1. Introduction
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy in the paediatric population. It is caused by various pathogenic mutations in the DMD gene, which mostly disrupt the open reading frame and thus lead to an absence of functional dystrophin protein. The exon-skipping approach has been developed to restore the reading frame of the gene and is based on the use of antisense oligonucleotides (ASO). ASOs aim to eliminate one or several exons by masking key splicing sites and therefore induce the expression of an internally deleted but functional dystrophin protein. Several ASO-based drugs have already been approved by the US FDA for the treatment of DMD (eteplirsen, golodirsen, viltolarsen and casimersen targeting exons 51, 53 and 45) [
1]. Approval was mostly based on safety, and a small percentage of dystrophin restoration was observed in muscle biopsies of treated patients. However, the clinical benefit is still marginal, and there is a critical need to improve the level of dystrophin being restored in DMD patients.
One of the most recognized challenges of ASO-mediated exon skipping is the delivery of ASOs to the nucleus of the target tissues [
2,
3]. To achieve this goal, second generations of ASO have been developed with alternative chemistries or various conjugates [
4,
5]. Amongst these, we have been working with one particular chemical substance named tricyclo-DNA (tcDNA), and we recently demonstrated that conjugation of palmitic acid to tcDNA significantly enhances its delivery to target tissues [
6]. Despite these improvements, the quantity of ASO ending up in the nucleus to bind their pre-mRNA target remains extremely low. Indeed, even when ASOs reach the correct target tissues/cells, most of them end up trapped in the endosomal compartment and are degraded in the lysosomes before they can enter the cytoplasm and the nucleus (e.g., the ultimate target compartment) [
2]. Previous studies have focused on ASO cellular trafficking to better characterize the mechanisms and improve the intracytoplasmic delivery of ASOs [
3,
7]. A number of studies have aimed at influencing ASO pharmacological effect by manipulating components of the endosomal machinery, specifically by targeting proteins involved in endomembrane trafficking, such as GCC2, M6PR, or COPII Golgi coat proteins [
8,
9,
10].
Overall, these studies confirmed that components of the endosomal trafficking machinery are intimately involved in the subcellular fate and thus the pharmacological effectiveness of ASOs [
11].
Several strategies have been considered to allow ASOs to cross the biological barriers and cell membranes. Among them, the conjugation of ASO to a cell penetration peptide (CPP) has been widely used. CPPs are relatively short peptides, from four to forty amino acids, which can induce endocytosis or promote the intracellular effects of ASO [
12]. Given that CPPs are charged molecules, they are often conjugated with charge neutral ASOs, such as phosphorodiamidate morpholino oligomers (PMO) [
13], and cannot be used with charged ASO like tcDNA. Other approaches based on the use of nanotechnology have been extensively studied, from DNA nanostructures to exosome-like nano-carriers to spherical nucleic acids or lipid nanoparticles (LNPs), all described in recent reviews [
5,
13,
14]. An alternative option is to use small molecules named oligonucleotide enhancing compounds (OEC), which were discovered through high throughput screening and which selectively release oligonucleotides from non-productive entrapment in endosomal compartments [
15,
16,
17]. Because they are not directly linked to the ASO, OECs may be used with all types of ASOs (charged or uncharged) to facilitate their access to the cytosol and thus improve the probability to reach the nucleus to substantially enhance pharmacological effects [
11].
Different types of OECs have been characterized, depending on their mechanism of action: the first one, which has been known for decades is a “proton sponge action”, characteristic of lysosomotropic drugs, leads to an influx of water molecules, swelling, and disruption of the endosomal compartment [
18]. Alternative mechanisms include disrupting the interaction with the proteins of the endosomal membrane (such as Retro-1, which affects Rab7/9 positive late endosomes) [
19]. The more recently identified OEC, named UNC7938, was shown to selectively release oligonucleotides from late endosomes, with only little effect on lysosomal pH, thus emphasizing the distinction between the OECs and typical lysosomotropic compounds [
11,
17].
In this study, we report the impact of OEC UNC7938 administration on exon-skipping efficacy mediated by tcDNA-ASO in mdx mice and characterize the kinetics of the effect. Adult mdx mice were treated with a tcDNA-ASO aiming at skipping the Dmd exon 23 with or without UNC7938, and these were analyzed at different time points. The combined therapy appeared to be particularly efficient at early time points, as well as in the cardiac muscle, leading to a normalization of cardiac function after three months of treatment.
