Microbiological Aspects of Pharmaceutical Manufacturing of Adipose-Derived Stem Cell-Based Medicinal Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Sterility Testing of the Biological Material
2.2. Environmental Monitoring of Microbiological Quality
2.3. Statistical Analysis
3. Results
3.1. Detection of Microbial Contamination in the Biological Material
3.2. Identification of Microbial Contamination in Biological Material
3.3. Environmental Monitoring of Microbiological Quality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F. Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef]
- Eom, Y.W.; Lee, J.E.; Yang, M.S.; Jang, I.K.; Kim, H.E.; Lee, D.H.; Kim, Y.J.; Park, W.J.; Kong, J.H.; Shim, K.Y.; et al. Rapid Isolation of Adipose Tissue-Derived Stem Cells by the Storage of Lipoaspirates. Yonsei Med. J. 2011, 52, 999–1007. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal Stem Cells Isolated from Adipose and Other Tissues: Basic Biological Properties and Clinical Applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Tabatabaei Qomi, R.T.; Sheykhhasan, M. Adipose-derived stromal cell in regenerative medicine: A review. World J. Stem Cells 2017, 9, 107–117. [Google Scholar] [CrossRef]
- Clauser, L.; Lucchi, A.; Tocco-Tussardi, I.; Gardin, C.; Zavan, B. Autologous Fat Transfer for Facial Augmentation and Regeneration: Role of Mesenchymal Stem Cells. Atlas Oral Maxillofac. Surg. Clin. North Am. 2018, 26, 25–32. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 6, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Coalson, E.; Bishop, E.; Liu, W.; Feng, Y.; Spezia, M.; Liu, B.; Shen, Y.; Wu, D.; Du, S.; Li, A.J. Stem cell therapy for chronic skin wounds in the era of personalized medicine: From bench to bedside. Genes Dis. 2019, 6, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Farge, D.; Loisel, S.; Lansiaux, P.; Tarte, K. Mesenchymal stromal cells for systemic sclerosis treatment. Autoimmun. Rev. 2021, 20, 102755. [Google Scholar] [CrossRef]
- Liau, L.L.; Looi, Q.H.; Chia, W.C.; Subramaniam, T.; Ng, M.H.; Law, J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020, 10, 112. [Google Scholar] [CrossRef]
- Detela, G.; Lodge, A. EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. Mol. Ther. Methods Clin. Dev. 2019, 13, 205–232. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two Decades of Global Progress in Authorized Advanced Therapy Medicinal Products: An Emerging Revolution in Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef]
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Hu, Z.; Yang, Y.-G.; Zhou, Q.; Sun, L.; Wu, J. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: A systematic review. Stem Cell Res. Ther. 2022, 13, 93. [Google Scholar] [CrossRef]
- Galipeau, J. Mesenchymal Stromal Cells for Graft-versus-Host Disease: A Trilogy. Biol. Blood Marrow Transplant. 2020, 26, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Ham, R.M.T.; Hoekman, J.; Hövels, A.M.; Broekmans, A.W.; Leufkens, H.G.; Klungel, O.H. Challenges in Advanced Therapy Medicinal Product Development: A Survey among Companies in Europe. Mol. Ther. Methods Clin. Dev. 2018, 11, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Fontana, L.I. A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges with Current Regulatory Expectations. Front. Med. 2022, 9, 855100. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off. J. Eur. Union 2007, 50, 121–137. [Google Scholar]
- Reflection Paper on Classification of Advanced Therapy Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-classification-advanced-therapy-medicinal-products_en-0.pdf (accessed on 30 January 2023).
- European Commission. EudraLex The Rules Governing Medicinal Products in the European Union Volume 4: Good Manufacturing Practice Guidelines on Good Manufacturing Practice Specific to Advanced Therapy Medicinal Products. 2017. Available online: https://health.ec.europa.eu/system/files/2017-11/2017_11_22_guidelines_gmp_for_atmps_0.pdf (accessed on 30 January 2023).
