Role of Astrocytes in Parkinson’s Disease Associated with Genetic Mutations and Neurotoxicants
Abstract
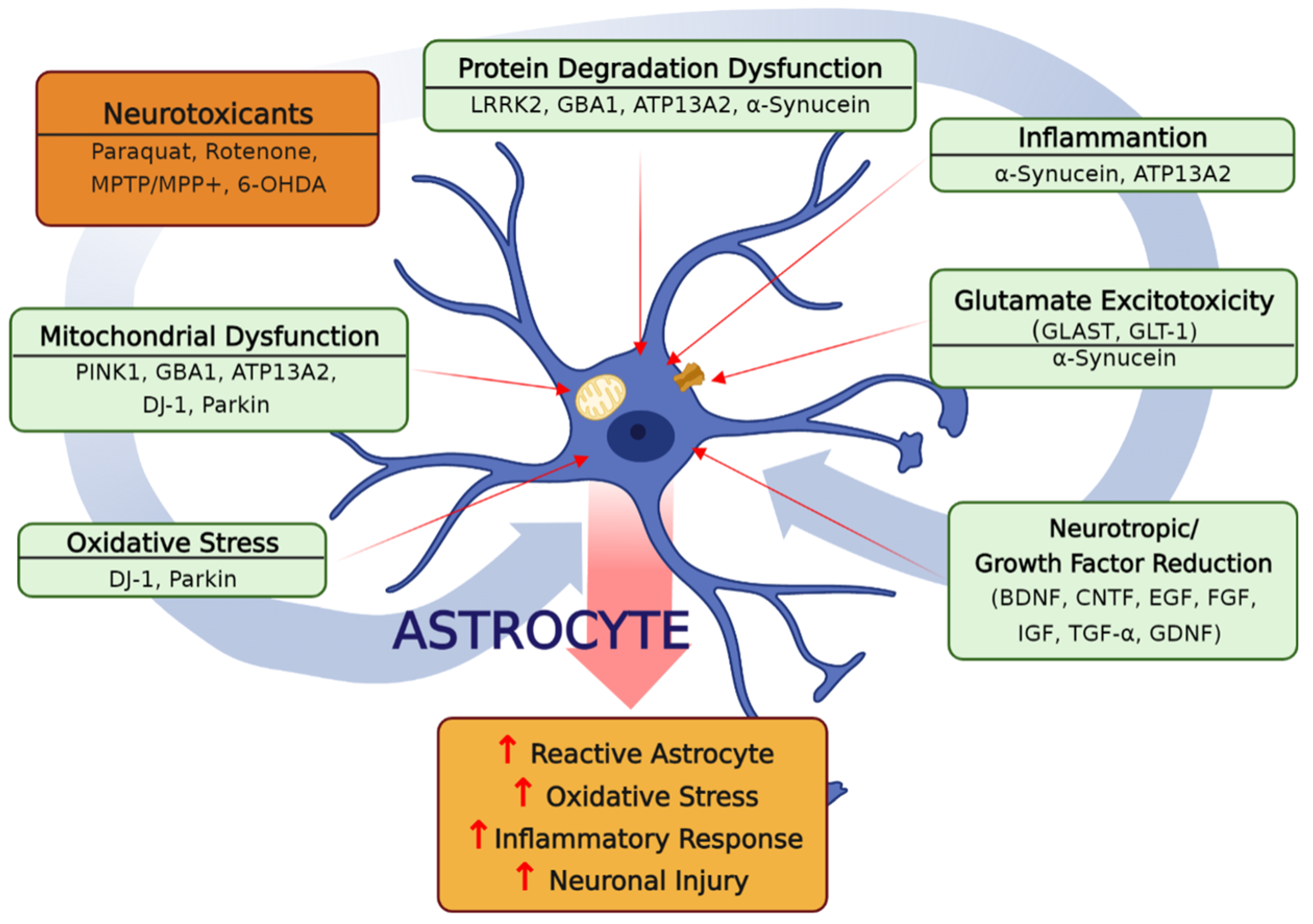
1. Introduction
2. Role of Astrocytes in PD-Related Gene Mutations-Induced Pathology
2.1. DJ-1
2.2. α-Synuclein
2.3. ATP13A2
2.4. LRRK2
2.5. PINK1 and Parkin
2.5.1. PINK1
2.5.2. Parkin
2.5.3. PINK1/Parkin Mitophagy
2.6. Glucocerebrosidase
2.7. Prospectives
3. The Dysregulations of Astrocytic Cellular and Molecular Mechanisms in Experimental PD Models
3.1. The Role of Astrocytic Oxidative Stress in PD Development
3.2. The Role of Astrocytic Inflammation in PD Development
3.3. Dysregulation of Astrocytic Degradation of Damaged Proteins in PD Development
3.4. The Role of Astrocytes in Inducing Excitotoxicity in PD Development
3.5. Dysregulation of Astrocytic Growth Factor Synthesis in PD Development
3.6. The Role of Astrocytic ER Stress in PD Development
3.7. The Role of Astrocytic Apoptosis in PD Development
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lieberman, A.; Dziatolowski, M.; Gopinathan, G.; Kupersmith, M.; Neophytides, A.; Korein, J. Evaluation of Parkinson’s disease. Adv. Biochem. Psychopharmacol. 1980, 23, 277–286. [Google Scholar]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef]
- Shadrina, M.; Slominsky, P. Modeling Parkinson’s Disease: Not Only Rodents? Front. Aging Neurosci. 2021, 13, 695718. [Google Scholar] [CrossRef]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous alpha-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef]
- Gu, X.L.; Long, C.X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain. 2010, 3, 12. [Google Scholar] [CrossRef]
- Diniz, L.P.; Araujo, A.P.B.; Matias, I.; Garcia, M.N.; Barros-Aragão, F.G.; Reis, R.A.D.M.; Foguel, D.; Braga, C.; Figueiredo, C.P.; Romão, L.; et al. Astrocyte glutamate transporters are increased in an early sporadic model of synucleinopathy. Neurochem. Int. 2020, 138, 104758. [Google Scholar] [CrossRef]
- Schmidt, S.; Linnartz, B.; Mendritzki, S.; Sczepan, T.; Lubbert, M.; Stichel, C.C.; Lubbert, H. Genetic mouse models for Parkinson’s disease display severe pathology in glial cell mitochondria. Hum. Mol. Genet. 2011, 20, 1197–1211. [Google Scholar] [CrossRef]
- Khasnavis, S.; Pahan, K. Cinnamon Treatment Upregulates Neuroprotective Proteins Parkin and DJ-1 and Protects Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2014, 9, 569–581. [Google Scholar] [CrossRef]
- Choi, I.; Kim, J.; Jeong, H.K.; Kim, B.; Jou, I.; Park, S.M.; Chen, L.; Kang, U.J.; Zhuang, X.; Joe, E.H. PINK1 deficiency attenuates astrocyte proliferation through mitochondrial dysfunction, reduced AKT and increased p38 MAPK activation, and downregulation of EGFR. Glia 2013, 61, 800–812. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.S.; Park, J.Y.; Suh, Y.H.; Jou, I.; Joe, E.H.; Park, S.M. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum. Mol. Genet. 2013, 22, 4805–4817. [Google Scholar] [CrossRef]
- Kim, J.M.; Cha, S.H.; Choi, Y.R.; Jou, I.; Joe, E.H.; Park, S.M. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci. Rep. 2016, 6, 28823. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hinkle, D.A. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J. Neurochem. 2011, 117, 375–387. [Google Scholar] [CrossRef]
- Manzoni, C.; Mamais, A.; Dihanich, S.; Abeti, R.; Soutar, M.P.M.; Plun-Favreau, H.; Giunti, P.; Tooze, S.A.; Bandopadhyay, R.; Lewis, P.A. Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochim. Biophys. Acta 2013, 1833, 2900–2910. [Google Scholar] [CrossRef]
- Henry, A.G.; Aghamohammadzadeh, S.; Samaroo, H.; Chen, Y.; Mou, K.; Needle, E.; Hirst, W.D. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 2015, 24, 6013–6028. [Google Scholar] [CrossRef]
- Qiao, C.; Yin, N.; Gu, H.-Y.; Zhu, J.-L.; Ding, J.-H.; Lu, M.; Hu, G. Atp13a2 Deficiency Aggravates Astrocyte-Mediated Neuroinflammation via NLRP3 Inflammasome Activation. CNS Neurosci. Ther. 2016, 22, 451–460. [Google Scholar] [CrossRef]
- Ran, C. Strong association between glucocerebrosidase mutations and Parkinson’s disease in Sweden. Neurobiol. Aging 2016, 45, 212.e5–212.e11. [Google Scholar] [CrossRef]
- Canet-Aviles, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef]
- Ahn, E.H.; Lei, K.; Kang, S.S.; Wang, Z.H.; Liu, X.; Hong, W.; Wang, Y.T.; Edgington-Mitchell, L.E.; Jin, L.; Ye, K. Mitochondrial dysfunction triggers the pathogenesis of Parkinson’s disease in neuronal C/EBPbeta transgenic mice. Mol. Psychiatry 2021, 26, 7838–7850. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Wang, B.; Abraham, N.; Gao, G.; Yang, Q. Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 19. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Li, R.; Yang, H. Mitophagy in Parkinson’s Disease: From Pathogenesis to Treatment. Cells 2019, 8, 712. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [CrossRef]