2. Materials and Methods
2.1. Antisense Oligonucleotides and Animal Experiments
Animal procedures were performed in accordance with national and European legislation, approved by the French government (Ministère de l’Enseignement supérieur et de la Recherche, Autorisation APAFiS #6518). Mdx (C57BL/10ScSc-Dmdmdx/J) mice were bred in our animal facility at the Plateforme 2Care, UFR des Sciences de la santé, Université de Versailles Saint Quentin and were maintained in a standard 12-h light/dark cycle with free access to food and water. Mice were weaned at week four to five (postnatal), and two to five individuals were housed per cage.
TcDNA-ASO, targeting the donor splice site of exon 23 of the mouse dystrophin pre-mRNA [
20], was synthesized by SQY Therapeutics (Montigny le Bretonneux, France). Palmitic acid was conjugated at the 5′end of tcDNA-PO via a C6-amino linker and a phosphorothioate bond as previously described (
Figure S1) [
6]. Mice were injected intravenously with 30 mg/kg/wk of the tcDNA-ASO (one intravenous injection per week under general anesthesia using 2% isoflurane). The oligonucleotide enhancing compound UNC7938 was produced by Initos Pharmaceuticals LLC (Chapel Hill, NC, USA).
Mdx mice were treated with UNC7938 at 15 mg/kg/wk under general anesthesia using 2% isoflurane 24 h after the ASO injection (every week or every four weeks, depending on the protocol). Age-matched
mdx groups receiving an equivalent volume of sterile saline were included as controls, and C57BL/10 mice were included as wild-type controls.
Animals were euthanized at different time points according to the different protocols and muscles and tissues were harvested and snap-frozen in liquid nitrogen-cooled isopentane and stored at −80 °C before further analysis.
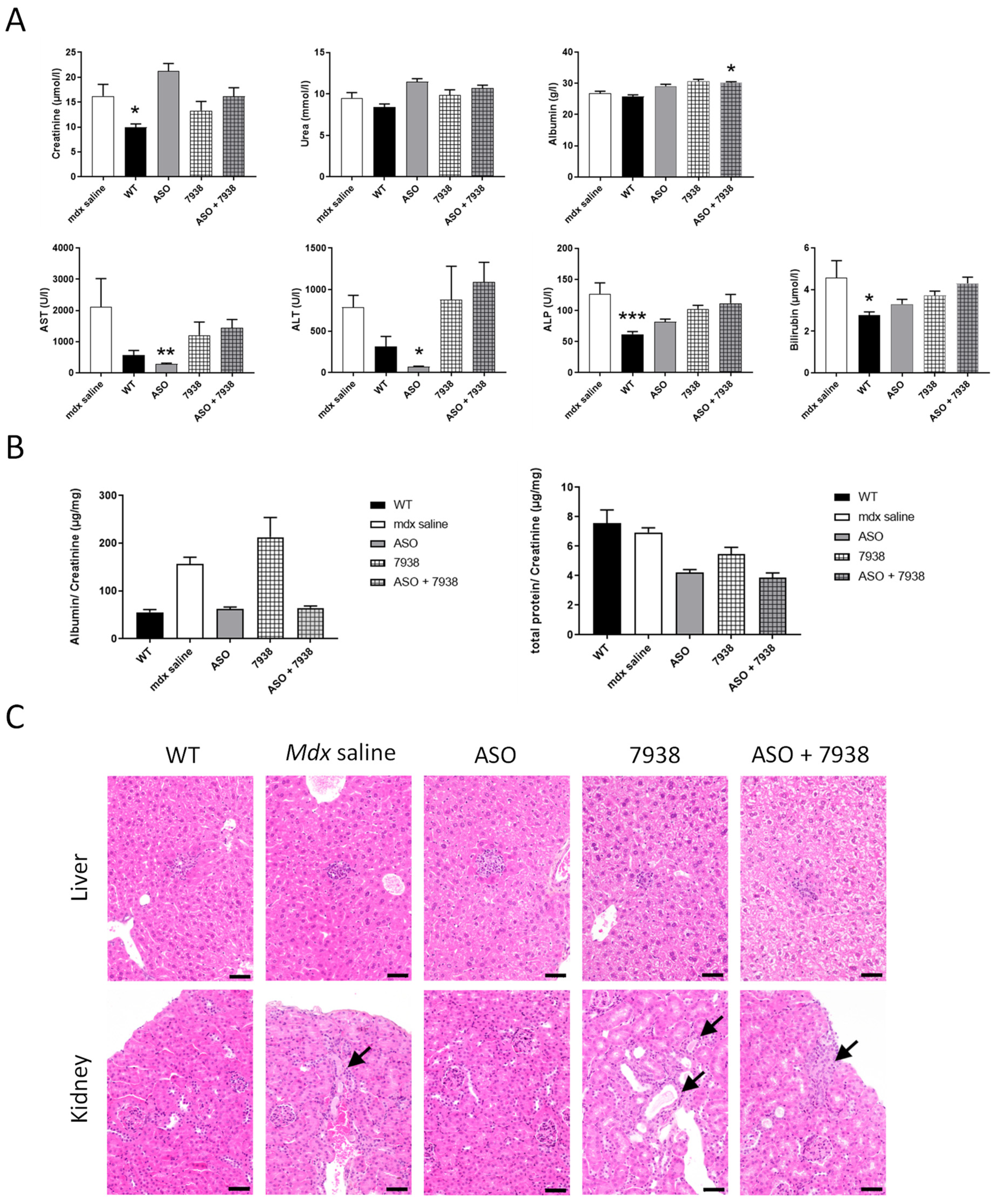
To assess the safety of UNC7938, liver and kidney were sampled at the end of the 12-wk protocol (two weeks after the last dose), fixed in 10% neutral buffered formalin, and embedded in paraffin wax. Thick sections of 4 µm were then routinely stained with hematoxylin-eosin-saffron (HES) for histopathological evaluation, which was further performed by a veterinary pathologist blind to treatment.
2.2. Echocardiography Procedure
