Surgical Models of Liver Regeneration in Pigs: A Practical Review of the Literature for Researchers
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search
2.2. Study Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection
2.5. Outcomes of Interest
2.6. Statistical Analysis
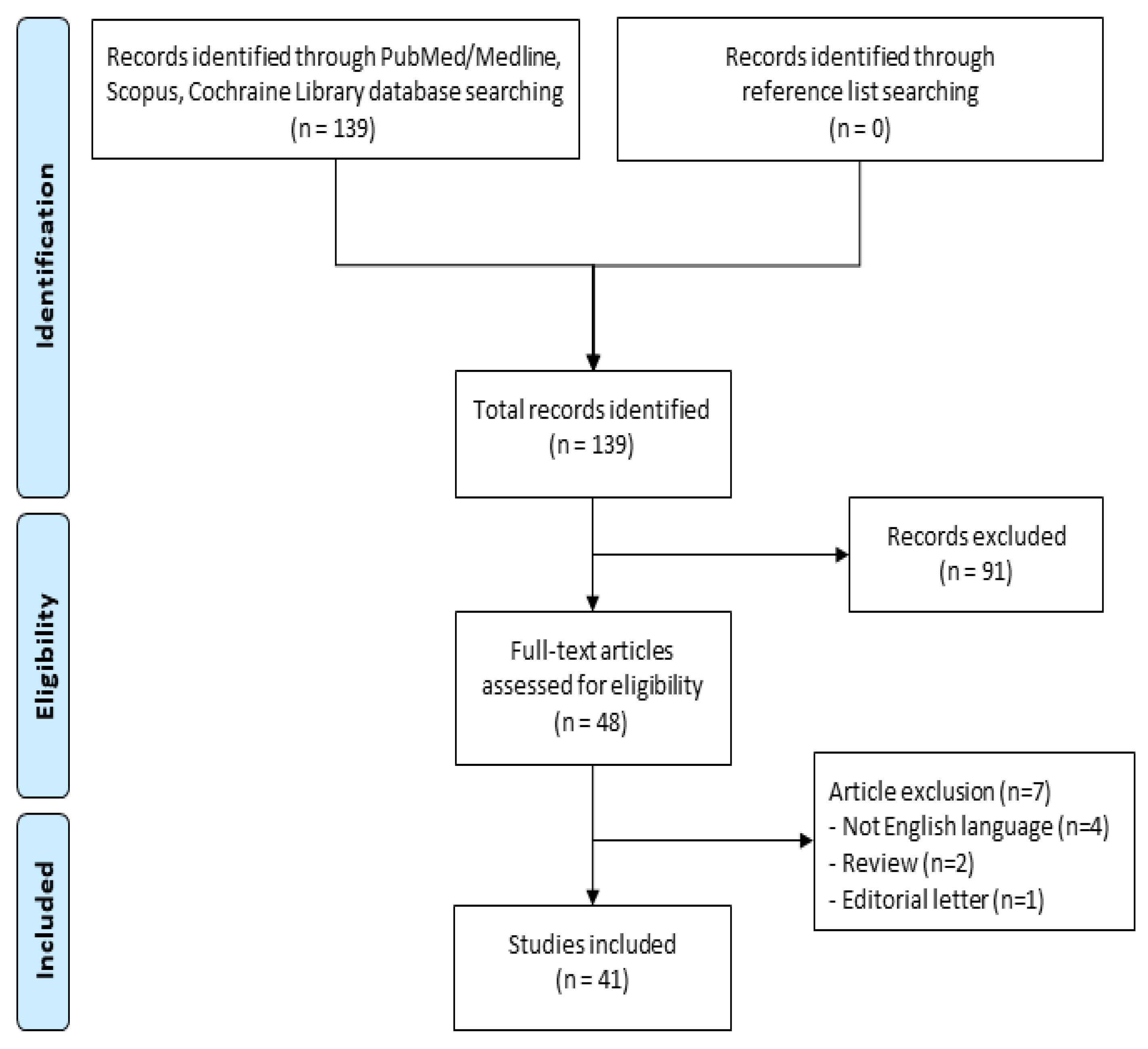
3. Results
3.1. Direct Hepatectomy and Staged Hepatectomy
3.2. Study Duration
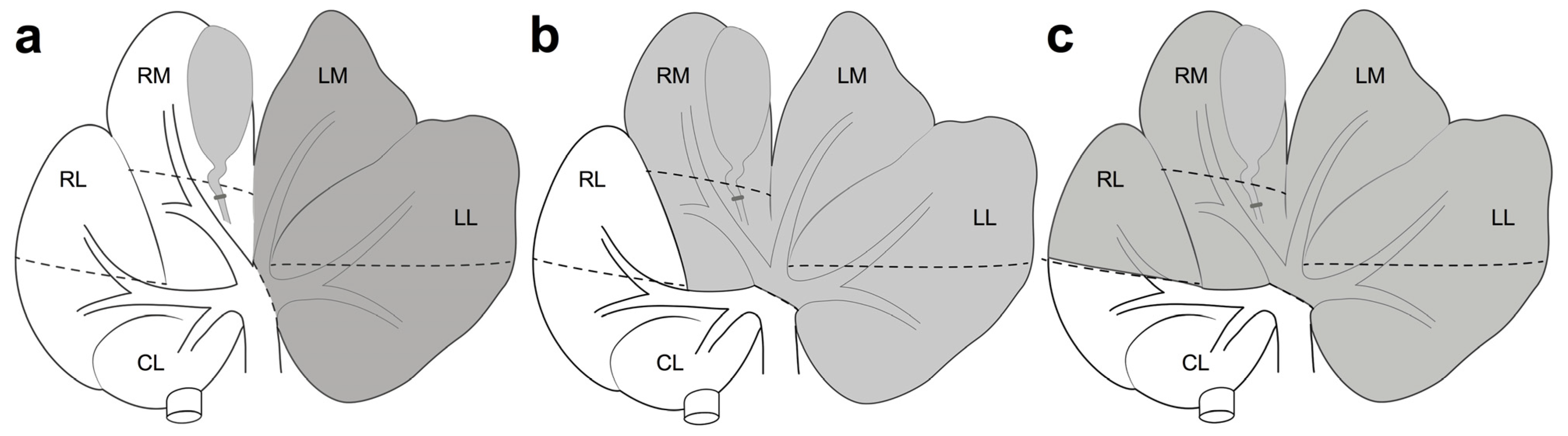
3.3. Remnant Liver Volume (RLV) and Surgical Procedures
3.4. Additional Procedures
3.4.1. Ischemia/Reperfusion Injury
3.4.2. Stem Cells Application
3.4.3. Venous Blood Flow Modulation in PH
3.4.4. Liver Regeneration Monitoring
Instrumental Functional Monitoring
Volumetric Analysis
4. Discussion
4.1. Anatomical Findings in Porcine Liver
4.2. Surgical Procedure
4.3. Recovery Time in the Regenerative Process
4.4. Staged Hepatectomy and New Perspectives
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma—a Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Albertsmeier, M.; Stintzing, S.; Guba, M.; Werner, J.; Angele, M. Multimodal treatment of colorectal liver metastases. MMW Fortschr. Der Med. 2015, 157, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, R.F., Jr.; Salvalaggio, P.; Rezende, M.B.; Evangelista, A.S.; Guardia, B.D.; Matielo, C.E.; Neves, D.B.; Pandullo, F.L.; Felga, G.E.; Alves, J.A.; et al. Liver transplantation: History, outcomes and perspectives. Einstein 2015, 13, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Vilca-Melendez, H.; Heaton, N.D. Paediatric liver transplantation: The surgical view. Postgrad. Med. J. 2004, 80, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, J.N.; Chaoui, A.; Do, K.A.; Bilimoria, M.M.; Fenstermacher, M.J.; Charnsangavej, C.; Hicks, M.; Alsfasser, G.; Lauwers, G.; Hawkins, I.F.; et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surgery 2000, 127, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Dahm, F.; Georgiev, P.; Clavien, P.A. Small-for-size syndrome after partial liver transplantation: Definition, mechanisms of disease and clinical implications. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2005, 5, 2605–2610. [Google Scholar] [CrossRef]
- Ray, S.; Mehta, N.N.; Golhar, A.; Nundy, S. Post hepatectomy liver failure—A comprehensive review of current concepts and controversies. Ann. Med. Surg. 2018, 34, 4–10. [Google Scholar] [CrossRef]
- Eshkenazy, R.; Dreznik, Y.; Lahat, E.; Zakai, B.B.; Zendel, A.; Ariche, A. Small for size liver remnant following resection: Prevention and management. Hepatobiliary Surg. Nutr. 2014, 3, 303–312. [Google Scholar]
- Michalopoulos, G.K.; DeFrances, M.C. Liver regeneration. Science 1997, 276, 60–66. [Google Scholar] [CrossRef]
- Grindlay, J.H.; Bollman, J.L. Regeneration of the liver in the dog after partial hepatectomy; role of the venous circulation. Surg. Gynecol. Obstet. 1952, 94, 491–496. [Google Scholar]
- Gallot, D.; Gouet, O.; Bidallier, M.; Coloigner, M.; Opolon, P.; Huguet, C. A simplified bloodless procedure for extensive hepatectomy. Experimental study in the pig. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 1976, 8, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Rous, P.; Larimore, L.D. Relation Of The Portal Blood To Liver Maintenance: A Demonstration Of Liver Atrophy Conditional On Compensation. J. Exp. Med. 1920, 31, 609–632. [Google Scholar] [CrossRef]
- Kinoshita, H.; Sakai, K.; Hirohashi, K.; Igawa, S.; Yamasaki, O.; Kubo, S. Preoperative portal vein embolization for hepatocellular carcinoma. World J. Surg. 1986, 10, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, F.; Marongiu, M.; Contini, A.; Serra, M.; Cadoni, E.; Murgia, R.; Laconi, E. Hyperplasia vs hypertrophy in tissue regeneration after extensive liver resection. World J. Gastroenterol. 2017, 23, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, M.; Chen, E.; Tang, H. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediat. Inflamm. 2017, 2017, 4256352. [Google Scholar] [CrossRef]