- Cessak, G. Polish Pharmacopoeia, Xth ed.; Office for Registration of Medicinal Products Medical Devices and Biocidal Products: Warsaw, Poland, 2014; Volume 1 and 2, ISBN 978-83-63724-47-4. [Google Scholar]
- Chiarini, A.; Palmeri, A.; Amato, T.; Immordino, R.; Distefano, S.; Giammanco, A. Detection of Bacterial and Yeast Species with the Bactec 9120 Automated System with Routine Use of Aerobic, Anaerobic, and Fungal Media. J. Clin. Microbiol. 2008, 46, 4029–4033. [Google Scholar] [CrossRef]
- Cessak, G. Polish Pharmacopoeia, XIIth ed.; Office for Registration of Medicinal Products Medical Devices and Biocidal Products: Warsaw, Poland, 2020; Volume 1, ISBN 978-83-8172-541-5. [Google Scholar]
- Labedz-Maslowska, A.; Szkaradek, A.; Mierzwinski, T.; Madeja, Z.; Zuba-Surma, E. Processing and Ex Vivo Expansion of Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells for the Development of an Advanced Therapy Medicinal Product for use in Humans. Cells 2021, 10, 1908. [Google Scholar] [CrossRef]
- Lechanteur, C.; Briquet, A.; Bettonville, V.; Baudoux, E.; Beguin, Y. MSC Manufacturing for Academic Clinical Trials: From a Clinical-Grade to a Full GMP-Compliant Process. Cells 2021, 10, 1320. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.