The procedure was performed under isoflurane anesthesia. Anesthesia doses were kept to the lowest possible levels, usually 5% isoflurane for induction and 2.5–3% isoflurane during measurements. Animals were placed on a heating pad to maintain a constant body temperature (37 °C), and their rectal temperature was monitored throughout the experiment. Doppler-echocardiography was performed using a high-resolution ultrasound system (Logiq 9, GE, France) with a 36-MHz scan head. Each animal was shaven from the left sternal border to the left axillary line with depilatory cream before the examination. Each set of measurements was obtained from the same cardiac cycle. At least three sets of measurements were obtained from three different cardiac cycles. The left ventricular end-diastolic diameter (LVEDD), end-diastolic posterior wall thickness, and end-diastolic interventricular septal wall thickness were measured using the leading-edge convention of the American Society of Echocardiography from M mode. The LVEDD was measured, from a M-mode short-axis view of the left ventricle at the papillary muscle level. Left ventricular shortening fraction (RF) and left ventricular ejection fraction (LVEF) were calculated from the M mode. Aortic velocity time integral (VTI) was recorded during the procedure from Doppler echocardiography. Mitral inflow Doppler pattern was recorded (peak E, peak A, and deceleration time) from a four-chamber apical view. The left ventricular systolic intervals of the isovolumic contraction time (IVCT), the ventricular ejection time (ET), and the diastolic interval of the isovolumic relaxation time (IVRT) were measured for the Tei index calculation. Measurements were made for aortic and mitral blood flows recorded from an apical four-chamber modified view using pulsed Doppler, and the sample was placed between the tip of the mitral and the Left ventricular outflow tract. The Tei index was calculated as the ratio of (IVCT + IVRT) to systolic ejection time. Cardiac output (CO) was defined as stroke volume x heart rate. The shortening fraction (%) was calculated by the formula: (LVEDD-LVESD)/LVEDD × 100. LV end-diastolic (EDV) and end-systolic (ESV) volumes were calculated using a half ellipsoid model of the LV. From these volumes, LV ejection fraction (%) was calculated using the formula: (EDV-ESV)/EDV × 100. These experiments were performed in blind.