- Kettenmann, H.; Verkhratsky, A. Neuroglia: The 150 years after. Trends Neurosci. 2008, 31, 653–659. [Google Scholar] [CrossRef]
- Jakel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef]
- Halliday, G.M.; Stevens, C.H. Glia: Initiators and progressors of pathology in Parkinson’s disease. Mov. Disord. 2011, 26, 6–17. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M.; Hertz, L. Why are astrocytes important? Neurochem Res. 2015, 40, 389–401. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Honbou, K.; Suzuki, N.N.; Horiuchi, M.; Niki, T.; Taira, T.; Ariga, H.; Inagaki, F. The crystal structure of DJ-1, a protein related to male fertility and Parkinson’s disease. J. Biol. Chem. 2003, 278, 31380–31384. [Google Scholar] [CrossRef]
- Ariga, H.; Takahashi-Niki, K.; Kato, I.; Maita, H.; Niki, T.; Iguchi-Ariga, S.M. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 683920. [Google Scholar] [CrossRef]
- Bandopadhyay, R.; Kingsbury, A.E.; Cookson, M.R.; Reid, A.R.; Evans, I.M.; Hope, A.D.; Pittman, A.M.; Lashley, T.; Canet-Aviles, R.; Miller, D.W.; et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain 2004, 127, 420–430. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hamilton, R.L.; Hinkle, D.A. DJ-1 immunoreactivity in human brain astrocytes is dependent on infarct presence and infarct age. Neuropathology 2009, 29, 125–131. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Invest. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Butchbach, M.E.; Tian, G.; Guo, H.; Lin, C.L. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: Importance for excitatory amino acid transporter localization and function. J. Biol. Chem. 2004, 279, 34388–34396. [Google Scholar] [CrossRef]
- Roberts, P.J.; Davies, S.W. Excitatory receptors and their role in excitotoxicity. Biochem. Soc. Trans. 1987, 15, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.K.; Hinds, A.I.; Hanneman, W.H.; Tjalkens, R.B.; Legare, M.E. DJ-1 mutation decreases astroglial release of inflammatory mediators. Neurotoxicology 2016, 52, 198–203. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hinkle, D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009, 33, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lev, N.; Barhum, Y.; Ben-Zur, T.; Melamed, E.; Steiner, I.; Offen, D. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013, 50, 542–550. [Google Scholar] [CrossRef]
- Larsen, N.J.; Ambrosi, G.; Mullett, S.J.; Berman, S.B.; Hinkle, D.A. DJ-1 knock-down impairs astrocyte mitochondrial function. Neuroscience 2011, 196, 251–264. [Google Scholar] [CrossRef]
- Bantle, C.M.; Hirst, W.D.; Weihofen, A.; Shlevkov, E. Mitochondrial Dysfunction in Astrocytes: A Role in Parkinson’s Disease? Front. Cell. Dev. Biol. 2020, 8, 608026. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Li, Y.; Zhou, Y.; Li, L.; Lei, S.; Yu, S.; Zhao, Y. Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury. J. Mol. Med. 2019, 97, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.J.; McCoy, M.K.; Blackinton, J.; Beilina, A.; van der Brug, M.; Sandebring, A.; Miller, D.; Maric, D.; Cedazo-Minguez, A.; Cookson, M.R. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 2011, 20, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Bjorkblom, B.; Adilbayeva, A.; Maple-Grodem, J.; Piston, D.; Okvist, M.; Xu, X.M.; Brede, C.; Larsen, J.P.; Moller, S.G. Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity. J. Biol. Chem. 2013, 288, 22809–22820. [Google Scholar] [CrossRef] [PubMed]
- Waak, J.; Weber, S.S.; Waldenmaier, A.; Gorner, K.; Alunni-Fabbroni, M.; Schell, H.; Vogt-Weisenhorn, D.; Pham, T.T.; Reumers, V.; Baekelandt, V.; et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009, 23, 2478–2489. [Google Scholar] [CrossRef]
- Choi, D.J.; Yang, H.; Gaire, S.; Lee, K.A.; An, J.; Kim, B.G.; Jou, I.; Park, S.M.; Joe, E.H. Critical roles of astrocytic-CCL2-dependent monocyte infiltration in a DJ-1 knockout mouse model of delayed brain repair. Glia 2020, 68, 2086–2101. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Bonini, N.M.; Giasson, B.I. Snaring the function of alpha-synuclein. Cell 2005, 123, 359–361. [Google Scholar] [CrossRef]
- Moore, S.A.; Yoder, E.; Murphy, S.; Dutton, G.R.; Spector, A.A. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J. Neurochem. 1991, 56, 518–524. [Google Scholar] [CrossRef]