-F.; Applegate, L.A.; Martin, R. Retrospective Analysis of Autologous Chondrocyte-Based Cytotherapy Production for Clinical Use: GMP Process-Based Manufacturing Optimization in a Swiss University Hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Pedrini, O.; Capelli, C.; Gotti, E.; Borleri, G.; Magri, M.; Vailati, F.; Passera, M.; Farina, C.; Rambaldi, A.; et al. Utility of routine evaluation of sterility of cellular therapy products with or without extensive manipulation: Best practices and clinical significance. Cytotherapy 2018, 20, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Santos, J.M.; de Almeida, J.M.; Filipe, M.A.; de Almeida, M.V.T.; Almeida, S.C.P.; Água-Doce, A.; Varela, A.; Gilljam, M.; Stellan, B.; et al. Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: Quality and safety data. Stem Cell Res. Ther. 2014, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Vériter, S.; André, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.-L.; Dufrane, D. Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption” Clinical Applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F.; Stacey, G.N.; Cortés, J.L.; Concha, A. Environmental monitoring in stem cell banks. Appl. Microbiol. Biotechnol. 2006, 70, 651–662. [Google Scholar] [CrossRef]
- Tršan, M.; Seme, K.; Srčič, S. The environmental monitoring in hospital pharmacy cleanroom and microbiota catalogue preparation. Saudi Pharm. J. 2019, 27, 455–462. [Google Scholar] [CrossRef]
