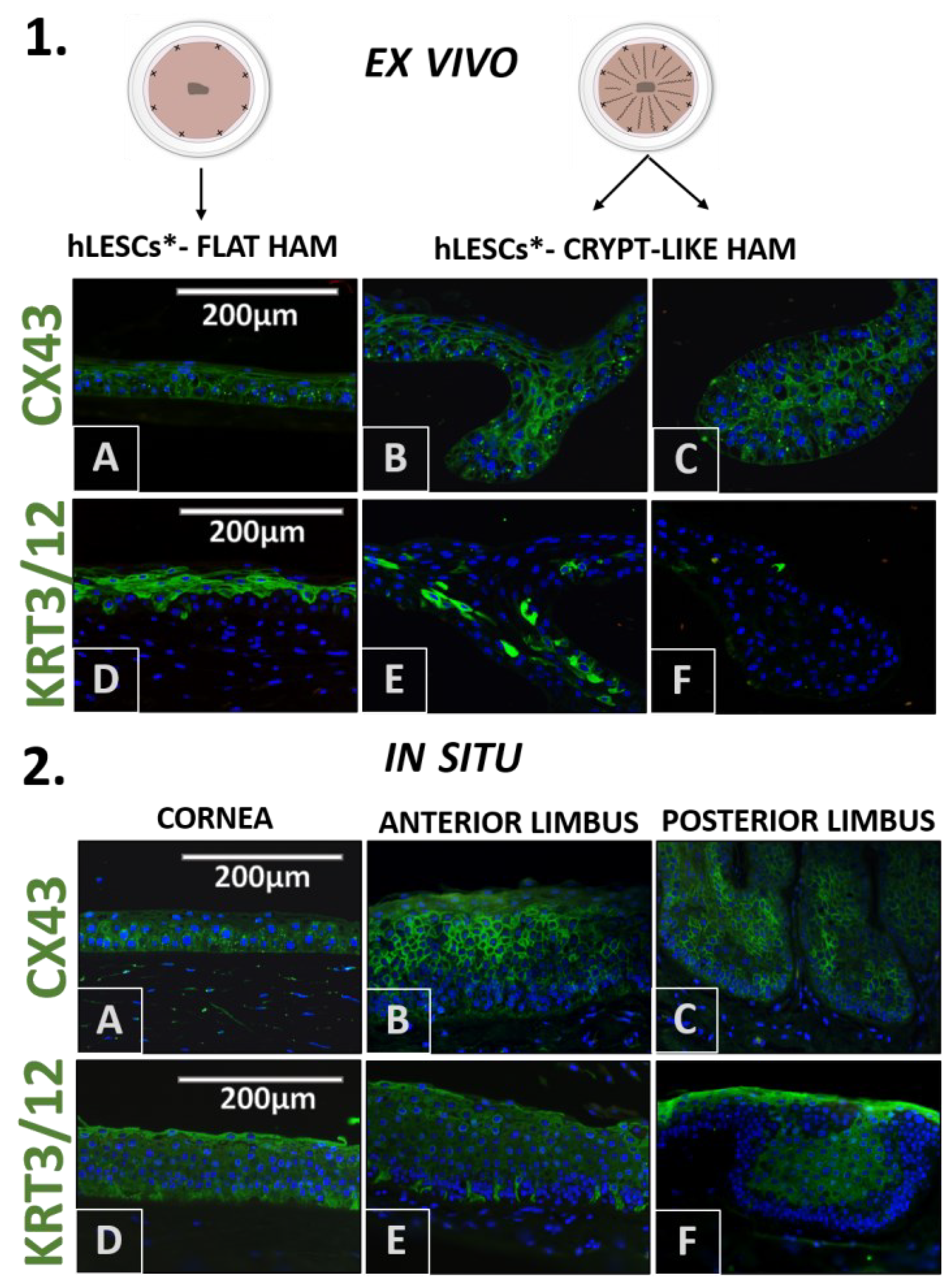
3. Results
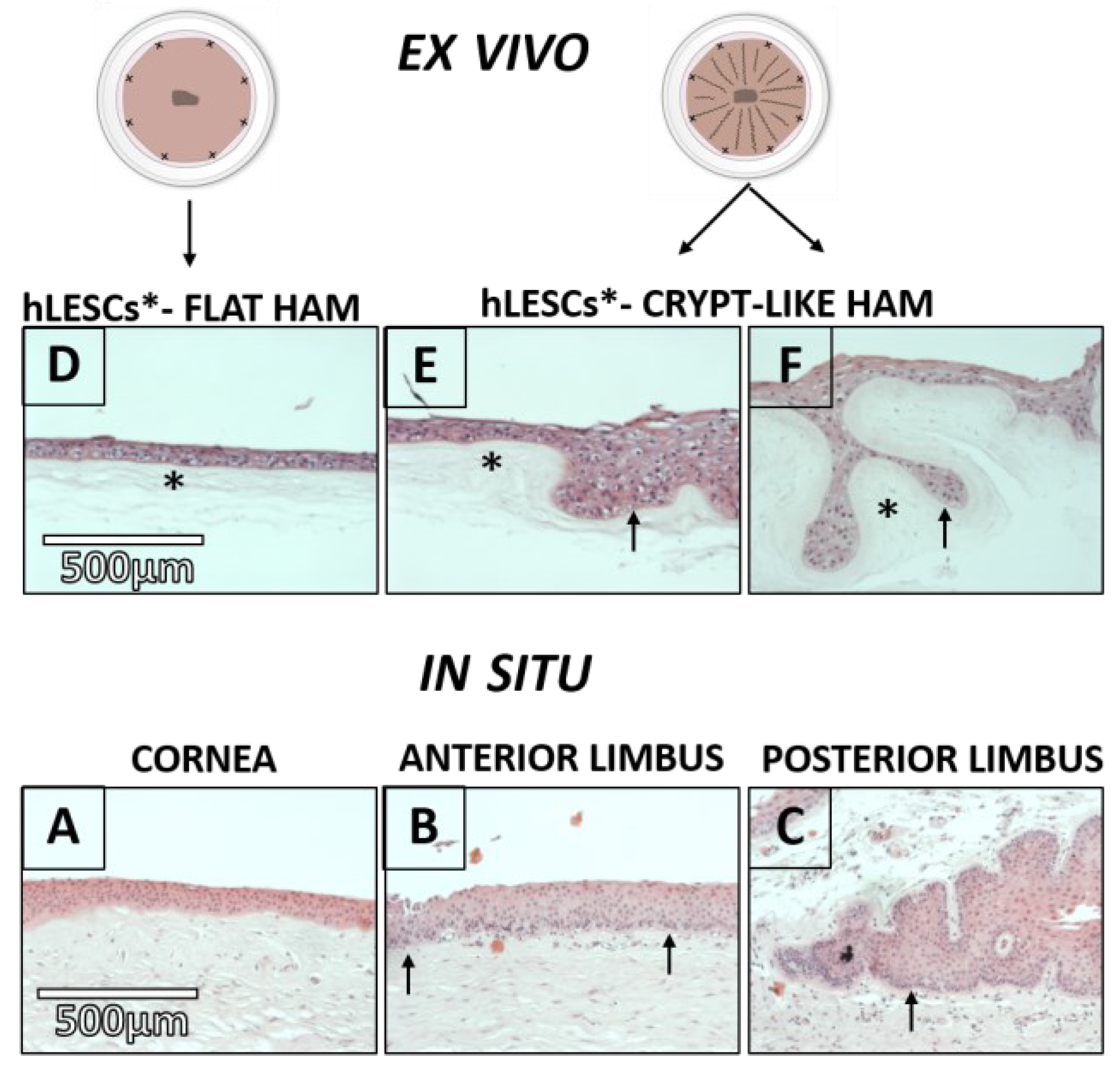
3.1. Epithelial and Basement Membrane (BM) Morphology in Corneal-limbal Tissue and Consequent Localization of hLESCs
3.2. Morphology of the hLESC Cultures Expanded on Conventional, Flat-sutured HAMs vs. hLESC Cultures Expanded on the Novel, Radially-sutured HAMs
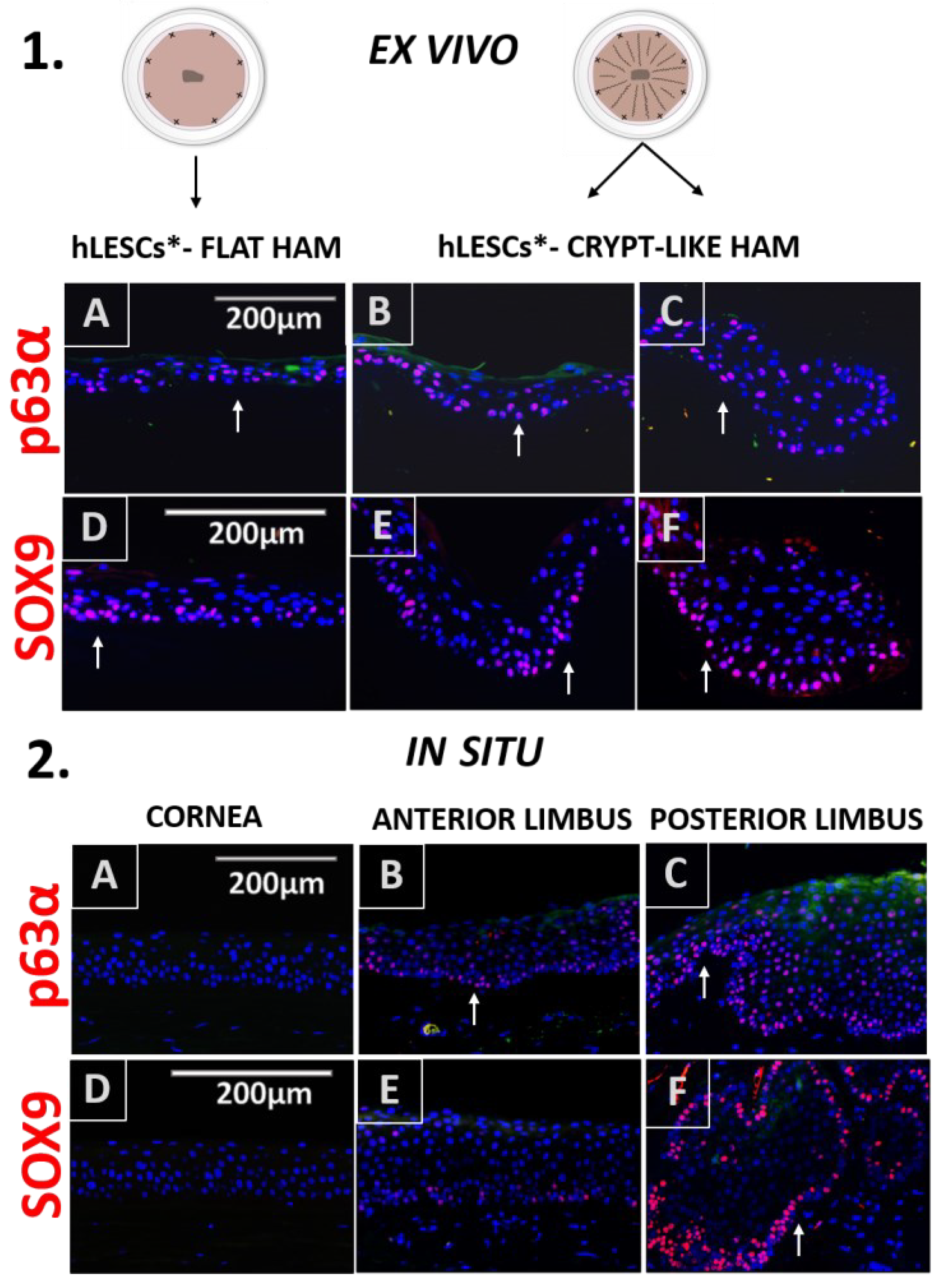
3.3. Distribution of the Progenitor Markers In Situ Versus In Vitro Study Conditions
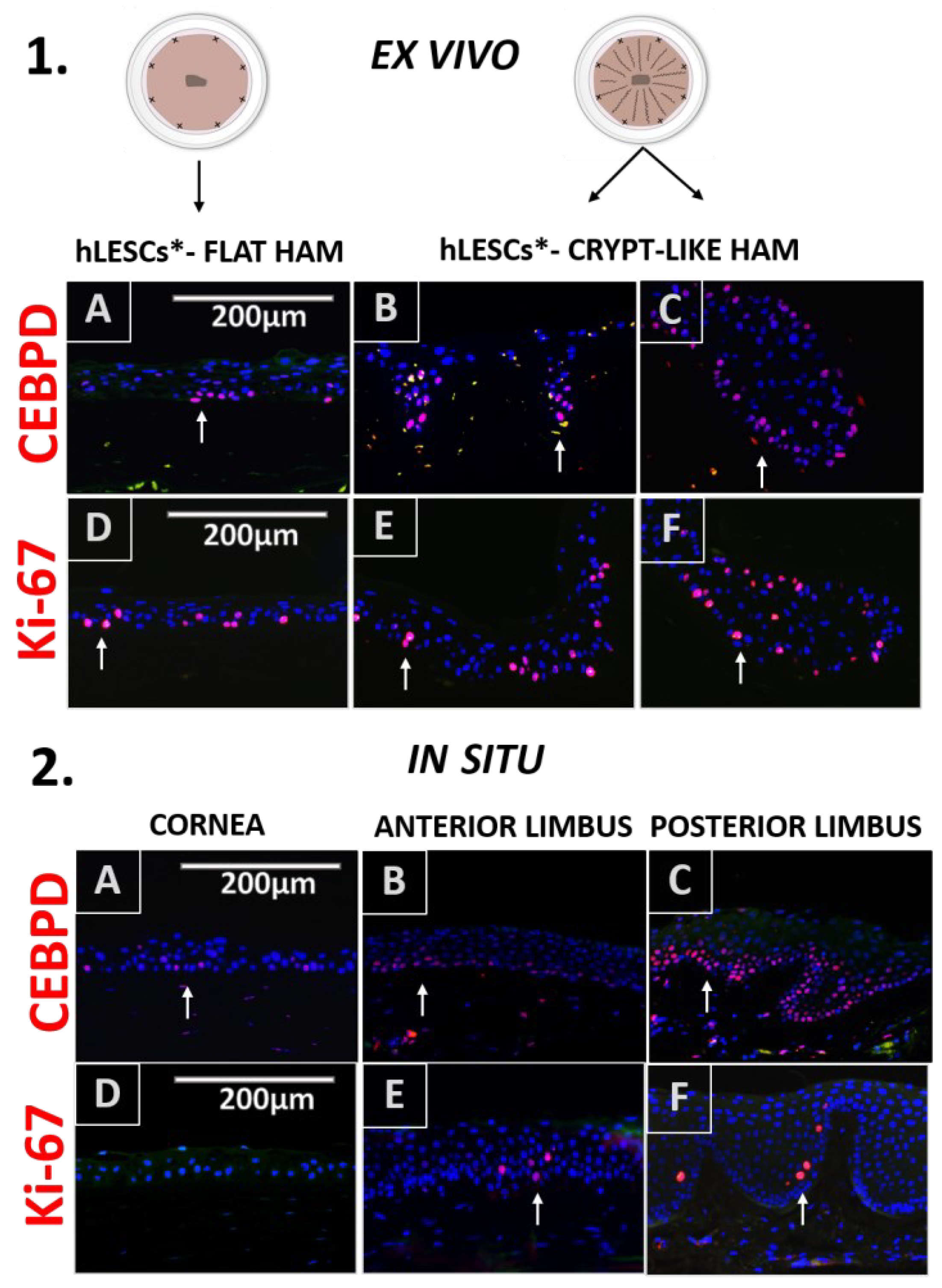
3.4. Expression Profile of the Proliferation and Quiescence Markers in the Corneal-limbal Epithelial Tissue Versus In Vitro Study Conditions
3.5. Differentiation Marker profile in the Epithelium of the Corneal-limbal Tissue, and hLESC Cultures on the Flat and Crypt-like HAMs
3.6. Presentation of Cell Adhesion Molecules in the Corneal-Limbal Epithelium and Expanded hLESC Cultures on Flat and Crypt-like HAMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar]
- Townsend, W.M. The limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1991, 89, 721–756. [Google Scholar]
- SDeng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Veréb, Z.; Albert, R.; Póliska, S.; Olstad, O.K.; Akhtar, S.; Moe, M.C.; Petrovski, G. Comparison of upstream regulators in human ex vivo cultured cornea limbal epithelial stem cells and differentiated corneal epithelial cells. BMC Genom. 2013, 14, 900. [Google Scholar] [CrossRef]
- Rama, P.; Ferrari, G.; Pellegrini, G. Cultivated limbal epithelial transplantation. Curr. Opin. Ophthalmol. 2017, 28, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Behaegel, J.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Safety of Cultivated Limbal Epithelial Stem Cell Transplantation for Human Corneal Regeneration. Stem Cells Int. 2017, 2017, 6978253. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Tiwari, A.; Kethiri, A.R.; Sangwan, V.S. Current perspectives of limbal-derived stem cells and its application in ocular surface regeneration and limbal stem cell transplantation. Stem Cells Transl. Med. 2021, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Pauklin, M.; Fuchsluger, T.A.; Westekemper, H.; Steuhl, K.P.; Meller, D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev. Ophthalmol. 2010, 45, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Chotikavanich, S.; Tesavibul, N. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin. Ophthalmol. 2012, 6, 1483–1492. [Google Scholar] [CrossRef]
- Sejpal, K.; Ali, M.H.; Maddileti, S.; Basu, S.; Ramappa, M.; Kekunnaya, R.; Vemuganti, G.K.; Sangwan, V.S. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013, 131, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Possemiers, T.; Dhubhghaill, S.N.; Leysen, I.; Rozema, J.; Koppen, C.; Timmermans, J.-P.; Berneman, Z.; Tassignon, M.-J. Results of a phase I/II clinical trial: Standardized, non-xenogenic, cultivated limbal stem cell transplantation. J. Transl. Med. 2014, 12, 58. [Google Scholar] [CrossRef]
- Ganger, A.; Vanathi, M.; Mohanty, S.; Tandon, R. Long-Term Outcomes of Cultivated Limbal Epithelial Transplantation: Evaluation and Comparison of Results in Children and Adults. Biomed. Res. Int. 2015, 2015, 480983. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Nikpoor, N.; Donthineni, P.R.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef]
- Sacchetti, M.; Rama, P.; Bruscolini, A.; Lambiase, A. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int. 2018, 2018, 8086269. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Cholidis, S.; Haug, K.; Shahdadfar, A.; Moe, M.C.; Nicolaissen, B.; Drolsum, L. Clinical transplantation of ex vivo expanded autologous limbal epithelial cells using a culture medium with human serum as single supplement: A retrospective case series. Acta Ophthalmol. 2013, 91, 769–775. [Google Scholar] [CrossRef]
