Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Primary Astrocyte Culture
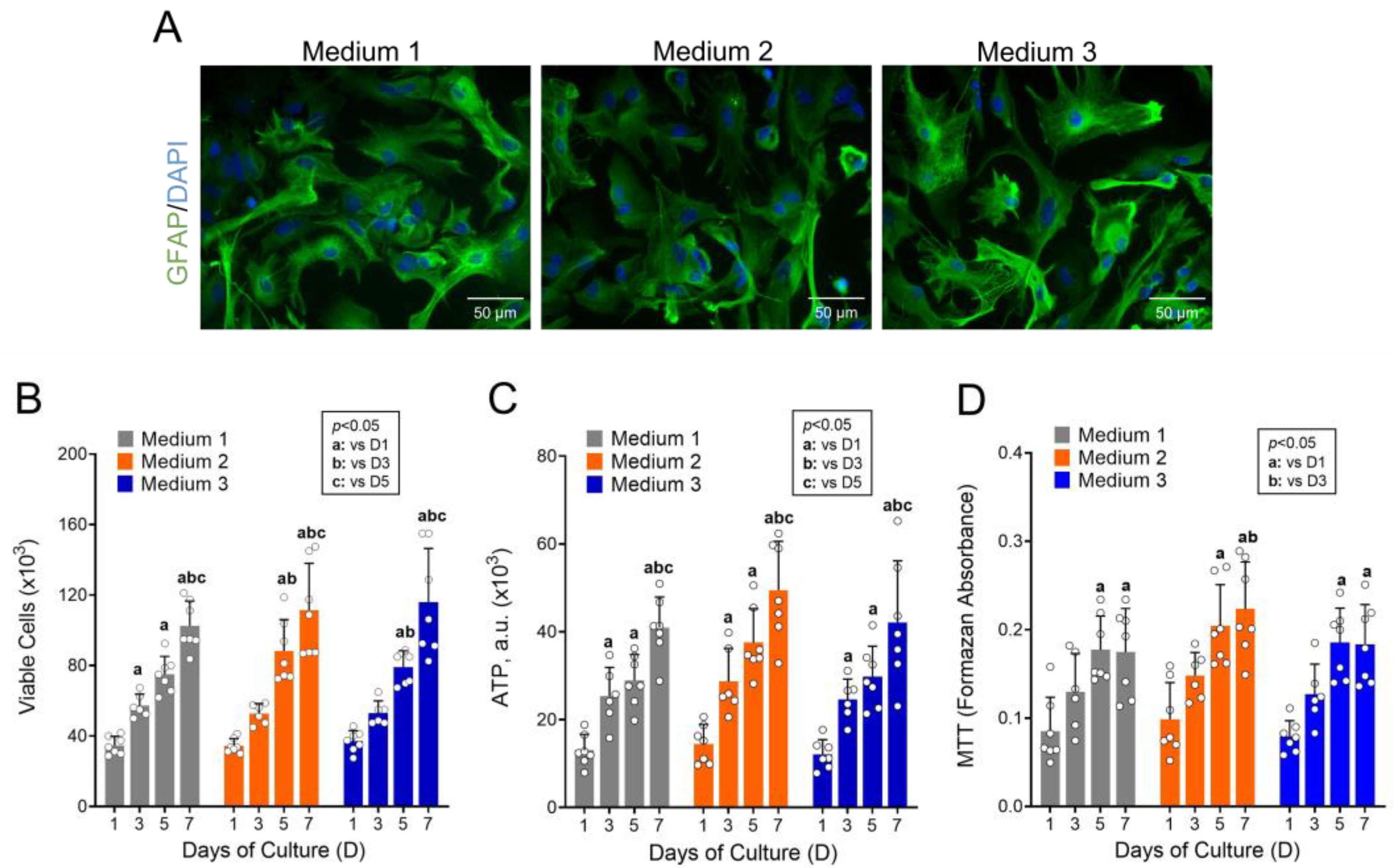
2.4. Culture Medium Optimization
2.5. Oxygen Consumption Rate (OCR) Assessment
2.6. Chronic Treatments of Cell Culture with Mitochondrial Inhibitors
2.7. Cell Counting
2.8. ATP Content
2.9. MTT Assay
2.10. Lactate Dehydrogenase (LDH) Release
2.11. Glucose Consumption and Lactate Release
2.12. Immunocytochemistry
2.13. Statistical Analysis
3. Results
3.1. High Concentrations of Metabolic Substrates Are Not Required to Maintain Astrocyte Growth and Viability
3.2. The Effect of the Mitochondrial Inhibitors Oligomycin and Piericidin A on the Rate of Astrocytes’ Oxygen Consumption (OCR)
3.3. Astrocytes Can Grow and Survive despite the Inhibition of ATP Synthase and Respiratory Complex I
3.4. Proliferating Astrocytes Consume Glucose and Release Lactate to Similar Extents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pellerin, L.; Magistretti, P.J. Sweet Sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, N.; Sonnewald, U.; Schousboe, A. Metabolic Trafficking between Neurons and Astrocytes: The Glutamate/Glutamine Cycle Revisited. Dev. Neurosci. 1995, 17, 203–211. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C. The Glutamate-Glutamine Cycle Is Not Stoichiometric: Fates of Glutamate in Brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Ransom, B.R. Astrocyte Glycogen and Brain Energy Metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neuronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, P.; Fernandez, E.; Bolan, J.P. Underestimation of the Pentose—Phosphate Pathway in Intact Primary Neurons as Revealed by Metabolic Flux Analysis. J. Cereb. Blood Flow Metab. 2013, 33, 1843–1845. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Francisco, A.; Figueira, T.R.; Castilho, R.F. Mitochondrial NAD(P)+ Transhydrogenase: From Molecular Features to Physiology and Disease. Antioxid. Redox Signal. 2022, 36, 864–884. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Weinhouse, S. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Silva, E.; Siqueira-Santos, E.S.; Ruas, J.S.; Ignarro, R.S.; Figueira, T.R.; Rogério, F.; Castilho, R.F. Evaluation of Mitochondrial Respiratory Function in Highly Glycolytic Glioma Cells Reveals Low ADP Phosphorylation in Relation to Oxidative Capacity. J. Neurooncol. 2017, 133, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of Lactate Dehydrogenase A Induces Oxidative Stress and Inhibits Tumor Progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chang, C.P.B.; Tsao, C.C.; Xu, J. Oligomycin-Induced Bioenergetic Adaptation in Cancer Cells with Heterogeneous Bioenergetic Organization. J. Biol. Chem. 2010, 285, 12647–12654. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Vander Heiden, M.G. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell 2015, 162, 552–563. [Google Scholar] [CrossRef]