- Ren, J.; Yu, D.; Wang, J.; Xu, K.; Xu, Y.; Sun, R.; An, P.; Li, C.; Feng, G.; Zhang, Y.; et al. Generation of immunodeficient pig with hereditary tyrosinemia type 1 and their preliminary application for humanized liver. Cell Biosci. 2022, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Archibald, A.L.; Bolund, L.; Churcher, C.; Fredholm, M.; Groenen, M.A.; Harlizius, B.; Lee, K.T.; Milan, D.; Rogers, J.; Rothschild, M.F.; et al. Pig genome sequence--analysis and publication strategy. BMC Genom. 2010, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Court, F.G.; Wemyss-Holden, S.A.; Morrison, C.P.; Teague, B.D.; Laws, P.E.; Kew, J.; Dennison, A.R.; Maddern, G.J. Segmental nature of the porcine liver and its potential as a model for experimental partial hepatectomy. Br. J. Surg. 2003, 90, 440–444. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hishikawa, S.; Teratani, T.; Lefor, A.T. The pig as a model for translational research: Overview of porcine animal models at Jichi Medical University. Transplant. Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, W.B.; Wan, Y.Y.; Li, W.G.; Huang, Z.Q.; Wu, X.T.; Yang, J.; Yang, L. Effects of partial portal vein arterialization on liver regeneration after hepatectomy in minipigs with obstructive jaundice. Chin. Med. J. 2012, 125, 2302–2305. [Google Scholar] [PubMed]
- Liska, V.; Treska, V.; Mirka, H.; Kobr, J.; Sykora, R.; Skalicky, T.; Sutnar, A.; Bruha, J.; Fiala, O.; Vycital, O.; et al. Tumour necrosis factor-alpha stimulates liver regeneration in porcine model of partial portal vein ligation. Hepato-Gastroenterol. 2012, 59, 496–500. [Google Scholar]
- Liška, V.; Třeška, V.; Mírka, H.; Vyčítal, O.; Brůha, J.; Haidingerová, L.; Beneš, J.; Tonar, Z.; Pálek, R.; Rosendorf, J. Experimental promotion of liver regeneration after portal vein branch ligation. Rozhl. V Chir. Mesic. Ceskoslovenske Chir. Spol. 2018, 97, 239–245. [Google Scholar]
- Liska, V.; Treska, V.; Mirka, H.; Kobr, J.; Sykora, R.; Skalicky, T.; Sutnar, A.; Vycital, O.; Bruha, J.; Pitule, P.; et al. Inhibition of transforming growth factor beta-1 augments liver regeneration after partial portal vein ligation in a porcine experimental model. Hepato-Gastroenterol. 2012, 59, 235–240. [Google Scholar]
- Huisman, F.; van Lienden, K.P.; Damude, S.; Hoekstra, L.T.; van Gulik, T.M. A review of animal models for portal vein embolization. J. Surg. Res. 2014, 191, 179–188. [Google Scholar] [CrossRef]
- Budai, A.; Fulop, A.; Hahn, O.; Onody, P.; Kovacs, T.; Nemeth, T.; Dunay, M.; Szijarto, A. Animal Models for Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS): Achievements and Future Perspectives. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2017, 58, 140–157. [Google Scholar] [CrossRef]
- Athanasiou, A.; Felekouras, E.; Moris, D. Mystery of Liver Regeneration After Portal Flow Changes: The Inductive Way of Thinking May Give the Answers. Ann. Surg. 2018, 268, e7–e8. [Google Scholar] [CrossRef]
- Gaillard, M.; Hornez, E.; Lecuelle, B.; Lilin, T.; Dubart-Kupperschmitt, A.; Dagher, I.; Tranchart, H. Liver Regeneration and Recanalization Time Course following Repeated Reversible Portal Vein Embolization in Swine. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2020, 61, 62–71. [Google Scholar] [CrossRef]
- Jiao, Z.; Liu, X.; Ma, Y.; Ge, Y.; Zhang, Q.; Liu, B.; Wang, H. Adipose-Derived Stem Cells Protect Ischemia-Reperfusion and Partial Hepatectomy by Attenuating Endoplasmic Reticulum Stress. Front. Cell Dev. Biol. 2020, 8, 177. [Google Scholar] [CrossRef]
- Lim, R.H.G.; Liew, J.X.K.; Wee, A.; Masilamani, J.; Chang, S.K.Y.; Phan, T.T. Safety Evaluation of Human Cord-Lining Epithelial Stem Cells Transplantation for Liver Regeneration in a Porcine Model. Cell Transplant. 2020, 29, 0963689719896559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Piao, C.; Xu, J.; Jiao, Z.; Ge, Y.; Liu, X.; Ma, Y.; Wang, H. Comparative study on protective effect of hydrogen rich saline and adipose-derived stem cells on hepatic ischemia-reperfusion and hepatectomy injury in swine. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 120, 109453. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Yagi, H.; Higashi, H.; Tajima, K.; Kuroda, K.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci. Rep. 2019, 9, 12543. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Moller, P.W.; Frey, S.; Tinguely, P.; Candinas, D.; Obrist, D.; Jakob, S.M.; Beldi, G. Portal hyperperfusion after major liver resection and associated sinusoidal damage is a therapeutic target to protect the remnant liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G264–G274. [Google Scholar] [CrossRef] [PubMed]