- Solano, S.M.; Miller, D.W.; Augood, S.J.; Young, A.B.; Penney, J.B., Jr. Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: Genes associated with familial Parkinson’s disease. Ann. Neurol. 2000, 47, 201–210. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in alpha-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Castagnet, P.I.; Golovko, M.Y.; Barcelo-Coblijn, G.C.; Nussbaum, R.L.; Murphy, E.J. Fatty acid incorporation is decreased in astrocytes cultured from alpha-synuclein gene-ablated mice. J. Neurochem. 2005, 94, 839–849. [Google Scholar] [CrossRef]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef]
- Braidy, N.; Gai, W.P.; Xu, Y.H.; Sachdev, P.; Guillemin, G.J.; Jiang, X.M.; Ballard, J.W.; Horan, M.P.; Fang, Z.M.; Chong, B.H.; et al. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl. Neurodegener. 2013, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhao, C.; Pang, S.; Lu, J.; Chan, P. Alpha-Synuclein aggregation causes muscle atrophy through neuromuscular junction degeneration. J. Cachexia Sarcopenia Muscle 2022, 14, 226–242. [Google Scholar] [CrossRef]
- Tsunemi, T.; Ishiguro, Y.; Yoroisaka, A.; Valdez, C.; Miyamoto, K.; Ishikawa, K.; Saiki, S.; Akamatsu, W.; Hattori, N.; Krainc, D. Astrocytes Protect Human Dopaminergic Neurons from alpha-Synuclein Accumulation and Propagation. J. Neurosci. 2020, 40, 8618–8628. [Google Scholar] [CrossRef]
- Ramirez, A.; Heimbach, A.; Grundemann, J.; Stiller, B.; Hampshire, D.; Cid, L.P.; Goebel, I.; Mubaidin, A.F.; Wriekat, A.L.; Roeper, J.; et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006, 38, 1184–1191. [Google Scholar] [CrossRef]
- Park, J.S.; Blair, N.F.; Sue, C.M. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov. Disord. 2015, 30, 770–779. [Google Scholar] [CrossRef]
- Dang, T.; Cao, W.J.; Zhao, R.; Lu, M.; Hu, G.; Qiao, C. ATP13A2 protects dopaminergic neurons in Parkinson’s disease: From biology to pathology. J. Biomed. Res. 2022, 36, 98–108. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Paisan-Ruiz, C.; Jain, S.; Evans, E.W.; Gilks, W.P.; Simon, J.; van der Brug, M.; Lopez de Munain, A.; Aparicio, S.; Gil, A.M.; Khan, N.; et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004, 44, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Herbst, S.; Gutierrez, M.G. LRRK2 in Infection: Friend or Foe? ACS Infect. Dis. 2019, 5, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Wallings, R.L.; Tansey, M.G. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019, 47, 1581–1595. [Google Scholar] [CrossRef]
- Madureira, M.; Connor-Robson, N.; Wade-Martins, R. LRRK2: Autophagy and Lysosomal Activity. Front. Neurosci. 2020, 14, 498. [Google Scholar] [CrossRef]
- Giasson, B.I.; Van Deerlin, V.M. Mutations in LRRK2 as a cause of Parkinson’s disease. Neurosignals 2008, 16, 99–105. [Google Scholar] [CrossRef]
- Greggio, E.; Jain, S.; Kingsbury, A.; Bandopadhyay, R.; Lewis, P.; Kaganovich, A.; van der Brug, M.P.; Beilina, A.; Blackinton, J.; Thomas, K.J.; et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006, 23, 329–341. [Google Scholar] [CrossRef]
- Di Maio, R.; Hoffman, E.K.; Rocha, E.M.; Keeney, M.T.; Sanders, L.H.; De Miranda, B.R.; Zharikov, A.; Van Laar, A.; Stepan, A.F.; Lanz, T.A.; et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Trans. Med. 2018, 10, eaar5429. [Google Scholar] [CrossRef]
- Miklossy, J.; Arai, T.; Guo, J.P.; Klegeris, A.; Yu, S.; McGeer, E.G.; McGeer, P.L. LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 2006, 65, 953–963. [Google Scholar] [CrossRef]
- di Domenico, A.; Carola, G.; Calatayud, C.; Pons-Espinal, M.; Munoz, J.P.; Richaud-Patin, Y.; Fernandez-Carasa, I.; Gut, M.; Faella, A.; Parameswaran, J.; et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem Cell Rep. 2019, 12, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Streubel-Gallasch, L.; Giusti, V.; Sandre, M.; Tessari, I.; Plotegher, N.; Giusto, E.; Masato, A.; Iovino, L.; Battisti, I.; Arrigoni, G.; et al. Parkinson’s Disease-Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance. Mol. Neurobiol. 2021, 58, 3119–3140. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigie, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, S.; Saiki, S.; Sato, S.; Hattori, N. Genetic mutations and functions of PINK1. Trends Pharmacol. Sci. 2011, 32, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Barodia, S.K.; McMeekin, L.J.; Creed, R.B.; Quinones, E.K.; Cowell, R.M.; Goldberg, M.S. PINK1 phosphorylates ubiquitin predominantly in astrocytes. NPJ Park. Dis. 2019, 5, 29. [Google Scholar] [CrossRef]
- Sun, L.; Shen, R.; Agnihotri, S.K.; Chen, Y.; Huang, Z.; Bueler, H. Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death. Sci. Rep. 2018, 8, 383. [Google Scholar] [CrossRef]