- Supplie, L.M.; Düking, T.; Campbell, G.; Diaz, F.; Moraes, C.T.; Götz, M.; Hamprecht, B.; Boretius, S.; Mahad, D.; Nave, K.A. Respiration-Deficient Astrocytes Survive as Glycolytic Cells in Vivo. J. Neurosci. 2017, 37, 4231–4242. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Malkov, A.E.; Waseem, T.; Mukhtarov, M.; Buldakova, S.; Gubkina, O.; Zilberter, M.; Zilberter, Y. Glycolysis and Oxidative Phosphorylation in Neurons and Astrocytes during Network Activity in Hippocampal Slices. J. Cereb. Blood Flow Metab. 2014, 34, 397–407. [Google Scholar] [CrossRef]
- Bicego, R.; Francisco, A.; Ruas, J.S.; Siqueira-Santos, E.S.; Castilho, R.F. Undesirable Effects of Chemical Inhibitors of NAD(P)+ Transhydrogenase on Mitochondrial Respiratory Function. Arch. Biochem. Biophys. 2020, 692, 108535. [Google Scholar] [CrossRef]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and Culture of Mouse Cortical Astrocytes. J. Vis. Exp. 2013, 71, e50079. [Google Scholar] [CrossRef]
- Ruas, J.S.; Siqueira-Santos, E.S.; Rodrigues-Silva, E.; Castilho, R.F. High Glycolytic Activity of Tumor Cells Leads to Underestimation of Electron Transport System Capacity When Mitochondrial ATP Synthase Is Inhibited. Sci. Rep. 2018, 8, 17383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Choudhury, G.R.; Winters, A.; Prah, J.; Lin, W.; Liu, R.; Yang, S.H. Hyperglycemia Alters Astrocyte Metabolism and Inhibits Astrocyte Proliferation. Aging Dis. 2018, 9, 674–684. [Google Scholar] [CrossRef]
- Arato-Oshima, T.; Matsui, H.; Wakizaka, A.; Homareda, H. Mechanism Responsible for Oligomycin-Induced Occlusion of Na+ within Na/K-ATPase. J. Biol. Chem. 1996, 271, 25604–25610. [Google Scholar] [CrossRef]
- Ruas, J.S.; Siqueira-Santos, E.S.; Amigo, I.; Rodrigues-Silva, E.; Kowaltowski, A.J.; Castilho, R.F. Underestimation of the Maximal Capacity of the Mitochondrial Electron Transport System in Oligomycin-Treated Cells. PLoS ONE 2016, 11, e0150967. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.F.; Hansson, O.; Ward, M.W.; Budd, S.L.; Nicholls, D.G. Mitochondrial Control of Acute Glutamate Excitotoxicity in Cultured Cerebellar Granule Cells. J. Neurosci. 1998, 18, 10277–10286. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D.; Ghelli, A.; Crimi, M.; Estornell, E.; Fato, R.; Lenaz, G. Complex I and Complex III of Mitochondria Have Common Inhibitorns Acting as Ubiquinone Antagonists. Biochem. Biophys. Res. Commun. 1993, 190, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Methods Mol. Biol. 2018, 1709, 107–127. [Google Scholar] [CrossRef]
- Amat, J.A.; Ishiguro, H.; Nakamura, K.; Norton, W.T. Phenotypic Diversity and Kinetics of Proliferating Microglia and Astrocytes Following Cortical Stab Wounds. Glia 1996, 16, 368–382. [Google Scholar] [CrossRef]