- Wittauer, E.M.; Oldhafer, F.; Augstein, E.; Beetz, O.; Kleine, M.; Schumacher, C.; Sieg, L.; Eismann, H.; Johanning, K.; Bleich, A.; et al. Porcine model for the study of liver regeneration enhanced by non-invasive 13C-methacetin breath test (LiMAx test) and permanent portal venous access. PLoS ONE 2019, 14, e0217488. [Google Scholar] [CrossRef]
- Fonouni, H.; Khajeh, E.; Ghamarnejad, O.; Kashfi, A.; Aydogdu, E.; Majlesara, A.; Mohammadi, S.; Gharabaghi, N.; Konstantinidis, L.; Longerich, T.; et al. Histopathological effects of modern topical sealants on the liver surface after hepatectomy: An experimental swine study. Sci. Rep. 2019, 9, 7088. [Google Scholar] [CrossRef]
- Bekheit, M.; Bucur, P.O.; Audebert, C.; Miquelestorena-Standley, E.; Vignon-Clementel, I.; Vibert, E. Kinetics of Hepatic Volume Evolution and Architectural Changes after Major Resection in a Porcine Model. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2019, 60, 31–44. [Google Scholar] [CrossRef]
- Orue-Echebarria, M.I.; Vaquero, J.; Lisbona, C.J.; Lozano, P.; Steiner, M.A.; Morales, Á.; López-Baena, J.; Laso, J.; Hernández, I.; Olmedilla, L.; et al. Comprehensive Characterization of a Porcine Model of The “Small-for-Flow” Syndrome. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2019, 23, 2174–2183. [Google Scholar] [CrossRef]
- Ge, Y.S.; Zhang, Q.Z.; Li, H.; Bai, G.; Jiao, Z.H.; Wang, H.B. Hydrogen-rich saline protects against hepatic injury induced by ischemia-reperfusion and laparoscopic hepatectomy in swine. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2019, 18, 48–61. [Google Scholar] [CrossRef]
- Schadde, E.; Guiu, B.; Deal, R.; Kalil, J.; Arslan, B.; Tasse, J.; Olthof, P.B.; Heil, J.; Schnitzbauer, A.A.; Jakate, S.; et al. Simultaneous hepatic and portal vein ligation induces rapid liver hypertrophy: A study in pigs. Surgery 2019, 165, 525–533. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Jiao, Z.; Li, H.; Bai, G.; Wang, H. Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 2018, 214, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Tajima, K.; Yagi, H.; Higashi, H.; Shimoda, H.; Matsubara, K.; Hibi, T.; Abe, Y.; Tsujikawa, H.; Kitago, M.; et al. A Pre-Clinical Large Animal Model of Sustained Liver Injury and Regeneration Stimulus. Sci. Rep. 2018, 8, 14987. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Joo, D.J.; Shaheen, M.; Li, Y.; Wang, Y.; Yang, J.; Nicolas, C.T.; Predmore, K.; Amiot, B.; Michalak, G.; et al. Randomized Trial of Spheroid Reservoir Bioartificial Liver in Porcine Model of Posthepatectomy Liver Failure. Hepatology 2019, 69, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Bartas, M.; Červeň, J.; Oppelt, J.; Peteja, M.; Vávra, P.; Zonča, P.; Procházka, V.; Brázda, V.; Pečinka, P. Liver regeneration during the associating liver partition and portal vein ligation for staged hepatectomy procedure in Sus scrofa is positively modulated by stem cells. Oncol. Lett. 2018, 15, 6309–6321. [Google Scholar] [PubMed]
- Brige, P.; Hery, G.; Palen, A.; Guilbaud, T.; Buffat, C.; Moyon, A.; Hardwigsen, J.; Guedj, E.; Guillet, B.; Vidal, V.; et al. Portal vein stenosis preconditioning of living donor liver in swine: Early mechanisms of liver regeneration and gain of hepatic functional mass. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G117–G125. [Google Scholar] [CrossRef]