2.3. ASO Quantification by Fluorescent Hybridization Assay
Tissues were homogenized using the Precellys 24 (Bertin Instruments, Montigny le Bretonneux, France) at a final concentration of 50 mg/mL of lysis buffer (100 mmol/L Tris–HCl, pH 8.5, 200 mmol/L NaCl, 5 mmol/L EDTA, 0.2% sodium dodecyl sulfate) containing 2 mg/mL of proteinase K (Invitrogen, Germany) and incubated overnight at 55 °C in a hybridization oven. After centrifugation at 7000×
g (Sorval ST 8R centrifuge, 75005719 rotor) for 15 min, the supernatant was used in the assay. Quantification of ASO was performed using a hybridization assay with a molecular beacon probe, as previously described [
21].
2.4. Cell Fractionation and Western Blot Validation
Cell fractionation from freshly dissected gluteus muscles and protein extraction were performed as described previously (Dimauro et al., 2012). Then, 35 μg of protein were loaded onto 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (BioRad, Carlsbad, CA, USA). The PVDF membranes were probed with primary polyclonal antibodies directed against histone H3 (Cell Signaling Technology, Danvers, MA USA, 1:1000) or primary monoclonal antibodies directed against EEA1 (Sigma Aldrich, Saint Louis, MI, USA, 1:1000) followed by incubation with a goat anti-mouse secondary antibody (IRDye 800 CW Goat anti-mouse IgG, Li-Cor, Germany, dilution 1/20,000) or goat anti-rabbit secondary antibody (IRDye 800 CW Goat anti-rabbit IgG, Li-Cor, Germany, dilution 1/20,000). Bands were visualized using the Odyssey CLx system (Li-Cor, Germany).
2.5. RNA Analysis
Total RNA was isolated from snap-frozen muscle tissues using TRIzol reagent, according to the manufacturer’s instructions (ThermoFisher Scientific, Carlsbad, CA USA). To visualize exon skipping levels, aliquots of 500 ng of total RNA were used for RT-PCR analysis using the Access RT-PCR System (Promega, Madison, WI, USA), as previously described [
6].
Exon 23 skipping levels were also quantified using real-time quantitative PCR using Taqman assays designed against the exon 23–24 junction and exon 22–24 junction, as previously described [
6].
2.6. Western Blot Analysis
Protein lysates were obtained from intervening muscle sections collected during cryosection using the Precellys 24 (Bertin Instruments, Montigny le Bretonneux, France) in RIPA buffer (ThermoFisher Scientific, Rockford, IL, USA) complemented with SDS powder (5% final) (Bio-Rad, Marnes-la-coquette, France) and protease inhibitor cocktail (ThermoFisher Scientific, Rockford, IL, USA). An amount of 25 μg of protein were loaded onto NuPAGE 3–8% Tris-Acetate Protein gels (Invitrogen, Carlsbad, CA, USA), following manufacturer instructions. Dystrophin protein was detected with NCL-DYS1 primary monoclonal antibody (NCL-DYS1; Novocastra, Newcastle, UK, dilution 1/200), as prevsiously described [
6]. Quantification was performed using the Empiria Studio software (Li-Cor, Bad Homburg, Germany) based on a standard curve made from pooled lysates from C57BL10 (WT) and
mdx control for each tissue.
2.7. Serum and Urine Analysis
Blood samples were collected at the end of the treatment for biochemistry analysis. Analyses of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, creatinine, urea, and albumin levels were performed by the pathology laboratory at Mary Lyon Centre, Medical Research Council, Harwell, Oxfordshire, UK.
Urine was collected using metabolic cages over 24 h, and urine creatinine and total protein were measured, as previously described [
22].
2.8. Immunohistochemistry Analysis
Sections of 10 µm at 120 µm intervals were cut from triceps, diaphragm, and heart and examined for dystrophin expression using a rabbit polyclonal antibody Dystrophin (dilution 1:500; cat. number RB-9024-P ThermoScientific, Fremont, CA, USA), which was then detected by goat anti-rabbit IgGs Alexa Fluor 488 (dilution 1/500; A11070, Invitrogen, Eugene, OR, USA). Images were cropped, and scale bars of 100 µm were added using ImageJ software.
2.9. Statistical Analysis
All in vivo data were analyzed with the GraphPad Prism8 software (San Diego, CA, USA) and expressed as means ± S.E.M. The “n” refers to the number of mice per group.