- Buffo, A.; Rite, I.; Tripathi, P.; Lepier, A.; Colak, D.; Horn, A.P.; Mori, T.; Götz, M. Origin and Progeny of Reactive Gliosis: A Source of Multipotent Cells in the Injured Brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Cheng, X.; Huang, X.; Yuan, Y.; Qin, S.; Tan, Z.; Wang, D.; Hu, X.; He, C.; Su, Z. Conditional Ablation of Reactive Astrocytes to Dissect Their Roles in Spinal Cord Injury and Repair. Brain. Behav. Immun. 2019, 80, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Birch, A.M.; Renziehausen, A.W.J.; Zach, C.; Aman, Y.; Steeds, H.; Bonsu, A.; Palmer, E.O.C.; Mirzaei, N.; Ries, M.; et al. Ablation of Reactive Astrocytes Exacerbates Disease Pathology in a Model of Alzheimer’s Disease. Glia 2020, 68, 1017–1030. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte Contribution to Dysfunction, Risk and Progression in Neurodegenerative Disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, R.S.; Bombeiro, A.L.; Chiarotto, G.B.; Cartarozzi, L.P.; Coser, L.d.O.; Ghizoni, E.; Tedeschi, H.; Cendes, F.; Lopes-Cendes, I.; Rogerio, F.; et al. Interferon-Beta Induces Major Histocompatibility Complex of Class I (MHC-I) Expression and a Proinflammatory Phenotype in Cultivated Human Astrocytes. Differentiation 2022, 128, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, J.; Hefti, F. Development of Septal Cholinergic Neurons in Culture: Plating Density and Glial Cells Modulate Effects of NGF on Survival, Fiber Growth, and Expression of Transmitter-Specific Enzymes. J. Neurosci. 1988, 8, 2967–2985. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.E.; Chiu, F.-C. Growth Kinetics, Cell Shape, and the Cytoskeleton of Primary Astrocyte Cultures. J. Neurochem. 1984, 42, 175–184. [Google Scholar] [CrossRef]
- Zhang, W.M.; Natowicz, M.R. Cerebrospinal Fluid Lactate and Pyruvate Concentrations and Their Ratio. Clin. Biochem. 2013, 46, 694–697. [Google Scholar] [CrossRef]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients with Probable Alzheimer’s Disease and Depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef]
- Aasly, J.; Gårseth, M.; Sonnewald, U.; Zwart, J.A.; White, L.R.; Unsgård, G. Cerebrospinal Fluid Lactate and Glutamine Are Reduced in Multiple Sclerosis. Acta Neurol. Scand. 1997, 95, 9–12. [Google Scholar] [CrossRef]
- Denker, N.; Harders, A.R.; Arend, C.; Dringen, R. Consumption and Metabolism of Extracellular Pyruvate by Cultured Rat Brain Astrocytes. Neurochem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Altea-Manzano, P.; Vandekeere, A.; Edwards-Hicks, J.; Roldan, M.; Abraham, E.; Lleshi, X.; Guerrieri, A.N.; Berardi, D.; Wills, J.; Junior, J.M.; et al. Reversal of Mitochondrial Malate Dehydrogenase 2 Enables Anaplerosis via Redox Rescue in Respiration-Deficient Cells. Mol. Cell 2022, 82, 4537–4547.e7. [Google Scholar] [CrossRef] [PubMed]
- Symersky, J.; Osowski, D.; Walters, D.E.; Mueller, D.M. Oligomycin Frames a Common Drug-Binding Site in the ATP Synthase. Proc. Natl. Acad. Sci. USA 2012, 109, 13961–13965. [Google Scholar] [CrossRef] [PubMed]
- Darrouzet, E.; Issartel, J.-P.P.; Lunardi, J.; Dupuis, A. The 49-KDa Subunit of NADH-Ubiquinone Oxidoreductase (Complex I) Is Involved in the Binding of Piericidin and Rotenone, Two Quinone-Related Inhibitors. FEBS Lett. 1998, 431, 34–38. [Google Scholar] [CrossRef]
- Bisbal, M.; Sanchez, M. Neurotoxicity of the Pesticide Rotenone on Neuronal Polarization: A Mechanistic Approach. Neural Regen. Res. 2019, 14, 762–766. [Google Scholar] [CrossRef]