- Martín, P.G.; González, M.B.; Martínez, A.R.; Lara, V.G.; Naveros, B.C. Isolation and characterization of the environmental bacterial and fungi contamination in a pharmaceutical unit of mesenchymal stem cell for clinical use. Biologicals 2012, 40, 330–337. [Google Scholar] [CrossRef]
- Ottria, G.; Dallera, M.; Aresu, O.; Manniello, M.; Parodi, B.; Spagnolo, A.; Cristina, M.L. Environmental monitoring programme in the cell therapy facility of a research centre: Preliminary investigation. J. Prev. Med. Hyg. 2010, 51, 133–138. [Google Scholar] [CrossRef]
- Cobo, F.; Concha, A. Environmental microbial contamination in a stem cell bank. Lett. Appl. Microbiol. 2007, 44, 379–386. [Google Scholar] [CrossRef]
- Napoli, C.; Marcotrigiano, V.; Montagna, M.T. Air sampling procedures to evaluate microbial contamination: A comparison between active and passive methods in operating theatres. BMC Public Health 2012, 12, 594. [Google Scholar] [CrossRef]
- Veronesi, L.; Colucci, M.E.; Napoli, C.; Castiglia, P.; Liguori, G.; Torre, I.; Righi, E.; Farruggia, P.; Tesauro, M.; Montagna, M.T.; et al. Air microbial contamination in dental clinics: Comparison between active and passive methods. Acta Biomed. 2020, 91, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Andon, B.M. Active air vs. passive air (settle plate) monitoring in routine environmental monitoring programs. PDA J. Pharm. Sci. Technol. 2006, 60, 350–355. [Google Scholar] [PubMed]