- Solomon, A.; Rosenblatt, M.; Monroy, D.; Ji, Z.; Pflugfelder, S.C.; Tseng, S.C. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 2001, 85, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Shimazaki, J.; Ohashi, Y.; Tsubota, K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 2001, 20, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Li, D.Q.; Tan, D.T.; Meller, D.C.; Tseng, S.C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr. Eye Res. 2000, 20, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Li, D.Q.; Ma, X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell Physiol. 1999, 179, 325–335. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Galask, R.P.; Snyder, I.S. Antimicrobial factors in amniotic fluid. Am. J. Obstet. Gynecol. 1970, 106, 59–65. [Google Scholar] [CrossRef]
- Gusdon, J.P. A bactericidin for Bacillus subtilis in pregnancy. J. Immunol. 1962, 88, 494–499. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014, 4, 111–121. [Google Scholar] [CrossRef]
- Drolsum, L.; Willoch, C.; Nicolaissen, B. Use of amniotic membrane as an adjuvant in refractory glaucoma. Acta Ophthalmol. Scand. 2006, 84, 786–789. [Google Scholar] [CrossRef]
- Chen, P.; Lu, M.; Wang, T.; Dian, D.; Zhong, Y.; Aleahmad, M. Human amniotic membrane as a delivery vehicle for stem cell-based therapies. Life Sci. 2021, 272, 119157. [Google Scholar] [CrossRef]
- Knoblich, J.A. Mechanisms of asymmetric stem cell division. Cell 2008, 132, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dai, J.; Zhang, X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta 2015, 1850, 1261–1266. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.D.; Chang, Y.; Hsu, S.T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef]
- Eberwein, P.; Reinhard, T. Concise reviews: The role of biomechanics in the limbal stem cell niche: New insights for our understanding of this structure. Stem Cells 2015, 33, 916–924. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Tan, Y.; Wang, Y. Unraveling the mechanobiology of cornea: From bench side to the clinic. Front. Bioeng. Biotechnol. 2022, 10, 953590. [Google Scholar] [CrossRef] [PubMed]