Group comparisons were performed using one and two-way analyses of variance (ANOVA) with repeated-measure comparisons when needed (effects in different muscle tissues for example), followed by post-hoc Dunnett’s or Sidak’s multiple comparisons when appropriate. The Kruskal-Wallis test was used to compare groups that do not follow a normal distribution (assessed with the Shapiro-Wilk test). Significance levels were set at * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
4. Discussion
In this study, we aimed to enhance the therapeutic potential of ASO-mediated exon skipping for DMD, which is still currently limited by several challenges. Our previous work has focused on improving the ASO compound itself, and we have optimized a tcDNA-based ASO, free of phosphorothioate linkages and conjugated to a palmitic acid, presenting a significantly higher therapeutic index [
6]. Besides the optimization of the ASO compound itself, which may address some of the delivery issues, it is possible to act on cellular mechanisms to facilitate the transport of ASO to their target organelle, i.e., the nucleus in the case of splice switching ASO. The inefficiency of endosomal escape is considered one of the biggest problem preventing the widespread use of RNA therapeutics [
24]. The chemical manipulation of the endosome trafficking machinery has been shown to positively impact ASO delivery and pharmacological effects [
11]. However, the proof of concept has never been demonstrated for DMD and the exon skipping approach in vivo. In the present study, we therefore evaluated the potential of such a combined therapy in vivo in the
mdx mouse model of DMD. We selected the UNC7938 compound that was discovered through high throughput screening and shown to selectively release ASO from non-productive entrapment in endosomal compartments [
15,
16,
17]. UNC7938 was recently shown to potentiate the effect of a peptide conjugated PMO (PPMO) in cystic fibrosis patients cells and in vivo in mouse lung [
23], but no data are available in muscle tissues.
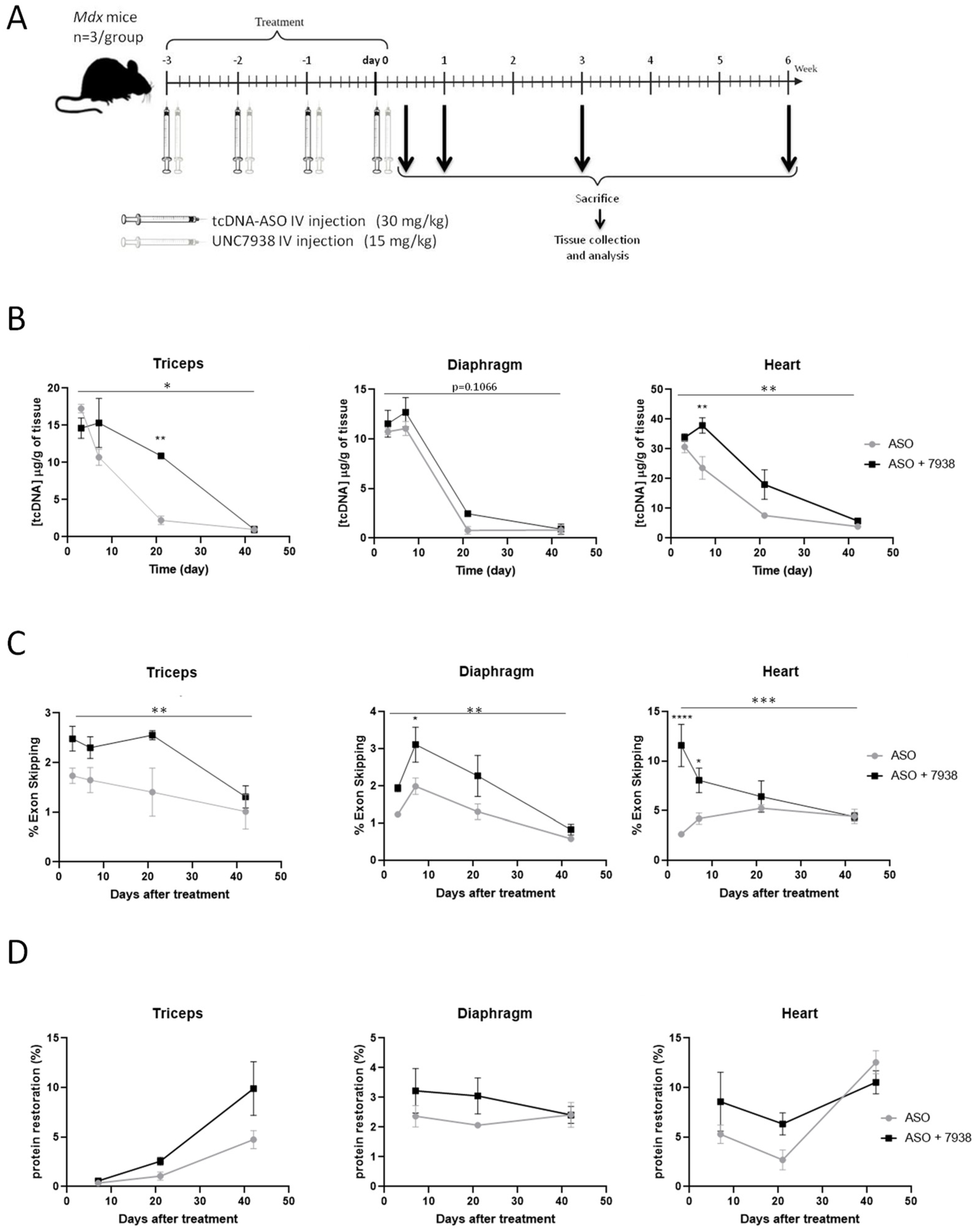