- Sandle, T. A Review of Cleanroom Microflora: Types, Trends, and Patterns. PDA J. Pharm. Sci. Technol. 2011, 65, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.M.; Lourenço, F.R. Rapid microbiological methods (RMMs) for evaluating the activity of cephalosporin antibiotics employing triphenyltetrazolium chloride. Talanta 2018, 185, 520–527. [Google Scholar] [CrossRef]
- McCormick, P.J.; Schoene, M.J.; Dehmler, M.A.; Kaiser, J.J.; Norton, S.E.; Pizon, A.; Fornalik, M.A. Evaluation of a Rapid Microbiological Method with a Mixed Culture Biofilm Model. PDA J. Pharm. Sci. Technol. 2013, 67, 512–532. [Google Scholar] [CrossRef]
- Bugno, A.; Saes, D.P.S.; Almodovar, A.A.B.; Dua, K.; Awasthi, R.; Ghisleni, D.D.M.; Hirota, M.T.; de Oliveira, W.A.; Pinto, T.D.J.A. Performance Survey and Comparison Between Rapid Sterility Testing Method and Pharmacopoeia Sterility Test. J. Pharm. Innov. 2017, 13, 27–35. [Google Scholar] [CrossRef]
- Parveen, S.; Kaur, S.; David, S.A.W.; Kenney, J.L.; McCormick, W.M.; Gupta, R.K. Evaluation of growth based rapid microbiological methods for sterility testing of vaccines and other biological products. Vaccine 2011, 29, 8012–8023. [Google Scholar] [CrossRef]
- Suessner, S.; Hennerbichler, S.; Schreiberhuber, S.; Stuebl, D.; Gabriel, C. Validation of an alternative microbiological method for tissue products. Cell Tissue Bank. 2014, 15, 277–286. [Google Scholar] [CrossRef]