- Lacorzana, J. Amniotic membrane, clinical applications and tissue engineering. Review of its ophthalmic use. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2020, 95, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Kaur, P.; Dhouailly, D.; Hengge, U.; Bickenbach, J. Epithelial stem cells in the skin: Definition, markers, localization and functions. Exp. Dermatol. 1999, 8, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Sun, H.; Zhang, Y.; Tighe, S.; Chen, S.; Su, C.-W.; Liu, Y.; Zhao, H.; Hu, M.; Zhu, Y. Limbal niche cells are a potent resource of adult mesenchymal progenitors. J. Cell Mol. Med. 2018, 22, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, J.; Nie, Q. Stem cell niche structure as an inherent cause of undulating epithelial morphologies. Biophys. J. 2013, 104, 237–246. [Google Scholar] [CrossRef]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Fukuda, K.; Chikama, T.; Nakamura, M.; Nishida, T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 1999, 18, 73–79. [Google Scholar] [CrossRef]
- Champliaud, M.F.; Lunstrum, G.P.; Rousselle, P.; Nishiyama, T.; Keene, D.R.; Burgeson, R.E. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J. Cell Biol. 1996, 132, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Le, Q.; Deng, S.X. The application of human amniotic membrane in the surgical management of limbal stem cell deficiency. Ocul. Surf. 2019, 17, 221–229. [Google Scholar] [CrossRef]
- Meller, D.; Pires, R.T.; Tseng, S.C. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br. J. Ophthalmol. 2002, 86, 463–471. [Google Scholar] [CrossRef]
- Grueterich, M.; Espana, E.; Tseng, S.C. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest. Ophthalmol. Vis. Sci. 2002, 43, 63–71. [Google Scholar]