- Wiederkehr, H.A.; Wiederkehr, J.C.; Collaço, L.M.; Sousa, E.L.; Salvalaggio, P.; Carvalho, C.A.; Wiederkehr, B.A.; Marques, C.A.M.; Rosa, F.F.D.; Nanni, F.N.; et al. Transection of the hepatic parenchyma associated or not with the contralateral portal vein branch ligature and its effect in liver regeneration. Einstein 2017, 15, 178–185. [Google Scholar] [CrossRef][Green Version]
- Deal, R.; Frederiks, C.; Williams, L.; Olthof, P.B.; Dirscherl, K.; Keutgen, X.; Chan, E.; Deziel, D.; Hertl, M.; Schadde, E. Rapid Liver Hypertrophy After Portal Vein Occlusion Correlates with the Degree of Collateralization Between Lobes-a Study in Pigs. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2018, 22, 203–213. [Google Scholar] [CrossRef]
- Bucur, P.O.; Bekheit, M.; Audebert, C.; Othman, A.; Hammad, S.; Sebagh, M.; Allard, M.A.; Decante, B.; Friebel, A.; Miquelestorena-Standley, E.; et al. Modulating Portal Hemodynamics With Vascular Ring Allows Efficient Regeneration After Partial Hepatectomy in a Porcine Model. Ann. Surg. 2018, 268, 134–142. [Google Scholar] [CrossRef]
- Asencio, J.M.; García-Sabrido, J.L.; López-Baena, J.A.; Olmedilla, L.; Peligros, I.; Lozano, P.; Morales-Taboada, Á.; Fernández-Mena, C.; Steiner, M.A.; Sola, E.; et al. Preconditioning by portal vein embolization modulates hepatic hemodynamics and improves liver function in pigs with extended hepatectomy. Surgery 2017, 161, 1489–1501. [Google Scholar] [CrossRef]
- Iguchi, K.; Hatano, E.; Nirasawa, T.; Iwasaki, N.; Sato, M.; Yamamoto, G.; Yamanaka, K.; Okamoto, T.; Kasai, Y.; Nakamura, N.; et al. Chronological Profiling of Plasma Native Peptides after Hepatectomy in Pigs: Toward the Discovery of Human Biomarkers for Liver Regeneration. PLoS ONE 2017, 12, e0167647. [Google Scholar] [CrossRef]
- Sang, J.F.; Shi, X.L.; Han, B.; Huang, X.; Huang, T.; Ren, H.Z.; Ding, Y.T. Combined mesenchymal stem cell transplantation and interleukin-1 receptor antagonism after partial hepatectomy. World J. Gastroenterol. 2016, 22, 4120–4135. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Huang, L.; Wang, X.; Zhao, Y.; Liu, Y.; Tan, J. How Much Portal Vein Flow Is Too Much for Liver Remnant in a Stable Porcine Model? Transplant. Proc. 2016, 48, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, P.; Mastoraki, A.; Papalois, A.; Nastos, C.; Kondi-Pafiti, A.; Kostopanagiotou, G.; Smyrniotis, V.; Arkadopoulos, N. Expression of Inflammatory and Regenerative Genes in a Model of Liver Ischemia/Reperfusion and Partial Hepatectomy. J. Investig. Surg. Off. J. Acad. Surg. Res. 2016, 29, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nygård, I.E.; Mortensen, K.E.; Hedegaard, J.; Conley, L.N.; Bendixen, C.; Sveinbjørnsson, B.; Revhaug, A. Tissue Remodelling following Resection of Porcine Liver. BioMed Res. Int. 2015, 2015, 248920. [Google Scholar] [CrossRef]
- Croome, K.P.; Mao, S.A.; Glorioso, J.M.; Krishna, M.; Nyberg, S.L.; Nagorney, D.M. Characterization of a porcine model for associating liver partition and portal vein ligation for a staged hepatectomy. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2015, 17, 1130–1136. [Google Scholar] [CrossRef]
- Bruha, J.; Vycital, O.; Tonar, Z.; Mirka, H.; Haidingerova, L.; Benes, J.; Palek, R.; Skala, M.; Treska, V.; Liska, V. Monoclonal antibody against transforming growth factor Beta 1 does not influence liver regeneration after resection in large animal experiments. In Vivo 2015, 29, 327–340. [Google Scholar] [PubMed]
- Wang, D.D.; Xu, Y.; Zhu, Z.M.; Tan, X.L.; Tu, Y.L.; Han, M.M.; Tan, J.W. Should temporary extracorporeal continuous portal diversion replace meso/porta-caval shunts in “small-for-size” syndrome in porcine hepatectomy? World J. Gastroenterol. 2015, 21, 888–896. [Google Scholar] [CrossRef]
- Gregoire, E.; Brige, P.; Barbier, L.; Buffat, C.; Coppola, A.; Hardwigsen, J.; Le Treut, Y.P.; Vidal, V.; Rolland, P.H. Minimal portal vein stenosis is a promising preconditioning in living donor liver transplantation in porcine model. J. Hepatol. 2014, 61, 59–66. [Google Scholar] [CrossRef]
- Wang, X.Q.; Xu, Y.F.; Tan, J.W.; Lv, W.P.; Liu, Z.; Zeng, J.P.; Dong, J.H. Portal inflow preservation during portal diversion in small-for-size syndrome. World J. Gastroenterol. 2014, 20, 1021–1029. [Google Scholar] [CrossRef]