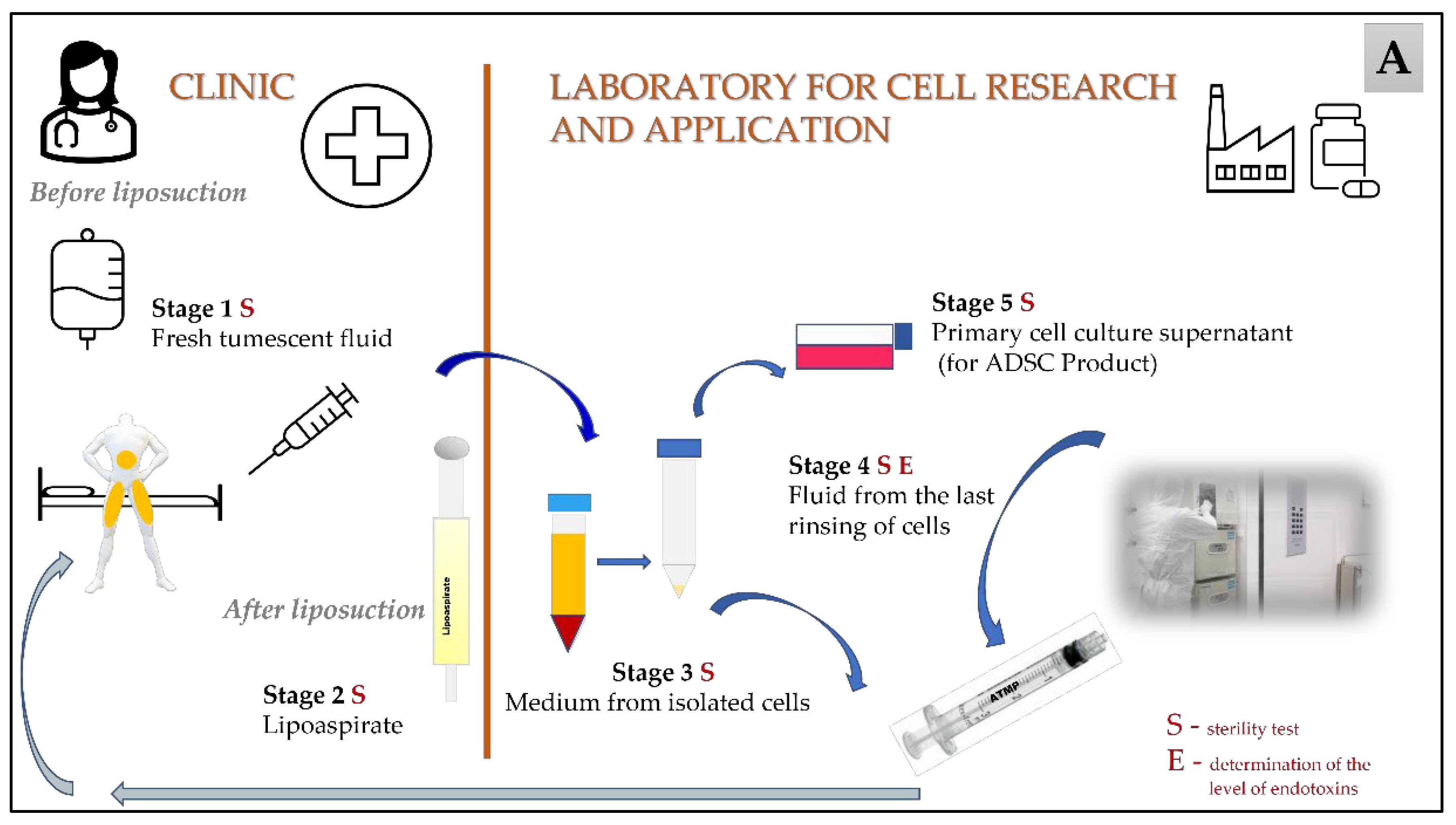
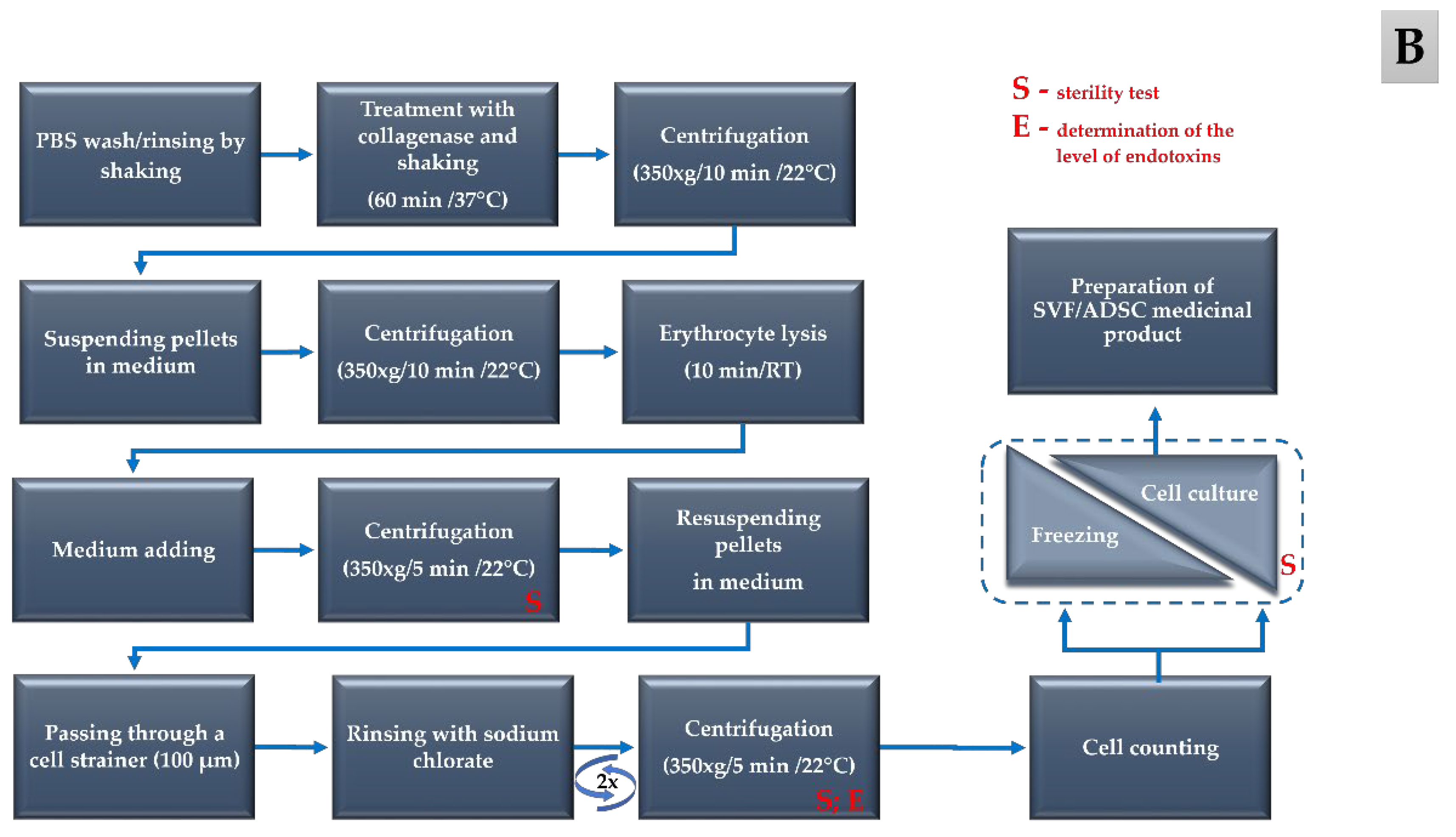

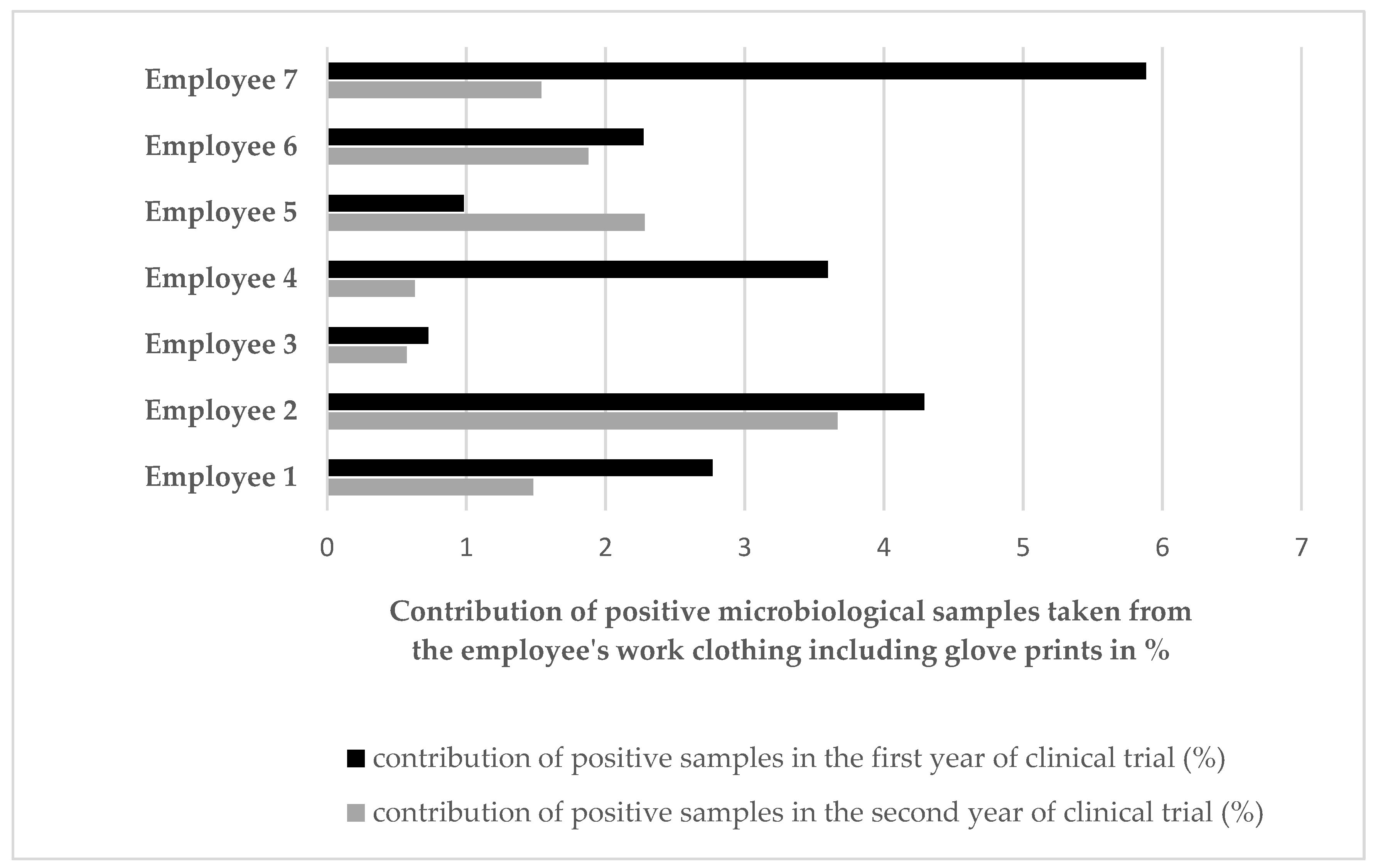
| Year of Clinical Trial | Total Number of Samples | Stage 1 Fresh Tumescent Fluid | Stage 2 Lipoaspirate | Stage 3 Medium from the Isolated Cells | Stage 4 Medium from the Last Wash of Cells | Stage 5 Cell Culture Supernatant | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Samples | Positive/% Contaminated | Number of Samples | Positive/% Contaminated | Number of Samples | Positive/% Contaminated | Number of Samples | Positive/% Contaminated | Number of Samples | Positive/% Contaminated | ||
| Year 1 | 276 | 56 | 1/1.8 | 56 | 25/44.6 | 56 | 8/14.3 | 56 | 3/5.4 | 52 | 0/0 |
| Year 2 | 222 | 44 | 1/2.3 | 44 | 16/36.4 | 44 | 4/9.1 | 44 | 3/6.8 | 46 | 0/0 |
| Total | 498 | 100 | 2 | 100 | 41 | 100 | 12 | 100 | 6 | 98 | 0 |
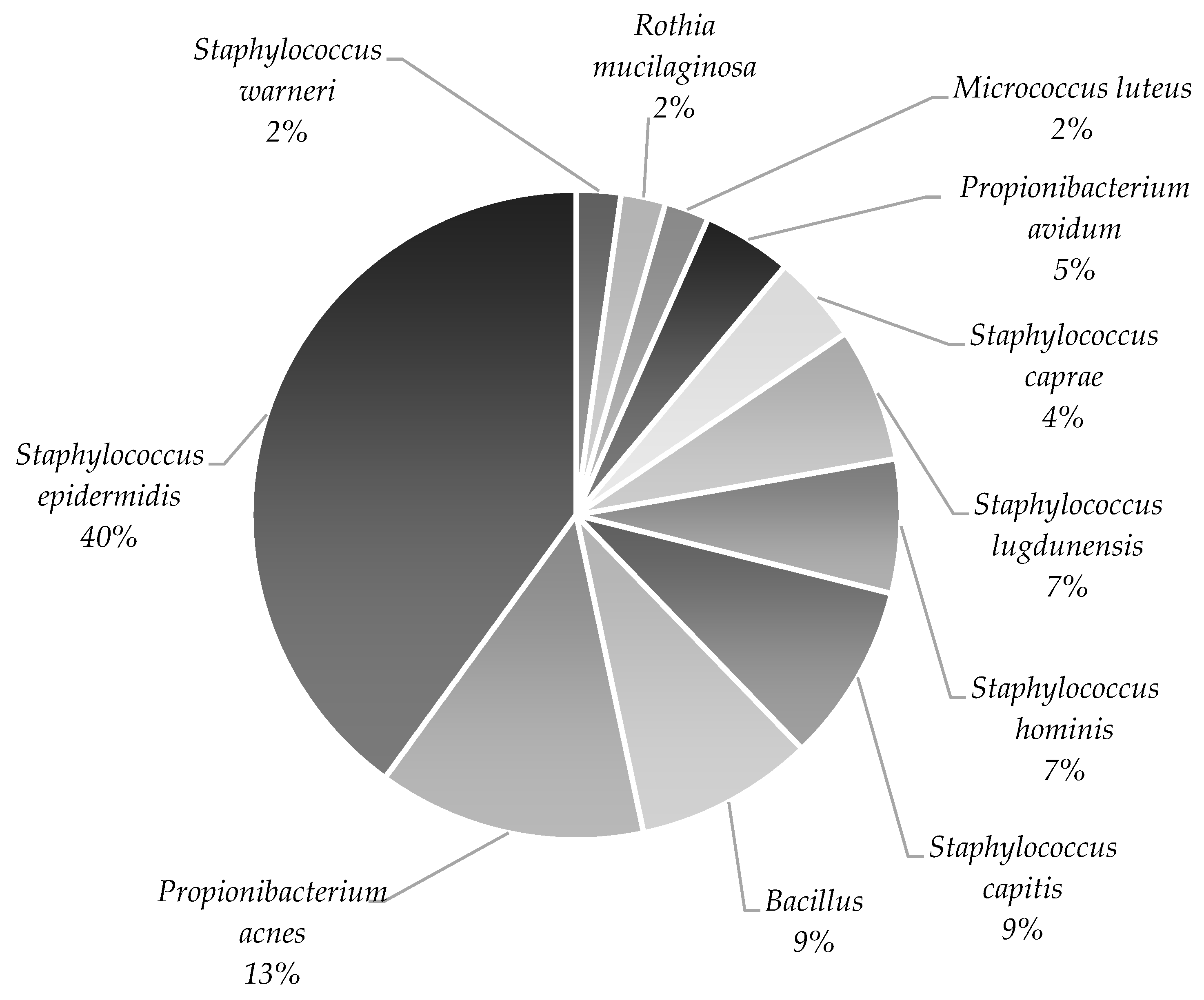
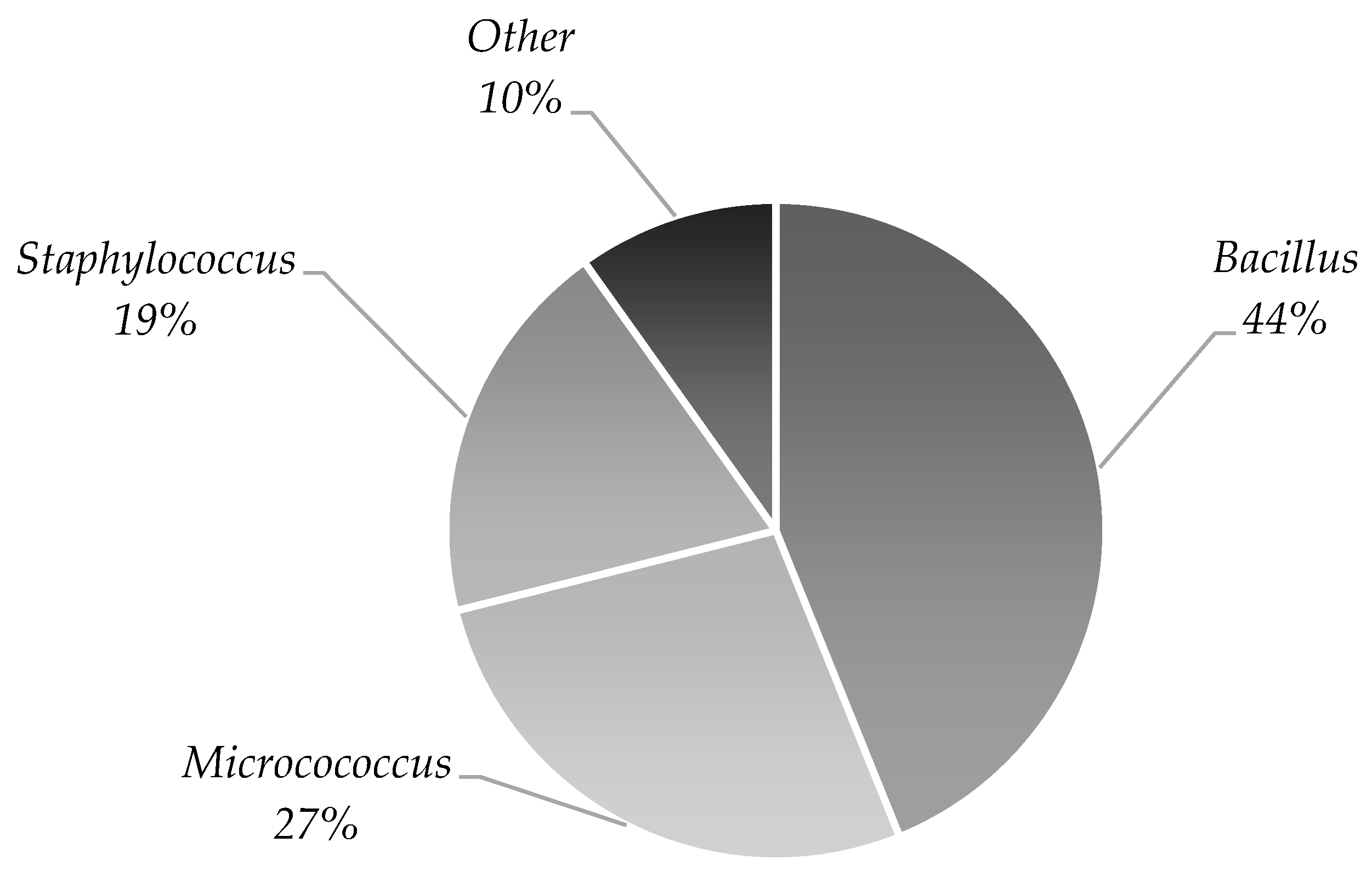
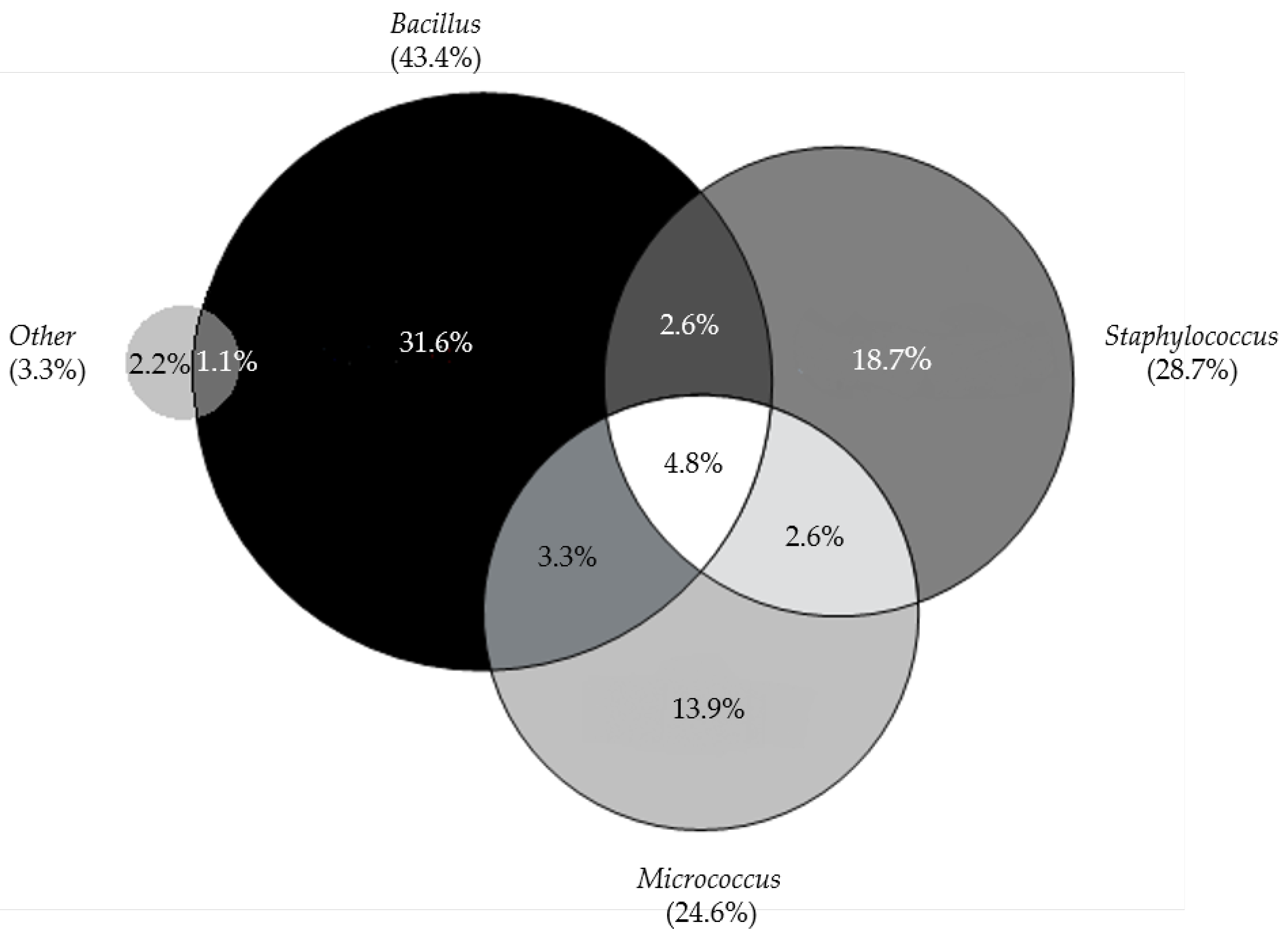
| Microorganism | Stage | ||
|---|---|---|---|
| Stage 2 Lipoaspirate | Stage 3 Medium from the Isolated Cells | Stage 4 Medium from the Last Cell Wash | |
| Staphylococcus epidermidis | 22 | 5 | 2 |
| Propionibacterium acnes | 6 | 4 | 2 |
| Bacillus | 4 | ||
| Staphylococcus capitis | 4 | 1 | 1 |