- Zdrazilova, L.; Hansikova, H.; Gnaiger, E. Comparable Respiratory Activity in Attached and Suspended Human Fibroblasts. PLoS ONE 2022, 17, e0264496. [Google Scholar] [CrossRef]
- Brookes, P.S. Mitochondrial H + Leak and ROS Generation: An Odd Couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Kennedy, J. Distribution, Subcellular Localization, and Product Inhibition of Dihydroorotate Oxidation in the Rat. Arch. Biochem. Biophys. 1973, 157, 369–373. [Google Scholar] [CrossRef]
- Miyake, S.; Masuda, S. Inhibition of Mitochondrial Complex III or Dihydroorotate Dehydrogenase (DHODH) Triggers Formation of Poly (A) RNA Foci Adjacent to Nuclear Speckles Following Activation of ATM (Ataxia Telangiectasia Mutated). RNA Biol. 2022, 19, 1244–1255. [Google Scholar] [CrossRef]
- Khutornenko, A.A.; Roudko, V.V.; Chernyak, B.V.; Vartapetian, A.B.; Chumakov, P.M.; Evstafieva, A.G. Pyrimidine Biosynthesis Links Mitochondrial Respiration to the P53 Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12828–12833. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate Metabolism in Human Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Lactate Metabolism: The Discoveries and the Controversies. J. Cereb. Blood Flow Metab. 2012, 32175, 1107–1138. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Z.; Yang, S.; Zhu, Y.; Xue, M.; Zhang, J.; Leng, L. Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases. CNS Neurosci. Ther. 2023, 29, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liang, J.; Zhou, B. Glucose Metabolic Dysfunction in Neurodegenerative Diseases—New Mechanistic Insights and the Potential of Hypoxia as a Prospective Therapy Targeting Metabolic Reprogramming. Int. J. Mol. Sci. 2021, 22, 5887. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Almeida, J.; Bolaños, J.P.; Moncada, S. Different Responses of Astrocytes and Neurons to Nitric Oxide: The Role of Glycolytically Generated ATP in Astrocyte Protection. Proc. Natl. Acad. Sci. USA 2001, 98, 15294–15299. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.A.; Dalla Costa, A.P.; Ruas, J.S.; Siqueira-Santos, E.S.; Francisco, A.; Castilho, R.F. Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation. Cells 2023, 12, 683. https://doi.org/10.3390/cells12050683
Silva EA, Dalla Costa AP, Ruas JS, Siqueira-Santos ES, Francisco A, Castilho RF. Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation. Cells. 2023; 12(5):683. https://doi.org/10.3390/cells12050683
Chicago/Turabian StyleSilva, Ellen A., Ana P. Dalla Costa, Juliana S. Ruas, Edilene S. Siqueira-Santos, Annelise Francisco, and Roger F. Castilho. 2023. "Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation" Cells 12, no. 5: 683. https://doi.org/10.3390/cells12050683
APA StyleSilva, E. A., Dalla Costa, A. P., Ruas, J. S., Siqueira-Santos, E. S., Francisco, A., & Castilho, R. F. (2023). Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation. Cells, 12(5), 683. https://doi.org/10.3390/cells12050683