- Szabó, D.J.; Noer, A.; Nagymihály, R.; Josifovska, N.; Andjelic, S.; Veréb, Z.; Facskó, A.; Moe, M.C.; Petrovski, G. Long-Term Cultures of Human Cornea Limbal Explants Form 3D Structures Ex Vivo-Implications for Tissue Engineering and Clinical Applications. PLoS ONE 2015, 10, e0143053. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Derby, A.; Elkodous, M.A.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Kang, M.; Choi, S.; Lee, A.R.C. Effect of freeze dried bovine amniotic membrane extract on full thickness wound healing. Arch. Pharm. Res. 2013, 36, 472–478. [Google Scholar] [CrossRef]
- Tehrani, F.D.; Firouzeh, A.; Shabani, I.; Shabani, A. A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 606982. [Google Scholar] [CrossRef]
- Sekiyama, E.; Nakamura, T.; Kurihara, E.; Cooper, L.J.; Fullwood, N.J.; Takaoka, M.; Hamuro, J.; Kinoshita, S. Novel sutureless transplantation of bioadhesive-coated, freeze-dried amniotic membrane for ocular surface reconstruction. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1528–1534. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, J.; Guan, L.; Zhao, M. Novel implantable composite biomaterial by fibrin glue and amniotic membrane for ocular surface reconstruction. J. Mater. Sci. Mater. Med. 2015, 26, 149. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Khan, J.; Mahata, D.; Saha, S.; Sengupta, J.; Silva, O.N.; Das, S.; Mandal, M.; Franco, O.L. A self-assembled clavanin A-coated amniotic membrane scaffold for the prevention of biofilm formation by ocular surface fungal pathogens. Biofouling 2017, 33, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, D.; Kumar, P.; Chacharkar, M.P. Development and evaluation of silver-impregnated amniotic membrane as an antimicrobial burn dressing. J. Burn. Care Res. 2008, 29, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Shimmura, S.; Miyashita, H.; Taguchi, T.; Kobayashi, H.; Shimazaki, J.; Tanaka, J.; Tsubota, K. Amniotic membrane immobilized poly(vinyl alcohol) hybrid polymer as an artificial cornea scaffold that supports a stratified and differentiated corneal epithelium. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ryzhuk, V.; Zeng, X.-X.; Wang, X.; Melnychuk, V.; Lankford, L.; Farmer, D. Human amnion extracellular matrix derived bioactive hydrogel for cell delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 191–202. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Z.; Lin, H.; Wu, J.; Ginn, B.; Choi, J.S.; Jiang, X.; Chung, L.; Elisseeff, J.H.; Yiu, S.; et al. Synthetic Nanofiber-Reinforced Amniotic Membrane via Interfacial Bonding. ACS Appl. Mater. Interfaces 2018, 10, 14559–14569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, D.; Hsu, C.-C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Westekemper, H.; Figueiredo, F.C.; Siah, W.F.; Wagner, N.; Steuhl, K.P.; Meller, D. Clinical outcomes of amniotic membrane transplantation in the management of acute ocular chemical injury. Br. J. Ophthalmol. 2017, 101, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Choi, J.S.; Joo, C.K. Effects of amniotic membrane suspension in the rat alkali burn model. Mol. Vis. 2011, 17, 404–412. [Google Scholar] [PubMed]
- Dudok, D.V.; Nagdee, I.; Cheung, K.; Liu, H.; Vedovelli, L.; Ghinelli, E.; Kenyon, K.; Parapuram, S.; Hutnik, C.M. Effects of amniotic membrane extract on primary human corneal epithelial and limbal cells. Clin. Exp. Ophthalmol. 2015, 43, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sheha, H.; Liang, L.; Hashem, H.; Ramzy, M.; ZaKi, A. Amniotic Membrane Extract for Acute Ocular Chemical Burns. Tech. Ophthalmol. 2010, 8, 146–150. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Asl, N.S.; Ebrahimi, M.; Jabbehdari, S.; Bamdad, S.; Roshandel, D.; Eslani, M.; Momeni, M. The role of amniotic membrane extract eye drop (AMEED) in vivo cultivation of limbal stem cells. Ocul. Surf. 2018, 16, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Paolin, A.; Cogliati, E.; Trojan, D.; Griffoni, C.; Grassetto, A.; Elbadawy, H.; Ponzin, D. Amniotic membranes in ophthalmology: Long term data on transplantation outcomes. Cell Tissue Bank. 2016, 17, 51–58. [Google Scholar] [CrossRef]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef]