- Nygård, I.E.; Mortensen, K.E.; Hedegaard, J.; Conley, L.N.; Kalstad, T.; Bendixen, C.; Revhaug, A. The genetic regulation of the terminating phase of liver regeneration. Comp. Hepatol. 2012, 11, 3. [Google Scholar] [CrossRef]
- Shimoda, M.; Iwasaki, Y.; Okada, T.; Kubota, K. Edaravone inhibits apoptosis caused by ischemia/reperfusion injury in a porcine hepatectomy model. World J. Gastroenterol. 2012, 18, 3520–3526. [Google Scholar] [CrossRef]
- Hisakura, K.; Murata, S.; Fukunaga, K.; Myronovych, A.; Tadano, S.; Kawasaki, T.; Kohno, K.; Ikeda, O.; Pak, S.; Ikeda, N.; et al. Platelets prevent acute liver damage after extended hepatectomy in pigs. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 855–864. [Google Scholar] [CrossRef]
- Arkadopoulos, N.; Kostopanagiotou, G.; Nastos, C.; Papalois, A.; Papoutsidakis, N.; Kalimeris, K.; Defterevos, G.; Kanna, T.; Polyzois, K.; Kampouroglou, G.; et al. Reversal of experimental posthepatectomy liver failure in pigs: A new application of hepatocyte bioreactors. Artif. Organs 2011, 35, 29–36. [Google Scholar] [CrossRef]
- Jo, H.S.; Park, H.J.; Choi, Y.Y.; Seok, J.I.; Han, J.H.; Yoon, Y.I.; Kim, D.S. Portal modulation effects of terlipressin on liver regeneration and survival in a porcine model subjected to 90% hepatectomy. Am. J. Transl. Res. 2021, 13, 5880–5891. [Google Scholar] [PubMed]
- Jo, H.S.; Han, J.H.; Choi, Y.Y.; Seok, J.I.; Yoon, Y.I.; Kim, D.S. The beneficial impacts of splanchnic vasoactive agents on hepatic functional recovery in massive hepatectomy porcine model. Hepatobiliary Surg. Nutr. 2021, 10, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Ma, Y.; Zhang, Q.; Wang, Y.; Liu, T.; Liu, X.; Piao, C.; Liu, B.; Wang, H. The adipose-derived mesenchymal stem cell secretome promotes hepatic regeneration in miniature pigs after liver ischaemia-reperfusion combined with partial resection. Stem Cell Res. Ther. 2021, 12, 218. [Google Scholar] [CrossRef]
- Oldhafer, F.; Wittauer, E.M.; Beetz, O.; Weigle, C.A.; Sieg, L.; Eismann, H.; Braubach, P.; Bock, M.; Jonigk, D.; Johanning, K.; et al. Supportive Hepatocyte Transplantation after Partial Hepatectomy Enhances Liver Regeneration in a Preclinical Pig Model. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2021, 62, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Vištejnová, L.; Liška, V.; Kumar, A.; Křečková, J.; Vyčítal, O.; Brůha, J.; Beneš, J.; Kolinko, Y.; Blassová, T.; Tonar, Z.; et al. Mesenchymal Stromal Cell Therapy in Novel Porcine Model of Diffuse Liver Damage Induced by Repeated Biliary Obstruction. Int. J. Mol. Sci. 2021, 22, 4304. [Google Scholar] [CrossRef]
- Xue, W.; Fu, Y.; Zhang, H.; Li, G.; Cao, P.; Li, Y.; Peng, Q.; Zhong, K.; Feng, S.; Gao, Y. A novel, simplified, and reproducible porcine model of acute ischemic liver failure with portal vein preservation. Exp. Anim. 2021, 71, 60–70. [Google Scholar] [CrossRef]
- Celinski, S.A.; Gamblin, T.C. Hepatic resection nomenclature and techniques. Surg. Clin. N. Am. 2010, 90, 737–748. [Google Scholar] [CrossRef]
- Osman, F.A.; Wally, Y.R.; El-Nady, F.A.; Rezk, H.M. Gross Anatomical Studies on the Portal Vein, Hepatic Artery and Bile Duct in the Liver of the Pig. J. Vet. Anat. 2008, 1, 59–72. [Google Scholar] [CrossRef]
- Boxenbaum, H. Interspecies variation in liver weight, hepatic blood flow, and antipyrine intrinsic clearance: Extrapolation of data to benzodiazepines and phenytoin. J. Pharm. Biopharm 1980, 8, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Vilei, M.T.; Granato, A.; Ferraresso, C.; Neri, D.; Carraro, P.; Gerunda, G.; Muraca, M. Comparison of pig, human and rat hepatocytes as a source of liver specific metabolic functions in culture systems--implications for use in bioartificial liver devices. Int. J. Artif. Organs 2001, 24, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Lu, T.F.; Zhou, Z.H.; Hu, L.X.; Ying, J.; Ding, D.Z.; Chen, X.S.; Zhang, J.J. Extended hepatectomy with segments I and VII as resection remnant: A simple model for small-for-size injuries in pigs. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2008, 7, 601–607. [Google Scholar] [PubMed]