In our preliminary four-wk treatment protocol, we showed that the combined ASO + UNC7938 therapy induced significantly higher exon skipping level (1.59 fold-changes) and dystrophin restoration levels (2.75 fold-changes) than ASO therapy alone, especially in the heart. This is in line with our hypothesis that UNC7938 treatment may help ASO to escape the endosome and diffuse to the nucleus where the pre-mRNA targets are located. However, the reasons underlying the higher effect in the heart in contrast with the other muscle tissues remain unclear and may simply be due to the fact that tcDNA is particularly efficient at targeting the heart (i.e., heart is the muscle with the highest content of ASO).
To better characterize the effects of UNC7938, we studied its impact at different time points after injection. The quantification of ASO in different tissues shows that ASO distribution is not affected by UNC7938 at the very early time point (72 h), suggesting that UNC7838 does not impact the distribution of ASO to the different tissues per se. However, ASO tissue content appeared higher between one and three weeks post-treatment in UNC7938 co-treated mice compared to mice treated with ASO alone. This suggests that ASOs are not eliminated as fast when the OEC is administrated, which would be in line with their mechanism of action. Endosomal escape would protect the ASO from a quick degradation/elimination. This was also confirmed by the pharmacological effect of ASO, which was significantly enhanced after the treatment with UNC7938. The effect was once again more important in the heart, which is the muscle tissue with the highest content of ASO, with up to a 4.4-increase in exon skipping levels at early time points. Interestingly, this ‘booster’ effect faded over time, and ASO content, as well as exon skipping levels, ended up being very similar six weeks after the treatment in both groups of mice (with or without UNC7938). This may be explained by the ‘depot effect’ occurring in mice treated with ASO alone, in which the ASO trapped in endosomes are actually slowly released over time. This phenomenon characterized by the fact that ~99% of ASO (or RNA therapeutics in general) are typically trapped inside endosomes, may indeed explain why ASO can achieve long pharmacological effects due to a constant low level of leakage into the cytoplasm over weeks or months [
24]. Using an OEC, such as UNC7938, may induce a short liberation of ASO from the endosomes, but might, in counterpart, affect the depot effect, which would no longer occur. This could explain why the overall exon skipping levels end up being similar several weeks after the treatment.