- Choi, I.; Choi, D.J.; Yang, H.; Woo, J.H.; Chang, M.Y.; Kim, J.Y.; Sun, W.; Park, S.M.; Jou, I.; Lee, S.H.; et al. PINK1 expression increases during brain development and stem cell differentiation, and affects the development of GFAP-positive astrocytes. Mol. Brain 2016, 9, 5. [Google Scholar] [CrossRef]
- Olszewska, D.A.; Lynch, T. Will crystal parkin help in understanding the future of Parkinson’s disease? Front. Neurol. 2015, 6, 35. [Google Scholar] [CrossRef]
- Ledesma, M.D.; Galvan, C.; Hellias, B.; Dotti, C.; Jensen, P.H. Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J. Neurochem. 2002, 83, 1431–1440. [Google Scholar] [CrossRef]
- Russ, K.; Teku, G.; Bousset, L.; Redeker, V.; Piel, S.; Savchenko, E.; Pomeshchik, Y.; Savistchenko, J.; Stummann, T.C.; Azevedo, C.; et al. TNF-alpha and alpha-synuclein fibrils differently regulate human astrocyte immune reactivity and impair mitochondrial respiration. Cell Rep. 2021, 34, 108895. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Kim, K.Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Sanchez, A.; Puertas-Avendano, R.; Rodriguez-Sabate, C.; Perez-Barreto, A.; Rodriguez, M. Neuroglial transmitophagy and Parkinson’s disease. Glia 2020, 68, 2277–2299. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J. Mitochondria-Striking a balance between host and endosymbiont. Science 2019, 365, eaaw9855. [Google Scholar] [CrossRef] [PubMed]
- Gkikas, I.; Palikaras, K.; Tavernarakis, N. The Role of Mitophagy in Innate Immunity. Front. Immunol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef]
- Kahns, S.; Kalai, M.; Jakobsen, L.D.; Clark, B.F.; Vandenabeele, P.; Jensen, P.H. Caspase-1 and caspase-8 cleave and inactivate cellular parkin. J. Biol. Chem. 2003, 278, 23376–23380. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, K.; Qi, W.; Huang, Z.; Ye, J.; Ma, Y.; Liao, M.; Ning, Z. Expression pattern of NLRP3 and its related cytokines in the lung and brain of avian influenza virus H9N2 infected BALB/c mice. Virol. J. 2014, 11, 229. [Google Scholar] [CrossRef]
- Neumann, J.; Bras, J.; Deas, E.; O’Sullivan, S.S.; Parkkinen, L.; Lachmann, R.H.; Li, A.; Holton, J.; Guerreiro, R.; Paudel, R.; et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 2009, 132, 1783–1794. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef]
- Aflaki, E.; Stubblefield, B.K.; McGlinchey, R.P.; McMahon, B.; Ory, D.S.; Sidransky, E. A characterization of Gaucher iPS-derived astrocytes: Potential implications for Parkinson’s disease. Neurobiol. Dis. 2020, 134, 104647. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Rahim, A.A.; Hargreaves, I.P.; Gegg, M.E.; Richard-Londt, A.; Brandner, S.; Waddington, S.N.; Schapira, A.H.V.; Duchen, M.R. Mitochondria and quality control defects in a mouse model of Gaucher disease--links to Parkinson’s disease. Cell Metab. 2013, 17, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Duchen, M.R. Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy 2013, 9, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; DeAndrade, M.P.; Novis, H.S.; Lin, S.; Chang, J.; Lengacher, N.; Tomlinson, J.J.; Tansey, M.G.; LaVoie, M.J. Lysosome and Inflammatory Defects in GBA1-Mutant Astrocytes Are Normalized by LRRK2 Inhibition. Mov. Disord. 2020, 35, 760–773. [Google Scholar] [CrossRef]
- Farfel-Becker, T.; Vitner, E.B.; Kelly, S.L.; Bame, J.R.; Duan, J.; Shinder, V.; Merrill, A.H., Jr.; Dobrenis, K.; Futerman, A.H. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum. Mol. Genet. 2014, 23, 843–854. [Google Scholar] [CrossRef]
- Ginns, E.I.; Mak, S.K.; Ko, N.; Karlgren, J.; Akbarian, S.; Chou, V.P.; Guo, Y.; Lim, A.; Samuelsson, S.; LaMarca, M.L.; et al. Neuroinflammation and alpha-synuclein accumulation in response to glucocerebrosidase deficiency are accompanied by synaptic dysfunction. Mol. Genet. Metab. 2014, 111, 152–162. [Google Scholar] [CrossRef]
- Goker-Alpan, O.; Giasson, B.I.; Eblan, M.J.; Nguyen, J.; Hurtig, H.I.; Lee, V.M.; Trojanowski, J.Q.; Sidransky, E. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology 2006, 67, 908–910. [Google Scholar] [CrossRef]
- Talifu, Z.; Liu, J.Y.; Pan, Y.Z.; Ke, H.; Zhang, C.J.; Xu, X.; Gao, F.; Yu, Y.; Du, L.J.; Li, J.J. In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: A narrative review. Neural. Regen. Res. 2023, 18, 750–755. [Google Scholar] [CrossRef]
- Wei, Z.D.; Shetty, A.K. Treating Parkinson’s disease by astrocyte reprogramming: Progress and challenges. Sci. Adv. 2021, 7, eabg3198. [Google Scholar] [CrossRef]