- Dua, H.S.; Joseph, A.; Shanmuganathan, V.A.; Jones, R.E. Stem cell differentiation and the effects of deficiency. Eye (Lond.) 2003, 17, 877–885. [Google Scholar] [CrossRef]
- Kawasaki, S.; Tanioka, H.; Yamasaki, K.; Connon, C.J.; Kinoshita, S. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp. Eye Res. 2006, 82, 293–299. [Google Scholar] [CrossRef]
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523–9528. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007, 177, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Menzel-Severing, J.; Zenkel, M.; Polisetti, N.; Sock, E.; Wegner, M.; Kruse, F.E.; Schlötzer-Schrehardt, U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018, 8, 10268. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girao, H. Role of connexin 43 in different forms of intercellular communication-gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef]
- Matic, M.; Petrov, I.N.; Chen, S.; Wang, C.; Dimitrijevich, S.D.; Wolosin, J.M. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 1997, 61, 251–260. [Google Scholar] [CrossRef]
- Wang, I.J.; Tsai, R.J.; Yeh, L.K.; Tsai, R.Y.; Hu, F.R.; Kao, W.W. Changes in corneal basal epithelial phenotypes in an altered basement membrane. PLoS ONE 2011, 6, e14537. [Google Scholar] [CrossRef] [PubMed]
- Grueterich, M.; Espana, E.M.; Touhami, A.; Ti, S.E.; Tseng, S.C. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology 2002, 109, 1547–1552. [Google Scholar] [CrossRef]
- Schermer, A.; Galvin, S.; Sun, T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986, 103, 49–62. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ben-Zvi, A.; Krachmer, J.; Schermer, A.; Sun, T.T. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation 1987, 34, 60–67. [Google Scholar] [CrossRef] [PubMed]