- Komorowski, A.L.; Mituś, J.W.; Sanchez Hurtado, M.A.; Sanchez Margallo, F.M. Porcine Model In The Laparoscopic Liver Surgery Training. Pol. J. Surg. 2015, 87, 425–428. [Google Scholar] [CrossRef]
- Torzilli, G.; Procopio, F.; Donadon, M.; Fabbro, D.D.; Cimino, M.; Montorsi, M. Safety of intermittent Pringle maneuver cumulative time exceeding 120 minutes in liver resection: A further step in favor of the “radical but conservative” policy. Ann. Surg. 2012, 255, 270–280. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Miyajima, A. To divide or not to divide: Revisiting liver regeneration. Cell Div. 2013, 8, 8. [Google Scholar] [CrossRef]
- Marubashi, S.; Sakon, M.; Nagano, H.; Gotoh, K.; Hashimoto, K.; Kubota, M.; Kobayashi, S.; Yamamoto, S.; Miyamoto, A.; Dono, K.; et al. Effect of portal hemodynamics on liver regeneration studied in a novel portohepatic shunt rat model. Surgery 2004, 136, 1028–1037. [Google Scholar] [CrossRef]
- Demetris, A.J.; Kelly, D.M.; Eghtesad, B.; Fontes, P.; Marsh, J.W.; Tom, K.; Tan, H.P.; Shaw-Stiffel, T.; Boig, L.; Novelli, P.; et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am. J. Surg. Pathol. 2006, 30, 986–993. [Google Scholar] [CrossRef]
- Preziosi, M.; Okabe, H.; Poddar, M.; Singh, S.; Monga, S.P. Endothelial Wnts regulate beta-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol. Commun. 2018, 2, 845–860. [Google Scholar] [CrossRef]
- Russell, J.O.; Monga, S.P. Wnt/beta-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. Hepatology 2006, 43, S45–S53. [Google Scholar] [CrossRef]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. J. Hepatol. 2012, 57, 692–694. [Google Scholar] [CrossRef]
- Yagi, S.; Hirata, M.; Miyachi, Y.; Uemoto, S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 8414. [Google Scholar] [CrossRef] [PubMed]
- Sparrelid, E.; Jonas, E.; Tzortzakakis, A.; Dahlen, U.; Murquist, G.; Brismar, T.; Axelsson, R.; Isaksson, B. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J. Gastrointest. Surg. 2017, 21, 967–974. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.R.; Hicks, M.E.; Cai, S.R.; Brunt, E.M.; Ponder, K.P. Embolization of portal vein branches induces hepatocyte replication in swine: A potential step in hepatic gene therapy. Radiology 1999, 210, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Tzeng, C.W.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Wei, S.H.; Huang, S.Y.; Gupta, S.; Wallace, M.J.; Vauthey, J.N. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br. J. Surg. 2013, 100, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Tzeng, C.-W.D.; Vauthey, J.N. Portal vein embolization for hepatocellular carcinoma. Liver Cancer 2012, 1, 159–167. [Google Scholar] [CrossRef]
- Schadde, E.; Ardiles, V.; Slankamenac, K.; Tschuor, C.; Sergeant, G.; Amacker, N.; Baumgart, J.; Croome, K.; Hernandez-Alejandro, R. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: Results of a multicenter analysis. World J. Surg. 2014, 38, 1510–1519. [Google Scholar] [CrossRef]
- Schlegel, A.; Lesurtel, M.; Melloul, E.; Limani, P.; Tschuor, C.; Graf, R.; Humar, B.; Clavien, P.A. ALPPS: From human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann. Surg. 2014, 260, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Aierken, Y.; Kong, L.-X.; Li, B.; Liu, X.-J.; Lu, S.; Yang, J.-Y. Liver fibrosis is a major risk factor for liver regeneration: A comparison between healthy and fibrotic liver. Medicine 2020, 99, e20003. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Swine Breed | Weight | Study Duration (Days) | No. | Liver Resection | Additional Procedures |
|---|---|---|---|---|---|---|---|
| Hisakura et al. [62] | 2010 | Chinese minipig Landrace white pig | 41.5 ± 9 30.1 ± 6.7 | 7 | 20 | PH | - |
| Arkadoupolos et al. [63] | 2011 | - | 35–40 | 1 | 12 | PH | IRI |
| Shimoda et al. [61] | 2012 | - | 23–26 | 30 | 12 | PH | IRI |
| Nygard et al. [60] | 2012 | Norwegian landrace pig | 31.7 ± 5.13 | 42 | 12 | PH | - |