- Kuter, K.; Olech, L.; Glowacka, U. Prolonged Dysfunction of Astrocytes and Activation of Microglia Accelerate Degeneration of Dopaminergic Neurons in the Rat Substantia Nigra and Block Compensation of Early Motor Dysfunction Induced by 6-OHDA. Mol. Neurobiol. 2018, 55, 3049–3066. [Google Scholar] [CrossRef]
- Kato, H.; Araki, T.; Imai, Y.; Takahashi, A.; Itoyama, Y. Protection of dopaminergic neurons with a novel astrocyte modulating agent (R)-(−)-2-propyloctanoic acid (ONO-2506) in an MPTP-mouse model of Parkinson’s disease. J. Neurol. Sci. 2003, 208, 9–15. [Google Scholar] [CrossRef]
- Himeda, T.; Kadoguchi, N.; Kamiyama, Y.; Kato, H.; Maegawa, H.; Araki, T. Neuroprotective effect of arundic acid, an astrocyte-modulating agent, in mouse brain against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity. Neuropharmacology 2006, 50, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Okumura-Torigoe, N.; Miyazaki, I.; Murakami, S.; Kitamura, Y.; Sendo, T. Region-Specific Neuroprotective Features of Astrocytes against Oxidative Stress Induced by 6-Hydroxydopamine. Int. J. Mol. Sci. 2019, 20, 598. [Google Scholar] [CrossRef]
- Bhatia, T.N.; Pant, D.B.; Eckhoff, E.A.; Gongaware, R.N.; Do, T.; Hutchison, D.F.; Gleixner, A.M.; Leak, R.K. Astrocytes Do Not Forfeit Their Neuroprotective Roles After Surviving Intense Oxidative Stress. Front. Mol. Neurosci. 2019, 12, 87. [Google Scholar] [CrossRef]
- Mullett, S.J.; Di Maio, R.; Greenamyre, J.T.; Hinkle, D.A. DJ-1 expression modulates astrocyte-mediated protection against neuronal oxidative stress. J. Mol. Neurosci. 2013, 49, 507–511. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, B.R.; Rocha, E.M.; Bai, Q.; El Ayadi, A.; Hinkle, D.; Burton, E.A.; Timothy Greenamyre, J. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol. Dis. 2018, 115, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Olesen, B.T.; Clausen, J.; Vang, O. Characterization of the transcriptional profile in primary astrocytes after oxidative stress induced by Paraquat. Neurotoxicology 2008, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Canovas-Jorda, D.; Zagoura, D.; Bal-Price, A. Nrf2 pathway activation upon rotenone treatment in human iPSC-derived neural stem cells undergoing differentiation towards neurons and astrocytes. Neurochem. Int. 2017, 108, 457–471. [Google Scholar] [CrossRef]
- Sundar Boyalla, S.; Barbara Victor, M.; Roemgens, A.; Beyer, C.; Arnold, S. Sex- and brain region-specific role of cytochrome c oxidase in 1-methyl-4-phenylpyridinium-mediated astrocyte vulnerability. J. Neurosci. Res. 2011, 89, 2068–2082. [Google Scholar] [CrossRef]
- Swarnkar, S.; Singh, S.; Goswami, P.; Mathur, R.; Patro, I.K.; Nath, C. Astrocyte activation: A key step in rotenone induced cytotoxicity and DNA damage. Neurochem. Res. 2012, 37, 2178–2189. [Google Scholar] [CrossRef]
- Rathinam, M.L.; Watts, L.T.; Narasimhan, M.; Riar, A.K.; Mahimainathan, L.; Henderson, G.I. Astrocyte mediated protection of fetal cerebral cortical neurons from rotenone and paraquat. Environ. Toxicol. Pharmacol. 2012, 33, 353–360. [Google Scholar] [CrossRef]
- Chen, P.C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, B.; Wang, X.; Smith, W.W.; Zhu, Y.; Liu, Z. Activation of Nrf2 in Astrocytes Suppressed PD-Like Phenotypes via Antioxidant and Autophagy Pathways in Rat and Drosophila Models. Cells 2021, 10, 1850. [Google Scholar] [CrossRef]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Kim, H.S.; Son, T.G.; Park, H.R.; Lee, Y.; Jung, Y.; Ishigami, A.; Lee, J. Senescence marker protein 30 deficiency increases Parkinson’s pathology by impairing astrocyte activation. Neurobiol. Aging 2013, 34, 1177–1183. [Google Scholar] [CrossRef]
- McNaught, K.S.; Jenner, P. Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J. Neurochem. 1999, 73, 2469–2476. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Long-Smith, C.M.; Sullivan, A.M.; Nolan, Y.M. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2009, 89, 277–287. [Google Scholar] [CrossRef]
- Sun, H.; Liang, R.; Yang, B.; Zhou, Y.; Liu, M.; Fang, F.; Ding, J.; Fan, Y.; Hu, G. Aquaporin-4 mediates communication between astrocyte and microglia: Implications of neuroinflammation in experimental Parkinson’s disease. Neuroscience 2016, 317, 65–75. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhang, S.; Ding, J.H.; Zhou, F.; Hu, G. Iptakalim protects against MPP+-induced degeneration of dopaminergic neurons in association with astrocyte activation. Int. J. Neuropsychopharmacol. 2009, 12, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Zhou, K.G.; Yin, Y.X.; Jin, L.J.; Tong, W.F.; Guo, J.; Yu, L.H.; Ye, X.C.; Jiang, M. Mesencephalic astrocyte-derived neurotrophic factor (MANF) prevents the neuroinflammation induced dopaminergic neurodegeneration. Exp. Gerontol. 2022, 171, 112037. [Google Scholar] [CrossRef] [PubMed]