- A Shanmuganathan, V.; Foster, T.; Kulkarni, B.B.; Hopkinson, A.; Gray, T.; Powe, D.G.; Lowe, J.; Dua, H.S. Morphological characteristics of the limbal epithelial crypt. Br. J. Ophthalmol. 2007, 91, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Shimmura, S.; Miyashita, H.; Kato, N.; Ogawa, Y.; Kawakita, T.; Shimazaki, J.; Tsubota, K. N-cadherin in the maintenance of human corneal limbal epithelial progenitor cells in vitro. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Yamato, M.; Sugiyama, H.; Sumide, T.; Yang, J.; Okano, T.; Tano, Y.; Nishida, K. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells 2007, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisevac, J.; Moe, M.C.; Drolsum, L.; Kristianslund, O.; Petrovski, G.; Noer, A. A Novel Technique of Amniotic Membrane Preparation Mimicking Limbal Epithelial Crypts Enhances the Number of Progenitor Cells upon Expansion. Cells 2023, 12, 738. https://doi.org/10.3390/cells12050738
Bisevac J, Moe MC, Drolsum L, Kristianslund O, Petrovski G, Noer A. A Novel Technique of Amniotic Membrane Preparation Mimicking Limbal Epithelial Crypts Enhances the Number of Progenitor Cells upon Expansion. Cells. 2023; 12(5):738. https://doi.org/10.3390/cells12050738
Chicago/Turabian StyleBisevac, Jovana, Morten Carstens Moe, Liv Drolsum, Olav Kristianslund, Goran Petrovski, and Agate Noer. 2023. "A Novel Technique of Amniotic Membrane Preparation Mimicking Limbal Epithelial Crypts Enhances the Number of Progenitor Cells upon Expansion" Cells 12, no. 5: 738. https://doi.org/10.3390/cells12050738
APA StyleBisevac, J., Moe, M. C., Drolsum, L., Kristianslund, O., Petrovski, G., & Noer, A. (2023). A Novel Technique of Amniotic Membrane Preparation Mimicking Limbal Epithelial Crypts Enhances the Number of Progenitor Cells upon Expansion. Cells, 12(5), 738. https://doi.org/10.3390/cells12050738