| Wang et al. [59] | 2014 | Bama minipig | 15–20 | 2 | 20 | PH | - |
| Gregoire et al. [58] | 2014 | Pietrain pig | 40–50 | 7 | 24 | - | PVM |
| Athanasopoulos et al. [53] | 2015 | Landrace pig | 30–35 | 1 | 12 | PH | IRI |
| Bruha et al. [56] | 2015 | - | - | 14 | 20 | PH | - |
| Nygard et al. [54] | 2015 | Norwegian Landrace pig | 31.7 ± 5.13 | 42 | 12 | PH | - |
| Wang et al. [57] | 2015 | Bama minipig | 15–20 | 2 | 14 | PH | - |
| Croome et al. [55] | 2015 | - | 31 ± 1 | 7 | 13 | ALPPS | - |
| Xiang et al. [52] | 2016 | Bama minipig | 15–20 | 14 | 30 | PH | - |
| Sang et al. [51] | 2016 | - | 15 ± 3 | 14 | 24 | PH | SCA |
| Bucur et al. [48] | 2017 | Large white pig | 32.9 ± 5.3 | 7 | 17 | PH | PVM |
| Asencio et al. [49] | 2017 | Minipig, Large white pig | 42 ± 2 | 1 | 20 | PH | PVE |
| Iguchi et al. [50] | 2017 | - | 20–22 | 7 | 5 | PH | - |
| Bartas et al. [44] | 2017 | Polish white pig | 30–50 | 9 | 6 | ALPPS | SCA |
| Wiederkehr et al. [46] | 2017 | - | - | 5 | 10 | ALPPS | - |
| Deal et al. [47] | 2017 | Yorkshire Landrace pig | - | 7 | 12 | ALPPS | - |
| Inomata et al. [42] | 2018 | Gottingen minipig | 14–20 | 28 | 34 | PH | - |
| Chen et al. [43] | 2018 | Large white pig | 28 ± 1.2 | 14 | 18 | PH | SRBAL |
| Ge et al. [39] | 2018 | Bama minipig | - | 7 | 21 | PH | IRI, SCA |
| Schadde et al. [40] | 2018 | Yorkshire landrace pig | - | 7 | 14 | - | PVM, HVL |
| Ge et al. [41] | 2018 | Bama minipig | - | 7 | 18 | PH | IRI, SCA |
| Brige et al. [45] | 2018 | Pietrain pig | - | 14 | 14 | - | PVM, PVE |
| Shimoda et al. [33] | 2019 | Large white pig | 20–25 | 28 | 6 | PH | Scaffolding |
| Zhang et al. [32] | 2019 | Bama minipig | 25–35 | 7 | 18 | PH | IRI, SCA |
| Fonouni et al. [36] | 2019 | Landrace minipig | 30.2 ± 2.1 | 6 | 36 | PH | - |
| Kohler et al. [34] | 2019 | Domestic minipig | 56–63 | 1 | 16 | PH | PVM |
| Bekheit et al. [37] | 2019 | Large white pig | 32.9 ± 5.3 | 27 | 19 | PH | - |
| Orue-Echebarria et al. [38] | 2019 | - | 42 [39.2–49.7] | 1 | 10 | PH | - |
| Wittauer et al. [35] | 2019 | Lewe minipig | 49.9 ± 2 | 30 | 7 | PH | - |
| Jiao et al. [30] | 2020 | Bama minipig | 20–25 | 21 | 18 | PH | IRI, SCA |
| Lim et al. [31] | 2020 | Yorkshire-Dutch Landrace pig | 40.5 | 21 | 16 | PH | SCA, Scaffolding |
| Gaillard et al. [29] | 2020 | - | 57.3 ± 5.7 | 28 | 12 | - | PVE |
| Jo et al. [64] | 2021 | Large white pig | 34.9 [28–39.4] | 7 | 20 | PH | Terlipressin |
| Jo et al. [65] | 2021 | Large white pig | 28–40 | 7 | 18 | PH | Terlipressin, Octreotide |
| Jiao et al. [66] | 2021 | Bama minipig | 20–25 | 7 | 24 | PH | IRI, SCA |
| Oldhafer et al. [67] | 2021 | Lewe minipig | 46 ± 3 | 30 | 16 | PH | HTx |
| Vištejnová et al. [68] | 2021 | Large white pig | 20 | 14 | 21 | PH | SCA, BDO |
| Xue et al. [69] | 2021 | Bama minipig | 35–45 | 15 | 18 | - | PVM |
| Author | Size of Pig | Removed Lobes | RLV (%) |
|---|---|---|---|
| Shimoda et al. [33] | - | LL | - |
| Zhang et al. [32] | Mini | LL, LM | - |
| Fonouni et al. [36] | Mini | LL, LM, RM | - |
| Kohler et al. [34] | Mini | LL, LM, RM | 30 |
| Inomata et al. [42] | Mini | LL, LM, RL | 40 |
| Bekheit et al. [37] | Mini | LL, LM, RM | 25 |
| Bucur et al. [48] | Large | LL, LM, RM | 25 |
| Chen et al. [43] | Large | LL, LM, RM, partial RL | 15 |
| Ge et al. [39] | Large | - | - |
| Orue-Echebarria et al. [38] | Mini | LL, LM, RM, RL | 10 |
| Asencio et al. [49] | Mini | LL, LM, RM, RL | 10 |
| Wittauer et al. [35] | Mini, Large | LL, LM | 50 |
| Athanasopoulos et al. [53] | - | LL, LM, RM | 30–20 |
| Sang et al. [51] | Mini | LL, LM, RM, partial RL | 15 |
| Iguchi et al. [50] | Mini | LL, LM, RM | 30 |
| Bruha et al. [56] | - | LL, LM | 60 |
| Xiang et al. [52] | Mini | LL, LM, RM | 20 |
| LL, LM, RM, 1/3RL | 15 | ||
| LL, LM, RM, 2/3RL | 10 | ||
| Nygard et al. [54] | Large | - | 40 |
| Wang et al. [57] | Mini | LL, LM, RM, partial RL | 15–10 |
| Wang et al. [59] | Mini | LL, LM, RM, partial RL | 15–10 |
| Jiao et al. [30] | Mini | Left hepatectomy | - |
| Arkadopoulos et al. [63] | - | LL, LM, RM | 30–25 |