- Michalovicz, L.T.; Kelly, K.A.; Vashishtha, S.; Ben-Hamo, R.; Efroni, S.; Miller, J.V.; Locker, A.R.; Sullivan, K.; Broderick, G.; Miller, D.B.; et al. Astrocyte-specific transcriptome analysis using the ALDH1L1 bacTRAP mouse reveals novel biomarkers of astrogliosis in response to neurotoxicity. J. Neurochem. 2019, 150, 420–440. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Town, T.; Tan, J.; Tateishi, N.; Asano, T. Modulation of Astrocytic Activation by Arundic Acid (ONO-2506) Mitigates Detrimental Effects of the Apolipoprotein E4 Isoform after Permanent Focal Ischemia in Apolipoprotein E Knock-in Mice. J. Cereb. Blood Flow Metab. 2005, 25, 748–762. [Google Scholar] [CrossRef]
- Kato, H.; Kurosaki, R.; Oki, C.; Araki, T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004, 1030, 66–73. [Google Scholar] [CrossRef]
- Wang, H.M.; Zhang, T.; Li, Q.; Huang, J.K.; Chen, R.F.; Sun, X.J. Inhibition of glycogen synthase kinase-3beta by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem. Int. 2013, 63, 345–353. [Google Scholar] [CrossRef]
- Gupta, S.; Goswami, P.; Biswas, J.; Joshi, N.; Sharma, S.; Nath, C.; Singh, S. 6-Hydroxydopamine and lipopolysaccharides induced DNA damage in astrocytes: Involvement of nitric oxide and mitochondria. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 22–36. [Google Scholar] [CrossRef]
- Zhang, J.; Culp, M.L.; Craver, J.G.; Darley-Usmar, V. Mitochondrial function and autophagy: Integrating proteotoxic, redox, and metabolic stress in Parkinson’s disease. J. Neurochem. 2018, 144, 691–709. [Google Scholar] [CrossRef]
- Mebratu, Y.A.; Negasi, Z.H.; Dutta, S.; Rojas-Quintero, J.; Tesfaigzi, Y. Adaptation of Proteasomes and Lysosomes to Cellular Environments. Cells 2020, 9, 2221. [Google Scholar] [CrossRef]
- Lee, H.J.; Patel, S.; Lee, S.J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 2005, 25, 6016–6024. [Google Scholar] [CrossRef]
- Deleidi, M.; Maetzler, W. Protein clearance mechanisms of alpha-synuclein and amyloid-Beta in lewy body disorders. Int. J. Alzheimers Dis. 2012, 2012, 391438. [Google Scholar] [CrossRef]
- Morales, I.; Sanchez, A.; Rodriguez-Sabate, C.; Rodriguez, M. Striatal astrocytes engulf dopaminergic debris in Parkinson’s disease: A study in an animal model. PLoS ONE 2017, 12, e0185989. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Cerf, L.; Dehay, B.; Ramos-Gonzalez, P.; De Giorgi, F.; Bourdenx, M.; Bessede, A.; Obeso, J.A.; Matute, C.; Ichas, F.; et al. In vitro alpha-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol. Dis. 2017, 103, 101–112. [Google Scholar] [CrossRef]
- Morales, I.; Puertas-Avendano, R.; Sanchez, A.; Perez-Barreto, A.; Rodriguez-Sabate, C.; Rodriguez, M. Astrocytes and retrograde degeneration of nigrostriatal dopaminergic neurons in Parkinson’s disease: Removing axonal debris. Transl. Neurodegener. 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Janda, E.; Lascala, A.; Carresi, C.; Parafati, M.; Aprigliano, S.; Russo, V.; Savoia, C.; Ziviani, E.; Musolino, V.; Morani, F.; et al. Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection. Autophagy 2015, 11, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and alpha-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 438. [Google Scholar] [CrossRef]
- Rostami, J.; Holmqvist, S.; Lindstrom, V.; Sigvardson, J.; Westermark, G.T.; Ingelsson, M.; Bergstrom, J.; Roybon, L.; Erlandsson, A. Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J. Neurosci. 2017, 37, 11835–11853. [Google Scholar] [CrossRef]
- van Veen, S.; Sorensen, D.M.; Holemans, T.; Holen, H.W.; Palmgren, M.G.; Vangheluwe, P. Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson’s disease and other neurological disorders. Front. Mol. Neurosci. 2014, 7, 48. [Google Scholar] [CrossRef]
- Tsunemi, T.; Krainc, D. Zn(2)(+) dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet. 2014, 23, 2791–2801. [Google Scholar] [CrossRef]
- Tsunemi, T.; Perez-Rosello, T.; Ishiguro, Y.; Yoroisaka, A.; Jeon, S.; Hamada, K.; Rammonhan, M.; Wong, Y.C.; Xie, Z.; Akamatsu, W.; et al. Increased Lysosomal Exocytosis Induced by Lysosomal Ca(2+) Channel Agonists Protects Human Dopaminergic Neurons from alpha-Synuclein Toxicity. J. Neurosci. 2019, 39, 5760–5772. [Google Scholar] [CrossRef]