| Staphylococcus hominis | 4 | ||
| Staphylococcus lugdunensis | 3 | 1 | |
| Staphylococcus caprae | 2 | 1 | |
| Propionibacterium avidum | 2 | 1 | 1 |
| Micrococcus luteus | 1 | ||
| Rothia mucilaginosa | 1 | ||
| Staphylococcus warneri | 1 | ||
| Year of Manufacturing for Clinical Trial | Number of Samples Collected from All Monitored Grade Areas | ||
|---|---|---|---|
| Surface Sampling (Contact Plates) | Passive Air Sampling (Settle Plates) | Active Air Sampling (Volumetric Sampling) | |
| Year 1 | 11,670 | 4731 | 128 |
| Year 2 | 7588 | 3389 | 128 |
| Total | 19,258 | 8120 | 256 |
| Sampling Surface | GMP Grade | Two-Year Manufacturing Period | Differences between Years | |||||
|---|---|---|---|---|---|---|---|---|
| Year One | Year Two | |||||||
| Total Number of Samples | Number of Positive Samples | % of Positive Samples | Total Number of Samples | Number of Positive Samples | % of Positive Samples | Statistical Significance | ||
| Cleanroom windows | B | 307 | 21 | 6.84 | 417 | 12 | 2.88 | p < 0.05 |
| Floor | B | 2200 | 45 | 2.05 | 1498 | 18 | 1.20 | ns |
| Door handles | B | 1300 | 30 | 2.31 | 875 | 3 | 0.34 | p < 0.001 |
| Walls | B | 1277 | 5 | 0.39 | 563 | 1 | 0.18 | ns |
| Devices | B | 1438 | 5 | 0.35 | 1005 | 1 | 0.10 | ns |
| Working surfaces | A + B | 2863 | 5 | 0.17 | 1901 | 0 | 0.00 | ns |