| Hisakura et al. [62] | Mini | LL, LM, RM, partial RL | 20 |
| Shimoda et al. [61] | - | LL, LM | 60 |
| Lim et al. [31] | Large | LL, LM | 50 |
| Nygard et al. [60] | Large | LL, LM, RM | 40 |
| Ge et al. [41] | Mini | Left hepatectomy | - |
| Bartas et al. [44] | Large | Left hepatectomy | - |
| Wiederkehr et al. [46] | - | LL, LM | - |
| Deal et al. [47] | Large | Left hepatectomy | - |
| Croome et al. [55] | - | LL, LM, RM, part of RL | 15–20 |
| Jo et al. [64] | Large | LLL + LML + RML + RLL | 10 |
| Jo et al. [65] | Large | LLL + LML + RML | 30 |
| Jiao et al. [66] | Mini | Left hepatectomy | - |
| Oldhafer et al. [67] | Mini | LLL + LML + RML | 50 |
| Vištejnová et al. [68] | - | LLL | - |
| Author | Lobe Volume % (Average) | |||||||
|---|---|---|---|---|---|---|---|---|
| Left Lateral | Left Medial | Right Medial | Right Lateral | Caudate | ||||
| Sg2 | Sg3 | Sg4 | Sg5 | Sg8 | Sg6 | Sg7 | Sg1 | |
| Xiang et al. [52] | 80 | 13.8–16.5 (14) | 4.9–7.5 (6) | |||||
| Inomata et al. [42] | 47.1–55.4 (51.3) | 20.6–29.3 (25) | 20.5–26.7 (23.4) | - | ||||
| Bucur et al. [48] | 75 | 25 | ||||||
| Orue-Echebarria et al. [38] | 90 | 10 | ||||||
| Asencio et al. [49] | 90 | 10 | ||||||
| Bruha et al. [56] | 40 | 60 | ||||||
| Nygård et al. [54,60] | 60 | 40 | ||||||
| Wang et al. [57,59] | 75–80 (77.5) | 20–25 (22.5) | ||||||
| Arkadopoulos et al. [63] | 70–75 (72.5) | 25–30 (27.5) | ||||||
| Kohler et al. [34] | 70 | 30 | ||||||
| Shimoda et al. [61] | 40 | 60 | ||||||
| Lim et al. [31] | 50 | 50 | ||||||
| Croome et al. [55] | 80–85 (82.5) | 15–20 (17.5) | ||||||
| Bekheit et al. [37] | 75 | 25 | ||||||
| Chen et al. [43] | 85 | 15 | ||||||
| Wittauer et al. [35] | 50 | 50 | ||||||
| Athanasopoulos et al. [53] | 70–80 (75) | 30–20 (25) | ||||||
| Sang et al. [51] | 85 | 15 | ||||||
| Iguchi et al. [50] | 70 | 30 | ||||||
| Hisakura et al. [62] | 80 | 20 | ||||||
| Jo et al. [64] | 90 | 10 | ||||||
| Jo et al. [65] | 70 | 30 | ||||||
| Oldhafer et al. [67] | 50 | 50 | ||||||
| Author | IL1β | IL6 | IL10 | HGF | TNFα | TGFβ | Liver Resection | Additional Treatments |
|---|---|---|---|---|---|---|---|---|
| Nygard et al. [60] | No | - | No | - | No | No | Yes | - |
| Sang et al. [51] | Yes | Yes | - | - | Yes | - | Yes | SCA |
| Inomata et al. [42] | - | Yes | - | Yes | - | - | Yes | RS |
| Chen et al. [43] | - | Yes | - | - | No | Yes | Yes | SRBAL |
| Brige et al. [45] | - | Yes | Yes | Yes | Yes | - | No | PVS |
| Jo et al. [64] | - | No * | - | No * | - | - | Yes | Terlipressin |
| Jo et al. [65] | - | No ** | - | - | - | - | Yes | Terlipressin, Octreotide |
| Jiao et al. [66] | No | Yes | Yes | - | No | No | Yes | IRI, SCA |
| Vištejnová et al. [68] | - | Yes | - | - | No | Yes | Yes | SCA, BDO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinelli, L.; Muttillo, E.M.; Felli, E.; Baiocchini, A.; Giannone, F.; Marescaux, J.; Mutter, D.; De Mathelin, M.; Gioux, S.; Felli, E.; et al. Surgical Models of Liver Regeneration in Pigs: A Practical Review of the Literature for Researchers. Cells 2023, 12, 603. https://doi.org/10.3390/cells12040603
Cinelli L, Muttillo EM, Felli E, Baiocchini A, Giannone F, Marescaux J, Mutter D, De Mathelin M, Gioux S, Felli E, et al. Surgical Models of Liver Regeneration in Pigs: A Practical Review of the Literature for Researchers. Cells. 2023; 12(4):603. https://doi.org/10.3390/cells12040603
Chicago/Turabian StyleCinelli, Lorenzo, Edoardo Maria Muttillo, Emanuele Felli, Andrea Baiocchini, Fabio Giannone, Jacques Marescaux, Didier Mutter, Michel De Mathelin, Sylvain Gioux, Eric Felli, and et al. 2023. "Surgical Models of Liver Regeneration in Pigs: A Practical Review of the Literature for Researchers" Cells 12, no. 4: 603. https://doi.org/10.3390/cells12040603
APA StyleCinelli, L., Muttillo, E. M., Felli, E., Baiocchini, A., Giannone, F., Marescaux, J., Mutter, D., De Mathelin, M., Gioux, S., Felli, E., & Diana, M. (2023). Surgical Models of Liver Regeneration in Pigs: A Practical Review of the Literature for Researchers. Cells, 12(4), 603. https://doi.org/10.3390/cells12040603