- Rocha, S.M.; Bantle, C.M.; Aboellail, T.; Chatterjee, D.; Smeyne, R.J.; Tjalkens, R.B. Rotenone induces regionally distinct alpha-synuclein protein aggregation and activation of glia prior to loss of dopaminergic neurons in C57Bl/6 mice. Neurobiol. Dis. 2022, 167, 105685. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic JWA deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Loschmann, P.A.; De Groote, C.; Smith, L.; Wullner, U.; Fischer, G.; Kemp, J.A.; Jenner, P.; Klockgether, T. Antiparkinsonian activity of Ro 25-6981, a NR2B subunit specific NMDA receptor antagonist, in animal models of Parkinson’s disease. Exp. Neurol. 2004, 187, 86–93. [Google Scholar] [CrossRef]
- Ferrarese, C.; Tremolizzo, L.; Rigoldi, M.; Sala, G.; Begni, B.; Brighina, L.; Ricci, G.; Albizzati, M.G.; Piolti, R.; Crosti, F.; et al. Decreased platelet glutamate uptake and genetic risk factors in patients with Parkinson’s disease. Neurol. Sci. 2001, 22, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Giusti, V.; Pischedda, F.; Giusto, E.; Plotegher, N.; Marte, A.; Battisti, I.; Di Iacovo, A.; Marku, A.; Piccoli, G.; et al. Trafficking of the glutamate transporter is impaired in LRRK2-related Parkinson’s disease. Acta Neuropathol. 2022, 144, 81–106. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Meng, X.; Wu, X.; Tong, H.; Zhang, X.; Qu, S. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death Dis. 2017, 8, e2574. [Google Scholar] [CrossRef]
- Chung, E.K.; Chen, L.W.; Chan, Y.S.; Yung, K.K. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J. Comp. Neurol. 2008, 511, 421–437. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Ding, L.; Mo, M.; Zou, J.; Lu, Z.; Li, H.; Wu, H.; Dai, Y.; Xu, P.; et al. Wnt1 Promotes EAAT2 Expression and Mediates the Protective Effects of Astrocytes on Dopaminergic Cells in Parkinson’s Disease. Neural. Plast. 2019, 2019, 1247276. [Google Scholar] [CrossRef]
- Xiao, N.; Le, Q.T. Neurotrophic Factors and Their Potential Applications in Tissue Regeneration. Arch. Immunol. Et Ther. Exp. 2016, 64, 89–99. [Google Scholar] [CrossRef]
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Komure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999, 270, 45–48. [Google Scholar] [CrossRef]
- Howells, D.W. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp. Neurol. 2000, 166, 127–135. [Google Scholar] [CrossRef]
- Du, F.; Li, R.; Huang, Y.; Li, X.; Le, W. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur. J. Neurosci. 2005, 22, 2422–2430. [Google Scholar] [CrossRef]
- Streifel, K.M.; Gonzales, A.L.; De Miranda, B.; Mouneimne, R.; Earley, S.; Tjalkens, R. Dopaminergic neurotoxicants cause biphasic inhibition of purinergic calcium signaling in astrocytes. PLoS ONE 2014, 9, e110996. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Neuron-Astrocyte Interactions in Parkinson’s Disease. Cells 2020, 9, 2623. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.; Kordower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010, 184, 237–264. [Google Scholar] [CrossRef]
- Yuan, L.J.; Zhang, M.; Chen, S.; Chen, W.F. Anti-inflammatory effect of IGF-1 is mediated by IGF-1R cross talk with GPER in MPTP/MPP(+)-induced astrocyte activation. Mol. Cell Endocrinol. 2021, 519, 111053. [Google Scholar] [CrossRef]
- Datta, I.; Ganapathy, K.; Razdan, R.; Bhonde, R. Location and Number of Astrocytes Determine Dopaminergic Neuron Survival and Function Under 6-OHDA Stress Mediated Through Differential BDNF Release. Mol. Neurobiol. 2018, 55, 5505–5525. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.K.; Gardaneh, M.; Iwasiow, R.; Lanthier, P.; Gangaraju, S.; Ribecco-Lutkiewicz, M.; Tremblay, R.; Kiuchi, K.; Sikorska, M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009, 33, 405–414. [Google Scholar] [CrossRef]
- Kordower, J.H.; Palfi, S.; Chen, E.Y.; Ma, S.Y.; Sendera, T.; Cochran, E.J.; Cochran, E.J.; Mufson, E.J.; Penn, R.; Goetz, C.G.; et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 1999, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Petrova, P.; Raibekas, A.; Pevsner, J.; Vigo, N.; Anafi, M.; Moore, M.K.; Peaire, A.E.; Shridhar, V.; Smith, D.I.; Kelly, J.; et al. MANF: A new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003, 20, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tong, W.; Sun, H.; Jiang, M.; Shen, Y.; Liu, Y.; Gu, H.; Guo, J.; Fang, J.; Jin, L. Nrf2-mediated neuroprotection by MANF against 6-OHDA-induced cell damage via PI3K/AKT/GSK3beta pathway. Exp. Gerontol. 2017, 100, 77–86. [Google Scholar] [CrossRef]
- Huang, J.; Chen, C.; Gu, H.; Li, C.; Fu, X.; Jiang, M.; Sun, H.; Xu, J.; Fang, J.; Jin, L. Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell. Biol. Int. 2016, 40, 803–811. [Google Scholar] [CrossRef]