| Laminar air flow chamber | A | 2708 | 3 | 0.11 | 1798 | 0 | 0.00 | ns |
| Tables | B | 155 | 2 | 1.29 | 103 | 0 | 0.00 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabłowska-Gadomska, I.; Humięcka, M.; Brzezicka, J.; Chróścicka, A.; Płaczkowska, J.; Ołdak, T.; Lewandowska-Szumiel, M. Microbiological Aspects of Pharmaceutical Manufacturing of Adipose-Derived Stem Cell-Based Medicinal Products. Cells 2023, 12, 680. https://doi.org/10.3390/cells12050680
Szabłowska-Gadomska I, Humięcka M, Brzezicka J, Chróścicka A, Płaczkowska J, Ołdak T, Lewandowska-Szumiel M. Microbiological Aspects of Pharmaceutical Manufacturing of Adipose-Derived Stem Cell-Based Medicinal Products. Cells. 2023; 12(5):680. https://doi.org/10.3390/cells12050680
Chicago/Turabian StyleSzabłowska-Gadomska, Ilona, Monika Humięcka, Joanna Brzezicka, Anna Chróścicka, Joanna Płaczkowska, Tomasz Ołdak, and Malgorzata Lewandowska-Szumiel. 2023. "Microbiological Aspects of Pharmaceutical Manufacturing of Adipose-Derived Stem Cell-Based Medicinal Products" Cells 12, no. 5: 680. https://doi.org/10.3390/cells12050680
APA StyleSzabłowska-Gadomska, I., Humięcka, M., Brzezicka, J., Chróścicka, A., Płaczkowska, J., Ołdak, T., & Lewandowska-Szumiel, M. (2023). Microbiological Aspects of Pharmaceutical Manufacturing of Adipose-Derived Stem Cell-Based Medicinal Products. Cells, 12(5), 680. https://doi.org/10.3390/cells12050680