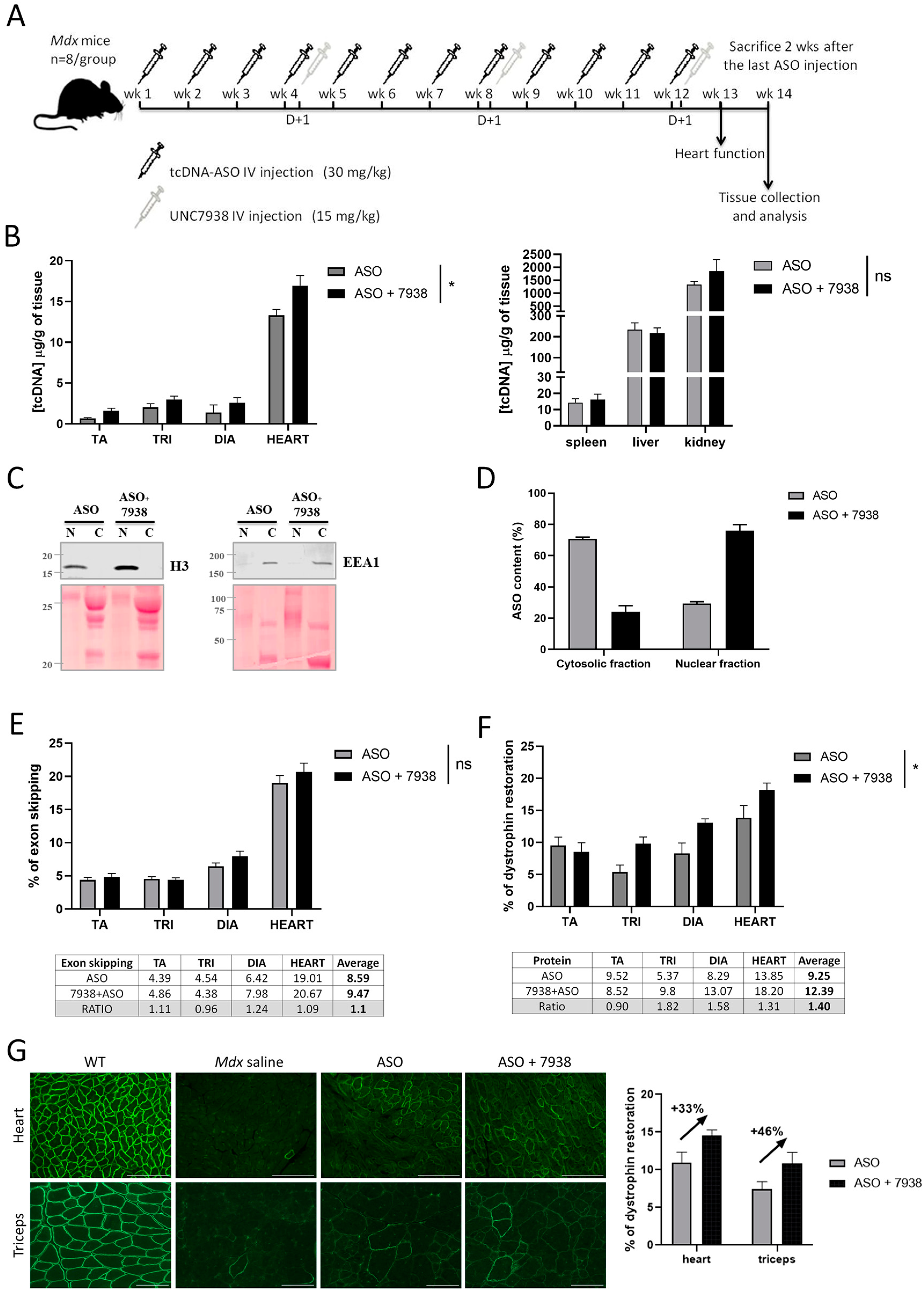
We next investigated the impact of UNC7938 on exon skipping therapy over a longer period of time and treated
mdx mice for 12 weeks, during which they received three injections of UNC7938 (every four weeks). We had previously validated that this dosing regimen induces similar effects that an injection every week. The ASO content in muscle tissues at the end of the 12-wk treatment was slightly higher in mice co-treated with UNC7938 compared to mice treated with ASO alone, as previously observed. In order to determine the ASO intracellular localization, we performed cell fractionation on freshly isolated skeletal muscles from treated
mdx mice and quantified the ASO content in enriched nuclear and cytoplasmic fractions. Interestingly, we found a higher proportion of ASO in the nuclear fraction of mice treated with ASO + UNC7938 compared to mice treated with ASO alone, which showed a higher proportion of ASO in the cytosolic fraction. We found approximately 71% of ASO in the cytosolic fractions in mice treated with ASO alone, which is higher that the commonly assumed 99% of ASO trapped in endosomes [
24]. This may be due to the repeated injections of ASO over 12 weeks, which may help accumulate more ASO in the nucleus than single injections, and also to the timing of analysis. Since muscles were analyzed two weeks after the last injection, a proportion of ASO that was trapped in the endosomal/lysosomal compartment may have already been cleared, thus lowering the quantified cytoplasmic fraction compared to the nuclear fraction. It would be interesting to perform similar fractionation analysis 24 h after a single injection as this may reveal different proportions. However, one should also keep in mind that cell fractionation protocol results in an enrichment of fractions rather that absolutely pure fractions, so it cannot be excluded that the ASO content in the nuclear fraction is slightly overestimated because of traces of cytosolic fraction. Nonetheless, the consistent higher proportion of ASO found in the nuclear fraction after UNC7938 treatment is in line with its mechanism of action and confirms that freeing ASO from endosomes helps them reach their ultimate target organelle, the nucleus.