- Voutilainen, M.H.; Back, S.; Porsti, E.; Toppinen, L.; Lindgren, L.; Lindholm, P.; Peranen, J.; Saarma, M.; Tuominen, R.K. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. 2009, 29, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Estebanez, B.; de Paz, J.A.; Cuevas, M.J.; Gonzalez-Gallego, J. Endoplasmic Reticulum Unfolded Protein Response, Aging and Exercise: An Update. Front. Physiol. 2018, 9, 1744. [Google Scholar] [CrossRef]
- Colla, E.; Coune, P.; Liu, Y.; Pletnikova, O.; Troncoso, J.C.; Iwatsubo, T.; Schneider, B.L.; Lee, M.K. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J. Neurosci. 2012, 32, 3306–3320. [Google Scholar] [CrossRef]
- Colla, E.; Jensen, P.H.; Pletnikova, O.; Troncoso, J.C.; Glabe, C.; Lee, M.K. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J. Neurosci. 2012, 32, 3301–3305. [Google Scholar] [CrossRef]
- Liu, M.; Qin, L.; Wang, L.; Tan, J.; Zhang, H.; Tang, J.; Shen, X.; Tan, L.; Wang, C. Alpha-synuclein induces apoptosis of astrocytes by causing dysfunction of the endoplasmic reticulum-Golgi compartment. Mol. Med. Rep. 2018, 18, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, J.H.; Kim, H.; Park, S.M.; Joe, E.H.; Jou, I. Parkinson’s disease-associated LRRK2-G2019S mutant acts through regulation of SERCA activity to control ER stress in astrocytes. Acta Neuropathol. Commun. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hui, Y.; Zhu, C.; Wu, J.; Taylor, D.H.; Ji, J.; Fan, W.; Huang, Z.; Hu, J. Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: Implications for Parkinson’s disease. Neuropharmacology 2015, 91, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Paquet, M.; Ribeiro, F.M.; Guadagno, J.; Esseltine, J.L.; Ferguson, S.S.; Cregan, S.P. Role of metabotropic glutamate receptor 5 signaling and homer in oxygen glucose deprivation-mediated astrocyte apoptosis. Mol. Brain 2013, 6, 9. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, R.; Zhou, F.; Huang, X.; Ding, J.H.; Hu, G. Reversal of rotenone-induced dysfunction of astrocytic connexin43 by opening mitochondrial ATP-sensitive potassium channels. Cell Mol. Neurobiol. 2011, 31, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Naus, C.C. Gap junctions and neurological disorders of the central nervous system. Biochim. Biophys. Acta 2004, 1662, 149–158. [Google Scholar] [CrossRef]
- Kielian, T. Glial connexins and gap junctions in CNS inflammation and disease. J. Neurochem. 2008, 106, 1000–1016. [Google Scholar] [CrossRef]
- Dermietzel, R.; Gao, Y.; Scemes, E.; Vieira, D.; Urban, M.; Kremer, M.; Bennett, M.V.; Spray, D.C. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Brain Res. Rev. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef]
- Martin, H.L.; Santoro, M.; Mustafa, S.; Riedel, G.; Forrester, J.V.; Teismann, P. Evidence for a role of adaptive immune response in the disease pathogenesis of the MPTP mouse model of Parkinson’s disease. Glia 2016, 64, 386–395. [Google Scholar] [CrossRef]
| Gene | Protein | Function | Mode of Inheritance | Time of Onset * | References |
|---|---|---|---|---|---|
| SNCA | α-synuclein | Glutamate transport, inflammatory response | Autosomal dominant | Early | [5,6,7] |
| PARK2 | Parkin | Inflammatory response, mitochondrial function | Autosomal recessive | Early | [8,9] |
| PARK6 | PINK1 | Mitochondrial function | Autosomal recessive | Early | [10] |
| PARK7 | DJ-1 | Glutamate uptake, inflammatory response, mitochondrial function | Autosomal recessive | Early | [11,12,13] |
| PARK8 | LRRK2 | Autophagy, Lysosome function | Autosomal dominant | Late | [14,15] |
| PARK9 | ATP13A2 | Inflammatory response, Lysosome function | Autosomal recessive | Early | [16] |
| GBA | GCase (Glucocerebrosidase) | Autophagy, Lysosome function | Autosomal recessive | Late | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Pajarillo, E.; Nyarko-Danquah, I.; Aschner, M.; Lee, E. Role of Astrocytes in Parkinson’s Disease Associated with Genetic Mutations and Neurotoxicants. Cells 2023, 12, 622. https://doi.org/10.3390/cells12040622
Kim S, Pajarillo E, Nyarko-Danquah I, Aschner M, Lee E. Role of Astrocytes in Parkinson’s Disease Associated with Genetic Mutations and Neurotoxicants. Cells. 2023; 12(4):622. https://doi.org/10.3390/cells12040622
Chicago/Turabian StyleKim, Sanghoon, Edward Pajarillo, Ivan Nyarko-Danquah, Michael Aschner, and Eunsook Lee. 2023. "Role of Astrocytes in Parkinson’s Disease Associated with Genetic Mutations and Neurotoxicants" Cells 12, no. 4: 622. https://doi.org/10.3390/cells12040622
APA StyleKim, S., Pajarillo, E., Nyarko-Danquah, I., Aschner, M., & Lee, E. (2023). Role of Astrocytes in Parkinson’s Disease Associated with Genetic Mutations and Neurotoxicants. Cells, 12(4), 622. https://doi.org/10.3390/cells12040622