The exon skipping levels and restoration of dystrophin protein were also slightly improved in co-treated mice, although to a lesser extent than after the four-wk treatment. This may be explained by the depot effect that would be more noticeable after several weeks. In mice treated with ASO alone, because most of ASO are trapped in endosomes and only slowly released over time, the pharmacological effects may be delayed compared to mice co-treated with UNC7938 in which the released ASO can act more rapidly after the injection.
Considering the clear increase in the amount of ASO detected in the nuclear fraction after treatment with UNC7938, the effect reported on exon skipping levels and dystrophin rescue may appear relatively low. This may be explained by the limited amount of ASO that overall reached the target tissues. Indeed, despite improvement in the design of our tcDNA ASO, conjugated to palmitic acid in order to increase its biodistribution to muscle tissues, the proportion of ASO actually reaching skeletal muscles remains low, as shown in
Figure 3B (between 0.6 and 3 µg/g of tissues). This highlights an important consideration to keep in mind—that the full potential of endosomal escape compounds can only be achieved in combination with efficient delivery of ASO to the target tissue.
Nonetheless, co-treatment of ASO + UNC7938 led to higher dystrophin restoration than treatment with ASO alone, especially in the heart (+31%), the diaphragm (+58%), and the triceps (+82%). This was also confirmed by immunostaining, which revealed a correct localization of dystrophin at the subsarcolemmal space of muscle fibers and higher intensity of staining. Given the particularly strong effect observed in the heart with UNC7938 and considering the importance of cardiac dysfunction in DMD patients, we investigated the cardiac function in treated
mdx mice.
Mdx mice typically show a progressive development of cardiac defects from six months of age [
25,
26], and electrocardiography investigations in the control
mdx mice from this study indeed revealed significant increase in the Tei index, as well as a reduction of the systolic pulse pressure (PP systole) of the left ventricular ejection fraction (LVEF) and of the fractional shortening (FS) compared with WT mice. We found that treatment with ASO alone improved all these parameters, although the difference did not reach statistical significance. It should be noted that we demonstrated, in a previous study, that treatment with tcDNA-ASO alone can significantly improve cardiac function in
mdx mice after 12 weeks of treatment at 50 mg/kg/wk [
6]. In the current study, we selected a lower dose of 30 mg/kg/wk on purpose to be able to detect differences with the UNC7938 co-treated mice. Remarkably,
mdx mice treated with both tcDNA-ASO and UNC7938 presented statistically significant improvement in all parameters, even reaching WT values (non-statistical difference from WT values for all parameters analyzed). This improved effect over the treatment with ASO alone at this dose likely results from higher dystrophin restoration levels in the heart of co-treated mice, but also from a possible earlier restoration. Indeed, as mentioned previously, one of the advantages of OEC molecules is their capacity to quickly release ASO from endosomes and therefore allow ASO to act more rapidly than those which would be slowly released through the depot effect. This may play an important role for cardiac function, which is more likely to be normalized when dystrophin expression has been restored for longer.
All together, our data confirm the potential of OECs, such as UNC7938, to enhance the therapeutic index of exon-skipping ASO for DMD. Yet, this may counterbalance the depot effect, which classically allows long-term effect of ASO. Ultimately, one should aim at keeping some depot effect while releasing sufficient ASO for rapid effect. Considering the high amount of ASO trapped in endosome compartments, believed to be around 99%, freeing up to 50% would suffice to maintain both rapid and long term effects.
Overall, this work establishes the proof of concept that using small molecules facilitating ASO endosomal escape enhances their efficacy in a DMD mouse model, thus opening new therapeutic avenues for combined therapies.